Abstract
Background
Retrospective studies have suggested a potential risk of hyperprogressive disease (HPD) in patients receiving immune checkpoint inhibitors (ICIs). We compared the incidence of HPD during treatment with nivolumab±ipilimumab versus natural tumor progression with placebo in post hoc analyses of two randomized, double-blind clinical trials.
Methods
ATTRACTION-2 randomized patients with advanced gastric or gastroesophageal junction cancer (GC/GEJC) and progression on ≥2 prior regimens to nivolumab 3 mg/kg Q2W or placebo. CheckMate 451 randomized patients with extensive-disease small cell lung cancer (ED SCLC) and ongoing complete/partial response or stable disease after first-line chemotherapy to nivolumab 240 mg Q2W, nivolumab 1 mg/kg+ipilimumab 3 mg/kg Q3W for four doses then nivolumab 240 mg Q2W, or placebo. Patients receiving ≥1 dose of study drug and with tumor scans at baseline and the first on-treatment evaluation were included in the HPD analyses. HPD definitions were ≥20%, ≥50%, and ≥100% increase in target lesion sum of the longest diameters (SLD) at the first on-treatment assessment.
Results
In the ATTRACTION-2 HPD-evaluable population, 243 patients received nivolumab and 115 placebo. Fewer patients receiving nivolumab versus placebo had increases in SLD ≥20% (33.7% vs 46.1%) and ≥50% (6.2% vs 11.3%); similar proportions had increases in SLD ≥100% (1.6% vs 1.7%). In the CheckMate 451 HPD-evaluable population, 177 patients received nivolumab, 179 nivolumab+ipilimumab, and 175 placebo. Fewer patients receiving nivolumab or nivolumab+ipilimumab versus placebo had increases in SLD ≥20% (27.1%, 27.4% vs 45.7%), ≥50% (10.2%, 11.2% vs 22.3%), and ≥100% (2.8%, 2.8% vs 6.3%).
Conclusions
Nivolumab±ipilimumab was not associated with an increased rate of progression versus placebo in patients with GC, GEJC, or ED SCLC, suggesting that previous reports of HPD may reflect the natural disease course in some patients rather than ICI-mediated progression.
Trial registration number
Keywords: gastrointestinal neoplasms, genetic markers, immunotherapy, lung neoplasms
Key messages.
What is already known on this topic
Possible hyperprogressive disease (HPD) has been reported in retrospective studies of patients treated with immune checkpoint inhibitors (ICIs); however, these analyses have been subject to a range of limitations, including use of various definitions of HPD.
What this study adds
Analyses of two phase III randomized, placebo-controlled clinical trials across two tumor types allowed tumor growth in patients receiving nivolumab monotherapy or nivolumab plus ipilimumab to be compared with natural tumor progression in the absence of treatment. Evaluation of HPD using a definition based on percentage change from baseline in target lesion sum of the longest diameters across multiple cut-offs showed that ICI treatment was not associated with an increased incidence of disease progression. These results suggest that reports of rapid tumor growth in patients treated with ICIs may reflect the natural course of disease in some patients.
How this study might affect research, practice or policy
Nivolumab-based treatment is not associated with HPD in the investigated tumor types; further investigation of this phenomenon in other indications is warranted.
Background
Pseudoprogression is a response pattern characterized by radiographic disease progression after initiation of treatment, per conventional response criteria, followed by prolonged clinical stabilization or partial/complete response.1 2 A second unique response pattern hypothesized to occur in patients receiving immune checkpoint inhibitor (ICI) therapy is an apparent acceleration in tumor growth, a phenomenon that has been named hyperprogressive disease (HPD).3 A number of single-arm retrospective studies have reported HPD in a subset of patients treated with ICIs1 4–12; however, no prospective studies of HPD have yet been performed, and it remains unclear whether reports of HPD represent the natural course of disease in some patients, or accelerated tumor growth induced by ICI treatment by some unknown mechanism.13
The term ‘hyperprogression’ was first defined by Champiat et al as (1) disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) at the first evaluation and (2) a twofold or greater increase in tumor growth rate (TGR) between the reference (before treatment) and experimental (on-treatment) periods.6 By assessing changes in tumor growth in each patient over time, this approach accounts for between-patient differences in disease course and has been used to evaluate the activity of numerous antineoplastic drugs in preclinical and clinical trials.6 Studies in patients with a range of malignancies, including advanced gastric cancer (GC), non-small cell lung cancer (NSCLC), and squamous cell carcinoma of the head and neck (SCCHN), have found that HPD in patients receiving ICIs was associated with poor overall survival (OS).1 4–12
Importantly, definitions of HPD and assessment methodologies have varied across studies,1 8 9 12 14 15 and rates of HPD in patients receiving programmed death-1/programmed death ligand 1 (PD-1/PD-L1) inhibitors have been reported to range from 9% to 37%, depending on the definition used.1 4 6 9 10 13 16 Various studies have also identified potential associations between HPD and a range of genomic (eg, MDM2 and EGFR mutations) and immune-cell (eg, PD-1–positive regulatory T cells) biomarkers, but there is no clear consensus as to their predictive values.17–24
The main limitation of previous studies of HPD is that they are based on retrospective analyses of small, nonrandomized, single-arm clinical trials and observational studies.1 6 9 10 13 16 The data from such studies do not allow an assessment of whether the HPD phenomenon is caused by ICI treatment, or whether it reflects variability in disease progression, which can be masked with alternative treatments such as cytotoxic chemotherapy. Thus, there is a need to include a control arm to determine whether HPD may be a manifestation of the natural course of disease progression in the absence of effective treatment. Furthermore, definitions of HPD used in previous studies may only be applied to those patients with multiple pretreatment tumor scans. To allow consistent assessments of HPD between treatment arms and across studies where pretreatment data are not available, there is a need for a standardized definition of HPD.
