Abstract
The objective of this study was to compare the immunogenicity and safety of a single-dose regimen and a two-dose regimen of a trivalent virosome influenza vaccine (Inflexal Berna V) with those of a trivalent subunit influenza vaccine (Influvac) in children and adolescents with cystic fibrosis (CF). In an open, randomized, multicenter study with parallel groups, 11 young children with CF (1 to 6 years old) and 53 older children and adolescents with CF (>6 years old) were randomly assigned to one of the following immunization regimens: virosome vaccine at 0.5 ml on study day 0 or 0.25 ml on days 0 and 28 or a standard regimen of subunit vaccine, i.e., 0.5 ml on day 0 for older children and 0.25 ml on days 0 and 28 for younger children. Safety assessments, i.e., recording of systemic and local adverse events (AEs) and vital signs, were made for a 5-day observation period after each immunization. Hemagglutination inhibition (HI) titers were determined at baseline and 4 weeks after the single-dose and the two-dose immunizations, respectively. Immunogenicity was assessed according to the criteria of the European Agency for the Evaluation of Medicinal Products (EMEA). Both vaccines induced comparable HI antibody titers. Seroconversion (≥4-fold rise in HI antibody titers, reaching a titer of ≥1:40) was achieved in 41 to 100% of the participants. Seroprotection (HI titer, ≥1:40) and a >2.5-fold increase in geometric mean titers were achieved in 100% of the participants. Thus, all three EMEA requirements for influenza vaccine efficacy were met by all treatment groups and for both vaccines. The virosome vaccine, when administered as a single dose, seemed to induce superior immunogenicity compared with the standard pediatric two-dose regimen. Totals of 42 and 57% of vaccinees receiving virosome and subunit vaccines, respectively, reported at least one local AE (predominantly pain). Totals of 84 and 71% of subjects receiving virosome and subunit vaccines, respectively, complained in response to questions of at least one systemic AE (mainly cough, fatigue, coryza, or headache). The majority of events were mild or moderate and lasted 1 or 2 days only. No obvious relationship was found between AE reporting rate and vaccine formulation, age group, or dose regimen. The relatively high AE reporting rate seemed to be partly related to the symptomatology of the underlying CF disease. In summary, the virosome and subunit vaccines induced in both age groups and against all three influenza strains an efficient immune response and were well tolerated by the children and adolescents with CF.
Influenza is a potentially serious disease in very young children due to no background immunity (3, 13), in the elderly due to innate decreased resistance to infections and a poorly functioning immune system (6, 20), and in individuals with an underlying disease which may render them unable to handle infections. Among the last group, patients with chronic pulmonary dysfunction, such as cystic fibrosis (CF) (19, 21, 26, 27), are at a particularly high risk for acquiring a severe influenza infection. Influenza disrupts the normal defense system of the respiratory tract and may lead to secondary bacterial pneumonia. Influenza vaccination has been shown to be beneficial to children (3, 13), healthy working adults (22), and elderly subjects with or without risk factors (23). Therefore, many public health authorities recommend routine annual immunization of high-risk individuals (24). In many countries, including Switzerland, these targeted vaccinations are reimbursed by health insurance or by public funds. However, despite these recommendations and incentives, at most half of Swiss citizens ≥65 years old are immunized annually against influenza (12). Recent surveys on influenza vaccination coverage showed these figures to be higher in France (≥70%), in The Netherlands (58 to 64%), and in the United States (55 to 75%), similar in Italy (26 to 49%), and lower in Austria (14%) (10, 25). For Switzerland, there are no figures on the influenza vaccination coverage of children, an age group which is, especially with regard to at-risk subjects, not adequately vaccinated worldwide (2, 17). Although patients with CF are at risk of developing complications following influenza, they are in general immunologically very competent, and a high percentage of such patients usually attain protective hemagglutination inhibition (HI) antibody levels (16, 17).
Currently, three main types of influenza vaccines are commercially available. The first is composed of intact virions inactivated by treatment with formalin. These whole-virus vaccines are considered to be the most reactinogenic and are recommended in many countries only for use in adults and older children (4). The two other types, the subunit and split vaccines, are composed of purified influenza antigens in which hemagglutinin (HA) predominates. These vaccines are recommended for individuals of all ages. Immunization of infants and young children requires two doses of vaccine spaced 1 or 2 months apart (4). However, current vaccines still do not result in a high, long-lasting protective immune response in this group (28, 29), and vaccines with improved immunogenicity are clearly needed (7, 8). Attempts to increase immunogenicity by increasing antigen content per dose does not always result in a better antibody response (7, 29). However, reformulation of either whole-virus vaccines or subunit vaccines with new adjuvants or antigen delivery systems has been shown to greatly potentiate the immune response in animals (1, 11).
