Abstract
Studies examining the overwintering behaviors of North American hibernating bats are limited to a handful of species. We deployed temperature-sensitive transmitters on four species of bat that exhibit differences in their susceptibility to white nose syndrome (WNS; Myotis grisescens, M. leibii, M. sodalis, and Perimyotis subflavus) to determine if these differences are correlated with behavior exhibited during hibernation (i.e., torpor expression and arousal frequency). Mean torpor skin temperature (Tsk) and torpor bout duration varied significantly among species (P ≤ 0.024), but arousal Tsk and duration did not (P ≥ 0.057). One of the species with low susceptibility to WNS, M. leibii, had significantly shorter torpor bout durations (37.67 ± 26.89 h) than M. sodalis (260.67 ± 41.33 h), the species with medium susceptibility to WNS. Myotis leibii also had significantly higher torpor Tsk (18.57 °C ± 0.20) than M. grisescens (13.33 °C ± 0.60), a second species with low WNS susceptibility. The high susceptibility species, Perimyotis subflavus, exhibited low torpor Tsk (14.42 °C ± 0.36) but short torpor bouts (72.36 ± 32.16 h). We demonstrate that the four cavernicolous species examined exhibit a wide range in torpid skin temperature and torpor bout duration. Information from this study may improve WNS management in multispecies hibernacula or individual species management by providing insight into how some species may differ in their techniques for overwinter survival.
Subject terms: Behavioural ecology, Conservation biology, Ecological epidemiology, Ecophysiology
Introduction
Many bat species in North America hibernate during winter to avoid seasonal climatic changes and periods of decreased prey availability1,2. Hibernation is characterized by a significant drop in body temperature (Tb) and metabolic processes that typically last several days and is sporadically punctuated by arousals to euthermic Tb2–4. To survive hibernation, bats must delicately balance an energy budget that is dependent on an individual’s metabolic rate, as well as the frequency and duration of torpor and arousal bouts5–7. Research on hibernation behavior in North American bats has focused on several species that are either common and easily accessible or endangered8–12. While studies that examine hibernation behavior in numerous cave-roosting species have been conducted, comparisons across multiple species in the wild are rare13. Further, research into hibernation behavior across a wide range of cavernicolous species in their natural environments is warranted due to the lack of baseline information on many species and persistent risk of anthropogenic and natural disturbances14.
One of the most detrimental disturbances affecting cavernicolous bats in North America is the disease white-nose syndrome (WNS). Since its discovery in 2006, this disease, caused by the fungal pathogen Pseudogymnoascus destructans (Pd), has led to population declines in numerous North American cave hibernating bat species15,16. The fungus erodes the epidermal tissue of hibernating bats, creating lesions on the skin, muzzle, forearms, and wing membranes that lead to the disruption of homeostatic processes, and increased morbidity and mortality among infected individuals17,18. Marked differences in Pd loads and prevalence have demonstrated that susceptibility to WNS varies among species15,19,20. Researchers have studied a number of mechanisms that may explain host variation in susceptibility, such as microclimate preference, clustering behavior, skin microbiome, and winter activity10,20–24. However, the true mechanisms underlying susceptibility and resistance and/or tolerance to the disease are still not fully understood21,22. Understanding the reasons for heterogeneity in species susceptibility to WNS can improve management outcomes, such as maximizing host persistence and minimizing Pd transmission25.
Periodic arousal from hibernation has been hypothesized as one mechanism that may influence a species’ susceptibility to WNS10,23,24,26. Although repeated arousals from torpor can be detrimental to individuals infected by Pd, occasional arousals in regions where winters are less severe may enable populations of some species to persist10,23,27. Episodic activity during winter raises body temperature, often above the optimal growth range of the fungus (~ 12.5–15.8 °C), and increases immune function, potentially inciting immune defense against Pd28–32. Additionally, repeated arousals throughout hibernation could allow bats to groom more frequently, thus removing Pd spores and reducing fungal loads33. Other activities such as drinking and foraging, may enable individuals to minimize some of the consequences of active WNS infection, like dehydration and starvation, by enabling individuals to supplement as needed26,31.
Understanding the nuances and patterns of hibernation behaviors is paramount in developing effective management recommendations targeting bat conservation. As WNS-induced mortality during hibernation can be significant, targeted intervention to minimize the effects of WNS may increase rates of survival for WNS-infected bats5,6,16. Data regarding the winter activity of bats may minimize the gap in knowledge regarding life history characteristics that influence susceptibility to WNS, and directly affect overwinter survival18,31. Therefore, we explored how hibernation behavior (i.e. torpor bout duration, arousal frequency and torpor and arousal skin temperature (Tsk)) varies among four species with differing susceptibility to this disease: Myotis grisescens, M. leibii, M. sodalis, and Perimyotis subflavus. We selected these four species because of their perceived differences in susceptibility to WNS. Our objectives were to investigate the frequency, duration and Tsk of torpor and arousal bouts for individuals in a natural cave environment. We predicted that species exhibiting medium–high susceptibility to WNS would maintain torpor Tsk at or near the optimal growth temperature of Pd and short torpor bouts punctuated by frequent arousals, as an artefact of WNS infection. Conversely, we predicted that species with low susceptibility to WNS would maintain torpor Tsk outside the optimal growth range of Pd and exhibit occasional arousals from torpor, as part of their natural life history strategies.