We performed post hoc analyses of the ATTRACTION-2 and CheckMate 451 studies to investigate the incidence of HPD in patients with advanced GC, gastroesophageal junction cancer (GEJC), or extensive-disease small cell lung cancer (ED SCLC) treated with nivolumab monotherapy or nivolumab plus ipilimumab versus placebo. As serial pretreatment scan data were not available for patients enrolled in ATTRACTION-2 and CheckMate 451, we used definitions of HPD based on change from baseline in target lesion sum of the longest diameters (SLD). In addition, we evaluated associations between MDM2/MDM4 and EGFR alterations and incidence of HPD based on previous reports of their hypothetical association with HPD.16 18 21
Methods
Study selection
Two nivolumab clinical trials (ATTRACTION-2 and CheckMate 451) had outcome data available for analysis of HPD and included placebo comparison groups, and were thus selected for post hoc analyses.
ATTRACTION-2
ATTRACTION-2 was a randomized, double-blind, multicenter, placebo-controlled study of nivolumab 3 mg/kg every 2 weeks (Q2W) versus placebo.25 The study included Japanese, Korean, and Taiwanese patients with unresectable advanced or recurrent GC or GEJC who had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and had disease progression or intolerance to two or more prior regimens.25
The primary endpoint of ATTRACTION-2 was OS.25 Tumor scans were scheduled to take place at baseline (within 1 week prior to the first dose of study treatment), every 6 weeks (Q6W) for 10 cycles, then every 12 weeks (Q12W) until disease progression.
CheckMate 451
CheckMate 451 was a randomized, double-blind, placebo-controlled study comparing nivolumab 240 mg Q2W, nivolumab 1 mg/kg every 3 weeks (Q3W) plus ipilimumab 3 mg/kg Q3W for four doses followed by nivolumab 240 mg Q2W, and placebo.26 The study included patients with ED SCLC and no symptomatic central nervous system metastases, ECOG PS of 0 or 1, and an ongoing complete response, partial response, or stable disease after four cycles of platinum-based, first-line chemotherapy.26
The primary endpoint of the study was OS for nivolumab plus ipilimumab versus placebo; secondary endpoints were OS for nivolumab versus placebo and progression-free survival for nivolumab plus ipilimumab and nivolumab monotherapy versus placebo.26 Tumor scans were scheduled to take place at baseline (within 3 weeks prior to the first dose of study treatment), Q6W (±5 days) for the first 36 weeks, then Q12W until disease progression.26
HPD analyses
Patients who had received at least one dose of study therapy and had tumor assessments at baseline and the first on-treatment evaluation, assessed by blinded independent central review, were included in the ATTRACTION-2 and CheckMate 451 HPD analysis populations.
Tumor progression was assessed using the RECIST V.1.1 definition of progressive disease, which comprises a ≥20% increase in SLD and a ≥5 mm absolute increase in SLD from nadir, unequivocal progression in nontarget lesions, and/or appearance of new lesions.27 Three definitions of HPD were investigated in the ATTRACTION-2 and CheckMate 451 HPD analysis populations: a ≥20% increase from baseline in target lesion SLD at first post-treatment tumor scan, as well as ≥50% and ≥100% increases in SLD. The lowest cut-off of ≥20% was chosen to align with the RECIST definition of progressive disease based on the hypothesis that patients with potential HPD would experience a tumor size increase of at least this magnitude at the first post-treatment scan. Sensitivity analyses using these definitions were also performed in the subpopulation of patients with non-response (inclusive of progressive disease or stable disease) at the first on-treatment assessment, based on the hypothesis that tumors exhibiting possible HPD behavior would comprise a higher proportion of the non-responder population than the overall population.
Genomic analyses were performed in a subgroup of patients randomized in ATTRACTION-2 and CheckMate 451 who had tumor tissue available for analysis. The relationship between MDM2/MDM4 amplifications and HPD was evaluated in both the ATTRACTION-2 and CheckMate 451 populations, based on their possible association with HPD in patients receiving ICIs in the published literature.16 18 21 The relationship between EGFR alterations and HPD was also evaluated in the ATTRACTION-2 population, based on potential associations identified in the literature.16 21 Patients whose tumors had EGFR mutations were excluded from CheckMate 451, precluding investigation of a potential association between EGFR alterations and HPD in this population. Genomic alterations were detected by next-generation sequencing using the Foundation Medicine FoundationOne CDx panel (Cambridge, Massachusetts, USA) according to a previously published methodology.16
Results
ATTRACTION-2
A total of 493 patients were randomized to receive nivolumab 3 mg/kg (n=330) or placebo (n=163), of whom 358 (243 treated with nivolumab and 115 placebo recipients) had both baseline and the first post-treatment tumor assessment and were thus included in the HPD analysis population. Reasons for patient exclusion from the HPD analysis population were balanced across both treatment groups, with the most common reason being missing data for target lesion size at baseline (71% and 67% of excluded patients in the nivolumab and placebo groups, respectively). Baseline patient demographics and clinical characteristics were similar between treatment groups (table 1). Previous treatment, previous gastrectomy, and sites of metastasis were similar between treatment groups in the intention-to-treat population and have been described previously.25 The demographics and clinical characteristics of patients who were excluded from the HPD analysis were similar to the included population, with the exception of a higher proportion of female patients (online supplemental table S1). Distribution of the time to first post-treatment scan was similar in the nivolumab and placebo groups (online supplemental figure S1).
Table 1.
