Abstract
Programmed cell death (PDCD) family of proteins includes at least 12 members, function of seven of them being more investigated. These members are PDCD1, PDCD2, PDCD4, PDCD5, PDCD6, PDCD7 and PDCD10. Consistent with the important roles of these proteins in the regulation of apoptosis, dysregulation of PDCDs is associated with diverse disorders ranging from intervertebral disc degeneration, amyotrophic lateral sclerosis, immune thrombocytopenia, type 1 diabetes, congenital hypothyroidism, Alzheimer’s disease to different types of cancers. More recently, the interaction between non-coding RNAs and different members of PDCD family is being discovered. In the current study, we described the functional interactions between PDCDs and two classes of non-coding RNAs, namely microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). miR-21 and miR-183 are two miRNAs whose interactions with PDCDs have been assessed in different contexts. The lncRNAs interaction with PDCDs is mainly assessed in the context of neoplasia indicating the role of MALAT1, MEG3, SNHG14 and LINC00473 in this process.
Keywords: lncRNA, miRNA, programmed cell death protein, expression, biomarker
1 Introduction
Programmed cell death (PDCD) has been observed as an important phenomenon during insect development about seven decades ago (1). Afterwards, several equivalent cell death phenomena have been recognized in vertebrates including human and this process has been retitled as apoptosis (2). This cellular process in contributes in eradication of foreign bodies and abnormal cells. Moreover, it has essential roles in the development of organisms, the homeostasis of the internal milieu and organ development. Abnormal regulation of apoptosis is strictly associated with immune disorders, developmental abnormalities, and cancers (3). Apoptosis is regulated by two types of proteins with one of them inhibiting cell death and the other group initiating this process. Genes regulating apoptosis are highly conserved between various species and genera (4). Different studies have shown wide expression of PDCD gene family members in normal adult tissues (4).
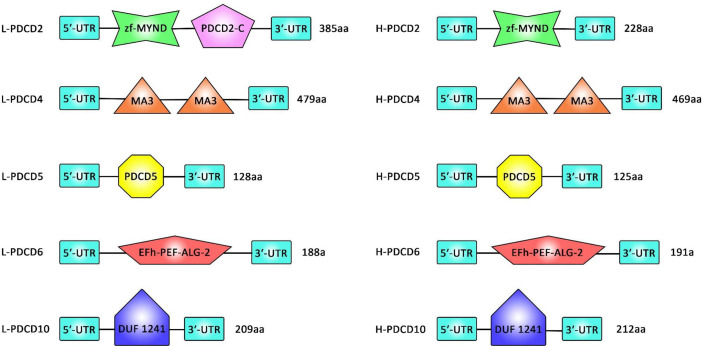
A comprehensive analysis of several PDCD gene family members in vertebrates and assessment of their sequences as well as alignment steps and 3D structure analyses has revealed no similarity in the structural domains between the PDCD family genes. In fact, PDCD4 family genes have no structural similarity but contain some conserved amino acid sequences (or so call motifs). Lamprey PDCD genes have been found to be highly homologous with the corresponding human PDCD genes. Figure 1 shows the conserved motifs between lamprey and human PDCD genes.
Figure 1.
A comparison between the sequence of the domains of PDCD family genes (PDCD2, PDCD4, PDCD5, PDCD6, and PDCD10) in Lamprey and human.
Assessment of motifs in the PDCD proteins has resulted in identification of 16 distinct motifs in these proteins. All lamprey PDCD proteins have been shown to contain motif 13. PDCD2 proteins have been found to contain motifs 7, 9, 13, 15, and 16. Besides, motifs 4, 8, 10, 11, 12, 13, and 14 have been present in PDCD4 proteins. Finally, motifs 1, 2, and 13 have been detected in PDCD5 proteins. This bioinformatics analysis has indicated that individual motifs might contribute in the biological activity of PDCD proteins. Phylogenetic studies have also verified the conserved evolution of each PDCD gene in vertebrates (4).
PDCD1 has an important role in the regulation of the immune system responses and induction of self-tolerance through inhibition of activity of T cells (5). PDCD2 participates in the development of embryo and differentiation of stem cells (6, 7). PDCD4 participates in tumor evolution, cancer progression and metastatic processes (8, 9). In fact, PDCD4 participates in the regulation of transcription, translation, apoptototic pathways, and regulation of various signal transduction pathways (10). Expression of PDCD5 has been found to be increased in TF-1 cells undertaking apoptosis (11). PDCD6 has been shown to be involved in cell proliferation and death (12). PDCD7 participates in apoptosis induced by glucocorticoids and staphylococci (13). PDCD10 has diverse roles in proteins synthesis, apoptotic pathways, cell proliferation, and induction of tumors (14, 15).
Moreover, expression levels of PDCD genes have been demonstrated to be altered in tumor samples and cancer cell lines (16, 17). At present, there is no evidence demonstrating a conclusive link between members of PDCD gene family (4). Yet, assessment of transcriptomic data has shown remarkable alterations in expression of the PDCD gene family following treatment with certain drugs (18). Although 12 members of this family have been identified in the human genome, seven main genes, namely PDCD1 (alternatively named as PD-1), PDCD2, PDCD4, PDCD5, PDCD6, PDCD7 and PDCD10 are in the focus of mechanistical investigations (4). PD-1 has two ligands, namely PD-L1 and PD-L2 which are present on the surface of dendritic cells or macrophages (19).
More recently, the interaction between non-coding RNAs and different members of PDCD family is being discovered. In the current study, we described the functional interactions between PDCDs and two classes of non-coding RNAs, namely microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). miRNAs are a group of small-sized non-coding RNAs that regulate gene expression at post-transcriptional level through base-pairing with mRNAs and inducing mRNA degradation or suppressing mRNA translation. LncRNAs can regulate gene expression at different levels through epigenetic mechanisms, modification of RNA stability and interaction with several types of biomolecules.
2 miRNAs and PDCD
miRNAs are transcripts with sizes about 22 nucleotides that regulate gene expression through binding with different regions of mRNAs, particularly their 3’ UTR (20). In addition to transcript degradation and translational suppression, miRNAs have been found to induce translation or modulate transcription (20). Most studies have shown that miRNAs regulate PDCD expression through binding with 3’ UTR of PDCD transcripts. This kind of interaction has been predicted by bioinformatics approaches and verified through luciferase assay. Binding of miRNAs with this region of PDCD transcript leads to down-regulation of expression of PDCDs. The degree of miRNA response elements complementarity defines whether the target mRNA is subjected to AGO2-dependent slicing or its translation is inhibited by miRNA-induced silencing complex and target mRNA decay (20). It has also been shown that in some situations, miRNAs may activate translation of target mRNAs or regulate transcription (20).
2.1 Interaction Between miRNAs and PDCD in Non-Neoplastic Disorders
2.1.1 Heart Diseases
Suppression of PDCD4 by miR-21 has been shown to contribute to the induction of fibroblastoid features during cardiac injury leading to cardiac fibrosis, since contributing in the pathogenesis of fibrogenic cardiac injury. In fact, pro-fibrogenic incitements, particularly TGF-β, can promote progression of epithelial-mesenchymal transition (EMT) in epicardial mesothelial cells, resulting in alterations in miRNAs signature, specifically expression of the pleiotropic miR-21. Ectopic expression of this miRNA has noticeably stimulated the fibroblast-like features causing by fibrogenic EMT, while miR-21 antagonism has suppressed this effect (21). Another study has shown up-regulation of miR-21 in macrophages in response to elevation of glucose levels. Elevation of glucose levels has been shown to stimulate apoptosis of macrophages. Concurrent inhibition of miR-21 and elevation of glucose concentrations results in enhancement of cell apoptosis (citation). Taken together, miR-21 levels have been changed by high glucose concentrations in macrophages, exerting a protective impact against glucose-induced macrophage apoptosis through suppression of PDCD4 expression (22). miR-208a-3p is another miRNA that could suppress expression of PDCD4, thus affecting autophagy in rat cardiomyoblasts (23). Meanwhile, the inhibitory effects of miR-499-5p on PDCD4 have been shown to decrease cardiomyocytes apoptosis and reduce myocardial infarct size (24). Finally, miR-613 through targeting PDCD10 could suppresses ischemia/reperfusion-induced cardiomyocyte apoptosis via regulating the PI3K/AKT signaling pathway (25). Table 1 shows the interaction between miRNAs and PDCD in heart diseases.
Table 1.
Interaction between miRNAs and PDCD in heart diseases.
| Disease | miRNA | Animal/human | Cell lines | Targets | Pathways | Function | Ref |
|---|---|---|---|---|---|---|---|
| Cardiac Fibrosis | miR-21 | Adult SD rats, female C57/BL6 mice | – | PDCD4, SPRY1, IL-1β, TNF-α, TGF-β, α-SMA, Slug, E-cadherin |
– | Upregulation of miR-21 by targeting PDCD4 and SPRY1 could increase fibrogenic EMT of epicardial mesothelial cells. | (21) |
| Cardiovascular Disease | miR-21 | – | Raw 264.7 | PDCD4, Caspase-3 |
– | Upregulation of miR-21 which is induced by high levels of glucose could reduce macrophage apoptosis by targeting PDCD4. | (22) |
| Cardiac Hypertrophy | miR-208a-3p | – | H9c2, 293T | PDCD4, ATG5, LC3BII/I, P62 |
miR-208a-3p could increase the autophagic activity via PDCD4-ATG5 pathway in Ang II-induced H9c2 cardiomyoblasts in rats. | (23) | |
| Myocardial I/R Injury | miR-613 | – | H9c2 | PDCD10, PDCD10, MDA, CHOP, GRP78, Bcl-2, Caspase-3/12, Bax, Cytochrome-c |
p-Akt, p-JNK |
Upregulation of miR-613 by targeting PDCD10 could suppress I/R-induced cardiomyocyte apoptosis via regulating the PI3K/AKT signaling pathway. | (25) |
| Acute Myocardial Infarction (AMI) | miR-499-5p | Male SD rats | – | PDCD4 | – | Upregulation of miR-499-5p could suppress cardiomyocytes apoptosis and myocardial infarct size of AMI in vitro and in vivo. | (24) |
2.1.2 Polycystic Ovary Syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is the utmost frequent female endocrine disease (26) being characterized by the presence of ovarian cysts, chronic anovulation, and clinical or biological signs of hyperandrogenism. People with PCOS may experience irregular menstrual periods, heavy periods, excess hair, acne, pelvic pain, difficulty getting pregnant, and patches of thick, darker, velvety skin (3) The primary characteristics of this syndrome include: hyperandrogenism, anovulation, insulin resistance, and neuroendocrine disruption Expression of miR-16 expression has been shown to be decreased in ovarian cortex tissues and serums of PCOS patients, parallel with up-regulation of PDCD4. Mechanistically, miR-16 enhances cell proliferation, facilitates cell cycle progression, and suppresses apoptosis in granulosa cells through inhibiting expression of PDCD4. Forced over-expression of PDCD4 has stopped the impact of miR-16 on growth and apoptosis of granulosa cells. Moreover, testosterone could reduce expression level of miR-16 and enhance PDCD4 levels, therefore hindering cell growth and enhancing apoptosis of granulosa cells (27). Another study in steroidogenic human ovarian granulose-like tumor cell line has shown that upregulation of miR-155 by targeting PDCD4 and regulating PI3K/AKT and JNK pathways could enhance proliferation, migration, and invasion of cells. Since miR-155 has been found to be up-regulated in PCOS samples, miR-155/PCDC4 axis might be involved in the pathogenesis of PCOS (28). Table 2 shows the interaction between miRNAs and PDCD in PCOS.
