Abstract
Background
Small single subcortical infarction (SSSI) may be classified as parent artery disease-related or only branch involved according to the stenosis of parent artery. The study aimed to evaluate short-term and long-term prognoses and the effectiveness of antiplatelet therapy in SSSI.
Methods
We prospectively enrolled 2890 patients with SSSI from the Third China National Stroke Registry (CNSR-III) database from August 2015 to March 2018. We assessed clinical outcomes and antiplatelet treatment effects in patients with SSSI with and without parent artery stenosis (PAS) identified by magnetic resonance angiography.
Results
Among 2890 patients with SSSI in the perforator territory of the middle cerebral artery and the basilar artery, there were 680 (23.53%) patients with PAS and 2210 (76.47%) patients without PAS, respectively. After adjusting for potential confounders, the PAS group had a greater initial stroke severity (OR 1.262, 95% CI 1.058 to 1.505; p=0.0097) and a higher risk of ischaemic stroke recurrence at 3 months (OR 2.266, 95% CI 1.631 to 3.149; p<0.0001) and 1 year (OR 2.054, 95% CI 1.561 to 2.702; p<0.0001), as well as composite vascular events at 3 months (OR 2.306, 95% CI 1.674 to 3.178; p<0.0001) and 1 year (OR 1.983, 95% CI 1.530 to 2.570; p<0.0001), compared with the non-PAS group. In both groups, dual antiplatelet therapy was not superior to single antiplatelet therapy in preventing stroke recurrence, composite vascular events and disability.
Conclusion
PAS related to significantly higher rates of short-term and long-term stroke recurrence and composite vascular events, suggesting heterogeneous mechanisms in SSSI subgroups. The effectiveness of antiplatelet therapy for SSSI needs further investigation.
Keywords: STROKE, MRI, CEREBROVASCULAR DISEASE
Key messages.
What is already known on this topic
Small single subcortical infarction (SSSI) in perforator territory has heterogeneous pathogenesis regarding the presence of parent artery disease.
What this study adds
In this study, parent artery stenosis (PAS) was related to significantly higher rates of short-term and long-term stroke recurrence and composite vascular events. For SSSI, dual antiplatelet therapy was not superior to single antiplatelet therapy in preventing stroke recurrence, composite vascular events and disability.
How this study might affect research, practice or policy
PAS can help predict the prognosis of SSSI and can be used as a solid standard in risk stratification. The antiplatelet therapy in second prevention for SSSI needs further investigation.
Introduction
Small single subcortical infarction (SSSI), commonly known as lacunar stroke, constitutes about 25% of ischaemic strokes.1 It is defined as a small (<20 mm transversal diameter) lesion in the territory of a penetrating arteriole,2 such as the basal ganglia, internal capsule and brainstem. Two major vascular pathologies of the brain damage have been suggested in patients with small-sized penetrating brain arteries and arterioles: (1) thickening of the arterial media and (2) obstruction of the origins of penetrating arteries by parent artery intimal plaques.3 Thus, not only small-vessel disease but also large-artery atherosclerosis could result in SSSI.
Generally, SSSI is thought to have a more favourable outcome compared with other subtypes of stroke, such as atherothrombotic stroke and cardiogenic stroke.4 Some studies on SSSI prognosis have produced inconsistent or incomplete results due to overestimating the role of infarct size rather than different arterial pathology.5 However, another study suggested no clinical and lesion-size differences between SSSI with or without parent artery stenosis (PAS); that is, there seemed to be no rationale for a specific size criterion for small-vessel infarction.6 In addition, aspirin plus clopidogrel is accepted as effective antiplatelet therapy for reducing stroke recurrence in minor stroke and high-risk transient ischaemic attack (TIA).7 8 However, in a Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) subgroup analysis, it is not significantly different from aspirin alone in preventing recurrent stroke for patients with and without intracranial artery stenosis (ICAS).9 Thus, less is known about the difference in prognosis and second prevention between the two aetiopathogeneses.
In this prespecified imaging substudy of the Third China National Stroke Registry (CNSR-III), we divided SSSI into parent artery-related and penetrating artery-related based on current imaging technology. The present study aimed to evaluate short-term and long-term prognoses and the effectiveness of dual antiplatelet therapy (DAPT) on preventing recurrent stroke in patients with SSSI of different aetiologies.
Methods
Cohort
We derived data from the CNSR-III database. The protocol for case identification and data collection has been previously reported elsewhere.10 Briefly, the CNSR-III is a nationwide clinical registry of ischaemic stroke or TIA based on aetiology, imaging and biological markers in China from August 2015 to March 2018. Consecutive patients were recruited consecutively if they were (1) aged >18 years, (2) patients with physician-diagnosed ischaemic stroke or TIA, (3) within 7 days from the onset of symptoms to enrolment and (4) patients who have provided consent to participant in the study. Patients were excluded if they had silent cerebral infarction with no symptoms or signs, or those who refused to participate in the registry.
Study population
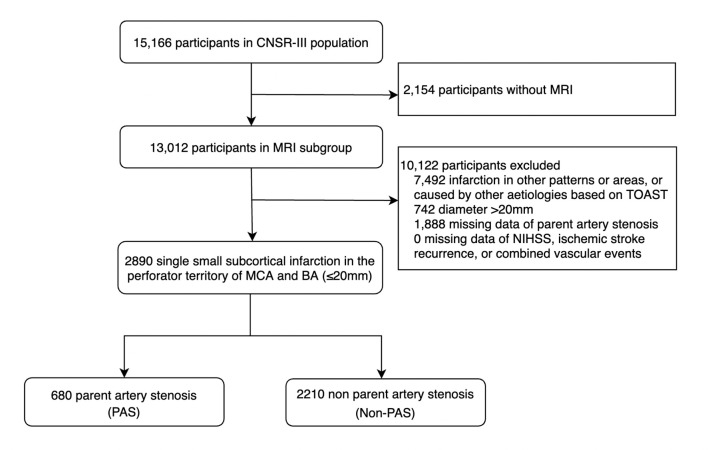
Patients were included in this study if they had a single small infarction in the perforator territory of middle cerebral artery (MCA) and basilar artery (BA) (diameter <20 mm) based on diffusion-weighted imaging (DWI) sequence in the MRI subgroup. Patients were excluded if the infarction caused by other aetiologies according to the Trial of Org 10 172 in Acute Stroke Treatment (TOAST) classification.11 We also excluded patients missing data of magnetic resonance angiography (MRA), modified Rankin Scale (mRS), ischaemic stroke recurrence and composite vascular events. After these exclusions, our primary study population consisted of 2890 patients with acute isolated infarction (shown in figure 1).
Figure 1.
Study population. BA, basilar artery; CNSR-III, Third China National Stroke Registry; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; PAS, parent artery stenosis; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Data collection and management
Patient information, including demographics, risk factors, comorbidities, medications, selected laboratory tests and hospital-level characteristics, was collected systematically during hospitalisation and at discharge by trained research coordinators at each participating hospital. National Institutes of Health Stroke Scale (NIHSS) score at admission, ischaemic stroke recurrence, composite vascular event and mRS score at 3 months and 1 year after stroke onset were also collected.
Antiplatelet regimen types included single antiplatelet therapy (SAPT), DAPT, and none or missing. The SAPT was defined as aspirin at a dose of 100 mg/day or clopidogrel at a dose of 75 mg/day for the first 21 days. The DAPT was defined as clopidogrel at an initial dose of 300 mg followed by 75 mg/day for the first 21 days, plus aspirin at a dose of 100 mg/day. Based on the PAS and antiplatelet use, patients were further divided into four subgroups: PAS+DAPT, PAS+SAPT, non-PAS+DAPT and non-PAS+SAPT.
