Abstract
The Symbiodiniaceae are a taxonomically and functionally diverse family of marine dinoflagellates. Their symbiotic relationship with invertebrates such as scleractinian corals has made them the focus of decades of research to resolve the underlying biology regulating their sensitivity to stressors, particularly thermal stress. Research to-date suggests that Symbiodiniaceae stress sensitivity is governed by a complex interplay between phylogenetic dependent and independent traits (diversity of characteristics of a species). Consequently, there is a need for datasets that simultaneously broadly resolve molecular and physiological processes under stressed and non-stressed conditions. Therefore, we provide a dataset simultaneously generating transcriptome, metabolome, and proteome data for three ecologically important Symbiodiniaceae isolates under nutrient replete growth conditions and two temperature treatments (ca. 26 °C and 32 °C). Elevated sea surface temperature is primarily responsible for coral bleaching events that occur when the coral-Symbiodiniaceae relationship has been disrupted. Symbiodiniaceae can strongly influence their host’s response to thermal stress and consequently it is necessary to resolve drivers of Symbiodiniaceae heat stress tolerance. We anticipate these datasets to expand our understanding on the key genotypic and functional properties that influence the sensitivities of Symbiodiniaceae to thermal stress.
Subject terms: Marine biology, Cellular microbiology
| Measurement(s) | RNA sequences and counts • Metabolite abundance • Protein abundance |
| Technology Type(s) | Illumina HiSeq. 2500 • QTOF MS system • Orbitrap Fusion Lumos |
| Sample Characteristic - Organism | Symbiodiniaceae |
| Sample Characteristic - Environment | In culture |
Background & Summary
The Symbiodiniaceae are a family of marine dinoflagellates that are genetically and functionally diverse. Recent advancements in molecular analysis have revealed immense, previously undetectable genetic diversity1–4. The family now has ten recognised genera, and five equivalent lineages yet to be formally described1,3–6. Symbiodiniaceae are found across a broad range of environments that span temperate and tropical biomes3. They also have various life-histories that include free-living7–9, diverse substrate attachment8, and a symbiotic state with invertebrates, e.g., scleractinian coral3. The symbiosis between Symbiodiniaceae and coral forms the foundation of coral reefs, aiding their survival and development in tropical, nutrient-poor waters10. In turn, dysbiosis of Symbiodiniaceae and corals under stressful environmental conditions, e.g., warming seawater from climate change, can compromise the survival of reef ecosystems. Loss of Symbiodiniaceae from the coral host compromises the host’s access to essential nutrients11–13 and metabolites14, and results in visual paling, commonly referred to as coral bleaching15. More frequent and intense marine heatwaves in combination with increasing mean sea surface temperatures are resulting in more frequent and severe global coral bleaching events that can result in mass coral mortality16,17. The species (or genotype) of Symbiodiniaceae hosted can significantly influence the coral response to thermal stress18, and so specificity of Symbiodiniaceae-coral host associations often reflects ecological patterns of thermally induced coral bleaching19,20. Consequently, the focus of many global research efforts has been on trying to understand the molecular, ecological, and biogeochemical factors influencing sensitivity of these symbioses.
Isolation and in vitro culturing of Symbiodiniaceae has become a widely adopted tool to further understanding of coral sensitivity to stressors18. In some instances, phylogenetic differences between isolates of Symbiodiniaceae have been documented to explain their stress response21. However, broader-scale studies inter-comparing multiple Symbiodiniaceae taxa are increasingly demonstrating the complex interplay between phylogenetic-dependent and -independent traits (diversity of characteristics of a species22) that govern Symbiodiniaceae stress responses. Functional “types” have thus been recognised as a key operational unit determining the stress response, and ultimate ecological success of Symbiodiniaceae23–25. Even so, the challenge remains identifying the most appropriate suite of both molecular and physiological traits to best define Symbiodiniaceae functional types. Furthermore, to truly understand the organismal response to stress, knowledge of the entire biological system is required, beyond just discrete trait properties26. This is where metabolic network analysis that applies multiple omics methods is beneficial, as it can begin to uncover any cross talk in traits and networks that ultimately govern the Symbiodiniaceae stress response.
To date, measuring photosynthetic functioning, particularly photosystem II (PSII) maximum photochemical efficiency (Fv/Fm), has been broadly applied to assess stress sensitivity of cultured Symbiodiniaceae18. Technological advancements and improved cost-effectiveness of omics analysis have led to more studies integrating omics techniques into their experimental designs to assess the stress response of cultured Symbiodiniaceae27. Studies have considered the transcriptional response of cultured Symbiodiniaceae to varying light regimes28, pH conditions29 and heat stress21,29–33. For example, Levin et al.21 ran a 13-day heat stress (32 °C) experiment on a thermo-tolerant Cladocopium sp. The authors observed no sign of physiological stress (e.g., stable Fv/Fm), but transcriptomics analysis revealed upregulation (by ≥ 4-fold) of reactive oxygen species (ROS) scavenging, and molecular chaperone genes21. Evidence that Symbiodiniaceae employ significant post-transcriptional and post-translational modification for gene regulation34,35 makes other omics techniques, such as proteomics and metabolomics essential. Recent proteomics analysis on heat stressed Breviolum psygmophilum found several hypothesised relevant surface proteins that were differentially expressed36. Integrating omics methods, e.g., transcriptomics and metabolomics14, is significantly advancing our understanding of both the molecular and physiological traits that ultimately govern the Symbiodiniaceae stress response.
In this study, we determined simultaneous changes in the transcriptome, metabolome, and proteome of three Symbiodiniaceae isolates under nutrient replete growth conditions and two temperature treatments (ca. 26 °C and 32 °C). Concurrent measurements of Symbiodiniaceae-specific bacteria were also made and have previously been published in Camp et al.37. While previous studies have considered the transcriptional e.g.21,29–33 and metabolite e.g.14,38,39 profiles of Symbiodiniaceae, the proteome of Symbiodiniaceae is still in its infancy despite its profound ability to resolve functional diversity in other microalgae36. Transcriptomics has furthered knowledge at a cellular level e.g.21,29–33, but it is metabolomics and proteomics techniques that provide knowledge on the outcomes of cellular processes36,38. No known datasets exist that contain uniformly generated and processed transcriptome, metabolome, and proteome datasets, required for a systems biology approach to resolving different functional responses occurring in Symbiodiniaceae across different temperature profiles. The presented datasets will provide a fundamental understanding on the genotypic and functional variance of Symbiodiniaceae isolates to thermal stress.
