Graphical Abstract
Keywords: hepatocellular carcinoma, microvascular invasion, liver transplantation, recurrence, delta AFP score
Abstract
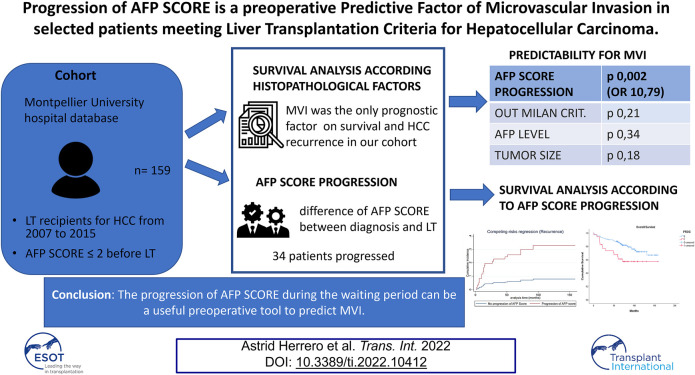
Microvascular invasion (MVI) is one of the main prognostic factors of hepatocellular carcinoma (HCC) after liver transplantation (LT), but its occurrence is unpredictable before surgery. The alpha fetoprotein (AFP) model (composite score including size, number, AFP), currently used in France, defines the selection criteria for LT. This study’s aim was to evaluate the preoperative predictive value of AFP SCORE progression on MVI and overall survival during the waiting period for LT. Data regarding LT recipients for HCC from 2007 to 2015 were retrospectively collected from a single institutional database. Among 159 collected cases, 34 patients progressed according to AFP SCORE from diagnosis until LT. MVI was shown to be an independent histopathological prognostic factor according to Cox regression and competing risk analysis in our cohort. AFP SCORE progression was the only preoperative predictive factor of MVI (OR = 10.79 [2.35–49.4]; p 0.002). The 5-year overall survival in the progression and no progression groups was 63.9% vs. 86.3%, respectively (p = 0.001). Cumulative incidence of HCC recurrence was significantly different between the progression and no progression groups (Sub-HR = 4.89 [CI 2–11.98]). In selected patients, the progression of AFP SCORE during the waiting period can be a useful preoperative tool to predict MVI.
Introduction
Hepatocellular carcinoma (HCC) is a major health problem worldwide, ranked sixth for cancer incidence and third for cancer-related deaths (1). Liver transplantation (LT) represents the only curative therapy being one of the few tumors treated by organ transplantation when diagnosed at an early stage (2).
Five-year overall survival (OS) ranges from 65 to 80% (3, 4, 5), but it is challenged by two events that cannot be avoided: the waiting period, with the growing risk of dropout (5–10%), and the risk of recurrence (6), highly influenced by the post-transplant immunosuppressive regimen. Due to organ shortage and to the ethical principle of equity, patients with HCC are constantly “competing” with cirrhotic patients, prioritizing patients with more severe disease according to the Model for End-Stage Liver Disease (MELD) score (7, 8, 9).
All these criteria must be integrated before LT for HCC to optimize benefit on survival and limit futile transplants due to tumor recurrence leading to rapid death and graft loss.
Milan criteria, based on tumor size and number of nodules, are considered the benchmark of transplant patients selection, but despite their use, HCC still has a recurrence of 10%–15% (10, 11).
HCC recurrence after LT significantly affects long-term patient survival (12, 13). Microvascular invasion is a well-known risk factor for recurrence, as well as poor differentiation, tumor size, tumor number, and satellite nodules (14, 15, 16). Unfortunately, histological data are only available after LT, and prediction of microvascular invasion before treatment remains one of the main challenges for physicians involved in LT. Alpha fetoprotein (AFP) is the main biomarker that has been shown to predict microvascular invasion, dropout, and recurrence (17, 18, 19). However, only half of patients with HCC have abnormal AFP levels and it cannot be the only variable taken into account (20); previous papers have always focused on cohorts of non-secreting tumors (21).
In France, HCC has become the first indication for liver transplantation, concerning 30% of patients on the waiting list (22). The French Study group for LT reported a new predictive model for HCC recurrence, called AFP SCORE, that was officially endorsed in 2013 by the French organ sharing organization (Agence de la Biomédecine, ABM) (23). The use of an AFP SCORE ≤2 in the last trimester preceding LT has been shown to reduce the risk of HCC recurrence up to 10% (24, 25). Only the last static AFP SCORE value is considered for decision-making in current practice.
While several studies analyzed the interest of dynamic AFP measurements as a possible prediction tool for dropout or post liver transplant recurrence (21), no studies investigating the variation effect on AFP SCORE exist. Yet, being assessed every 3 months during the waiting period by the French organ sharing organization, it could be a relevant and an easy-to-use tool.
The main objective of our study was to evaluate the impact of the AFP SCORE variation during the waiting period to preoperatively predict the microvascular invasion risk in a selected population of LT recipients with a histologically proven AFP SCORE ≤2.
