Abstract
Ramichloridium obovoideum (“Ramichloridium makenziei”) is a rare cause of lethal cerebral phaeohyphomycosis. It has been, so far, geographically restricted to the Middle East. BALB/c mice were inoculated with two strains of R. obovoideum intracranially. Therapy with amphotericin B, itraconazole, or the investigational triazole SCH 56592 was conducted for 10 days. Half the mice were monitored for survival and half were killed for determination of the fungal load in brain tissue. Recipients of SCH 56592 had significantly prolonged survival and lower brain fungal burden, and this result was found for mice infected with both of the fungal strains tested. Itraconazole reduced the brain fungal load in mice infected with one strain but not the other, while amphotericin B had no effect on brain fungal concentrations. This study indicates a possible role of SCH 56592 in the treatment of the serious cerebral phaeohyphomycosis due to R. obovoideum.
Dematiaceous fungi, the agents of phaeohyphomycosis, cause a variety of clinical syndromes (9). These vary from superficial skin infection to lethal cerebral disease (4, 6, 8). Several species of the dematiaceous fungi are neurotropic, i.e., have a predilection for central nervous system tissue, causing brain lesions and/or abscesses. These include Cladophialophora bantiana, Wangiella dermatitidis, Chaetomium spp., Exophiala spp., Curvularia spp., Bipolaris spp., and a few other species (1, 2, 4, 5).
Ramichloridium obovoideum (“Ramichloridium mackenziei”) is a rare cause of cerebral phaeohyphomycosis. It seems to be geographically restricted, as all cases have been reported from the Middle East or natives of the Middle East who lived in other countries (3, 7, 11). Reported cases have involved both immunocompromised and apparently immunocompetent patients. The infection was lethal for almost all patients with reported cases of infection, despite surgery and, in some cases, antifungal therapy. In the study described here we developed a murine model of infection with R. obovoideum and tested currently available (amphotericin B, itraconazole) and investigational (SCH 56592; Schering-Plough Research Institute Inc., Kenilworth, N.J.) antifungal agents in vitro and in an experimental murine model.
MATERIALS AND METHODS
Pathogen.
R. obovoideum (“R. mackenziei”) 653 (gift from S. Al-Hedaithy, Riyadh, Saudi Arabia) and 95-1147 (Fungus Testing Laboratory, San Antonio, Tex.) were used for the in vitro testing and the experimental infection of BALB/c nu/+ mice. R. obovoideum 653 was also used to establish infection in ICR mice. R. obovoideum strains were grown on potato dextrose agar (PDA) medium plates (BBL, Baltimore Biologics, Cockeysville, Md.) for 14 days at 37°C. Surface growth was washed with sterile 0.85% normal saline and was filtered through layers of sterile glass wool. Homogeneous suspensions of the appropriate conidial concentration were prepared by using hemacytometer counts. The conidial suspension was administered in 0.2 ml for the intravenous inoculations and in 0.06 ml for the intracranial inoculations. The actual inoculation dose was determined by quantitative cultures.
In vitro susceptibility.
R. obovoideum clinical isolates were grown on PDA medium plates for 7 to 14 days at 35°C. Mature cultures on PDA plates were overlaid with sterile normal saline, and suspensions were made by gently scraping the colonies with the tip of a Pasteur pipette. Heavy hyphal fragments were allowed to settle, and the upper, homogeneous conidial suspensions were removed. Conidial suspensions were adjusted spectrophotometrically to 95% transmittance at 530 nm and were further diluted 1:10 in medium. SCH 56592 and itraconazole were dissolved in polyethylene glycol (PEG 400). Fluconazole was dissolved in sterile distilled water, and amphotericin B deoxycholate was diluted in sterile water. Drugs were diluted in medium, and the final drug concentrations were as follows: for amphotericin B, 0.03 to 16 μg/ml; for SCH 56592, 0.03 to 8 μg/ml; for fluconazole, 0.125 to 64 μg/ml; and for itraconazole, 0.015 to 8 μg/ml. The antifungal agents were tested in RPMI 1640 with l-glutamine and morpholinepropanesulfonic acid buffer at a concentration of 165 mM (American Biorganics, Inc., Niagara Falls, N.Y.). Tubes containing 0.1 ml of drug were inoculated with 0.9 ml of the conidial suspension. A drug-free growth control tube was included for each isolate. The drug-free tubes received the same concentration of PEG 400 as the drug-containing tubes. Tubes were incubated at 35°C, and MICs were read at the first 24-h interval when growth was observed in the drug-free growth control tube. MICs were defined in terms of the first tube that gave a score of 0 (optically clear) for amphotericin B and a score of 2 (reduction in turbidity of >80% in contrast to that for the drug-free control tube) for SCH 56592, fluconazole, and itraconazole.
