Abstract
Mantle cell lymphoma (MCL) is a B-cell lymphoma featuring an aggressive course and a progressive relapsing pattern. International guidelines recommend early consolidative autologous stem cell transplant (auto-SCT) for eligible patients while reserving allogeneic SCT (allo-SCT) as therapy for refractory cases. Since data describing the implementation of transplants in the Asian population with MCL are limited, we aimed to analyze post-SCT outcomes of 99 MCL patients from the Taiwan Bone Marrow Transplant Registry database. The median age was 56 years, and 11% of the patients had blastoid variant MCL. Ninety-four patients received auto-SCT, while 13 patients received allo-SCT, eight of which received allo-SCT after failing auto-SCT. Before auto-SCT, 52% of the patients were in their first complete remission (CR1). Overall, 37 patients (39%) relapsed after auto-SCT. The median post-auto-SCT progression-free survival and overall survival (OS) were 43.6 months and not reached, respectively. Blastoid variant MCL, transplant not received in CR1, and disease progression within 12 months post-auto-SCT independently predicted inferior OS in multivariable analysis. The median post-allo-SCT OS was 74 months. Two patients (15%) died of MCL recurrence post-allo-SCT. Three patients with refractory diseases were salvaged with ibrutinib or venetoclax to allo-SCT. Treatment strategies incorporating novel agents warrant further optimization.
Subject terms: Cancer, Stem cells, Medical research, Oncology, Cancer, Haematological diseases, Prognosis, Stem-cell therapies
Introduction
Mantle cell lymphoma (MCL) is a B-cell lymphoma that mostly presents with an advanced stage and frequent extranodal involvement, including bone marrow and gastrointestinal tract involvement1–5. The clinical course is typically aggressive, with a progressive shortening of response duration and disease-free survival after each relapse6,7. High-dose chemotherapy with autologous stem cell transplant (auto-SCT) has become the standard of care for eligible patients8–10. Nevertheless, the high chance of disease recurrence within 3–5 years, particularly in high-risk populations (i.e., patients with blastoid variant MCL or TP53 mutation), often affects the post-SCT course and makes long-term survival challenging11–17. Allogeneic SCT (allo-SCT), in the meantime, remains an option for those who fail auto-SCT or have refractory disease18,19. Recently, in line with the promising responses to small molecule targeted agents, such as BTK and BCL-2 inhibitors, treatment strategies combining conventional therapy, auto-SCT or allo-SCT, and targeted therapies may be warranted20–25.
The current risk stratification and global treatment guidelines were primarily developed based on Western studies26–29. Data that describe the epidemiology, use of transplantation, and outcomes in Asian populations are relatively scarce30–36. Herein, we present a registry-based study and delineate the post-SCT outcome of 94 MCL patients in Taiwan. Pre-SCT parameters and therapy modalities were also analyzed for risk stratification and survival prediction.
Results
Patient characteristics
In total, 94 patients received auto-SCT, while 13 patients received allo-SCT (8 of which received the allo-SCT after relapsing after auto-SCT). The median age of the 94 MCL patients who received only auto-SCT was 55 years (Table 1). There were 77 male patients and 17 female patients. The Ann Arbor stage at diagnosis in most patients was stage 4 (79.8%). Approximately three-fourths of patients (74.5%) had bone marrow involvement at diagnosis. Regarding morphology subtypes, 76 patients (87.2%) had a classic type, 10 (10.6%) had blastoid variant MCL, and 2 (2.2%) had pleomorphic variant MCL. In terms of MIPI classification, patients were stratified into low (40.7%), intermediate (39.5%), and high (19.8%) risk groups. In 18 patients with available Ki-67% data from the registry, eight patients (44.4%) had a Ki-67% equal to or higher than 30%.
Table 1.
Clinical and laboratory features of 94 mantle cell lymphoma patients who received autologous stem cell transplant.
| Clinical parameter | Number* (%) |
|---|---|
| Age† | 55 (25–73) |
| Sex | |
| Male | 77 (81.9) |
| Female | 17 (18.1) |
| Advanced stage (III/IV) | 87 (92.6) |
| Bone marrow involvement | 70 (74.5) |
| Laboratory data† | |
| WBC, × 109 /L | 10.7 (2–19) |
| LDH, U/L‡ | 244 (97–4082) |
| MIPI | |
| Low | 37 (40.7) |
| Intermediate | 36 (39.5) |
| High | 18 (19.8) |
| Unavailable | 3 |
| Blastoid variant | 10 (10.6) |
| Ki-67 | |
| < 30% | 10 (55.6) |
| ≥30% | 8 (44.4) |
| Unavailable | 76 |
MIPI mantle cell lymphoma international prognostic index, WBC white blood cell.
*Eight patients received allogeneic stem cell transplant due to relapse/progression of disease after autologous stem cell transplant.
†Median (range).
‡LDH, normal range was 140–271 U/L.
The characteristics of the 13 patients who received allo-SCT are presented in Table 2. The median age was 49.6 years. Three of the 13 (23%) patients had blastoid variant MCL, whereas 11 of the 13 (85%) patients had bone marrow involvement of MCL at diagnosis. Overall, 7 (54%), 2 (15%), and 4 (31%) patients had low-, intermediate-, and high-risk disease according to the MIPI classification.
Table 2.
Clinical characteristics of 13 mantle cell lymphoma patients who received allogeneic stem cell transplant.
