Abstract
Adverse childhood experiences (ACEs) may have a critical influence on adult outcomes and subsequent offspring development, but few data have explored the effects of ACEs in low-resource settings where the burdens of childhood adversity and HIV are high. Among mothers living with HIV in Cape Town, we examined the effects of ACEs on maternal psychosocial and HIV-related outcomes, as well as early child development in their offspring aged 36–60 months. The World Health Organization’s Adverse Childhood Experiences International Questionnaire was used to measure maternal report of ACEs, and the Ages & Stages Questionnaire to screen for developmental delays in their offspring. Among 353 women (median age: 32 years), 84% reported ≥1 ACEs. Increased report of ACEs was strongly associated with depressive symptoms, hazardous alcohol use, intimate partner violence, and self-reported suboptimal adherence to antiretroviral therapy. These associations were driven by more severe childhood experiences, including abuse, neglect, and exposure to collective violence. Among 255 women who reported on their child’s development, maternal ACEs were associated with poorer socioemotional development. These data suggest that childhood adversity has long-term effects on maternal outcomes as well as their children’s socioemotional development, and point to ACEs that might be targeted for screening and intervention.
Keywords: Adverse childhood experiences, mental health, HIV, early child development, South Africa
Introduction
Pregnant and postpartum women living with HIV (WLHIV) are a vulnerable population, with high levels of poor psychosocial and HIV-related outcomes. In South Africa, where the antenatal HIV prevalence is around 30% (Woldesenbet et al., 2019), depression, alcohol use and intimate partner violence are commonly reported (Davis et al., 2017). In addition, suboptimal adherence to antiretroviral therapy (ART) and elevated HIV viral load are well-documented concerns during pregnancy and postpartum in this setting (Matthews et al., 2020; Rotheram-Borus et al., 2019; Woldesenbet et al., 2020). Notably, poor maternal psychosocial and HIV-related outcomes frequently co-occur, and both negatively influence outcomes among their offspring. In South African children, high levels of developmental delay have been observed (Donald et al., 2019; le Roux et al., 2018), exacerbating intergenerational cycles of adversity.
To inform intervention development, factors associated with poor maternal and child outcomes must be identified. Adverse childhood experiences (ACEs), defined as potentially traumatic events that occur during the first 18 years of life, have received little attention in low-resource settings. These events include childhood abuse and neglect, family dysfunction, and experience of bullying, community violence, and collective violence (World Health Organization, 2015). Studies from high-income settings suggest that ACEs can lead to a trajectory which includes adverse experiences during adulthood (LaNoue et al., 2012; Mersky et al., 2018), as well as subsequent adult mental health problems such as poor mental well-being (Hughes et al., 2016) and depression or psychological distress (Hughes et al., 2017; Lee & Chen, 2017; Merrick et al., 2017). Further, ACEs have been associated with experiencing violence during adulthood (Hughes et al., 2017), including intimate partner violence (IPV; Gartland et al., 2016; McMahon et al., 2015), as well as increased substance use (Hughes et al., 2017; Lee & Chen, 2017; Merrick et al., 2017).
Other research from high-income settings has focussed on ACEs among pregnant and postpartum women specifically. Studies from these settings suggest that ACEs may be associated with depression in this population (Gartland et al., 2016; McDonnell & Valentino, 2016; Mersky & Janczewski, 2018; Schury et al., 2017) and may have additional adverse effects on women’s offspring. Maternal ACEs have been associated with poor parenting practices during adulthood (Fuchs et al., 2015) as well as poor mother-infant bonding (Farré-Sender et al., 2018). Further, research from high-income settings suggests that maternal ACEs may be associated with infant developmental difficulties (Folger et al., 2018; Racine et al., 2018) and poor socioemotional functioning (Madigan et al., 2017; McDonnell & Valentino, 2016) including internalizing and externalizing problems (Plant et al., 2018).
As noted above, there are few data exploring the effects of ACEs on maternal and child outcomes in low- and middle-income countries such as South Africa, despite the high burden of childhood adversity in these settings (Manyema et al., 2018; Richter et al., 2018; Shields et al., 2008), with the potential for significant mental health problems. In addition, few studies have explored the impact of ACEs on HIV-related outcomes during adulthood, including adherence to ART and HIV viral suppression. The limited available evidence suggests that childhood trauma is associated with suboptimal adherence to ART among adults living with HIV in the United States (Pence et al., 2012) and in Tanzania (Whetten et al., 2013). As noted above, postpartum WLHIV are a particularly vulnerable population, and additional data to explore the effects of ACEs on a wide range of outcomes in low-resource settings may inform interventions to break intergenerational cycles of adversity.
Using data from an intervention study evaluating strategies for delivering HIV care in Cape Town, South Africa, we examined the associations between maternal report of ACEs and maternal psychosocial and HIV-related health outcomes among mothers with young children, as well as the effect of reported ACEs on early child development in their offspring. Specifically, we hypothesised that an increasing number of ACEs would be associated with (i) maternal depression, experience of IPV and alcohol use; (ii) suboptimal self-reported ART adherence and elevated HIV viral load; and (iii) poor early child development in their offspring.
Materials and methods
Pregnant WLHIV were recruited as part of the MCH-ART study, which evaluated strategies for delivering HIV care and treatment services during pregnancy and the postpartum period (ClinicalTrials.gov NCT01933477). The design, methods and primary outcomes of the study have been previously described (Myer et al., 2016; Myer et al., 2018). Briefly, pregnant women aged 18 years or older were enrolled at a large primary care clinic in the former township of Gugulethu. This low socioeconomic community is characterised by high levels of poverty and HIV, with an antenatal HIV prevalence of around 30% (Myer et al., 2015). Women who were eligible to initiate ART were followed through delivery; and women who opted to breastfeed were followed through 18 months postpartum as part of an intervention study. Women followed as part of the intervention study (n=471) were contacted again to complete one additional study visit with the child born during the study between 36–60 months postpartum in order to assess longer term effects of the intervention (Mogoba et al., 2019); viral load outcomes at 12 months postpartum did not differ by attendance at the additional study visit (Phillips et al., 2020). All women provided written informed consent prior to participation, and the study was approved by the University of Cape Town’s Faculty of Health Sciences Human Research Ethics Committee and by the Institutional Review Board of the Columbia University Medical Center.
Measures
This analysis uses data from the 36–60 month postpartum visit, which was conducted separately from any routine maternal or child care. While they were followed during pregnancy for the MCH-ART study, women had self-reported the date on which they initiated ART, and gestation was assessed using ultrasound. At the 36–60 month postpartum visit, women completed a battery of questionnaires drawing on Social Action Theory (Ewart, 1991; Traube et al., 2011), as well as a child developmental assessment. In addition, women underwent phlebotomy for HIV viral load testing (Abbott RealTime HIV-1) conducted by the South African National Health Laboratory Service. All questionnaires and the child developmental assessment were translated into isiXhosa, the predominant local language, and were back-translated into English to ensure accuracy (Preciago & Henry, 1997). Questionnaires included an assessment of demographic characteristics, and a composite socioeconomic status score was calculated based on maternal educational attainment, current employment status, housing type and access to household resources in order to assess relative levels of disadvantage in this sample.
