Abstract
Immune-checkpoint inhibitors (ICI) have revolutionized cancer therapy but are associated with infrequent but lethal myocarditis, for which management remains uncertain. Abatacept, a CTLA-4 fusion protein targeting CD86 on antigen presenting cells and leading to global T-cell anergy, has been described as a potential treatment in individual reports. Yet, abatacept treatment dosage, schedule and optimal combination with other immunosuppressive therapies are unclear. We describe a 25-year-old man who developed pembrolizumab (anti-PD1)-induced myocarditis 14 days after first injection for thymoma treatment, which deteriorated into cardiogenic shock, with sustained ventricular arrhythmia, requiring urgent extracorporeal life support implantation, despite prompt initiation of corticosteroids and mycophenolate-mofetil. Using a strategy of serial measurement ensuring with a target of >80% CD86 receptor occupancy on circulating monocytes, abatacept dose was adjusted and combined with ruxolitinib and methylprednisolone. This strategy resulted in high-dose of abatacept: 60 mg/kg in three doses (20 mg/kg each) within the first 10 days, followed by two doses. Clinical improvement occurred within 7 days, with resolution of systolic cardiac dysfunction, and ventricular arrhythmias resulting in successful discharge from hospital. We reversed a case of nearly lethal ICI-myocarditis, using specific patient-dose adjusted abatacept, which may serve as basis for personalized treatment of patients with severe ICI-adverse events. Trial registration number: NCT04294771.
Keywords: immunotherapy, autoimmunity, case reports
Background
Immune-checkpoint inhibitors (ICIs) are indicated for a growing number of cancer types.1 ICIs are antibodies targeting the programmed cell-death-protein-1 (PD1), its ligand (PDL1), or cytotoxic-T-lymphocyte-associated-protein-4 (CTLA4) and restore T-cell cytotoxicity against cancer cells.1 However, ICI therapies may be hampered by fulminant myocarditis, which is often fatal and results from T-cells and macrophages infiltration into the myocardium.2
Salem et al have recently shown that abatacept, a CTLA4 immunoglobulin fusion protein, is a promising treatment strategy for life-threatening glucocorticoid-refractory ICI-myocarditis in a case report.3 4 These findings were supported using a genetic mouse model which recapitulates clinical and pathological features of ICI-myocarditis.4 In this model, CTLA4 and PD1 functionally interact for myocarditis development and intervention with CTLA4-Ig (abatacept) attenuates myocarditis.4 However, optimal abatacept dosing scheme and combination with other synergistic immunosuppressants for glucocorticoid-refractory ICI-myocarditis is unknown. By binding CD80/CD86, CTLA4 agonists (abatacept, belatacept) act on antigen-presenting-cells (eg, macrophages) and lead to global T-cell anergy with limited off-target effects and reverse pathways activated by ICI.1 4 In their approved indications (rheumatic arthritis for abatacept, kidney graft rejection prophylaxis for belatacept), the circulating monocytes CD86 receptor occupancy (CD86RO, ie, CTLA4 agonists receptors), is a relevant pharmacodynamic biomarker of their clinical activity, with CD86RO ≥80% as a target for full efficacy.5 6 However, such measurements have not been made with abatacept during use for ICI-myocarditis.
Here, we detail the management of a patient with thymoma treated by pembrolizumab (anti-PD1) who developed fulminant corticosteroid and mycophenolate mofetil-resistant myocarditis requiring circulatory mechanical support. This event was reversed using high-dose abatacept guided by CD86RO immune-monitoring and abatacept circulating levels. Additionally, ruxolitinib, a JAK (Janus-Kinase) inhibitor which impairs T-cell activation via blockade of pro-inflammatory cytokines and is used to treat graft vs host disease (a condition in which donor T-cells attack host tissue), was also administrated,7 given its expected rapid synergistic effect with abatacept (acting downstream in the immunological synapse) and the severity of initial presentation.1
Methods for immune monitoring
Blood samples were collected in EDTA tubes. Monocytes and T-lymphocytes were analyzed by flow cytometry using monoclonal antibodies(mAb) as previously described8: CD45-PacificBlue, CD14-APC Alexafluor700, CD86-Phycoerythrin, or an isotype control-Phycoerythrin (all Beckman-Coulter) and CD279(PD1)-BrilliantViolet510(Biolegend). Monocytes were identified as CD45+CD14+cells, CD86 mean fluorescent intensity (MFI) was analyzed on gated monocytes and compared with labeling with an isotypic control (online supplemental figure 1). CD86RO was calculated as follows: (1) MFIs-MFIns=ΔMFI, where MFIs is MFI of the Phycoerythrin-labeled anti-CD86 mAb; MFIns is the non-specific MFI with an isotype control; ΔMFI is the difference between these two values, representing a relative measurement of specific binding and (2) CD86RO at each timepoint was calculated using: [1-(ΔMFItimepoint/ ΔMFIbaseline)] where ΔMFIbaseline is calculated from a sample extracted within 1 hour of first abatacept dose (online supplemental figure 1).5 6 For PD1 expression, the gating strategy is described in online supplemental figure 2; CD3+T-lymphocytes were gated in lymphocytes, followed by CD4+ and CD8+T-cells gating within CD3+T-cells. The % of PD1+cells were then analyzed in each population.8 Methods for assessment of abatacept and pembrolizumab circulating levels are in online supplemental data.