Patient demographics and baseline characteristics for the ATTRACTION-2 HPD analysis population
| Characteristic | Placebo (n=115) | Nivolumab 3 mg/kg (n=243) |
| Age, years, median (range) | 61 (26–83) | 63 (20–83) |
| Female, n (%) | 28 (24.4) | 60 (24.7) |
| Race, n (%) | ||
| Asian | 115 (100.0) | 242 (99.6) |
| Native Hawaiian/other Pacific Islander | 0 | 1 (0.4) |
| ECOG PS, n (%) | ||
| 0 | 28 (24.4) | 64 (26.3) |
| 1 | 71 (61.7) | 161 (66.3) |
| Baseline tumor size, cm | ||
| Mean (SD) | 7.9 (5.1) | 8.2 (5.8) |
| Median (range) | 6.6 (1.3–25.3) | 6.7 (1–31.3) |
ECOG PS, Eastern Cooperative Oncology Group performance status; HPD, hyperprogressive disease.
jitc-2021-004273supp001.pdf (426.5KB, pdf)
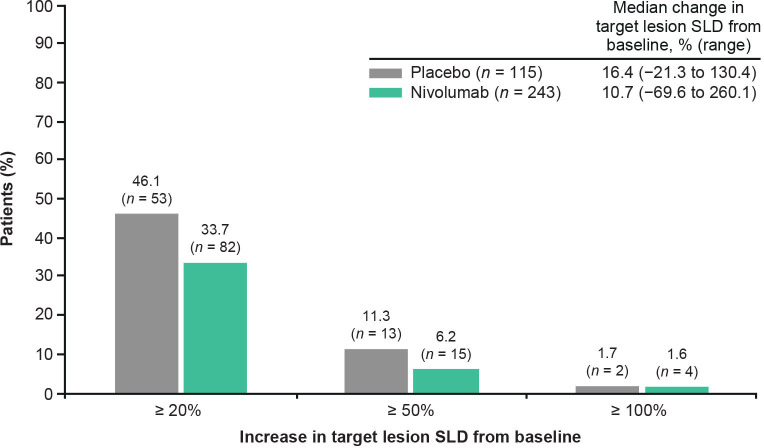
The proportions of patients with ≥50% and ≥20% increases in SLD at the first assessment were lower in the nivolumab group than the placebo group. The proportions of patients with ≥100% increases in SLD were similar in the nivolumab and placebo groups, although only two patients in the placebo group and four patients in the nivolumab group had tumor growth of this magnitude (figure 1). Findings in the non-responder sensitivity analysis were similar to those in the overall HPD analysis population (online supplemental figure S2). Reduced proportions of patients with ≥20%, ≥50%, and ≥100% increases in target lesion SLD were observed in the nivolumab group relative to the placebo group regardless of timing of first on-treatment scan (online supplemental figure S3). Median percentage increase in SLD from baseline was 10.7% (range, −69.6% to 260.1%) in the nivolumab group vs 16.4% (range, −21.3% to 130.4%) in the placebo group (figure 1). Three patients had a percentage increase in SLD ≥200%, all of whom were in the nivolumab group. Of note, all three patients had ECOG PS 1. Two of the patients had relatively small stage IIIC tumors, with both having a baseline target lesion SLD of 2.2 cm and metastasis to one organ. The remaining patient had stage IV disease with a baseline target lesion SLD of 5.4 cm and metastasis to three organs. Demographic and clinical characteristics for these patients are shown in online supplemental table S2.
Figure 1.
Proportions of patients with ≥20%, ≥50%, and ≥100% increases from baseline in target lesion SLD at the first on-treatment scan in ATTRACTION-2. SLD, sum of the longest diameters.
MDM2 copy number alterations (CNAs) were identified in four nivolumab-treated patients. TGR at the first scan in these four patients ranged from −16.2% to 58.1%, and OS follow-up was ongoing for two patients at the time of database lock (online supplemental table S3). EGFR alterations were identified in six nivolumab-treated patients. TGR at the first scan in these patients ranged from –31% to 92%; OS follow-up was ongoing for one patient at the time of database lock (online supplemental table S4). No samples for genomic analysis were collected from the three nivolumab-treated patients with increases in target lesion SLD ≥200%.
CheckMate 451
A total of 834 patients were randomized to receive nivolumab (n=280), nivolumab plus ipilimumab (n=279), or placebo (n=275) in CheckMate 451. In total, 531 patients had baseline and first post-treatment tumor assessments and were included in the HPD analysis population (177 randomized to nivolumab monotherapy, 179 to nivolumab plus ipilimumab, and 175 to placebo). Reasons for patient exclusion from the HPD analysis population were balanced between treatment groups, with the most common reason being missing data for target lesion size at baseline (78%–81% of excluded patients across treatment arms). Patient demographics and baseline characteristics appeared to be similar across all three treatment groups, with the exception of a higher proportion of patients with ECOG PS 1 in the placebo group compared with the nivolumab and nivolumab plus ipilimumab groups (65.1% vs 56.4%–56.5%; table 2). The demographics and clinical characteristics of patients who were excluded from the HPD analysis were similar to the included population (online supplemental table S5). As expected, time to first post-treatment scan was similarly distributed in all three treatment groups (online supplemental figure S4).
Table 2.
Patient demographics and baseline characteristics for the CheckMate 451 HPD analysis population
| Characteristic | Placebo (n=175) | Nivolumab 240 mg (n=177) | Nivolumab 1 mg/kg+ipilimumab 3 mg/kg (n=179) |
| Age, years, median (range) | 64 (44–81) | 65 (34–84) | 64 (39–85) |
| Female, n (%) | 60 (34.3) | 62 (35.0) | 60 (33.5) |
| Race, n (%) | |||
| White | 127 (72.6) | 138 (78.0) | 141 (78.8) |
| Black or African American | 1 (0.6) | 2 (1.1) | 1 (0.6) |
| Asian | 42 (24.0) | 35 (19.8) | 34 (19.0) |
| Other | 5 (2.9) | 2 (1.1) | 3 (1.7) |
| ECOG PS, n (%) | |||
| 0 | 61 (34.9) | 77 (43.5) | 78 (43.6) |
| 1 | 114 (65.1) | 100 (56.5) | 101 (56.4) |
| Region, n (%) | |||
| US/Canada | 35 (20.0) | 38 (21.5) | 38 (21.2) |
| Asia | 41 (23.4) | 34 (19.2) | 33 (18.4) |
| Europe | 68 (38.9) | 81 (45.8) | 76 (42.5) |
| Rest of world | 31 (17.7) | 24 (13.6) | 32 (17.9) |
| Type of first-line, platinum-based therapy, n (%)* | |||
| Carboplatin | 103 (58.9) | 118 (66.7) | 107 (59.8) |
| Cisplatin | 78 (44.6) | 62 (35.0) | 82 (45.8) |
| Baseline tumor size, cm | |||
| Mean (SD) | 5.7 (4.2) | 5.2 (3.7) | 5.1 (4.3) |
| Median (range) | 4.4 (1.2–21.9) | 4.0 (1.0–18.5) | 3.6 (1.0–28.6) |
*Patients may have received more than one type of platinum compound.