Methods
We conducted our study at the entrances of four cave hibernacula in east Tennessee, United States (U.S.), during three hibernation periods (November 1–March 31) of 2016–2019. Cave 1 is in Blount County in the Great Smoky Mountains National Park (GRSM) and is managed by the National Park Service (NPS). Prior to WNS, this cave was the largest known M. sodalis hibernaculum in the state with an estimated population of 9500 individuals34. White-nose-syndrome was detected in this colony during the hibernation season of 2009/10 and M. sodalis numbers have since declined to ~ 750 individuals34. Myotis leibii and P. subflavus are also encountered at this cave. Cave 2 is in Hawkins County and is managed by the Tennessee Wildlife Resources Agency (TWRA). It is one of the largest M. grisescens hibernacula in the state, with an estimated population of ~ 350,000 as of January 201934. This cave also contains a small hibernating population of M. sodalis and low numbers of a few other bat species. Cave 3 is in White County and is managed by TWRA. It contains approximately 400 bats, including ~ 30 M. sodalis and numerous P. subflavus34. Cave 4 is also in White County and is managed by TWRA. This cave is a winter hibernaculum for 100 + M. sodalis and several hundred M. grisescens34. Our study includes M. leibii from Cave 1, M. grisescens from Cave 2, M. sodalis from Caves 2 and 4, and P. subflavus from Caves 1 and 3.
Definitions
Perimyotis subflavus were classified as having high susceptibility to WNS because in natural settings they exhibit average fungal loads of ≥ 10–3 log10 nanograms (ng) during winter and prevalence rates ≥ 80%15,20,35. Myotis sodalis were considered medium–high susceptibility because they carry average fungal loads of 10–3–10–4 log10 ng and show prevalence levels of only 40–80%. Lastly, M. grisescens and M. leibii were classified as exhibiting low susceptibility to WNS because average winter fungal loads on these species are < 10–4 log10 ng and prevalence rates are < 40%19,20,35,36.
Capture methods
We captured bats emerging from each cave up to four times a month using mist nets (Avinet Inc., Dryden, NY, U.S.; mesh diameter: 75/2, 2.6 m high, 4 shelves, 4–9 m wide) or harp traps (Austbat, Bat Conservation and Management Inc., Carlisle, PA, USA). We deployed mist nets and harp traps 30 min before civil sunset on nights with no rain and when temperatures were above 0 °C and left them open for up to 5 h or until temperatures fell below 0 °C. After capture, we placed individual bats into separate paper bags and held them for up to 30 min in an insulated cooler with four hand-warmers (HotHands, Dalton, GA, USA). For all individuals captured, we recorded species, sex, reproductive condition, forearm length (mm), and weight (g). Prior to release, we banded all cave hibernating species with a unique 2.4 mm or 2.9 mm numbered, lipped, alloy forearm band (Porzana, Ltd., Icklesham, East Sussex, UK).
To determine torpor and arousal bout duration and frequency, we attached a 0.27 g or 0.32 g temperature-sensitive VHF radio transmitter (LB-2X, Holohil Systems Ltd., Isanti, Ontario, Canada) to individuals of our focal bat species. We attached transmitters in the interscapular region, away from concentrations of brown adipose tissue, using surgical adhesive8 (Perma-Type, Plainville, CT, USA). Transmitter weight did not exceed 5–7% of an individual’s body weight37. In 2016/17 and 2018/19, we also entered each cave to assist TWRA and NPS with their biannual endangered species surveys, and applied radio transmitters to our focal species.
Capture, handling, sample collection, and radio-transmitter application methods were approved by the University of Tennessee Institutional Animal Care and Use Committee (IACUC 2253-0317), the American Society of Mammalogists38 and authorized under scientific collection permits from the U.S. Fish and Wildlife Service (TE35313B-3), NPS (GRSM-2018-SCI-1253), and TWRA (3742).
Torpor and arousal bout data collection
We used data-logging radio-telemetry receivers (R4500SD, Advanced Telemetry Systems, Inc., Isanti, MN, USA) and dipole omnidirectional antennas (Model 13861, Advanced Telemetry Systems, Inc., Isanti, MN, USA) to record the radio signal (i.e., pulse rate) of each transmitter. Depending on the size and configuration of our cave sites, we deployed up to three antennae inside each cave, with an additional antenna stationed outside to provide radio signal detection if tagged individuals emerged. We positioned radio receivers and external batteries outside each cave to minimize disturbance of hibernating bats when downloading data. We collected data from each receiver weekly, when possible, and at a minimum biweekly.
The manufacturer calibrated each transmitter and provided a unique polynomial equation that we used to convert transmitter pulse rate to bat Tsk. We used Tsk as a proxy for Tb to determine periods of torpor and arousal39. We used a conservative estimate of torpor as Tsk < 22 °C and arousal as Tsk > 22°C40,41. We defined arousal bouts as the period when Tsk > 22 °C, however individuals that gradually increased Tsk but did not rise to arousal Tsk (> 22 °C) were not included in arousal bout calculations42. We defined torpor bout duration as the period between two arousal bouts when Tsk, < 22 °C42. Both torpor and arousal bout duration were calculated to the nearest minute.
We calculated the mean Tsk during each torpor and arousal bout. A torpor bout index, i.e., the number of torpor bouts divided by the number of days active, was calculated to compare among species and control for variation in transmitter lifespan. We used the same method to calculate an arousal frequency index, i.e., the number of arousals per day43. We characterized activity during arousals into five categories: (1) departed cave indefinitely, (2) departed cave and returned, (3) moved location in cave, (4) no movement, or (5) arrival/transmitter attachment. The activity categories were based on movement (or lack thereof) of the transmitter signal between antenna (i.e., detection of signal by different antenna) placed within and outside caves. “No movement” was defined as a transmitter that was consistently picked up on the same antenna with no gap in data collection before, during, and after an arousal bout. “Departed cave” indicated that the transmitter signal was recorded on antenna outside the cave followed by the loss of signal. “Arrival/transmitter attachment” signified when a transmitter was deployed on an individual or was detected by an antenna at a site upon reentry into the cave.
Due to small sample size, our analyses were limited to exploring differences in hibernation behavior among species only. All individuals in our study were captured at caves during hibernation, therefore natural infection of Pd was possible. However, due to weather limitations and the low rate of bat activity during hibernation, we were unable to recapture individuals during the life of the transmitter to determine Pd status. All data analyses were conducted in R44. We used linear mixed-effects models using the lme4 package45 to compare torpor bout duration, arousal bout duration, torpor temperature, and arousal temperature among species, with individual bat as a random effect. We included individuals as a random effect to correct for variation within individuals, as most bats had multiple torpor bouts and arousal events within the life of a transmitter43,46. For torpor bout and arousal frequency index, we used linear models with species as our explanatory variable. We log transformed all index data prior to analysis to meet assumptions of normality and homogeneity of variance47,48. Post-hoc tests were conducted using least square means using the emmeans package49.