Methods
Symbiodiniaceae culture conditions
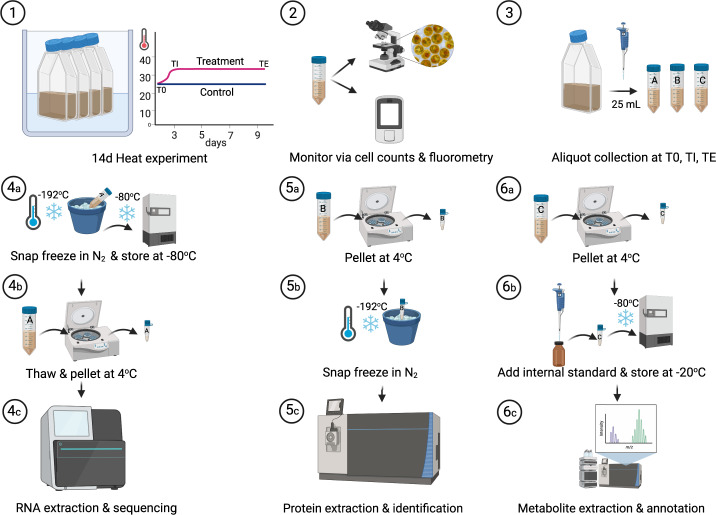
Three Symbiodiniaceae cultures, ITS2 isolate C1 (Cladocopium goreaui, culture identifier: C1-124), ITS2 isolate D1a (Durusdinium trenchii), and ITS2 isolate B1 (Breviolum sp.) were obtained from the long-term laboratory stock at the University of Technology Sydney (UTS) (see Table 1 for further information) grown under 20.0 °C. All isolates had been maintained in culture for over a decade. Isolates were moved to 26.0 °C and were grown for three months prior to the experimentation. Isolates were cultured in Daigo IMK medium (Nihon Pharmaceutical Co. Ltd.), kept in 750 mL culture flasks grown under a white light of 207 ± 0.05 µmol photons m−2 s−1 on a 12:12 h light:dark cycle. Light was provided by three Hydra 52 HD LED units (Aqua Illumination, Ames). Cultures were grown for ten days under two temperature conditions (mean ± SE), 26.0 ± 0.5 °C (Control) and 32.4 ± 0.01 °C (Treatment). Temperature was ramped 2 °C per day for three consecutive days to achieve 32.4 °C. Temperatures were achieved by water baths controlled with Julabo heaters (JULABO GmbH). All cultures were maintained in exponential growth for subsequent sample analysis, and culture cell densities were maintained via regular dilutions at ca. 150,000 cells mL−1 to prevent carbon or nutrient limitation39. Health of the isolates was monitored via cell counts and fluorometry (maximum quantum yield of PSII (Fv/Fm)), with both cell counts and Fv/Fm declining in the heat treatment37 (Table 2). Samples were collected at three points in time, prior to temperature ramping (day 0, T0), after temperature ramping (day 3, TI), and then at the end of seven days (day 10, TE, Fig. 1). All cultures had four biological replicates across each temperature condition (Table 3), however not all samples were able to be processed for their respective analyses due to cost, time, and availability of biological material; final numbers are presented in Table 4. During sample processing, culture flasks were systematically removed from the water baths to minimise time out of the experimental conditions. One replicate flask of each isolate for control and treatment was taken at a time, creating four sampling blocks of six culture flasks, each sampled an hour apart. For each of the four sampling blocks, the order in which a culture was sampled first was rotated (see Table 3 and Camp et al.37).
Table 1.
Isolate information.
| Genus | Species | ITS2 Major type profile | Culture isolate identity | Internal isolate label | Geographic origin | Host isolate |
|---|---|---|---|---|---|---|
| Breviolum | sp. | B1 | B (UTS) | B1-UTS-B | South Taiwan (Indo-Pacific) | Euphyllia glabrescens (coral) |
| Cladocopium | goreaui | C1 | SCF055-06 | C1-124/SCF124 | Magnetic Island (Pacific) | Acropora tenuis (coral) |
| Durusdinium | trenchii | D1a | amur‐D‐MI, UTS-D, (UTS_D) | SCF082 | Magnetic Island (Pacific) | Acropora muricata (coral) |
Information on the Symbiodiniaceae origin (host isolate or free-living and geographic location) and ITS2 major type profile. Culture isolate identification is also provided as found in the literature and as labelled internally at the University of Technology of Sydney (UTS).
Table 2.
Cell counts and maximum quantum yield of PSII (Fv/Fm).
| Isolate | Time Point | Treatment | Cell mL−1 | Fv/Fm | ||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| D1a | T0 | Control | 164134 | 8436 | 0.55 | 0.01 |
| TE | 126282 | 12680 | 0.43 | 0.01 | ||
| T0 | Treatment | 173596 | 17762 | 0.53 | 0.00 | |
| TE | 78151 | 7263 | 0.36 | 0.00 | ||
| B1 | T0 | Control | 156750 | 8413 | 0.53 | 0.00 |
| TE | 155405 | 19992 | 0.51 | 0.01 | ||
| T0 | Treatment | 131500 | 19506 | 0.53 | 0.01 | |
| TE | 72441 | 4191 | 0.10 | 0.03 | ||
| C1-124 | T0 | Control | 138675 | 8405 | 0.59 | 0.02 |
| TE | 121877 | 18468 | 0.50 | 0.00 | ||
| T0 | Treatment | 122000 | 19877 | 0.58 | 0.02 | |
| TE | 49925 | 3544 | 0.33 | 0.02 | ||
Data is expressed as means ± SE (n = 4) for each Symbiodiniaceae isolate (Breviolum sp. (B1), Cladocopium goreaui (C1-124), and Durusdinium trenchii (D1a)) at day 0, (T0), and at the end of the experiment (day 10, TE).