Patients and Methods
This is a single institution observational retrospective study, conducted according to the Strengthening and the Reporting of Observational Studies in Epidemiology (STROBE) guidelines of the EQUATOR network (26). All consecutive adult recipients who underwent LT for HCC from January 2007 to December 2015 were reviewed. All patients gave their informed consent prior to their inclusion in the study. The study was registered in the institutional review board of the Montpellier University Hospital (N° 2018_IRB-MTP_11-23). The inclusion criteria were defined according to the AFP model (23, 24), in accordance with French national guidelines, considering LT for patients with HCC with an AFP SCORE ≤2 at the last trimester preceding LT. HCC was histologically proven on the native liver. Patients with an AFP SCORE >2 at HCC diagnosis but were down-staged by locoregional therapies to fit transplantation criteria and patients who underwent LT for recurrence after a first liver resection or ablation (salvage transplantation) were also included. The exclusion criteria included presence of cholangiocarcinoma, incidental finding of HCC on the explant, and patients transplanted within 3 months after the diagnosis of HCC.
Data regarding LT recipients’ age, gender, BMI, primary etiology of cirrhosis, and Child Pugh and MELD scores were collected. Tumor characteristics collected at the diagnosis before any treatment were number of nodules, size of the largest nodule, AFP level, the AFP SCORE, and grading according to the Milan criteria. Histopathology data collected on the explant were size of the largest nodule (mm), number of nodules, tumor differentiation according to the WHO classification, microvascular invasion, macrovascular invasion, and satellite nodules (27).
Management During the Waiting Period and the Follow up
All variables of interest were evaluated every 3 months during the waiting period by CT scan or MRI and blood sample analysis until liver transplantation (based on the national protocol for patients on the waiting list for liver transplantation). Any bridging therapies during the waiting period were decided by the institutional weekly Multi-Disciplinary Team in HCC of the Montpellier University Hospital, in accordance with the European and French guidelines (28, 29). All bridging therapies performed were reported. The delay between HCC diagnosis and the inscription on the waiting list and the delay between the inscription and the liver transplantation was recorded. Over the study follow-up period, the same standard immunosuppressive regimen was followed by LT recipients, consisting of tacrolimus (plus steroids for the first 3–6-month period post-LT) ± mycophenolate mofetil. Follow-up was scheduled every 3 months during the first year after LT, then every 6 months until May 2020. Tumor recurrence was screened performing serum AFP levels and chest and abdominal CT scans or hepatic ultrasounds every 3 months during the 2 first post-operative years, and then twice a year and/or when clinically indicated.
Definition of AFP SCORE Progression
The AFP SCORE (0–9 points) was calculated depending on largest tumor diameter (≤3 cm = 0 points, 3–6 cm = 1 point, >6 cm = 4 points), number of HCC nodules (1–3 nodules = 0 points, ≥4 nodules = 2 points), and pre-LT AFP levels ng/ml (≤100 = 0 points, 101–1,000 = 2 points, and >1,000 = 3 points) (23).
The variation of AFP SCORE was calculated from the difference between AFP SCORE at the diagnosis of HCC and AFP SCORE 3 months before LT, regardless of pre-transplant therapy (radiofrequencies, chemo-embolization, or others), as it is part of the natural history of HCC patients on the waiting list. Patients were classified into two groups: progression (ΔAFP ≥ +1) and non-progression (Δ < 1).
Endpoints
The primary outcome was the preoperative prediction of MVI on the explant. MVI was defined as the presence of tumor cells in portal veins, in large capsule vessels, or in a vascular space lined by endothelial cells on microscopy. Pathological specimen were evaluated on 5 mm slices, observed by two expert pathologists, blinded from clinical data (30).
Secondary outcomes were OS and HCC recurrence risk after LT according to AFP SCORE progression.
Statistical Analysis
The categorical data were described by frequencies and percentages, whereas continuous data were described by mean ± standard deviation (SD) or median ± interquartile range (IQR) depending on whether or not they showed a normal distribution. Categorical variables were compared by using the χ 2 test or Fisher exact test, while continuous variables were compared by applying Student’s t-test or Mann-Whitney test, when appropriate. Median follow-up (and 95% CI) was computed using the reverse Kaplan-Meier method. Overall and disease-free survival were calculated using the Kaplan-Meier method. First, we aimed to confirm the prognostic impact of MVI on OS through a Cox regression analysis incorporating histopathological data determined from the native liver. Secondly, predictive factors associated with MVI were identified using uni- and multivariate logistic regression models. Owing to the relatively limited number of events, relevant variables with a p value of less than 0.1 were selected for multivariate analysis via a backward procedure, and an internal validation of the model was performed with 150 bootstrap samples to prevent overfitting (cf Supplementary Material) (31). In addition, the area under the ROC (AUROC) curve was computed to capture the predicting ability of the model. Finally, OS was compared in patients with vs. without AFP SCORE progression, using a log-rank test. HCC recurrence after LT was analyzed in a competing risks framework with HCC recurrence and death as competing events. Cumulative incidence curves for HCC recurrence using Fine-Gray proportional sub-distribution hazards models according to the AFP SCORE progression were performed. Log-linearity was checked and continuous variables were transformed whenever necessary (32).
All analyses were performed with the Stata software, version 17 (Stata Corporation, College Station, TX, United States). A p-value < 0.05 was considered significant.
Results
Patient and Tumor Characteristics
From 2007 to 2015, among 484 liver transplantations performed in our hospital, 192 patients presented with HCC on the native liver and 159 patients met the inclusion criteria (Figure 1).
FIGURE 1.
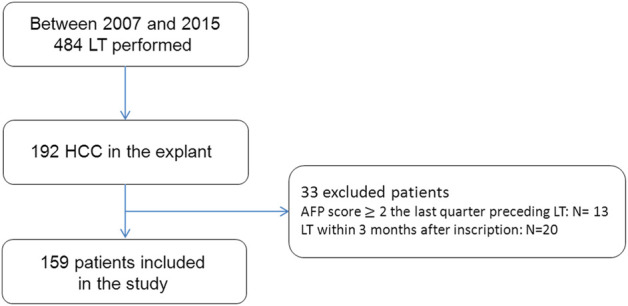
Flow chart of the study population.