| UPN | Sex | Age | Morphology | Stage | BM involvement | MIPI | Relapse after ASCT | Salvage therapy bridging to allo-SCT | State before allo-SCT | Survival at last follow up | Overall survival* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 44 | Classic | IV | Yes | Low | Yes | 4 cycles of bortezomib + rituximab + bendamustine | CR | Alive in remission | 88 | |
| 8 | Male | 61 | Classic | IV | Yes | Low | Yes | 3 cycles of bortezomib + rituximab + bendamustine | CRu | Death due to infection | 26 | |
| 13 | Male | 52 | Blastoid | IV | Yes | Low | Nil | ibrutinib | PR | Alive in remission | 28 | |
| 14 | Male | 49 | Classic | III | Nil | High | Nil | 1 cycle of asparaginase + paclitaxel + gemcitabine | PR | Alive in remission | 140 | |
| 34 | Male | 48 | Classic | IV | Yes | Low | Yes | 8 cycles of bortezomib + rituximab + bendamustine | CRu | Alive in remission | 82 | |
| 36 | Female | 66 | Blastoid | IV | Yes | High | Yes | Nil | Refractory | Death due to infection | 18 | |
| 41 | Female | 52 | Blastoid | IV | Yes | Low | Yes | Venetoclax | PR | Alive in remission | 25 | |
| 46 | Male | 63 | Classic | IV | Yes | High | Nil | 3 cycles of Ibrutinib + rituximab + bendamustine | CR | Alive in remission | 11 | |
| 47 | Female | 36 | Classic | IV | Yes | intermediate | Yes | 2 cycles of R-BOMES | CR | Death due to MCL | 68 | |
| 52 | Male | 52 | Classic | IV | Yes | Low | Nil | Not reported | PR | Alive in remission | 61 | |
| 82 | Male | 42 | Classic | III | Nil | intermediate | Yes | 1 cycle of bortezomib + mitoxantrone + dexamethasone | PR | Death due to infection | 132 | |
| 85 | Male | 47 | Classic | IV | Yes | Low | Nil | Not reported | PR | Death due to MCL | 61 | |
| 89 | Male | 50 | Classic | IV | Yes | High | Yes | 2 cycles of rituximab + bendamustine | CR | Alive in remission | 24 | |
CR complete remission, Cru complete remission, unconfirmed, MIPI mantle cell lymphoma international prognostic index, PR partial remission, PD progression of disease, R-BOMES Rituximab, carmustine (BCNU), Vincristine, Methotrexate Etoposide, Methylprednisolone, UPN unlinked patient number.
*Months.
Forty-five (45.5%) of the patients had available data regarding the induction treatments for MCL. The CHOP-based (cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone) therapy was given to 73.3% of patients37–39. Other induction modalities included VR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) with or without alternating with R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin)40,41 in 6 (13.3%) patients, RB-based therapy (bendamustine plus rituximab)42,43 with or without cytarabine44,45 in 5 (11.1%) patients, and Hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with high-dose methotrexate-cytarabine46–48 in 1 (2.2%) patient. By and large, 8 (17.8%) patients received high dose cytarabine, while 5 (11.1%) received bendamustine in their induction chemotherapies.
Stem cell harvest and transplantation procedures
In Taiwan, the most commonly used regimen for stem cell harvest is etoposide, methylprednisolone, cytarabine, and cisplatin (ESHAP) with or without rituximab, followed by granulocyte colony-stimulating factor (G-CSF) administration49. A minority of patients received high-dose cytarabine in combination with dexamethasone and cisplatin (DHAP) or other cytarabine-based regimens before harvest50. All except for ten patients received BCNU, etoposide, cytarabine, and melphalan (BEAM) as the conditioning chemotherapy regimen before auto-SCT51. Ten patients received the Benda-EAM regimen (which substitutes the BCNU in the BEAM regimen with bendamustine) as the conditioning regimen52,53. The mostly employed infection prophylaxis for auto-SCT is levofloxacin 750 mg or ceftibuten 400 mg daily, administered from D0 until the day absolute neutrophil counts reached 1000 cells/mm3. The most used conditioning regimens for allo-SCT in Taiwan are generally categorized into myeloablative and reduced-intensity regimens. The myeloablative regimens are usually backboned with busulfan IV 3.2 mg/kg/day consecutively from day − 8 to day − 5 and cyclophosphamide IV 60 mg/kg/day on day − 3 and day − 2. In contrast, the reduced-intensity regimens are based on the following scheme: fludarabine 30 mg/m2/day consecutively from day − 8 to day -4, busulfan IV 3.2 mg/kg/day on day − 5 and day − 4, and cyclophosphamide IV 60 mg/kg/day on day − 2. Anti-thymocyte globulin (ATG) 4–6 mg/kg can be added as a part of the conditioning regimen in HLA-mismatched allo-SCT scenarios. Usually, cyclosporin with methotrexate is used for graft-versus-host disease (GVHD) prevention in myeloablative allo-SCT, and cyclosporin with mycophenolate mofetil is used for reduced-intensity protocols.
Stem cell transplantation
Before auto-SCT, 49 patients (52.1%) were in their first complete remission (CR1), and 16 (17%) were in their second complete remission (CR2). Twenty-nine (30.9%) patients were in partial remission (PR), of whom 16 were in PR after 1st line therapy while 13 reached PR after two or more lines of treatment. Overall, 37 patients (39.4%) had a disease recurrence after auto-SCT at a median follow-up time of 20.9 months. Among them, 23 (62.2%) patients experienced disease recurrence within 24 months after auto-SCT whereas 18 (48.6%) has patients relapsed within 12 months post-SCT. The median time to post-auto-SCT relapse was 13.1 months (range: 0.7–84).
Among the 13 patients who received allo-SCT, 8 experienced relapses after prior auto-SCT (Table 2). Five patients had primary refractory diseases and were salvaged with various regimens to receive frontline allo-SCT. Only two (15.4%) patients had recurrence of MCL after allo-SCT and eventually succumbed to the disease. However, three patients died of infections after allo-SCT. Patient unlinked patient number (UPN) 13 had disease progression before the scheduled auto-SCT, and he was salvaged with ibrutinib and achieved PR prior to subsequent allo-SCT. Patient UPN 14 had a primary disease that was refractory to multiple lines of therapy and finally achieved PR after treatment with a combination of asparaginase, paclitaxel, and gemcitabine. He then received allo-SCT and was disease-free and alive until the last follow-up. Patient UPN 36 experienced relapse one month after her auto-SCT. Allo-SCT was used as salvage due to the rapid progression of the disease. She had acute GVHD and ensuing infection and died of uncontrolled infection; imaging and bone marrow examinations before death showed no evidence of disease recurrence. Patient UPN 41 had blastoid variant MCL and relapsed eight months after auto-SCT. Her lymphoma was refractory to ibrutinib; rituximab, BCNU, vincristine, methotrexate etoposide, and methylprednisolone (R-BOMES); and rituximab, bendamustine, and cytarabine (R-BAC). She finally attained PR with venetoclax and underwent allo-SCT. Patient UPN 46 had primary refractory disease, although we confirmed that he had nonblastoid MCL. He received ibrutinib with bendamustine and rituximab and was bridged to allo-SCT. Other details are shown in Table 2.
Interestingly, a trend of growing transplantation activity with time (52% of patients were transplanted after 2016) was observed despite the increasing age at transplant (before vs. after 2016: 51.8 years vs. 56.1 years, p = 0.087), which partly reflects the evolution of MCL management. Although similar proportions of patients were transplanted in CR before and after 2016 (66.7% vs. 66.6%), transplantation implemented in CR1 increased by 5% after 2016 (47.9% vs. 52.9%).