Recently, a novel vaccine antigen delivery system, so-called virosomes, has been developed by incorporating the HA from an influenza virus A strain into liposomes composed of phosphatidylcholine (14, 15, 18). The influenza virus surface glycoprotein HA guides the virosomes specifically to antigen-presenting cells and leads to fusion with their endosomal membrane. This process provides optimal processing and presentation of the antigens to immunocompetent cells. The T lymphocytes are activated to produce cytokines, which in turn stimulate the B lymphocytes to form large amounts of specific antibodies. The stimulation of B lymphocytes also occurs through direct contact with the antigen-virosome complex (14, 18). When tested for safety and immunogenicity in elderly nursing home residents in comparison to whole-virion and subunit vaccines, the virosome-formulated influenza vaccine was found to be superior with regard to reactogenicity and immunogenicity (5, 15). This trivalent virosome influenza vaccine (Inflexal Berna V) is currently licensed in several European countries (e.g., Switzerland since 1997 and Italy since 1998). Therefore, we investigated the safety and comparative immunogenicity of this new virosome influenza vaccine in comparison with a standard subunit influenza vaccine for a pediatric group at risk, i.e., children and adolescents with CF.
(This paper was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, U. B. Schaad et al., abstr. H-136, 1997.)
MATERIALS AND METHODS
Patients and study design.
The study was performed in the winter of 1994–1995 in five pediatric centers in Switzerland with an open, randomized, parallel-group design. All children and adolescents enrolled had clinically stable CF and received either the trivalent virosome influenza vaccine Inflexal Berna V or the trivalent subunit influenza vaccine Influvac as an intramuscular injection into the upper arm. The study was conducted in accordance with the principles of the Declaration of Helsinki III (as amended in Tokyo, Venice, and Hong Kong). The trial protocol was approved prior to the study start by local ethical review boards, and written informed consent was obtained from the parents of the participating children or adolescents before the start. Screening prior to the study start included a physical examination and recording of medical history and demographic data. All subjects had to meet carefully selected inclusion and exclusion criteria. At baseline, adverse events, body temperature, and pulse rate were recorded; in addition, a preimmunization blood sample was taken for serology testing.
The eligible children (Table 1) were randomized (i) to be immunized with Inflexal Berna V, either as a single dose of 0.5 ml on study day 0 in the younger (≤6 years; group A1) and the older (>6 years; group A2) age groups or as two doses of 0.25 ml (groups B1 and B2) on study days 0 and 28, or (ii) to receive two 0.25-ml doses of Influvac on study days 0 and 28 (younger children; group C) or a single 0.5-ml dose of Influvac on day 0 (older children; group D). Blood samples for serology testing were taken 4 weeks after the single or the second immunization, i.e., on study day 28 or study day 56, respectively. Systemic and local adverse events were reported by the parents of the children and adolescents during a 5-day observation period following the immunizations. A further assessment of health status and vaccine tolerance and collection of the adverse event report forms were performed at the follow-up visit 4 weeks after the single-dose or two-dose immunization.
TABLE 1.
Demographic characteristics of populations and dose regimens
| Vaccine | Group | Dosage (ml) | No. of subjects in the following population:
|
Mean ± SD (range) age of subjects, in yr | |||
|---|---|---|---|---|---|---|---|
| Safety | Efficacy | Men | Women | ||||
| Virosome (Inflexal Verna V) | A1 | 0.5, once | 4 | 4 | 1 | 3 | 4.1 ± 1.6 (1.9–5.6) |
| A2 | 0.5, once | 15 | 14 | 6 | 9 | 14.5 ± 5.7 (6.4–26.1) | |
| B1 | 0.25, twice | 5 | 5 | 1 | 4 | 3.8 ± 1.7 (1.6–6.0) | |
| B2 | 0.25, twice | 19 | 17 | 8 | 11 | 16.4 ± 6.3 (8.4–33.1) | |
| Subunit (Influvac) | C | 0.25, twice | 2 | 1 | 0 | 2 | 2.9 (2.9–2.9) |
| D | 0.5, once | 19 | 18 | 7 | 12 | 15.7 ± 7.3 (6.1–31.9) | |
Forty-three subjects were randomized to receive Inflexal Berna V, and 21 were randomized to receive Influvac. Eleven young children 1 to 6 years old and with CF were randomly assigned to immunization groups A1 (n = 4), B1 (n = 5), and C (n = 2). Fifty-three older children and adolescents >6 years old and with CF were randomly assigned to immunization groups A2 (n = 15), B2 (n = 19), and D (n = 19). Dosing regimens and the age and gender of these groups are shown in Table 1.