Table 2.
Interaction between miRNAs and PDCD in polycystic ovary syndrome (PCOS).
| miRNA | Animal-human | Cell lines | Targets | Pathways | Function | Ref |
|---|---|---|---|---|---|---|
| miR-16 | Female Wistar rats/Human: 19 pairs of PCOs and normal healthy women | GCs | PDCD4, PCNA, caspase-3 | – | Upregulation of miR-16 by targeting PDCD4 could enhance ovarian GCs proliferation and suppress apoptosis in PCOS. | (27) |
| miR-155 | 20 pairs of PCOS tissues and normal tissues | KGN | PDCD4, c-Myc, p21 Cyclin-D1, Vimentin, Caspase-3/9, p53, MMP-2/9 |
PI3K/AKT, JNK | Upregulation of miR-155 by targeting PDCD4 and regulating PI3K/AKT and JNK pathways could enhance proliferation, migration, and invasion in KGN cells. | (28) |
2.1.3 Other Non-Neoplastic Disorders
The impact of miRNAs on expression of PDCD is also involved in a variety of other non-neoplastic disorders. For instance, in the context of intervertebral disc degeneration, up-regulation of miR-21 could promote proliferation of human degenerated nucleus pulposus cells through regulating PDCD4 expression, enhancing phosphorylation of c-Jun protein, and activating AP-1-dependent transcription of MMP-2/9 (29). miR-183-5p, a highly expressed miRNA in neurons has been shown to inhibit expression of PDCD4. Neuronal expression of this miRNA is instantly increased in response to treatment with hydrogen peroxide, tunicamycin or TNF-α. Its up-regulation enhances survival of neurons under stress situations, while its silencing leads to death of neurons. In fact, miR-183-5p synchronizes apoptosis and necroptosis mechanisms through its direct interactions with PDCD4 and RIPK3. This function shields neurons against cell death under stress situations. Consistently, expression of miR-183-5p has been found to be reduced in amyotrophic lateral sclerosis patients and animal models enhances supporting the role of this miRNA in regulation of motor neuron survival (30). Another set of in vivo and in vitro experiments has shown that down-regulation of miR-21 could increase fibroblast apoptosis and prevent knee scar adhesion though influencing PDCD4 expression. In fact, miR-21 attenuates the impact of mitomycin on decreasing the number of fibroblasts through down-regulating PDCD4 levels (31). miR-16 is another miRNA that targets PDCD4. Through this route, miR-16 can inhibit activation of inflammatory macrophages during the atherosclerotic process through the MAPK and NF-κB pathways (32). In the context of steroid-induced avascular necrosis of femor, miR−206 by targeting PDCD4 could reduce cell viability and proliferation, and enhance apoptosis (33).
hsa-miR-424-5p has been found to bind with PD-1 stimulating immune responses through the mTORC signal transduction, thus participating in the pathoetiology of type I diabetes (34). Similarly, up-regulation of miR-28 by targeting PD-1 and regulating cytokine secretion could modulate exhaustive differentiation of T cells (35). Table 3 shows interaction between miRNAs and PDCD in diverse non-neoplastic disorders. Figure 2 demonstrates that aberrant expression of PDCD-interacting ncRNAs could play an effective role in causing several non-neoplastic disorders.
Table 3.
Interaction between miRNAs and PDCD in other non-neoplastic disorders.
| Disorder | miRNA | Animal-human | Cell lines | Targets | Pathways | Function | Ref |
|---|---|---|---|---|---|---|---|
| – | miR-21-5p | – | L02 | PDCD4, ROS, MMP, MRCC I/II | – | Upregulation of miR-21-5p by ROS through targeting PDCD4 could regulate the proliferation and apoptosis in L02 hepatocytes. | (36) |
| – | miR-28 | C57BL/6 mice | B16F10 | PD1, Foxp3+, BTLA, TIM3, IL-2, TNF-α |
– | Upregulation of miR-28 by targeting PD-1 and regulating cytokine secretion could modulate exhaustive differentiation of T cells. | (35) |
| Intervertebral Disc Degeneration (IDD) | miR-21 | 20 IDD patients and 5 healthy control | – | PDCD4, MMP-2, MMP-9 | c-Jun | Upregulation of miR-21 by regulating PDCD4 expression, enhancing phosphorylation of c-Jun protein, and activating AP-1-dependent transcription of MMP-2/9 could promote the proliferation of human degenerated NP cells. | (29) |
| Amyotrophic Lateral Sclerosis (ALS) | miR-183-5p | – | Neuro2a, NSC-34 | PDCD4, TNF-α, RIPK3, Caspase-3 |
– | Upregulation of miR-183-5p by targeting PDCD4 could enhance the survival rate of neurons under stress conditions in ALS cell lines. | (30) |
| Knee Scar Adhesion | miR-21 | Rabbit | 293T | PDCD4, PARP, Bax, Bcl-2 | – | Downregulation of miR-21 could increase fibroblast apoptosis and prevent knee scar adhesion through influencing expression of PDCD4 in vivo and in vitro. | (31) |
| Steroid−Induced Avascular Necrosis Of Femoral Head (SANFH) | miR-206 | 15 SANFH and 15 healthy control specimens | hFOB1.19, 293T | PDCD4, ALP, Bax, Bcl-2 | – | Upregulation of miR−206 by targeting PDCD4 could reduce cell viability and proliferation, and enhance apoptosis in hFOB1.19 cells. | (33) |
| Immune Thrombocytopenia (ITP) | miR-155-5p | Female CBA mice/Human: 42 patients with ITP and 30 healthy volunteers | PBMCs, 293T | PD1, PDL1, SOCS1, IL-4, IL-10, IL-17A, TGF-β1 | – | Downregulation of miR-155-5p could induce the PD1/PDL1 pathway-mediated macrophage M2 polarization and suppress ITP progression via targeting SOCS1. | (37) |
| Contrast-Induced Acute Kidney Injury (CI-AKI) | miR-21 | – | HK-2, 293T |
PDCD4, Bcl-2, Bax | – | Upregulation of miR-21 by targeting PDCD4 could suppress HK-2 cell apoptosis. | (38) |
| Type 1 Diabetes (T1D) | miR-424-5 | Male SD rats | – | PD-1, T-bet, CXCR3, STING, IGF-1, SHP2, Rheb, Rictor | mTORC | Upregulation of miR-424-5p by targeting PD-1 signaling molecules could result in the immune response in T1D. | (34) |
| Congenital Hypothyroidism (CH) | miR-124-3p | Pregnant SD rats | – | PDCD6, PARP, Caspase-3, Bcl−2, Bax | – | Upregulation of miR-124-3p by targeting PDCD6 could suppress the progression of CH. | (39) |
| Alzheimer’s Disease (AD) | miR-21 | – | SH-SY5Y | PDCD4, amyloid-β, GSK-3β, Bcl-2, Bax |
PI3K/AKT | Upregulation of miR-21 by targeting the PDCD4/PI3K/AKT/GSK-3β pathway could reduce apoptosis in SH-SY5Y cells. | (40) |
| Atherosclerosis | miR-16 | ApoE-/- mice with a C57BL/6 background | RAW264.7, 293T | PDCD4, TNF-α, IL-10, IL-6, TGF-β, NF-κB, p38 |
MAPK, ERK, JNK | Upregulation of miR-16 by targeting PDCD4 could inhibit inflammatory macrophages activation in atherosclerosis through the MAPK and NF-κB pathways. | (32) |
| Cholesteatomas | miR-21 | 7 pairs of cholesteatoma and normal skin samples | – | PDCD4, IL-6R, gp130, PTEN | STAT | Upregulation of miR-21 by targeting PTEN and PDCD4 could regulate apoptosis, proliferation, invasion, and migration of Cholesteatoma. | (41) |
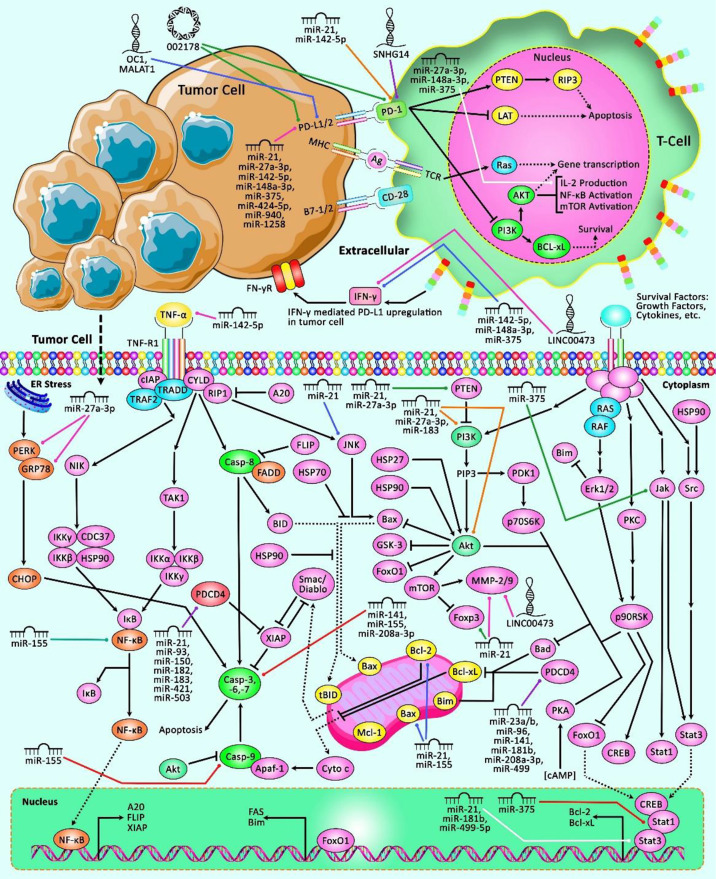
Figure 2.
A schematic diagram of the functional interactions between PDCDs proteins and various ncRNAs in non-neoplastic disorders. Apoptosis, a gene-controlled process of programmed cell death (PCD), is a cascade which is induced in normal cells under physiological conditions and pathological stress. Dysregulation of apoptosis can result in uncontrolled cell proliferation that can play a significant role in causing various non-neoplastic disorders including heart diseases, polycystic ovary syndrome, and several immune disorders. The death receptors include Fas receptors, TNF receptors, and TRAIL receptors. As a surface receptor, TNF-R1 can interact with TNF to induce the recruitment of adaptor proteins FADD and TRADD, which can recruit a series of downstream factors, such as Caspase-8, that is a key modulator of the extrinsic cascade, eventually leading to cell apoptosis. In the intrinsic cascade, the functional outcome of the pro-apoptotic pathway is mitochondrial membrane perturbation and release of cytochrome c in the cytoplasm, where it can create a complex with APAF1 and the inactive form of caspase-9. In order to trigger the activation of caspase-9, this complex hydrolyzes adenosine triphosphate. Consequently, the initiator caspase-9 can cleave and upregulate the executioner caspases-3/6/7, leading to cell apoptosis (42, 43). Growing evidence suggests that dysregulation of PDCDs is associated with several non-neoplastic disorders and importantly their interaction with different ncRNAs (miRNAs and lncRNAs) is also detected.
