Abstract
Kidney organoid technology has led to a renaissance in kidney developmental biology. The complex underpinnings of mammalian kidney development have provided a framework for the generation of kidney cells and tissues from human pluripotent stem cells. Termed kidney organoids, these 3-dimensional structures contain kidney-specific cell types distributed similarly to in vivo architecture. The adult human kidney forms from the reciprocal induction of two disparate tissues, the metanephric mesenchyme (MM) and ureteric bud (UB), to form nephrons and collecting ducts, respectively. Although nephrons and collecting ducts are derived from the intermediate mesoderm (IM), their development deviates in time and space to impart distinctive inductive signaling for which separate differentiation protocols are required. Here we summarize the directed differentiation protocols which generate nephron kidney organoids and collecting duct kidney organoids, making note of similarities as much as differences. We discuss limitations of these present approaches and discuss future directions to improve kidney organoid technology, including a greater understanding of anterior IM and its derivatives to enable an improved differentiation protocol to collecting duct organoids for which historic and future developmental biology studies will be instrumental.
Keywords: kidney development, stem cell, kidney organoids, metanephric mesenchyme, ureteric bud, nephron
Introduction
Developmental biology has informed the derivation of human stem cell-derived cells and tissues. Directed differentiation protocols, involving the sequential application of small molecules and growth factors to mimic signals governing organogenesis, have been deciphered to generate the major organs of the human body, including the kidney1. The adult kidney develops from the reciprocal induction of the MM and UB. Derived from the posterior intermediate mesoderm (pIM), the MM is a heterogenous populations of nephron progenitor cells (NPCs), stromal progenitor cells (SPCs), and endothelial progenitor cells (EPCs), which give rise to nephron structures and their surrounding interstitium. Derived from the anterior intermediate mesoderm (aIM), UB progenitor cells differentiate into the branched collecting duct network that drains individual nephrons to the bladder via a solitary ureter. Importantly, the formation of nephron structures is predicated upon inductive signals from developing collecting duct, and vice versa, in a process termed reciprocal induction. Given their disparate origins, separate directed differentiation protocols have been required to generate nephron kidney organoids and collecting duct kidney organoids.
Historically, studies in kidney developmental biology have been enriched by knowledge of congenital anomalies of the kidney and urinary tract (CAKUT), a group of quite common genetic disorders often stemming from the malunion of the MM and UB. Mutations involving growth factors and their receptors responsible for CAKUT has shed light on the signaling pathways involved in the separate formation of the MM and UB, as well as their reciprocal induction. Drawing from these studies, kidney organoids have been generated from modulated Wnt, FGF, TGF-β/BMP, and RA signaling, as discussed in detail below. Due to the cessation of nephrogenesis by birth in humans2, kidney development has historically been of little clinical importance, in terms of mitigatable disease. However, the advent of kidney organoids has re-invigorated kidney developmental biology, which should be instrumental in improving organoid technology towards revolutionizing the fields of drug development and regenerative medicine.
Intermediate mesoderm/kidney development
Three germ layer and primitive streak
Mammalian development begins with the union of male and female haploid gametes, forming a single diploid pluripotent stem cell which undergoes iterative proliferation and differentiation. After approximately 7 cell doublings, a blastula consists of a hollow spheroid of ~128 (2^7) undifferentiated cells. The primitive streak forms in the caudal and mid-line aspect of the blastula to determine the site of gastrulation, initiate germ layer formation, and establish bilateral symmetry via cranial-caudal and midline-lateral body axes3. The outer cell layer surrounding the primitive streak forms ectoderm destined to predominantly become the integumentary and nervous systems. The inner cell layer that involutes through and lines the primitive streak forms endoderm destined to primarily form the lining of the digestive and respiratory systems, as well as the liver and pancreas. In a process termed gastrulation, mesodermal progenitor cells involute through the primitive streak and migrate cranially and laterally to form a middle layer sandwiched between an outer ectoderm and inner endoderm. The mesoderm gives rise to the musculoskeletal, circulatory, reproductive, and urinary systems, including the mammalian kidney4.
Mesoderm differentiation
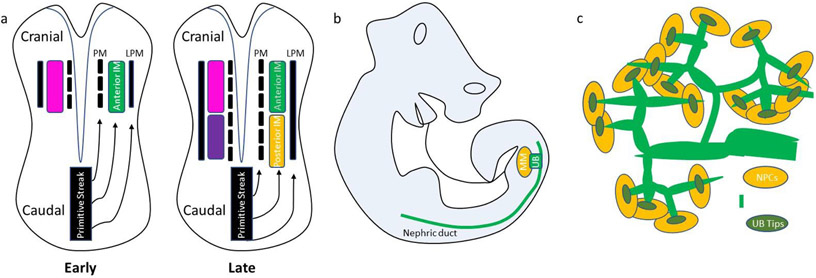
The kidney primordia arises from spatiotemporal patterning of the mesoderm. The cranial-caudal axis of the mesoderm is temporally patterned based on the duration of gastrulation, resulting in differing lengths of exposure to Wnt signaling in the primitive streak5. The midline-lateral axis of the mesoderm is spatially patterned across the anterior-posterior axis of the primitive streak, due to an increasing BMP4 gradient. Fate mapping in the mouse has demonstrated that mesodermal progenitors involuting through the anterior primitive streak become the paraxial mesoderm (PM), mid-primitive streak become intermediate mesoderm (IM), and posterior primitive streak become lateral plate mesoderm (LPM)6-9. From a signaling perspective, the duration of Wnt signaling and the degree of BMP signaling are major determinants governing differentiation from pluripotency to mesodermal organ primordia10. As the kidney arises from the anterior and posterior IM, it derives from mesodermal progenitors involuting through a similar region of mid-primitive streak over time (Figure 1a).
Figure 1. Diagrams of mammalian kidney development.
(a) A cranial-caudal axis in the primitive streak determines the laterality of gastrulating mesoderm, PM = Paraxial Mesoderm, LP = Lateral Plate Mesoderm. Early primitive streak forms anterior mesoderm while the late differentiates into posterior mesoderm.
(b) The ureteric bud (UB) originates from the anterior intermediate mesoderm (aIM) and develops as a caudal outpouching of the nephric duct to invade adjacent metanephric mesenchyme (MM), which is derived from the posterior intermediate mesoderm (pIM).
(c) Branching UB stalks terminate in distinct clusters of UB tip cells which invade nephron progenitor cells (NPCs) of the cap mesenchyme to initiate reciprocal induction.