Fig. 1.
Experimental scheme. Three Symbiodiniaceae isolates (C1, Cladocopium goreaui, (identifier: C1-124), D1a, Durusdinium trenchii, B1, Breviolum sp.) were grown in replicate (n = 4) at 26 °C and 32 °C (1). Culture health was regularly monitored via cell counts and fluorometry (see Table 2) (2). At three time points, 25 mL × 3, per culture was removed for subsequent analysis (3). For RNA extraction and sequencing, the culture aliquot was immediately snap-frozen and stored at −80 °C (4a), prior to thawing and pelleting (4b), and RNA extraction (4c). For protein extraction, culture aliquots were pelleted at 4 °C (5a), before snap-freezing in liquid nitrogen (5b) ahead of subsequent extraction protocols (5c). For metabolite extraction, culture aliquots were pelleted at 4 °C (6a). Pellets were then re-suspended with an internal standard (analytical grade 0.005 mM 2-Aminoanthracene) and stored at −80 °C (6b) prior to metabolite extraction protocols (see methods text) (6c).
Table 3.
Sample identification and processing order.
| Sub-sample order | Sample ID | Experimental condition | Isolate |
|---|---|---|---|
| Round-1 | 1 | Control | D1a |
| 17 | Treatment | D1a | |
| 9 | Control | B1 | |
| 25 | Treatment | B1 | |
| 13 | Control | C1-124 | |
| 29 | Treatment | C1-124 | |
| Round-2 | 10 | Control | B1 |
| 26 | Treatment | B1 | |
| 14 | Control | C1-124 | |
| 30 | Treatment | C1-124 | |
| 2 | Control | D1a | |
| 18 | Treatment | D1a | |
| Round-3 | 11 | Control | B1 |
| 27 | Treatment | B1 | |
| 15 | Control | C1-124 | |
| 31 | Treatment | C1-124 | |
| 3 | Control | D1a | |
| 19 | Treatment | D1a | |
| Round-4 | 16 | Control | C1-124 |
| 32 | Treatment | C1-124 | |
| 4 | Control | D1a | |
| 20 | Treatment | D1a | |
| 12 | Control | B1 | |
| 28 | Treatment | B1 |
A systematic design was used to process samples in four rounds to ensure one replicate of each Symbiodiniaceae isolate and experimental condition. This design was undertaken to account for processing time. The sample ID is used throughout as an identifier, along with the experimental condition (control maintained at ca. 26 °C and treatment maintained at ca. 32 °C) and time points (day 0, (T0), after temperature ramping (day 3, TI), and then at the end of seven days (day 10, TE) (see methods and Table 3).
Table 4.
Information on the data reposited for each method within the experimental set-up.
| Isolate | Time-point | Treatment or Control | Transcriptomics | Metabolomics | Proteomics |
|---|---|---|---|---|---|
| Cladocopium goreaui | T0 | Control | n = 4* | n = 4 | n = 3 |
| Durusdinium trenchii | n = 4* | n = 4 | n = 2 | ||
| Breviolum sp. | n = 4* | n = 4 | n = 3 | ||
| Cladocopium goreaui | Treatment | n = 4* | n = 4 | n = 1 | |
| Durusdinium trenchii | n = 4* | n = 4 | n = 1 | ||
| Breviolum sp. | n = 4* | n = 4 | n = 1 | ||
| Cladocopium goreaui | TI | Control | n = 4 | n = 4 | n = 1 |
| Durusdinium trenchii | n = 4 | n = 4 | n = 2 | ||
| Breviolum sp. | n = 4 | n = 4 | n = 1 | ||
| Cladocopium goreaui | Treatment | n = 4 | n = 4 | n = 4 | |
| Durusdinium trenchii | n = 4 | n = 4 | n = 4 | ||
| Breviolum sp. | n = 4 | n = 4 | n = 4 | ||
| Cladocopium goreaui | TE | Control | n = 4 | n = 4 | n = 1 |
| Durusdinium trenchii | n = 4 | n = 4 | n = 1 | ||
| Breviolum sp. | n = 4 | n = 4 | n = 0 | ||
| Cladocopium goreaui | Treatment | n = 4 | n = 4 | n = 4 | |
| Durusdinium trenchii | n = 4 | n = 4 | n = 4 | ||
| Breviolum sp. | n = 4 | n = 4 | n = 4 |
*Indicates that the raw sequence files were uploaded but they are not included in the paper analyses. Samples were collected at three points in time, prior to temperature ramping (day 0, T0), after temperature ramping (day 3, TI), and then at the end of seven days (day 10, TE).
RNA extraction and sequencing
For each replicate, a 25 mL aliquot of culture was removed in a sterile culturing facility at UTS and immediately snap frozen in liquid nitrogen. Preliminary testing by microscopy analysis confirmed no visual signs of cell lysis. After collection, the frozen cells were thawed at room temperature in a water bath and pelleted through centrifugation for 5 min at 3,000 g and at 4°C. The supernatant was discarded, and the remaining pellet re-suspended using 450 µL of RLT buffer solution (RNeasy Plant Mini Kit, Qiagen, Hilden, Germany) and 4.5 µL of β-mercaptoethanol (Sigma-Aldrich, Australia). The pelleted cells were lysed by bead beating with 0.2 g of 0.5 mm sterile acid-washed glass beads (Biospec, OK, USA) in the TissueLyser II (Qiagen, Australia) at 50 Hz for 2 min. The lysate was then used for RNA extraction and purification using the Rneasy mini plant kit (Qiagen, Australia). A total volume of 5 µL of extracted sample RNA was eluted into Nuclease-free water (Sigma-Aldrich, Australia) and subsequently stirred at −80 °C before sequencing.
RNA quality and yield (150–500 ng) were checked using a Bioanalyser Agilent 2100 (Agilent Technologies, CA, USA). RNA was polyA-enriched and sequencing libraries were prepared using Illumina’s TruSeq stranded library preparation kit and sequenced on an HiSeq2500 Sequencer (Illumina, CA, USA) resulting in a total of 793,531,730 paired-end sequencing reads (Supplementary Tables 1–3). Sequencing was performed at the Australian Genome Research Facility, Melbourne, Australia.