ECOG PS, Eastern Cooperative Oncology Group performance status; HPD, hyperprogressive disease.
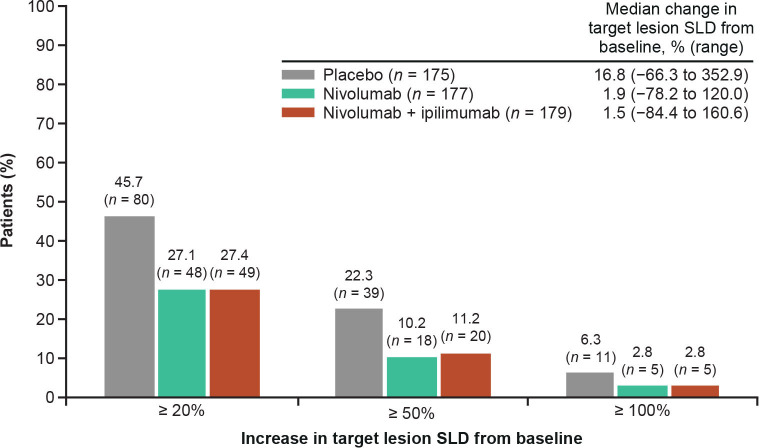
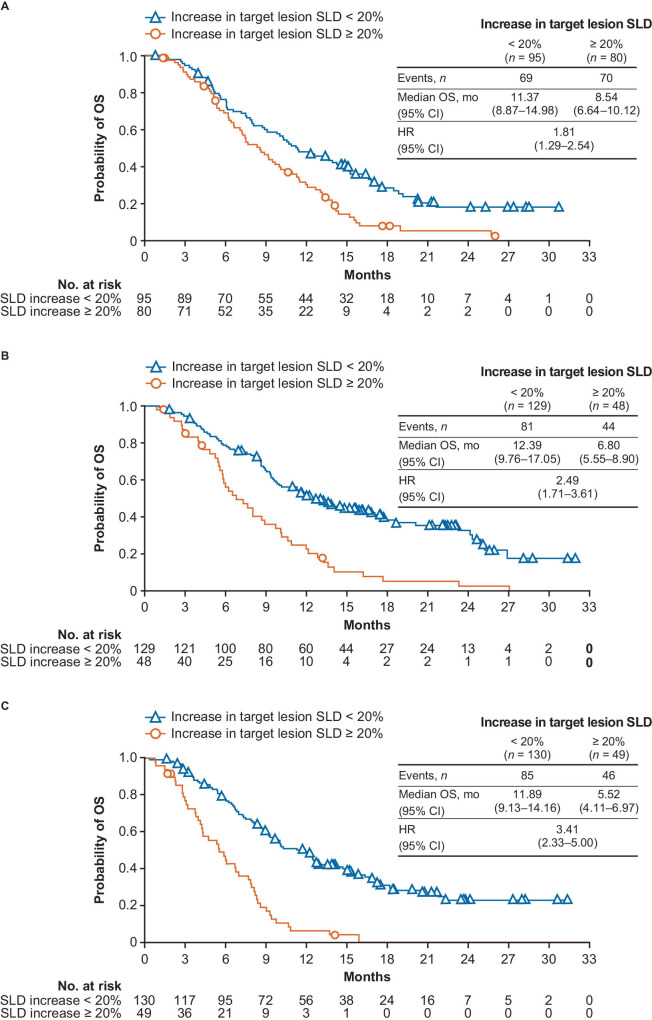
The proportions of patients with ≥20%, ≥50%, and ≥100% increases in SLD at the first assessment were lower in the nivolumab and nivolumab plus ipilimumab groups than the placebo group (figure 2). Findings in the non-responder sensitivity analysis were similar to those in the overall HPD analysis population (online supplemental figure S5). Timing of first scan relative to the start of treatment did not appear to affect the observed differences in the proportions of patients with ≥20%, ≥50%, or ≥100% increases in target lesion SLD across the nivolumab, nivolumab plus ipilimumab, and placebo groups (online supplemental figure S6). The median increase in target lesion SLD from baseline was 1.9% (range, −78.2% to 120.0%) in the nivolumab monotherapy group, 1.5% (range, −84.4% to 160.6%) in the nivolumab plus ipilimumab group, and 16.8% (range, −66.3% to 352.9%) in the placebo group (figure 2). Analysis of OS in patient subgroups with <20% and ≥20% increases in SLD found that a ≥20% increase in SLD was associated with worse prognosis in all three treatment groups (figure 3). Evaluation of OS in patients excluded from the HPD analysis showed that the placebo recipients excluded from the analysis had a poor prognosis, with a median OS of 9.43 months (95% CI 6.21 to 12.22), comparable to the median OS in placebo recipients with ≥20% increase in SLD in the HPD analysis population. In contrast, median OS was 10.55 months (95% CI 9.43 to 15.11) in excluded patients who received nivolumab monotherapy and 10.55 (95% CI 7.62 to 15.51) in excluded patients who received nivolumab plus ipilimumab, markedly longer than the corresponding populations with ≥20% increase in SLD in the HPD analysis.