Kidney development: Disparate MM and UB development
The vertebrate kidney develops in structurally distinct stages, from a nonfunctional pronephros to a rudimentary mesonephros, and ultimately to the metanephros, which persists as the adult kidney11. The functional stages contain nephron structures that interact with the nephric duct for drainage to the cloaca, a common excretory chamber of the urinary, digestive, and reproductive tracts prior to partitioning into the urogenital sinus and the rectum12. The nephric duct contributes to each nephric stage, as the pronephric duct, mesonephric duct, and metanephric duct, also known as the ureteric bud (UB). The pronephros and mesonephros are anterior IM derivatives, while the metanephros arises from structures originating from both anterior IM (aIM) and posterior IM (pIM)4. Studies in mice have traced the origins of the metanephros to the reciprocal induction of the pIM-derived metanephric mesenchyme (MM) and aIM-derived UB13,14 (Figure 1b,c).
The MM is a heterogenous collection of nephron, stromal, and endothelial progenitor cells. The contiguous epithelial compartments of the nephron, including podocytes of glomeruli, their surrounding parietal epithelial cells, and proximal, loop of Henle, distal, and connecting tubules, arise from SIX2+SALL1+CITED1+HOXD11+GDNF+ nephron progenitor cells (NPCs) that form pretubular aggregates in cap mesenchyme (CM)15. The CM is surrounded by FOXD1+MEIS1+ stromal progenitor cells (SPCs) which give rise to peritubular fibroblasts and perivascular cells, namely mesangial cells supporting glomerular capillaries and pericytes supporting interstitial capillaries16,17. These capillary beds and their contributories are derived from FLK1+TIE2+ endothelial progenitor cells (EPCs) that infiltrate an avascular cap mesenchyme during the early stages of nephrogenesis18,19. Collectively, MM-derived NPCs, SPCs, and EPCs form the majority of the kidney cortex, consisting of nephrons surrounded by supporting stroma and penetrating blood vessels20. Following gastrulation, MM-fated cells receive TGF-β/BMP signaling21,22 and FGF signaling23 to establish and maintain the NPC niche. As examples in mice, the BMP7 knockout is a postnatal lethal mutation associated with markedly reduced nephron number24, from massive apoptosis of NPCs and early defects in nephrogenesis25, and the FGF receptor 1/2 conditional knockout in the kidney primordia do not develop MM26. Moreover, FGF9 and FGF20 signaling through these receptors has been shown to be necessary and sufficient to maintain the stemness of the NPC niche23.
The UB, or metanephric duct, generates the branched urinary collecting system of the kidney, which initially develops as a solitary caudal outpouching of the common nephric duct27. The origin of the nephric duct, and its derivatives, from the aIM suggests development from early mid-primitive streak, with fate determined by relatively brief Wnt and intermediate BMP4 signaling during gastrulation, as previously described. Following gastrulation, the aIM contributes to significantly more structures than the nephric duct alone, including the pronephros, mesonephros, and gonadal structures from the Wolffian duct in males and Mullerian duct in females28. A retinoic acid (RA) gradient patterns the anterior to posterior axis of the developing IM, such that RA signaling is required for the formation of the aIM and its derivatives.29-31 The origins of the UB have been traced to aIM-derived RET+GFRA1+ cells, with these markers highly expressed in mesenchymal UB tip cells and diminished in trailing epithelial UB stalk cells32. Of major consequence towards kidney development, the nephric duct initially forms as a discontinuous tubular structure destined to connect MM-derived nephrons with the cloaca, or primitive bladder. The initial stalk of the UB connects to the developing bladder as the ureter, while UB tip cells undergo iterative branching to form collecting ducts which drain individual nephrons during nephrogenesis. Although there is variability, nephrogenesis ceases after forming approximately 1,000,000 nephrons by birth in humans and around 16,000 nephrons by the early postnatal period in mice33. Genetic studies in mice have demonstrated that Wnt, Fgf, and Ret signaling is required for branching morphogenesis of the ureteric bud to form the urinary collecting system34.
Kidney development: Reciprocal induction of the MM and UB
The adult mammalian kidney, or metanephros, is generated by the reciprocal induction of pIM-derived MM and aIM-derived UB. In the mid-1950s, Clifford Grobstein developed assays of kidney development to find that microdissected MM and UB in coculture are necessary and sufficient to reconstitute kidney structures ex vivo, demonstrating that these populations are sufficiently programed at this stage to drive kidney organogenesis35. He further demonstrated that MM separated from UB fails to undergo nephrogenesis, but that UB could be replaced by a distal portion of the embryonic spinal cord to provide sufficient signaling to induce nephrogenesis in MM36. In fact, developing spinal cord was a stronger inducer of MM than UB. In the decades since, multiple members of the Wnt, FGF, and TGF-β/BMP signaling pathways are required for the reciprocal induction between the MM and UB for proper kidney development. It was found that UB-derived Wnt expression is required for mesenchymal-epithelial transition (MET) of the MM to form contiguous nephron epithelial structures, as mice lacking Wnt4 activity fail to form pretubular cell aggregates11. Unsurprisingly, Wnt4 is highly expressed in the developing spinal cord37. In addition to Wnt-4, the UB maintains self-renewal of NPCs by providing Wnt9b and the TGF-β superfamily member, Activin A, as differentiation factors for the MM15,38. In turn, the MM produces factors which govern both UB budding and subsequent branching morphogenesis. Disruption of budding of the UB from the nephric duct can result in renal agenesis, duplex kidneys, and improper positioning of the ureter27. As branching morphogenesis of the UB is integral to nephrogenesis, defects have been associated with reduced nephron number39. BMP4 expressed by the MM signals through BMP type receptors in the nephric duct to ensure a solitary UB develops, as heterozygotic Bmp4 knockout mice demonstrate hypo/dysplastic kidneys with ectopic and accessory ureteral budding40. FGF signaling also plays a role in UB budding as Fgf10 mutant mice exhibit renal agenesis associated with absence of the UB41. Perhaps best described, the MM produces GDNF, which signals through its receptor, RET, and coreceptor, GFRA1, in the UB to govern branching morphogenesis27. Gdnf expression in the mouse is regulated by expression of other NPC markers, Six242, Sall143, and Eya144, reflecting its production by future nephron epithelia to signal to the developing UB. Lack of Gdnf, Gfra1, or Ret in mice is perinatally lethal due to bilateral renal aplasia or agenesis45. Due to a multitude of paracrine signaling, the UB buds and infiltrates adjacent CM. Responsive aggregates of NPCs undergo MET and sequential morphogenesis through comma-shaped and S-shaped bodies comprised of nephron epithelia, patterned in a proximal to distal orientation 15,46-48. The proximal portion develops into clusters of podocytes surrounded by parietal epithelial cells of Bowman’s capsule. FLK1+ EPCs and FOXD1+ SPCs invade the glomerular cleft of the S-shaped body to establish the glomerular vascular pole and give rise to glomerular capillaries and mesangial cells, respectively49,50. The connecting tubules of individual nephrons converge in a branched collecting system derived from the UB, following reciprocal induction between the two tissues outlined above51.