MRI analysis and Interpretation
All patients underwent MRI on a 3 T MR scanner. Imaging sequences obtained included three-dimensional time-of-flight MRA (repetition time, 20–25 ms; echo time, 3.3–3.9 ms; flip angle, 15°–20°; slice thickness, 0.65–1.0 mm); axial T2-weighted imaging (repetition time, 4500 ms; echo time, 8 ms); T1-weighted imaging (repetition time, 1200 ms; echo time, 11 ms); fluid-attenuated inversion recovery sequences (repetition time, 7000 ms; echo time, 94 ms); and diffusion-weighted imaging (repetition time, 3000 ms; echo time, 75 ms). All aforementioned sequences except MRA had 5 mm slice thickness and a 1.5 mm interslice gap.
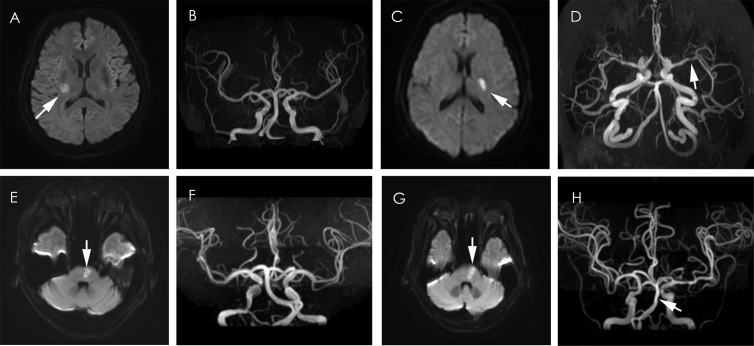
MRIs were collected from individual centres in digital format and were reviewed centrally by two readers (JJ and Y-YX) blinded to the patients’ clinical details. They reached a consensus if they disagreed on interpretations (shown in figure 2A–H).
Figure 2.
SSSI with and without parent artery stenosis in the MCA and BA perforator Territory. (A, B) SSSI (arrow) without disease of MCA. (C, D) SSSI (arrow) with MCA stenosis (arrow). (E, F) SSSI (arrow) without disease of BA (arrow). (G, H) SSSI (arrow) with BA stenosis (arrow). BA, basilar artery; MCA, middle cerebral artery; SSSI, small single subcortical infarction.
On DWI, SSSIs were required to meet MRI criteria that included a lesion measuring less than 20 mm in diameter. Intracranial arteries were analysed with three-dimensional time-of-flight MRA. PAS was defined as any degree of MCA stenosis for basal ganglia infarcts or BA stenosis for pons infarcts. Infarctions and PAS were in the same sagittal plane (for infarction in the perforator territory of MCA) or axial plane (for infarction in the perforator territory of BA) according to multiweighted sequences.
Follow-up and clinical outcome evaluations
Patients were followed up for clinical outcomes at 3 months and 1 year annually. Information including functional status, cardiovascular/cerebrovascular events, recommended secondary prevention medication compliance and risk factor control was collected at each follow-up.
NIHSS score on administration was used as the index of stroke severity, and mRS score at discharge was used as the index of functional outcome. Disability was defined as an mRS score of ≥3. Composite vascular events were defined as ischaemic stroke, haemorrhagic stroke, myocardial infarction or vascular death. Our study examined patient-relevant outcomes of mRS score, haemorrhagic stroke, ischaemic stroke recurrence, and composite vascular events at 3 months and 1 year.
Ischaemic stroke was defined as an acute focal infarction of the brain or retina with one of the following: sudden onset of a new focal neurological deficit lasting fewer than 24 hours with clinical or imaging evidence of infarction, or rapid worsening of an existing focal neurological deficit lasting 24 hours or more, with imaging evidence of new ischaemic changes clearly distinct from the index ischaemic event. Haemorrhagic stroke was defined as acute extravasation of blood into the brain parenchyma or subarachnoid space with associated neurological symptoms.
Statistical analysis
Categorical variables were reported as absolute numbers with percentages, and continuous variables were reported as mean along with SD. Multivariable logistic regression analyses were used to investigate the associations between parental artery stenosis and outcomes (NIHSS score, mRS score, recurrent ischaemic stroke and composite vascular events). Age, sex, hypertension, diabetes mellitus and smoking were included in the model. Cumulative event curves were constructed with the Kaplan-Meier method for 3 month and 1 year recurrent ischaemic stroke and composite vascular events. In addition, we assessed whether outcomes differed in certain prespecified subgroups by different treatment with the use of multivariable logistic regression. The ‘PAS+DAPT’ subgroup was selected as the reference. Patients without antiplatelet therapy or missing data (n=72) were excluded in this model. Adjust factors included age, sex, hypertension, diabetes mellitus and smoking. All p values were two-sided, with p<0.05 considered statistically significant. All statistical analyses were performed using SAS V.9.4 software.
Results
Characteristics of study participants
Among 2890 patients with SSSI (shown in figure 1), 680 (23.53%) patients had PAS and 2210 (76.47%) did not have PAS. Table 1 displayed the demographic, clinical and hospital characteristics according to the stenosis of the parent artery.
Table 1.
Baseline characteristics
| Characteristics | Total N=2890 |
Non-PAS n=2210 (76.47%) |
PAS n=680 (23.53%) |
P value |
| Age | 61.68±10.79 | 60.90±10.80 | 64.23±10.35 | <0.0001 |
| BMI | 24.84±3.30 | 24.85±3.29 | 24.82±3.35 | 0.4375 |
| Sex (female), n (%) | 874 (30.24) | 608 (27.51) | 266 (39.12) | <0.0001 |
| Medical history, n (%) | ||||
| Ischaemic stroke | 544 (18.82) | 400 (18.10) | 144 (21.18) | 0.0727 |
| TIA | 42 (1.45) | 31 (1.40) | 11 (1.62) | 0.6821 |
| Myocardial infarction | 39 (1.35) | 26 (1.18) | 13 (1.91) | 0.1462 |
| Atrial fibrillation | 71 (2.46) | 48 (2.17) | 23 (3.38) | 0.0746 |
| Hypertension | 1924 (66.57) | 1411 (63.85) | 513 (75.44) | <0.0001 |
| Hyperlipidaemia | 210 (7.27) | 157 (7.10) | 53 (7.79) | 0.5444 |
| Diabetes mellitus | 693 (23.98) | 487 (22.04) | 206 (30.29) | <0.0001 |
| Peripheral artery disease | 23 (0.80) | 17 (0.77) | 6 (0.88) | 0.7716 |
| Smoking, n (%) | 955 (33.04) | 787 (35.61) | 168 (24.71) | <0.0001 |
| HbA1c (%) | 6.55±1.72 | 6.45±1.67 | 6.89±1.82 | <0.0001 |
| Missing | 1175 | 896 | 279 | |
| LDL (mmol/L) | 2.56±1.07 | 2.51±1.05 | 2.71±1.13 | <0.0001 |
| Missing | 116 | 82 | 34 | |
| Antiplatelet therapy, n (%) | ||||
| Single antiplatelet | 1196 | 929 (42.04) | 267 (39.26) | 0.3449 |
| Dual antiplatelet | 1622 | 1229 (55.61) | 393 (57.79) | |
| None or missing | 72 | 52 (2.35) | 20 (2.94) | |
| Statin therapy, n (%) | ||||
| Statin standard treatment | 727 (25.16) | 551 (24.93) | 176 (25.88) | 0.6843 |
| Statin intensive treatment | 2163 (74.84) | 1659 (75.07) | 504 (74.12) | 0.6175 |
| Thrombolytic therapy, n (%) | 176 (6.09) | 139 (6.29) | 37 (5.44) | 0.4185 |
| MRI features | ||||
| Diameter of infarction | 11.51±4.41 | 11.47±4.36 | 11.69±4.60 | 0.2017 |
| Outcomes, n (%) | ||||
| NIHSS score | ||||
| <4 | 1511 (52.28) | 1195 (54.07) | 316 (46.47) | 0.0005 |
| ≥4 | 1379 (47.72) | 1015 (45.93) | 364 (53.53) | |
| 3 months | ||||
| Ischaemic stroke | 152 (5.26) | 87 (3.94) | 65 (9.56) | <0.0001 |
| Composite vascular event | 160 (5.54) | 91 (4.12) | 69 (10.15) | <0.0001 |
| Haemorrhagic stroke | 4 (0.14) | 2 (0.09) | 2 (0.29) | 0.2117 |
| mRS score | ||||
| 0–2 | 2547 (89.34) | 1973 (90.50) | 574 (85.54) | 0.0003 |
| 3–6 | 304 (10.66) | 207 (9.50) | 97 (14.46) | |
| Missing | 39 | 30 | 9 | |
| 12 months | ||||
| Ischaemic stroke | 225 (7.79) | 137 (6.20) | 88 (12.94) | <0.0001 |
| Composite vascular events | 254 (8.79) | 157 (7.10) | 97 (14.26) | <0.0001 |
| Haemorrhagic stroke | 19 (0.66) | 14 (0.63) | 5 (0.74) | 0.7739 |
| mRS score | ||||
| 0–2 | 2561 (90.78) | 1986 (92.03) | 575 (86.73) | <0.0001 |
| 3–6 | 260 (9.22) | 172 (7.97) | 88 (13.27) | |
| Missing | 69 | 52 | 17 | |
BMI, body mass index; HbA1c, haemoglobin A1c; LDL, low-density lipoprotein; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; PAS, parent artery stenosis; TIA, transient ischaemic attack.