Infections
Within 180 days of auto-SCT, 46 infection episodes were documented. Seven (15.2%) patients experienced gram-negative bacilli bacteremia, and five had gram-positive cocci bacteremia. Three patients had invasive fungal infection, and one had Pneumocystis jiroveci pneumonia. Notably, 12 (26.1%) patients had cytomegalovirus antigenemia, while another two patients had cytomegalovirus colitis after auto-SCT. Approximately one-tenth of patients experienced a flare-up of herpes simplex virus and subsequent varicella-zoster virus infection. Other details are provided in Supplementary Table S1.
Maintenance therapy
After proceeding with auto-SCT, 22 (23.4%) patients received maintenance therapy. Twelve (54.5%) patients received rituximab (median duration, 14.6 months, range: 4–60.8), two of whom received rituximab and alternating bortezomib with a duration of 46.8 and 36.7 months, respectively; six (27.3%) patients used bortezomib for maintenance therapy (median duration, 12.2 months, range: 4–48.6); and four used ibrutinib (median duration, 23.1 months, range: 21.8–47).
Ibrutinib
Ibrutinib was used at a dose of 560 mg/day in twelve patients as salvage therapy: in ten patients, ibrutinib was administered because of relapse after prior auto-SCT, and in two patients, ibrutinib was given as salvaging and bridging therapy to allo-SCT. The median duration of salvaging ibrutinib for post-auto-SCT relapse was 4.6 months (range: 1.9–19.7). Of the eight evaluable patients, the overall response rate (ORR) was 62.5%. Two (25%) patients achieved PR, 3 (37.5%) patients attained CR, 2 patients (25%) maintained stable disease, and one patient (12.5%) had disease progression despite treatment. The median time to response and the response duration were 2.6 months (range: 1.9–4.4) and 6.6 months (range: 1.3–18.6), respectively. Two patients (25%) lost their response after three months and 18 months, respectively. The most frequently encountered adverse events were bleeding (37.5%), pulmonary infection (25%), including Pneumocystis jiroveci pneumonia (12.5%) and pulmonary nontuberculous mycobacterial infection (12.5%), and cytopenia (25%).
Survival
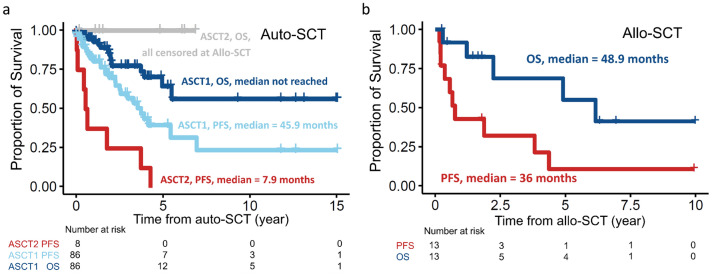
The median post-auto-SCT PFS and OS of the of 86 patients receiving auto-SCT only (ASCT1) were 45.9 months and not reached (NR), respectively (Fig. 1a). The median PFS of the 8 patients who underwent auto-SCT and subsequent allo-SCT (ASCT2) due to post-auto-SCT relapse was 7.9 months (Fig. 1a). Furthermore, the median post-allo-SCT PFS and OS of the 13 patients receiving allo-SCT were 36 months and 48.9 months, respectively (Fig. 1b).
Figure 1.
Kaplan–Meier plots of MCL patients receiving autologous (auto-) or allogeneic stem cell transplant (allo-SCT). (a) Progression-free survival (PFS) and overall survival (OS) of 86 patients receiving auto-SCT only (ASCT1) and 8 patients receiving auto-SCT and subsequent allo-SCT due to post-auto-SCT relapse (ASCT2). OS of ASCT2 patients were censored at the time of allo-SCT. (b) PFS and OS of 13 MCL patients receiving allo-SCT.
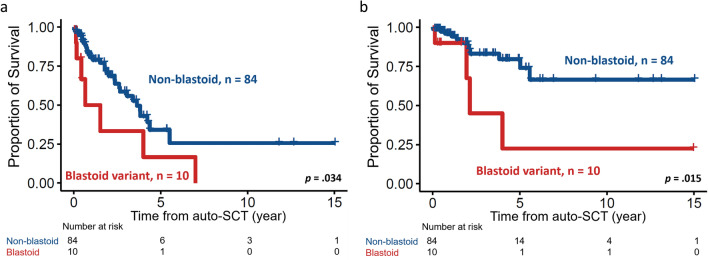
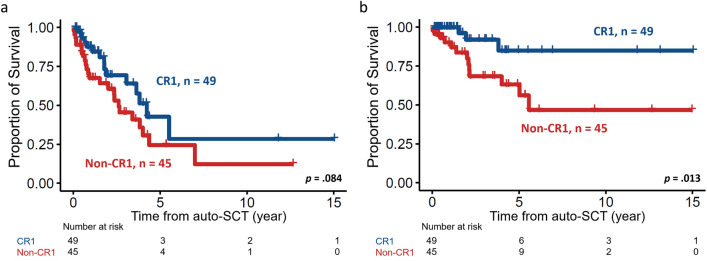
Specifically, for auto-SCT, patients with blastoid variant MCL had significantly shorter PFS and OS than those without blastoid variant MCL (median PFS, 7.9 months vs. 43.6 months, p < 0.001, Fig. 2a; and median OS, 25.5 months vs. NR, p = 0.015, Fig. 2b). Furthermore, disease status before auto-SCT also had an impact on survival, as expected. Patients who underwent auto-SCT in CR1 (n = 49, 52.1%) had more prolonged survival than those who underwent auto-SCT in CR2 or PR (PFS, 50.8 vs. 31.3 months, p = 0.084; and OS, NR vs. 66.8 months, p = 0.013, Fig. 3a,b, respectively).
Figure 2.
Kaplan–Meier plots stratified by morphologic variant of MCL. (a) PFS and (b) OS of 94 MCL patients with blastoid or non-blastoid variant. Patients with blastoid variant had significantly inferior PFS and OS.
Figure 3.
Kaplan–Meier plots stratified by disease status before autologous stem cell transplant. (a) PFS and (b) OS of patients with different disease status before receiving transplant. Patients receiving transplant at their CR1 state had better PFS and OS.