Results
We deployed 77 temperature-sensitive radio transmitters and successfully recorded Tsk data from 22 individuals (28.6% of bats tagged). After initial transmitter attachment, the transmitter signals of 40 individuals (51.9% of tagged bats) were never detected on cave antenna during the life of their transmitters (15–21 days). Fifteen individuals (19.5% of bats tagged) were detected by cave antennas after the night of transmitter attachment but did not remain in antennae range long enough for data collection.
Mean torpor and arousal temperature
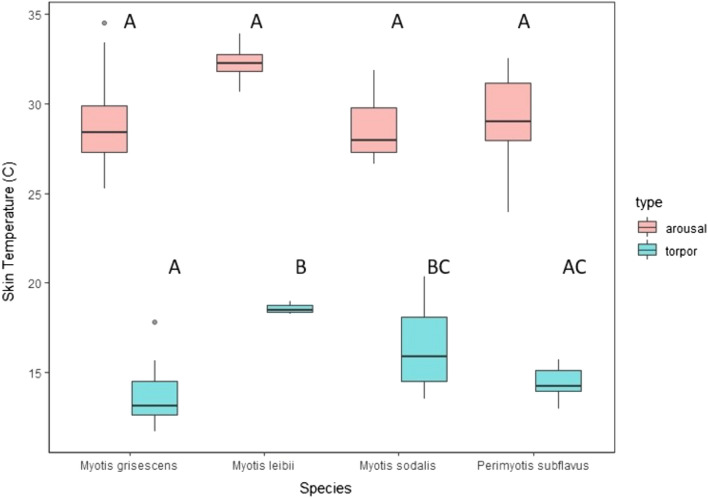
There was considerable variation in the mean Tsk during torpor and arousal of the four species studied (Table 1). There was an effect of species on mean torpor Tsk (P = 0.004; Fig. 1). Myotis leibii had the highest mean torpor Tsk (18.57 ± 0.20 °C), whereas M. grisescens had the lowest mean torpor Tsk (13.72 ± 0.60 °C). Post-hoc tests indicated that M. leibii had a higher mean torpor Tsk than P. subflavus (14.44 ± 0.36 °C; P = 0.021), and M. grisescens had a lower mean torpor Tsk compared to M. leibii (P = 0.005) and M. sodalis (16.48 ± 0.79 °C; P = 0.019).
Table 1.
The number of transmitters detected, mean skin temperature (Tsk) during torpor and arousal, and mean torpor (hours) and arousal (minutes) bout durations of four bat species tracked with temperature-sensitive radio transmitters at four cave hibernacula in Tennessee during hibernation (November 1–March 31) of 2016–2019.
| Species | Number of transmitters detected (no.) | Torpor Tsk (°C; ± SE) | Arousal Tsk (°C; ± SE) | Torpor bout duration (h; ± SE)a | Arousal bout duration (min; ± SE) a |
|---|---|---|---|---|---|
| Myotis grisescens | 8 | 13.72 ± 0.60 A | 29.01 ± 0.64 A | 162.13 ± 48.79AB | 84.88 ± 15.08 A |
| Myotis leibii | 2 | 18.57 ± 0.20 B | 32.29 ± 0.67 A | 37.67 ± 26.89A | 103.75 ± 60.62 A |
| Myotis sodalis | 9 | 16.48 ± 0.79 BC | 28.59 ± 0.38 A | 260.67 ± 41.33B | 66.94 ± 6.82 A |
| Perimyotis subflavus | 3 | 14.44 ± 0.36 AC | 28.99 ± 0.84 A | 72.36 ± 32.16A | 79.27 ± 21.04 A |
a ± SE in the same column followed by the same uppercase letter are not significantly different (P > 0.05).
Figure 1.
Mean skin temperature (℃; Tsk) during torpor and arousal of four bat species tracked with temperature-sensitive radio transmitters at four cave hibernacula in Tennessee during hibernation (November 1–March 31) of 2016–2019. Dots above plots represent outlying data. *Boxplot data followed by the same uppercase letter not significantly different (P > 0.05).
Myotis leibii had the highest mean arousal Tsk (32.29 ± 0.67 °C) and M. sodalis had the lowest mean arousal Tsk (28.59 ± 0.38 °C; Table 1; Fig. 1). However, there was no effect of species on mean arousal Tsk (P = 0.057).