Patients with PAS were more likely to be female (39.12% vs 27.51%, p<0.0001) and older (64.23±10.35 vs 60.90±10.80, p<0.0001), and had a greater prevalence of cardiovascular risk factors (hypertension, diabetes mellitus and smoking) (p<0.0001) and a higher level of haemoglobin A1c (HbA1c) and low-density lipoprotein (LDL) (p<0.0001). The types of antiplatelet and lipid-lowering therapy for second prevention showed no difference between the two groups. In addition, there were significant differences in stroke recurrence, composite vascular events and function dependence at both 3 months and 1 year. However, haemorrhagic stroke was very rare and of no significant difference in both groups.
Association between PAS and outcomes
The number and rates of NIHSS at admission, stroke recurrence and other outcomes are listed in table 2 according to groups with and without PAS. After adjusting for potential confounders, we found that patients with PAS had a greater initial stroke severity (OR 1.262, 95% CI 1.058 to 1.505; p=0.0097) and a higher risk of ischaemic stroke recurrence at 3 months (OR 2.266, 95% CI 1.631 to 3.149; p<0.0001) and 1 year (OR 2.054, 95% CI 1.561 to 2.702; p<0.0001), as well as composite vascular events at 3 months (OR 2.306, 95% CI 1.674 to 3.178; p<0.0001) and 1 year (OR 1.983, 95% CI 1.530 to 2.570; p<0.0001), compared with the non-PAS group. However, no significant association between stenosis and mRS was observed at 3 months and 1 year after adjustment.
Table 2.
Associations of stenosis and outcomes
| Total | Events, n (%) | Unadjusted | P value | Adjusted | P value | ||
| OR (95% CI) | OR (95% CI) | ||||||
| NIHSS score ≥4 | Non-PAS | 2210 | 1015 (45.93) | Reference | – | Reference | – |
| PAS | 680 | 364 (53.53) | 1.356 (1.142 to 1.611) | 0.0005 | 1.262 (1.058 to 1.505) | 0.0097 | |
| 3 months | |||||||
| mRS score 3–6* | Non-PAS | 2210 | 207 (9.50) | Reference | – | Reference | – |
| PAS | 680 | 97 (14.46) | 1.611 (1.244 to 2.086) | 0.0003 | 1.200 (0.902 to 1.598) | 0.2112 | |
| Ischaemic stroke recurrence | Non-PAS | 2210 | 87 (3.94) | Reference | – | Reference | – |
| PAS | 680 | 65 (9.56) | 2.475 (1.795 to 3.414) | <0.0001 | 2.266 (1.631 to 3.149) | <0.0001 | |
| Composite vascular events | Non-PAS | 2210 | 91 (4.12) | Reference | – | Reference | – |
| PAS | 680 | 69 (10.15) | 2.516 (1.840 to 3.441) | <0.0001 | 2.306 (1.674 to 3.178) | <0.0001 | |
| 1 year | |||||||
| mRS score 3–6* | Non-PAS | 2210 | 172 (7.97) | Reference | – | Reference | – |
| PAS | 680 | 88 (13.27) | 1.767 (1.345 to 2.322) | <0.0001 | 1.266 (0.939 to 1.708) | 0.1225 | |
| Ischaemic stroke recurrence | Non-PAS | 2210 | 137 (6.20) | Reference | – | Reference | – |
| PAS | 680 | 88 (12.94) | 2.163 (1.655 to 2.827) | <0.0001 | 2.054 (1.561 to 2.702) | <0.0001 | |
| Composite vascular events | Non-PAS | 2210 | 157 (7.10%) | Reference | – | Reference | – |
| PAS | 680 | 97 (14.26%) | 2.090 (1.623 to 2.693) | <0.0001 | 1.983 (1.530 to 2.570) | <0.0001 | |
Adjustment for baseline characteristics includes age, sex, history of hypertension, history of diabetes and smoking.
*(mRS) Adjustment for baseline characteristics includes age, sex, history of hypertension, history of diabetes, smoking and NIHSS score.
CI, confidence interval; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratios; PAS, parent artery stenosis.
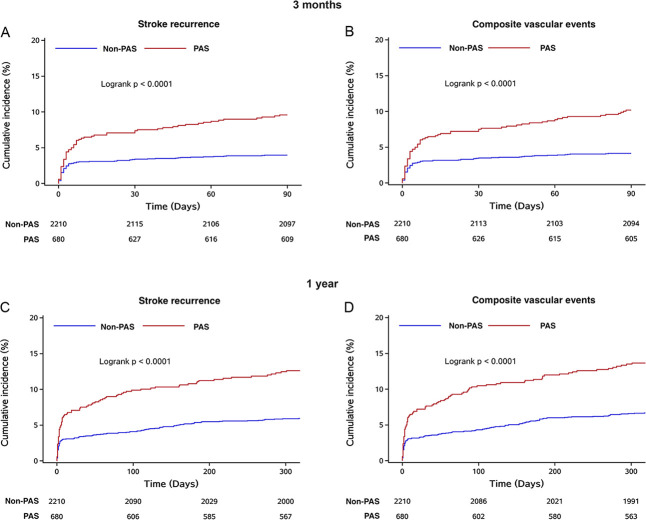
Risk of stroke recurrence and composite vascular events at 3 months and 1 year
Figure 3 showed Kaplan-Meier curves describing the time to event for the stroke recurrence, composite vascular events at 3 months and 1 year in the PAS and non-PAS groups. Kaplan-Meier survival curves showed that the 3-month rate of freedom from stroke recurrence was 89.56% of the PAS group and 94.89% of the non-PAS group (p<0.0001); the 1-year rate was 83.38% of the PAS group and 90.49% of the non-PAS group (p<0.0001) (shown in figure 3A, C).
Figure 3.
Probability of survival free of stroke recurrence, composite vascular events in 3 months and 1 year. PAS, parent artery stenosis.
The same result could be observed in composite vascular events. The curves indicated that composite vascular events increased for patients with stenosis at 3 months (p<0.0001) and 1 year (p<0.0001) (shown in figure 3B, D).
Antiplatelet therapy
Table 3 showed the regression model results respectively, by PAS and the therapy. The rates of ischaemic stroke recurrence and composite vascular events were significantly different between PAS and non-PAS groups. In PAS group, after considering for multiple testing, DAPT was not superior to SAPT in preventing composite vascular events (OR 0.563, 95% CI 0.330 to 0.963; p=0.0358) and improving functional outcome (OR 0.561, 95% CI 0.337 to 0.937; p=0.0270). There was no statistically significant evidence on the effects of DAPT versus SAPT on stroke recurrence and disability in the non-PAS group.
Table 3.