Owing to the complexity of the union between the MM and UB in forming the adult kidney, urinary tract anomalies are among the most common birth defects in humans, with estimated occurrence as high as 1 in 250 live births. These heterogenous malformations are commonly referred to as CAKUT52. RET mutations have been reported to be found in 5-30% of CAKUT patients, noting that varied Ret mutants in the mouse display a spectrum of urinary tract defects similar to CAKUT in humans53. More recently, whole-exome sequencing applied to 232 familial cohorts manifesting CAKUT identified causative mutations in 30% of known or highly suspected monogenic CAKUT genes54. Notably, no known or suspected mutation was detected in more than 50% of the families, which illustrates our ongoing knowledge gaps in human kidney development. Some of these gaps may be due to inter-species differences. Recent studies have deciphered convergent and divergent features between human and mouse kidney development, including progenitor niche organization, kidney lobe formation, and the expression of cell type-defining markers55. In such studies, human kidney has been obtained from abortive embryonic tissue, permitting insights rather than experimentation. Using mammalian kidney development as an archetype, protocols to generated kidney cells and tissues from human pluripotent stem cells (hPSC) may enable testable hypotheses in human kidney development. Towards this end, the determination that MM is derived from the posterior IM, and UB from the anterior IM14, along with characteristic marker expression, affords a roadmap for the specific induction of these two kidney lineages. We will discuss the deciphered methods to efficiently induce MM-derived nephron epithelia and UB-derived collecting duct epithelia from human pluripotent stem cells, whose combination may represent the optimal method to developing human kidney tissue ex vivo for developmental studies and translational applications towards drug development and regenerative medicine.
In vitro differentiation of stem cells
Drawing from our knowledge of mammalian kidney development, multiple reproducible methods have been deciphered which turn human pluripotent stem cells (hPSCs) into kidney cells and tissues, termed kidney organoids56. While there is some debate to its definition, we accept Hans Clevers’ definition of an organoid as a 3-dimensional structure grown from stem cells and consisting of organ-specific cell types that self-organizes through spatially restricted lineage commitment57. Such organoids exhibit high similarities with their in vivo counterparts in terms of tissue morphology, cell types and content, and cellular proliferation and differentiation58.
Differentiation towards nephron kidney organoids
Many of the established protocols, and subsequent analysis, have been dedicated to the generation of nephron kidney organoids. In elegantly designed experiments, Taguchi et al performed microarrays on developing embryonic tissue to determine which signalling pathways were active during the formation of the MM at key time points. They used an Osr1-GFP reporter mouse, noting that Osr1 is one of the earliest IM markers and persists in the renal precursor populations through MM59,60, to flow sort renal precursors and perform re-aggregation assays informed by their microarray-based signalling pathway analysis. This seminal work was the first to demonstrate the differential cell fate between the aIM and pIM outlined above14. They attributed their success in generating NPCs from mouse and human PSCs to their backwards approach, first determining the factors which induce NPCs from pIM, then determining those governing pIM induction from late primitive streak and late primitive streak induction from hPSCs. Rather than starting by differentiating ESCs, their backwards approach was deemed necessary as reliable markers and detection assays were best known for the MM stage. Moreover, the intermediate cell types were often multipotent populations which may give rise to a variety of tissues, and thus do not manifest specific markers towards the generation of MM14. In the end, or at the start, OCT3/4+SOX2+ hPSC spheroids were grown on suspension culture and administered extended Wnt activation with the glycogen synthase kinase 3 (GSK-3) inhibitor, CHIR99021 (CHIR)61, to form T+TBX6+ late primitive streak, which is destined to contribute to posterior mesodermal structures. The medial-lateral axis of the mesoderm was directed towards IM formation by concurrent treatment with BMP4. Addition of Activin and RA induced WT1+HOXD11+OSR1+ posterior IM (pIM). Next, FGF9 was added to support the development of SIX2+SALL1+WT1+ NPCs, with a reported induction efficiency of 62%. While NPCs were generated from both mouse and human PSCs, co-culture with mouse embryonic spinal cord was necessary to further differentiate them into proximal tubules, distal tubules, and podocyte clusters14.
The following year, Morizane et al published a differentiation protocol that generated SIX2+SALL1+WT1+ NPCs with up to 90% efficiency by differentiation day 8-962. hPSCs were treated with an extended period of CHIR to mimic Wnt signaling and BMP4 activity was modulated to induce T+TBX6+ late primitive streak. Next, Activin was applied to induce OSR1+WT1+HOXD11+ pIM, which further differentiated into SIX2+SALL1+WT1+ NPCs in response to FGF9. These NPCs were then transitioned to 3-dimensional suspension culture by centrifugation in ultra-low adhesion plates. Then exposure to a CHIR pulse, simulating UB-derived Wnt signaling, catalyzed MET in NPCs, which are subject to FGF9 to expand and maintain the stemness of the NPC niche23. After the CHIR pulse, and in the presence of ongoing FGF9, NPCs differentiated into LAM1+ renal vesicles by day 14 through a pre-tubular aggregate intermediate. In the absence of further factors, renal vesicles self-patterned into nephron structures of sequentially connected structures that included NPHS1+PODXL+ podocytes, LTL+CDH2+ proximal tubules, CDH1+UMOD+ loop of Henle, CDH1+UMOD− distal tubules, and CDH1+AQP2+ connecting tubules63. Both the protocols of Taguchi and Morizane shared induction of late primitive streak with extended Wnt and modulated BMP4 signaling, followed by induction of pIM with Activin A, and then FGF9 was applied to induced MM which was subject to Wnt signaling using either CHIR or embryonic spinal cord to catalyze MET.