RNASeq data processing and bioinformatic analysis
All samples were processed as described in21. In summary, raw fastq files of all samples (NCBI SRA bioproject PRJNA723630) were processed with Trim Galore! (v0.6.0; Babraham Bioinformatics) with default settings to remove low quality sequences as well as sequencing adapters. Trimmed fastq files were combined into a single fastq for each Symbiodiniaceae isolate and assembled into transcripts using Trinity (v2.8.4) in de novo mode with default parameters40. Gene expression quantification for each sample was performed using kallisto v0.43.141 in conjunction with the Trinity script align_and_estimate_abundance.pl. Counts for each gene were calculated by combining the read counts for each gene isoform. Open Reading Frames (ORFs) were predicted in the assembled transcripts using Transdecoder v5.5.0 (https://github.com/Transdecoder)40 with default settings. Predicted protein sequences were filtered by length and proteins <100 amino acids were removed prior to functional annotation. The remaining protein sequences were annotated using InterproScan v5.2742. Differential expression analysis was performed in R using voom43 and limma44 on gene read counts. Prior to normalisation, genes were filtered by read count. We analysed control and treatment samples of each time point together. For each time point, genes with less than ten reads in at least four samples were removed from the analysis. Reads were normalised using voom, with normalisation factors being calculated with calcNormFactors(). For each comparison (Treatment TE vs Control TE, and Treatment TI vs Control TI), linear models were fitted using lmFit(), contrasts for each gene estimated using contrasts.fit(), and empirical Bayes smoothing of standard errors performed using eBayes().
Protein extraction, identification, and quantification
For each replicate, a 25 mL aliquot of culture was removed in a sterile culturing facility at UTS. Cells were pelleted through centrifugation for 5 min at 3,000 g and at 4 °C. The medium was discarded, and the pellet stored at −80 °C. For each species, the control consisted of all samples from the initial timepoint (T0) as well as the 26 °C samples from later timepoints (TI and TE). Proteins were extracted, digested, and purified using a modified filter-aided sample preparation protocol45, as follows. Salt was removed by centrifugation and resuspension of the cells in liquid chromatography-grade water twice, followed by transfer to low protein binding tubes (Eppendorf LoBind, Eppendorf SE) and freezing at −80 °C until further processing. The algal pellet was then resuspended in 500 μL 5% w/v sodium deoxycholate and the cells lysed by 20 × 2 s pulses with an ultrasonicator probe. Proteins were reduced with 1% final concentration β-mercaptoethanol and denatured by incubation at 85 °C for 30 min. Photosynthetic pigments were partially removed by twice adding two volumes of ethyl acetate, vortexing for 1 min, and centrifugation at 10,000 g × 1 min, discarding the upper organic fraction each time. Residual ethyl acetate was removed by centrifugation under vacuum for 15 min. The supernatant was then transferred to a centrifugation filter (Amicon Ultra 30 kDa 0.5 mL, Merck Millepore) and concentrated by centrifugation (14,000 g × 20 min). Proteins were then resuspended and washed twice by the addition of 380 μL 50 mM Tris buffer, pH 8.1, followed by centrifugation (14,000 g × 15 min). After a third and final addition of 380 μL 50 mM Tris buffer, a 10 μL subsample was transferred to a new tube, diluted with 90 μL water (Optima LC/MS Grade, ThermoFisher), acidified with 1 μL 100% formic acid to precipitate deoxycholate, centrifuged (16,000 g × 5 min) and the protein quantified by protein-binding dye fluorescence (Qubit 2.0, ThermoFisher Scientific, USA). A total of 100 μg total protein were reduced by incubation with 10 mM β-mercaptoethanol at 37 °C for 10 min, alkylated by incubation for 20 min at room temperature with 20 mM acrylamide, the alkylation quenched by a second equal addition of β-mercaptoethanol, and digested with 2 μg trypsin for 18 h in the filter unit. Digested peptides were collected by centrifugation (14,000 g × 20 min) through the filter and any remaining deoxycholate removed by the addition of formic acid to 1% final concentration, followed by centrifugation (16,000 g × 1 min). The supernatant, containing digested peptides, was transferred to a new tube, concentrated by vacuum centrifugation to approximately 200 μL and desalted using C18-packed pipette tips (Omix Bond Elut, Agilent Technologies). Peptides were dried by centrifugation under vacuum and stored at 4 °C until analysis, upon which they were dissolved in 70 μL 0.1% formic acid and the peptide content quantified by fluorescence as before. Unless otherwise noted, all reagents were obtained from Sigma-Aldrich New Zealand, and all samples were handled in low protein binding tubes (Eppendorf).
Peptide samples were separated by a 75 min linear gradient from 5%/95% to 35%/65% buffer A/B (buffer A: 0.1% formic acid; buffer B: 80% acetonitrile, 0.1% formic acid) at 300 nL min−1 on an Acclaim PepMap C18, 3 μm, 100 Å column (Thermo Scientific, Auckland, New Zealand) and Ultimate 3000 liquid chromatograph system (Dionex, Sunnyvale, CA). Peptides were ionised by electrospray at 1.8 kV and analysed by an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Scientific). Precursor mass spectra were acquired in the Orbitrap at a resolution of 120,000, rejecting singly-charged ions, with quadrupole isolation enabled and an automatic gain target of 7.0e5 and a maximum injection time of 50 ms. The 20 most intense precursor spectra were fragmented by higher-energy collision dissociation and analysed in the ion trap with an automatic gain target of 5.0e3 and a maximum injection time of 50 ms. Dynamic exclusion was enabled with a duration of 60 s. Samples were each analysed 1–3 times (depending on available biomass) as technical replicates in a stratified random order.