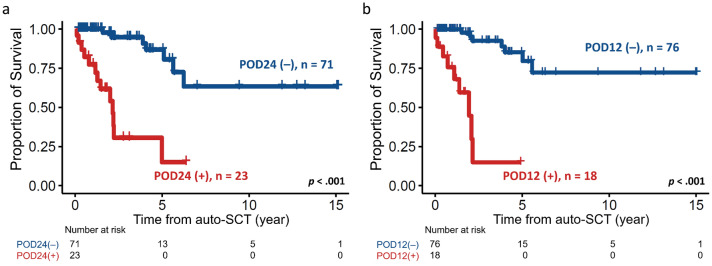
Since time to progression (progression of disease within 24 months, POD24)16,17 has been established as a prognostic factor for MCL, we subsequently explored the impact of POD24 on patients’ outcomes. Conceivably, patients with POD24 had exceptionally worse survival than those without POD24 (median: 24.9 months vs. NR, p < 0.001, Fig. 4a). We next inquired whether progression of disease within 12 months (POD12)14 also affect post-SCT survival. Strikingly, patients with POD12 also had an inferior OS compared without POD12 (median: 23.3 months vs. NR, p < 0.001, Fig. 4b). In the multivariable analysis, blastoid variant MCL, transplant not received in CR1, and POD12 independently predicted adverse post-auto-SCT survival (Table 3).
Figure 4.
Kaplan–Meier plots stratified by progression of disease post-auto-SCT or not. (a) Patients who had progression of disease within 24 months (POD24) post-auto-SCT had a significantly shorter survival. (b) POD12 also predicted an inferior post-auto-SCT outcome.
Table 3.
Univariate and multivariable analyses for OS of the 94 MCL patients receiving autologous stem cell transplant.
| Variable | Progression-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| MIPI* | 1.4 (0.9–2.1) | 0.131 | 1.3 (0.8–2.0) | 0.291 | 1.1 (0.6–2.1) | 0.848 | 0.8 (0.3–1.9) | 0.597 |
| Bone marrow involvement | 1.5 (0.7–3.2) | 0.294 | 3.1 (0.7–13.6) | 0.141 | ||||
| Blastoid variant | 2.4 (1.1–5.5) | 0.039 | 1.7 (0.7–4.2) | 0.260 | 3.7 (1.2–11.2) | 0.023 | 3.8 (1.1–12.8) | 0.033 |
| Status before transplant† | 0.6 (0.3–1.1) | 0.088 | 0.6 (0.3–1.2) | 0.179 | 0.2 (0.1–0.8) | 0.023 | 0.3 (0.1–0.9) | 0.049 |
| Bendamustine in conditioning | 0.6 (0.1–4.6) | 0.641 | 0.1 (0–65,272) | 0.666 | ||||
| Maintenance therapy | 0.9 (0.4–1.9) | 0.712 | 0.2 (0.1–1.4) | 0.102 | ||||
| Progression within 12 months | 14.3 (4.6–44.9) | < 0.001 | 12.7 (3.9–40.9) | < 0.001 | ||||
P values < .05 are considered statistically significant.
Only variables with P value less than 0.10 in univariate analysis were incorporated into the multivariable Cox proportional hazard regression analysis.
CI confidence interval, HR hazard ratios, MIPI mantle cell lymphoma international prognostic index.
*MIPI: stratified into low-, intermediate-, and high-risk groups.
†Transplant at first complete remission versus others.
Moreover, as the recruitment of patients spanned an extended period, we further analyzed patients receiving transplantation in different eras to examine the possible chronologic effect. Patients were separated by the median calendar year of transplantation, 2016. The post-SCT outcomes of patients who received transplantation more contemporarily were better than their counterparts regarding PFS and OS (NR vs. 40.8 months, p = 0.039, and NR vs. 73.9 months, p = 0.087, respectively, Supplementary Fig. S1).
Discussion
International guidelines recommend various induction regimens, mainly cytarabine-containing regimens, for transplant-eligible MCL patients and underscore the importance of frontline auto-SCT28,29. The survival benefit of early consolidation followed by auto-SCT was first reported in a prospective randomized trial of the European MCL Network, notwithstanding that there was only a PFS, but not an OS, benefit10. In Asia, Miura et al. analyzed the outcomes of 64 newly diagnosed Japanese patients with MCL54. Sixteen patients in the study received various intensive chemotherapies and ensuing auto-SCT. Before auto-SCT, nine patients were in CR1, one was in CR2, and six were refractory. The survival was rather promising, with a 5-year OS of 93%, compared to the 5-year OS of less than 50% in their counterparts. In another retrospective study, 16% of 501 Japanese patients received auto-SCT following induction chemotherapy with a rituximab-high-dose cytarabine combination55. This approach yielded HRs of 0.24 and 0.43 for PFS and OS, respectively, compared to R-CHOP treatment. A survival benefit of frontline auto-SCT was also observed in 97 transplant-eligible patients in a recently published Taiwanese study32. This multi-institutional study identified auto-SCT, gastric MCL involvement, blastoid variant MCL, and POD12 as independent prognostic factors. Patients who underwent auto-SCT in CR1 also had a better OS than those who underwent auto-SCT in CR2 or PR (median OS: NR vs. 71 months, p = 0.027). In accordance with previously mentioned studies, approximately half of the patients in the present study received auto-SCT in CR1 and had a superior post-SCT survival than those who received auto-SCT in CR2 or PR.
While early auto-SCT improved the long-term outcome of MCL patients, survival plateaus have not been observed. Disease recurrence frequently intercedes in the post-transplantation course, which imposes a challenge against a durable remission for MCL patients28. Recently, Visco et al. modelled the relationship between early POD (POD24)16 and risk of death in 188 relapsed or refractory MCL patients from the Fondazione Italiana Linfomi series. The early POD patients had significantly shorter OS than the late POD group (median, 12 months vs. NR, p < 0.0001). Early POD was also confirmed prognostically detrimental in multivariable analysis with a hazard ratio (HR) of 3.9 (95% confidence interval, 2.16–7.06) and then validated in 93 patients from the MCL Younger study. Interestingly, in 41 patients that received allo-SCT at any time during the study period, patients with early POD had comparable OS to patients with a late POD (p = 0.72), implying that detrimental effects of early POD might be ameliorated by allo-SCT. In a retrospective analysis of the European Society for Blood & Marrow Transplantation (EBMT) registry56, there was no significant difference in post-allo-SCT PFS (p = 0.173) and OS (p = 0.336) between patients with and without POD24, further demonstrating the potential of allo-SCT overcoming the poor prognosis of POD24. In the present cohort, patients with POD24 also had a reduced survival than those without, corresponding to that in Visco et al.’s study. On the other hand, although POD12 was associated with worse survival in our study, further validation in larger cohorts may be warranted.