Liver transplantation was performed using full grafts or partial grafts from a split procedure (n = 2) from deceased donors. At the diagnosis, the tumor number was 1 in 45% of patients (n = 73), 2 in 28% of patients (n = 44), and ≥3 in 27% of them (n = 42). Median tumor size was 18.5 mm [±17]. The median value of AFP level was 6 ng/ml (range:1–1,584 ng/ml), while AFP SCORE was 0 in 70% of patients (n = 111), 1 in 17% (n = 27), 2 in 8% (n = 13), and ≥3 in 5% of patients (n = 8). All patient and tumor characteristics are detailed in Table 1. The mean time on the waiting list was 7.32 months (±5.75). Overall, 119 patients (75%) received one or more bridging therapies to control the disease during the waiting period, according to the institutional multidisciplinary team indications (as detailed in the Supplementary Material).
TABLE 1.
Comparative analysis according to the AFP SCORE progression during the waiting period.
| Characteristics | Total | Progression | No progression | p-value |
|---|---|---|---|---|
| (n = 159) | (n = 34) | (n = 125) | ||
| Age, mean (range) | 57.9 (39–72) | 59.3 (46–69) | 57.5 (39–72) | 0.18 |
| Sex, male/Female | 136/23 | 31/7 | 105/16 | 0.85 |
| BMI, kg/m2 median (IQR) | 26 (±6) | 26 (±6) | 26 (±7) | 0.58 |
| Etiology of cirrhosis (%) | ||||
| Hepatitis C | 55 (34) | 6 (18) | 49 (40) | 0.01 |
| Hepatitis B | 10 (6) | 2 (6) | 8 (6.5) | 0.91 |
| Alcohol | 75 (47) | 21 (62) | 54 (43) | 0.055 |
| NASH | 11 (7) | 4 (12) | 7 (5.6) | 0.20 |
| Other | 8 (6) | 1 (2) | 7 (5.6) | 0.52 |
| MELD score, Median (IQR) | 11 (±9) | 10 (±5.25) | 12 (±9) | 0.13 |
| MELD >30 (%) | 2 (1) | 0 | 2 (99) | 0.45 |
| MELD <30 (%) | 157 (99) | 34 (100) | 123 (1) | |
| Child- Pugh score (%) | ||||
| A | 79 (49) | 19 (56) | 60 (49) | 0.49 |
| B | 52 (32) | 10 (30) | 42 (34) | 0.64 |
| C | 26 (19) | 5 (14) | 21 (17) | 0.61 |
| Time on waiting list, mean (SD) | 7.32 (±5.75) | 7.85 (±4.95) | 7.18 (±5.9) | 0.50 |
| Pre-LT tumor treatment (%) | 119 (78) | 24 (64) | 95 (76) | 0.51 |
| Tumor number, (%) | ||||
| 1 | 73 (45) | 19 (50) | 67 (50) | 0.81 |
| 2 | 44 (28) | 8 (21) | 36 (27) | 0.55 |
| ≥3 | 42 (27) | 11 (29) | 31 (23) | 0.37 |
| Tumor size, mm, Median (IQR) | 18.5 (±17) | 24 (±11) | 22 (±13) | 0.68 |
| Diameter of largest nodule (%) | ||||
| ≤30 mm | 124 (78) | 95 (76) | 29 (85) | 0.28 |
| 30–60 mm | 32 (20) | 27 (22) | 5 (15) | 0.37 |
| >60 mm | 3 | 3 (2) | 0 | |
| AFP at diagnosis, ng/ml, median (range) | 6 [1–1,584] | 5.95 (2–607) | 6 (1–1,584) | 0.27 |
| AFP at diagnosis, ng/ml | ||||
| ≤100 | 153 (96.5) | 120 (96) | 33 (97) | 0.77 |
| 100–1,000 | 5 (3) | 4 (3) | 1 | 0.93 |
| >1,000 | 1 (0.5) | 1 | 0 | |
| AFP SCORE at diagnosis | ||||
| 0 | 111 (70) | 28 (82) | 83 (66) | 0.07 |
| 1 | 27 (17) | 5 (15) | 22 (18) | 0.69 |
| 2 | 13 (8) | 1 (3) | 12 (10) | 0.20 |
| ≥3 | 8 (5) | 0 | 8 (6) | |
Pathological examination of the explanted liver showed that the median tumor size was 30 mm (±20 mm). The HCC nodule was solitary in 55 patients (22%), while 64 LT recipients had three or more lesions (40%), and 89 patients (56%) were within the Milan criteria. Among the 70 LT recipients (44%) beyond the Milan criteria, histological analysis showed a tumor size >50 mm in six patients, four or more nodules in 35 patients; 29 patients had less than three nodules but with a tumor size >30 mm. According to the WHO classification, the tumor was well, moderate, or poorly differentiated in 28, 62, and 10% of patients, respectively. MVI was found in 31 (19.5%) explants. Among them, 14 presented satellite nodules. The histopathological results are shown in Table 2.
TABLE 2.