Proteins were identified from raw spectrum files using the Andromeda search algorithm in MaxQuant 1.6.10.4346,47. Protein search databases were generated for each Symbiodiniaceae species from transcripts obtained from samples from the same experiment. Trypsin was the selected digest enzyme with a maximum of two missed cleavages allowed. Oxidation of methionine and acetylation of the protein n-terminus were considered as variable modifications and carbamidomethylation of cysteine considered as a fixed modification. The first search and main search peptide tolerances were 20 ppm and 4.5 ppm, respectively, and a mass tolerance of 0.5 Da for the ion trap MS2 search parameter. Label-free quantification was enabled with a minimum of two unique plus razor peptides for quantification and match between runs enabled. Peptide spectrum match and protein false discovery rates were both set to 1%, with a minimum of two peptides required for annotation. Three MaxQuant analyses, one for each species, were conducted separately using the same settings and the search database specific to each species.
Metabolomic extraction, annotation, and quantification
For each replicate, a 25 mL aliquot of culture was removed in a sterile culturing facility at UTS. Cells were pelleted through centrifugation (5 min at 3,000 g and at 4 °C). To each sample, 150 µL of 100% cold MeOH (spiked with the internal standard 0.005 mM 2-aminoanthracene (technical grade, Sigma Aldrich, Australia) were added and the pellet re-suspended in a scintillation vial. Vials were covered in tin foil to prevent light degradation and placed for 24 h in −80 °C storage. Samples were then homogenised using a TissueLyser LT (Qiagen Inc., Hilden, Germany) with 20 mg acid washed glass beads (425–600 µm; Sigma Aldrich, Australia). Visual counts (via a haemocytometer) of trial samples confirmed effective cellular disruption of over 90% of cells48. Samples were pelleted (5 min at 3,000 g and at 4 °C) and the supernatant collected and placed on ice. The remaining pellet was resuspended with 100 µL of 100% MeOH (again spiked with the internal standard) and again pelleted (5 min at 3,000 g and at 4 °C). The subsequent supernatant was combined with the supernatant from the first round and stored in a tin foil covered scintillation vial at −80 °C for 7 days prior to analysis.
Untargeted LC-MS profiling analysis of samples was carried out by Metabolomics Australia (School of BioSciences, University of Melbourne). Instrument and LC-MS setup were as follows: Agilent 6520 QTOF MS system (Agilent Technologies, Santa Clara, CA, USA) with a dual sprayer ESI source and attached to Agilent 1200 series HPLC system comprised of a vacuum degasser, binary pump, thermostated auto-sampler and column oven. The MS was operated in positive mode using the following conditions: nebulizer pressure 30 psi, gas flow-rate 10 L min−1, gas temperature 300 °C, capillary voltage 4000 V, fragmentor 150 and skimmer 65 V. Instrument was operated in the extended dynamic range mode with data collected in m/z range 70–1700. Chromatography was carried out using an Agilent Zorbax Eclipse XDB-C18, 2.1 × 100 mm, 1.8 µm column maintained at 40 °C ( ± 1 °C) at a flow rate of 400 µL min−1 with a 20 min run time. A gradient LC method was used with mobile phases comprised of (A) 0.1% formic acid in deionized water and (B) 0.1% formic acid in acetonitrile: 5 min linear gradient from 5% to 30% mobile phase B, followed by a 5 min gradient to 100% mobile phase B and then a 5 min hold, followed by a 5 min re-equilibration at 5% mobile phase B. Molecular feature extraction (MFE) was conducted in R using Bioconductor49.
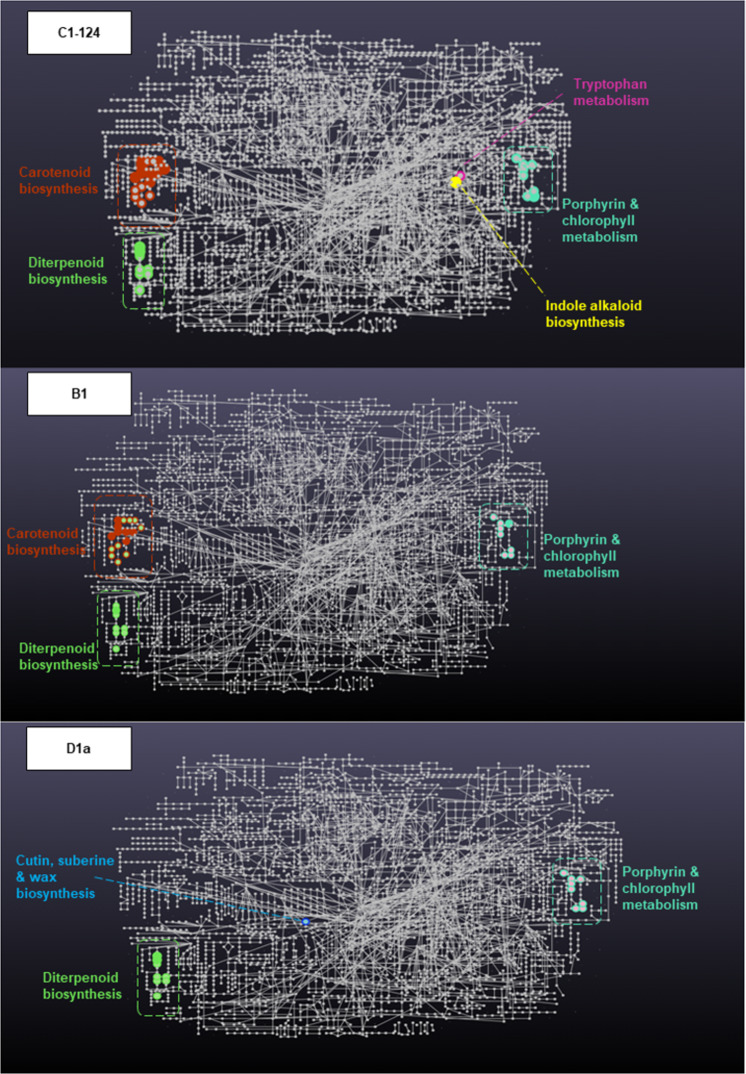
Confident annotation of metabolites from reverse phase untargeted LC-MS profiling is a significant challenge for non-model organisms50. We thus eschewed a direct annotation, and utilised the mummichog-style analysis in Metaboanalyst 5.051 to provide a glimpse of pathways amenable to reverse phase metabolomic profiling. In contrast to direct annotation, such functional analysis leverages the organization of metabolic networks to predict functional activity directly from feature tables, bypassing metabolite identification. Thus, high-quality hypotheses can be quickly generated from an LC-MS peak table52. Functional analysis using mummichog and a top-10% peak cut-off was undertaken in Metaboanalyst51. Data were median-normalised and log-transformed. For each isolate, TE control (n = 4) versus treatment (n = 4) were analysed. Results were visualised in a KEGG format pathway analysis that were searched against Arabidopsis thaliana (the best photosynthetic organism match based on available options and high pathway coverage). The coloured pathways indicated significant differences between the two treatments (Fig. 2).