Outcomes at 3 months by PAS and antiplatelet therapy
| Outcomes at 3 months | Total | Events, n (%) | Unadjusted | Adjusted | |||
| N=2818 | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Recurrence of ischaemic stroke | PAS+DAPT | 393 | 44 (11.2) | Reference | – | Reference | – |
| PAS+SAPT | 267 | 19 (7.12) | 0.627 (0.366 to 1.073) | 0.0887 | 0.605 (0.352 to 1.039) | 0.0687 | |
| Non-PAS+DAPT | 1229 | 51 (4.15) | 0.361 (0.241 to 0.541) | <0.0001 | 0.392 (0.260 to 0.590) | <0.0001 | |
| Non-PAS+SAPT | 929 | 36 (3.88) | 0.338 (0.218 to 0.526) | <0.0001 | 0.363 (0.232 to 0.567) | <0.0001 | |
| Composite vascular events | PAS+DAPT | 393 | 47 (11.96) | Reference | – | Reference | – |
| PAS+SAPT | 267 | 19 (7.12) | 0.586 (0.344 to 0.998) | 0.0493 | 0.563 (0.330 to 0.963) | 0.0358 | |
| Non-PAS+DAPT | 1229 | 54 (4.39) | 0.358 (0.242 to 0.529) | <0.0001 | 0.389 (0.262 to 0.579) | <0.0001 | |
| Non-PAS+SAPT | 929 | 37 (3.98) | 0.325 (0.211 to 0.500) | <0.0001 | 0.347 (0.225 to 0.537) | <0.0001 | |
| Haemorrhagic stroke | PAS+DAPT | 393 | 1 (0.25) | Reference | – | Reference | – |
| PAS+SAPT | 267 | 0 | 0 | 0.9980 | 0 | 0.9982 | |
| Non-PAS+DAPT | 1229 | 2 (0.16) | 0.638 (0.058 to 7.036) | 0.7136 | 0.753 (0.066 to 8.658) | 0.8200 | |
| Non-PAS+SAPT | 929 | 0 | 0 | 0.9963 | 0 | 0.9962 | |
| mRS 3–6 (missing 25)* |
PAS+DAPT | 388 | 60 (15.46) | Reference | – | Reference | – |
| PAS+SAPT | 263 | 33 (12.55) | 0.784 (0.497 to 1.239) | 0.2975 | 0.561 (0.337 to 0.937) | 0.0270 | |
| Non-PAS+DAPT | 1220 | 106 (8.69) | 0.520 (0.370 to 0.731) | 0.0002 | 0.672 (0.465 to 0.972) | 0.0350 | |
| Non-PAS+SAPT | 912 | 99 (10.86) | 0.666 (0.471 to 0.940) | 0.0209 | 0.731 (0.501 to 1.066) | 0.1034 | |
Adjustment for baseline characteristics includes age, sex, history of hypertension, history of diabetes and smoking.
*(mRS) Adjustment for baseline characteristics includes age, sex, history of hypertension, history of diabetes, smoking and NIHSS.
CI, confidence interval; DAPT, dual antiplatelet therapy; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratios; PAS, parent artery stenosis; SAPT, single antiplatelet therapy.
Discussion
This national hospital-based study indicated that over three quarters of infarction occurred in SSSI patients without PAS. Furthermore, we found that the diagnosis of PAS could efficiently stratify the risk of recurrent stroke and composite vascular events within 3 months and 1 year of SSSI, while the parent disease was not associated with disability in both short and long terms. Our study may have important clinical implications with the large sample size of patients with SSSI included and comprehensive prognostic characteristics recorded.
In this study, we described the epidemiological characteristics of the short-term and long-term prognoses of SSSI. Our data showed that the characteristics of SSSI are heterogeneous between PAS and non-PAS groups. Compared with the non-PAS, PAS was more likely to be related to atherosclerosis indicators. Our observation was consistent with the previous studies that diabetes and coronary heart disease were more prevalent in patients with large-artery atherosclerosis than in those with small-vessel disease,12 13 while in a study on subcortical infarction in MCA territory, there were no clinical differences between infarctions caused by MCA and small vessels.6 Atherosclerosis indicators, including hypertension, diabetes and smoking, could cause not only intracranial atherosclerosis but also microatheroma and lipohyalinosis.14 15 However, it remains unknown which pathological change is dominant and symptomatic in certain high-risk populations.
The proportion of minor strokes at admission (NIHSS score <4) was 52.28% in our study. Three-month and 1-year ischaemic stroke recurrences were 5.26% and 7.79%, respectively. Nearly 90% of patients had favourable functional outcomes in both the short and long terms. Similarly, a systematic review in the UK indicated that the risk of recurrence among lacunar patients during the first month ranged from 0% to 4%, and that from 1 month to 12 months was 5%–8%.16 In a CHANCE subgroup study, stroke recurrence rates of the single acute infarction group was 8.68%.17 Our study confirmed these general findings but also indicated a differentiation by infarct aetiology.
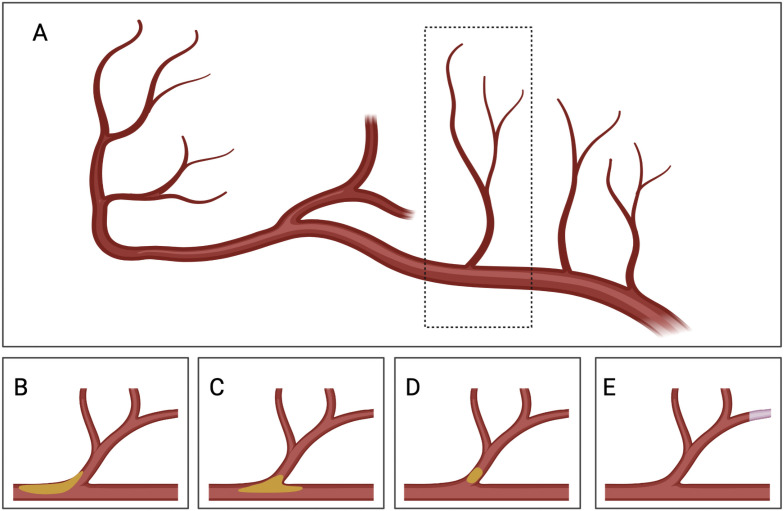
Louis Caplan first used branch atheromatous disease to describe an occlusion or stenosis at the origin of a deep penetrating artery of the brain,18 either isolated microatheromata in the branch’s orifice or plaques seated in the wall of the parent artery, leading to a small internal capsule or pontine infarct (shown in figure 4). Thus, PAS could help to predict the prognosis of SSSI and be used as a solid standard in risk stratification. In this study, we found that the PAS predicts higher NIHSS score at admission, ischaemic stroke recurrence and composite vascular events after adjusting for age and other known predictors of poor outcome in stroke. In a subgroup study of CHANCE, severe ICAS or occlusion doubles the risk of recurrent stroke in minor stroke and TIA.9 Our study was consistent with previous studies and indicated that PAS increased short-term and long-term risk in stroke recurrence and composite vascular events in SSSI. We also examined the relationship between PAS and functional disability at 3 months and 1 year. However, it did not provide differences regarding disability in the short and long terms after considering the NIHSS score at admission and related risk factors. Therefore, PAS was a crucial aetiology of SSSI and plays a significant role in stroke recurrence, despite not being related to the severity of disability.
Figure 4.
Presumed mechanism of infarcts in penetrating artery territory. (A) Plaque in parent artery obstructing a branch. (B) Junctional plaque extending into the branch. (C) Microatheroma formed at the orifice of a branch. (D) Emboli from unstable microatheromatous plaque. (E) Fibrinoid degeneration or lipohyalinosis of the distal perforating artery. Y-YZ drew and created this figure with full permission. The authors confirm that this figure was not a reuse of previously published work.