Animals.
Age-matched 4- to 6-week-old female inbred BALB/c nu/+ mice (Veterinary Medical Unit breeding colony of the Audie Murphy Veterans Administration Hospital, San Antonio, Tex.) were used in the therapeutic studies. Ten mice were included in each treatment or control group for each survival and tissue burden study and were housed at up to five mice per cage with free access to water and food. Forty ICR female mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were used to establish the murine model for infection with R. obovoideum.
Brain fungal burden.
Surviving mice were euthaniatized under methoxyfluorine anesthesia. The brains were harvested and a small piece was removed and fixed in 10% formalin for histopathology. The brains then were reweighed and homogenized in 2 ml of sterile normal saline containing piperacillin and amikacin antibiotics. The homogenate was diluted by serial 10-fold dilutions in saline. Each dilution (0.1 ml) and the undiluted homogenate were plated onto PDA in duplicate, and the plates were incubated at 37°C. The numbers of CFU were determined, and the numbers of CFU per gram of organ were calculated.
Drugs.
SCH 56592 is an investigational triazole antifungal agent provided by Schering-Plough Research Institute Inc. The drug was reconstituted from powder in 0.3% Noble agar and given in a 0.2-ml volume orally by gavage. Amphotericin B was purchased commercially (Bristol-Myers Squibb, Princeton, N.J.) and injected intraperitoneally in 0.2 ml. Itraconazole in cyclodextrin solution (Janssen Pharmaceutica, Titusville, N.J.) was diluted in sterile water to the appropriate concentration and was administered in a 0.2-ml volume by gavage.
R. obovoideum infection in ICR mice.
Two groups (10 per group) of ICR mice were immunosuppressed with cortisone acetate at 100 mg/kg of body weight given subcutaneously 1 day before the day of infection and 1 day postinfection. Two other groups did not receive immunosuppression. Half the mice were inoculated intravenously with 4 × 105 CFU of R. obovoideum 653 in a 0.2-ml volume through the lateral tail vein. The other half were inoculated intracranially with 5.8 × 105 CFU. Intracranial injection was done as follows: under methoxyfluorine anesthesia, the scalp of the mouse was swabbed with 70% alcohol. By using a 1-ml syringe and a 27-gauge needle, 0.06 ml of conidial suspension was injected through the skull at a midpoint between the two ears. The mice were monitored for survival for up to 40 days postchallenge. Brain fungus concentrations were determined at the time of death or at day 40 for surviving animals. Brain fungal burdens were determined as described above. Specimens for histopathological examination were obtained prior to homogenization of the tissue. Pieces from the liver, spleen, and kidneys were examined histopathologically for evidence of fungal involvement.
Experimental therapy of R. obovoideum 653 infection.
Eight groups of 10 BALB/c nu/+ mice (four groups for survival and the other four for brain fungal burden) were selected randomly to receive SCH 56592 at a dose of 50 mg/kg/day orally, amphotericin B at a dose of 3 mg/kg/day intraperitoneally, itraconazole at a dose of 30 mg/kg three times daily (interval of at least 4 h between doses), or 0.3% Noble agar orally.
A total of 102 CFU of R. obovoideum 653 in a 0.06-ml volume was directly injected into the brains of the mice as described above. Treatments were started 24 h postinfection and were continued for 10 days. Half the mice were monitored for survival for up to 35 days postchallenge, and the other half were killed at day 11 postchallenge for brain fungal burden determination.
Experimental therapy of R. obovoideum 95-1147 infection.
Eight groups (10 per group for survival determination and 8 per group for brain fungal burden determination) of randomly selected female BALB/c nu/+ mice received SCH 56592 at a dose of 50 mg/kg/day orally, amphotericin B at a dose of 3 mg/kg/day intraperitoneally, itraconazole at a dose of 30 mg/kg three times daily, and 0.3% Noble agar orally. Mice were inoculated intracranially with 7.8 × 104 CFU of R. obovoideum 95-1147. Treatments were started 24 h postinfection and were continued for 10 days. Four groups were monitored for survival, and another four groups were killed at day 18 postchallenge (when mice started to have neurological signs like unstable gait or extremity weakness) and the brain fungal burden was determined.
Statistical analysis.