Fig. 2.
Metabolite pathway analysis at the end time-point (TE) for the three Symbiodiniaceae isolates (C1, Cladocopium goreaui, (identifier: C1-124), D1a, Durusdinium trenchii, B1, Breviolum sp.). Functional analysis using mummichog and a top-10% peak cut-off was undertaken in Metaboanalyst51. Data were median-normalised and log-transformed. For each isolate, TE control (n = 4 maintained at ca. 26 °C) versus treatment (n = 4 maintained at ca. 32 °C) were analysed. Results are visualised in a KEGG format pathway analysis that were searched against Arabidopsis thaliana (the best photosynthetic organism match based on available options and high pathway coverage). The coloured pathways indicated significant differences between the two treatments.
Data Records
All data are reposited with an identification format that includes the sample ID (indicated in Table 3), and experimental time point of collection prior to temperature ramping (day 0, T0), after temperature ramping (day 3, TI), and then at the end of seven days (day 10, TE). Table 3 denotes whether a sample is a control (C) or treatment (T).
Mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository53 with the dataset identifiers PXD025080 (Breviolum sp.)54, PXD025051 (C. goreaui)55 and PXD025050 (D. trenchii)56. For proteomic data, the PRIDE data repository also has dates indicated, e.g. “18_11–19” that represent the dates (Day-Month_Year) that the peptide sample were analysed by the mass spectrometer.
Raw RNASeq data has been deposited into the Sequence Read Archive (SRA) at the National Center for Biotechnology Information under bioproject PRJNA72363057.
Furthermore, differential gene expression results, normalised read counts, functional annotation of assembled RNA transcripts and raw metabolomics data sets of all time points have been deposited into the Open Science Forum project “Metabolomics data and differential gene expression results or three coral symbionts (Cladocopium goreaui, Durusdinium trenchii, and Breviolum sp.) under elevated temperature stress.58”.
Technical Validation
Re-verification of the Symbiodiniaceae culture genotypes was conducted on the stock aliquots prior to sub-culturing. A 5 mL aliquot was removed and pelleted through centrifugation for 5 min at 3,000 g. The supernatant was discarded, and the pellet went through two wash steps with PBS and repeat centrifugation. The excess PBS was removed, and the remaining pellet resuspended in 410 µL of Bead Solution (Qiagen, Australia) and 40 µL of Phenolic separation solution (Qiagen, Australia). Cells were subsequently lysed by bead beating with 0.2 g of 0.5 mm sterile acid-washed glass beads (Biospec, OK, USA) in the TissueLyser II (Qiagen, Australia) at 50 Hz for 2 min. The lysate was then used for DNA extraction and purification using the PowerPlant kit (Qiagen, Australia). Polymerase chain reaction (PCR) amplification of the ITS2 region was performed using the primers ITSintfor2 and ITS2-reverse following the PCR conditions of Arif et al.59. Purified PCR products were sequenced by the Australian Genome Research Facility.
The transcript-based protein search databases were analysed with BUSCO (v5.2.2), see Table 5. The complete BUSCO values range from 77.3–80.8% (Table 5), which are comparable to other datasets in non-model organisms (70–92% in 49 taxa across multiple invertebrate animal phyla60). From this analysis and the high number of identified proteins, we conclude that these search databases are appropriate and of high quality.
Table 5.
The transcript-based protein search databases results analysed with BUSCO (v5.2.2).
| Breviolum sp. | Cladocopium goreaui | Durusdinium trenchii | ||||
|---|---|---|---|---|---|---|
| Complete BUSCOs | 200 | 78.4% | 197 | 77.3% | 206 | 80.8% |
| Complete and single-copy BUSCOs | 160 | 62.7% | 169 | 66.3% | 152 | 59.6% |
| Complete and duplicated BUSCOs | 40 | 15.7% | 28 | 11.0% | 54 | 21.2% |
| Fragmented BUSCOs | 13 | 5.1% | 14 | 5.5% | 13 | 5.1% |
| Missing BUSCOs | 42 | 16.5% | 44 | 17.2% | 36 | 14.1% |
| Total searched | 255 | 255 | 255 | |||
The complete BUSCO values range from 77.3–80.8%, which are comparable to other datasets in non-model organisms (70–92% in 49 taxa across multiple invertebrate animal phyla59). From this analysis and the high number of identified proteins we conclude that these search databases are appropriate and of high quality.
Supplementary information
Acknowledgements
The authors wish to thank Dr Nerissa Fisher, Dr David Hughes, Dr Lisa Fujise, Dr Matthew Nitschke, Dr Samantha Goyen, Dr Lifeng Peng, and Dr Mickael Ros for their assistance during the data collection. Thanks is given to the Technical Service staff at the University of Technology Sydney for their logistical support, particularly, Mr Paul Brooks. Thanks to Ms. Imogen Ashley for BUSCO analysis.
Author contributions
W.L. and D.J.S. secured funding for the project. E.F.C., D.J.S., S.K.D. and W.L. designed the data collection. E.F.C. and D.J.S. collected the data. E.F.C. conducted the RNA extractions, while T.K. and B.S. undertook the RNA sequencing bioinformatics. C.A.O. conducted the protein extraction, proteomics mass spectrometry and protein annotation. A.L. and E.F.C. conducted the metabolite extraction and annotation. E.F.C., T.K. and C.A.O. led the manuscript preparation, with all authors contributing to the final version of the manuscript. All experimentation was supported by an ARC Discovery Grant (DP160100271 to D.J.S., W.L. and T.K.). The contribution of E.F.C. to manuscript writing and final preparation was through the University of Technology Sydney Chancellor’s Postdoctoral Research Fellowship and ARC Discovery Early Career Research Award (DE190100142). S.K.D. and C.A.O. were supported by the Marsden Fund of the Royal Society Te Apārangi (#1202).