In our study, dual antiplatelet use had no absolute advantage over single antiplatelet use on stroke recurrence, composite vascular events and disability. Previous research has indicated that DAPT was more efficient in treating ischaemic stroke caused by large-artery atherosclerosis.19 Despite the finding that the PAS group could be more related to intracranial atherosclerosis, DAPT was not superior to SAPT on preventing composite vascular events and improving functional outcome. The fact that patients with PAS in our study did not have significantly more indicators of atherosclerosis than non-PAS ones might be one underlying cause. In addition, antiplatelet regimes were not related to good outcomes in patients without PAS. The results confirmed the findings of previous studies that DAPT was not associated with the reduction in recurrent strokes in single small strokes. In a subgroup analysis of the CHANCE study, patients who had minor stroke with single acute infarction seem to benefit less from dual antiplatelet treatment.17 The Secondary Prevention of Small Subcortical Strokes trial indicated that dual antiplatelet treatment did not reduce the risk of ischaemic stroke.20 This finding indicates that in different types of ischaemic cerebrovascular disease, the role of platelets and the components of thrombosis are different. The reason might be that thrombosis may have a minimal role in precipitating occlusions of small penetrating cerebral arteries.2 A study indicating that procoagulant platelets are lower in lacunar stroke than non-lacunar stroke confirmed the suggestion.21
Meanwhile, in this study, DAPT did not increase haemorrhagic stroke. Our result was consistent with previous studies. In the CHANCE study and the Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilisation Management, and Avoidance (CHARISMA) trial, the risk of intracranial bleeding did not increase when clopidogrel was added to aspirin.8 22 While in the Platelet-Oriented Inhibition in New TIA and Minor Ischaemic Stroke trial, the increase of haemorrhagic stroke in the dual antiplatelet group might be due to the prolonged usage of clopidogrel.7
Therefore, the effective therapy for secondary prevention in SSSI needs further investigation.
Limitations
First, parent artery disease might be underdiagnosed in our study.23 24 High-resolution and ultrafield MRI has been proven capable of revealing cerebral perforating arteries25 26 and describing in much more detail intracranial vessel wall changes.27 That might enhance our understanding of the mechanism of stroke in the territory of perforating arteries.28
Second, it seems very difficult to identify the aetiologies more detailed by current clinical routine imaging examinations. Isolated atherosclerosis in the branch’s orifice or mild plaque occluding the orifice cannot be detected even by high-resolution MRI. White matter intensity and other MRI markers could be evidence for cerebral small-vessel disease. However, atherosclerotic lesions, microatheroma and fibrohyalinosis often coexist, and the aetiologies are challenging to distinguish. Thus, the distinction criteria of aetiologies we used were practical and critical in clinical practice.
Third, in this study, we were unable to evaluate early neurological deterioration, which is a characteristic clinical manifestation in acute pontine or basilar ganglia infarction and might relate to unfavourable outcomes.14 29
Fourth, we did not have detailed data about TOAST classification of recurrent ischaemic strokes in follow-up. One study suggested that cardiogenic embolism might contribute to stroke recurrence in patients who had lacunar stroke.30 This could explain the non-ideal efficacy of DAPT in our study. However, there was evidence that recurrent strokes were more likely to be lacunar if the index event was lacunar.16 Thus, the mechanism of SSSI and the recurrence needs further investigation.
Conclusion
In conclusion, PAS is related significantly to higher short-term and long-term stroke recurrence and composite vascular events, suggesting heterogeneous mechanisms in SSSI subgroups. The antiplatelet therapy in the second prevention of SSSI needs further investigation.
Acknowledgments
We thank Dr Luanluan Sun (Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, UK) for her important intellectual contribution to the article. We thank all participating hospitals, their physicians and nurses. We appreciate all the patients who took part in the Third China National Stroke Registry trial.
Footnotes
Collaborators: The complete list of CNSR-III members and sites Yongjun Wang, MD (Beijing Tiantan Hospital Capital Medical University, Beijing, China); Jilai Li, MD (Aerospace Central Hospital, Beijing, China); Jian Wu, MD (Beijing Qinghua Changgeng Hospital, Beijing, China); Mei Zhang, MD (Beijing Longfu Hospital, Beijing, China); Maolin He, MD (Beijing Shijitan Hospital Capital Medical University, Beijing, China); Tao Gong, MD (Beijing Hospital, Beijing, China); Quping Ouyang, MD (The Hospital of Shunyi District Beijing, Beijing, China); Guang Huang, MD (Fuxing Hospital Capital Medical University, Beijing, China); Fengchun Yu, MD (Beijing Haidian Hospital, Beijing, China); Chenlong Wang, MD (Civil Aviation General Hospital, Beijing, China); Jinli Zhang, MD (Chinese People's Liberation Army 263 Hospital, Beijing, China); Wenqing Wu, MD; phD (Beijing Ditan Hospital Capital Medical University, Beijing, China); Yi Wang, MD; Yaoyu Yu, MD (Affiliated Hospital of the Chinese People's Armed Police Force Logistics College, Tianjin, China); Meiyun Zhang, MD (Tianjin People's Hospital, Tianjin, China); Zhongping An, MD (Tianjin Huanhu Hospital, Tianjin, China); Junyan Liu, MD (The Third Hospital of Hebei Medical University, Shijiazhuang, China); Wanying Shi, MD (Shijiazhuang First Hospital, Shijiazhuang, China); Baoquan Lu, MD (Tangshan Gongren Hospital, Tangshan, China); Lijun Geng, MD (Luannan County Hospital, Tangshan, China); Shujuan Wang, MD (Kailuan General Hospital, Tangshan, China); Xu Zhang, MD (Yutian County Hospital, Tangshan, China); Ruifang Liu, MD (Zunhua People's Hospital, Tangshan, China); Fengli Zhao, MD (The NO.2 Hospital of Baoding, Baoding, China); Jie Lin, MD (Handan City First Hospital, Handan, China); Xinping Liu, MD (Handan Central Hospital, Handan, China); Xuebing Sun, MD (Wu'an First People's Hospital, Handan, China); Tianyuan Li, MD (Shexian Hospital, Handan, China); Youming Wang, MD (Affiliated Hospital of Hebei University of Engineering, Handan, China); Xinxia He, MD (Hengshui People's Hospital, Hengshui, China); Weiqiang Yuan, MD (Hengshui Fifth People Hospital, Hengshui, China); Ronghua Dou, MD (Cangzhou Hospital of Integrated TCM-WM. Hebei, Cangzhou, China); Lihai Liu, MD; Yanling Wang, MD (Cangzhou People's Hospital, Cangzhou, China); Junling Zhang, MD (Cangzhou Central Hospital, Cangzhou, China); Haisong Du, MD (Chengde Central Hospital, Chengde, China); Yuqing Wei, MD (Xingtai Third Hospital, Xingtai, China); Cunrui Wang, MD (Weixian People's Hospital, Xingtai, China); Limin Wang, MD (First Hospital of Zhangjiakou, Zhangjiakou, China); Yu'an Zou, MD (The First Affiliated Hospital of Hebei North University, Zhangjiakou, China); Xiaofei Chen, MD (Shanxi Cardiovascular Hospital, Taiyuan, China); Fengyun Hu, MD (Shanxi Provincial People's Hospital, Taiyuan, China); Jinfeng Liu, MD (Yangquan Coalmine Group General Hospital, Yangquan, China); Lili Zhao, MD (Changzhi People's Hospital, Changzhi, China); Fanping He, MD (The Second People's Hospital of Jinzhong, Jinzhong, China); Xingchen