Additional differentiation protocols, often predicated on maximal induction of PAX2+ or OSR1+ IM cells as opposed to SIX2+ NPCs, have generated nephron kidney organoids, as well. 64-66. After reporting the paradoxical simultaneous induction of aIM-derived UB and pIM-derived MM progenitors, based on non-specific ureteric epithelial markers PAX2+GATA3+ 67, Takasato et al demonstrated that varied duration of initial CHIR treatment, prior to the addition of FGF9, was one of the critical factors for the differential induction of aIM-derived structures versus those of the pIM. Shorter duration Wnt signaling induced LHX1+GATA3+ aIM and longer duration induced HOXD11+EYA1+ pIM. Along with duration of Wnt signaling, they found that RA also controls the anterior-posterior patterning of the IM with RA signaling promoting aIM-derived UB markers. This anterior-posterior RA gradient has been attributed to the inactivation of RA via CYP26B1 expression in the posteriorly located primitive streak68. Using this knowledge, they modified their kidney differential protocol to favor MM induction to find that extended CHIR for 4 days, followed by FGF9 for 3 days co-induced ureteric epithelium and metanephric mesenchyme. Despite reportedly containing both UB and MM progenitors, the MM required a CHIR pulse (to simulate UB-derived WNT9b) to undergo MET and form segmented nephrons containing 4 components: WT1+ glomeruli, LTL+ECAD− early proximal tubule, GATA3−LTL−ECAD+ early distal tubule, and GATA3+ECAD+ collecting duct. However, the Takasato protocol has been demonstrated to induce a significant neuronal lineage69, for which GATA3 is an important developmental marker70. While GATA3 specificity for the UB may be demonstrated by coupled expression with the non-neuronal marker, CDH1, and the off-target neuronal population explained by batch-to-batch heterogeneity71, GATA3 is not specific to the UB and its derivatives but expressed in distal tubules in mammalian kidney samples72.
Notably, Freedman et al employed a sandwich Matrigel method to form cavitated epiblast spheroids by transitioning hPSCs to adherent 3-dimensional culture. A directed differentiation protocol based on one initially designed for cardiomyocyte generation in 2-dimensional culture was employed. In a two-step protocol, epiblast spheroids were first subject to relatively short duration CHIR (1.5 days) followed by extended B27-supplemented media to form tubular epithelial structures via MET. Immunostaining confirmed the induction of nephron structures consisting of peripheral PODXL+WT1+SYNPO+ podocyte clusters which aggregated at the termini of LTL+ proximal tubular structures in series with CDH1+ loop of Henle/distal tubular structures73. Additionally, CD31+vWF+ endothelial cords arose within organoids to associate with tubular structures and podocyte clusters suggestive of vascular structures.
The outlined differentiation protocols to nephron kidney organoids were published by 2015. In the years since, many groups have followed similar approaches to direct the differentiation of hPSCs through intermediate stages of late primitive streak, pIM, and MM to form contiguous nephron structures in 3-dimensional tissue69,74-76. Many of these studies were performed to characterize constituents and their maturity. Wu et al. performed transcriptional profiling of the Morizane and Takasato protocols at the single cellular level, comparing them against each other as well as similar datasets from 16-week gestation human fetal kidney and 62-year old human adult kidney. Major findings included i) the identification of at least 12 distinct kidney cell types in both protocols, ii) distal tubules in both protocols expressed GATA3, iii) that nephron epithelia were more similar to fetal kidney than adult at day 26 in both protocols, iv) the Morizane protocol induced more mature podocytes, proximal tubules, and loop of Henle (based on reduced developmental and higher differentiation marker expression), v) the Morizane protocol induced half as many non-renal specific cell types (i.e. muscle, neurons) compared to the Takasato protocol (11% vs 21%), vi) the neuronal population in Takasato organoids was reduced by inhibiting the brain-derived neurotrophic factor (BDNF) pathway, and vii) Takasato organoids demonstrated less differentiation across most cell types, lost the endothelial cluster, and had emergence of a muscle cluster in extended culture to day 3469.
Subsequent single cell transcriptomics of Morizane nephron kidney organoids from Tran et al. supported the application of organoid-derived podocytes for glomerular filtration and disease modeling. On subcapsular transplantation, organoid-derived podocytes recruited host vasculature to enable further maturation74. Garreta et al progressed the transcriptional profile of organoids to second trimester human fetal kidney by day 16 through cell-cell and cell-matrix interactions facilitated by embedment in a soft hydrogel76. Homan and Gupta et al coupled organoid and organ-on-a-chip technologies to demonstrate fluidic shear stress enhances the functional maturation and vascularization of nephron kidney organoids from the Morizane protocol. Tubular maturation was demonstrated by increased polarization and solute transporter expression, while glomerular maturation was evidenced by enhanced podocyte foot processes, increased expression of slit diaphragm proteins, and formation of vascularized glomeruli manifesting early glomerular basement membranes77 Applying single cell transcriptomics to a similar nephron organoid protocol, Low et al suggested a divergent source of kidney vasculature between mouse and human, with a subset of human NPCs as a source for FLK1+ EPCs75 . Here it is worthwhile to note that increasing differences have been identified between human and mouse kidney development33,78.
Lastly, nephron kidney organoids have been produced by direct reprogramming to NPCs by Hiratsuka et al. An initial transcriptional factor combination of FIGLA, PITX2, ASCL1, and TFAP2C differentiated hPSCs into SIX2+SALL1+ NPCs with 92% efficiency in 2 days. Thereafter, a second set consisting of HNF1A, GATA3, GATA1, and EMX2 differentiated the NPCs into PAX8+LHX1+ pretubular aggregates in another 2 days. In extended 3-dimensional culture, the pretubular aggregates stochastically differentiated into nephron structures, whose global gene expression profile was similar to human adult kidney79. Importantly, the authors chose to induce the multipotent NPC population in efforts to generate the entirety of nephron epithelia. A second set of transcription factors was required as a relatively pure population of NPCs lacked inductive signaling from other kidney progenitor populations.
Taken together, the published protocols for the generation of nephron kidney organoids have many commonalities. While it is easy to focus attention of the differences, most protocols share i) extended Wnt signaling towards the induction of posterior mesoderm, ii) modulated BMP4 activity (added BMP4, BMP4 inhibition, or BMP4 present in basal media) to pattern to IM, iii) FGF9 addition to induce and maintain the NPC niche in the MM. Additionally, Activin A was employed to induce pIM in the protocols which have yielded the highest induction efficiency of SIX2+ NPCs. Notably activin signaling activates Hoxd11, which regulates the posterior specification of the metanephros80. Since this finding, it has been reported that SIX2+ NPCs generated without Activin form aIM-derived mesonephric kidney organoids, with lower SIX2 induction (~34%) and absent HOXD11, while similarly induced SIX2+ NPCs generated with Activin formed pIM-derived metanephric kidney organoids with greater SIX2 and HOXD11 induction81. Mesonephric kidney organoids were confirmed to lack expression of two typical loop of Henle markers, UMOD and NKCC2, which were present in the metanephric kidney organoids81, as the mammalian mesonephros has been reported to lack a loop of Henle82. As the mesonephric kidney ultimately degrades, perhaps it is unsurprising that nephron kidney organoids generated without Activin lost differentiation markers in extended culture69.