Figure 2.
Proportions of patients with ≥20%, ≥50%, and ≥100% increases from baseline in target lesion SLD at the first on-treatment scan in CheckMate 451. SLD, sum of the longest diameters.
Figure 3.
OS in patients randomized to (A) placebo, (B) nivolumab 240 mg Q2W, and (C) nivolumab 1 mg/kg plus ipilimumab 3 mg/kg Q2W with increases in target lesion SLD of <20% or ≥20% in CheckMate 451. OS, overall survival; Q2W, every 2 weeks; SLD, sum of the longest diameters.
Of the 538 nivolumab or nivolumab plus ipilimumab–treated patients for whom genomic data were available, 23 had short variants of MDM2/MDM4 and one had an MDM4 CNA. No further analysis was performed due to the small number of patients with MDM2/MDM4 variants.
Discussion
HPD is a relatively new phenomenon first reported in patients receiving ICI therapies. Evaluation of the relationship between ICIs and HPD remains difficult, as patients with advanced cancers generally receive cytotoxic chemotherapy or targeted therapies as standard of care, potentially masking changes in tumor growth over the course of the disease and preventing evaluation of off-treatment biological growth. To our knowledge, this is the first analysis of HPD in patients treated with ICIs to use data from randomized, placebo-controlled clinical trials, thereby allowing the effects of treatment to be compared with the natural course of tumor progression in the absence of treatment. On evaluating the incidence of HPD based on increase from baseline in target lesion SLD at multiple cutoffs, as opposed to using a single measure that may not capture all manifestations of increased aggressiveness of tumor growth, we did not find evidence of HPD in patients receiving nivolumab or nivolumab plus ipilimumab in the overall HPD analysis population or in the non-responder population.
Much of the information on HPD published to date has been drawn from retrospective analyses of clinical data mostly from single institutions.1 6 12 14 28 In particular, definitions of HPD used in previous studies rely on the availability of multiple pretreatment scans to evaluate TGR during the pretreatment period, precluding their use in patients for whom suitable pretreatment data are not available. In addition, the lack of a placebo control group suggests that such longitudinal assessments may not account for potential tumor growth acceleration as part of the natural course of disease.1 6 Published studies have reported variable rates of HPD across tumor types, with relatively low rates in melanoma (1%–9%) and higher rates in SCCHN (14%–29%).6 10 11 29 A wide range of HPD rates (5%–37%) have been reported for NSCLC, which could be related to differences in HPD definitions and other methodological aspects across studies.1 9 13 16 30 31
Five definitions of HPD were compared in a retrospective analysis of 406 patients with NSCLC by Kas et al.30 The study found incidences of HPD ranging from 5.4% to 18.5%, depending on the definition used, with only 19 patients classified as having HPD by all five definitions.30 Of note, although HPD rates varied considerably between definitions in the analysis population, HPD rates with four of the five definitions were consistent with previous studies in which those definitions were used.1 6 16 21 30 Similar findings were reported in a study by Abbar et al which found a wide range of HPD incidence across five definitions (5.7%–31.7%) in a population of 169 patients with advanced NSCLC, with variable agreement between definitions (Cohen’s kappa 29%–77%) and only a small proportion of patients meeting all definitions for HPD.32 These findings highlight the need for a standardized approach to evaluation of HPD to enable interstudy comparisons and provide a more reliable indication of the extent of the HPD phenomenon.33 In our analyses, the proportion of nivolumab-treated patients with ≥20% and ≥50% percentage increases in SLD appeared consistent with rates of HPD reported in the literature, although rates at the most inclusive cut-off (≥20% increase in SLD) were aligned with the upper range of previously reported incidence rates. However, additional analyses are needed to investigate the utility of a 20% increase in SLD at the first on-treatment assessment as a definition of HPD.
By evaluating multiple cutoffs of change in SLD, our analyses allowed for the investigation of different rates of tumor growth. Similar rates of increase in target lesion SLD ≥20%, ≥50%, and ≥100% at the first on-treatment assessment were reported with nivolumab and nivolumab plus ipilimumab versus placebo across both studies, despite the differences in tumor types and treatment settings. The trend for similar or lower rates of ≥50% and ≥100% increase in SLD with nivolumab and nivolumab plus ipilimumab versus placebo did not support the hypothesis that nivolumab treatment led to accelerated tumor growth in a subset of patients and suggests that the acceleration in tumor growth seen in previous retrospective studies may reflect the natural course of disease progression in the absence of effective treatment in some patients. Moreover, there was no apparent difference in the rates of SLD increase between nivolumab monotherapy and nivolumab plus ipilimumab in the CheckMate 451 population. Although three nivolumab-treated patients, but no placebo patients, in ATTRACTION-2 had percentage increases in SLD ≥200% at the first on-treatment scan, this result should be interpreted with caution due to the 2:1 randomization ratio for nivolumab:placebo and the limited information available to further investigate the characteristics of these tumors. Although the possibility of treatment-related hyperprogression occurring in a small minority of patients cannot be completely excluded, such a phenomenon is not consistent with current understanding of the mechanisms of action of ICIs. Data from CheckMate 451 also showed that tumor growth ≥20% at the first post-treatment scan was strongly prognostic for reduced OS across all three treatment groups, a finding that is consistent with other studies in which the effects of HPD on survival have been evaluated.1 4–10
The demographics and characteristics of patients excluded from the HPD analysis were generally comparable to the HPD-evaluable population. Most exclusions were due to missing data for target lesion SLD at baseline rather than missing data for on-treatment target lesion SLD, and the reasons for exclusion were balanced across treatment arms in both studies. Furthermore, investigation of OS in the patients excluded from the CheckMate 451 HPD analysis population showed that excluded placebo recipients had a prognosis comparable to that of placebo recipients with ≥20% increase in SLD in the HPD-evaluable population. In contrast, excluded patients who received nivolumab monotherapy or nivolumab plus ipilimumab had a prognosis more similar to that of HPD-evaluable patients receiving nivolumab monotherapy or nivolumab plus ipilimumab who had <20% increase in SLD. Together, these data give no cause to suggest that patients with possible ICI-related HPD were excluded from the analysis.