Wang, MD (The Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China); Qingwei Zhao, MD (Shandong Police General Hospital, Jinan, China); Xiaohong Li, MD (Jinan Central Hospital, Jinan, China); Jun Zhao, MD (Leling People's Hospital, Leling, China); Zhangyong Xia, MD (Liaocheng People's Hospital, Liaocheng, China); Hongjin Li, MD (Dong'a County People's Hospital, Liaocheng, China); Mingzong Yan, MD (Penglai Traditional Chinese Medicine Hospital, Penglai, China); Guiru Zhang, MD (Penglai People's Hospital, Penglai, China); Hui Liang, MD (Yantaishan Hospital of Yantai City, Yantai, China); Yunlin Liu, MD (Taian City Central Hospital, Taian, China); Jun Xu, MD (Zhengzhou Yihe Hospital affiliated to Henan University, Zhengzhou, China); Runqing Wang, MD (Zhengzhou Central Hospital, Zhengzhou, China); Yuhui Han, MD (Gongyi City People's Hospital, Gongyi, China); Xianghong Meng, MD; Mingzhen Li, MD (Qixian People's Hospital, Hebi, China); Ting Wang, MD (Jiyuan People's Hospital, Jiyuan, China); Xinsheng Han, MD (Kaifeng Central Hospital, Kaifeng, China); Hongtian Zhang, MD (Yucheng County People's Hospital, Luoyang, China); Congmin Ma, MD (Luoyang Central Hospital, Luoyang, China); Wenjun Xue, MD (Pingdingshan First People's Hospital, Pingdingshan, China); Chun Wang, MD (Ruzhou First People's Hospital, Ruzhou, China); Yan Fang, MD (Shangqiu First People's Hospital, Shangqiu, China); Gexia Liu, MD (Changge Municipal People's Hospital, Xuchang, China); Jianfeng Wang, MD (Dalian Central Hospital, Dalian, China); Qiang Ma, MD (affiliated Zhongshan Hospital of Dalian University, Dalian, China); Xiaohong Li, MD; Wenxu Zheng, MD (Dalian Friendship Hospital, Dalian, China); Haitao Chi, MD (Xinhua Hospital affiliated to Dalian University, Dalian, China); Lianbo Gao, MD (The Fourth Affiliated Hospital of China Medical University, Shenyang, China); Jin Zhou, MD (First People's Hospital of Shenyang, Shenyang, China); Huisheng Chen, MD (People's Liberation Army Shenyang Military Region General Hospital, Shenyang, China); Juan Feng, MD (Shengjing Hospital of China Medical University, Shenyang, China); Hongbo Xiao, MD (Anshan Central Hospital, Anshan, China); Lijun Xiao, MD (Third People Hospital of Liaoyang, Liaoyang, China); Yi Yang, MD (Jilin University First Hospital, Changchun, China); Guozhong Li, MD (The First Affiliated Hospital of Harbin Medical University, Harbin, China); Yulan Zhu, MD; phD; Lihua Wang, MD (The Second Affiliated Hospital of Harbin Medical University, Harbin, China); Yindong Yang, MD (Hongqi Hospital affiliated to Mudanjiang Medical College, Mudanjiang, China); Xuerong Qiu, MD (Qiqihar City Rongjian Stroke Prevention and Treatment Institute, Qiqihar, China); Xuhai Gong, MD (Daqing Oilfield General Hospital, Daqing, China); Guohua Chen, MD (Wuhan First Hospital, Wuhan, China); Xiaoxiang Peng, MD (Hubei Third People's Hospital, Wuhan, China); Qunhui Liu, MD (The Central Hospital of Enshi Autonomous Prefecture, Wuhan, China); Shiping Gong, MD (Zhongxiang Hospital of Renmin Hospital of Wuhan University, Jingmen, China); Hongbin Zhou, MD (Xiangyang Central Hospital, Xiangyang, China); Haipeng Li, MD (Zhangzhou First People's Hospital, Chenzhou, China); Yong You, MD (The First Affiliated Hospital of Nanhua University, Hengyang, China); Jinsheng Lin, MD (Xiangtan Central Hospital, Xiangtan, China); Yun Xu, MD (Nanjing Drum Tower Hospital, Nanjing, China); Lei Sheng, MD (The Second Chinese Medicine Hospital of Jiangsu, Nanjing, China); Heqing Zhao, MD (The Second Affiliated Hospital of Suzhou University, Suzhou, China); Aixia Zhuang, MD (The Second Affiliated Hospital of Lianyungang, Lianyungang, China); Kaifu Ke, MD (Affiliated Hospital of Nantong University, Nantong, China); Qi Fang, MD (The First Affiliated Hospital of Suzhou University, Nantong, China); Zhengxie Dong, MD (The First People's Hospital of Nantong, Nantong, China); Guiyun Cui, MD; Deqin Geng, MD (The Affiliated Hospital of Xuzhou Medical College, Xuzhou, China); Liangqun Rong, MD (The Second Affiliated Hospital of Xuzhou Medical College, Xuzhou, China); Junfeng Shi, MD (Yixing People's Hospital, Yixing, China); Ming Yu, MD (Affiliated Hospital of Jiangsu University, Zhenjiang, China); Jun Xu, MD, phD (Subei People's Hospital of Jiangsu, Yangzhou, China); Yu Geng, MD (Zhejiang Provincial People's Hospital, Hangzhou, China); Benyan Luo, MD (The First Affiliated Hospital of Zhejiang University, Hangzhou, China); Xueli Cai, MD (Lishui Center Hospital, Lishui, China); Jun Zhou, MD (Shaoxing Central Hospital, Shaoxing, China); Yi Wu, MD (Yiwu Hospital affiliated to Wenzhou Medical University, Yiwu, China); Weiguo Tang, MD (Zhoushan Hospital, Zhoushan, China); Zhimin Wang, MD (Taizhou First People's Hospital, Taizhou, China); Yangmei Chen, MD (The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China); Yanjiang Wang, MD (Third Affiliated Hospital of the Third Military Medical University of the Chinese People's Liberation Army, Chongqing, China); Kangning Chen, MD (Affiliated Hospital of the Third Military Medical University, Chongqing, China); Shizheng Wu, MD (Qinghai Provincial People's Hospital, Qinghai, China); Wenguang Bu, MD (Huainan Chaoyang Hospital, Huainan, China); Xiaohua Cheng, MD (Huangshan People's Hospital, Huangshan, China); Zhengqi Lu, MD (The Third Affiliated Hospital of Zhongshan University, Guangzhou, China); An'ding Xu, MD (The First Affiliated Hospital of Jinan University, Guangzhou, China); Jia Yin, MD (Southern Hospital of Southern Medical University, Guangzhou, China); Jifu Cai, MD (The University of Hong Kong. Shenzhen Hospital, Shenzhen, China); Yi Guo, MD (Shenzhen People's Hospital, Shenzhen, China); Jun Wu, MD (Shenzhen Hospital of Peking University, Shenzhen, China); Lvli Li, MD (The People's Hospital of Guangxi Zhuang Autonomous Region, Nanning, China); Li Pan, MD (Wuzhou People's Hospital, Wuzhou, China); Yinzhou Wang, MD (Fujian Provincial Hospital, Fuzhou, China); Ning Wang, MD; phD (The First Affiliated Hospital of Fujian Medical University, Fuzhou, China); Jianping Niu, MD (The Second Hospital of Xiamen, Xiamen, China); Qing Li, MD (Xiamen Haicang Hospital, Xiamen, China); Hong Wang, MD (The First Affiliated Hospital of Medical College, Shihezi University, Shihezi, China); Hongyan Li, MD (Xinjiang Uygur Autonomous Region People's Hospital, Urumqi, China); Xiaoying Zhang, MD (Xinjiang Production and Construction Corps Hospital, Urumqi, China); Liping Zhan, MD (Kunming Yan'an Hospital, Kunming, China); Yongming Chen, MD (Wuyuan County People's Hospital, Bayannaoer, China); Baojun Wang, MD (Baotou Central Hospital, Baotou, China); Li'e Wu, MD (First Affiliated Hospital of Baotou Medical University, Baotou, China); Li Liu, MD (Chifeng Municipal Hospital, Chifeng, China); Yanru Zhao, MD (Affiliated Hospital of Chifeng University, Chifeng, China); Yingchun Wu, MD (Ordos Center Hospital, Ordos, China); Runxiu Zhu, MD (Inner Mongolia People's Hospital, Hohhot, China); Yanhui Du, MD (Ningxia Medical University General Hospital, Yinchuan, China); Yongxia Wen, MD (The Third People's Hospital of Ningxia, Yinchuan, China); Ye Tian, MD (Xi'an Central Hospital, Xi'an, China); Songdi Wu, MD (Xi'an First Hospital, Xi'an, China); Yongcai Qu, MD (Yan'an University Affiliated Hospital, Yan'an, China); Yuncheng Wu, MD (First People's Hospital Affiliated to Shanghai Jiaotong University, Shanghai, China); Jianren Liu, MD (The Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China); Qiang Dong, MD (Huashan Hospital affiliated to Fudan