The World Health Organization’s Adverse Childhood Experiences International Questionnaire (ACE-IQ) was used to assess maternal report of ACEs prior to the age of 18 years. This tool has been field tested globally, including in South Africa, and an adapted version has been found to perform well among mothers in the United States (Murphy et al., 2014). The questionnaire assesses ACEs across 13 categories: childhood abuse (physical, emotional and sexual) and neglect (physical and emotional), family dysfunction (experience of mental illness or substance abuse in the household, imprisonment of household member(s), violence against household member(s), or parental death/separation), and experience of bullying, community violence, and collective violence (World Health Organization, 2015). The total number of categories in which women reported experiencing childhood adversity were summed for a maximum of 13, as recommended in the original development of the tool (World Health Organization, 2015).
Psychosocial outcomes of interest included depressive symptoms, hazardous alcohol use, and IPV. The Edinburgh Postnatal Depression Scale [EPDS; Cronbach’s alpha (α)=0.90] was used to measure self-reported depressive symptoms during the past week. Each of the 10 items were assessed on a frequency scale ranging from 0 to 3 and items were summed for a maximum score of 30, with a score of ≥13 used to suggest elevated depressive symptoms (Cox et al., 1987). This tool has been validated for use among postpartum women in South Africa (Lawrie et al., 1998). The Alcohol Use Disorders Identification Test-Consumption (AUDIT-C; α=0.85) was used to assess hazardous alcohol use during the past 12 months. The 3 items of this scale assess the frequency of any alcohol use and binge drinking, respectively, as well as the quantity of alcohol consumed when drinking; each item was scored between 0 and 4 and a score of ≥3 was used to indicate hazardous drinking (Bush et al., 1998; Bradley et al., 2003). The AUDIT has been validated among adults living with HIV in South Africa (Myer et al., 2008). The World Health Organization’s Violence Against Women questionnaire (α=0.96), which has been validated in South Africa (Dunkle et al., 2003), was used to assess psychological, physical and sexual IPV experienced during the past 12 months (Garcia-Moreno et al., 2005). In analyses, we categorised women as reporting any versus no violence. HIV-related outcomes of interest included self-reported ART adherence and HIV viral load. We defined suboptimal ART adherence as report of missed doses on ≥1 day during the past 30 days, and ≥2 days in sensitivity analyses. Elevated viral load was defined as ≥50 copies/mL, and ≥1000 copies/mL in sensitivity analyses.
The Ages & Stages Questionnaire: Third Edition (ASQ-3) was used to measure child development (Squires & Bricker, 2009). This standardised, parent-reported assessment was designed to screen for developmental delays across 5 domains: communication, gross motor, fine motor, problem solving and personal-social functioning. Scores in each domain range from 0 to 60, with higher scores indicating better development. In addition, scores can be categorised as normal development or as falling within established cut-offs which suggest that the child should be monitored (1–2 standard deviations below the mean) or below the cut-off for suspected delay (2 standard deviations below the mean; Squires & Bricker, 2009). This tool has been found to be feasible in and applicable to the South African context (Hsiao et al., 2017; van Heerden et al., 2017). The Ages & Stages Questionnaire-Socioemotional (ASQ-SE) was used to assess infant socioemotional development across a range of behaviours (Squires et al., 2015). Items on this parent-reported tool are summed, with higher scores indicating higher levels of maladaptive socioemotional development and established cut-offs suggestive of risk. The Ages & Stages tools include assessments of whether the child can perform certain tasks and were thus only administered to mothers who attended the study visit with their child, while all other measures were administered regardless of child attendance at the visit.
Data analysis
Data were analysed using Stata 14 (StataCorp Inc, College Station, Texas, USA). Reported ACEs, maternal psychosocial and HIV-related outcomes, and child developmental scores were summarised using frequencies and proportions for categorial variables, or medians with interquartile ranges (IQRs) for continuous variables. We used multivariable logistic regression models to examine the associations between ACEs and each maternal psychosocial and HIV-related outcome, using the threshold values described above. For each domain of the ASQ-3 and ASQ-SE, we explored the associations between maternal ACEs and child developmental outcomes in their offspring in both linear and logistic regression models, using total scores and the cut-off scores described above. Throughout, we examined maternal report of ACEs using the sum of ACE categories reported, and present results for a one-category increase in reported ACEs. In exploratory analyses of excess risk, we explored the impact of individual ACEs on each maternal outcome by calculating the prevalence difference between women reporting versus not reporting each ACE (prevalence among women reporting the ACE minus prevalence among those not reporting the ACE).
Results
A total of 353 women (median age: 32.1 years) attended the study visit, and 266 women were accompanied by their child (median age: 44.0 months). Table 1 presents maternal and child characteristics, maternal psychosocial and HIV-related outcomes, and child developmental outcomes. Overall, the majority of women reported low levels of educational attainment and 38% reported a married/cohabiting relationship status; approximately half of women were employed, and half lived in informal housing. A total of 4% of mothers reported elevated depressive symptoms during the past week; 4% and 14% reported experiencing IPV and hazardous alcohol use, respectively, during the past 12 months. After all initiating the local first-line ART regimen during pregnancy, women had been on ART for a median of 47.4 months, and suboptimal adherence and elevated viral load were common: 29% of women reported missing ART dose(s) during the past 30 days, and 44% had a viral load ≥50 copies/mL. Report of missed doses was strongly associated with elevated viral load (p<0.001).
Table 1.