Differentiation towards collecting duct kidney organoids
While 2015 was an influential year for the development of nephron kidney organoids, the time since has seen a dramatic degree of publications towards hPSC-derived collecting duct kidney organoids. Yet one of the first demonstrations of directed differentiation to kidney tissue of any sort was published in 2013, prior to the demonstration that MM originates from the pIM and UB from the aIM. Xia et al. published a two-step protocol over the course of 4 days to generate hPSCs capable of differentiation into the renal lineage. In the first step, mesodermal fate was induced by a combination of FGF2 and BMP4 for 2 days. Thereafter, RA and Activin A was applied for 2 days with the resultant hPSC-derived cellular population(s) simultaneously expressed nephron lineage markers (SIX2, CITED1, SALL1, GDNF) and collecting duct lineage markers (RET, GFRA1, HOXB7), suggesting the stochastic differentiation of induced aIM into both mesonephros and UB progenitors. Termed UB progenitors, these cells were combined with E11.5 developing murine kidney to mature into chimeric ureteric bud structures in 3-dimensional culture. Limitations of this early work include the dependency on chimeric reaggregation to facilitate the maturation of hPSC-derived cells past a progenitor cell state and a lack of in vitro branching of the induced cells. Such chimeric tissue may have limited translation to human development and disease modeling, while being poorly suitable for regenerative medicine strategies, due to xenogenic contamination. Moreover, the presence of significant undefined factors derived from the developing mouse kidney may limit reproducibility of the protocol. While their methodology has not served as the foundation for ensuing studies of UB induction, there were notable findings when viewed retrospectively. While performed prior to identifying that disparate duration of Wnt signaling determines the anterior-posterior axis of the IM, Xia’s finding of a shortened first step with modulated BMP4 activity is consistent with early mid-primitive streak induction destined for aIM fate. Meanwhile, use of RA in the second step have been implicated in aIM development, as previously described, and is a common UB-inducing factor in the ensuing improved directed differentiation protocols towards collecting duct epithelium83,84.
Employing a similar reverse induction approach to their protocol towards nephron progenitor cells, Taguchi et al first identified factors which induce the maturation of primary murine WD into UB through microarray analysis. Through reaggregation assays of flow sorted primary murine WD, the combination of RA, Wnt signaling, and Fgf9 enriched critical markers of UB maturation (namely Wnt9b, Hnf1b, Pax2 and Emx2), while inducing the UB tip cell marker, Ret. The addition of Gdnf was required to support Wnt11 expression, which facilitated bud-like formation. Using similar methodology, they found that the same factors at varying concentrations matured WD precursors into WD. In an ongoing backwards approach, they sought to identify faithful markers of WD precursor cells. Interrogating in vivo microarray data, Cxcr4 and Kit were identified as cell surface markers highly expressed in WD precursors which permit FACS isolation. To maximize the induction of Cxcr4+/Kit+ WD progenitors from mESCs, they optimized their protocol for the induction of aIM (as opposed to pIM14) by reducing the duration of the initial mesoderm-inducing Wnt signaling and finding that ongoing Wnt agonism, coupled to RA and FGF9 signaling, enhanced the induction of aIM markers. Additional major findings included i) Activin suppressed aIM induction (suggesting that Activin/Tgfb signaling is a primary driver that differentiates pIM from aIM), ii) addition or inhibition of BMP4 signaling suppressed aIM (suggesting that an optimal BMP4 concentration is required for aIM induction), and iii) removal of Wnt markedly reduced the Cxcr4+Kit+ population (suggesting a crucial role for Wnt signaling towards WD precursors). Flow-sorted Cxcr4+Kit+ cells (35.6% induction efficiency) were sequential matured from WD precursors to WD and induced UB (iUB) using factors determined by the above backwards approach. iUB formed branched structures on extended culture in defined media in the absence of nephron organoids. In coculture with primary murine MM (including SPCs), an isolated bud from the iUB underwent dichotomous branching with ureteric tips connecting to the distal end of MM-derived nephron structures. Subsequent reaggregation assays between primary and induced populations demonstrated that both SPCs and NPCs were required to induce sufficient UB branching. The necessity of mouse primary stromal progenitors highlights a detrimental effect of cell purification by flow-sorting; the restriction of progenitor populations required for kidney development through multilineage induction. Adapting the differentiation protocol to hPSCs enabled the formation of branched UB organoids in the absence of additional cell types. However, coculture of flow-sorted human UB organoids with MM failed to undergo reciprocal induction due to premature cessation of UB branching, likely due to the lack of human stromal progenitors85. Perhaps the cause of incomplete branching could be discerned from the use of primary human stromal progenitors, or their hPSC-derived equivalent, yet improved induction of RET+ UB tip cells concurrently with a SPCs population may be of greatest benefit.
Mae et al had two relevant publications, from the Osafune lab, towards the generation of early collecting duct kidney organoids. First they established a protocol to induce nephric duct progenitor cells in 2-dimensions, which formed branched ureteric epithelial structures in 3-dimensional culture86. In a subsequent publication from the same group, their initial protocol was modified, particularly adding RA to an extended protocol step to induce nephric duct aggregates and removing off-target cells by pipetting. Importantly, the addition of 2% Matrigel to the nephric duct aggregates induced polarized and luminal UB-like structures with basal LAMININ and apical EZRIN expression. Resultant UB organoids contained Cytokeratin-8 (CK8)+ trunk domains capped by distinct RET+ tip domains, which when microdissected led to expansion of UB tips with the reconstitution of UB organoids through epidermal growth factor (EGF) and FGF1-induced branching morphogenesis. As Wnt signaling maintains the UB tip niche87, UB organoids were subject to Wnt inhibition which promoted differentiation into AQP2+ tubular epithelia, termed collecting duct progenitors, which manifest few markers of principal cells and lacked intercalated cell markers in RNAseq. While promising, these results suggested immature collecting duct organoids which lacked the segmentation into discrete units seen with nephron kidney organoids derived from SIX2+ NPCs88.
Rather than separately inducing MM and UB, Uchimura et al. combined hPSC-derived aIM and pIM with intentions of forming kidney organoids containing both nephron and collecting duct elements. Treatment with circulating hormones important for collecting duct function in vivo induced segmentation of a tubular structure bearing markers of the collecting duct. The Takasato protocol was employed to induce pIM65 in parallel with a modified Taguchi protocol to induce aIM, both by day 785. Monolayer cultures of aIM and pIM were mixed, subject to an exogenous CHIR pulse, and then cultured in FGF9, GDNF, RA, and EGF until day 12. Following stochastic differentiation until day 26, CK8+ branched structures formed which required hormonal stimulation to further mature. Applying single cellular transcriptomics to treated samples, aldosterone and arginine vasopressin drove the segmentation and maturation of UB cells into principal and intercalated cells by pseudotime trajectories, as well as the first demonstration of Urothelial cells present in kidney organoids89. Their methodology deviates from kidney organogenesis, as the aIM and pIM develop into UB and MM separately with significant spatial deviations prior to reciprocal induction. As the aIM is a multipotent population that gives rise to pronephros, mesonephros, male and female gonadal structures, and the nephric duct, the hope would be that pIM would induce aIM fated to preferential differentiate into nephric duct. However, the requirement for both Wnt signaling (CHIR pulse) and GDNF, factors produced by aIM-derived UB and pIM-derived MM respectively, depicts a lack of reciprocal induction. The result of generating aIM and pIM separately, dissociating to single cell, and aggregating the populations causes loss of polarity between the invading UB into the CM. Additionally, a response to aldosterone and arginine vasopressin suggest the presence of the mineralocorticoid receptor and vasopressin receptors, neither of which are specific to the kidney and are expressed in other mesodermal tissue90,91.