University, Shanghai, China); Qingke Bai, MD (Shanghai Pudong New Area People's Hospital, Shanghai, China); Yuwu Zhao, MD (The Sixth People's Hospital affiliated to Shanghai Jiaotong University, Shanghai, China); Xu Chen, MD (The Eighth People's Hospital of Shanghai Province, Shanghai, China); Chaoming He, MD (The Third People's Hospital of Hainan Province, Sanya, China); Lijie Ren, MD (Shenzhen Second People's Hospital, Shenzhen, China); Weiwen Qiu, MD (Lishui People's Hospital, Lishui, China); Shufang Yao, MD (Sanmenxia Central Hospital, Sanmenxia, China); Xuwen Sun, MD (Yantai Yuhuangding Hospital, Yantai, China); Hainan Zhang, MD (The Second Xiangya Hospital of Central South University, Changsha, China); Weirong Li, MD (Taiyuan Central Hospital, Taiyuan, China); Ligong Gao, MD (Zhumadian Central Hospital, Zhumadian, China); Xianglin Chen, MD (Qingyuan People's Hospital, Qingyuan, China); Jianhua Li, MD (The First Hospital of Beijing Fangshan District, Beijing, China); Qiuyan Shi, MD (Affiliated Hospital of North China University of Technology, Tangshan, China); Yan Wang, MD (Tangshan People's Hospital, Tangshan, China); Mingzhi Zhao, MD (Zhongli County People's Hospital, Zhengzhou, China); Jinsheng Zeng, MD (First Affiliated Hospital of Zhongshan University, Guangzhou, China); Liping Wang, MD (Kaifeng Second People's Hospital, Kaifeng, China); Wei Wang, MD (People's Liberation Army No. 309 Hospital, Beijing, China); Feng Qiu, MD (Naval general hospital, Beijing, China); Zhaochen Li, MD (Beijing Huairou Hospital of University of Chinese Academy of Sciences, Beijing, China); Liang Zhao, MD (Affiliated Hospital of Chengde Medical College, Chengde, China); Tianbao Chen, MD (Yingyang City People's Hospital, Xingyang, China); Lei Xia, MD (Zhoukou Central Hospital, Zhoukou, China); SuYun Yang, MD (Changzi People's Hospital, Changzhi, China); Yazhou Han, MD (Qinyang City People's Hospital, Qinyang, China); Liyan Liu, MD (Lianyungang Municipal Oriental Hospital, Lianyungang, China); Xinxiao Wu, MD (Qingyuan County People's Hospital, Lishui, China); Beihai Jiang, MD (Xunxian People's Hospital, Hebi, China); Lizhong Li, MD (The People's Hospital of Hebi, Hebi, China); Weidong Lou, MD (Longquan People's Hospital, Longquan, China:; Hospital of Jianshui, Jianshui, China); Ping Zhang, MD (Affiliated Nanhua Hospital, University of South China, Hengyang, China); Weiming Lan, MD (Jingning County People's Hospital, Lishui, China); Aihu Zheng, MD (Jinzhong City First People's Hospital, Jinzhong, China); Qifu Bai, MD (Shanxi Qixian People's Hospital, Jinzhong, China); Lifang Luan, MD (Jiangsu Jiangbei People's Hospital, Nanjing, China); Lin Chen, MD (The Second Affiliated Hospital of Nanhua University, Hengyang, China); Liqing Yan, MD (Fenxi Mining Bureau Hospital, Jinzhong, China); Yanxia Wang, MD (Hejian City People's Hospita, Hejian, China); Xuerong Huang, MD (Wenzhou City Third People's Hospital, Wenzhou, China); Xiangting Chai, MD (Huangdao District People's Hospital, Qingdao, China); Yanshu Liu, MD (Anyang County People's Hospital, Jiyuan, China); Liangjun You, MD (Nanyang Second People's Hospital, Nanyang, China); Hongqin Yang, MD (Jiyuan City Hospital of Traditional Chinese Medicine, Jiyuan, China); Dongfang Li, MD (The Second Affiliated Hospital of Shanxi Medical University, Taiyuan, China); Huijuan Wang, MD (The Second Hospital of Hebei Medical University, Shijiazhuang, China); Linying Gui, MD (Hengshui City Yinzhou District Hospital, Hengshui, China); Aisheng Wu, MD; Jianling Zhang, MD (The No.4 People' Hospital of Hengshui, Hengshui, China); Dengling Wang, MD (Huimin County People's Hospital, Binzhou, China); Qinghua Zhang, MD (The Western Hospital of Shandong Provincial Hospital, Jinan, China); Yunhong He, MD (The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou, China); Ruiyou Guo, MD (Qingdao City, Hai Ci Hospital, Qingdao, China); Jijun Teng, MD (the Affiliated Hospital of Qingdao University, Qingdao, China); Ping Lou, MD (The Zhengzhou First People's Hospital, Zhengzhou, China).
Contributors: Conception and design: Y-YX and JJ; administrative support: XM and JJ; provision of study materials or patients: XM and Z-XL; collection and assembly of data: XM, X-QZ, L-PL and Y-LW; data analysis and interpretation: A-XW and Y-JZ; manuscript writing: Y-YX; responsible for the overall content as the guarantor: Y-JW; final approval of the manuscript: all authors.
Funding: This work was supported by the National Key R&D Programme of China (number 2018YFC1312903), the National Science and Technology Major Project (number 2017Z×09304018) and the National Natural Science Foundation of China (numbers 81870905 and 81801139).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
the CNSR-III Investigators:
Yongjun Wang, Jilai Li, Jian Wu, Mei Zhang, Maolin He, Tao Gong, Quping Ouyang, Guang Huang, Fengchun Yu, Chenlong Wang, Jinli Zhang, Wenqing Wu, Yi Wang, Yaoyu Yu, Meiyun Zhang, Zhongping An, Junyan Liu, Wanying Shi, Baoquan Lu, Lijun Geng, Shujuan Wang, Xu Zhang, Ruifang Liu, Fengli Zhao, Jie Lin, Xinping Liu, Xuebing Sun, Tianyuan Li, Youming Wang, Xinxia He, Weiqiang Yuan, Ronghua Dou, Lihai Liu, Yanling Wang, Junling Zhang, Haisong Du, Yuqing Wei, Cunrui Wang, Limin Wang, Yu'an Zou, Xiaofei Chen, Fengyun Hu, Jinfeng Liu, Lili Zhao, Fanping He, Xingchen Wang, Qingwei Zhao, Xiaohong Li, Jun Zhao, Zhangyong Xia, Hongjin Li, Mingzong Yan, Guiru Zhang, Hui Liang, Yunlin Liu, Jun Xu, Runqing Wang, Yuhui Han, Xianghong Meng, Mingzhen Li, Ting Wang, Xinsheng Han, Hongtian Zhang, Congmin Ma, Wenjun Xue, Chun Wang, Yan Fang, Gexia Liu, Jianfeng Wang, Qiang Ma, Xiaohong Li, Wenxu Zheng, Haitao Chi, Lianbo Gao, Jin Zhou, Huisheng Chen, Juan Feng, Hongbo Xiao, Lijun Xiao, Yi Yang, Guozhong Li, Yulan Zhu, Lihua Wang, Yindong Yang, Xuerong Qiu, Xuhai Gong, Guohua Chen, Xiaoxiang Peng, Qunhui Liu, Shiping Gong, Hongbin Zhou, Haipeng Li, Yong You, Jinsheng Lin, Yun Xu, Lei Sheng, Heqing Zhao, Aixia Zhuang, Kaifu Ke, Qi Fang, Zhengxie Dong, Guiyun Cui, Deqin Geng, Liangqun Rong, Junfeng Shi, Ming Yu, Jun Xu, Yu Geng, Benyan Luo, Xueli Cai, Jun Zhou, Yi Wu, Weiguo Tang, Zhimin Wang, Yangmei Chen, Yanjiang Wang, Kangning Chen, Shizheng Wu, Wenguang Bu, Xiaohua Cheng, Zhengqi Lu, An'ding Xu, Jia Yin, Jifu Cai, Yi Guo, Jun Wu, Lvli Li, Li Pan; Yinzhou Wang, Ning Wang, Jianping Niu, Qing Li, Hong Wang, Hongyan Li, Xiaoying Zhang, Liping Zhan, Yongming Chen, Baojun Wang, Li'e Wu, Li Liu; Yanru Zhao, Yingchun Wu, Runxiu Zhu, Yanhui Du, Yongxia Wen, Ye Tian, Songdi Wu, Yongcai Qu, Yuncheng Wu, Jianren Liu, Qiang Dong, Qingke Bai, Yuwu Zhao, Xu Chen, Chaoming He, Lijie Ren, Weiwen Qiu, Shufang Yao, Xuwen Sun, Hainan Zhang, Weirong Li, Ligong Gao, Xianglin Chen, Jianhua Li, Qiuyan Shi, Yan Wang, Mingzhi Zhao, Jinsheng Zeng, Liping Wang, Wei Wang, Feng Qiu, Zhaochen Li, Liang Zhao, Tianbao Chen, Lei Xia, SuYun Yang, Yazhou Han, Liyan Liu, Xinxiao Wu, Beihai Jiang, Lizhong Li, Weidong Lou, Ping Zhang, Weiming Lan, Aihu Zheng, Qifu Bai, Lifang Luan, Liqing Yan, Yanxia Wang, Xuerong Huang, Xiangting Chai, Yanshu Liu, Liangjun You, Hongqin Yang, Dongfang Li, Huijuan Wang, Linying Gui, Aisheng Wu, Jianling Zhang, Dengling Wang, Qinghua Zhang, Yunhong He, Ruiyou Guo, Jijun Teng, and Ping Lou
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the protocol of the Third China National Stroke Registry study at Beijing Tiantan Hospital (institutional review board approval number: KY2015-001-01) and all participating centres. Participants gave informed consent to participate in the study before taking part.