Maternal and child characteristics, maternal psychosocial and HIV-related outcomes, and child developmental outcomes
| Variable | Total sample – n (%) |
|---|---|
| Maternal characteristics (n=353) | |
| Median [IQR] age in years | 32.1 [28.6, 36.2] |
| Educational attainment | |
| Less than secondary | 260 (74) |
| Completed secondary/any tertiary | 93 (26) |
| Employed | 170 (48) |
| Informal housing | 168 (48) |
| Married and/or cohabiting | 134 (38) |
| Median [IQR] months postpartum | 44.0 [41.6, 46.5] |
| Median [IQR] months since antiretroviral therapy (ART) initiation | 47.4 [45.3, 49.9] |
| Maternal psychosocial outcomes (n=353) | |
| Median [IQR] depressive symptoms score (Edinburgh Postnatal Depression Scale; EPDS) | 0 [0, 1] |
| Elevated depressive symptoms during the past week (score ≥13) | 13 (4) |
| Median [IQR] alcohol consumption score (Alcohol Use Disorders Identification Test – Consumption; AUDIT-C) | 0 [0, 1] |
| Hazardous alcohol use during the past 12 months (score ≥3) | 51 (14) |
| Any intimate partner violence during the past 12 months | 14 (4) |
| Maternal HIV-related outcomes (n=353) | |
| Missed antiretroviral therapy dose(s) on ≥1 day during the past 30 days | 104 (29) |
| Missed antiretroviral therapy doses on ≥2 days during the past 30 days | 91 (26) |
| HIV viral load ≥50 copies/mL | 153 (44) |
| HIV viral load ≥1000 copies/mL | 123 (35) |
| Birth and infant characteristics (n=255) 1 | |
| Median [IQR] age in months | 44.3 [41.6, 46.7] |
| Child born in study was mother’s first child | 48 (19) |
| Median [IQR] gestational age at delivery in weeks | 39 [38, 40] |
| Preterm delivery (<37 weeks) | 34 (13) |
| Male | 126 (49) |
| Currently lives with mother | 246 (96) |
| Child development using the Ages & Stages screening tool (n=255) 2 | |
| Median [IQR] communication score | 60 [60, 60] |
| Communication delay | 10 (4) |
| Median [IQR] gross motor score | 60 [50, 60] |
| Gross motor delay | 21 (8) |
| Median [IQR] fine motor score | 50 [40, 50] |
| Fine motor delay | 49 (19) |
| Median [IQR] problem solving score | 55 [45, 60] |
| Problem solving delay | 38 (15) |
| Median [IQR] personal-social score | 60 [55, 60] |
| Personal-social delay | 18 (7) |
| Median [IQR] socioemotional score | 25 [10, 35] |
| Socioemotional risk | 7 (3) |
Restricted to children who were included in the child development analysis;
Higher scores indicate better development in all domains except socioemotional, where higher scores indicate higher levels of maladaptive development.
Among the 87/353 women who attended the study visit without their child, 68% reported that their child does not live with them; 24% reported that their child was at home or elsewhere; and 8% reported that their child had died. Eleven additional children were excluded from the analysis of child development: 9 mothers completed the incorrect questionnaire for the child’s age, and 2 children were receiving intensive developmental and physical support for severe, diagnosed developmental delay due to reasons other than maternal ACEs. Compared to those whose children did not attend the study visit or who were excluded from analysis, mothers of children included in this analysis were less likely to be employed and to live in informal housing and were somewhat more likely to report that the child was their first child; no differences in reported ACEs were observed. Of the 255 children who were included in analyses, 49% were male, 13% were born <37 weeks gestation, and 19% were the first child born to their mother. Almost all children included currently lived with their mother. Across the five domains of the ASQ-3 assessment, median scores were within the normative range overall, but 19% and 15% of children scored below the normal range for fine motor and problem solving, respectively. Using the ASQ-SE assessment, 3% of children scored above threshold for socioemotional risk.
Maternal report of ACEs
Overall, 84% of mothers reported having experienced one or more ACEs, with mothers reporting a median of 2 (IQR: 1–3). The most commonly reported ACEs were parental separation/death and experience of community violence, each reported by 52% of women. Emotional and physical neglect were reported by 22% and 6% of women, respectively, and reports of abuse were less common (physical: 7%; emotional: 6%; sexual: 4%). Report of household dysfunction was higher, with 13% reporting witnessing violence within the household, and 9% and 13% reporting that a household member abused substances or was incarcerated, respectively. Figure 1 presents the prevalence of each reported ACE, as well as the median number of ACEs experienced among women reporting each ACE versus those not reporting the ACE. These data suggest that women who report having experienced less common but more severe experiences report higher levels of cumulative adversity overall. For example, the median number of ACEs reported among women who have experienced abuse or collective violence, which are relatively uncommon experiences, is significantly higher than among those who have not experienced each of these ACEs.
Figure 1.
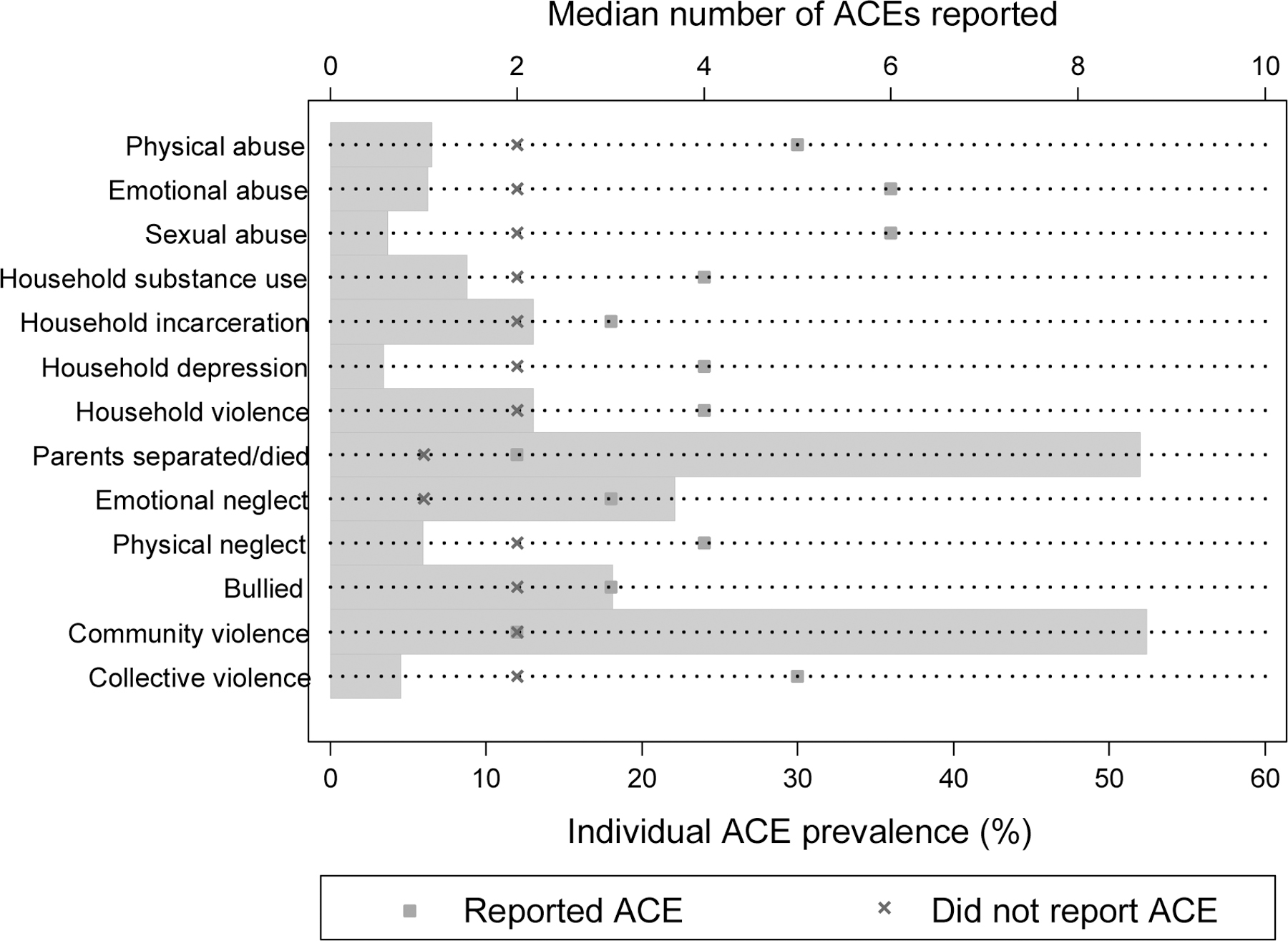
Reported adverse childhood experiences (ACEs): grey bars indicate the proportion of women who reported each ACE, with the prevalence indicated on the bottom axis; markers indicate the median number of ACE categories reported among women reporting each individual ACE versus those not reporting the ACE, with the median number of categories indicated on the top axis.