Code availability
The version and parameter of all bioinformatics tools used in this work are described in the Methods section.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-022-01258-w.
References
- 1.Nitschke MR, et al. Description of Freudenthalidium gen. nov. and Halluxium gen. nov. to Formally Recognize Clades Fr3 and H as Genera in the Family Symbiodiniaceae (Dinophyceae) J. Phycol. 2020;56:923–940. doi: 10.1111/jpy.12999. [DOI] [PubMed] [Google Scholar]
- 2.LaJeunesse, T. C. & Nitschke, M. R. In Memoriam: Hugo David Freudenthal. J. Eukaryot. Microbiol. e12848 (2021).
- 3.LaJeunesse TC, et al. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018;28:2570–2580.e6. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Pochon, X., & LaJeunesse, T. C. Miliolidium n. gen, a New Symbiodiniacean Genus Whose Members Associate with Soritid Foraminifera or are Free‐living. J. Eukaryotic Microbio. e12856 (2021). [DOI] [PubMed]
- 5.LaJeunesse, T. C. et al. Revival of Philozoon Geddes for host-specialized dinoflagellates,‘zooxanthellae’, in animals from coastal temperate zones of northern and southern hemispheres.” European J. Phycol. 1–15 (2021).
- 6.González-Pech RA, et al. Comparison of 15 dinoflagellate genomes reveals extensive sequence and structural divergence in family Symbiodiniaceae and genus Symbiodinium. BMC Biol. 2021;19:73. doi: 10.1186/s12915-021-00994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunning R, Yost DM, Guarinello ML, Putnam HM, Gates RD. Variability of Symbiodinium Communities in Waters, Sediments, and Corals of Thermally Distinct Reef Pools in American Samoa. PLoS One. 2015;10:e0145099. doi: 10.1371/journal.pone.0145099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takabayashi M, Adams LM, Pochon X, Gates RD. Genetic diversity of free-living Symbiodinium in surface water and sediment of Hawai’i and Florida. Coral Reefs. 2012;31:157–167. [Google Scholar]
- 9.Fujise L, et al. Unlocking the phylogenetic diversity, primary habitats, and abundances of free‐living Symbiodiniaceae on a coral reef. Molecular Ecol. 2021;30(1):343–360. doi: 10.1111/mec.15719. [DOI] [PubMed] [Google Scholar]
- 10.Muscatine L, Porter JW. Reef Corals: Mutualistic Symbioses Adapted to Nutrient-Poor Environments. Bioscience. 1977;27:454–460. [Google Scholar]
- 11.Falkowski PG, Dubinsky Z, Muscatine L, Porter JW. Light and the Bioenergetics of a Symbiotic Coral. Bioscience. 1984;34:705–709. [Google Scholar]
- 12.Ros M, et al. Unlocking the black‐box of inorganic carbon‐uptake and utilization strategies among coral endosymbionts (Symbiodiniaceae) Limnol. and Oceanography. 2020;65(8):1747–1763. [Google Scholar]
- 13.Rädecker, N. et al. Heat stress destabilizes symbiotic nutrient cycling in corals. Proc. Natl. Acad. Sci. USA118(5) (2021). [DOI] [PMC free article] [PubMed]
- 14.Matthews, et al. Optimal nutrient exchange and immune responses operate in partner specificity in the cnidarian-dinoflagellate symbiosis. Proc. Natl. Acad. Sci. USA. 2017;114(50):13194–13199. doi: 10.1073/pnas.1710733114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suggett DJ, Smith DJ. Interpreting the sign of coral bleaching as friend vs. foe. Glob. Chang. Biol. 2011;17(1):45–55. [Google Scholar]
- 16.Oliver ECJ, et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018;9:1324. doi: 10.1038/s41467-018-03732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frieler K, et al. Limiting global warming to 2 °C is unlikely to save most coral reefs. Nat. Clim. Chang. 2013;3:165–170. [Google Scholar]
- 18.Suggett DJ, Warner ME, Leggat W. Symbiotic Dinoflagellate Functional Diversity Mediates Coral Survival under Ecological Crisis. Trends Ecol. Evol. 2017;32:735–745. doi: 10.1016/j.tree.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O. Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl. Acad. Sci. USA. 2008;105:10444–10449. doi: 10.1073/pnas.0708049105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howells EJ, et al. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Chang. 2011;2:116–120. [Google Scholar]
- 21.Levin RA, et al. Sex, Scavengers, and Chaperones: Transcriptome Secrets of Divergent Symbiodinium Thermal Tolerances. Mol. Biol. Evol. 2016;33:3032. doi: 10.1093/molbev/msw201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacArthur, R. H. Geographical Ecology: Patterns in the Distribution of Species. Princeton University Press (1984).