The role of maintenance therapy with rituximab after auto-SCT has been established in the randomized, phase III LyMA trial, in which the rituximab group had superior event-free survival, PFS, and OS at 4 years post randomization57. A systematic review and meta-analysis also indicated that rituximab maintenance therapy improved post-auto-SCT PFS and OS in MCL patients58. However, post-SCT maintenance therapy in our analysis did not seem to affect post-SCT survival as much as it did in the previous studies. Admittedly, since the Taiwan National Health Insurance does not reimburse maintenance rituximab for MCL, the rate of patients receiving post-auto-SCT maintenance rituximab in this study was limited, thereby precluding a sound comparison.
While real-world data have potentially demonstrated the benefit of upfront auto-SCT in some patients, a high recurrence rate, even after consolidation treatment, has been observed in patients harboring high-risk factors, including blastoid variant MCL or TP53 mutation15,59,60. Hence, an approach with intensified chemotherapy followed by frontline auto-SCT for young and fit patients might be justified, particularly in the era of novel agents and improved safety and accessibility of allo-SCT.
Although current data supporting allo-SCT as upfront therapy are lacking, earlier studies suggested at least some benefits for patients with chemorefractory MCL. Hamadani et al. investigated the outcomes of 202 patients from the Center for International Blood and Marrow Transplant Research (CIBMTR) database and found that approximately 25% of patients with refractory MCL could attain durable remission after allo-HCT18. In a study by Fenske et al., 519 chemotherapy-sensitive MCL patients received auto-SCT or allo-SCT with reduced-intensity conditioning at different time points during the disease course61. Auto-SCT and allo-SCT resulted in comparable 5-year OS rates (61% vs. 62%, p = 0.951). Both auto-SCT and allo-SCT in frontline settings were demonstrated to be beneficial for survival in multivariable analysis. In a recent prospective and multicenter study, 24 of 25 patients received upfront allo-SCT and were engrafted without therapy-related mortality by day 10062. With a median follow-up of 60.5 months, there were three (12.5%) deaths from MCL. The PFS and OS were 56% and 76% at 5 years, implying that frontline allo-SCT is feasible and should be considered for selected patients, such as those with refractory disease or a high risk of progression (i.e., POD24).
In this study, two patients had a relapse after allo-SCT, and 80% of the patients receiving upfront allo-SCT were still alive at a median follow-up of 21.5 months. Nonetheless, too few patients in this cohort received allo-SCT, hindering the results regarding the impact of allo-SCT. Therefore, larger-scale, prospective, and randomized controlled trials are warranted to support this approach.
In our cohort, the ORR of patients receiving ibrutinib for post-auto-SCT relapsed MCL was 62.5%, in agreement with that in trial settings63–65 as well as in real-world analysis66. There were no unexpected adverse events, and all events were controlled by tapering of doses. Nevertheless, the limited number of patients using BTK inhibitor could make the data much less convincing than it could be. Moreover, in the scenario where patients who remain in remission with ibrutinib and hence hesitate about SCT, it may also render bias in this transplantation registry-based analysis.
Ibrutinib has been shown efficacious in relapsed MCL after first-line treatment and auto-SCT, with ORRs spawning from 69 to 74% and CR rates ranging from 27 to 32%56,66. Nonetheless, its effectiveness in early-POD patients has not been conclusive. In a real-world analysis, 211 patients received ibrutinib as second-line therapy for relapsed or refractory MCL66. POD24 was a significant adverse predictor of PFS and OS in a univariable regression model, yet this association was lost in multivariable analysis. In Burney et al.’s study, there was no difference in the duration of response to ibrutinib between patients with and without POD24 (median: 10.4 months vs. 9.8, p = 0.9). After 2 years of initiating ibrutinib, the POD24 group had markedly shorter PFS and OS than patients without POD24 (p = 0.017 and p = 0.019, respectively). In the MANTLE-FIRST study17, curiously, ibrutinib was associated with significantly better OS in patients with POD24 compared with other second-line therapies (HR 2.41 for RB, 2.78 for R-BAC, and 2.17 for others). By contrast, in patients without POD24, such improvement in survival was not observed. As novel agents have become more broadly used in real-world practice, a treatment strategy that incorporates conventional chemotherapies, stem cell transplants, and targeted therapies needs to be optimized for this heterogeneous disease.
This study's limitations lie in its registry-based nature and, crucially, its lack of TP53 genotyping data. The study could also be improved with more data, including data regarding the regimens and responses of each line therapy. Furthermore, this study enrolled patients over 20 years, thereby introducing various inherent confounding factors into our analysis. While early transplantation has been evoked since the early 2010s8,14, the lack of consensus on treatment for MCL in Taiwan makes the decision to an upfront transplantation more heterogeneous in real-world practice. Although there isn't a comparison arm for patients who did not receive transplantation in this registry-based study, a previous Taiwanese multi-institutional study indicated that 14 of 38 (37%) transplant-eligible patients received transplantation between 2006 and 2012, whereas 29 of 59 (49%) patients did between 2013 and 201932. The expansion of transplantation activity for MCL, despite elder ages, reflected the remarkable integration of transplantations into the treatment strategy for MCL.
Nowadays, allo-SCT remains the curable potential in patients with relapsed/refractory MCL61,67. Aside from transplantation, the chimeric antigen receptor T-cells (CAR -T) may represent an alternative for long-term survival, notably after BTK inhibitors have failed68. In the ZUMA-2 study68, the ORR of 60 patients evaluated for response was 93%, with a CR rate of 67%. Remissions were durable in a majority of patients while only 15% of patients experienced grade 3 or higher but non-fatal cytokine release syndrome. In the TRANSCEND-NHL-001 Trial69, the ORR was 84% in 32 evaluable patients, and 59% achieved a CR. Notably, patients with a history of auto-SCT or allo-SCT were eligible in the TRANSCEND-NHL-001 study while those with prior allo-HCT was not included in the Zuma-2 study. While many trials are currently enrolling patients, the above data suggest the high efficacy of CD19-targeted CAR-T therapy for relapsed or refractory MCL, further underpinning the importance of integrating novel agents, cellular therapies, and transplantation. Consideration of disease refractoriness following relapse after transplantation or novel agents, and importantly, cost-effectiveness, is also required. Although tisagenlecleucel has been recently approved in Taiwan for the treatment of B-cell acute lymphoblastic leukemia in children and young adults70 and relapsed or refractory diffuse large B-cell lymphoma71, CAR-T therapies for MCL patients are still restricted within a clinical trial setting. However, with the impressive results from previous studies, a profound change in the treatment landscape for MCL is anticipated following the introduction of CAR-T therapy.