Impact of ACEs on maternal and child outcomes
Table 2 presents the associations between ACEs and maternal and child outcomes. The total number of ACEs reported was strongly associated with women’s psychosocial outcomes in both unadjusted models and after adjustment for maternal age, socioeconomic status and relationship status: after adjustment, each additional ACE category was associated with a higher odds of elevated depressive symptoms [odds ratio (OR): 1.68; 95% confidence interval (CI): 1.33–2.12], hazardous alcohol use (OR: 1.20; 95% CI: 1.03–1.40) and past year IPV (OR: 1.40; 95% CI: 1.12–1.75). Similarly, the total number of ACEs was associated with report of suboptimal adherence (OR: 1.25; 95% CI: 1.10–1.43), although the association with elevated viral load was weaker (OR: 1.11; 95% CI: 0.98–1.26); results were consistent when suboptimal adherence was defined as missed ART dose(s) on ≥2 days, and elevated viral load as ≥1000 copies/mL.
Table 2.
Associations between maternal adverse childhood experiences (ACEs) and (A) maternal psychosocial and HIV-related outcomes in logistic regression models, and offspring development in (B) logistic and (C) linear regression models.
| (A) Maternal psychosocial and HIV-related outcomes (n=353) | Unadjusted OR [95% CI]1 | P-value | Adjusted OR [95% CI]2 |
P-value |
|---|---|---|---|---|
|
| ||||
| Psychosocial outcomes | ||||
| Elevated depressive symptoms during the past week | 1.68 [1.35, 2.11] | <0.001 | 1.68 [1.33, 2.12] | <0.001 |
| Hazardous alcohol use during the past 12 months | 1.21 [1.04, 1.40] | 0.012 | 1.20 [1.03, 1.40] | 0.019 |
| Intimate partner violence during the past 12 months | 1.42 [1.15, 1.76] | 0.001 | 1.40 [1.12, 1.75] | 0.003 |
| HIV-related outcomes | ||||
| Missed antiretroviral therapy dose(s) on ≥1 day during the past 30 days | 1.24 [1.09, 1.41] | 0.001 | 1.25 [1.10, 1.43] | 0.001 |
| HIV viral load ≥50 copies/mL | 1.11 [0.98, 1.25] | 0.089 | 1.11 [0.98, 1.26] | 0.086 |
| (B) Offspring developmental delay in logistic regression models (n=255) | Unadjusted OR [95% CI] | P-value | Adjusted OR [95% CI] |
P-value |
|
| ||||
| Communication delay | 0.84 [0.54, 1.29] | 0.427 | 0.84 [0.55, 1.30] | 0.443 |
| Gross motor delay | 0.75 [0.54, 1.06] | 0.101 | 0.77 [0.57, 1.05] | 0.099 |
| Fine motor delay | 1.01 [0.85, 1.19] | 0.952 | 1.01 [0.85, 1.19] | 0.943 |
| Problem solving delay | 1.05 [0.88, 1.25] | 0.608 | 1.08 [0.89, 1.31] | 0.419 |
| Personal-social delay | 0.80 [0.57, 1.12] | 0.195 | 0.82 [0.59, 1.13] | 0.226 |
| Socioemotional risk | 1.47 [1.12, 1.92] | 0.005 | 1.57 [1.15, 2.14] | 0.004 |
| (C) Offspring development in linear regression models (n=255)4 | Unadjusted β [95% CI]3 |
P-value | Adjusted β [95% CI] |
P-value |
|
| ||||
| Communication score | −0.02 [−0.58, 0.53] | 0.934 | −0.05 [−0.60, 0.51] | 0.869 |
| Gross motor score | 0.29 [−0.27, 0.85] | 0.308 | 0.31 [−0.25, 0.87] | 0.276 |
| Fine motor score | 0.24 [−0.61, 1.08] | 0.584 | 0.16 [−0.68, 1.00] | 0.710 |
| Problem solving score | −0.33 [−1.09, 0.44] | 0.400 | −0.46 [−1.18, 0.26] | 0.213 |
| Personal-social score | 0.04 [−0.52, 0.60] | 0.881 | 0.05 [−0.50, 0.59] | 0.864 |
| Socioemotional score | 1.52 [−0.02, 3.05] | 0.052 | 1.57 [0.04, 3.10] | 0.044 |
OR: odds ratio for each additional ACE category reported; 95% CI: 95% confidence interval;
Psychosocial models adjusted for maternal age, socioeconomic status and relationship status; HIV-related models additionally adjusted for time since antiretroviral therapy initiation; offspring development models adjusted for maternal age, socioeconomic status, infant gender and infant age;
β: regression coefficient;
Higher scores indicate better development in all domains except socioemotional, where higher scores indicate higher levels of maladaptive development.
We observed no significant associations between maternal ACEs and child development in the domains of communication, gross motor, fine motor, problem solving or personal-social functioning in either logistic regression models using thresholds for developmental delay or linear regression models using continuous developmental scores. However, the total number of ACEs was associated with maternal report of maladaptive socioemotional development: when examining continuous socioemotional scores, each additional ACE category was associated with increased report of maladaptive development [regression coefficient (β): 1.57; 95% CI: 0.04–3.10], independent of maternal age and socioeconomic status, and infant gender and age; consistent results were observed in logistic regression models examining child socioemotional risk using threshold values.
Finally, we explored the associations between report of individual ACEs and maternal psychosocial and HIV-related outcomes, examining the excess risk of these outcome among women reporting each ACE versus those not reporting the ACE (Figure 2a and 2b). Overall, the experiences that appear to be the primary drivers of poor psychosocial and HIV-related outcomes in this sample include physical and emotional abuse, physical and emotional neglect, and exposure to collective violence.
Figure 2a.