Mean torpor and arousal bout duration
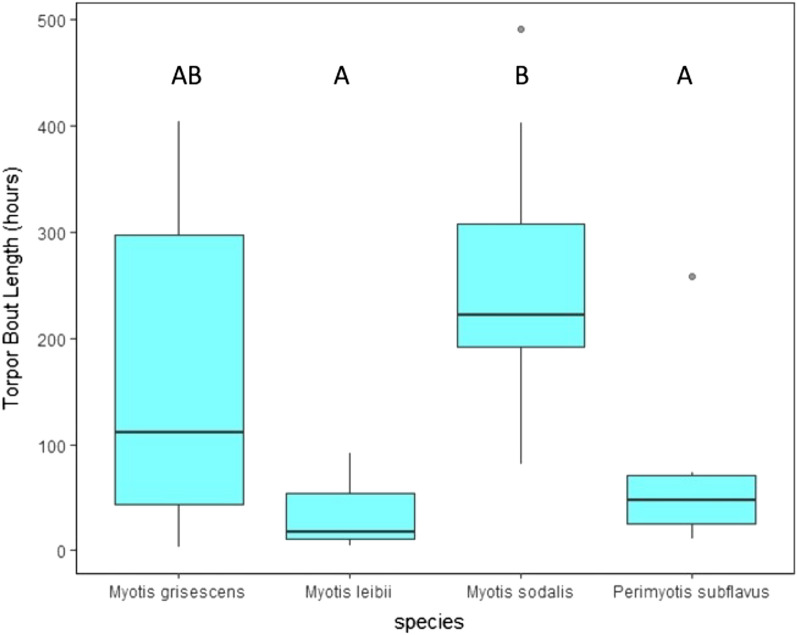
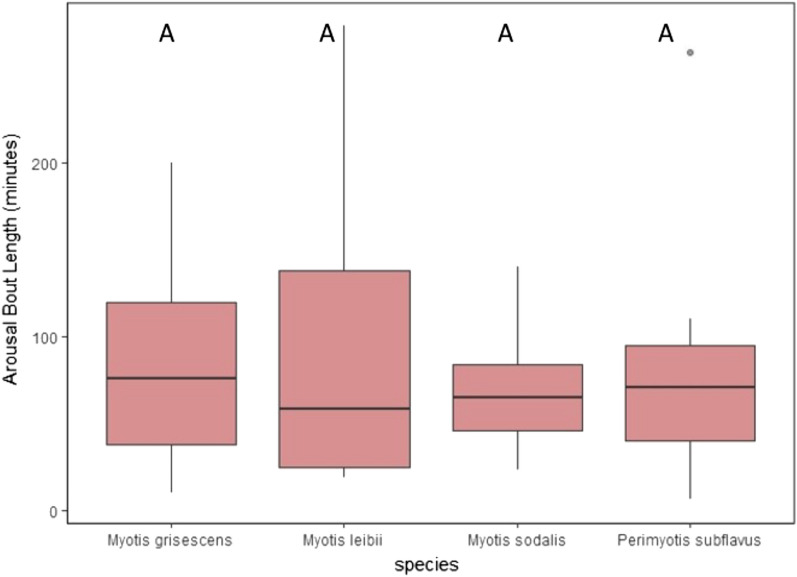
Mean torpor bout duration across all species was less than 11 days (Table 1; Fig. 2). There was an effect of species on mean torpor bout duration (P = 0.024). Myotis sodalis had a significantly longer mean torpor bout duration (260.67 ± 41.33 h) than P. subflavus (72.36 ± 32.16 h; P = 0.029) and M. leibii (37.67 ± 26.89 h; P = 0.029). Myotis leibii had the longest mean arousal bout duration (103.75 ± 60.62 min), whereas M. sodalis had the shortest mean arousal bout duration (66.94 ± 6.82 min; Table 1; Fig. 3). However, there was no effect of species on mean arousal bout duration (P = 0.655).
Figure 2.
Mean torpor bout duration (hours) of four bat species tracked with temperature-sensitive radio transmitters at four cave hibernacula in Tennessee during hibernation (November 1–March 31) of 2016–2019. Dots above plots represent outlying data. *Boxplot data followed by the same uppercase letter not significantly different (P > 0.05).
Figure 3.
Mean arousal bout duration (min) of four bat species tracked with temperature-sensitive radio transmitters at four cave hibernacula in Tennessee during hibernation (November 1–March 31) of 2016–2019. Dots above plots represent outlying data. *Boxplot data followed by the same uppercase letter not significantly different (P > 0.05).
Activity throughout hibernation
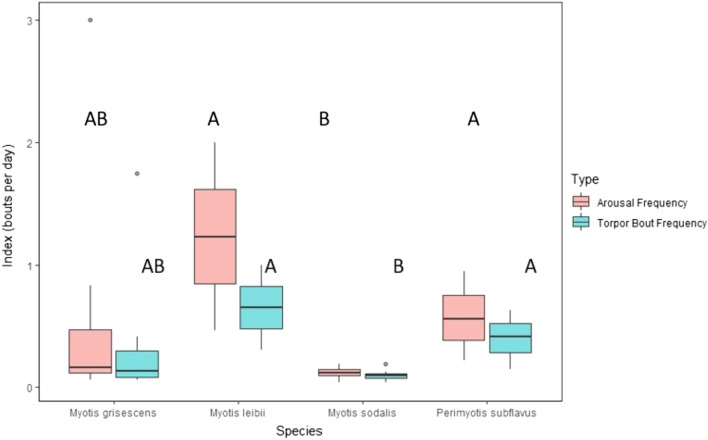
The number of torpor and arousal bouts per species during hibernation varied significantly across species (Fig. 4). Myotis leibii had the highest torpor bout (0.654 ± 0.346 torpor bouts/day) and arousal frequency indices (1.231 ± 0.769 arousals/day), whereas M. sodalis had the lowest indices (torpor bout index: 0.097 ± 0.015; arousal frequency index: 0.117 ± 0.016). There was an effect of species on both torpor bout index (P = 0.026) and arousal frequency index (P = 0.023). Post hoc tests indicate M. leibii and P. subflavus had a greater torpor bout index and arousal frequency index than M. sodalis (P ≤ 0.028). However, the torpor bout index and arousal bout index of M. leibii and P. subflavus were similar to that of M. grisescens (P ≥ 0.087).
Figure 4.
Torpor bout and arousal bout frequency indices (bouts/day) of four target bat species tracked with temperature-sensitive radio transmitters at four cave hibernacula in Tennessee during hibernation (November 1–March 31) of 2016–2019. Dots above plots represent outlying data. *Boxplot data followed by the same uppercase letter not significantly different (P > 0.05).
Activity during arousals varied, with 34.69% of arousals resulting in bats moving out of range of the antennas and returning within five hours (n = 17/49 arousal events). In 12.24% of arousal events, bats left the cave and were never recorded again during the life of the transmitter (n = 6/49). There was no indication of movement within or out of the cave in 20.41% of recorded arousal events (n = 10/49).