In summary, this study depicted the implementation of transplantation for MCL patients in an Asian population, emphasizing the survival benefit conferred by early consolidative auto-SCT compared to later auto-SCT and the prognostic impact of blastoid variant MCL and POD24. In addition, novel agents such as ibrutinib or venetoclax may play a role in bridging high-risk patients to subsequent allo-SCT, which was also demonstrated to be feasible in the frontline setting for selected patients. While the results presented are mainly confirmational, these data simultaneously convey the need for additional Asian population-based registry and clinical trials to understand this currently incurable disease and improve patient outcomes.
Patients and methods
Data source
This retrospective observational study reviewed and analyzed data from the Taiwan Blood and Marrow Transplantation Registry (TBMTR). The TBMTR is maintained by the Taiwan Society of Blood and Marrow Transplantation (TSBMT), which has been tasked with registering clinical information of blood and bone marrow transplant recipients in Taiwan since 2009. Currently, 17 hospitals contribute to the registry, and the collection and analysis of data from the TBMTR is approved by the institutional review board of each participating hospital. All contributing centers that were registered with the TBMTR Data Center are listed in Supplementary data S1. All experimental protocols were approved by Institutional review board or Ethical committee of Taiwan Society of Blood and Marrow Transplantation. All methods were carried out in accordance with relevant guidelines and regulations, and the Declaration of Helsinki. Informed consent was obtained from all subjects and/or their legal guardian.
Patient selection
We recruited MCL patients consecutively from the TBMTR aged > 20 years who received one or more auto-SCT or allo-SCT for MCL between September 1999 and August 2020. A total of 99 MCL patients were identified. Data on prognosis-relevant variables, such as age, Mantle Cell Lymphoma International Prognostic Index (MIPI) classification, stage at diagnosis, morphologic type of MCL, disease status before SCT, preparation regimens, and post-SCT outcome, were extracted. Treatment response was evaluated per Response evaluation criteria in solid tumors (RECIST)72,73.
Statistical analysis
We utilized the Mann–Whitney U test to compare the medians and distributions of continuous variables. Fisher’s exact test or the χ2 test was performed to examine the differences between discrete variables. Progression-free survival (PFS) was the duration from the date of reinfusion of autologous stem cells to the date of first documented disease progression, last follow-up, allo-SCT, or death from any cause, whichever occurred first. Overall survival (OS) was the duration from the date of reinfusion of autologous stem cells to the date of last follow-up, allo-SCT, or death from any cause, whichever occurred first. We plotted the survival curves with Kaplan–Meier analysis and calculated the statistical significance with the log-rank test. The Cox proportional hazards model was used in the univariate and multivariable regression analyses. P values < 0.05 were considered statistically significant. We considered biologically relevant factors (MIPI classification) and parameters with p < 0.1 in the univariate Cox regression analysis as covariates in the multivariable analysis. All statistical analyses and imaging were performed with IBM SPSS Statistics 23 for Windows and R software.
Supplementary Information
Acknowledgements
We would like to acknowledge the service provided by the Taiwan Blood and Marrow Transplantation Registry (TBMTR). We also thank additional colleagues who provide patient information to TBMTR in this study: Dr. Yeu-Chin Chen of Tri-Service General Hospital, Taipei, Taiwan; Dr. Chieh-Lin Jerry Teng of Taichung Veterans General Hospital, Taichung, Taiwan; Dr. Tsai-Yun Chen of National Cheng Kung University Hospital, Tainan, Taiwan; Dr. Shih-Chiang Lin of Far Eastern Memorial Hospital, New Taipei City, Taiwan; Dr. Tso-Fu Wang of Buddhist Tzu-Chi General Hospital, Hualien, Taiwan; Dr. Ming-Chun Ma of Kaohsiung Chang-Gung Memorial Hospital, Kaohsiung, Taiwan; Dr. Shyh-Jer Lin of Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan; Dr. Chih-Cheng Chen of Chang Gung Memorial Hospital, Chiayi, Taiwan; and Dr. Tsu-Yi Chao of Taipei Medical University-Shuang Ho Hospital, New Taipei City, Taiwan. This research was funded by Ministry of Science and Technology, Taiwan (Grant number MOST-110-2314-B-002-076).
Author contributions
Y.H.W. contributed to research design, data collection and management, statistical analysis and interpretation, literature research, and manuscript writing. C.Y.H., L.T.H., T.L.L., Y.C.L. and M.Y. contributed to data collection and management, statistical analysis and interpretation. T.D.T. and B.S.K. contributed to research design and supervision, data collection and management, statistical analysis and interpretation and manuscript writing.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tran-Der Tan, Email: trander@kfsyscc.org.
Bor-Sheng Ko, Email: bskomd@ntu.edu.tw.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-09539-5.