Our findings of no apparent HPD in the current analysis contrast with those of a nonrandomized, retrospective, placebo-controlled study by Kim et al which evaluated HPD in patients with hepatocellular carcinoma who received nivolumab, regorafenib, or placebo/best supportive care (BSC).4 HPD was assessed using three definitions based on change in tumor growth dynamics between the pretreatment and post-treatment period. Importantly, the study found that criteria for all three definitions of HPD were met in 12.7% of patients who received nivolumab, while no patients who received regorafenib or placebo/BSC had changes in tumor growth dynamics meeting criteria for HPD under any of the definitions.4 Evaluation of change in target lesion SLD, using RECIST V.1.1, found that nivolumab-treated patients who met all three definitions of HPD also displayed more rapid tumor progression than patients without HPD.4
Previous studies have identified a variety of patient and tumor characteristics that may be associated with HPD, including tumor genomic alterations.1 6 7 10 16 19 In particular, potential associations of MDM2 CNAs and EGFR alterations with HPD have been identified in some studies.16 18 21 Patients whose tumors had EGFR mutations were excluded from CheckMate 451, so the association of EGFR alterations with HPD was examined in the ATTRACTION-2 analysis only.24 Although the mechanisms through which MDM2 CNAs could promote tumor growth are yet to be confirmed, it has been proposed that signaling pathways activated during ICI treatment increase expression of MDM2, which in turn inhibits the tumor suppressor p53. In tumors with MDM2 amplification, overexpression of MDM2 could contribute to HPD following ICI treatment by increasing p53 suppression, resulting in increased tumor cell proliferation.34 35 Studies investigating the association between MDM2/MDM4 mutations and HPD have had conflicting results.16 19 21 No apparent relationship between MDM2/MDM4 amplification or EGFR alterations and HPD were identified in our analysis of the ATTRACTION-2 and CheckMate 451 populations; however, the number of tumors harboring MDM2/MDM4 amplification and EGFR alterations in our analysis was small, limiting the generalizability of this finding. Further studies are needed to characterize the relationship between MDM2/MDM4 amplification or EGFR alterations and HPD, as well as to identify additional genomic alterations associated with HPD in patients receiving ICIs.
No clear mechanisms by which PD-1/PD-L1 inhibitors could induce HPD in solid tumors have been identified, although a number of potential mechanisms have been suggested. In addition to the hypothesized effects of MDM2 amplification and EGFR alterations discussed above, another proposed mechanism for HPD is the presence of tumor-associated macrophages with a protumor, immunosuppressive phenotype.19 Such macrophages could be triggered by interaction with the Fc region of anti-PD-1 antibodies, promoting tumor growth. At present, this finding is based on data from patient-derived xenograft models and has not yet been directly assessed in humans.19 Another possibility is that tumors classified as hyperprogressive may represent intrinsic or acquired resistance to anti–PD-1/PD-L1 therapies through a variety of mechanisms, such as an immunosuppressive tumor microenvironment, or clonal evolution of tumor immunophenotypes with low immunogenicity.36–38 Further studies are needed to investigate the various mechanisms of resistance that have been proposed and to identify markers to predict tumor response to ICI treatment. However, it should be noted that HPD has been reported in nivolumab-treated patients with adult T-cell lymphoma.39 40 Expression of PD-1 on T-cell lymphoma cells, where it is thought to act as a suppressor of tumor growth,41 is a plausible mechanism linking anti–PD-1 therapy to HPD that appears unique to this tumor type and is not generalizable to solid tumors.
Limitations of these analyses include their post hoc and descriptive design. Additionally, patients whose tumor size could not be measured at baseline and patients with clinical disease progression who did not have their first tumor assessment were excluded from the HPD analyses. As tumor size data were not available for the period before study enrollment, evaluation of changes in TGR before and after ICI treatment was not possible. Extrapolation of data from the CheckMate 451 and ATTRACTION-2 studies to other treatment settings should be done with caution due to the inherent nature of each tumor type, the stage of disease, and previous treatment. Specifically, patients enrolled in CheckMate 451 had extensive-stage disease and disease control with first-line chemotherapy at study entry. In contrast, patients enrolled in ATTRACTION-2 had advanced disease previously treated with at least three lines of chemotherapy. Given these limitations, the analyses presented here should be considered hypothesis-generating only, and prospective, randomized, placebo-controlled studies are needed to draw definitive conclusions on whether there is a subset of patients who experience HPD directly caused by ICI treatment.
This analysis does, however, have important strengths, including the use of placebo comparators to control for the natural history of tumor growth in the absence of effective treatment, as well as the evaluation of a broader, more inclusive definition of HPD using multiple cutoffs of percentage increase in target lesion SLD to analyze a greater range of TGRs. This definition is consistent with that used in an analysis performed by the FDA.42 Finally, the consistent proportions of patients with rapid tumor progression observed in placebo and active treatment groups across both studies, despite the differences in tumor type (GC/GEJC vs ED SCLC) and treatment setting (third- or later-line treatment vs first-line maintenance therapy), support the conclusion that cases classified as HPD represent the natural history of some tumors. The rapid tumor progression in a minority of patients could be attributed to tumor biology, given that rapid tumor growth appeared to occur more frequently in patients receiving placebo than nivolumab or nivolumab plus ipilimumab in our analysis, and shows a consistently similar or lower rate with nivolumab-containing therapy versus placebo across the cutoffs evaluated.