Discussion
We found significant differences in torpor Tsk, torpor bout duration, torpor bout index, and arousal frequency index among four bat species that exhibit variation in their susceptibility to WNS. In contrast, we found no significant difference in arousal Tsk or arousal bout duration among species. Although we were unable to gather data from all deployed transmitters, we were able to fill in knowledge gaps regarding the hypothesized differences in hibernation behavior across multiple bat species in a natural environment. By understanding the differences in these winter torpor and arousal characteristics in bat species with varying degrees of susceptibility to WNS, we can continue to improve our understanding of why some species experience high rates of morbidity and mortality from the disease. Given that Pd grows and colonizes hosts more efficiently in certain microclimates50 identifying Tsk (as a proxy for Tb) and winter activity of bats that hibernate in natural cave environments can help elucidate factors that may increase the risk of Pd infection.
Prior to our study, we expected P. subflavus and M. sodalis, the species with medium–high susceptibility to WNS, to maintain torpor Tsk at or near the optimal growing temperature of Pd, with M. grisescens and M. leibii, our species with seemingly low susceptibility, at torpid Tsk levels outside the optimal Pd growth range (~ 12.5–15.8 °C)32. Interestingly, M. leibii maintained the highest Tsk during both torpor and arousal and were consistently active throughout the life of the transmitter. Data from winter captures and diet analysis from this region indicate M. leibii arouse from torpor regularly and engage in foraging throughout the hibernation period, frequently leaving hibernacula on winter nights, when temperatures are greater than − 1 ℃20,27. This species is known to occupy a large portion of eastern North America, with the northern extent of its limit in southern Canada50. Given this species’ ability to survive severe winters throughout southern Canada and the northern US, M. leibii in Tennessee may be physiologically adept at handling winters in the southern US, where temperatures are comparably mild and rarely remain below freezing for extended periods23,51. Therefore, the frequent winter activity documented by M. leibii in our study may largely be influenced by the species’ inherent cold tolerance. Such tolerance may be necessary for survival in the northern portion of their range and may play an important role in the species apparent low susceptibility in the southern US. In areas where winter temperatures are rarely below freezing, M. leibii may be able to regularly maintain both torpor and arousal Tsk above the optimal growth temperature of Pd, which may reduce exposure to and minimize serious Pd infection32. Short torpor bouts punctuated by regular foraging activity may also help the species maintain immune system function, as well as supplemental energy reserves, allowing them to persist with low level infections without significant mortality15,19,20,39,52,53. Additionally, our findings are consistent with pre-WNS data from this latitude, suggesting that this activity regime is not an artifact of WNS, but an inherent life history characteristic52,54,55. While activity during winter does not completely explain the persistence of M. leibii throughout the WNS epizootic, it is possible that their rates of frequent activity are beneficial to their survival10,20,24,26,36.
Conversely, P. subflavus maintained short torpor bout durations similar to M. leibii, but low torpor Tsk, akin to M. grisescens and within the optimal Pd growth range32. These short torpor bouts at low Tsk could explain some of the mortality seen in P. subflavus due to WNS. Compared to higher Tsk, bats expend more energy arousing from torpor when they hibernate at low Tsk, which is otherwise useful for maintaining long, energy-saving torpor bouts56. However, in our system, P. subflavus was arousing (on average) every 3 days from a low torpor Tsk, which likely puts a severe and recurring drain on energy reserves and may contribute to mortality in this species42. Data from the region also suggests that while P. subflavus maintain a low level of activity during much of the hibernation period, activity increases in February, months earlier than the documented emergence from hibernation in pre-WNS literature for this longitude57,58. The increase in activity, which correlates with a rise in fungal loads and prevalence of Pd on P. subflavus, and associated energy drain may be a product of sickness behavior in infected P. subflavus20,36,42,59. It is possible that the characteristics of winter activity in P. subflavus documented during our study are WNS-induced behaviors, rather than a healthy life history strategy, and could greatly reduce the overwinter survival rate of P. subflavus in this area.
In contrast to M. leibii and P. subflavus, M. grisescens and M. sodalis had longer torpor bouts, with M. grisescens torpor Tsk the lowest across our focal species and M. sodalis torpor Tsk just below M. leibii. Given the limited similarities in hibernation behavior between these two species, aspects other than winter activity likely influence their risk of Pd infection and WNS manifestation. Myotis grisescens are year-round cave dwellers that are large-bodied and highly gregarious, hibernate in cold microclimates, and can be active throughout winter20,60. Dietary analysis of M. grisescens guano collected from active bats during winter indicates that this species is regularly eating aquatic insects through the hibernation period, likely from waterways in or near hibernacula that provide ample foraging opportunities year-round27. Coupled with adequate energy intake throughout winter, the physical and behavioral traits of M. grisescens may enable this species to resist or tolerate Pd infections with minimal adverse effects on survival, much like M. leibii20,53. While M. leibii may be resisting Pd infection via regular arousals and shallow torpor bouts, M. grisescens may tolerate Pd due to their preference for long torpor bouts at cold temperatures.
Alternatively, we found that M. sodalis maintained a relatively high torpid Tsk, similar to M. leibii, yet had significantly longer torpor bouts and less frequent arousals than seen in M. leibii. Previous research on the fungal load and infection intensity of WNS for M. sodalis indicates that hibernating populations of the species experience mid-range intensity of fungal load with relatively high prevalence15,20,36. Although we found the species maintains similar torpor Tsk as M. leibii, the difference in activity regimes between the two species may allow for increased fungal growth on M. sodalis, despite maintaining torpid Tsk slightly above the optimal growth temperature of Pd. While there are likely myriad factors influencing susceptibility, it is possible that higher Tsk, coupled with low activity, is not enough to reduce Pd growth on this species36. More research is needed to explore the drivers of WNS susceptibility in this species, given that there are highly variable patterns in pathogen dynamics, but still a marked decline in their population16.