References
- 1.Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romaguera JE, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer. 2003;97:586–591. doi: 10.1002/cncr.11096. [DOI] [PubMed] [Google Scholar]
- 3.Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J. Clin. Oncol. 2016;34:1256–1269. doi: 10.1200/jco.2015.63.5904. [DOI] [PubMed] [Google Scholar]
- 4.Vose JM. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am. J. Hematol. 2017;92:806–813. doi: 10.1002/ajh.24797. [DOI] [PubMed] [Google Scholar]
- 5.Maddocks K. Update on mantle cell lymphoma. Blood. 2018;132:1647–1656. doi: 10.1182/blood-2018-03-791392. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: Progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019;9:50. doi: 10.1038/s41408-019-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo JC, et al. Clinical features and treatment outcomes of limited-stage mantle cell lymphoma: CONSORTIUM for Improving Survival of Lymphoma report. Ann. Hematol. 2020;99:223–228. doi: 10.1007/s00277-019-03803-x. [DOI] [PubMed] [Google Scholar]
- 8.Delarue R, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: A phase 2 study from the Groupe d'Etude des Lymphomes de l'Adulte. Blood. 2013;121:48–53. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- 9.Eskelund CW, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): Prolonged remissions without survival plateau. Br. J. Haematol. 2016;175:410–418. doi: 10.1111/bjh.14241. [DOI] [PubMed] [Google Scholar]
- 10.Dreyling M, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: Results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 11.Budde LE, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J. Clin. Oncol. 2011;29:3023–3029. doi: 10.1200/jco.2010.33.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar SK, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisler CH, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: Still very long survival but late relapses do occur. Br. J. Haematol. 2012;158:355–362. doi: 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich S, et al. Outcome and prognostic factors in patients with mantle-cell lymphoma relapsing after autologous stem-cell transplantation: A retrospective study of the European Group for Blood and Marrow Transplantation (EBMT) Ann. Oncol. 2014;25:1053–1058. doi: 10.1093/annonc/mdu097. [DOI] [PubMed] [Google Scholar]
- 15.Dreyling M, Klapper W, Rule S. Blastoid and pleomorphic mantle cell lymphoma: Still a diagnostic and therapeutic challenge! Blood. 2018;132:2722–2729. doi: 10.1182/blood-2017-08-737502. [DOI] [PubMed] [Google Scholar]
- 16.Visco C, et al. Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br. J. Haematol. 2019;185:940–944. doi: 10.1111/bjh.15643. [DOI] [PubMed] [Google Scholar]
- 17.Visco C, et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: Results from the MANTLE-FIRST study. Leukemia. 2021;35:787–795. doi: 10.1038/s41375-020-01013-3. [DOI] [PubMed] [Google Scholar]
- 18.Hamadani M, et al. Allogeneic hematopoietic cell transplantation for chemotherapy-unresponsive mantle cell lymphoma: A cohort analysis from the center for international blood and marrow transplant research. Biol. Blood Marrow Transpl. 2013;19:625–631. doi: 10.1016/j.bbmt.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin RJ, et al. Allogeneic haematopoietic cell transplantation impacts on outcomes of mantle cell lymphoma with TP53 alterations. Br. J. Haematol. 2019;184:1006–1010. doi: 10.1111/bjh.15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davids MS, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J. Clin. Oncol. 2017;35:826–833. doi: 10.1200/jco.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam CS, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N. Engl. J. Med. 2018;378:1211–1223. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): A single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–667. doi: 10.1016/s0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portell CA, et al. Bendamustine and rituximab plus venetoclax in untreated mantle cell lymphoma over 60 years of age (PrE0405): A phase II study. Blood. 2019;134:5243–5243. doi: 10.1182/blood-2019-122759. [DOI] [Google Scholar]
- 24.Wang ML, et al. Frontline treatment with ibrutinib plus rituximab (IR) followed by short course R-Hypercvad/MTX Is extremely potent and safe in patients (age ≤ 65 years) with mantle cell lymphoma (MCL)—results of phase-II window-1 clinical trial. Blood. 2019;134:3987–3987. doi: 10.1182/blood-2019-126044. [DOI] [Google Scholar]
- 25.Reem Karmali, J. S. A., Deborah Marie Stephens, Jeffrey A. Barnes, Jason B. Kaplan, Jane N. Winter, Shuo Ma, Adam Matthew Petrich, Ephraim P. Hochberg, Tak Takvorian, Valerie Nelson, Leo I. Gordon, Barbara Pro. Ibrutinib maintenance following induction for untreated mantle cell lymphoma (MCL): Initial safety report. J. Clin. Oncol.37. 10.1200/JCO.2019.37.15_suppl.7542 (2019).
- 26.Hoster E, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 27.Hoster E, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European mantle cell lymphoma network. J. Clin. Oncol. 2016;34:1386–1394. doi: 10.1200/jco.2015.63.8387. [DOI] [PubMed] [Google Scholar]
- 28.Dreyling, M. et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.28, iv62–iv71. 10.1093/annonc/mdx223 (2017). [DOI] [PubMed]
- 29.McKay P, Leach M, Jackson B, Robinson S, Rule S. Guideline for the management of mantle cell lymphoma. Br. J. Haematol. 2018;182:46–62. doi: 10.1111/bjh.15283. [DOI] [PubMed] [Google Scholar]
- 30.Lee MY, Tan TD, Feng AC, Liu MC. Clinicopathological analysis of 598 malignant lymphomas in Taiwan: Seven-year experience in a single institution. Am. J. Hematol. 2006;81:568–575. doi: 10.1002/ajh.20649. [DOI] [PubMed] [Google Scholar]
- 31.Chuang SS, Huang WT, Hsieh PP, Tseng HH, Chang HM. Striking male predominance of mantle cell lymphoma in Taiwan. J. Clin. Pathol. 2006;59:780. doi: 10.1136/jcp.2005.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YH, et al. Correlative analysis of overall survival with clinical characteristics in 127 patients with mantle cell lymphoma: A multi-institutional cohort in Taiwan. Int. J. Hematol. 2020;112:385–394. doi: 10.1007/s12185-020-02903-z. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi H, Ohshima K. Epidemiology of malignant lymphoma and recent progress in research on adult T-cell leukemia/lymphoma in Japan. Int. J. Hematol. 2018;107:420–427. doi: 10.1007/s12185-018-2430-6. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, et al. Nationwide statistical analysis of lymphoid malignancies in Korea. Cancer Res. Treat. 2018;50:222–238. doi: 10.4143/crt.2017.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chim CS, et al. Mantle cell lymphoma in the Chinese: Clinicopathological features and treatment outcome. Am. J. Hematol. 1998;59:295–301. doi: 10.1002/(sici)1096-8652(199812)59:4<295::aid-ajh5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 36.Yoon DH, et al. Treatment of mantle cell lymphoma in Asia: A consensus paper from the Asian Lymphoma Study Group. J. Hematol. Oncol. 2020;13:21. doi: 10.1186/s13045-020-00855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher RI, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N. Engl. J. Med. 1993;328:1002–1006. doi: 10.1056/nejm199304083281404. [DOI] [PubMed] [Google Scholar]