Histopathological features on surgical specimens after liver explant.
| MVI (%) | 31 (20) |
| Macrovascular invasion (%) | 4 (2.5) |
| Satellite nodules (%) | 19 (11.9%) |
| Median tumor size (IQR) | 30 (20) |
| Median tumor number (IQR) | 2 (2) |
| Poor differentiation (%) | 15 (9.4) |
MVI, microvascular invasion; IQR, inter-quartile range; poor differentiation, G3 sec. Edmonson.
Survival Analysis According to Post-Operative Histopathologic Factors on the Native Liver
After a median follow-up of 94 months [95% CI: 83–105], a total of 43 patients died (28.1%). Among them, 29 did not recur. HCC recurrence was observed in 19 patients (12%) within a median delay of 13 (range 2–92) months, and 14 of them died after the recurrence (73.6%). The 90-day post-operative mortality was 2.5% (4 patients). Three- and 5-year OS was 86.1% and 81.5%.
Cox regression analysis showed MVI as the only histopathological prognostic factor (among tumor differentiation, tumor size, number of nodules, and the presence of satellite nodules) of overall survival (HR 3.85 [95% CI 1.98–7.49]; p < 0.0001) (Table 3). Competing risk analysis for HCC recurrence identified MVI as an independent prognostic factor (SHR 8.11 [CI 3.13–20.96]; p < 0.0001) (Table 4).
TABLE 3.
Cox regression model for overall survival, univariate analysis.
| Cox regression model for overall survival according to histopathological features | ||
|---|---|---|
| HR (CI 95%) | p-value | |
| MVI | 3.85 (1.98–7.49) | 0.000 |
| Poor differentiation | 0.89 (0.34–2.29) | 0.815 |
| Satellite nodules | 0.85 (0.31–2.30) | 0.460 |
| Tumor size >30 mm | 1.63 (0.85–3.14) | 0.139 |
| Tumor nodule >3 | 1.31 (0.63–2.69) | 0.460 |
Bold values represent statistically significant results
MVI, microvascular invasion; SHR, sub-distribution hazard ratio; CI, confidence interval.
TABLE 4.
competing risk analysis for HCC recurrence according to post-operative histopathological factors.
| Competing risk analysis for HCC recurrence | ||
|---|---|---|
| p-value multivariate SHR [CI] | p-value univariate | |
| MVI | 0.000 8.11 [CI 3.13–20.96] | 0.000 |
| Poor differentiation | 0.403 | 0.056 |
| Satellite nodules | 0.72 | |
| Tumor size >30 mm | 0.47 | |
| Tumor nodule >3 | 0.08 | |
Bold values represent statistically significant results
MVI, microvascular invasion; SHR, sub-distribution hazard ratio; CI, confidence interval.
AFP SCORE Variation During the Waiting Period
According to the AFP SCORE, 34 LT recipients showed progression during the waiting period.
Progressed and non-progressed patients were statistically comparable with respect to all patient and tumor characteristics, as well as time on the waiting list and bridging therapies. The only statistically significative difference was in cirrhosis etiology where the incidence of progression was lower in the HCV group (Table 1).
Primary Outcome: Preoperative Predictive Risk Factors on Microvascular Invasion
Tumor size larger than 30 mm, beyond Milan criteria, AFP value pre-LT, and AFP SCORE progression were associated (i.e., p-value <0.10) with MVI in univariate analysis (Table 5). When tested with multivariate analysis, the AFP SCORE progression was the only independent preoperative risk factor of MVI (OR = 10.79 [95% CI = 2.35–49.4]; p 0.002). A 0.74 AUROC confirmed the good predictive ability of the multivariate model.
TABLE 5.
Multivariate logistic regression analysis for prediction of MVI in patients undergoing LT for HCC.
| Tot | MVI | No MVI | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | ||||
| Overall n (%) | 159 (100) | 31 (19.5) | 128 (80.5) | ||||
| Age >60 (%) | 81 (50.9) | 14 (17.3) | 67 (82.7) | 0.74 (0.34–1.64) | 0.47 | ||
| BMI >30 (%) | 37 (23.2) | 27 (73) | 10 (27) | 1.78 (0.74–4.22) | 0.17 | ||
| Cirrhosis etiology (%) | |||||||
| HCV | 55 (34) | 6 (11) | 49 (89) | 0.38 (0.14–1.02) | 0.10 | ||
| HBV | 10 (6) | 3 (30) | 7 (70) | 1.85 (0.45–7.61) | 0.28 | ||
| Alcoholic | 75 (47) | 14 (18.6) | 61 (81.4) | 0.90 (0.41–1.98) | 0.74 | ||
| NASH | 11 (7) | 2 (18.2) | 9 (81.8) | 0.91 (0.18–4.44) | 0.95 | ||
| Other | 8 (6) | 3 (37.5) | 5 (62.5) | 2.63 (0.59–11.6) | 0.13 | ||
| Waiting time, mean (SD) | 6.78 (±5.75) | 7.07 (±5.77) | 7.38 (±5.79) | 1.003 (0.96–1.04) | 0.78 | ||
| No treatments during waiting time (%) | 40 (25.1) | 10 (25) | 30 (75) | 1.55 (0.66–3.66) | 0.31 | ||
| Tumor size >30 mm (%) | 17 (10.7) | 6 (35) | 11 (65) | 2.55 (0.86–7.55) | 0.08 | 1.49 (0.39–5.7) | 0.18 |
| Tumor nodules pre-LT ≥3 (%) | 53 (33.3) | 10 (19) | 43 (81) | 0.94 (0.40–2.17) | 0.30 | ||
| Child- Turgot- Pugh (%) | |||||||
| A | 79 (49.6) | 18 (22.7) | 61 (77.7) | 1.52 (0.68–3.36) | 0.29 | ||
| B | 52 (32.7) | 7 (13.4) | 45 (86.6) | 0.53 (0.21–1.34) | 0.18 | ||
| C | 28 (17.6) | 6 (21.5) | 22 (78.5) | 1.15 (0.42–3.15) | 0.77 | ||
| MELD, median (IQR) | 11 (±9) | 11 (±6) | 11 (±9) | 0.97 (0.90–1.04) | 0.42 | ||
| AFP pre-LT ng/ml, median (range) | 6.65 (1–1,170) | 12.7 (1.2–1,170) | 5.3 (1–373) | 1.02 (0.99–1.03) | 0.07 | 1.03 (0.98–1.05) | 0.34 |
| Within Milan criteria (%) | 114 (71.6) | 19 (16.6) | 98 (83.4) | 0.48 (0.21–1.11) | 0.08 | 0.17 (0.01–1.18) | 0.21 |
| Delta AFP SCORE progression | 34 (21.3) | 18 (53) | 16 (47) | 9.69 (3.9–23.4) | <0.0001 | 10,79 (CI = 2.35–49.4) | 0.002 |
Bold values represent statistically significant results
BMI, body mass index; HCV, hepatitis C virus; HBV, hepatitis B virus; NASH, non-alcoholic steatohepatitis; LT, liver transplantation; MELD, model for end stage liver Disease; AFP, alpha fetoprotein; MVI, microvascular invasion. OR, odds ratio; CI, confidence interval. Variables with p-value <0.10 underwent multivariate analysis.