Conclusions
Treatment with nivolumab, alone or in combination with ipilimumab, was not associated with an increased incidence of disease progression at the first on-treatment assessment versus placebo in patients with GC/GEJC or ED SCLC. Our analyses, using a placebo control group and a broader measure of HPD based on percentage increase in SLD at multiple cutoffs, suggest that previous reports of rapid tumor growth with ICI treatment may mostly reflect the natural course of disease.
Acknowledgments
The authors thank the patients, their families, and all investigators involved in the ATTRACTION-2 and CheckMate 451 studies. Professional medical writing and editorial assistance were provided by Bernard Kerr, PGDipSci, and Jay Rathi, MA, of Spark Medica Inc, funded by Bristol Myers Squibb, according to Good Publication Practice 3 guidelines.
Footnotes
Twitter: @MartinReck2
Contributors: Y-KK and MR contributed equally to this paper and are co-lead authors. MR, HRK, and TKO contributed to the development of the CheckMate 451 protocol and data analysis and interpretation and have been active investigators. Y-KK, NB, and L-TC contributed to the development of the ATTRACTION-2 protocol and data analysis and interpretation and have been active investigators. ML and HC contributed to data analysis. JS, YF, WHL, GP, PN, AR, and AB contributed to the study design, data analysis, and data interpretation. All authors contributed to drafting and critical review of the manuscript. JS is the guarantor of the manuscript and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. YF and WHL were employed by Bristol Myers Squibb at the time of the analysis. NB was employed by NCCH at the time of the analysis.
Funding: This work was supported by Bristol Myers Squibb. ATTRACTION-2 and CheckMate 451 were funded by Bristol Myers Squibb. PN was supported by the National Institutes of Health (P01-CA225517). The sponsor was involved in the study design, collection, analysis, and interpretation of data and information provided in the manuscript. However, the ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Competing interests: Y-KK received honoraria for consultancy from ALX Oncology, Amgen, Bristol Myers Squibb, DAE HWA, MacroGenics, Novartis, Surface Oncology, and Zymeworks. MR received honoraria for lectures and consultancy from Amgen, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Lilly, Merck, Merck Sharp & Dohme, Mirati, Novartis, Pfizer, Roche, and Samsung. PN served as a paid consultant to EMD Serono and Pfizer; his institution received research support from Bristol Myers Squibb and EMD Serono. YF was employed by Bristol Myers Squibb at the time of manuscript preparation and owns Bristol Myers Squibb stock. HRK declares no conflicts of interest. TKO served as a paid consultant to Bristol Myers Squibb, Merck, Amgen, AstraZeneca, Roche/Genentech, Novartis, BeiGene, Lilly, and Oncocyte. His institution received grants from Bristol Myers Squibb, Merck, Amgen, AstraZeneca, Roche/Genentech, and Novartis. NB received honoraria for lectures from Ono and Taiho and received research funding from Ono and Takeda. L-TC is employed by Kaohsiung Medical University Hospital, Kaohsiung Medical University, and the National Institute of Cancer Research, National Health Research Institutes in Taiwan, received honoraria to their institution from PharmaEngine, Taivex, and OBI, received research grants to their institution from SynCore Biotechnology, TTY, Polaris, ACT Genomics, Pfizer, Bristol Myers Squibb, Novartis, and Merck Serono, served as a paid consultant to ONO, Bristol Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Ipsen, TTY, SynCore Biotechnology, Novartis, AstraZeneca, and Stone, reports royalties by HuniLife, and is part of the board of directors of ScinoPharm Taiwan, Ltd. AB, HC, ML, WHL, GP, AR, and JS are employees of Bristol Myers Squibb and own Bristol Myers Squibb stock.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Bristol Myers Squibb’s policy on data sharing can be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
An institutional review board or independent ethics committee at each site approved all versions of the protocols for ATTRACTION-2 and CheckMate 451. An independent data monitoring committee provided safety and efficacy oversight for each study. The trials were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent. A separate written consent was required in ATTRACTION-2 for the collection of tumor tissue.
References
- 1.Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 2018;4:1543–52. 10.1001/jamaoncol.2018.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borcoman E, Nandikolla A, Long G, et al. Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educ Book 2018;38:169–78. 10.1200/EDBK_200643 [DOI] [PubMed] [Google Scholar]
- 3.Ferrara R, Pilotto S, Caccese M, et al. Do immune checkpoint inhibitors need new studies methodology? J Thorac Dis 2018;10:S1564–80. 10.21037/jtd.2018.01.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CG, Kim C, Yoon SE, et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol 2021;74:350–9. 10.1016/j.jhep.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 5.Karabajakian A, Garrivier T, Crozes C, et al. Hyperprogression and impact of tumor growth kinetics after PD1/PDL1 inhibition in head and neck squamous cell carcinoma. Oncotarget 2020;11:1618–28. 10.18632/oncotarget.27563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920–8. 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 7.Kanjanapan Y, Day D, Wang L, et al. Hyperprogressive disease in early-phase immunotherapy trials: clinical predictors and association with immune-related toxicities. Cancer 2019;125:1341–9. 10.1002/cncr.31999 [DOI] [PubMed] [Google Scholar]
- 8.Kim Y, Kim CH, Lee HY, et al. Comprehensive clinical and genetic characterization of hyperprogression based on volumetry in advanced non-small cell lung cancer treated with immune checkpoint inhibitor. J Thorac Oncol 2019;14:1608–18. 10.1016/j.jtho.2019.05.033 [DOI] [PubMed] [Google Scholar]
- 9.Kim CG, Kim KH, Pyo K-H, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 2019;30:1104–13. 10.1093/annonc/mdz123 [DOI] [PubMed] [Google Scholar]
- 10.Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605–11. 10.1093/annonc/mdx178 [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Chun SH, Lee Y-G, et al. Hyperprogressive disease and its clinical impact in patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with immune-checkpoint inhibitors: Korean cancer Study Group HN 18-12. J Cancer Res Clin Oncol 2020;146:3359–69. 10.1007/s00432-020-03316-5 [DOI] [PubMed] [Google Scholar]