The Wilcoxon matched pairs test was used to determine the difference between survival groups, and the Mann-Whitney U test was used to determine the significance in brain tissue burden between treatment and control groups. A P value of ≤0.03 was required for statistically significant differences because of several studies in which more than two groups were compared.
RESULTS
In vitro data.
The first noticed growth in the drug-free tube was at 96 h. In vitro, both strains of R. obovoideum were susceptible to itraconazole and SCH 56592. Strain 653 was resistant to amphotericin B, and fluconazole MICs were high for both strains (Table 1).
TABLE 1.
MICs of four antifungal drugs for two strains of R. obovoideum
| Drug | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Strain 653
|
Strain 95-1147
|
|||
| At 96 h | At 120 h | At 96 h | At 120 h | |
| Amphotericin B | >16 | >16 | 0.5 | 1 |
| Fluconazole | 32 | 32 | 16 | 16 |
| SCH 56592 | 0.06 | 0.25 | 0.03 | 0.03 |
| Itraconazole | <0.015 | <0.015 | <0.015 | <0.015 |
Infection of ICR mice.
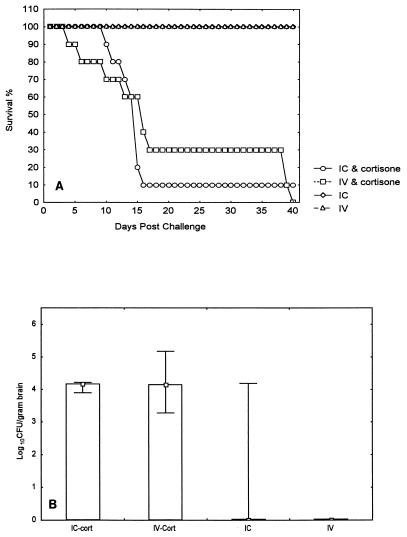
Animals immunosuppressed with cortisone acetate had lethal infections after either intravenous or intracranial challenge. All mice that did not receive steroid survived 40 days after inoculation with R. obovoideum 653 conidia given intravenously and intracranially (Fig. 1A). The median survival time for cortisone recipients that were challenged intravenously was 16 days (range, 4 to 40 days). The median survival time for cortisone recipients that were challenged intracranially was 15 days (range, 10 to 17 days). The brains of immune-competent intravenously infected mice were sterile. The brains of 4 of 10 immune-competent mice that had been inoculated IC intracranially were positive by culture. The median counts were 4.73 log10 CFU/g of brain (range, 3.28 to 5.18 log10 CFU/g), 4.19 log10 CFU/g (range, 3.12 to 4.22 log10 CFU/g), and 0 log10 CFU/g (range, 0.0 to 4.19 log10 CFU/g) for cortisone recipients challenged intravenously, cortisone recipients challenged intracranially, and immune-competent mice challenged intracranially, respectively (Fig. 1B). Histopathological examination of random samples from the brain showed mixed acute and granulomatous necrotizing encephalitis. Histopathology of other organs indicated that dissemination to the liver, spleen, and kidneys occurred only in immunosuppressed mice following both intravenous and intracranial challenges.
FIG. 1.
Survival times for immunosuppressed and immunocompetent ICR mice infected with R. obovoideum 653 intravenously (IV) or intracranially (IC) (A) and brain fungal concentrations at day 11 postinfection (B).
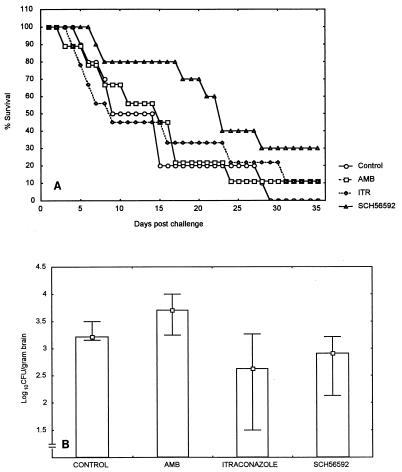
Therapy of R. obovoideum 653 infection.