2.2 Interaction Between miRNAs and PDCD in Neoplastic Disorders
2.2.1 Cervical, Ovarian and Breast Cancers
A number of dysregulated miRNAs in female cancers have been shown to exert regulatory roles on PDCD genes. For instance, the oncogenic miRNAs miR-150 (44) and miR-21 (45) have been shown to inhibit expression of PDCD4, thus enhancing cell proliferation and malignant behaviors of cervical cancer cells. miR-21 has similar role in ovarian (46) and breast cancers (47). miR-421/PDCD4, miR-27a-3p/PD-L1, miR-424/PD-L1, miR-26a-5p/PDCD10 and miR-26b-5p/PDCD10 are other routes of participation of miRNAs in the pathogenesis of breast cancer ( Table 4 ).
Table 4.
Interaction between miRNAs and PDCD in female cancers.
| Cancer | miRNA | Animal-human | Cell lines | Targets | Pathways | Function | Ref |
|---|---|---|---|---|---|---|---|
| Cervical Cancer (CC) |
miR-150 | 50 pairs of CC and adjacent normal tissues | HeLa, SiHa | PDCD4 | – | Upregulation of miR-150 by targeting PDCD4 could enhance cell migration, invasion, and proliferation. | (44) |
| CC | miR-21 | – | C−33A, CaSki, SiHa, HeLa, ME−180, H8 | PDCD4, Bcl-2, Bax, c-myc, PTEN |
AKT | Downregulation of miR−21 by targeting PDCD4 and regulating the PTEN/AKT could inhibit cell proliferation and colony formation in CC cells. | (48) |
| CC | miR-21 | – | HeLa | PDCD4 | – | Upregulation of miR-21 by targeting PDCD4 could enhance cell proliferation in CC cell lines. | (45) |
| Ovarian Serous Carcinoma (OSC) | miR-21 | 14 OSC, 14 serous cystadenoma (CA), and 14 normal ovaries from cases of uterine prolapse | – | PDCD4 | – | Upregulation of miR-21 by targeting PDCD4 could result in OSC oncogenesis. | (46) |
| Ovarian Cancer (OC) |
miR-182 | 13 OC tissues and 2 normal control tissues | T29, T80, OVCAR3, SKOV3, OV2008, HEY, 3AO, A2780, HO8910, C13 | PDCD4 | – | Upregulation of miR-182 by targeting PDCD4 could enhance cell growth, invasion, and chemoresistance in human OC. | (49) |
| Breast Cancer (BCa) | miR-21 | 60 BCa tissues and blood samples and blood samples from 30 normal volunteers | – | PDCD4 | – | Upregulation of miR-21 by translational repression of the PDCD4 could promote breast cell transformation and the development of BCa. | (47) |
| BCa | miR-421 | 52 BCa tissue samples and 52 normal tissue samples | MCF-7, MDA-MB-231, Hs578bst | PDCD4 | – | Downregulation of miR−421 by targeting PDCD4 could reduce cell proliferation, migration potential, and invasiveness, and enhance apoptosis in BCa cell lines. | (50) |
| BCa | miR-27a-3p | 26 pairs of BCa and adjacent normal tissues | MCF-7, BT474, MDA-MB-23, MCF-10A, THP-1 | PD-L1, MAGI2, PTEN, GRP78, PERK, ATF6, IRE1α, IL-2 | PI3K/AKT | Upregulation of miR-27a-3p by targeting PD-L1 through MAGI2/PTEN/PI3K axis could enhance immune evasion in BCa. | (51) |
| BCa | miR-26a-5p, miR-26b-5p | Male nude mice/Human: 20 pairs of BCa and adjacent normal tissues | T24, 5637 | PDCD10 | – | Upregulation of miR-26a-5p and miR-26b-5p by regulating PDCD10 could suppress the BCa cell lines proliferation. | (52) |
| Triple-Negative Breast Cancer (TNBC) | miR-424-5p | Female Balb/c athymic nude mice | MCF10A, BT474, HCC1500, 293T, HCC1806, HCC1954, MM231 | PD-L1 | – | Upregulation of miR-424-5p by targeting PD-L1 could enhance the secretion of pro-inflammatory cytokines, reduce the secretion of anti-inflammatory cytokines and boost the apoptosis of tumor cells. | (53) |
2.2.2 Gastrointestinal Cancers
Two independent studies in esophageal squamous cell carcinoma have shown up-regulation of the PDCD4-interacting miRNA miR-183 in this type of cancer (54, 55). Up-regulation of this miRNA has resulted in down-regulation of PDCD4 expression, thus promoting proliferation and invasion of esophageal squamous cell carcinoma cells. Suppression of the PI3K/Akt signaling by LY294002 could increase PDCD4 expression and decrease miR-183 level in these cells (54). In this type of cancer, the inhibitory role of miR-21 on PDCD4 has been verified both in vitro and in vivo (56).
In gastric cancer, miR-21 has been found to affect carcinogenesis through interacting with PDCD4 (57) as well as PD-1/PD-L1 pathway (58). miR-940, miR-208a-3p, miR-23a/b, miR-129-1-3p, miR−46146, miR-503, miR-181b and miR-148a-3p are other miRNAs that participate in the pathogenesis of gastrointestinal cancers via regulation of expression of PDCD genes ( Table 5 ).
Table 5.
Interaction between miRNAs and PDCD in gastrointestinal cancers.
| Cancer | miRNA | Animal-human | Cell lines | Targets | Pathways | Function | Ref |
|---|---|---|---|---|---|---|---|
| Oesophageal Squamous Cell Carcinoma (ESCC) | miR-183 | 32 pairs of primary ESCC, 9 pairs of oesophageal low-grade intraepithelial neoplasia (LG-IEN), 21 pairs of high-grade intraepithelial neoplasia (HG-IEN), and normal controls |
Eca109, TE13 |
PDCD4 | PI3K/AKT | Upregulation of miR-183 by reducing PDCD4 enhances ESCC cell proliferation and invasion. | (54) |
| ESCC | miR-183 | 81 pairs of ESCC and adjacent non-tumor tissues | EC109, EC9706, Het-1A |
PDCD4 | – | Upregulation of miR-183 by targeting PDCD4 inhibits apoptosis and enhance proliferation in esophageal cancer. | (55) |
| ESCC | miR-21 | Male BALB/c-nu mice/Human: 50 pairs of ESCC and adjacent normal tissues | Eca109 | PDCD4 | – | Upregulation of miR-21 by targeting PDCD4 enhances proliferation, migration of ESCC both in vitro and in vivo. | (56) |
| ESCC | miR-21 | 70 pairs of ESCC and adjacent normal tissues | EC9706 | PDCD4, MMP-2, MMP-9 | JNK | Upregulation of miR-21 by targeting PDCD4 could increase migration and invasion of the cell in esophageal cancer. | (59) |
| Pancreatic Ductal Adenocarcinoma (PDAC) |
miR-21 | 25 pairs of PDAC and adjacent normal tissues | MIA-Pa-Ca-2, HUP-T3, PSN-1 |
PDCD4 | – | Downregulation of miR-21 could suppress proliferation and enhance cell death in PDAC. | (60) |
| Pancreatic Cancer | miR-142-5p | Female C57BL/6 mice | Panc02, 293T |
PD-L1, IFN-γ, TNF-α, IL-10 | – | Upregulation of miR-142-5p by targeting the PD-L1/PD-1 pathway could increase anti-tumor immunity and suppress mice pancreatic cancer growth. | (61) |
| Hepatocellular Carcinoma (HCC) | miR-183-5p | Male BALB/C Nude Mice/Human: 50 pairs of HCC and adjacent normal tissues | L02, Huh-6, Huh-7, SNU-449, Li-7 |
PDCD4 | – | Downregulation of miR-183-5p could inhibit proliferation, survival, migration, and invasion of HCC cells. | (62) |
| HCC | miR-93 | 64 pairs of HCC and adjacent normal tissues | 293T, mmcc-7721, Huh-7 |
PDCD4, A-Catenin, Ɣ-Catenin, N-Cadherin |
– | Upregulation of miR-93 by Targeting Pdcd4 enhances invasion and metastasis by EMT in HCC. | (61) |
| HCC | miR-21 | 16 pairs of HCC and adjacent normal tissues | L02, Hepg2, Mhcc97h, Bel7402, Huh7 | Pdcd4, Ap-1, Mmp-2/9 | C-Jun | Upregulation of miR-21 by targeting PDCD4 and AP-1 could enhance migration and invasion in human HCC. | (63) |
| Gastric Cancer (GC) | miR-499-5p | – | SGC-7901, 293T | PDCD4 | STAT | Upregulation of miR-499-5p via STAT3 signaling pathway could increase gastric cancer cell proliferation and invasion by targeting PDCD4. | (64) |
| GC | miR-21 | – | SGC-7901, MKN-45 | PDCD4, PTEN | – | Upregulation of miR-21 by targeting PTEN and PDCD could regulate cell growth, migration, invasion, and apoptosis in gastric cancer. | (57) |
| GC | miR-21 | 16 pairs of GC and adjacent normal tissues | Th17, Treg, PBMCs | PD-1/PD-L1, RORγt, IL-17, Foxp3, TGF-β1 |
– | Upregulation of miR-21 could by targeting PD-1/PD-L1 Pathway regulate the percentages of Th17 and Treg cells and the expression of RORγt and Foxp3 In Vitro. | (58) |
| GC | miR-21 | 105 pairs of GC and adjacent normal tissues | MKN1, MKN7, MKN45, MKN74, NUGC3, NUGC4, AZ521, KATOIII | PDCD4 | – | Upregulation of miR-21 by targeting PDCD4 could result in biological aggressiveness in human GC. | (65) |
| GC | miR-940 | Female BALB/c nude mice | MGC803, AGS, Jurkat, NCI-N87, MKN74 | PDL1, Cbl-b | STAT5a | Upregulation of miR-940 by targeting PDL1 and Cbl-b/STAT5a could enhance the proliferation and migration of GC cells. | (66) |
| GC | miR-208a-3p | Male SCID mice/Human: 16 pairs of GC and adjacent normal tissues | MKN45, HGC-27, AGS |
PDCD4, Caspase-3 | – | Upregulation of miR-208a-3p by targeting PDCD4 could inhibit apoptosis in GC cell lines and enhance tumor growth in xenograft mice. | (67) |
| GC | miR-23a/b | Male SCID mice/Human:10 pairs of GC and adjacent normal tissues | MKN-45, AGS |
PDCD4 | – | Upregulation of miR-23a/b by targeting PDCD4 could increase tumor growth and inhibit apoptosis in GC. | (38) |
| GC | miR-129-1-3p | – | BGC-823, 293T | PDCD2 | – | Upregulation of miR-129-1-3p by targeting PDCD2 could enhance BGC-823 cell proliferation. | (68) |
| Colorectal Cancer (CRC) | miR−46146 | – | HCT116, HT29 | PDCD10 | – | miR-46146 acts as a mediator of oxaliplatin resistance via targeting PDCD10. | (69) |
| CRC | miR-503 | 30 pairs of CRC and adjacent normal tissues | SW480, FHC, HT-29, 293T, HCT116, SW620 |
PDCD4 | – | Overexpression of miR-503 by targeting PDCD4 increases CRC cell migration and invasion. | (23) |
| CRC | miR-181b | 14 pairs of CRC and adjacent normal tissues | SW480, Caco2, HT-29 |
PDCD4, IL-6 |
STAT | Upregulation of miR-181b by targeting PDCD4 could increase CRC cell proliferation and migration to enhance tumorigenesis and suppresses apoptosis. | (70) |
| CRC | miR-208a-3p | 40 pairs of CRC and adjacent normal tissues | HCT116, SW480, SW620, HT-29, NCM460 |
PDCD4 | – | Upregulation of miR-208a-3p by targeting PDCD4 could enhance CRC cell proliferation and invasion. | (71) |
| CRC | miR-21 | Chicken embryo | LS174T | PDCD4 | – | Downregulation of miR-21 by targeting PDCD4 could inhibit metastatic features of CRC cells. | (72) |
| CRC | miR-148a-3p | – | HCT116, SW837 | PD-L1, IFNγ, IL-2 |
– | Upregulation of miR-148a-3p by targeting PD-L1 could restore T-cell viability in the tumor microenvironment. | (73) |
2.2.3 Head and Neck Cancers
In head and neck squamous cell carcinoma, miR-21 has been reported to increase cell proliferation through decreasing expression of PDCD4 (74). Another study in this type of cancer has revealed that miR-375 inhibits IFN-γ-associated surface expression of PD-L1. Moreover, JAK2 has been identified as a valid target of miR-375. In fact, the suppressive effects of miR-375 on PD-L1 have been found to depend on the JAK2/STAT1 pathway. Taken together, through attenuation of PD-1/PD-L1 axis, miR-375 can enhance cellular immune response against tumor cells (75).