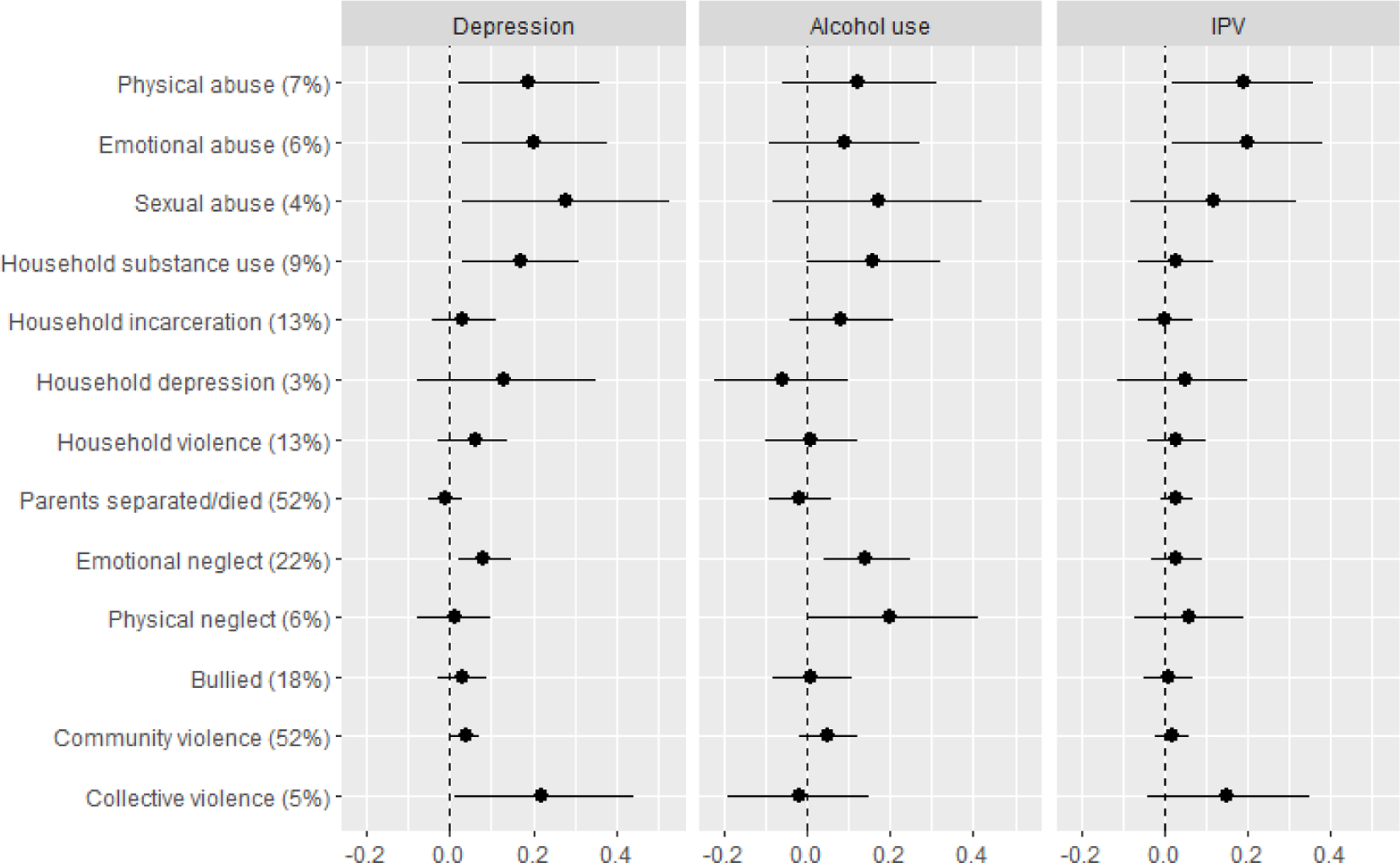
Prevalence difference for each of depression, hazardous alcohol use and intimate partner violence (IPV), reflecting the excess risk of each outcome among women reporting each adverse childhood experience (ACE) versus those not reporting the ACE1
Figure 2b.
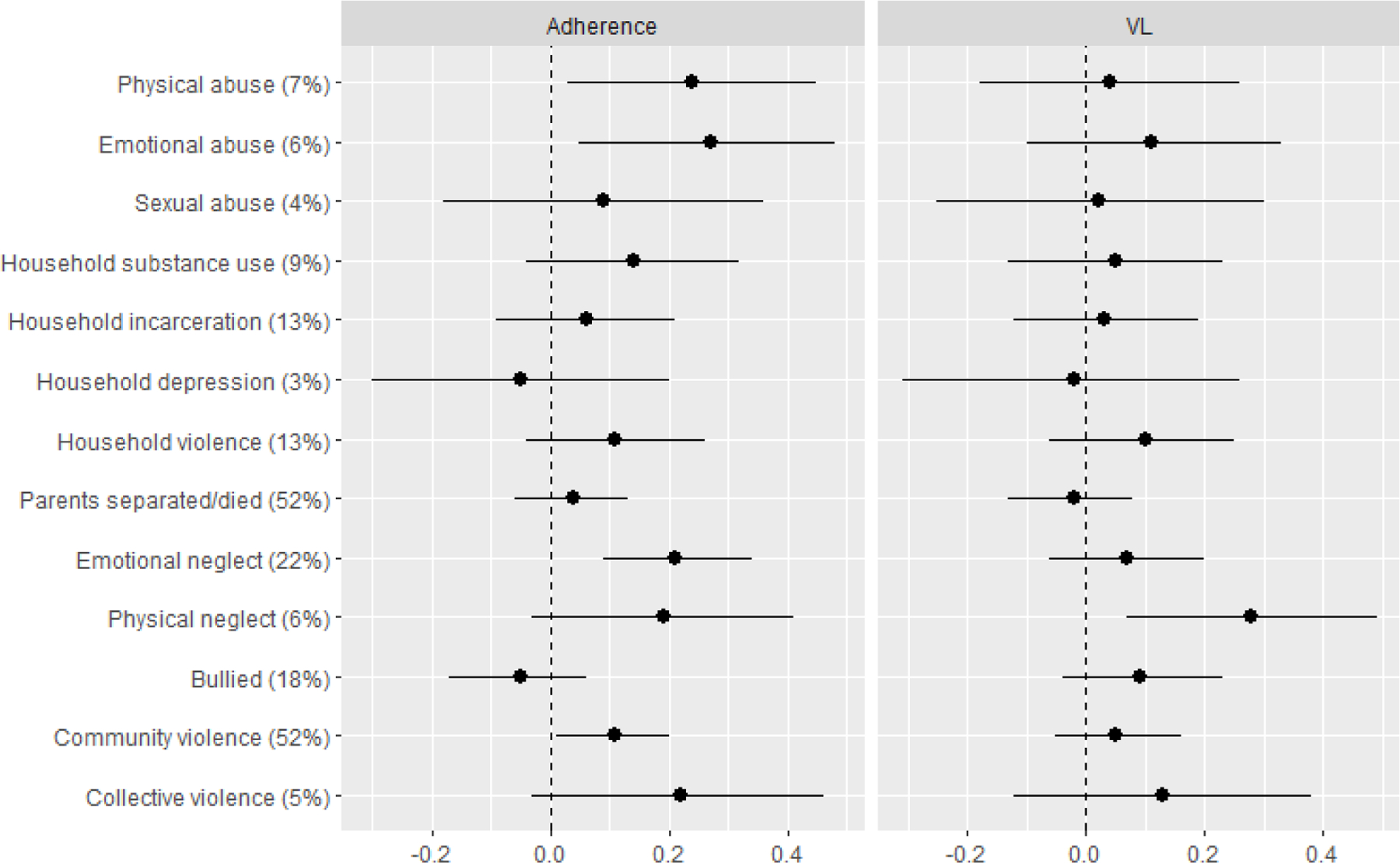
Prevalence difference for each of suboptimal adherence (missed dose(s) on ≥1 days during the past 30 days) and elevated viral load (VL ≥50 copies/mL), reflecting the excess risk of each outcome among women reporting each adverse childhood experience (ACE) versus those not reporting the ACE1