While our success at documenting the hibernation behaviors of cavernicolous bats in their natural environments was limited, our methods illuminated issues with the long-held belief that many hibernating bat species practice strict roost fidelity during winter55,61,62. Over 50% of tagged bats did not stay within range of the cave antenna that would have allowed for the collection of temperature data. Specifically, several M. grisescens and M. leibii were recorded leaving the hibernacula via aerial radiotelemetry and either roosting on the landscape and never returning to the cave during the life of the transmitter or disappearing completely, several kilometers away from the cave capture site. While the reason for signal disappearance is not wholly understood, aerial telemetry experts believe that bats with disappearing signals most likely moved to other subterranean sites, outside the range of the antenna (S. Samoray, pers. comm.). Although roost fidelity is known to fluctuate based on availability of roosts, hibernacula fidelity has long been either unknown or assumed to be high55,61,63. Our results provide definitive proof that hibernacula fidelity largely varies among individuals, especially for species like M. grisescens and M. leibii. Many agencies may base proposed management actions on previous assumptions, possibly missing secondary hibernacula important for overwinter survival64. Therefore, many researchers may mistakenly assume high hibernacula fidelity in their study species and develop study methodology based on this assumption. More work is needed to understand winter roost fidelity in cavernicolous species and what it may mean for WNS management.
Due to small sample size for M. leibii and P. subflavus, our ability to analyze characteristics that may have influenced hibernation behavior within species, such as age, sex, body condition, time of year, cave microclimate, or Pd status is limited.However, even with these limitations, our study is the first to directly compare hibernation behavior across several species of cavernicolous bats in their natural overwintering environment. The information we collected is an important step towards understanding the intricacies of hibernation behavior of cavernicolous bats, although more work is obviously needed to identify the drivers of the differences we found. Recognizing the variations in hibernation behavior is important to both researchers and managers as strategies aimed at maintaining bat populations affected by WNS may be more effective if specifically targeted to species with the highest known susceptibility. Some low-cost actions may be to reduce anthropogenic disturbances at or near hibernacula used by M. sodalis and P. subflavus. Additionally, improving, or augmenting habitat for increased prey availability around hibernation sites may be beneficial to species that remain active on the landscape during winter, such as M. grisescens and M. leibii. Given that two of our four focal species are listed as Endangered in the U.S. (M. grisescens and M. sodalis) and one (P. subflavus) is petitioned for listing, any management efforts that can increase survival of individuals and persistence of populations are crucial for successful conservation of these species.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
We acknowledge that the lands where our study was conducted are located on the ancestral home of the Eastern Band of the Cherokee, S’atsoyaha (Yuchi), and Shawandasse Tula (Shawnee) Indigenous peoples. We thank Nigel House for his technical support in helping design and build our antenna systems. We also thank Zack David, Keith Dreyer, D.S. Hollis, Mallory Tate, and Laura Vining for their field assistance. We wish to acknowledge the National Park Service, The Nature Conservancy, and the Tennessee Wildlife Resources Agency for assistance and cave access. We also thank the U.S Fish and Wildlife Service and National Park Service for funding. Finally, we would like to thank Dr. Justin Boyles and another anonymous reviewer for their thoughtful and constructive comments on our manuscript.
Author contributions
R.F.B. and E.V.W. designed the study and received funding. R.T.J. performed field work and conducted data analysis. R.T.J. wrote the initial draft of the manuscript. R.T.J., R.F.B., and E.V.W. contributed equally to the final editing and draft of the manuscript.
Funding
U.S. Fish and Wildlife Service (Grant No. F15AP00955-03) and National Park Service (Grant No. P18AC01279).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang, L.C.H. Ecological, physiological, and biochemical aspects of torpor in mammals and birds in Advances in comparative and environmental physiology (ed. Wang, L.C.H.) 361–401 (Springer, 1989).
- 2.Humphries, M.M., Speakman, J.R., & Thomas, D.W. Temperature, hibernation energetics, and the cave and continental distributions of little brown myotis in Functional and Evolutionary Ecology ofBats (ed. Zubaid, A., McCracken, G.F., & Kunz, T.H.) 23–37 (Oxford Press, 2005).
- 3.Hudson, J.W. Torpidity in mammals in Comparative physiology of thermoregulation. (ed. Whittow, G.C., Hudson, J.W., & Deavers, D.R.) 97–165 (Academic Press, 1973).
- 4.Geiser F, Ruf T. Hibernation versus daily torpor in mammals and birds: Physiological variables and classification of torpor patterns. Phys. Zool. 1995;68:935–966. doi: 10.1086/physzool.68.6.30163788. [DOI] [Google Scholar]
- 5.Davis WH, Hitchcock HB. Biology and migration of the bat, Myotis lucifugus, New England. J. Mamm. 1965;46:296–313. doi: 10.2307/1377850. [DOI] [Google Scholar]
- 6.Speakman JR, Rowland A. Preparing for inactivity: How insectivorous bats deposit a fat store for hibernation. Proc. Nutr. Soc. 1999;58:123–131. doi: 10.1079/PNS19990017. [DOI] [PubMed] [Google Scholar]
- 7.Boyles JG, Johnson JS, Blomberg A, Lilley TM. Optimal hibernation theory. Mammal Rev. 2020;50:91–100. doi: 10.1111/mam.12181. [DOI] [Google Scholar]
- 8.Britzke ER, Sewell P, Hohmann MG, Smith R, Scott R. Use of temperature-sensitive transmitters to monitor the temperature profiles of hibernating bats affected with white-nose syndrome. Northeast. Nat. 2010;17:239–246. doi: 10.1656/045.017.0207. [DOI] [Google Scholar]
- 9.Halsall AL, Boyles JG, Whitaker JO., Jr Body temperature patterns of big browns during winter in a building hibernaculum. J. Mamm. 2012;93:497–503. doi: 10.1644/11-MAMM-A-262.1. [DOI] [Google Scholar]