- 12.Aoki M, Shoji H, Nagashima K, et al. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open 2019;4:e000488. 10.1136/esmoopen-2019-000488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018;15:748–62. 10.1038/s41571-018-0111-2 [DOI] [PubMed] [Google Scholar]
- 14.Al-ezzi EM, Alshammari K, Kanjanapan Y, et al. Impact of immunotherapy and targeted therapy on tumour growth rate in sarcoma. Ann Oncol 2019;30:v700–1. 10.1093/annonc/mdz283.043 [DOI] [Google Scholar]
- 15.Sasaki A, Nakamura Y, Mishima S, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer 2019;22:793–802. 10.1007/s10120-018-00922-8 [DOI] [PubMed] [Google Scholar]
- 16.Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017;23:4242–50. 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamada T, Togashi Y, Tay C, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A 2019;116:9999–10008. 10.1073/pnas.1822001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato S, Kurzrock R. Genomics of immunotherapy-associated hyperprogressors-response. Clin Cancer Res 2017;23:6376. 10.1158/1078-0432.CCR-17-1990 [DOI] [PubMed] [Google Scholar]
- 19.Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res 2019;25:989–99. 10.1158/1078-0432.CCR-18-1390 [DOI] [PubMed] [Google Scholar]
- 20.Tachihara M, Nishimura Y. Who will suffer from hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. J Thorac Dis 2019;11:S1289–91. 10.21037/jtd.2019.04.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singavi AK, Menon S, Kilari D, et al. Predictive biomarkers for hyper-progression (HP) in response to immune checkpoint inhibitors (ICI) – analysis of somatic alterations (SAs). Ann Oncol 2017;28:v405. 10.1093/annonc/mdx376.006 [DOI] [Google Scholar]
- 22.Chen Y, Hu J, Bu F, et al. Clinical characteristics of hyperprogressive disease in NSCLC after treatment with immune checkpoint inhibitor: a systematic review and meta-analysis. BMC Cancer 2020;20:707. 10.1186/s12885-020-07206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi YJ, Kim T, Kim EY, et al. Prediction model for hyperprogressive disease in non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer 2020;11:2793–803. 10.1111/1759-7714.13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Wu Q, Wu S, et al. Investigation on potential biomarkers of hyperprogressive disease (HPD) triggered by immune checkpoint inhibitors (ICIs). Clin Transl Oncol 2021;23:1782–93. 10.1007/s12094-021-02579-9 [DOI] [PubMed] [Google Scholar]
- 25.Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–71. 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 26.Owonikoko TK, Park K, Govindan R, et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J Clin Oncol 2021;39:1349–59. 10.1200/JCO.20.02212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 28.Refae S, Gal J, Brest P, et al. Hyperprogression under immune checkpoint inhibitor: a potential role for germinal immunogenetics. Sci Rep 2020;10:3565. 10.1038/s41598-020-60437-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuiveling M, Tonk EHJ, Verheijden RJ, et al. Hyperprogressive disease rarely occurs during checkpoint inhibitor treatment for advanced melanoma. Cancer Immunol Immunother 2021;70:1491–6. 10.1007/s00262-020-02716-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kas B, Talbot H, Ferrara R, et al. Clarification of definitions of hyperprogressive disease during immunotherapy for non-small cell lung cancer. JAMA Oncol 2020;6:1039–46. 10.1001/jamaoncol.2020.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuo N, Azuma K, Kojima T, et al. Comparative incidence of immune-related adverse events and hyperprogressive disease in patients with non-small cell lung cancer receiving immune checkpoint inhibitors with and without chemotherapy. Invest New Drugs 2021;39:1150–8. 10.1007/s10637-021-01069-7 [DOI] [PubMed] [Google Scholar]
- 32.Abbar B, De Castelbajac V, Gougis P, et al. Definitions, outcomes, and management of hyperprogression in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Lung Cancer 2021;152:109–18. 10.1016/j.lungcan.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 33.Frelaut M, Le Tourneau C, Borcoman E. Hyperprogression under immunotherapy. Int J Mol Sci 2019;20:2674. 10.3390/ijms20112674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Z, Peng Z, Gong J, et al. Hyperprogression after immunotherapy in patients with malignant tumors of digestive system. BMC Cancer 2019;19:705. 10.1186/s12885-019-5921-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camelliti S, Le Noci V, Bianchi F, et al. Mechanisms of hyperprogressive disease after immune checkpoint inhibitor therapy: what we (don't) know. J Exp Clin Cancer Res 2020;39:236. 10.1186/s13046-020-01721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong D, Wang Y, Singavi AK, et al. Immunogenomic landscape contributes to hyperprogressive disease after anti-PD-1 immunotherapy for cancer. iScience 2018;9:258–77. 10.1016/j.isci.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zang H, Peng J, Zheng H, et al. Hyperprogression after immune-checkpoint inhibitor treatment: characteristics and hypotheses. Front Oncol 2020;10:515. 10.3389/fonc.2020.00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adashek JJ, Subbiah IM, Matos I, et al. Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer 2020;6:181–91. 10.1016/j.trecan.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennani NN, Pederson LD, Atherton P, et al. A phase II study of nivolumab in patients with relapsed or refractory peripheral T-cell lymphoma. Blood 2019;134:467. 10.1182/blood-2019-126194 [DOI] [Google Scholar]
- 40.Rauch DA, Conlon KC, Janakiram M, et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood 2019;134:1406–14. 10.1182/blood.2019002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wartewig T, Kurgyis Z, Keppler S, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 2017;552:121–5. 10.1038/nature24649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Association for Cancer Research. Understanding hyperprogression in cancer. Cancer Discov 2019;9:821.31023698 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-004273supp001.pdf (426.5KB, pdf)
Data Availability Statement
Bristol Myers Squibb’s policy on data sharing can be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.