Secondary Outcomes: Survival Analysis and Recurrence According to Preoperative AFP SCORE Progression
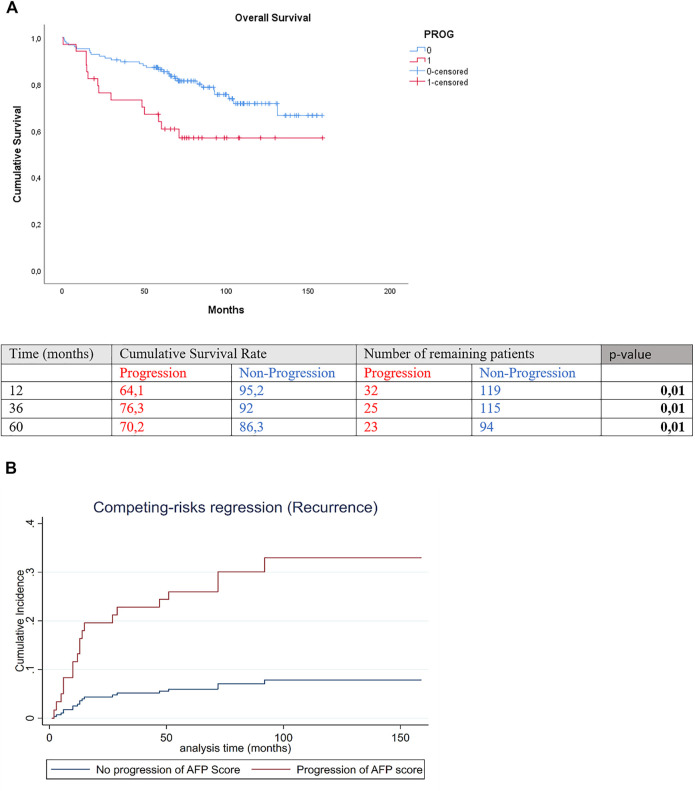
Three-year and 5-year overall survival was significantly lower in the progression than the non-progression group [(3-year OS 73.2% vs. 89.6%, 5-year OS 63.9% vs. 86.3%; p 0.01] (Figure 2A). Cumulative incidence of recurrence significantly differed between the groups of progression and no progression in AFP SCORE (SHR = 4.89 [CI 2–11.98]; p = 0.001 (Figure 2B).
FIGURE 2.
(A) Overall survival and (B) competing risk regression for HCC recurrence of patients with and without delta AFP SCORE progression.
Discussion
In the present study, for the first time in literature, we analyzed the predictability of AFP SCORE progression on MVI in a homogeneous population of LT recipients with histologically proven HCC who had AFP SCORE ≤2. Our results showed that AFP SCORE progression, for those on the waiting list for liver transplantation, was the only preoperative factor that enabled prediction of MVI. In contrast, the absolute value of AFP, Milan criteria, number of tumor nodules, and tumor size were not associated with MVI, emphasizing the need to use a composite score as defined by the AFP model.
Furthermore, variation of the AFP SCORE can be easily calculated in clinical practice and could be a relevant preoperative tool for predicting tumor aggressiveness and other related outcomes.
Notably, our patient cohort had already undergone a stringent selection process, thus, a low rate of recurrence was expected. Despite this, 19 recurrences were observed and among them 14 died shortly after. In both overall survival and HCC recurrence, MVI was shown to be a strong prognostic factor in our study population, with HR greater than 3. Despite the strict cohort selection, the augmentation of AFP SCORE by one point, even from 0 to 1—expected to be an irrelevant variation—was shown to enable prediction of MVI, with OR greater than 10.
These results could lead to an optimization of pre- or post-transplantation strategies in terms of prevention and surveillance, enabling adapted treatment strategies, radiological monitoring of patients on waiting lists, and adjustment of immunosuppressive therapy after LT.