Immunogenicity assessment.
HI antibody titers were determined at baseline and 4 weeks after the single-dose or the two-dose immunizations by a specific HI test. HI titers were used to calculate seroconversion rates, seroprotection rates, and increase in geometric mean titers (GMTs) for each immunization group. Immunogenicity was assessed according to the criteria of the European Agency for the Evaluation of Medicinal Products (EMEA) (9). In order to confirm protective immunogenicity, at least one of the following three requirements have to be met in subjects ≥18 to <60 years old for each influenza virus strain: (i) seroconversion, i.e., a ≥4-fold increase in HI antibody titer, reaching a titer of ≥1:40, in >40% of subjects; (ii) an increase in GMTs of >2.5-fold; and (iii) seroprotection, i.e., achievement of an HI titer of ≥1:40 in >70% of subjects. As no criteria are given by the EMEA for subjects younger than 18 years, the above-mentioned requirements were applied for all participants in this trial.
Safety assessment.
The following systemic reactions were evaluated by use of “solicited questions” during the 5-day observation period: headache, fatigue, nausea, cough, coryza, vertigo, and irritability. The body temperature was measured daily. In addition, any other systemic symptoms were recorded. The intensity of the adverse events was graded on a three-point scale as mild (presence of mild symptoms), moderate (symptoms which have an impact on normal daily activities), or severe (symptoms which prevent normal activities). Local reactions evaluated were pain, swelling, induration, and redness. The intensity of pain was graded as mentioned above. Redness at the injection site was measured in millimeters, and swelling and induration were recorded as present or absent.
Vaccines. (i) Virosome influenza vaccine Inflexal Berna V.
The novel vaccine Inflexal Berna V was developed by the Swiss Serum and Vaccine Institute, Berne, Switzerland. HA was extracted from the 1994–1995 recommended influenza virus strains A/Singapore/6/86-like (H1N1), A/Shangdong/9/93-like (H3N2), and B/Panama/45/90-like and incorporated into phosphatidylcholine bilayer liposomes, yielding unilamellar, so-called virosomes with an average diameter of 150 nm (28). The final vaccine contained 15 μg of HA of each virus strain in a volume of 0.5 ml for intramuscular injection.
(ii) Subunit influenza vaccine Influvac.
The commercially available subunit vaccine Influvac was purchased from Solvay, Berne, Switzerland, and contained 15 μg of HA of each of the influenza virus strains A/Singapore/6/86-like (H1N1), A/Shangdong/9/93-like (H3N2), and B/Panama/45/90-like per 0.5-ml dose for intramuscular injection.
RESULTS
All 64 subjects enrolled were evaluable for safety, and 59 were evaluable for immunogenicity. Five subjects had no postvaccination blood samples taken and had to be excluded from the immunogenicity analysis. Due to difficulties in recruiting young children (less than 6 years old) with CF, the originally planned number of 20 subjects was not reached for groups A1, B1, and C.
Immunogenicity assessment.