Howden et al. demonstrated that the distal nephron epithelia of developing nephron organoids harbor sufficient plasticity to be coaxed into ureteric epithelial fate. Isolated distal nephron segments from organoids were cultured in ureteric epithelial media to form induced ureteric stalks and tips. Separated UB tips could be expanded, serially passaged, and recombined with NPCs to form combined nephron and collecting duct kidney organoids92. However, AQP2+ tubular structures in the distal nephron, which may be interpreted as collecting duct, may be connecting tubules62. Importantly, connections made by connecting tubules may be interpreted as branching. It may be difficult to discern the connecting tubule from collecting duct, as both express mineralocorticoid receptor-expressing principal cells93 and intercalated cells94. Additionally, as ureteric epithelia are generated from the aIM, it remains unclear whether the distal nephron epithelia formed from pIM-derived MM would maintain plasticity to adopt a connecting tubule or collecting duct phenotype.
More recently, Zeng et al. first identified culture conditions (FGF9, EGF,Y27632) which supported the expansion and differentiation of primary UB progenitor cells isolated from human fetal kidney, to guide the differentiation of hPSC-derived UB progenitors into collecting duct kidney organoids. To generate hPSC-derived UB progenitors, they used a dual reporter hPSC line for PAX2-mCherry and WNT11-GFP to test the ability of existing differentiation protocols for generating PAX2+WNT11+ UB progenitor cells. The induction efficiency for UB progenitors in the above protocols by Taguchi et al, Xia et al, and Mae et al was determined based on the induction of GFP+ positivity reflecting WNT11+ expression. While WNT11 was expressed at the mRNA level in some of the tested protocols85,88, the authors reported limited induction of WNT11+ based on GFP positivity. Using the dual reporter line, they developed a novel 7-day directed differentiation protocol to PAX2+SOX9+GATA3+PAX8+KIT+KRT8+ aIM cells, however, required flow sorting of the 13.2% PAX2-mCherry+ cells due to low induction efficiency. Purified PAX2+ cells were subject to the culture conditions that supported their initial testing in primary UB progenitor cells. Subsequent WNT11+ branched structures were induced at ~3 weeks, with these UB organoids demonstrating expansion of UB tip cells95. To determine whether their results extended to induced pluripotent stem cell lines, they employed a SOX9-GFP hiPSC line, flow-sorted a 60.2% SOX9-GFP population on day 7, and subject to the same culture conditions, yet WNT11 induction was not demonstrated. Importantly, they report a protocol to induce a PAX2+SOX9+GATA3+PAX8+KIT+KRT8+ population, yet the induction efficiency for PAX2 in embryonic stem cells was discordant with SOX9 induction in induced pluripotent stem cells, with unknown induction efficiency for cells collectively bearing the 6 reported markers. The reciprocal induction of hPSC-derived UB organoids with MM organoids remains a challenge, noting that the authors generated a RET knock-out in this work.
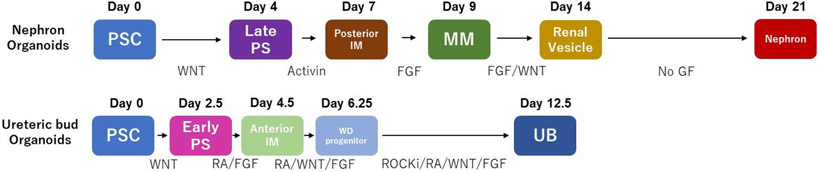
Taken together, the published protocols for the generation of ureteric bud organoids share many commonalities (Figure 2), particularly protocols derived work from the lab of Nishinakamura in 2017. These include i) shortened Wnt signaling towards the induction of anterior mesoderm, ii) addition of RA and Wnt agonists to simulate anterior mesodermal signaling, and iii) lack of Activin (shown to promote pIM differentiation). As suggested by prior work, early steps of kidney differentiation to nephron kidney organoids is dependent on fine-tuning BMP4 signaling62. Similarly, a specific degree of BMP4 signaling appears to provide optimal conditions to induce the UB organoids85. Perhaps differing cell lines, maintained in stem cell media of varying BMP4 activity, then subject to differentiation media of varying BMP4, may need to be optimized for improved generation of UB organoids.
Figure 2. A developmental timeline diagram of in vitro differentiation of PSCs.
PSC: pluripotent stem cell. PS: primitive streak. IM: intermediate mesoderm. MM: metanephric mesenchyme. WD: Wolffian duct. UB: ureteric bud. FGF: fibroblast growth factor. RA: retinoic acid. ROCKi: ROCK inhibitor.
Conclusion:
Kidney developmental studies have enabled the generation of hPSC-derived human kidney ex vivo. Significant strides have been made to first develop, and further modify, protocols to generate kidney organoids bearing nephron or collecting duct structures. Numerous groups have published independent directed differentiation protocols to kidney which collectively share similar sequential signaling pathway activation through intermediate cells bearing characteristic markers. The utility of one organoid protocol over another is dependent on the application.
Future milestones in progressing organoid technology include i) an efficient directed differentiation protocol for UB progenitor cells, ii) improved resolution of aIM and its derivatives, iii) hPSC-derived MM and UB capable of reciprocal induction to form nephrons interconnected via a unifying collecting duct, and iv) patterning of kidney tissue for appropriate polarity/organization/function.
Many of the current protocols towards hPSC-derived UB organoids are often predicated upon flow sorting85,95, have failed to demonstrate reciprocal induction with MM85,88,89,95, and lack reported differentiation into principal and intercalated cells of the collecting duct88,92. Despite significant efforts by international leaders in the field, a demonstration of reciprocal induction between hPSC-derived MM and UB tissue remains elusive. Inductive signals between the MM and UB would be required to model CAKUT, may progress the maturation of kidney organoids beyond embryologic stages for translational purposes, and may permit the scale of functional nephrons as an organ building block for regenerative medicine.