In oral squamous cell carcinoma cells, miR-21 could enhance tumor cell invasion through targeting PDCD4 (76). This miRNA has a similar effect in the carcinogenic process in salivary adenoid cystic carcinoma (77). In oropharyngeal cancer cells, the primary inhibition of PDCD4 is facilitated by miR-21 while continuous inhibition of its expression is facilitated by miR-499. Besides, the single miR-21 site could provoke the same degree of expression inhibition as the three miR-499 sites (32).
In tongue cancer, miR-155 has been found to target Pdcd4 transcript and inhibit its expression. Forced up-regulation of Pdcd4 or miR-155 silencing in these neoplastic cancer cells could reduce AP-1-associated transcription of the BIC promoter and reduces expression of miR-155. In fact, expression of miR-155 is regulated by a feedback circuit between Pdcd4, AP-1, and miR-155. Up-regulation of miR-155 results in progression of this type of cancer (78). Table 6 shows the interaction between miRNAs and PDCD in head and neck cancers.
Table 6.
Interaction between miRNAs and PDCD in head and neck cancers.
| Cancer | miRNA | Animal-human | Cell lines | Targets | Pathways | Function | Ref |
|---|---|---|---|---|---|---|---|
| Head & Neck Squamous Cell Carcinoma (HNSCC) | miR-21 | – | UD-SCC-1/2, UM-SCC-9/11B/47/104, PCS-200-011 | PDCD4 | – | Upregulation of miR-21 by targeting PDCD4 could increase proliferation in HNSCC cell lines. | (74) |
| HNSCC | miR-375 | – | Hp-2, FaDu | PD-L1, IFN-γ, IL-2 |
JAK, STAT |
Upregulation of miR-375 by targeting PD-L1and JAK2/STAT1 signaling could enhance the cellular immune responses to HNSCC. | (75) |
| Oral Squamous Cell Carcinomas (OSCCs) | miR-21 | 50 OSCCs and 25 normal oral tissues | UT-SCC-15, 20A, 24A, 74A, 87, HOK |
PDCD4 | – | Upregulation of miR-21 by targeting PDCD4 could enhance tumor cell invasion in oral squamous cell carcinoma. | (76) |
| Tongue Cancer | miR-155 | Female BALB/c athymic nude mouse |
Hep3B, SiHa, MCF-7, SCC172, MDA-MB231, H1299, HCT116, SCC131, SCC745, SCC969, AWL |
NF-κB, Bax, Bcl-2, Caspase-3/9 |
Upregulation of miR-155 by targeting PDCD4 is involved in the progression of tongue cancer. | (78) | |
| Salivary Adenoid Cystic Carcinoma (SACC) | miR-21 | 27 SACC and 20 healthy controls | SACC-LM, SACC-83 |
PDCD4, STAT3 | – | Downregulation of miR-21 by targeting PDCD4 could reduce tumor growth and invasion in SACC. | (77) |
| Oropharyngeal Squamous Cell Carcinoma | miR-499, miR-21 | 43 patients treated for tonsillar cancer and 17 matched normal tissues | HNSCC, SCC089, SCC003, SCC099, SCC029b, 293T | PDCD4, Dicer1, Drosha, DDX5, DGCR8 | – | miR-499 and miR-21 by regulating PDCD4 could participate in the pathogenesis of oropharyngeal cancers. | (32) |
| Laryngeal Squamous Cell Carcinoma (LSCC) | miR-503 | 48 pairs of LSCC and adjacent normal tissues | AMC-HN-8, Tu-177, HaCaT, 293T |
PDCD4 | – | Upregulation of miR−503 by targeting PDCD4 could enhance tumor growth and invasion in LSCC. | (79) |
| Thyroid Cancer | miR-183 | 38 pairs of papillary thyroid cancer and adjacent normal tissues | TPC-1, BCPAP, K1, NPA PTC, Nthy-ori 3-1, 293T |
PDCD4 | – | Upregulation of miR-183 by targeting PDCD4 could enhance cell proliferation, migration, invasion, and inhibit apoptosis in TPC-1 cells. | (80) |
2.2.4 Lung Cancer
The inhibitory role of miR-182 on PDCD4 is involved in the modulation of sensitivity of lung cancer cells to cisplatin (81). Moreover, resistance to this chemotherapeutic agent has been shown to be reversed through suppression of miR-141 (82). Similarly, miR-21 silencing could inhibit proliferation and migration of lung cancer cells via changing expression of PDCD4 (83). miR-182 silencing has inhibited growth and invasive properties of lung cancer cells through modulation of PDCD4 levels (55). On the other hand, miR-103 has been shown to exert its tumor suppressive roles in lung cancer through targeting PDCD10 (84). Table 7 shows the interaction between miRNAs and PDCD in lung cancer.
Table 7.
Interaction between miRNAs and PDCD in lung cancer.
| Cancer | miRNA | Animal-human | Cell lines | Targets | Pathways | Function | Ref |
|---|---|---|---|---|---|---|---|
| Non-Small Cell Lung Carcinoma (NSCLC) |
miR-182 | – | A549 | PDCD4 | – | Upregulation of miR-182 by targeting PDCD4 could induce chemoresistance to cisplatin in NSCLC cells. | (81) |
| NSCLC | miR-141 | – | A549, A549/DDP |
PDCD4, Caspase-3 | – | Downregulation of miR-141 via targeting PCDP could reverse cisplatin resistance in NSCLC cells. | (82) |
| NSCLC | miR-21 | 17 patients with NSCLC and 16 matched healthy volunteers | A549 | PDCD4 | – | Downregulation of miR-21 by targeting PDCD4 could reduce NSCLC cell proliferation and migration. | (83) |
| NSCLC | miR-103 | Male BALB/c nude mice/Human: 32 pairs of NSCLC and adjacent lung tissues | A549, SPC-A1, NCI-H460, H1299, PC9, 293T, 16HBE | PDCD10 | – | Upregulation of miR-103 by targeting PDCD10 could suppress cell proliferation and migration in the A549 cell line and NSCLC growth in vivo. | (84) |
| Lung Cancer | miR-182 | – | A549, NCI-H1299, LTEP-a-2, SPC-A-1, NHBE | PDCD4 | – | miR-182 silencing could suppress cell growth and invasion in human lung adenocarcinoma cells. | (55) |
2.2.5 Nervous System Cancers
miR-21 has been shown to affect the carcinogenesis of malignant peripheral nerve sheath tumors (85), glioblastoma (86) and neuroblastoma (87) through modulation of PDCD4. Moreover, over-expression the PDCD4-targeting miR-96 has been shown to promote resistance of glioblastoma cells to radiotherapy (88). Table 8 shows the interaction between miRNAs and PDCD nervous system cancers.
Table 8.
Interaction between miRNAs and PDCD nervous system cancers.
| Cancer | miRNA | Animal-human | Cell lines | Targets | Pathways | Function | Ref |
|---|---|---|---|---|---|---|---|
| Malignant Peripheral Nerve Sheath Tumor (MPNST) | miR-21 | 12 MPNSTs, 11 neurofibroma, 5 normal nerves | HS-Sch-2, YST-1, NMS-2 |
PDCD4 | – | Downregulation of miR-21 by targeting PDCD4 could induce cell apoptosis of MPNST cells. | (85) |
| Glioblastoma (GBM) | miR-96 | – | U87-MG, T98G U251-MG, A172 |
PDCD4 | Upregulation of miR-96 by targeting PDCD4 could improve radioresistance in GBM cells. | (88) | |
| GBM | miR-21 | 13 pairs of GBM and normal brain tissue samples |
SNB19, U251, U87, SF767 | PDCD4 | – | Downregulation of miR-21 could reduce proliferation, enhance apoptosis, and suppress anchorage-independent growth in glioblastoma-derived cell lines. | (86) |
| Neuroblastoma (NB) | miR-21 | – | SK-N-SH, SH-SY5Y, BE2C, 293T |
PDCD4, PTEN | – | Downregulation of miR-21 by targeting PTEN/PDCD4 could result in SK-N-SH cell apoptosis and suppress proliferation in NB. | (87) |
| Retinoblastoma | miR-181b | – | HXO-RB44, HUVECs | PDCD10, HIF-1α, GATA6 |
– | Upregulation of miR-181b which is induced by hypoxia could increase angiogenesis of retinoblastoma cells by regulating PDCD10 and GATA6. | (89) |
2.2.6 Other Malignancies
The interaction between miRNAs and PDCD has been verified in other malignancies such as hematological malignancies, bladder and kidney cancers as well as melanoma. miR-21 has been the most assessed miRNA in this regard ( Table 9 ). Figure 3 represents the role of several ncRNAs in several human cancers via interacting with PDCD genes.
Table 9.
Interaction between miRNAs and PDCD in other cancers.
| Cancer | miRNA | Animal-human | Cell lines | Targets | Function | Ref |
|---|---|---|---|---|---|---|
| Acute Myeloid Leukemia (AML) | miR-183 | 106 pairs of pediatric AML and normal controls | HL60, K562 | PDCD6 | Upregulation of miR-183 by targeting PDCD6 could enhance cell proliferation and inhibit apoptosis in pediatric AML. | (90) |
| Multiple Myeloma | miR-1258 | 20 MGUS patients, 63 with myeloma at diagnosis, and 30 myeloma patients at relapse/progression | KMS-12-PE, MOLP-8, OPM-2, U-266, NCI-H929, OCI-MY5, KMS-27, KMS-11/BTZ, OPM-2/BTZ, LP-1, RPMI-8226, JJN-3, XG-1, RPMI-8226R, WL-2, MMKKF |
PDL1 | Overexpression of miR-1258 could lead to reducing the expression of PD-L1 during myeloma progression. | (91) |
| Bladder Carcinoma (BC) | miR-21 | 22 patients with BC and 3 corresponding normal urothelial tissue | – | PDCD4 | Upregulation of miR-21 could reduce the expression of PDCD4 in BC. | (92) |
| Renal Cell Carcinoma (RCC) | miR-21 | – | 786−O, A498, HMEC-1 | PDCD4 | Upregulation of miR-21 by targeting the PDCD4/c-Jun signaling pathway could increase the migration, invasion, and angiogenic abilities of RCC cells. | (93) |
| Malignant Melanoma | miR-21 | 67 pairs of human cutaneous malignant melanoma and normal nonmalignant control skin | – | PDCD4 | Upregulation of miR-21 by targeting PDCD4 could enhance tumor size and metastasis in malignant melanoma. | (94) |
Figure 3.