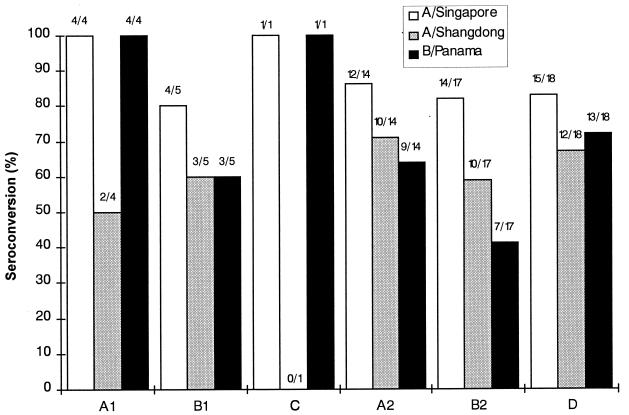
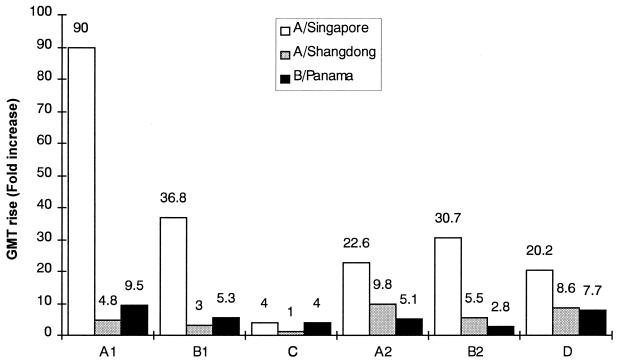
The results of the evaluation of seroconversion, seroprotection, and fold increase in GMTs are presented in Fig. 1 and 2. In group C, only one 2-year-old child was evaluable. This child met the EMEA requirements for seroprotection against all three viral strains and for seroconversion and fold increase in GMTs against strains A/Singapore and B/Panama. The data for this child were not compared with those for the other groups.
FIG. 1.
Seroconversion rates determined 4 weeks after single-dose or two-dose immunizations of pediatric CF patients with the virosome influenza vaccine Inflexal Berna V or with the subunit influenza vaccine Influvac. See Table 1 for details.
FIG. 2.
Fold-increase in GMTs determined 4 weeks after single-dose or two-dose immunizations of pediatric CF patients with the virosome influenza vaccine Inflexal Berna V or with the subunit influenza vaccine Influvac. See Table 1 for details.
In the other five immunization groups, the rates of seroconversion against the three influenza virus strains tested ranged between 41 and 100% (>40% required by EMEA [9]) (Fig. 1). Seroprotection was reached by 100% of the vaccinees for all three influenza virus strains (EMEA requirement, >70%), and the increase in GMTs was 2.8- to 90.5-fold (>2.5-fold increase required by EMEA) (Fig. 2). Thus, all three EMEA criteria needed to confirm immunogenicity were fulfilled by the virosome and subunit vaccines against all three strains. Although the small number of subjects in the immunization groups prevents a comparative analysis, the results indicate that both vaccines induced comparable HI antibody titers and that Inflexal Berna V may have superior immunogenicity when given as a single dose than as the two-dose pediatric regimen (groups A1 versus B1 and groups A2 versus B2, respectively, in Fig. 1 and 2). Immune responses in the younger children were comparable to those observed in the older vaccinees.
In the majority of vaccinees, especially in those more than 6 years old, protective prevaccination HI titers of ≥1:40 were found against all strains, either following previous influenza immunization (32% of the participants had been vaccinated in 1992–1993 and/or 1993–1994) or through natural infection in the past. Preexisting HI titers of ≥1:40 in the different immunization groups ranged from 0 to 61% against strain A/Singapore, from 50 to 100% against strain A/Shangdong, and from 50 to 88% against strain B/Panama. Therefore, it was not surprising to find 100% seroprotection rates in all groups for all influenza strains at day 29.
Safety assessment.
After injection of Inflexal Berna V, 19 vaccinees (44%) reported one or more local adverse reactions (Table 2). After injection of Influvac, 12 vaccinees (57%) experienced local adverse reactions. All local reactions were classified as mild or moderate and disappeared after 1 or 2 days. In the younger age groups, A1 and B1, fewer local adverse reactions were reported. However, no relationship was found between frequency of adverse events and dose regimen or vaccine formulation.
TABLE 2.
Local adverse events experienced in the 5-day observation period following immunization of pediatric CF patients with the virosome or subunit influenza vaccine
| Vaccine | Dose (ml) | Group (no. of subjects) | Mean age of subjects (yr) | Total no. (%) of vaccines with at least one adverse event | % of vaccines with:
|
|||
|---|---|---|---|---|---|---|---|---|
| Pain | Induration | Redness | Swelling | |||||
| Inflexal Berna V | 0.5, once | A1 (4) | 4.1 | 1 | 25 | 0 | 0 | 0 |
| 0.5, once | A2 (15) | 14.5 | 10 (67) | 53 | 13 | 20 | 27 | |
| 0.25, twice | B1 (5) | 3.8 | 0 | 0 | 0 | 0 | 0 | |
| 0.25, twice | B2 (19) | 16.4 | 8 (42) | 31 | 5.3 | 21 | 5.3 | |
| Influvac | 0.25, twice | C (2) | 2.9 | 1 | 50 | 0 | 0 | 0 |
| 0.5, once | D (19) | 15.7 | 11 (58) | 53 | 21 | 21 | 21 | |
After injection of Inflexal Berna V, 36 vaccinees (84%) reported one or more systemic adverse events (Table 3). After injection of Influvac, 15 vaccinees (71%) experienced systemic adverse events. Most of these events were classified by the investigators as mild or moderate. As expected, the younger study participants did not report any headache or vertigo and, as for the local reactions, there was a tendency for lower reporting rates regarding the other symptoms among the younger children.