As coculture of microdissected primary MM and UB sufficiently reconstitutes kidney ex vivo35, similar reciprocal induction between hPSC-derived MM and UB should be possible. A notable major milestone was the demonstration such interaction between murine ESC-derived tissues85. The initial differentiation to kidney from hPSCs may have performed well in chimeric reaggregation with developing mouse kidney because it bore markers of both nephron and collecting duct precursors83, seemingly relying on reciprocal inductive signals derived from the mouse. Current protocols that efficiently induce either human nephron or ureteric organoids have not been shown to generate chimeric tissue when combined with their murine counterparts. Perhaps this is due to developmental species differences78,96 or the need for improved protocols. As induction efficiency is predicated upon tissue-specific markers, an improved understanding of markers that differentiate aIM-derived tissues (pronephros, mesonephros, nephric duct, male and female gonadal structures) would provide critical insight to improving UB organoid protocols, determine whether nephron organoid protocols generate pronephros/mesonephros vs metanephros, and prevent the prior misidentification of ureteric tissue based on non-specific markers. Importantly, gonadal structures should default to the female gender even in male PSC lines, due to a lack of Mullerian inhibitory hormone (MIH). Regardless of the gender of the hPSC line, use of MIH and a lack of testosterone should limit male and female gonadal structures, respectively. Given differences between mouse and human kidney development, tissue specific markers of aIM derivatives may best be identified in abortive human tissue.
Acknowledgements:
The authors thank the support from the following grants: National Institutes of Health (NIH) T32 fellowship training grant (DK007527, to N.G.), Harvard Stem Cell Institute interdisciplinary grant (to N.G.), Brigham and Women’s Hospital Research Excellence Award (to N.G. and R.M.), Brigham and Women’s Hospital Faculty Career Development Award (R.M.), Harvard Stem Cell Institute Seed Grant (R.M.), DiaComp Pilot & Feasibility Program (R.M.), NIH DP2EB029388 award (R.M.), NIH U01EB028899 grant (R.M.), and NIH UC2DK126023 grant (R.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kim J, Koo B-K & Knoblich JA Human organoids: model systems for human biology and medicine. Nature Reviews Molecular Cell Biology 21, 571–584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan D, et al. Development of the Human Fetal Kidney from Mid to Late Gestation in Male and Female Infants. EBioMedicine 27, 275–283 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downs KM The enigmatic primitive streak: prevailing notions and challenges concerning the body axis of mammals. Bioessays 31, 892–902 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dressler GR Advances in early kidney specification, development and patterning. Development (Cambridge, England) 136, 3863–3874 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morizane R & Bonventre JV Kidney Organoids: A Translational Journey. Trends in molecular medicine 23, 246–263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimelman D & Griffin KJ Vertebrate mesendoderm induction and patterning. Curr Opin Genet Dev 10, 350–356 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Yiangou L, et al. Cell cycle regulators control mesoderm specification in human pluripotent stem cells. J Biol Chem 294, 17903–17914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinder SJ, et al. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126, 4691–4701 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Parameswaran M & Tam PP Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev Genet 17, 16–28 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Gupta N, Dilmen E & Morizane R 3D kidney organoids for bench-to-bedside translation. Journal of Molecular Medicine 99, 477–487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark K, Vainio S, Vassileva G & McMahon AP Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Kruepunga N, et al. The development of the cloaca in the human embryo. J Anat 233, 724–739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mugford JW, Sipilä P, McMahon JA & McMahon AP Osr1 expression demarcates a multipotent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Developmental Biology 324, 88–98 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taguchi A, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi A, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi A, et al. Identification of a Multipotent Self-Renewing Stromal Progenitor Population during Mammalian Kidney Organogenesis. Stem Cell Reports 3, 650–662 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quaggin SE & Kreidberg JA Development of the renal glomerulus: good neighbors and good fences. Development 135, 609–620 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Abrahamson DR Development of kidney glomerular endothelial cells and their role in basement membrane assembly. Organogenesis 5, 275–287 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahamson DR, Robert B, Hyink DP, St John PL & Daniel TO Origins and formation of microvasculature in the developing kidney. Kidney Int Suppl 67, S7–11 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Hendry C, Rumballe B, Moritz K & Little MH Defining and redefining the nephron progenitor population. Pediatr Nephrol 26, 1395–1406 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeshima A, Nojima Y & Kojima I The role of the activin-follistatin system in the developmental and regeneration processes of the kidney. Cytokine Growth Factor Rev 12, 289–298 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Dudley AT, Godin RE & Robertson EJ Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13, 1601–1613 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barak H, et al. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell 22, 1191–1207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jena N, Martín-Seisdedos C, McCue P & Croce CM BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res 230, 28–37 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Tomita M, et al. Bmp7 maintains undifferentiated kidney progenitor population and determines nephron numbers at birth. PLoS One 8, e73554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poladia DP, et al. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291, 325–339 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Nagalakshmi VK & Yu J The ureteric bud epithelium: morphogenesis and roles in metanephric kidney patterning. Mol Reprod Dev 82, 151–166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullen RD & Behringer RR Molecular genetics of Müllerian duct formation, regression and differentiation. Sex Dev 8, 281–296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima T, Iguchi T & Sato T Retinoic acid signaling determines the fate of uterine stroma in the mouse Müllerian duct. Proceedings of the National Academy of Sciences 113, 14354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannema SE & Hughes IA Regulation of Wolffian Duct Development. Hormone Research in Paediatrics 67, 142–151 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Marlier A & Gilbert T Expression of retinoic acid-synthesizing and -metabolizing enzymes during nephrogenesis in the rat. Gene Expr Patterns 5, 179–185 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Sainio K, et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124, 4077–4087 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Lindström NO, et al. Conserved and Divergent Features of Human and Mouse Kidney Organogenesis. Journal of the American Society of Nephrology 29, 785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krause M, Rak-Raszewska A, Pietilä I, Quaggin SE & Vainio S Signaling during Kidney Development. Cells 4, 112–132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rak-Raszewska A, Hauser PV & Vainio S Organ In Vitro Culture: What Have We Learned about Early Kidney Development? Stem Cells Int 2015, 959807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll TJ & McMahon AP Secreted Molecules in Metanephric Induction. Journal of the American Society of Nephrology 11, S116 (2000). [PubMed] [Google Scholar]