References
- 1. Kolominsky-Rabas PL, Weber M, Gefeller O, et al. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001;32:2735–40. 10.1161/hs1201.100209 [DOI] [PubMed] [Google Scholar]
- 2. Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry 2005;76:617–9. 10.1136/jnnp.2004.039982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. J Stroke 2015;17:2–6. 10.5853/jos.2015.17.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryoo S, Lee MJ, Cha J, et al. Differential vascular pathophysiologic types of intracranial atherosclerotic stroke: a high-resolution wall magnetic resonance imaging study. Stroke 2015;46:2815–21. 10.1161/STROKEAHA.115.010894 [DOI] [PubMed] [Google Scholar]
- 5. Suto Y, Nakayasu H, Maeda M, et al. Long-Term prognosis of patients with large subcortical infarctions. Eur Neurol 2009;62:304–10. 10.1159/000235943 [DOI] [PubMed] [Google Scholar]
- 6. Cho A-H, Kang D-W, Kwon SU, et al. Is 15 mm size criterion for lacunar infarction still valid? A study on strictly subcortical middle cerebral artery territory infarction using diffusion-weighted MRI. Cerebrovasc Dis 2007;23:14–19. 10.1159/000095753 [DOI] [PubMed] [Google Scholar]
- 7. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379:215–25. 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 9. Liu L, Wong KSL, Leng X, et al. Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of chance. Neurology 2015;85:1154–62. 10.1212/WNL.0000000000001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Jing J, Meng X, et al. The third China national stroke registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol 2019;4:158–64. 10.1136/svn-2019-000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams HPJ, Bendixen BH, Kappelle LJ, et al. Marsh ee 3rd. classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 12. Khan U, Porteous L, Hassan A, et al. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry 2007;78:702–6. 10.1136/jnnp.2006.103549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim BJ, Lee S-H, Kang BS, et al. Diabetes increases large artery diseases, but not small artery diseases in the brain. J Neurol 2008;255:1176–81. 10.1007/s00415-008-0864-0 [DOI] [PubMed] [Google Scholar]
- 14. Petrone L, Nannoni S, Del Bene A, et al. Branch atheromatous disease: a clinically meaningful, yet unproven concept. Cerebrovasc Dis 2016;41:87–95. 10.1159/000442577 [DOI] [PubMed] [Google Scholar]
- 15. Men X, Wu A, Zhang B, et al. Leukoaraiosis and NIHSS score help to differentiate subtypes of intracranial branch atheromatous disease in southern Han Chinese patients with stroke. Neurol Sci 2013;34:1727–33. 10.1007/s10072-013-1322-z [DOI] [PubMed] [Google Scholar]
- 16. Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain 2005;128:2507–17. 10.1093/brain/awh636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jing J, Meng X, Zhao X, et al. Dual antiplatelet therapy in transient ischemic attack and minor stroke with different infarction patterns: subgroup analysis of the chance randomized clinical trial. JAMA Neurol 2018;75:711–9. 10.1001/jamaneurol.2018.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology 1989;39:1246–50. 10.1212/WNL.39.9.1246 [DOI] [PubMed] [Google Scholar]
- 19. Wong KSL, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (clair study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–97. 10.1016/S1474-4422(10)70060-0 [DOI] [PubMed] [Google Scholar]
- 20. SPS3 Investigators, Benavente OR, Hart RG, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817–25. 10.1056/NEJMoa1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prodan CI, Joseph PM, Vincent AS, et al. Coated-platelets in ischemic stroke: differences between lacunar and cortical stroke. J Thromb Haemost 2008;6:609–14. 10.1111/j.1538-7836.2008.02890.x [DOI] [PubMed] [Google Scholar]
- 22. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–17. 10.1056/NEJMoa060989 [DOI] [PubMed] [Google Scholar]
- 23. Chung J-W, Kim BJ, Sohn CH, et al. Branch atheromatous plaque: a major cause of lacunar infarction (high-resolution MRI study). Cerebrovasc Dis Extra 2012;2:36–44. 10.1159/000341399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim BJ, Lee DH, Kang D-W, et al. Branching patterns determine the size of single subcortical infarctions. Stroke 2014;45:1485–7. 10.1161/STROKEAHA.114.004720 [DOI] [PubMed] [Google Scholar]
- 25. Kang C-K, Park C-A, Park C-W, et al. Lenticulostriate arteries in chronic stroke patients visualised by 7 T magnetic resonance angiography. Int J Stroke 2010;5:374–80. 10.1111/j.1747-4949.2010.00464.x [DOI] [PubMed] [Google Scholar]
- 26. Zhang Z, Fan Z, Kong Q, et al. Visualization of the lenticulostriate arteries at 3T using black-blood T1-weighted intracranial vessel wall imaging: comparison with 7T TOF-MRA. Eur Radiol 2019;29:1452–9. 10.1007/s00330-018-5701-y [DOI] [PubMed] [Google Scholar]
- 27. Harteveld AA, van der Kolk AG, van der Worp HB, et al. Detecting intracranial vessel wall lesions with 7T-Magnetic resonance imaging: patients with posterior circulation ischemia versus healthy controls. Stroke 2017;48:2601–4. 10.1161/STROKEAHA.117.017868 [DOI] [PubMed] [Google Scholar]
- 28. Liang J, Liu Y, Xu X, et al. Cerebral Perforating Artery Disease : Characteristics on High-Resolution Magnetic Resonance Imaging. Clin Neuroradiol 2019;29:533–41. 10.1007/s00062-018-0682-4 [DOI] [PubMed] [Google Scholar]
- 29. Li H, Dai Y, Wu H, et al. Predictors of early neurologic deterioration in acute pontine infarction. Stroke 2020;51:637–40. 10.1161/STROKEAHA.119.027239 [DOI] [PubMed] [Google Scholar]
- 30. Kazui S, Levi CR, Jones EF, et al. Lacunar stroke: transoesophageal echocardiographic factors influencing long-term prognosis. Cerebrovasc Dis 2001;12:325–30. 10.1159/000047729 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.