TABLE 3.
Systemic adverse events experienced in the 5-day observation period following immunization of pediatric CF patients with the virosome or subunit influenza vaccine
| Vaccine | Dose (ml) | Group (no. of subjects) | Mean age of subjects (yr) | Total no. (%) of vaccinees with at least one adverse event | % of vaccines with:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Headache | Fatigue | Nausea | Cough | Coryza | Vertigo | Irritability | Othersa | |||||
| Inflexal Berna V | 0.5, once | A1 (4) | 4.1 | 4 | 0 | 50 | 25 | 75 | 25 | 0 | 0 | 75 |
| 0.5, once | A2 (15) | 14.5 | 14 (93) | 53 | 60 | 6.7 | 53 | 47 | 6.7 | 13 | 33 | |
| 0.25, twice | B1 (5) | 3.8 | 5 | 0 | 20 | 20 | 40 | 80 | 0 | 20 | 20 | |
| 0.25, twice | B2 (19) | 16.4 | 13 (68) | 37 | 42 | 21 | 37 | 37 | 16 | 16 | 16 | |
| Influvac | 0.25, twice | C (2) | 2.9 | 2 | 0 | 100 | 0 | 100 | 100 | 0 | 0 | 0 |
| 0.5, once | D (19) | 15.7 | 13 (68) | 32 | 47 | 5.3 | 47 | 26 | 11 | 21 | 26 | |
Insomnia, vomiting, rigors, pharyngitis, thirst, apathy, abdominal pain, palpitation, laryngitis, increased sweating, euphoria, and earache.
A total of 13 systemic adverse events observed in eight vaccinees (5 of 43, or 12%, in the Inflexal Berna V group, and 3 of 21, or 14%, in the Influvac group) were judged by the vaccinees or the parents as severe, i.e., preventing normal activity. The predominant symptoms judged severe were cough and fatigue. The majority of these events were of short duration and disappeared within 1 or 2 days. No relationship between frequency of systemic events and dose regimen or vaccine formulation could be detected.
Body temperature measured after the immunizations revealed no major changes from the baseline. There was no single body temperature value of ≥38.5°C, fulfilling the definition of fever. Four weeks after each immunization, tolerance of the vaccination was assessed by the investigators and by the vaccinees themselves (or their parents or legal guardians). In general, the tolerance was classified as “very good” or “good,” only one vaccinee had a “bad” impression, and one investigator rated one case as “intermediate.” This favorable judgment is also reflected by the high acceptance for revaccination: 98% of the vaccinees agreed to be revaccinated.
DISCUSSION
The primary objective of this study was to investigate the immunogenicity and safety of a virosome influenza vaccine (Inflexal Berna V) in comparison with those of a subunit vaccine (Influvac) in pediatric patients with clinically stable CF. In addition, we planned to compare the efficacy of single-dose immunization of the virosome influenza vaccine with that of the current standard pediatric two-dose regimen, spaced 1 month apart.
The occurrence of new antigenic influenza virus variants prevents long-lasting protection conferred by either vaccination or infection. Therefore, the development of new, highly efficient, and well-tolerated vaccines is mandatory. A single-dose regimen might increase compliance for basic immunization, especially in children. However, yearly revaccination currently consists of one booster injection with all influenza vaccines.
The recent development and first clinical investigation of liposomes containing HA from influenza virus strains (immunopotentiating reconstituted influenza virosomes) revealed promising results. In a comparative study with elderly nursing home residents, a prototype trivalent virosome influenza vaccine was compared with commercial whole-virion and subunit vaccines for safety and immunogenicity (15). All three vaccines were well tolerated and induced a significant increase in GMTs to all three vaccine strains. Moreover, the virosome vaccine was shown to be less reactogenic and at the same time more immunogenic than either the whole-virus or the subunit influenza vaccine. Of additional importance was the finding that the virosome vaccine engendered protective titers in a significantly higher percentage of the study subjects than the commercial subunit vaccine. This finding was also observed for a subset of patients who had nonprotective antibody titers at baseline. These good results were confirmed in a second clinical trial (5).