A schematic representation of the role of ncRNAs in modulating the PDCD genes in multiple human cancers. The loss of apoptotic control could cause tumor cells to survive longer and give more time for the accumulation of mutations that could, in turn, enhance invasiveness during tumor cell progression, stimulate angiogenesis, deregulate cell proliferation and interfere with differentiation (95). Apoptosis could be mediated via elevation or suppression of caspases. Besides, the PD-1/PD-L1 interaction could suppress T lymphocyte proliferation, survival and effector functions including cytotoxicity and cytokine release, and thereby could trigger apoptosis of tumor-specific T cells (96, 97). Previous studies have authenticated that several ncRNAs could have a crucial role in human cancers via interacting with various PDCD transcripts. In fact, aberrant expression of these ncRNAs could result in dysregulation of PDCDs, and therefore is associated with different kinds of cancer.
2.3 Association Between PDCD-Interacting miRNAs and Cancer Prognosis
Among PDCD-related miRNAs, overexpression of miR-183-5p, miR-21 and miR-93 has been correlated with shorter overall survival of patients with different types of neoplasms. However, miR-26a-5p, miR-103 and miR-183 have been revealed to have opposite effect ( Table 10 ).
Table 10.
Association between PDCD-interacting miRNAs and cancer prognosis.
| Sample | Results of Kaplan-Meier analysis | Ref |
|---|---|---|
| 50 pairs of tumorous tissue samples and normal tissue samples | Overexpression of miR-183-5p was correlated with shorter overall survival of HCC patients. | (62) |
| 27 cases of resected SACC and 20 healthy controls | Overexpression of miR-21 in SACC tissues was correlated with poor prognosis. | (77) |
| 64 pairs of human primary HCC tissues and the corresponding normal tissues | Overexpression of miR-93 was correlated with shorter overall survival of HCC patients. | (61) |
| Male BALB/c-nu mice/Human: 50 pairs of primary ESCC and adjacent normal tissues | Overexpression of miR-21 was correlated with shorter overall survival of ESCC patients. | (56) |
| 105 pairs of GC samples and matched controls | Overexpression of miR-21 was correlated with shorter overall survival of GC patients. | (65) |
| Male nude mice/Human: 20 pairs of BC tissues and corresponding adjacent bladder tissues |
Low expression of miR-26a-5p was correlated with shorter overall survival of BC patients. | (52) |
| Male BALB/c nude mice/Human: 32 pairs of fresh primary NSCLC tissues and matched adjacent noncancerous lung tissues | Low expression of miR-103 was correlated with shorter overall survival of NSCLC patients. | (84) |
| 106 pairs of pediatric AML and normal controls | Low expression of miR-183 was correlated with better overall survival of AML patients. | (90) |
3 LncRNAs and PDCD
LncRNAs are transcripts with a wide range of length, i.e. 200 to thousands of nucleotides. They exert regulatory roles in different phases of gene expression through diverse action modalities (98). Limited numbers of lncRNAs have been found to affect expression of PDCDs. In fact, lncRNAs that participate in the regulation of PDCDs mostly affect expression of miRNAs and through this route they exert their function. For instance, MALAT1 has been shown to affect apoptosis of regulates cardiomyocytes through serving as a sponge for miR-200a-3p, a miRNA that inhibits PDCD4 expression. This function of MALAT1 has impacts in the cardiac injury produced by hypoxia/reperfusion in the course of myocardial infarction (98). Meanwhile, this lncRNA has been shown to affect progression of lung cancer through miR-200a-3p/PD-L1 axis (99). The known miR-21/PDCD4 axis has been shown to be controlled by two lncRNAs, namely MEG3 (90) and CiRS-126 (100), in the contexts of ischemic neuron death and PCOS, respectively.
The impact of Lnc-OC1 on PDCD-related pathways has been verified in endometrial cancer, where suppression of this lncRNA has been shown to stimulate apoptosis and decreased viability of neoplastic cells through influencing miR-34a/PD-L1 axis (69). Moreover, the lncRNA SNHG14 has been found to serve as a sponge for miR-5590-3p and enhance expression of ZEB1 to promote progression of certain type of B cell malignancy and facilitate evasion of malignant cell from immune responses through modulation of PD-1/PD-L1 checkpoint (101). Expression of this axis has also been shown to be regulated by a circular RNA, namely circRNA-002178 in lung cancer (69). Finally, LINC00473 can facilitate pathogenic course of pancreatic cancer through sponging miR-195-5p and enhancing expression of PD-L1 (102). In colorectal cancer cells, Linc00472 has been found to act as a sponge for miR-196a to release PDCD4. Besides, up-regulation of miR-196a or down-regulation of PDCD4 could reverse Linc00472-mediated suppression of cell proliferation and activation of apoptosis in these cells. Forced over-expression of Linc00472 could hinder tumor growth in animal models. Taken together, Linc00472 could suppress proliferation and induce apoptosis via enhancement of PDCD4 expression by decoying miR-196a (103). Table 11 shows the interaction between lncRNAs and PDCD in different conditions.
Table 11.
Interaction between lncRNAs and PDCD in different conditions.
| Disease | LncRNA | Animal-human | Cell lines | Targets | Function | Ref |
|---|---|---|---|---|---|---|
| Myocardial Infarction (MI) | MALAT1 | Female C57BL/6 mice | AC16 | PDCD4, miR-200a-3p, IL-1, IL-8, TNF-α, Bcl-2, Bax, Cyclin-D1 |
Downregulation of MALAT1 by targeting the miR-200a-3p/PDCD4 axis could improve cell viability and suppress cell apoptosis in the hypoxia-induced myocardial cells. | (98) |
| Stroke | MEG3 | Male C57BL/6 J mice | N2a | PDCD4, miR-21 |
Downregulation of MEG3 could protect against ischemic damages and enhance overall neurological functions in vivo. | (90) |
| Polycystic Ovarian Syndrome (PCOS) | CiRS-126 | Female CF1 mice/Human: 18 PCOS patients and 5 healthy controls | – | PDCD4, miR-21, ROS, Bcl-2, Bax |
Downregulation of ciRS-126 by targeting the miR-21/PDCD4 axis could decrease proliferation and enhance apoptosis in PCOS granulosa cells. | (100) |
| Endometrial Carcinoma (EC) | Lnc-OC1 | 28 pairs of EC and adjacent normal tissues | Ishikawa, HESCs | PD-L1, miR-34a |
Downregulation of Lnc-OC1 by targeting PD-L1 could suppress cell growth and enhance cell apoptosis of EC cells. | (69) |
| Non-Small Cell Lung Carcinoma (NSCLC) |
MALAT1 | 113 pairs of NSCLC and adjacent normal tissues | A549, CAL-12T |
PD-L1, miR-200a-3p |
Upregulation of MALAT1 by targeting the miR-200a-3p-PD-L1 axis could increase proliferation, mobility, migration, and invasion of NSCLC cells. | (99) |
| Diffuse Large B Cell Lymphoma (DLBCL) | SNHG14 | BALB/c mice/Human: 38 pairs of DLBCL and adjacent normal tissues |
GM12878, 293T, A20), OCI-LY7, DB, U2932, FARAGE | PD-1, ZEB1, miR-5590-3p, E-cadherin, N-cadherin |
Upregulation of SNHG14 by targeting PD-1-miR-5590 axis could enhance proliferation, invasion, and EMT in DLBCL. | (101) |
| Lung Adenocarcinoma (LUAD) | circRNA-002178 | 105 pairs of LUAD and adjacent normal tissues | A549, PC9, 95D, BEAS-2B |
PDL1/PD1 | Upregulation of circRNA-002178 could enhance PDL1/PD1 expression in LUAD. | (69) |
| Pancreatic Cancer (PC) | LINC00473 | 134 PC patients and 20 healthy controls | SW‐1990, Panc‐1, BxPC‐3, AsPC‐1, CAPAN‐2, H6C7 |
PD‐L1, miR‐195‐5p, Bcl‐2, Bax, IFN‐γ, IL‐4, MMP‐2/9/10 |
Downregulation of LINC00473 by targeting PD‐L1 could increase apoptosis and decrease proliferation, invasion, and migration of the PC cells. | (102) |
4 Discussion
As important regulators of apoptosis, PDCDs participate in normal development as well as several pathological conditions. Expression and function of PDCDs are under controls of several non-coding RNAs, especially miRNAs. In fact, miRNAs can affect pathoetiology of human disorders through suppression of expression of PDCDs. This type of interaction has been assessed in the context of cardiac disorders, PCOS, intervertebral disc degeneration, amyotrophic lateral sclerosis, immune thrombocytopenia, type 1 diabetes, congenital hypothyroidism, Alzheimer’s disease as well as different types of cancers. PCDC4 is the top member of this family in terms of interaction with miRNAs. In fact, miR-21, miR-208a-3p, miR-499-5p, miR-16, miR-155, miR-182, miR-141, miR-96, miR-182, miR-499, miR-503 and miR-183 have been verified as modulators of expression of PCDC4 in different tissues. Particularly, dysregulation of miR-21/PDCD4 axis has been identified as an important pathway in many diseases.
Although the roles of several PDCD-related miRNAs have been appraised in development of human cancers, data regarding their impact on patients’ survival is limited. In fact, miR-183-5p, miR-21, miR-93, miR-26a-5p, miR-103 and miR-183 are the only PDCD-related miRNAs whose association with cancer prognosis has been verified.
Since PDCDs affect an important hallmark of carcinogenesis, i.e. decreased response of cancer cells to pro-apoptotic stimuli, PDCD-related miRNAs represent an ideal group of therapeutic targets for cancers. Knock-down experiments in cell lines and animal models have revealed promising results, since down-regulation of PDCD-targeting miRNAs has substantially reduced viability of cancer cells and enhanced the response to apoptosis. These miRNAs have also been found to affect radio/chemoresistance of cancer cells, representing a novel avenue for enhancement of effectiveness of routine anti-cancer therapies.
The impact of lncRNAs on modulation of expression of PDCDs has been less studied. In fact, lncRNAs that affect expression of these proteins mainly act as molecular sponges for miRNAs. MALAT1/miR-200a-3p/PDCD4, ciRS-126/miR-21/PDCD4 and SNHG14/PD-1-miR-5590 are among lncRNA/miRNA/PDCD axes which contribute in the pathoetiology of human disorders. Thus, lncRNAs mainly affect expression of PDCD-interacting miRNAs and through this route they regulate expression of PDCDs.
5 Future Perspectives
Understanding the important roles of ncRNAs in regulation of function of PDCDs is expected to lead to design of specific treatments targeting these proteins via modulation of expression of ncRNAs. The role of PDCD5 and 7 in conjunction with ncRNAs is still unclear. These kinds of treatments can be theoretically used in a wide range of human disorders, including malignancies and disorders related with cell senescence or apoptosis. Considering the orchestrated effects of several miRNAs and lncRNAs on expressions of these proteins, targeting multiples points in this interaction network seems to be an efficient way for modulation of expressions of PDCDs.