jitc-2022-004699supp001.pdf (4.1MB, pdf)
Case-report
A 25-year-old man was admitted to the intensive care unit (ICU) with fulminant ICI-myocarditis. Six months earlier, he was diagnosed with non-operable thymoma extending to lung and pleura (figure 1). First-line chemotherapy included cyclophosphamide, doxorubicin, and cisplatin, with partial response; but still unsuitable for surgical removal. Thymoma-cells expressed high levels of PDL1, prompting pembrolizumab treatment after expert panel consultation. Baseline troponin levels were normal and echocardiography showed normal left ventricular ejection fraction (LVEF:65%).
Figure 1.
Timechart of the evolution of tumor size, PD1 +T cells and pembrolizumab plasma concentration after injection, and through the myocarditis event. Thoracic CT-scanner images showing the thymoma 48 days before myocarditis (ie, 34 days before pembrolizumab) and 1-year follow-up after pembrolizumab (maximal tumor size in mm) with the different anticancer treatment’s subsequent sequences. Evolution over time of pembrolizumab circulating levels associated with proportion of T-cells expression PD1 (%) is also represented.
Two weeks after pembrolizumab initiation, the patient presented with chest pain, subtle myalgia and was admitted to cardiology (index event, day 0 (D0)). Coronary angiography was normal with cardiac MRI showing decreased LVEF, mild pericardial effusion, edema on T2-mapping affecting LV inferior wall and junctional late gadolinium enhancement; and LV endomyocardial biopsy identifying a pathological inflammatory infiltrate mainly composed of CD3+ T-cells (20/mm²) and CD68+ macrophages (7 /mm²) compatible with ICI-myocarditis. Muscular biopsy confirmed the concurrent ICI-myositis with dense focal inflammatory infiltrates also mainly composed of T-cells and macrophages with necrotic myofibers on pathology. Troponin-T and creatine-kinase rose, respectively, to 3000 ng/L (upper-limit-normal-value:14 ng/L) and 3714 IU/L (upper-limit-normal- value <190 IU/L), while LVEF deteriorated to <10%, despite prompt administration of intravenous boluses of methylprednisolone(1 g/day)×3 doses and addition of mycophenolate-mofetil (figure 2). He was transferred to ICU on day 8, with cardiogenic shock, persistent ventricular tachycardia, and severe intraventricular conduction blockade, requiring implantations of an extracorporeal life support (ECLS) (figure 2).