- 38.Lenz G, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J. Clin. Oncol. 2005;23:1984–1992. doi: 10.1200/jco.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi, Y., Mori, J. & Tanimoto, T. Treatment of older patients with mantle-cell lymphoma. N. Engl. J. Med.367, 1765; author reply 1765–1766. 10.1056/NEJMc1210783 (2012). [DOI] [PubMed]
- 40.Robak T, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N. Engl. J. Med. 2015;372:944–953. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 41.Robak T, et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19:1449–1458. doi: 10.1016/s1470-2045(18)30685-5. [DOI] [PubMed] [Google Scholar]
- 42.Lipsky A, Martin P. Bendamustine-rituximab in mantle cell lymphoma. Lancet Haematol. 2017;4:e2–e3. doi: 10.1016/s2352-3026(16)30187-9. [DOI] [PubMed] [Google Scholar]
- 43.Czuczman MS, et al. Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: Efficacy, tolerability, and safety findings. Ann. Hematol. 2015;94:2025–2032. doi: 10.1007/s00277-015-2478-9. [DOI] [PubMed] [Google Scholar]
- 44.Visco C, et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J. Clin. Oncol. 2013;31:1442–1449. doi: 10.1200/jco.2012.45.9842. [DOI] [PubMed] [Google Scholar]
- 45.Visco C, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: A multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017;4:e15–e23. doi: 10.1016/s2352-3026(16)30185-5. [DOI] [PubMed] [Google Scholar]
- 46.Chihara D, et al. Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15-year follow-up of a phase II study from the MD Anderson Cancer Center. Br. J. Haematol. 2016;172:80–88. doi: 10.1111/bjh.13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merli F, et al. Rituximab plus HyperCVAD alternating with high dose cytarabine and methotrexate for the initial treatment of patients with mantle cell lymphoma, a multicentre trial from Gruppo Italiano Studio Linfomi. Br. J. Haematol. 2012;156:346–353. doi: 10.1111/j.1365-2141.2011.08958.x. [DOI] [PubMed] [Google Scholar]
- 48.Romaguera JE, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J. Clin. Oncol. 2005;23:7013–7023. doi: 10.1200/jco.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 49.Watts, M. J. et al. ESHAP and G-CSF is a superior blood stem cell mobilizing regimen compared to cyclophosphamide 1.5 g m(-2) and G-CSF for pre-treated lymphoma patients: A matched pairs analysis of 78 patients. Br. J. Cancer82, 278–282. 10.1054/bjoc.1999.0915 (2000). [DOI] [PMC free article] [PubMed]
- 50.Lefrere F, et al. Sequential chemotherapy by CHOP and DHAP regimens followed by high-dose therapy with stem cell transplantation induces a high rate of complete response and improves event-free survival in mantle cell lymphoma: A prospective study. Leukemia. 2002;16:587–593. doi: 10.1038/sj.leu.2402406. [DOI] [PubMed] [Google Scholar]
- 51.Mills W, et al. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin's lymphoma. J. Clin. Oncol. 1995;13:588–595. doi: 10.1200/jco.1995.13.3.588. [DOI] [PubMed] [Google Scholar]
- 52.Visani G, et al. Bendamustine, etoposide, cytarabine, melphalan, and autologous stem cell rescue produce a 72% 3-year PFS in resistant lymphoma. Blood. 2014;124:3029–3031. doi: 10.1182/blood-2014-08-596668. [DOI] [PubMed] [Google Scholar]
- 53.Hueso T, et al. Bendamustine-EAM versus BEAM regimen in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in the frontline setting: a multicenter retrospective study from Lymphoma Study Association (LYSA) centers. Bone Marrow Transpl. 2020;55:1076–1084. doi: 10.1038/s41409-020-0783-y. [DOI] [PubMed] [Google Scholar]
- 54.Miura K, et al. Does more intensive therapy have effects on mantle cell lymphoma? A clinical experience from the Lymphoma Treatment Study Group in Japan. Int. J. Hematol. 2011;93:684–686. doi: 10.1007/s12185-011-0845-4. [DOI] [PubMed] [Google Scholar]
- 55.Chihara D, et al. Prognostic model for mantle cell lymphoma in the rituximab era: A nationwide study in Japan. Br. J. Haematol. 2015;170:657–668. doi: 10.1111/bjh.13486. [DOI] [PubMed] [Google Scholar]
- 56.Burney C, et al. Ibrutinib for relapsed mantle cell lymphoma after standard first line therapy and ASCT is efficacious but does not overcome the impact of POD24—a retrospective study from the LWP-EBMT. Blood. 2019;134:701–701. doi: 10.1182/blood-2019-123442. [DOI] [Google Scholar]
- 57.Le Gouill S, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N. Engl. J. Med. 2017;377:1250–1260. doi: 10.1056/NEJMoa1701769. [DOI] [PubMed] [Google Scholar]
- 58.Hilal T, et al. Rituximab maintenance therapy for mantle cell lymphoma: A systematic review and meta-analysis. Am. J. Hematol. 2018;93:1220–1226. doi: 10.1002/ajh.25226. [DOI] [PubMed] [Google Scholar]
- 59.Cohen JB. TP53 mutations in MCL: More therapy is not better. Blood. 2017;130:1876–1877. doi: 10.1182/blood-2017-08-803551. [DOI] [PubMed] [Google Scholar]
- 60.Eskelund CW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903–1910. doi: 10.1182/blood-2017-04-779736. [DOI] [PubMed] [Google Scholar]
- 61.Fenske TS, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: Analysis of transplantation timing and modality. J. Clin. Oncol. 2014;32:273–281. doi: 10.1200/jco.2013.49.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rule S, et al. Allogeneic stem cell transplantation as part of front line therapy for Mantle cell lymphoma. Br. J. Haematol. 2019;184:999–1005. doi: 10.1111/bjh.15723. [DOI] [PubMed] [Google Scholar]
- 63.Wang ML, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dreyling M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet. 2016;387:770–778. doi: 10.1016/s0140-6736(15)00667-4. [DOI] [PubMed] [Google Scholar]
- 65.Rule S, et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: A pooled analysis from three open-label studies. Br. J. Haematol. 2017;179:430–438. doi: 10.1111/bjh.14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCulloch R, et al. Ibrutinib for mantle cell lymphoma at first relapse: A United Kingdom real-world analysis of outcomes in 211 patients. Br. J. Haematol. 2021;193:290–298. doi: 10.1111/bjh.17363. [DOI] [PubMed] [Google Scholar]
- 67.Robinson S, et al. The EBMT/EMCL consensus project on the role of autologous and allogeneic stem cell transplantation in mantle cell lymphoma. Leukemia. 2015;29:464–473. doi: 10.1038/leu.2014.223. [DOI] [PubMed] [Google Scholar]
- 68.Wang M, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palomba ML, et al. Safety and preliminary efficacy in patients with relapsed/refractory mantle cell lymphoma receiving lisocabtagene maraleucel in transcend NHL 001. Blood. 2020;136:10–11. doi: 10.1182/blood-2020-136158. [DOI] [Google Scholar]
- 70.Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schuster SJ, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large b-cell lymphoma. N. Engl. J. Med. 2018;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 72.Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer45, 228–247. 10.1016/j.ejca.2008.10.026 (2009). [DOI] [PubMed]
- 73.From the RECIST committee Schwartz, L. H. et al. RECIST 1.1-Update and clarification. Eur. J. Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.