1 Prevalence of each ACE reported in brackets.
Discussion
This novel study sought to examine the associations between maternal report of ACEs and maternal psychosocial and HIV-related outcomes among WLHIV in South Africa, as well as the effect of reported ACEs on early child development in their offspring. Our findings suggest that the negative trajectory initiated by ACEs may include effects that persist into adulthood in this population, with additional effects on the early child development of the next generation. Specifically, the total number of ACEs experienced was strongly associated with adverse maternal psychosocial outcomes and self-reported suboptimal ART adherence, although the association with elevated HIV viral load was weaker. Furthermore, maternal ACEs were associated with higher reported levels of maladaptive socioemotional development in their offspring.
The proportion of women reporting ≥1 ACEs (84%) is consistent with other estimates among general populations of young adults in South Africa (Manyema et al., 2018) and women in the United States (Mersky et al., 2018). A meta-analysis of studies from mostly high- and middle-income countries has reported that 57% of participants report having experienced ≥1 ACEs, with a range between 33–78% (Hughes et al., 2017); report of ≥1 ACEs in this sample is thus on the higher end of this spectrum. Over half of mothers reported experiencing parental separation or death, and we hypothesise that these high levels may reflect the history of HIV treatment in South Africa, where more than a decade of limited or no ART devastated families. In addition, 52% of mothers reported experiencing community violence, consistent with the staggering levels of violence in Cape Town and other areas of South Africa (Richter et al., 2018; Shields et al., 2008). The high levels of emotional neglect reported (22%) are consistent with reports from young adults in Johannesburg, South Africa (Manyema et al., 2018). These levels of adversity are a clear public health crisis which, in the absence of widespread community-level change, will continue to affect subsequent generations of South African children. Change will require a multi-sectoral approach, including the involvement of the health system, law enforcement, and social development agencies.
The adverse effects of ACEs on mental health have been well-documented in high-income settings (Gartland et al., 2016; Hughes et al., 2016; Lee & Chen, 2017; McDonnell & Valentino, 2016; Merrick et al., 2017; Mersky & Janczewski, 2018; Schury et al., 2017), with additional evidence that ACEs affect adherence to ART among adults living with HIV (Pence et al., 2012). Here, we demonstrated consistent effects in a sample of women from a low-resource setting with a high burden of HIV. Given the high levels of ACEs observed, these adverse impacts are a major public health concern. Notably, the more severe, less common forms of adversity, which occurred among mothers who reported a higher number of ACEs overall (Schilling et al., 2008), appeared to be the primary drivers of adverse outcomes in this sample, suggesting a need for additional screening for more severe but less common experiences. Given that women in this population routinely access antenatal and HIV care, services are well placed to screen for high impact experiences and to support families and communities (Black et al., 2017; Woods-Jaeger et al., 2018). This will require a focus on increasing healthcare provider and community expertise in identifying and addressing early adversity.
Although maternal ACEs were not associated with maternal report of their offspring’s development in most domains, we argue that ACEs may have other harmful effects alongside the effects on their offspring’s socioemotional development in these settings. In particular, poor maternal mental health and HIV-related outcomes may have detrimental impacts on other aspects of child development which, coupled with growing up in a low-socioeconomic environment where levels of violence are high, may increase the vulnerability of this group (Walker et al., 2007; Walker et al., 2011). In the absence of community-level interventions to reduce the prevalence of ACEs, these children will likely experience many of the same early-life adversities as their mothers, perpetuating intergenerational cycles of trauma.
Our findings should be interpreted in light of several limitations. These data arise from a peri-urban setting in South Africa in the context of a research study and may not be generalisable to other settings. Maternal ACEs were self-reported retrospectively, as is the case with the majority of ACEs research, but the potential for social desirability and recall bias should be considered. The low prevalence of the more severe ACEs led to wide confidence intervals when examining the effect of individual ACEs on outcomes, and may have reduced our power to detect significant effects. In addition, more common but less severe ACEs may have a larger population-level impact. Our outcome variables were predominantly based on self-report and are subject to reporting bias, but we used validated tools to assess both maternal mental health and child developmental outcomes in their offspring. Importantly, their children’s developmental outcomes were assessed via maternal report using a screening tool, rather than a clinician assessment. Finally, 1 in 4 mothers did not attend the study visit with their child. As such, our findings regarding child developmental outcomes are based on a subset of mothers who differed in terms of some socioeconomic factors as well as number of previous pregnancies, both of which may affect child developmental outcomes, although no differences were observed in reported ACEs.
Despite some limitations, these findings are notable in demonstrating long-term effects of childhood adversity on a range of outcomes, including maternal psychosocial and HIV-related outcomes, as well as socioemotional development among their offspring. While the majority of research on ACEs has been conducted in high-income countries, these findings arise from a low-resource setting where women and children face significant challenges. Considering the high prevalence of ACEs in this population and the long-term negative impacts on a range of maternal and child outcomes, interventions to prevent and to provide support to those who have experienced ACEs are urgently needed to break intergenerational cycles of adversity, and future research which explores possible mediators and modifiers of the effects observed may guide intervention development.
Acknowledgements
The authors would like to thank the women who participated in this study, as well as the study staff for their support of this research.
source of funding
This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the National Institute of Child Health and Human Development (NICHD), grant numbers 1R01HD074558 and 1R01HD080465. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation. Dr. Mellins is supported by a grant from the National Institute of Mental Health (NIMH) to the HIV Center for Clinical and Behavioral Studies (P30-MH45320).
Footnotes
Conflicts of interest
No conflicts of interest declared.