Author Contributions
SG-F wrote the draft and revised it. MT designed and supervised the study. HS, BH, MM, and AB collected the data and designed the figures and tables. All the authors read the draft and approved the submission version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Lockshin RA, Williams CM. Programmed Cell Death–I. Cytology of Degeneration in the Intersegmental Muscles of the Pernyi Silkmoth. J Insect Physiol (1965) 11:123–33. doi: 10.1016/0022-1910(65)90099-5 [DOI] [PubMed] [Google Scholar]
- 2. Fadeel B, Orrenius S. Apoptosis: A Basic Biological Phenomenon With Wide-Ranging Implications in Human Disease. J Internal Med (2005) 258(6):479–517. doi: 10.1111/j.1365-2796.2005.01570.x [DOI] [PubMed] [Google Scholar]
- 3. Cookson BT, Brennan MA. Pro-Inflammatory Programmed Cell Death. Trends Microbiol (2001) 9(3):113–4. doi: 10.1016/S0966-842X(00)01936-3 [DOI] [PubMed] [Google Scholar]
- 4. Guan X, Lu J, Sun F, Li Q, Pang Y. The Molecular Evolution and Functional Divergence of Lamprey Programmed Cell Death Genes. Front Immunol (2019) 10:1382. doi: 10.3389/fimmu.2019.01382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Syn NL, Teng MW, Mok TS, Soo RA. De-Novo and Acquired Resistance to Immune Checkpoint Targeting. Lancet Oncol (2017) 18(12):e731–41. doi: 10.1016/S1470-2045(17)30607-1 [DOI] [PubMed] [Google Scholar]
- 6. Kawakami T, Furukawa Y, Sudo K, Saito H, Takami S, Takahashi E, et al. Isolation and Mapping of a Human Gene (PDCD2) That Is Highly Homologous to Rp8, a Rat Gene Associated With Programmed Cell Death. Cytogenetic Genome Res (1995) 71(1):41–3. doi: 10.1159/000134058 [DOI] [PubMed] [Google Scholar]
- 7. Mu W, Munroe RJ, Barker AK, Schimenti JC. PDCD2 Is Essential for Inner Cell Mass Development and Embryonic Stem Cell Maintenance. Dev Biol (2010) 347(2):279–88. doi: 10.1016/j.ydbio.2010.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation Inhibitor Pdcd4 Is Targeted for Degradation During Tumor Promotion. Cancer Res (2008) 68(5):1254–60. doi: 10.1158/0008-5472.CAN-07-1719 [DOI] [PubMed] [Google Scholar]
- 9. Hayashi A, Aishima S, Miyasaka Y, Nakata K, Morimatsu K, Oda Y, et al. Pdcd4 Expression in Intraductal Papillary Mucinous Neoplasm of the Pancreas: Its Association With Tumor Progression and Proliferation. Hum Pathol (2010) 41(11):1507–15. doi: 10.1016/j.humpath.2010.02.019 [DOI] [PubMed] [Google Scholar]
- 10. Zhao M, Zhu N, Hao F, Song Y, Wang Z, Ni Y, et al. The Regulatory Role of Non-Coding RNAs on Programmed Cell Death Four in Inflammation and Cancer. Front Oncol (2019) 9:919. doi: 10.3389/fonc.2019.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen G, et al. TFAR19, a Novel Apoptosis-Related Gene Cloned From Human Leukemia Cell Line TF-1, Could Enhance Apoptosis of Some Tumor Cells Induced by Growth Factor Withdrawal. Biochem Biophys Res Commun (1999) 254(1):203–10. doi: 10.1006/bbrc.1998.9893 [DOI] [PubMed] [Google Scholar]
- 12. Lo KW-H, Zhang Q, Li M, Zhang M. Apoptosis-Linked Gene Product ALG-2 Is a New Member of the Calpain Small Subunit Subfamily of Ca2+-Binding Proteins. Biochemistry (1999) 38(23):7498–508. doi: 10.1021/bi990034n [DOI] [PubMed] [Google Scholar]
- 13. Peng S-Y, Chang K-W, Lin S-C, Tu H-F. miR-134 Targets Programmed Cell Death 7 (PDCD7) Gene to Modulate the Pathogenesis of Head and Neck Carcinoma. AACR; (2015). [Google Scholar]
- 14. Dibble CF, Horst JA, Malone MH, Park K, Temple B, Cheeseman H, et al. Defining the Functional Domain of Programmed Cell Death 10 Through Its Interactions With Phosphatidylinositol-3, 4, 5-Trisphosphate. PLoS One (2010) 5(7):e11740. doi: 10.1371/journal.pone.0011740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu L, Ying K, Wei S, Liu Y, Lin H, Mao Y. Dermal Fibroblast-Associated Gene Induction by Asiaticoside Shown In Vitro by DNA Microarray Analysis. Br J Dermatol (2004) 151(3):571–8. doi: 10.1111/j.1365-2133.2004.06146.x [DOI] [PubMed] [Google Scholar]
- 16. Madera S, Chervo MF, Chiauzzi VA, Pereyra MG, Venturutti L, Izzo F, et al. Nuclear PDCD4 Expression Defines a Subset of Luminal B-Like Breast Cancers With Good Prognosis. Horm Cancer (2020) 11(5-6):218–39. doi: 10.1007/s12672-020-00392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen C, Feng X, Yuan H, Gong Y, Wang G. Circ_0003266 Sponges miR-503-5p to Suppress Colorectal Cancer Progression via Regulating PDCD4 Expression. BMC Cancer (2021) 21(1):284. doi: 10.1186/s12885-021-07997-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kars MD, Işeri ÖD, Gündüz U. A Microarray Based Expression Profiling of Paclitaxel and Vincristine Resistant MCF-7 Cells. Eur J Pharmacol (2011) 657(1-3):4–9. doi: 10.1016/j.ejphar.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 19. Ghiotto M, Gauthier L, Serriari N, Pastor S, Truneh A, Nunès JA, et al. PD-L1 and PD-L2 Differ in Their Molecular Mechanisms of Interaction With PD-1. Int Immunol (2010) 22(8):651–60. doi: 10.1093/intimm/dxq049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (2018) 9:402. doi: 10.3389/fendo.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bronnum H, Andersen DC, Schneider M, Sandberg MB, Eskildsen T, Nielsen SB, et al. miR-21 Promotes Fibrogenic Epithelial-to-Mesenchymal Transition of Epicardial Mesothelial Cells Involving Programmed Cell Death 4 and Sprouty-1. PLoS One (2013) 8(2):e56280. doi: 10.1371/journal.pone.0056280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shang YY, Fang NN, Wang F, Wang H, Wang ZH, Tang MX, et al. MicroRNA-21, Induced by High Glucose, Modulates Macrophage Apoptosis via Programmed Cell Death 4. Mol Med Rep (2015) 12(1):463–9. doi: 10.3892/mmr.2015.3398 [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Ye N, Lian X, Peng F, Zhang H, Gong H. MiR-208a-3p Aggravates Autophagy Through the PDCD4-ATG5 Pathway in Ang II-Induced H9c2 Cardiomyoblasts. BioMed Pharmacother (2018) 98:1–8. doi: 10.1016/j.biopha.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Lu J, Bao X, Wang X, Wu J, Li X, et al. MiR-499-5p Protects Cardiomyocytes Against Ischaemic Injury via Anti-Apoptosis by Targeting PDCD4. Oncotarget (2016) 7(24):35607–17. doi: 10.18632/oncotarget.9597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Z, Qi Y, Guo Z, Li P, Zhou D. miR-613 Suppresses Ischemia-Reperfusion-Induced Cardiomyocyte Apoptosis by Targeting the Programmed Cell Death 10 Gene. Biosci Trends (2016) 10(4):251–7. doi: 10.5582/bst.2016.01122 [DOI] [PubMed] [Google Scholar]
- 26. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovary Syndrome–Part 1. Endocrine Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol (2015) 21(11):1291–300. [DOI] [PubMed] [Google Scholar]
- 27. Fu X, He Y, Wang X, Peng D, Chen X, Li X, et al. MicroRNA-16 Promotes Ovarian Granulosa Cell Proliferation and Suppresses Apoptosis Through Targeting PDCD4 in Polycystic Ovarian Syndrome. Cell Physiol Biochem (2018) 48(2):670–82. doi: 10.1159/000491894 [DOI] [PubMed] [Google Scholar]
- 28. Xia H, Zhao Y. miR-155 Is High-Expressed in Polycystic Ovarian Syndrome and Promotes Cell Proliferation and Migration Through Targeting PDCD4 in KGN Cells. Artif Cells Nanomed Biotechnol (2020) 48(1):197–205. doi: 10.1080/21691401.2019.1699826 [DOI] [PubMed] [Google Scholar]
- 29. Chen B, Huang SG, Ju L, Li M, Nie FF, Zhang Y, et al. Effect of microRNA-21 on the Proliferation of Human Degenerated Nucleus Pulposus by Targeting Programmed Cell Death 4. Braz J Med Biol Res (2016) 49(6). doi: 10.1590/1414-431x20155020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li C, Chen Y, Chen X, Wei Q, Ou R, Gu X, et al. MicroRNA-183-5p Is Stress-Inducible and Protects Neurons Against Cell Death in Amyotrophic Lateral Sclerosis. J Cell Mol Med (2020) 24(15):8614–22. doi: 10.1111/jcmm.15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen H, Wang S, Sun Y, Wang J. Mitomycin C Induces Fibroblast Apoptosis and Reduces Intra-Articular Scar Adhesion by Regulating miR-21 and Its Target Programmed Cell Death 4. Fitoterapia (2020) 142:104392. doi: 10.1016/j.fitote.2019.104392 [DOI] [PubMed] [Google Scholar]
- 32. Liang X, Xu Z, Yuan M, Zhang Y, Zhao B, Wang J, et al. MicroRNA-16 Suppresses the Activation of Inflammatory Macrophages in Atherosclerosis by Targeting PDCD4. Int J Mol Med (2016) 37(4):967–75. doi: 10.3892/ijmm.2016.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Z, Jin A, Yan D. MicroRNA206 Contributes to the Progression of Steroidinduced Avascular Necrosis of the Femoral Head by Inducing Osteoblast Apoptosis by Suppressing Programmed Cell Death 4. Mol Med Rep (2018) 17(1):801–8. doi: 10.3892/mmr.2017.7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang G, Yan Y, Zheng Z, Zhang T. The Mechanism of hsa-miR-424-5 Combining PD-1 Through mTORC Signaling Pathway to Stimulate Immune Effect and Participate in Type 1 Diabetes. Biosci Rep (2020) 40(3). doi: 10.1042/BSR20193800 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Smith MC, Mader MM, Cook JA, Iversen P, Ajamie R, Perkins E, et al. Characterization of LY3023414, a Novel PI3K/mTOR Dual Inhibitor Eliciting Transient Target Modulation to Impede Tumor Growth. Mol Cancer Ther (2016) 15(10):2344–56. doi: 10.1158/1535-7163.MCT-15-0996 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Xiao Y, Ma Y, Liang N, Liang Y, Lu C, et al. ROS-Mediated miR-21-5p Regulates the Proliferation and Apoptosis of Cr(VI)-Exposed L02 Hepatocytes via Targeting PDCD4. Ecotoxicol Environ Saf (2020) 191:110160. doi: 10.1016/j.ecoenv.2019.110160 [DOI] [PubMed] [Google Scholar]