Survival of SCH 56592-treated mice was significantly prolonged. The median survival time was 9 days (range, 5 to 29 days) for the untreated group, 15 days (range, 3 to 35 days) for amphotericin B recipients, 9 days (range, 4 to 35 days) for itraconazole recipients, and 23 days (range, 7 to 35 days) for SCH 56592-treated mice (P values for comparison of treated mice and untreated controls were 0.05, 0.48, and 0.005 for respectively) (Fig. 2A). SCH 56592 was superior to amphotericin B and itraconazole (P values, 0.012 and 0.010, respectively). Four other groups of mice (10 per group) were killed at day 11 postinfection, and the brain fungal burdens were determined. Itraconazole- and SCH 56592-treated mice had lower brain fungal concentrations than amphotericin B-treated mice and untreated controls. The median counts were 3.22 log10 CFU/g of brain (range, 3.16 to 3.5 log10 CFU/g) for the untreated group, 3.70 log10 CFU/g (range, 3.25 to 4.00 log10 CFU/g) for amphotericin B recipients, 2.63 log10 CFU/g (range, 1.50 to 3.27) for itraconazole recipients, and 2.70 log10 CFU/g (range, 2.13 to 3.22 log10 CFU/g) for SCH 56592 recipients (by comparison of the results for treated animals to the results for untreated controls, P values were 0.5, 0.01, and 0.03, respectively) (Fig. 2B). SCH 56592 was superior to amphotericin B but equivalent to itraconazole in reducing brain fungal concentrations.
FIG. 2.
Survival times (A) and brain fungal burdens (B) for BALB/c mice infected with R. obovoideum 653 intracranially and treated with amphotericin B (AMB) at 3 mg/kg/day, itraconazole (ITR) at 30 mg/kg three times daily, and SCH 56592 at 50 mg/kg/day for 10 days.
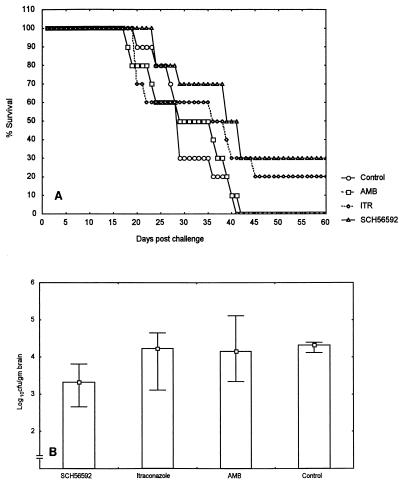
Therapy of R. obovoideum 95-1147 infection.
In the study with strain 95-1147 the infective dose was 7.8 × 104 CFU/mouse, which was administered intracranially. Prolongation of survival was significantly achieved only in SCH 56592-treated mice (Fig. 3A). The median survival times were 29 days (range, 20 to 41 days) for the untreated group, 29 days (range, 18 to 42 days) for amphotericin B recipients, 36 days (range, 20 to 60 days) for itraconazole recipients, and 39 days (range, 24 to 60 days) for SCH 56592-treated mice (P values, 0.88, 0.06, and 0.008 respectively). SCH 56592 was superior to itraconazole in prolonging animals' survival times (P value, 0.012). Four other groups of mice (10 per group) were killed at day 18 postinfection for brain fungal burden determinations. SCH 56592-treated mice had lower brain fungal burdens than amphotericin B and itraconazole recipients or untreated controls. The median counts were 4.33 log10 CFU/g of brain (range, 3.24 to 4.39 log10 CFU/g) for the untreated group, 4.15 log10 CFU/g (range, 3.34 to 5.11 log10 CFU/g) for amphotericin B recipients, 4.17 log10 CFU/g (range, 3.11 to 4.65 log10 CFU/g) for itraconazole recipients, and 3.29 log10 CFU/g (range, 2.66 to 3.81 log10 CFU/g) for SCH 56592-treated mice by comparison of the results for treated animals to the results for untreated controls, P values were 0.96, 1.0, and 0.003, respectively) (Fig. 3B). SCH 56592 recipients had significantly less brain fungal loads than itraconazole recipients (P value, 0.009).
FIG. 3.
Survival times (A) and brain fungal burdens (B) for BALB/c mice infected with R. obovoideum 95-1147 intracranially and treated with amphotericin B (AMB) at 3 mg/kg/day, itraconazole (ITR) at 30 mg/kg three times daily, and SCH 56592 at 50 mg/kg/day for 10 days.
DISCUSSION
R. obovoideum has been reported to cause exclusively cerebritis with brain abscesses in both immunocompetent and immunocompromised patients from the Middle East. Descriptions of nine cases have been published (3, 11). The outcomes for all patients for whom there have been sufficient follow-up periods have been dismal. All patients succumbed to the disease, despite surgery and antifungal therapy. Antifungal therapy consisted of amphotericin B for most patients and itraconazole for one patient described in a recent report (11). Some reports of other agents of phaeohyphomycosis have suggested that itraconazole and flucytosine may be the best available drugs for the treatment of infections caused by these resistant fungi (4, 10). In this study we established a model of cerebral infection due to R. obovoideum in two strains of mice. Immunosuppression with steroids clearly enhanced the infection and promoted dissemination to other organs. BALB/c mice seemed to be more susceptible to this infection than ICR mice, as the establishment of infection did not need immunosuppression with steroids.