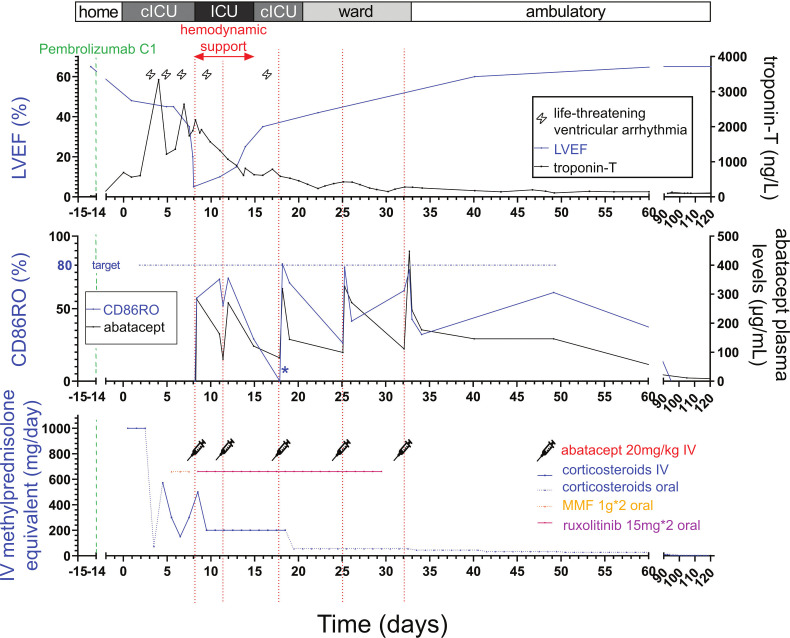
Figure 2.
ICI-myocarditis case report evolution on immunossupressant drugs. Timechart evolution of the patient (103 kg) with the main clinical events, treatments received and abatacept immune-monitoring results (circulating levels and CD86RO). CD86RO, CD86 receptor occupancy on circulating monocytes; (C) ICU, (cardiac). 99th percentile normal upper value of troponin-T was 14 ng/L; Hemodynamic support included extracorporeal life support associated with norepinephrine for the first 48hours. *For graphical representation, negative values of CD86RO were represented as null (see the Methods section for CD86RO value computation). ICI, immune checkpoint inhibitor; ICU, intensive care unit; LVEF, left ventricular ejection fraction; MMF, mycophenolate mofetil.
Baseline level of CD86 expression on circulating monocytes were evaluated to allow for subsequent immune-monitoring on abatacept to reach CD86RO≥80% (figure 2).5 6 This threshold has been demonstrated to be relevant in current indications of CTLA4 fusion proteins, particularly belatacept.1 5 6 This strategy resulted in a loading dose of intravenous abatacept 20 mg/kg (similar to belatacept loading dose equivalent given in graft rejection prophylaxis),1 in combination with oral ruxolitinib 15 mg two times daily and intravenous methylprednisolone 2 mg/kg starting on day 8. Within a week, ventricular arrhythmias resolved, LVEF recovered to 35%–40%, and troponin progressively decreased to ~1000 ng/L. CD86RO increased near 80% after each abatacept injection, but monitoring showed initially (the first three doses) very fast consumption of abatacept with maximal abatacept plasma concentration dropping from ~300 to ~150 µg/mL (50% clearance) consistent with maximal CD86RO decreasing from ~80% to below 40% in ~2–3 days vs a known half-life of ~13 days1 (figure 2). All decisions regarding timing and dosage of abatacept injections were made on CD86RO assessment, clinical (including ECG with ventricular patterns, online supplemental figure 3), LVEF and biological evolution (troponin-T). Patient was successfully weaned from inotropes within 48 hours and from ECLS within 7 days after abatacept/ruxolitinib start. After ECLS weaning, the only significant recurrences in sustained ventricular arrhythmia occurred on day 17 and was concomitant with a negative CD86RO, prompting an additional abatacept dosing. Corticosteroids and ruxolitinib were progressively stopped over a month. The patient ultimately required 5 injections of abatacept 20 mg/kg (day 8, day 11, day 18, day 25, and day 32).
On day 40, the patient fully recovered clinically, with LVEF restoring to 60%. A wearable defibrillator showed no significant arrhythmic events to day 106. Ramipril and beta blockers were started on day 18–day 20 and were maintained after discharge. Pembrolizumab was permanently discontinued. Further CT-scan assessment showed tumor shrinkage compatible with a surgical procedure; however, the patient refused surgery. 4 months after pembrolizumab dosing, the tumor relapsed, concomitant to restoration of lymphocytes expressing PD1 (15% vs. <1% just after pembrolizumab), correlating with pembrolizumab circulating levels being undetectable (figure 1). The patient was then treated with combination of carboplatin and paclitaxel.