The downstaging of the tumor burden in HCC patients awaiting transplantation has been widely shown to be advantageous in terms of survival, both in patients still on waiting lists and in those who dropped out of the criteria (33, 34, 35). Therefore, having a dynamic tool for identifying patients at high risk of MVI while in the waiting period could further identify a subgroup of candidates that could benefit from a different strategy. We suggest that further studies should explore this direction.
Several studies have reported that baseline or follow-up AFP levels are correlated with survival and/or tumor recurrence (36, 37). However, increased AFP levels are inconstant in HCC patients, with only 30%–40% of patients having abnormal values (38). Furthermore, previous studies on the topic have always selected the study population by eliminating non-secreting tumors (21). In contrast, our study included all patients transplanted for HCC who had an AFP SCORE ≤2, regardless of the levels of AFP and other parameters.
In our study, 93% of patients had an AFP level <100 ng/ml, with the median value being 6 ng/ml, which for some reason challenged the tools used to select the correct follow-up strategy. In such circumstances, AFP SCORE progression could identify patients who would benefit from a stricter follow-up. Eventually, contrast-enhanced (18)F-choline or (11)C-choline PET/CT could be useful, alone or combined with (18F)-FDG PET/CT (39, 40).
Actually, in current practice, we do not dispose of any specific tool that can predict MVI preoperatively. The originality of our study is that it demonstrates the utility of a simple preoperative dynamic score evaluation that can strongly predict MVI without performing a biopsy or radiological exam. Previous studies demonstrated the potential utility of MRI or circulating cell free DNA (cfDNA) as dynamic preoperative biomarkers. These biomarkers were found to be independent predictors of MVI (41, 42). However, cfDNA use is not widespread, as it is expensive and difficult to perform regularly in all centers due to the technical procedures necessary for genetic analysis. In contrast, AFP SCORE can be measured easily and without additional cost, as no additional exams are needed. Future studies exploring the association between cfDNA and the dynamic assessment of AFP SCORE may provide physicians with an effective tool and consequently help guide the selection of individualized therapies or treatment monitoring before radiologic and/or biologic progression.
Finally, the parameter of AFP SCORE progression can be integrated into a new predictive score for identifying the risk of tumor recurrence. The interest in creating, validating, and developing such a model is demonstrated by the increasing number of interesting similar papers (43, 44, 45). At the same time, previous efforts have not enabled identification of the best model. We believe that AFP progression could be associated with parameters related to the total tumor burden and tumor aggressiveness (pre-LT AFP levels, total tumor volume). All previous hypotheses require prospective studies on larger populations to be corroborated, but we believe that they must be considered in light of the importance of HCC recurrence in LT recipients and the relevance of MVI to recurrence and survival (46).
Our study’s limitations include its retrospective design and relatively small number of events. Therefore, further large-scale, multicenter studies are needed. Another limitation is its use of the AFP SCORE, which at the moment is widely used only in France. Nevertheless, the strengths of our study include its long median follow-up of 94 months, its monocentric character, which ensured the homogeneous management of all patients using the same surgical and medical teams, and its selection of a patient cohort with no known preoperative MVI or recurrence factors.
To conclude, this study highlights the potentially high relevance of AFP SCORE progression as a simple, dynamic, preoperative predictive factor for MVI in patients undergoing LT for HCC. These findings could lead LT units to adopt new strategies before or after LT to optimize the management of such subgroups of patients.
Acknowledgments
We are grateful to Dr. J. Butterworth for proofreading assistance.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional review board of the Montpellier University Hospital (N° 2018_IRB-MTP_11-23). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AH and G-PP were responsible for study conception and design. LB, GC, and BG drafted the manuscript. BR, SF, and JB collected and analyzed data. FN, EA, and FP were responsible for the critical revision of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10412/full#supplementary-material
Abbreviations
AFP, alpha fetoprotein; DFS, disease-free survival; HCC, hepatocellular carcinoma; LT, liver transplantation; MVI, microvascular invasion; OS, overall survival.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J Clinicians (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Forner A, Reig M, Bruix J. Hepatocellular Carcinoma. Lancet (2018) 391(10127):1301–1314. 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 3. Schwartz M. Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology (2004) 127:S268–S276. 10.1053/j.gastro.2004.09.041 [DOI] [PubMed] [Google Scholar]