In the present study, evaluation of the HI antibody titers of 59 pediatric patients with CF showed that the virosome vaccine and the subunit vaccine induced in all vaccinees an efficient immune response against all three viral strains. Comparable HI antibody titers were determined for both vaccines. All three EMEA criteria needed to prove immunogenicity regarding each influenza virus strain (required is the fulfillment of at least one criterion) were reached, i.e., seroconversion, seroprotection, and a fold increase in GMTs (9). As no EMEA criteria are provided for subjects less than 18 years old, the more restrictive criteria valid for subjects >18 to <60 years old were applied to the results obtained in the present study for pediatric patients. Although the number of young subjects was too small to perform a comparative statistical analysis of the treatment groups, Inflexal Berna V seemed to induce superior immunogenicity when given as a single dose compared to the two-dose pediatric regimen. However, since the majority of vaccinees already had prevaccination HI antibody titers, a comparison of single- versus two-dose immunization schedules must be interpreted with caution.
Based on the safety evaluation, both vaccines were found to be safe and well tolerated by both age groups independent of the dose regimen. Most of the local reactions and the systemic adverse events were classified as mild or moderate and lasted 1 or 2 days only. The tolerance assessment made by the investigators and the vaccinees themselves was very favorable, and almost all of the vaccinees agreed to be revaccinated. The high rate of systemic adverse events reported in response to questions for both vaccines contrasts with the excellent tolerance self-assessment of the vaccinees and may be explained by the fact that the children had CF. One has to assume that the assessment regarding fatigue, cough, coryza, and so forth (rates of >50% reported for both vaccines) was confounded by the symptomatology of the underlying CF. No serious adverse event occurred, and none of the participants dropped out for safety reasons. Due to the small number of young children, no comparison between the virosome and the standard subunit vaccines could be made.
In conclusion, both vaccine formulations were safe and induced an efficient immune response against all three viral strains in both the children and the adolescents with CF.
ACKNOWLEDGMENT
This work was supported in part by a grant from the Swiss Serum and Vaccine Institute.
REFERENCES
- 1.Allison A C, Greggoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252:252–255. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 2.Barnett E D. Influenza immunization for children. N Engl J Med. 1998;338:1459–1461. doi: 10.1056/NEJM199805143382010. [DOI] [PubMed] [Google Scholar]
- 3.Brady M T. Influenza virus infections in children. Semin Pediatr Infect Dis. 1998;9:92–102. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbid Mortal Weekly Rep. 1997;46:1–25. [PubMed] [Google Scholar]
- 5.Conne P, Gauthey L, Vernet P, Althaus B, Que J U, Finkel B, Glück R, Cryz S J. Immunogenicity of trivalent subunit versus virosome-formulated influenza vaccines in geriatric patients. Vaccine. 1997;15:1675–1679. doi: 10.1016/s0264-410x(97)00087-x. [DOI] [PubMed] [Google Scholar]
- 6.Couch R B, Kasel J A, Glezen W P, Cate T R, Six H R, Taber C H. Influenza: its control in persons and populations. J Infect Dis. 1986;153:431–440. doi: 10.1093/infdis/153.3.431. [DOI] [PubMed] [Google Scholar]
- 7.Couch R B, Keitel W A, Cate T R. Improvement of inactivated influenza vaccines. J Infect Dis. 1997;176(Suppl. 1):S38–S44. doi: 10.1086/514173. [DOI] [PubMed] [Google Scholar]
- 8.Ershler W B. Influenza vaccination in the elderly: can efficacy be enhanced? Geriatrics. 1988;43:79–83. [PubMed] [Google Scholar]
- 9.European Agency for the Evaluation of Medicinal Products (EMEA) Ad Hoc Working Party on Influenza Vaccines: summary record of meeting on the choice of strains for influenza vaccine for the 1995–1996 vaccination campaign. Brussels, 27 February 1995. 1995. [Google Scholar]