- 37. Chang Y, Chen X, Tian Y, Gao X, Liu Z, Dong X, et al. Downregulation of microRNA-155-5p Prevents Immune Thrombocytopenia by Promoting Macrophage M2 Polarization via the SOCS1-Dependent PD1/PDL1 Pathway. Life Sci (2020) 257:118057. doi: 10.1016/j.lfs.2020.118057 [DOI] [PubMed] [Google Scholar]
- 38. Hu X, Wang Y, Liang H, Fan Q, Zhu R, Cui J, et al. miR-23a/B Promote Tumor Growth and Suppress Apoptosis by Targeting PDCD4 in Gastric Cancer. Cell Death Dis (2017) 8(10):e3059. doi: 10.1038/cddis.2017.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li W, Song D, Sun Y, Lv Y, Lv J. microRNA-124-3p Inhibits the Progression of Congenital Hypothyroidism via Targeting Programmed Cell Death Protein 6. Exp Ther Med (2018) 15(6):5001–6. doi: 10.3892/etm.2018.6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feng MG, Liu CF, Chen L, Feng WB, Liu M, Hai H, et al. MiR-21 Attenuates Apoptosis-Triggered by Amyloid-Beta via Modulating PDCD4/ PI3K/AKT/GSK-3beta Pathway in SH-SY5Y Cells. BioMed Pharmacother (2018) 101:1003–7. doi: 10.1016/j.biopha.2018.02.043 [DOI] [PubMed] [Google Scholar]
- 41. Friedland DR, Eernisse R, Erbe C, Gupta N, Cioffi JA. Cholesteatoma Growth and Proliferation: Posttranscriptional Regulation by microRNA-21. Otol Neurotol (2009) 30(7):998–1005. doi: 10.1097/MAO.0b013e3181b4e91f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Loreto C, La Rocca G, Anzalone R, Caltabiano R, Vespasiani G, Castorina S, et al. The Role of Intrinsic Pathway in Apoptosis Activation and Progression in Peyronie’s Disease. BioMed Res Int (2014) 2014. 10.1155/2014/616149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicologic Pathol (2007) 35(4):495–516. doi: 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Z, Wang J, Li J, Wang X, Song W. MicroRNA-150 Promotes Cell Proliferation, Migration, and Invasion of Cervical Cancer Through Targeting PDCD4. BioMed Pharmacother (2018) 97:511–7. doi: 10.1016/j.biopha.2017.09.143 [DOI] [PubMed] [Google Scholar]
- 45. Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 Promotes Cell Proliferation and Down-Regulates the Expression of Programmed Cell Death 4 (PDCD4) in HeLa Cervical Carcinoma Cells. Biochem Biophys Res Commun (2009) 388(3):539–42. doi: 10.1016/j.bbrc.2009.08.044 [DOI] [PubMed] [Google Scholar]
- 46. Cappellesso R, Tinazzi A, Giurici T, Simonato F, Guzzardo V, Ventura L, et al. Programmed Cell Death 4 and microRNA 21 Inverse Expression Is Maintained in Cells and Exosomes From Ovarian Serous Carcinoma Effusions. Cancer Cytopathol (2014) 122(9):685–93. doi: 10.1002/cncy.21442 [DOI] [PubMed] [Google Scholar]
- 47. Abdulhussain MM, Hasan NA, Hussain AG. Interrelation of the Circulating and Tissue MicroRNA-21 With Tissue PDCD4 Expression and the Invasiveness of Iraqi Female Breast Tumors. Indian J Clin Biochem (2019) 34(1):26–38. doi: 10.1007/s12291-017-0710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Du G, Cao D, Meng L. miR-21 Inhibitor Suppresses Cell Proliferation and Colony Formation Through Regulating the PTEN/AKT Pathway and Improves Paclitaxel Sensitivity in Cervical Cancer Cells. Mol Med Rep (2017) 15(5):2713–9. doi: 10.3892/mmr.2017.6340 [DOI] [PubMed] [Google Scholar]
- 49. Wang YQ, Guo RD, Guo RM, Sheng W, Yin LR. MicroRNA-182 Promotes Cell Growth, Invasion, and Chemoresistance by Targeting Programmed Cell Death 4 (PDCD4) in Human Ovarian Carcinomas. J Cell Biochem (2013) 114(7):1464–73. doi: 10.1002/jcb.24488 [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Liu Z, Shen J. MicroRNA-421-Targeted PDCD4 Regulates Breast Cancer Cell Proliferation. Int J Mol Med (2019) 43(1):267–75. doi: 10.3892/ijmm.2018.3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yao X, Tu Y, Xu Y, Guo Y, Yao F, Zhang X. Endoplasmic Reticulum Stress-Induced Exosomal miR-27a-3p Promotes Immune Escape in Breast Cancer via Regulating PD-L1 Expression in Macrophages. J Cell Mol Med (2020) 24(17):9560–73. doi: 10.1111/jcmm.15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu K, Mu XY, Jiang JT, Tan MY, Wang RJ, Zhou WJ, et al. Mirna26a5p and Mir26b5p Inhibit the Proliferation of Bladder Cancer Cells by Regulating PDCD10. Oncol Rep (2018) 40(6):3523–32. doi: 10.3892/or.2018.6734 [DOI] [PubMed] [Google Scholar]
- 53. Zhou Y, Yamamoto Y, Takeshita F, Yamamoto T, Xiao Z, Ochiya T. Delivery of miR-424-5p via Extracellular Vesicles Promotes the Apoptosis of MDA-MB-231 TNBC Cells in the Tumor Microenvironment. Int J Mol Sci (2021) 22(2). doi: 10.3390/ijms22020844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ren LH, Chen WX, Li S, He XY, Zhang ZM, Li M, et al. MicroRNA-183 Promotes Proliferation and Invasion in Oesophageal Squamous Cell Carcinoma by Targeting Programmed Cell Death 4. Br J Cancer (2014) 111(10):2003–13. doi: 10.1038/bjc.2014.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang M, Liu R, Li X, Liao J, Pu Y, Pan E, et al. miRNA-183 Suppresses Apoptosis and Promotes Proliferation in Esophageal Cancer by Targeting PDCD4. Molecules Cells (2014) 37(12):873–80. doi: 10.14348/molcells.2014.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu T, Liu Q, Zheng S, Gao X, Lu M, Yang C, et al. MicroRNA-21 Promotes Cell Growth and Migration by Targeting Programmed Cell Death 4 Gene in Kazakh's Esophageal Squamous Cell Carcinoma. Dis Markers (2014) 2014:232837. doi: 10.1155/2014/232837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li L, Zhou L, Li Y, Lin S, Tomuleasa C. MicroRNA-21 Stimulates Gastric Cancer Growth and Invasion by Inhibiting the Tumor Suppressor Effects of Programmed Cell Death Protein 4 and Phosphatase and Tensin Homolog. J BUON (2014) 19(1):228–36. [PubMed] [Google Scholar]
- 58. Zheng X, Dong L, Wang K, Zou H, Zhao S, Wang Y, et al. MiR-21 Participates in the PD-1/PD-L1 Pathway-Mediated Imbalance of Th17/Treg Cells in Patients After Gastric Cancer Resection. Ann Surg Oncol (2019) 26(3):884–93. doi: 10.1245/s10434-018-07117-6 [DOI] [PubMed] [Google Scholar]
- 59. Liao J, Liu R, Shi YJ, Yin LH, Pu YP. Exosome-Shuttling microRNA-21 Promotes Cell Migration and Invasion-Targeting PDCD4 in Esophageal Cancer. Int J Oncol (2016) 48(6):2567–79. doi: 10.3892/ijo.2016.3453 [DOI] [PubMed] [Google Scholar]
- 60. Bhatti I, Lee A, James V, Hall RI, Lund JN, Tufarelli C, et al. Knockdown of microRNA-21 Inhibits Proliferation and Increases Cell Death by Targeting Programmed Cell Death 4 (PDCD4) in Pancreatic Ductal Adenocarcinoma. J Gastrointest Surg (2011) 15(1):199–208. doi: 10.1007/s11605-010-1381-x [DOI] [PubMed] [Google Scholar]
- 61. Zhang R, Sui L, Hong X, Yang M, Li W. MiR-448 Promotes Vascular Smooth Muscle Cell Proliferation and Migration in Through Directly Targeting MEF2C. Environ Sci Pollution Res (2017) 24(28):22294–300. doi: 10.1007/s11356-017-9771-1 [DOI] [PubMed] [Google Scholar]
- 62. Duan X, Li W, Hu P, Jiang B, Yang J, Zhou L, et al. MicroRNA-183-5p Contributes to Malignant Progression Through Targeting PDCD4 in Human Hepatocellular Carcinoma. Biosci Rep (2020) 40(10). doi: 10.1042/BSR20201761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu Q, Wang Z, Hu Y, Li J, Li X, Zhou L, et al. miR-21 Promotes Migration and Invasion by the miR-21-PDCD4-AP-1 Feedback Loop in Human Hepatocellular Carcinoma. Oncol Rep (2012) 27(5):1660–8. doi: 10.3892/or.2012.1682 [DOI] [PubMed] [Google Scholar]
- 64. Yang YL, Liu P, Li D, Yang Q, Li B, Jiang XJ. Stat-3 Signaling Promotes Cell Proliferation and Metastasis of Gastric Cancer Through PDCD4 Downregulation. Kaohsiung J Med Sci (2020) 36(4):244–9. doi: 10.1002/kjm2.12159 [DOI] [PubMed] [Google Scholar]
- 65. Motoyama K, Inoue H, Mimori K, Tanaka F, Kojima K, Uetake H, et al. Clinicopathological and Prognostic Significance of PDCD4 and microRNA-21 in Human Gastric Cancer. Int J Oncol (2010) 36(5):1089–95. doi: 10.3892/ijo_00000590 [DOI] [PubMed] [Google Scholar]
- 66. Fan Y, Che X, Hou K, Zhang M, Wen T, Qu X, et al. MiR-940 Promotes the Proliferation and Migration of Gastric Cancer Cells Through Up-Regulation of Programmed Death Ligand-1 Expression. Exp Cell Res (2018) 373(1-2):180–7. doi: 10.1016/j.yexcr.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 67. Yin K, Liu M, Zhang M, Wang F, Fen M, Liu Z, et al. miR-208a-3p Suppresses Cell Apoptosis by Targeting PDCD4 in Gastric Cancer. Oncotarget (2016) 7(41):67321–32. doi: 10.18632/oncotarget.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Du Y, Wang D, Luo L, Guo J. miR-129-1-3p Promote BGC-823 Cell Proliferation by Targeting PDCD2. Anat Rec (Hoboken) (2014) 297(12):2273–9. doi: 10.1002/ar.23003 [DOI] [PubMed] [Google Scholar]