- 4. Mazzaferro V, Chun YS, Poon RTP, Schwartz ME, Yao FY, Marsh JW, et al. Liver Transplantation for Hepatocellular Carcinoma. Ann Surg Oncol (2008) 15:1001–7. 10.1245/s10434-007-9559-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N Engl J Med (1996) 334:693–700. 10.1056/nejm199603143341104 [DOI] [PubMed] [Google Scholar]
- 6. Toso C, Dupuis-Lozeron E, Majno P, Berney T, Kneteman NM, Perneger T, et al. A Model for Dropout Assessment of Candidates with or without Hepatocellular Carcinoma on a Common Liver Transplant Waiting List. Hepatology (2012) 56:149–56. 10.1002/hep.25603 [DOI] [PubMed] [Google Scholar]
- 7. Persad G, Wertheimer A, Emanuel EJ. Principles for Allocation of Scarce Medical Interventions. Lancet (2009) 373(9661):423–31. 10.1016/S0140-6736(09)60137-9 [DOI] [PubMed] [Google Scholar]
- 8. Vitale A, Farinati F, Burra P, Trevisani F, Giannini EG, Ciccarese F, et al. Utility-based Criteria for Selecting Patients with Hepatocellular Carcinoma for Liver Transplantation: A Multicenter Cohort Study Using the Alpha-Fetoprotein Model as a Survival Predictor. Liver Transpl (2015) 21:1250–8. 10.1002/lt.24214 [DOI] [PubMed] [Google Scholar]
- 9. Vitale A, Farinati F, Pawlik TM, Frigo AC, Giannini EG, Napoli L, et al. The Concept of Therapeutic Hierarchy for Patients with Hepatocellular Carcinoma: A Multicenter Cohort Study. Liver Int (2019) 39:1478–89. 10.1111/liv.14154 [DOI] [PubMed] [Google Scholar]
- 10. Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan Criteria in Liver Transplantation for Hepatocellular Carcinoma: An Evidence-Based Analysis of 15 Years of Experience. Liver Transpl (2011) 17:S44–S57. 10.1002/lt.22365 [DOI] [PubMed] [Google Scholar]
- 11. Martin P, Dimartini A, Feng S, Brown R, Fallon M. Evaluation for Liver Transplantation in Adults: 2013 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology (2014) 59:1144–65. 10.1002/hep.26972 [DOI] [PubMed] [Google Scholar]
- 12. Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, et al. Recurrence of Hepatocellular Carcinoma Following Liver Transplantation. Arch Surg (2008) 143:182. 10.1001/archsurg.2007.39 [DOI] [PubMed] [Google Scholar]
- 13. Hoffman D, Mehta N. Recurrence of Hepatocellular Carcinoma Following Liver Transplantation. Expert Rev Gastroenterol Hepatol (2020) 15:91–102. 10.1080/17474124.2021.1823213 [DOI] [PubMed] [Google Scholar]
- 14. Kluger MD, Salceda JA, Laurent A, Tayar C, Duvoux C, Decaens T, et al. Liver Resection for Hepatocellular Carcinoma in 313 Western Patients: Tumor Biology and Underlying Liver rather Than Tumor Size Drive Prognosis. J Hepatol (2015) 62:1131–40. 10.1016/j.jhep.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 15. Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of Hepatocellular Carcinoma after Liver Transplant: Patterns and Prognosis. Liver Transpl (2004) 10:534–40. 10.1002/lt.20128 [DOI] [PubMed] [Google Scholar]
- 16. Costentin CE, Amaddeo G, Decaens T, Boudjema K, Bachellier P, Muscari F, et al. Prediction of Hepatocellular Carcinoma Recurrence after Liver Transplantation: Comparison of Four Explant-Based Prognostic Models. Liver Int (2017) 37:717. 10.1111/liv.13388 [DOI] [PubMed] [Google Scholar]
- 17. Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, et al. Diagnostic and Prognostic Role of Alpha-Fetoprotein in Hepatocellular Carcinoma: Both or Neither? Am J Gastroenterol (2006) 101:524–32. 10.1111/j.1572-0241.2006.00443.x [DOI] [PubMed] [Google Scholar]
- 18. Charrière B, Maulat C, Suc B, Muscari F. Contribution of Alpha-Fetoprotein in Liver Transplantation for Hepatocellular Carcinoma. Wjh (2016) 8:881. 10.4254/wjh.v8.i21.881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai Q, Melandro F, Pinheiro RS, Donfrancesco A, Fadel BA, Levi Sandri GB, et al. Alpha-Fetoprotein and Novel Tumor Biomarkers as Predictors of Hepatocellular Carcinoma Recurrence after Surgery: A Brilliant Star Raises Again. Int J Hepatol (2012) 2012:1–9. 10.1155/2012/893103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujioka M, Nakashima Y, Nakashima O, Kojiro M. Immunohistologic Study on the Expressions of [alpha ]-fetoprotein and Protein Induced by Vitamin K Absence or Antagonist II in Surgically Resected Small Hepatocellular Carcinoma. Hepatology (2001) 34:1128–34. 10.1053/jhep.2001.29202 [DOI] [PubMed] [Google Scholar]
- 21. Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, et al. Progression of Alphafetoprotein before Liver Transplantation for Hepatocellular Carcinoma in Cirrhotic Patients: A Critical Factor. Am J Transpl (2010) 10:129–37. 10.1111/j.1600-6143.2009.02750.x [DOI] [PubMed] [Google Scholar]
- 22. Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of Liver Disease in Europe: Epidemiology and Analysis of Risk Factors to Identify Prevention Policies. J Hepatol (2018) 69:718–35. 10.1016/j.jhep.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 23. Duvoux C, Roudot–Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology (2012) 143:986–94. 10.1053/j.gastro.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 24. Notarpaolo A, Layese R, Magistri P, Gambato M, Colledan M, Magini G, et al. Validation of the AFP Model as a Predictor of HCC Recurrence in Patients with Viral Hepatitis-Related Cirrhosis Who Had Received a Liver Transplant for HCC. J Hepatol (2017) 66:552–9. 10.1016/j.jhep.2016.10.038 [DOI] [PubMed] [Google Scholar]