The two strains used in this study, strain 653 reported by Campble and Al-Hedaithy (3) and strain 95-1147 reported by Sutton et al. (11), had different in vitro profiles of susceptibility to various antifungal drugs. The latter was more susceptible to the antifungal agents tested. In addition, there have been differences in virulence, as untreated controls infected with strain 653 died earlier than mice infected with strain 95-1147, despite a lower infecting dose. In experimental infections with both of these strains, SCH 56592 was consistently more potent than amphotericin B or itraconazole in prolonging the survival of infected animals. Although in vitro susceptibility testing has shown that itraconazole was more active, SCH 56592 was more efficacious in vivo than itraconazole. This may be related to the better bioavailability of SCH 56592 in mice and/or a lack of a correlation between in vitro and in vivo responses for R. obovoideum. In future studies, measurements of the levels of these two agents in the serum and/or brain would be useful for assessments of the potencies of these drugs.
We conclude that SCH 56592 may offer a better choice than the currently available antifungal agents for the treatment of cerebral phaeohyphomycosis due to R. obovoideum.
ACKNOWLEDGMENT
We thank S. Al-Hedaithy for providing R. obovoideum 653.
REFERENCES
- 1.Adam R D, Paquin M L, Petersen E A, Saubolle M A, Rinaldi M G, Corcoran J G, Galgiani J N, Sobonya R E. Phaeohyphomycosis caused by the fungal genera Bipolaris and Exserohilum. A report of 9 cases and review of the literature. Medicine. 1986;65:203–217. doi: 10.1097/00005792-198607000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Anandi V, John T J, Walter A, Shastry J C, Lalitha M K, Padhye A A, Ajello L, Chandler F W. Cerebral phaeohyphomycosis caused by Chaetomium globosum in a renal transplant recipient. J Clin Microbiol. 1989;27:2226–2229. doi: 10.1128/jcm.27.10.2226-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell C L, Al-Hedaithy S S. Phacohyphomycosis of the brain caused by Ramichloridiwn mackenziei sp. nov. in Middle Eastern countries. J Med Vet Mycol. 1993;31:325–332. [Google Scholar]
- 4.Dixon D M, Walsh T J, Merz W G, McGinnis M R. Infections due to Xylohypha bantiana (Cladosporium trichoides) Rev Infect Dis. 1989;11:515–525. doi: 10.1093/clinids/11.4.515. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto T, Matsuda T, McGinnis M R, Ajello L. Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses. 1993;36:145–155. doi: 10.1111/j.1439-0507.1993.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 6.Mirza S H, Hannan A, Ahmad A, Ahmad M. Subcutaneous phaeohyphomycosis. J Infect. 1993;27:75–78. doi: 10.1016/0163-4453(93)93908-m. [DOI] [PubMed] [Google Scholar]
- 7.Naim-ur-Rahman, Mahgoub E S, Chagla A H. Fatal brain abscesses caused by Ramichloridium obovoideum: report of three cases. Acta Neurochirurg. 1988;93:92–95. doi: 10.1007/BF01402887. [DOI] [PubMed] [Google Scholar]
- 8.Rao A, Forgan-Smith R, Miller S, Haswell H. Phaeohyphomycosis of the nasal sinuses caused by Bipolaris species. Pathology. 1989;21:280–281. doi: 10.3109/00313028909061075. [DOI] [PubMed] [Google Scholar]
- 9.Rinaldi M G. Phaeohyphomycosis. Dermatol Clin. 1996;14:147–153. doi: 10.1016/s0733-8635(05)70335-1. [DOI] [PubMed] [Google Scholar]
- 10.Sharkey P K, Graybill J R, Rinaldi M G, Stevens D A, Tucker R M, Peterie J D, Hoeprich P D, Greer D L, Frenkel L, Counts G W. Itraconazole treatment of phaeohyphomycosis. J Am Acad Dermatol. 1990;23:577–586. doi: 10.1016/0190-9622(90)70259-k. [DOI] [PubMed] [Google Scholar]
- 11.Sutton D A, Slifkin M, Yakulis R, Rinaldi M G. U.S. case report of cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (R. mackenziei): criteria for identification, therapy, and review of other known dematiaceous neurotropic taxa. J Clin Microbiol. 1998;36:708–715. doi: 10.1128/jcm.36.3.708-715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]