References
- Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, McCoy DC, Fink G, Shawar YR, Shiffman J, Devercelli AE, Wodon QT, Vargas-Barón E, Grantham-McGregor S, & the Lancet Early Childhood Development Series Steering Committee. (2017). Early childhood development coming of age: science through the life course. The Lancet, 389(10064), 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, Maynard C, Burman ML, & Kivlahan DR (2003). Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Archives of Internal Medicine, 163(7), 821–829. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, & Bradley KA (1998). The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Archives of Internal Medicine, 158(16), 1789–1795. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150(6), 782–786. [DOI] [PubMed] [Google Scholar]
- Davis EC, Rotheram-Borus MJ, Weichle TW, Rezai R, & Tomlinson M (2017). Patterns of alcohol abuse, depression, and intimate partner violence among township mothers in South Africa over 5 years. AIDS and Behavior, 21(Suppl 2), S174–S182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Wedderburn CJ, Barnett W, Nhapi RT, Rehman AM, Stadler JAM, Hoffman N, Koen N, Zar HJ, & Stein DJ (2019). Risk and protective factors for child development: an observational South African birth cohort. PLoS Medicine, 16(9), e1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle K, Jewkes R, Brown H, McIntyre J, Gray G, & Harlow S (2003). Gender-based violence and HIV infection among pregnant women in Soweto. A technical report to the Australian Agency for International Development. Pretoria: Medical Research Council. [Google Scholar]
- Ewart CK Social action theory for a public health psychology. (1991). American Psychologist, 46(9), 931–946. [DOI] [PubMed] [Google Scholar]
- Farré-Sender B, Torres A, Gelabert E, Andrés S, Roca A, Lasheras G, Valdés M, & Garcia-Esteve L (2018). Mother-infant bonding in the postpartum period: assessment of the impact of pre-delivery factors in a clinical sample. Archives of Women’s Mental Health, 21(3), 287–297. [DOI] [PubMed] [Google Scholar]
- Folger AT, Eismann EA, Stephenson NB, Shapiro RA, Macaluso M, Brownrigg ME, & Gillespie RJ (2018). Parental adverse childhood experiences and offspring development at 2 years of age. Pediatrics, 141(4), e20172826. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Möhler E, Resch F, & Kaess M (2015). Impact of a maternal history of childhood abuse on the development of mother-infant interaction during the first year of life. Child Abuse & Neglect, 48, 179–189. [DOI] [PubMed] [Google Scholar]
- Garcia-Moreno C, Jansen HAFM, Ellsberg M, Heise L, & Watts C (2005). WHO multi-country study on women’s health and domestic violence against women. Geneva: World Health Organization. [Google Scholar]
- Gartland D, Woolhouse H, Giallo R, McDonald E, Hegarty K, Mensah F, Herrman H, & Brown SJ (2016). Vulnerability to intimate partner violence and poor mental health in the first 4-year postpartum among mothers reporting childhood abuse: an Australian pregnancy cohort study. Archives of Women’s Mental Health, 19(6), 1091–1100. [DOI] [PubMed] [Google Scholar]
- Hsiao C, Richter L, Makusha T, Matafwali B, van Heerden A, & Mabaso M (2017). Use of the ages and stages questionnaire adapted for South Africa and Zambia. Child: Care, Health and Development, 43(1), 59–66. [DOI] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, & Dunne MP (2017). The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health, 2(8), e356–366. [DOI] [PubMed] [Google Scholar]
- Hughes K, Lowey H, Quigg Z, & Bellis MA (2016). Relationships between adverse childhood experiences and adult mental well-being: results from an English national household survey. BMC Public Health, 16, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaNoue M, Graeber D, Urquieta de Hernandez B, Warner TD, & Helitzer DL (2012). Direct and indirect effects of childhood adversity on adult depression. Community Mental Health Journal, 48(2), 187–192. [DOI] [PubMed] [Google Scholar]
- Lawrie TA, Hofmeyr GJ, de Jager M, & Berk M (1998). Validation of the Edinburgh Postnatal Depression Scale on a cohort of South African women. South African Medical Journal, 88(10), 1340–1344. [PubMed] [Google Scholar]
- le Roux SM, Donald KA, Brittain K, Phillips TK, Zerbe A, Nguyen KK, Strandvik A, Kroon M, Abrams EJ, & Myer L (2018). Neurodevelopment of breastfed HIV-exposed uninfected and HIV-unexposed children in South Africa. AIDS, 32(13), 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RD, & Chen J (2017). Adverse childhood experiences, mental health, and excessive alcohol use: examination of race/ethnicity and sex differences. Child Abuse & Neglect, 69, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan S, Wade M, Plamondon A, Maguire JL, & Jenkins JM (2017). Maternal adverse childhood experience and infant health: biomedical and psychosocial risks as intermediary mechanisms. The Journal of Pediatrics, 187, 282–289. [DOI] [PubMed] [Google Scholar]
- Manyema M, Norris SA, & Richter LM (2018). Stress begets stress: the association of adverse childhood experiences with psychological distress in the presence of adult life stress. BMC Public Health, 18(1), 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LT, Orrell C, Bosco Bwana M, Tsai AC, Psaros C, Asiimwe S, Amanyire G, Musinguzi N, Bell K, Bangsberg DR, Haberer JE, & the META Study Investigators. (2020). Adherence to HIV antiretroviral therapy among pregnant and postpartum women during the Option B+ era: 12-month cohort study in urban South Africa and rural Uganda. Journal of the International AIDS Society, 23(8), e25586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell CG, & Valentino K (2016). Intergenerational effects of childhood trauma: evaluating pathways among maternal ACEs, perinatal depressive symptoms, and infant outcomes. Child Maltreatment, 21(4), 317–326. [DOI] [PubMed] [Google Scholar]
- McMahon K, Hoertel N, Wall MM, Okuda M, Limosin F, & Blanco C (2015). Childhood maltreatment and risk of intimate partner violence: a national study. Journal of Psychiatric Research, 69, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MT, Ports KA, Ford DC, Afifi TO, Gershoff ET, & Grogan-Kaylor A (2017). Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse & Neglect, 69, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersky JP, & Janczewski CE (2018). Adverse childhood experiences and postpartum depression in home visiting programs: prevalence, association, and mediating mechanisms. Maternal and Child Health Journal, 22(7), 1051–1058. [DOI] [PubMed] [Google Scholar]
- Mersky JP, Janczewski CE, & Nitkowski JC (2018). Poor mental health among low-income women in the U.S.: the roles of adverse childhood and adult experiences. Social Science & Medicine, 206, 14–21. [DOI] [PubMed] [Google Scholar]