- 37.Hollyday M, McMahon JA & McMahon AP Wnt expression patterns in chick embryo nervous system. Mech Dev 52, 9–25 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Maeshima A, Yamashita S, Maeshima K, Kojima I & Nojima Y Activin A Produced by Ureteric Bud Is a Differentiation Factor For Metanephric Mesenchyme. Journal of the American Society of Nephrology 14, 1523 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Ishibe S, et al. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development 136, 337–345 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazaki Y, Oshima K, Fogo A, Hogan BL & Ichikawa I Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. The Journal of clinical investigation 105, 863–873 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michos O, et al. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet 6, e1000809 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodbeck S, Besenbeck B & Englert C The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech Dev 121, 1211–1222 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Kiefer SM, et al. Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development 137, 3099–3106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sajithlal G, Zou D, Silvius D & Xu PX Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol 284, 323–336 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain S The many faces of RET dysfunction in kidney. Organogenesis 5, 177–190 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyle S, et al. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313, 234–245 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Hinchliffe SA, Sargent PH, Howard CV, Chan YF & van Velzen D Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Laboratory investigation; a journal of technical methods and pathology 64, 777–784 (1991). [PubMed] [Google Scholar]
- 49.Boyle SC, Liu Z & Kopan R Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development (Cambridge, England) 141, 346–354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schell C, Wanner N & Huber TB Glomerular development – Shaping the multi-cellular filtration unit. Seminars in Cell & Developmental Biology 36, 39–49 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Costantini F & Kopan R Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18, 698–712 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grote D, et al. Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet 4, e1000316–e1000316 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis TK, Hoshi M & Jain S To bud or not to bud: the RET perspective in CAKUT. Pediatr Nephrol 29, 597–608 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Ven AT, et al. Whole-Exome Sequencing Identifies Causative Mutations in Families with Congenital Anomalies of the Kidney and Urinary Tract. Journal of the American Society of Nephrology 29, 2348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindstrom NO, et al. Conserved and Divergent Features of Human and Mouse Kidney Organogenesis. J Am Soc Nephrol 29, 785–805 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta N, Susa K & Morizane R Regenerative Medicine, Disease Modeling, and Drug Discovery in Human Pluripotent Stem Cell-derived Kidney Tissue. Eur Med J Reprod Health 3, 57–67 (2017). [PMC free article] [PubMed] [Google Scholar]
- 57.Clevers H Modeling Development and Disease with Organoids. Cell 165, 1586–1597 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Justice BA, Badr NA & Felder RA 3D cell culture opens new dimensions in cell-based assays. Drug Discov Today 14, 102–107 (2009). [DOI] [PubMed] [Google Scholar]
- 59.James RG, Kamei CN, Wang Q, Jiang R & Schultheiss TM Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133, 2995–3004 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Mugford JW, Sipilä P, McMahon JA & McMahon AP Osr1 expression demarcates a multipotent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol 324, 88–98 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennett CN, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem 277, 30998–31004 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Morizane R, et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nature biotechnology (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morizane R & Bonventre JV Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nature protocols 12, 195–207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang M & Han Y-M Differentiation of Human Pluripotent Stem Cells into Nephron Progenitor Cells in a Serum and Feeder Free System. PLOS ONE 9, e94888 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takasato M, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Toyohara T, et al. Cell Therapy Using Human Induced Pluripotent Stem Cell-Derived Renal Progenitors Ameliorates Acute Kidney Injury in Mice. STEM CELLS Translational Medicine 4, 980–992 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takasato M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nature cell biology 16, 118–126 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Duester G Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu H, et al. Single-Cell Transcriptomics of a Human Kidney Allograft Biopsy Specimen Defines a Diverse Inflammatory Response. Journal of the American Society of Nephrology 29, 2069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao GY, et al. Expression of the transcription factor GATA3 in the postnatal mouse central nervous system. Neurosci Res 61, 420–428 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Phipson B, et al. Evaluation of variability in human kidney organoids. Nat Methods 16, 79–87 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Combes AN, et al. Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk. Development 146(2019). [DOI] [PubMed] [Google Scholar]
- 73.Freedman BS, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nature communications 6, 8715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tran T, et al. In Vivo Developmental Trajectories of Human Podocyte Inform In Vitro Differentiation of Pluripotent Stem Cell-Derived Podocytes. Dev Cell 50, 102–116.e106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Low JH, et al. Generation of Human PSC-Derived Kidney Organoids with Patterned Nephron Segments and a De Novo Vascular Network. Cell Stem Cell 25, 373–387.e379 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garreta E, et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater 18, 397–405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Homan KA, et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nature Methods 16, 255–262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindstrom NO, et al. Conserved and Divergent Features of Mesenchymal Progenitor Cell Types within the Cortical Nephrogenic Niche of the Human and Mouse Kidney. J Am Soc Nephrol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hiratsuka K, et al. Induction of human pluripotent stem cells into kidney tissues by synthetic mRNAs encoding transcription factors. Scientific reports 9, 913 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaunt SJ, George M & Paul Y-L Direct activation of a mouse Hoxd11 axial expression enhancer by Gdf11/Smad signalling. Developmental Biology 383, 52–60 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Tsujimoto H, et al. A Modular Differentiation System Maps Multiple Human Kidney Lineages from Pluripotent Stem Cells. Cell Reports 31, 107476 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Wintour EM, et al. Ontogeny of hormonal and excretory function of the meso- and metanephros in the ovine fetus. Kidney international 50, 1624–1633 (1996). [DOI] [PubMed] [Google Scholar]
- 83.Xia Y, et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nature cell biology 15, 1507–1515 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Xia Y, et al. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor–like cells. Nat Protoc 9, 2693–2704 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Taguchi A & Nishinakamura R Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell 21, 730–746.e736 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Mae SI, et al. Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochem Biophys Res Commun 495, 954–961 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Yuri S, Nishikawa M, Yanagawa N, Jo OD & Yanagawa N In Vitro Propagation and Branching Morphogenesis from Single Ureteric Bud Cells. Stem Cell Reports 8, 401–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mae S-I, et al. Expansion of Human iPSC-Derived Ureteric Bud Organoids with Repeated Branching Potential. Cell Reports 32, 107963 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Uchimura K, Wu H, Yoshimura Y & Humphreys BD Human Pluripotent Stem Cell-Derived Kidney Organoids with Improved Collecting Duct Maturation and Injury Modeling. Cell Rep 33, 108514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holmes CL, Landry DW & Granton JT Science review: Vasopressin and the cardiovascular system part 1--receptor physiology. Crit Care 7, 427–434 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bauersachs J, Jaisser F & Toto R Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 65, 257–263 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Howden SE, et al. Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell 28, 671–684.e676 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pearce D, et al. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10, 135–146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roy A, Al-bataineh MM & Pastor-Soler NM Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol 10, 305–324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeng Z, et al. Generation of patterned kidney organoids that recapitulate the adult kidney collecting duct system from expandable ureteric bud progenitors. Nature communications 12, 3641 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindstrom NO, et al. Conserved and Divergent Features of Human and Mouse Kidney Organogenesis. J Am Soc Nephrol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]