- 10.Johnson JS, Lacki MJ, Thomas SC, Grider JF. Frequent arousals from winter torpor in Rafinesque’s big-eared bat (Corynorhinus rafinesquii) PLoS ONE. 2012;7:e49754. doi: 10.1371/journal.pone.0049754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonasson KA, Willis CKR. Hibernation energetics of free-ranging little brown bats. J. Exp. Bio. 2012;215:2141–2149. doi: 10.1242/jeb.066514. [DOI] [PubMed] [Google Scholar]
- 12.Day KM, Tomasi TE. Winter energetics of female Indiana bats Myotis sodalis. Physiol. Biochem. Zool. 2014;87:56–64. doi: 10.1086/671563. [DOI] [PubMed] [Google Scholar]
- 13.Meierhofer MB, et al. Winter habitats of bats in Texas. PLoS ONE. 2019;14:e0220839. doi: 10.1371/journal.pone.0220839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyles JG. Benefits of knowing the costs of disturbance to hibernating bats. Wildl. Soc. Bull. 2017;41:388–392. doi: 10.1002/wsb.755. [DOI] [Google Scholar]
- 15.Frick WF, et al. Pathogen dynamics during invasion and establishment of white-nose syndrome explain mechanisms of host persistence. Ecology. 2017;98:624–631. doi: 10.1002/ecy.1706. [DOI] [PubMed] [Google Scholar]
- 16.Cheng TL, et al. The scope and severity of white-nose syndrome on hibernating bats in North America. Conserv. Biol. 2021;35:1586–1597. doi: 10.1111/cobi.13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cryan PM, Meteyer CU, Boyles JG, Blehert DS. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 2010;8:1–8. doi: 10.1186/1741-7007-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cryan PM, et al. Electrolyte depletion in white-nose syndrome bats. J. Wild. Dis. 2013;49:398–402. doi: 10.7589/2012-04-121. [DOI] [PubMed] [Google Scholar]
- 19.Frick WF, et al. Disease alters macroecological patterns of North American bats. Glob. Ecol. Biogeogr. 2015;24:741–749. doi: 10.1111/geb.12290. [DOI] [Google Scholar]
- 20.Bernard, R.F., Willcox, E.V., Parise, K.L., Foster, J.T., & McCracken, G.F. White-nose syndrome fungus, Pseudogymnoascus destructans, on bats captured emerging from caves during winter in the southeastern United States. BMC Zool.10.1186/s40850-017-0021-2 (2017).
- 21.Davy CM, et al. The other white-nose syndrome transcriptome: Tolerant and susceptible hosts respond differently to the pathogen Pseudogymnoascus destructans. Ecol. Evol. 2015;7:7161–7170. doi: 10.1002/ece3.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilley TM, et al. Resistance is futile: RNA-sequencing reveals differing responses to bat fungal pathogen in Nearctic Myotis lucifugus and Palearctic Myotis myotis. Oecologia. 2019;191:295–309. doi: 10.1007/s00442-019-04499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernard RF, McCracken GF. Winter behavior of bats and the progression of white-nose syndrome in the southeastern United States. Ecol. Evol. 2017;7:1487–1496. doi: 10.1002/ece3.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moosman, P.R., Warner, D.P., Hendren, R.H., & Hosler, M.J. Potential for monitoring eastern small-footed bats on talus slopes. Northeast. Nat.https://www.jstor.org/stable/26453719 (2015).
- 25.Bernard RF, et al. Identifying research needs to inform white-nose syndrome management decisions. Cons. Sci. Prac. 2020;2(e220):2020. doi: 10.1111/csp2.220. [DOI] [Google Scholar]
- 26.Reynolds DS, Shoemaker K, Oettingen SV, Najjar S. High rates of winter activity and arousals in two New England bat species: implications for a reduced white-nose syndrome impact? Northeast. Nat. 2017;24:B188–B208. doi: 10.1656/045.024.s720. [DOI] [Google Scholar]
- 27.Bernard RF, Willcox EV, Jackson RT, Brown VA, McCracken GF. Feasting, not fasting: Winter diets of cave hibernating bats in the United States. Front. Zool. 2021;18:1–13. doi: 10.1186/s12983-021-00434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am. J. Phys. 2002;282:1054–1062. doi: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- 29.Dobony CA, et al. Little brown myotis persist despite exposure to white-nose syndrome. J. Fish Wild. 2011;2:190–195. doi: 10.3996/022011-JFWM-014. [DOI] [Google Scholar]
- 30.Rowley JJ, Alford RA. Hot bodies protect amphibians against chytrid infection in nature. Sci. Rep. 2013;3:1–4. doi: 10.1038/srep01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verant ML, et al. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 2014;14:10. doi: 10.1186/s12899-014-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verant ML, et al. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS ONE. 2012;7:e46280. doi: 10.1371/journal.pone.0046280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brownlee-Bouboulis SA, Reeder DM. White-nose syndrome-affected little brown Myotis (Myotis lucifugus) increase grooming and other active behaviors during arousals from hibernation. J. Wildl. Dis. 2013;49:850–859. doi: 10.7589/2012-10-242. [DOI] [PubMed] [Google Scholar]
- 34.Campbell, J. Tennessee winter bat population and white-nose syndrome monitoring report for 2018–2019. TWRA Wildlife Technical Report 16-4. http://www.tnbwg.org/2019%20Annual%20Monitoring%20Report.pdf (2019).
- 35.Langwig KE, et al. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Eco. Let. 2012;15:1050–1057. doi: 10.1111/j.1461-0248.2012.01829.x. [DOI] [PubMed] [Google Scholar]
- 36.Langwig, K.E., et al. Drivers of variation in species impacts for a multi-host fungal disease of bats. Philos. Trans. R. Soc. B Biol. Sci.371, 20150456. 10.1098/rstb.2015.0456 (2016). [DOI] [PMC free article] [PubMed]
- 37.Aldridge, H.D.J.N., & Brigham, R.M. Load carrying and maneuverability in an insectivorous bat: A test of the 5% ‘rule’ of radio-telemetry. J. Mamm.69, 379–382. 10.2307/1381393 (1988)
- 38.Sikes RS, et al. Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mamm. 2016;97:663–688. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barclay RMR, et al. Can external radiotransmitters be used to assess body temperature and torpor in bats? J. Mammal. 1996;77:1102–1106. doi: 10.2307/1382791. [DOI] [Google Scholar]
- 40.Turbill, C., & Geiser, F. Hibernation by tree-roosting bats. J. Comp. Physiol. B.178, 597–605. 10.1007/s00360-007-0249-1 (2008) [DOI] [PubMed]
- 41.Park, K.J., Jones, G., & Ransome, R.D. Torpor, arousal and activity of hibernating greater horseshoe bats (Rhinolophus ferrumequinum). Funct. Ecol.14, 580–588 (2000).