- 69. Wang J, Zhao X, Wang Y, Ren F, Sun D, Yan Y, et al. circRNA-002178 Act as a ceRNA to Promote PDL1/PD1 Expression in Lung Adenocarcinoma. Cell Death Dis (2020) 11(1):32. doi: 10.1038/s41419-020-2230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu Y, Uzair Ur R, Guo Y, Liang H, Cheng R, Yang F, et al. miR-181b Functions as an oncomiR in Colorectal Cancer by Targeting PDCD4. Protein Cell (2016) 7(10):722–34. doi: 10.1007/s13238-016-0313-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu H, Xu L, Chen Y, Xu C. MiR-208a-3p Functions as an Oncogene in Colorectal Cancer by Targeting PDCD4. Biosci Rep (2019) 39(4). doi: 10.1042/BSR20181598 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72. Nedaeinia R, Sharifi M, Avan A, Kazemi M, Nabinejad A, Ferns GA, et al. Inhibition of microRNA-21 via Locked Nucleic acid-anti-miR Suppressed Metastatic Features of Colorectal Cancer Cells Through Modulation of Programmed Cell Death 4. Tumour Biol (2017) 39(3):1010428317692261. doi: 10.1177/1010428317692261 [DOI] [PubMed] [Google Scholar]
- 73. Ashizawa M, Okayama H, Ishigame T, Thar Min AK, Saito K, Ujiie D, et al. miRNA-148a-3p Regulates Immunosuppression in DNA Mismatch Repair-Deficient Colorectal Cancer by Targeting PD-L1. Mol Cancer Res (2019) 17(6):1403–13. doi: 10.1158/1541-7786.MCR-18-0831 [DOI] [PubMed] [Google Scholar]
- 74. Sun Z, Li S, Kaufmann AM, Albers AE. miR-21 Increases the Programmed Cell Death 4 Gene-Regulated Cell Proliferation in Head and Neck Squamous Carcinoma Cell Lines. Oncol Rep (2014) 32(5):2283–9. doi: 10.3892/or.2014.3456 [DOI] [PubMed] [Google Scholar]
- 75. Wu Q, Zhao Y, Sun Y, Yan X, Wang P. miR-375 Inhibits IFN-Gamma-Induced Programmed Death 1 Ligand 1 Surface Expression in Head and Neck Squamous Cell Carcinoma Cells by Blocking JAK2/STAT1 Signaling. Oncol Rep (2018) 39(3):1461–8. doi: 10.3892/or.2018.6177 [DOI] [PubMed] [Google Scholar]
- 76. Reis PP, Tomenson M, Cervigne NK, Machado J, Jurisica I, Pintilie M, et al. Programmed Cell Death 4 Loss Increases Tumor Cell Invasion and Is Regulated by miR-21 in Oral Squamous Cell Carcinoma. Mol Cancer (2010) 9:238. doi: 10.1186/1476-4598-9-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jiang LH, Ge MH, Hou XX, Cao J, Hu SS, Lu XX, et al. miR-21 Regulates Tumor Progression Through the miR-21-PDCD4-Stat3 Pathway in Human Salivary Adenoid Cystic Carcinoma. Lab Invest (2015) 95(12):1398–408. doi: 10.1038/labinvest.2015.105 [DOI] [PubMed] [Google Scholar]
- 78. Zargar S, Tomar V, Shyamsundar V, Vijayalakshmi R, Somasundaram K, Karunagaran D. A Feedback Loop Between MicroRNA 155 (miR-155), Programmed Cell Death 4, and Activation Protein 1 Modulates the Expression of miR-155 and Tumorigenesis in Tongue Cancer. Mol Cell Biol (2019) 39(6). doi: 10.1128/MCB.00410-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shuang Y, Zhou X, Li C, Huang Y, Zhang L. MicroRNA503 Serves an Oncogenic Role in Laryngeal Squamous Cell Carcinoma via Targeting Programmed Cell Death Protein 4. Mol Med Rep (2017) 16(4):5249–56. doi: 10.3892/mmr.2017.7278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wei C, Song H, Sun X, Li D, Song J, Hua K, et al. miR-183 Regulates Biological Behavior in Papillary Thyroid Carcinoma by Targeting the Programmed Cell Death 4. Oncol Rep (2015) 34(1):211–20. doi: 10.3892/or.2015.3971 [DOI] [PubMed] [Google Scholar]
- 81. Ning FL, Wang F, Li ML, Yu ZS, Hao YZ, Chen SS. MicroRNA-182 Modulates Chemosensitivity of Human Non-Small Cell Lung Cancer to Cisplatin by Targeting PDCD4. Diagn Pathol (2014) 9:143. doi: 10.1186/1746-1596-9-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fu WF, Chen WB, Dai L, Yang GP, Jiang ZY, Pan L, et al. Inhibition of miR-141 Reverses Cisplatin Resistance in Non-Small Cell Lung Cancer Cells via Upregulation of Programmed Cell Death Protein 4. Eur Rev Med Pharmacol Sci (2016) 20(12):2565–72. [PubMed] [Google Scholar]
- 83. Yang Y, Meng H, Peng Q, Yang X, Gan R, Zhao L, et al. Downregulation of microRNA-21 Expression Restrains Non-Small Cell Lung Cancer Cell Proliferation and Migration Through Upregulation of Programmed Cell Death 4. Cancer Gene Ther (2015) 22(1):23–9. doi: 10.1038/cgt.2014.66 [DOI] [PubMed] [Google Scholar]
- 84. Yang D, Wang JJ, Li JS, Xu QY. miR-103 Functions as a Tumor Suppressor by Directly Targeting Programmed Cell Death 10 in NSCLC. Oncol Res (2018) 26(4):519–28. doi: 10.3727/096504017X15000757094686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Itani S, Kunisada T, Morimoto Y, Yoshida A, Sasaki T, Ito S, et al. MicroRNA-21 Correlates With Tumorigenesis in Malignant Peripheral Nerve Sheath Tumor (MPNST) via Programmed Cell Death Protein 4 (PDCD4). J Cancer Res Clin Oncol (2012) 138(9):1501–9. doi: 10.1007/s00432-012-1223-1 [DOI] [PubMed] [Google Scholar]
- 86. Gaur AB, Holbeck SL, Colburn NH, Israel MA. Downregulation of Pdcd4 by Mir-21 Facilitates Glioblastoma Proliferation In Vivo. Neuro Oncol (2011) 13(6):580–90. doi: 10.1093/neuonc/nor033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang Z, Yao W, Li K, Zheng N, Zheng C, Zhao X, et al. Reduction of miR-21 Induces SK-N-SH Cell Apoptosis and Inhibits Proliferation via PTEN/Pdcd4. Oncol Lett (2017) 13(6):4727–33. doi: 10.3892/ol.2017.6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guo P, Yu Y, Tian Z, Lin Y, Qiu Y, Yao W, et al. Upregulation of miR-96 Promotes Radioresistance in Glioblastoma Cells via Targeting PDCD4. Int J Oncol (2018) 53(4):1591–600. doi: 10.3892/ijo.2018.4498 [DOI] [PubMed] [Google Scholar]
- 89. Xu X, Ge S, Jia R, Zhou Y, Song X, Zhang H, et al. Hypoxia-Induced miR-181b Enhances Angiogenesis of Retinoblastoma Cells by Targeting PDCD10 and GATA6. Oncol Rep (2015) 33(6):2789–96. doi: 10.3892/or.2015.3900 [DOI] [PubMed] [Google Scholar]
- 90. Wang X, Zuo D, Yuan Y, Yang X, Hong Z, Zhang R. MicroRNA-183 Promotes Cell Proliferation via Regulating Programmed Cell Death 6 in Pediatric Acute Myeloid Leukemia. J Cancer Res Clin Oncol (2017) 143(1):169–80. doi: 10.1007/s00432-016-2277-2 [DOI] [PubMed] [Google Scholar]
- 91. Wang LQ, Kumar S, Calin GA, Li Z, Chim CS. Frequent Methylation of the Tumour Suppressor miR-1258 Targeting PDL1: Implication in Multiple Myeloma-Specific Cytotoxicity and Prognostification. Br J Haematol (2020) 190(2):249–61. doi: 10.1111/bjh.16517 [DOI] [PubMed] [Google Scholar]
- 92. Fischer N, Goke F, Splittstosser V, Lankat-Buttgereit B, Muller SC, Ellinger J. Expression of Programmed Cell Death Protein 4 (PDCD4) and miR-21 in Urothelial Carcinoma. Biochem Biophys Res Commun (2012) 417(1):29–34. doi: 10.1016/j.bbrc.2011.11.035 [DOI] [PubMed] [Google Scholar]
- 93. Fan B, Jin Y, Zhang H, Zhao R, Sun M, Sun M, et al. MicroRNA21 Contributes to Renal Cell Carcinoma Cell Invasiveness and Angiogenesis via the PDCD4/cJun (AP1) Signalling Pathway. Int J Oncol (2020) 56(1):178–92. doi: 10.3892/ijo.2019.4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jiao J, Fan Y, Zhang Y. Expression and Clinicopathological Significance of microRNA-21 and Programmed Cell Death 4 in Malignant Melanoma. J Int Med Res (2015) 43(5):672–8. doi: 10.1177/0300060515583707 [DOI] [PubMed] [Google Scholar]
- 95. Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and Molecular Targeting Therapy in Cancer. BioMed Res Int (2014) 2014. doi: 10.1155/2014/150845 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96. Chowdhury I, Tharakan B, Bhat GK. Caspases—An Update. Comp Biochem Physiol Part B: Biochem Mol Biol (2008) 151(1):10–27. doi: 10.1016/j.cbpb.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 97. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol (2017) 8:561. doi: 10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sun R, Zhang L. Long Non-Coding RNA MALAT1 Regulates Cardiomyocytes Apoptosis After Hypoxia/Reperfusion Injury via Modulating miR-200a-3p/PDCD4 Axis. BioMed Pharmacother (2019) 111:1036–45. doi: 10.1016/j.biopha.2018.12.122 [DOI] [PubMed] [Google Scholar]
- 99. Wei S, Wang K, Huang X, Zhao Z, Zhao Z. LncRNA MALAT1 Contributes to Non-Small Cell Lung Cancer Progression via Modulating miR-200a-3p/Programmed Death-Ligand 1 Axis. Int J Immunopathol Pharmacol (2019) 33:2058738419859699. doi: 10.1177/2058738419859699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lu J, Xue Y, Wang Y, Ding Y, Zou Q, Pan M, et al. CiRS-126 Inhibits Proliferation of Ovarian Granulosa Cells Through Targeting the miR-21-PDCD4-ROS Axis in a Polycystic Ovarian Syndrome Model. Cell Tissue Res (2020) 381(1):189–201. doi: 10.1007/s00441-020-03187-9 [DOI] [PubMed] [Google Scholar]
- 101. Zhao L, Liu Y, Zhang J, Liu Y, Qi Q. LncRNA SNHG14/miR-5590-3p/ZEB1 Positive Feedback Loop Promoted Diffuse Large B Cell Lymphoma Progression and Immune Evasion Through Regulating PD-1/PD-L1 Checkpoint. Cell Death Dis (2019) 10(10):731. doi: 10.1038/s41419-019-1886-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhou WY, Zhang MM, Liu C, Kang Y, Wang JO, Yang XH. Long Noncoding RNA LINC00473 Drives the Progression of Pancreatic Cancer via Upregulating Programmed Death-Ligand 1 by Sponging microRNA-195-5p. J Cell Physiol (2019) 234(12):23176–89. doi: 10.1002/jcp.28884 [DOI] [PubMed] [Google Scholar]
- 103. Ye Y, Yang S, Han Y, Sun J, Xv L, Wu L, et al. Linc00472 Suppresses Proliferation and Promotes Apoptosis Through Elevating PDCD4 Expression by Sponging miR-196a in Colorectal Cancer. Aging (2018) 10(6):1523–33. doi: 10.18632/aging.101488 [DOI] [PMC free article] [PubMed] [Google Scholar]