- Mogoba P, Gomba Y, Brittain K, Phillips TK, Zerbe A, Myer L, & Abrams EJ (2019). Re-recruiting postpartum women living with HIV into a follow-up study in Cape Town, South Africa. BMC Research Notes, 12(1), 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A, Steele M, Dube SR, Bate J, Bonuck K, Meissner P, Goldman H, & Steele H (2014). Adverse Childhood Experiences (ACEs) Questionnaire and Adult Attachment Interview (AAI): implications for parent child relationships. Child Abuse & Neglect, 38(2), 224–233. [DOI] [PubMed] [Google Scholar]
- Myer L, Phillips TK, Hsiao N-Y, Zerbe A, Petro G, Bekker L-G, McIntyre JA, & Abrams EJ (2015). Plasma viraemia in HIV-positive pregnant women entering antenatal care in South Africa. Journal of the International AIDS Society, 18(1), 20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao N-Y, Remien RH, Mellins CA, McIntyre JA, & Abrams EJ (2018). Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: a randomised controlled trial. PLoS Medicine, 15(3), e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer L, Phillips TK, Zerbe A, Ronan A, Hsiao N-Y, Mellins CA, Remien RH, le Roux SM, Brittain K, Ciaranello A, Petro G, McIntyre JA, & Abrams EJ (2016). Optimizing antiretroviral therapy (ART) for maternal and child health (MCH): rationale and design of the MCH-ART study. Journal of Acquired Immune Deficiency Syndromes, 72(Suppl 2), S189–S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer L, Smit J, Le Roux L, Parker S, Stein DJ, & Seedat S (2008). Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care & STDs, 22(2), 147–158. [DOI] [PubMed] [Google Scholar]
- Pence BW, Mugavero MJ, Carter TJ, Leserman J, Thielman NM, Raper JL, Proeschold-Bell RJ, Reif S, & Whetten K (2012). Childhood trauma and health outcomes in HIV-infected patients: an exploration of causal pathways. Journal of Acquired Immune Deficiency Syndromes, 59(4), 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TK, Mogoba P, Brittain K, Gomba Y, Zerbe A, Myer L, & Abrams EJ (2020). Long-term outcomes of HIV-infected women receiving antiretroviral therapy after transferring out of an integrated maternal and child health service in South Africa. Journal of Acquired Immune Deficiency Syndromes, 83(3): 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant DT, Pawlby S, Pariante CM, & Jones FW (2018). When one childhood meets another – maternal childhood trauma and offspring child psychopathology: a systematic review. Clinical Child Psychology, 23(3), 483–500. [DOI] [PubMed] [Google Scholar]
- Preciago J, & Henry M (1997). Linguistic barriers in health education and services. In: Garcia JG & Zea MC (Eds.), Psychological interventions and research with Latino populations. Boston: Allyn and Bacon. [Google Scholar]
- Racine N, Plamondon A, Madigan S, McDonald S, & Tough S (2018). Maternal adverse childhood experiences and infant development. Pediatrics, 141(4), e20172495. [DOI] [PubMed] [Google Scholar]
- Richter LM, Mathews S, Kagura J, & Nonterah E (2018). A longitudinal perspective on violence in the lives of South African children from the Birth to Twenty Plus cohort study in Johannesburg-Soweto. South African Medical Journal, 108(3), 181–186. [DOI] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Weichle TW, Wynn A, Almirol E, Davis E, Stewart J, Gordon S, Tubert J, & Tomlinson M (2019). Alcohol, but not depression or IPV, reduces HIV adherence among South African mothers living with HIV over 5 years. AIDS and Behavior, 23(12), 3247–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, & Gore S (2008). The impact of cumulative childhood adversity on young adult mental health: measures, models, and interpretations. Social Science & Medicine, 66(5), 1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schury K, Zimmermann J, Umlauft M, Hulbert AL, Guendel H, Ziegenhain U, & Kolassa I-T (2017). Childhood maltreatment, postnatal distress and the protective role of social support. Child Abuse & Neglect, 67, 228–239. [DOI] [PubMed] [Google Scholar]
- Shields N, Nadasen K, & Pierce L (2008). The effects of community violence on children in Cape Town, South Africa. Child Abuse & Neglect, 32(5), 589–601. [DOI] [PubMed] [Google Scholar]
- Squires J, & Bricker D (2009). Ages & Stages Questionnaires (ASQ-3): A Parent-Completed Child Monitoring System (3rd ed.). Baltimore: Paul H. Brookes Publishing Co. [Google Scholar]
- Squires J, Bricker D, & Twombly E (2015). Ages & Stages Questionnaires: Social-Emotional (ASQ:SE-2): A Parent-Completed Child-Monitoring System for Social-Emotional Behaviors (2nd ed.). Baltimore: Paul H. Brookes Publishing Co. [Google Scholar]
- Traube DE, Holloway IW, & Smith L (2011). Theory development for HIV behavioral health: empirical validation of behavior health models specific to HIV risk. AIDS, 23(6), 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerden A, Hsiao C, Matafwali B, Louw J, & Richter L (2017). Support for the feasibility of the ages and stages questionnaire as a developmental screening tool: a cross-sectional study of South African and Zambian children aged 2–60 months. BMC Pediatrics, 17(1), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SP, Wachs TD, Grantham-McGregor S, Black MM, Nelson CA, Huffman SL, Baker-Henningham H, Chang SM, Hamadani JD, Lozoff B, Meeks Gardner JM, Powell CA, Rahman A, & Richter L (2011). Inequality in early childhood: risk and protective factors for early child development. The Lancet, 378(9799), 1325–1338. [DOI] [PubMed] [Google Scholar]
- Walker SP, Wachs TD, Meeks Gardner J, Lozoff B, Wasserman GA, Pollitt E, Carter JA, & the International Child Development Steering Group. (2007). Child development: risk factors for adverse outcomes in developing countries. The Lancet, 369(9556), 145–157. [DOI] [PubMed] [Google Scholar]
- Whetten K, Shirey K, Pence BW, Yao J, Thielman N, Whetten R, Adams J, Agala B, Ostermann J, O’Donnell K, Hobbie A, Maro V, Itemba D, Reddy E, & the CHAT Research Team. (2013). Trauma history and depression predict incomplete adherence to antiretroviral therapies in a low income country. PLoS ONE, 8(10), e74771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldesenbet SA, Kufa T, Barron P, Chirombo BC, Cheyip M, Ayalew K, Lombard C, Manda S, Diallo K, Pillay Y, & Puren AJ (2020). Viral suppression and factors associated with failure to achieve viral suppression among pregnant women in South Africa. AIDS, 34(4), 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldesenbet SA, Kufa T, Lombard C, Manda S, Ayalew K, Cheyip M, & Puren A (2019). The 2017 National Antenatal Sentinel HIV Survey. South Africa: National Department of Health. [Google Scholar]
- Woods-Jaeger BA, Cho B, Sexton CC, Slagel L, & Goggin K (2018). Promoting resilience: breaking the intergenerational cycle of adverse childhood experiences. Health Education & Behavior, 45(5), 772–780. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2015). Adverse Childhood Experiences International Questionnaire. In Adverse Childhood Experiences International Questionnaire (ACE-IQ). [website]: Geneva: WHO. http://www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/ [Google Scholar]