- 10.Fedson D S, Hirota Y, Shin H K, et al. Influenza vaccination in 22 developed countries: an update to 1995. Vaccine. 1997;15:1506–1511. doi: 10.1016/s0264-410x(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 11.Garcon N M, Six H R. Universal vaccine carrier. J Immunol. 1991;146:3697–3702. [PubMed] [Google Scholar]
- 12.Gauthey L, Toscani L, Schira J C. Vaccination contra la grippe en Suisse. Soz Praeventivmed. 1997;42(Suppl. 2):107–111. doi: 10.1007/BF01365164. [DOI] [PubMed] [Google Scholar]
- 13.Glezen W P, Taber L H, Frank A L, Gruber W C, Piedra P A. Influenza virus infections in infants. Pediatr Infect Dis J. 1997;16:1065–1068. doi: 10.1097/00006454-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Glück R, Mischler R, Brantschen S, Just M, Althaus B, Cryz S J., Jr Immunopotentiating reconstituted influenza virosome vaccine delivery system for immunization against hepatitis A. J Clin Investig. 1992;90:2491–2495. doi: 10.1172/JCI116141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glück R, Mischler R, Finkel B, Que J U, Scarpa B, Cryz S J., Jr Immunogenicity of new virosome influenza vaccine in elderly people. Lancet. 1994;344:160–163. doi: 10.1016/s0140-6736(94)92758-8. [DOI] [PubMed] [Google Scholar]
- 16.Gross P A, Denning C R, Gaerlen P F. Annual influenza vaccination: immune response in patients over 10 years. Vaccine. 1996;14:1280–1284. doi: 10.1016/s0264-410x(96)00004-7. [DOI] [PubMed] [Google Scholar]
- 17.Gruber W C, Campbell P W, Thompson J M, et al. Comparison of live and inactivated vaccines in cystic fibrosis patients and their families: results of a 3-year study. J Infect Dis. 1994;169:241–247. doi: 10.1093/infdis/169.2.241. [DOI] [PubMed] [Google Scholar]
- 18.Holm K J, Goa K L. Liposomal influenza vaccine. BioDrugs. 1999;11:137–144. doi: 10.2165/00063030-199911020-00007. [DOI] [PubMed] [Google Scholar]
- 19.Hordvik N L, König P, Hamory B, Cooperstock M, Kreutz C, Gayer D, Barbero G. Effects of acute viral respiratory tract infections in patients with cystic fibrosis. Pediatr Pulmonol. 1989;7:217–222. doi: 10.1002/ppul.1950070406. [DOI] [PubMed] [Google Scholar]
- 20.Kohn R P. Cause of death in very old people. JAMA. 1982;247:2793–2797. [PubMed] [Google Scholar]
- 21.Marks M I. Respiratory viruses in cystic fibrosis. N Engl J Med. 1984;311:1695–1696. doi: 10.1056/NEJM198412273112610. [DOI] [PubMed] [Google Scholar]
- 22.Nichol K L, Lind A, Margolis K L, et al. The effectiveness of vaccination against influenza in healthy working adults. N Engl J Med. 1995;333:889–893. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- 23.Nichol K L, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch Intern Med. 1998;158:1769–1776. doi: 10.1001/archinte.158.16.1769. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson K G, Snacken R, Palache A M. Influenza immunization policies in Europe and the United States. Vaccine. 1995;13:365–369. doi: 10.1016/0264-410x(95)98258-c. [DOI] [PubMed] [Google Scholar]
- 25.Pregliasco F, Sodano L, Mensi C, et al. Influenza vaccination among the elderly in Italy. Bull W H O. 1999;77:127–131. [PMC free article] [PubMed] [Google Scholar]
- 26.Pribble C G, Black P G, Bosso J A, Turner R B. Clinical manifestations of exacerbations of cystic fibrosis associated with nonbacterial infections. J Pediatr. 1990;117:200–204. doi: 10.1016/S0022-3476(05)80530-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang E E L, Prober C G, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;311:1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 28.Wright P F, Cherry J D, Foy H M. Antigenicity and reactogenicity of influenza A/USSR/77 virus vaccine in children—a multicentered evaluation of dosage and safety. Rev Infect Dis. 1983;5:758–764. doi: 10.1093/clinids/5.4.758. [DOI] [PubMed] [Google Scholar]
- 29.Zei T, Neri M, Iorio A M. Immunogenicity of trivalent subunit and split influenza vaccines (1989–1990 winter season) in volunteers of different age groups. Vaccine. 1991;9:613–617. doi: 10.1016/0264-410x(91)90184-8. [DOI] [PubMed] [Google Scholar]