- 25. Piñero F, Tisi Baña M, de Ataide EC, Hoyos Duque S, Marciano S, Varón A, et al. Liver Transplantation for Hepatocellular Carcinoma: Evaluation of the Alpha-Fetoprotein Model in a Multicenter Cohort from Latin America. Liver Int (2016) 36:1657–67. 10.1111/liv.13159 [DOI] [PubMed] [Google Scholar]
- 26. Cuschieri S. The STROBE Guidelines. Saudi J Anaesth (2019) 13:31. 10.4103/sja.SJA_543_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. In: International Agency for Research on Cancer. 4th ed. (2010). Geneva, Switzerland: WHO Press, World Health Organization; 3:417. [Google Scholar]
- 28. Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J Hepatol (2018) 69:182–236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 29. Burra P, Burroughs A, Graziadei I, Pirenne JC, Valdecasas P, Muiesan D, et al. EASL Clinical Practice Guidelines: Liver Transplantation. J Hepatol (2016) 64:433–85. 10.1016/j.jhep.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 30. Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A Systematic Review of Microvascular Invasion in Hepatocellular Carcinoma: Diagnostic and Prognostic Variability. Ann Surg Oncol (2013) 20:325–39. 10.1245/s10434-012-2513-1 [DOI] [PubMed] [Google Scholar]
- 31. Harrell FE, Lee KL, Mark DB. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med (1996) 15:361–87. [DOI] [PubMed] [Google Scholar]
- 32. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc (1999) 94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 33. Ravaioli M, Odaldi F, Cucchetti A, Trevisani F, Piscaglia F, De Pace V, et al. Long Term Results of Down-Staging and Liver Transplantation for Patients with Hepatocellular Carcinoma beyond the Conventional Criteria. Sci Rep (2019) 9:3781. 10.1038/s41598-019-40543-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, et al. Liver Transplantation in Hepatocellular Carcinoma after Tumour Downstaging (XXL): a Randomised, Controlled, Phase 2b/3 Trial. Lancet Oncol (2020) 21(7):947–956. 10.1016/S1470-2045(20)30224-2 [DOI] [PubMed] [Google Scholar]
- 35. Toso C, Meeberg G, Andres A, Shore C, Saunders C, Bigam DL, et al. Downstaging Prior to Liver Transplantation for Hepatocellular Carcinoma: Advisable but at the price of an Increased Risk of Cancer Recurrence - a Retrospective Study. Transpl Int (2019) 32:163–72. 10.1111/tri.13337 [DOI] [PubMed] [Google Scholar]
- 36. McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R. Alpha-fetoprotein and Tumour Size Are Associated with Microvascular Invasion in Explanted Livers of Patients Undergoing Transplantation with Hepatocellular Carcinoma. HPB (2010) 12:56–61. 10.1111/j.1477-2574.2009.00128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Özdemir F, Baskiran A. The Importance of AFP in Liver Transplantation for HCC. J Gastrointest Canc (2020) 51:1127–32. 10.1007/s12029-020-00486-w [DOI] [PubMed] [Google Scholar]
- 38. Chan SL, Mo FKF, Johnson PJ, Hui EP, Ma BBY, Ho WM, et al. New Utility of an Old Marker: Serial α-Fetoprotein Measurement in Predicting Radiologic Response and Survival of Patients with Hepatocellular Carcinoma Undergoing Systemic Chemotherapy. Jco (2009) 27:446–52. 10.1200/JCO.2008.18.8151 [DOI] [PubMed] [Google Scholar]
- 39. Wu H-b., Wang Q-s., Li B-y., Li H-s., Zhou W-l., Wang Q-y. F-18 FDG in Conjunction with 11C-Choline PET/CT in the Diagnosis of Hepatocellular Carcinoma. Clin Nucl Med (2011) 36:1092–7. 10.1097/RLU.0b013e3182335df4 [DOI] [PubMed] [Google Scholar]
- 40. Castilla-Lièvre M-A, Franco D, Gervais P, Kuhnast B, Agostini H, Marthey L, et al. Diagnostic Value of Combining 11C-Choline and 18F-FDG PET/CT in Hepatocellular Carcinoma. Eur J Nucl Med Mol Imaging (2016) 43:852–9. 10.1007/s00259-015-3241-0 [DOI] [PubMed] [Google Scholar]
- 41. Ono A, Fujimoto A, Yamamoto Y, Akamatsu S, Hiraga N, Imamura M, et al. Circulating Tumor DNA Analysis for Liver Cancers and its Usefulness as a Liquid Biopsy. Cell Mol Gastroenterol Hepatol (2015) 1:516–34. 10.1016/j.jcmgh.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang P, Lo YMD. The Long and Short of Circulating Cell-free DNA and the Ins and Outs of Molecular Diagnostics. Trends Genet (2016) 32:360–71. 10.1016/j.tig.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 43. Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, et al. A Novel Prognostic Nomogram Accurately Predicts Hepatocellular Carcinoma Recurrence after Liver Transplantation: Analysis of 865 Consecutive Liver Transplant Recipients. J Am Coll Surgeons (2015) 220:416–27. 10.1016/j.jamcollsurg.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 44. Feng J, Wu J, Zhu R, Feng D, Yu L, Zhang Y, et al. Simple Risk Score for Prediction of Early Recurrence of Hepatocellular Carcinoma within the milan Criteria after Orthotopic Liver Transplantation. Sci Rep (2017) 7:44036. 10.1038/srep44036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ma KW, She WH, Chan ACY, Cheung TT, Fung JYY, Dai WC, et al. Validated Model for Prediction of Recurrent Hepatocellular Carcinoma after Liver Transplantation in Asian Population. Wjgo (2019) 11:322–34. 10.4251/wjgo.v11.i4.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sapisochin G, Bruix J. Liver Transplantation for Hepatocellular Carcinoma: Outcomes and Novel Surgical Approaches. Nat Rev Gastroenterol Hepatol (2017) 14:203–17. 10.1038/nrgastro.2016.193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.