- 42.Reeder DM, et al. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS ONE. 2012;7:e38920. doi: 10.1371/journal.pone.0038920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sirajuddin, P. Vulnerability of tri-colored bats (Perimyotis subflavus) to white-nose syndrome in the southeastern United States. M.S thesis at https://www.proquest.com/dissertations-theses/vulnerability-tri-colored-bats-i-perimyotis/docview/2185672601/se-2?accountid=8361 (2018).
- 44.R Development Core Team 3.6.1. A Language and Environment for Statistical Computing. http://www.r-project.org (2019).
- 45.Bates, D., Maechler, M., Bolker, B., & Walker, S. lme4: Linear mixed-effects models using ‘Eigen’ and S4. R Packag. version 1.1-13. ftp://cran.r-project.org/pub/R/web/packages/lme4/lme4.pdf. (2017)
- 46.Czenze ZJ, Willis CKR. Warming up and shipping out: Arousal and emergence timing in hibernating little brown bats (Myotis lucifugus) J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 2015;185:575–586. doi: 10.1007/s00360-015-0900-1. [DOI] [PubMed] [Google Scholar]
- 47.Conover, W.J. Practical Nonparametric Statistics. (Wiley, 1999).
- 48.Crawley, M.J. The R Book: Second Edition. (Wiley, 2013)
- 49.Lenth, R., Singmann, H., Love, J., Buerkner, P., & Herve, M. Package ‘emmeans’. R Packag. version 1.7.0.https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (2021)
- 50.Best, T.L. & Jennings, J.B. Myotis leibii. Mammalian Species 547, 1–6. 10.2307/3504255 (1997)
- 51.Frank, C.L., Herzog, C.,. Laske, J.P., & Cardino V. The role of skin temperature in the resistance of Myotis leibii to white-nose syndrome presented at the 49th Symposium of the North American Society for Bat Research, Kalamazoo, Michigan https://www.nasbr.org/resources/docs/meetings/year/2019/NASBR-2019-Abstracts-Draft-191001.pdf (2019)
- 52.Johnson, J.S., Scafini, M.R., Sewall, B.J., & Turner, G.G. Hibernating bat species in Pennsylvania use colder winter habitats following the arrival of white-nose syndrome in Conservation and Ecology of Pennsylvania’s Bats (ed. Butchkoski, C.M., Reeder, D.M., Turner, G.G., & Whidden, H.P) 181–199 (The Pennsylvania Academy of Science, 2016)
- 53.Haase, C.G., et al. Body mass and hibernation microclimate may predict bat susceptibility to white‐nose syndrome. Eco. Evo.11, 506–515 10.1002/ece3.7070 (2021) [DOI] [PMC free article] [PubMed]
- 54.Veilleux, J.P. A Noteworthy Hibernation Record of Myotis leibii (Eastern Small-footed Bat) in Massachusetts. Northeast. Nat.14, 501–502 10.1656/1092-6194(2007)14[501:ANHROM]2.0.CO;2 (2007)
- 55.Boyles JG, Dunbar MB, Whitaker JO. Activity following arousal in winter in North American vespertilionid bats. Mamm. Rev. 2006;36:267–280. doi: 10.1111/j.1365-2907.2006.00095.x. [DOI] [Google Scholar]
- 56.Webb PI, Speakman JR, Racey PA. How hot is a hibernaculum? A review of the temperatures at which bats hibernate. Can. J. Zool. 1996;74:761–765. doi: 10.1139/z96-087. [DOI] [Google Scholar]
- 57.Jackson, R.T., Willcox, E.V., Zobel, J.M., & Bernard, R.F. Hibernation behavior of four bat species with differing susceptibility to white-nose syndrome. In review (2021).
- 58.Fujita MS, Kunz TH. Pipstrellus subflavus. Mamm. Spec. 1984;228:1–6. doi: 10.2307/3504021. [DOI] [Google Scholar]
- 59.Bohn SJ, et al. Evidence of ‘sickness behaviour’ in bats with white-nose syndrome. Behaviour. 2016;152:981–1003. doi: 10.1163/1568539X-00003384. [DOI] [Google Scholar]
- 60.Tuttle MD. Status, causes of decline, and management of endangered gray bats. J. Wild. Manag. 1979;43:1–17. doi: 10.2307/3800631. [DOI] [Google Scholar]
- 61.Harvey, M.J., Altenbach, J.S., & Best, T.L. Bats of the Eastern United States. (JHU Press, 2011).
- 62.Klüg-Baerwald BJ, Lausen CL, Willis CK, Brigham RM. Home is where you hang your bat: Winter roost selection by prairie-living big brown bats. J. Mamm. 2017;98:752–760. doi: 10.1093/jmammal/gyx039. [DOI] [Google Scholar]
- 63.Sandel JK, et al. Use and selection of winter hibernacula by the eastern pipistrelle (Pipistrellus subflavus) in Texas. J. Mamm. 2001;82:173–178. doi: 10.1644/1545-1542(2001)082<0173:UASOWH>2.0.CO;2. [DOI] [Google Scholar]
- 64.Hayes, J. P., Ober, H. K., Sherwin, R. E. Survey and monitoring of bats in Ecological and behavioral methods for the study of bats (ed. Kunz, T. H., Parsons, S.). 120–129 (JHU Press, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.