- 23.Suggett DJ, et al. Functional diversity of photobiological traits within the genus Symbiodinium appears to be governed by the interaction of cell size with cladal designation. New Phytol. 2015;208:370–381. doi: 10.1111/nph.13483. [DOI] [PubMed] [Google Scholar]
- 24.Parkinson JE, Baums IB. The extended phenotypes of marine symbioses: ecological and evolutionary consequences of intraspecific genetic diversity in coral–algal associations. Front. Microbiol. 2014;5:445. doi: 10.3389/fmicb.2014.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker DM, Andras JP, Jordán-Garza AG, Fogel ML. Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J. 2013;7:1248–1251. doi: 10.1038/ismej.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suggett DJ, Smith DJ. Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob. Chang. Biol. 2020;26:68–79. doi: 10.1111/gcb.14871. [DOI] [PubMed] [Google Scholar]
- 27.Meyer E, Weis VM. Study of Cnidarian-Algal Symbiosis in the ‘Omics’ Age. Biol. Bull. 2012;223:44–65. doi: 10.1086/BBLv223n1p44. [DOI] [PubMed] [Google Scholar]
- 28.Xiang T, Nelson W, Rodriguez J, Tolleter D, Grossman AR. Symbiodinium transcriptome and global responses of cells to immediate changes in light intensity when grown under autotrophic or mixotrophic conditions. Plant J. 2015;82:67–80. doi: 10.1111/tpj.12789. [DOI] [PubMed] [Google Scholar]
- 29.Gong S, Jin X, Xiao Y, Li Z. Ocean Acidification and Warming Lead to Increased Growth and Altered Chloroplast Morphology in the Thermo-Tolerant Alga Symbiochlorum hainanensis. Front. Plant Sci. 2020;11:585202. doi: 10.3389/fpls.2020.585202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gierz SL, Forêt S, Leggat W. Transcriptomic analysis of thermally stressed Symbiodinium reveals differential expression of stress and metabolism genes. Front. Plant Sci. 2017;8:271. doi: 10.3389/fpls.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyen S, et al. A molecular physiology basis for functional diversity of hydrogen peroxide production amongst Symbiodinium spp. (Dinophyceae) Mar. Biol. 2017;164:46. [Google Scholar]
- 32.Bellantuono AJ, Dougan KE, Granados-Cifuentes C, Rodriguez-Lanetty M. Free-living and symbiotic lifestyles of a thermotolerant coral endosymbiont display profoundly distinct transcriptomes under both stable and heat stress conditions. Mol. Ecol. 2019;28:5265–5281. doi: 10.1111/mec.15300. [DOI] [PubMed] [Google Scholar]
- 33.Chakravarti LJ, Buerger P, Levin RA, van Oppen MJH. Gene regulation underpinning increased thermal tolerance in a laboratory-evolved coral photosymbiont. Mol. Ecol. 2020;29:1684–1703. doi: 10.1111/mec.15432. [DOI] [PubMed] [Google Scholar]
- 34.Roy, S., Jagus, R. & Morse, D. Translation and Translational Control in Dinoflagellates. Microorganisms6 (2018). [DOI] [PMC free article] [PubMed]
- 35.Liew YJ, Li Y, Baumgarten S, Voolstra CR, Aranda M. Condition-specific RNA editing in the coral symbiont Symbiodinium microadriaticum. PLoS Genet. 2017;13:e1006619. doi: 10.1371/journal.pgen.1006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci CA, et al. The cell-surface protein composition of a coral symbiont, Breviolum psygmophilum, reveals a mechanism for host specificity and displays dynamic regulation during temperature stress. Mar. Biol. 2020;167:66. [Google Scholar]
- 37.Camp EF, et al. Revealing changes in the microbiome of Symbiodiniaceae under thermal stress. Environ. Microbiol. 2020;22:1294–1309. doi: 10.1111/1462-2920.14935. [DOI] [PubMed] [Google Scholar]
- 38.Tchernov D, et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA. 2004;101:13531–13535. doi: 10.1073/pnas.0402907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klueter A, Crandall JB, Archer FI, Teece MA, Coffroth MA. Taxonomic and environmental variation of metabolite profiles in marine dinoflagellates of the genus. symbiodinium. Metabolites. 2015;5:74–99. doi: 10.3390/metabo5010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas BJ, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 42.Jones P, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Law CW, Chen Y, Shi W, Smyth G. K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47–e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 46.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 47.Cox J, et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillyer KE, et al. Metabolite profiling of symbiont and host during thermal stress and bleaching in the coral Acropora aspera. Coral Reefs. 2017;36:105–118. [Google Scholar]
- 49.Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching and identification. Analytical Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 50.Blaženović I, Kind T, Ji J, Fiehn O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites. 2018;8(2):31. doi: 10.3390/metabo8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucl. Acids Res. 2009;37:W652–660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S, et al. Predicting network activity from high throughput metabolomics. PLoS Comp. Biol. 2013;9(7):e1003123. doi: 10.1371/journal.pcbi.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Riverol Y, et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oakley C, Davy S. 2021. Proteome of the coral symbiont Breviolum sp. under elevated temperature stress. PXD025080
- 55.Oakley C, Davy S. 2021. Proteome of the coral symbiont Cladocopium goreaui under elevated temperature stress. PXD025051
- 56.Oakley C, Davy S. 2021. Proteome of the coral symbiont Durusdinium trenchii under elevated temperature stress. PXD025050
- 57.Kahlke T, Camp EF. 2021. Heat Stress response of three Symbiodiniaceae genera NCBI. SRP315798
- 58.Kahlke T, Camp EF. 2021. Metabolomics data and Differential Gene Expression results for three coral symbionts (Cladocopium goreaui, Durusdinium trenchii, and Breviolum sp.) under elevated temperature stress. Open Science Framework. [DOI]
- 59.Arif C, et al. Assessing Symbiodinium diversity in scleractinian corals via pyrosequencing-based genotyping of the ITS2 rDNA region. Mol Ecol. 2014;23(17):4418–4433. doi: 10.1111/mec.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rivera-Vicens RE, et al. TransPi-a comprehensive Transcriptome Analysis Pipeline for de novo transcriptome assembly. Mol Ecol Resources. 2022 doi: 10.1111/1755-0998.13593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Oakley C, Davy S. 2021. Proteome of the coral symbiont Breviolum sp. under elevated temperature stress. PXD025080
- Oakley C, Davy S. 2021. Proteome of the coral symbiont Cladocopium goreaui under elevated temperature stress. PXD025051
- Oakley C, Davy S. 2021. Proteome of the coral symbiont Durusdinium trenchii under elevated temperature stress. PXD025050
- Kahlke T, Camp EF. 2021. Heat Stress response of three Symbiodiniaceae genera NCBI. SRP315798
- Kahlke T, Camp EF. 2021. Metabolomics data and Differential Gene Expression results for three coral symbionts (Cladocopium goreaui, Durusdinium trenchii, and Breviolum sp.) under elevated temperature stress. Open Science Framework. [DOI]
Supplementary Materials
Data Availability Statement
The version and parameter of all bioinformatics tools used in this work are described in the Methods section.