Discussion
This case shows the potential of reversal of a fulminant ICI-myocarditis using receptor occupancy guided and tailored dosing of abatacept, combined with ruxolitinib and corticosteroids. Abatacept (a CTLA4 fusion-protein), antagonizes the activation of ICI pathways by inhibiting the upstream antigen-presenting-cells (monocytes); meanwhile, the association with corticosteroids and ruxolitinib, a JAK inhibitor, inhibits T-cell signals either directly (corticosteroids), or indirectly through cytokine pathways inhibition (ruxolitinib).1 9 As T-cells and macrophages are critical in the development of ICI-myocarditis, specifically targeting them is important for treating this condition.1 3 4
Abatacept dosing was assessed using threshold chosen for CD86RO (CD86RO≥80%) similar to those used in allograft rejection with belatacept and in rheumatoid arthritis with abatacept.1 5 6 Using this strategy, abatacept dose used to reach CD86RO≥80% in our case was 4–6 times compared with dose used in RA within the two first weeks (three doses of 20 mg/kg herein vs 10 mg/kg in RA) and similar to that used for allograft rejection (three doses of belatacept 10 mg/kg within 2 weeks in rejection, with belatacept being twice more potent than abatacept in term of CD86RO).1
Frequent immune-profiling revealed association between CD86RO and evolution of bio-clinical phenotypes (troponin-T, ECG, figure 2). We observed recurrences in sustained ventricular arrhythmias in the early management phases when CD86RO was <40%, emphasizing the link between CD86RO and cardiac arrhythmias, a hallmark of ICI-myocarditis.10 Assessing CD86RO helped to precisely time the injections and dose of abatacept. Yet, numerous questions remain such as potential interacting elements altering abatacept-CD86RO relationship in ICI-myocarditis including concomitant intake of ipilimumab (anti-CTLA4) and remain to be determined in a future trial (NCT05195645).
Lastly, a major challenge to consider while treating patient with severe immune-related adverse events is how to mitigate the potential lethality associated with this side effect while preserving antitumor beneficial effects. Most immunossupressant have been flagged with a potential risk for protumorigenicity particularly when used chronically for years.1 11 In our case, use of abatacept and ruxolitinib was the shortest possible and limited to less than a month; though associated with high-dose corticosteroids. We did observe a relapse of thymoma ~4 months after last anti-PD1 dose; and ~3 months after last abatacept/ruxolitinib dosing. This relapse was concurrent with re-expression of PD1 on T-cells and pembrolizumab blood level being undetectable suggesting a progressive vanish of ICI effect; but it is unclear to which extent the various immunossupressant drugs used to treat the ICI-myocarditis have contributed to this observation. Further research is indeed required to assess the question of the optimal drug mix, dosage and duration to be used to preserve ICI therapeutic effect while treating a severe ICI-related adverse event.
Conclusion
We present a case of quick reversal of pembrolizumab-induced fulminant life-threatening myocarditis, using personalized dose adapted immunosuppression based on abatacept, ruxolitinib and corticosteroids.
Footnotes
MR and J-ES contributed equally.
Correction notice: Since this article was first published, a typo has been corrected in the third paragraph of the 'Case report' section.
Contributors: J-ES designed the research. J-ES and LSN wrote the first draft. MR established the immune monitoring methods. All authors contributed to data collection and editing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: Written consent for publication was obtained from the patient. J-ES have participated to advisory boards or consultancy from BMS, Novartis, Banook, AstraZeneca and Beigene. Other authors have nothing to disclose regarding this manuscript. SE received modest consultant fees from AstraZeneca, Amgen, BMS, Banook, Celgene and EISAI. J-ES, JM and YA have patents related to the treatment of ICI related immune adverse events. JM has served on advisory boards for Bristol Myers Squibb, Takeda, Audentes, Deciphera, Janssen, Immuno-Core, Boston Biomedical, Amgen, Myovant, Kurome Therapeutics, Star Therapeutics, ProtinQure, Pharmacyclics, Pfizer, Mallinckrodt Pharmaceuticals, Silverback Therapeutics, Cytokinetics, and AstraZeneca. JM was supported by National Institutes of Health grants (R01HL141466, R01HL155990, and R01HL156021).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by APHP-CSE-20-37_JOCARDITE.
References
- 1.Geraud A, Gougis P, Vozy A, et al. Clinical pharmacology and interplay of immune checkpoint agents: a yin-yang balance. Annu Rev Pharmacol Toxicol 2021;61:85–112. 10.1146/annurev-pharmtox-022820-093805 [DOI] [PubMed] [Google Scholar]
- 2.Salem J-E, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–89. 10.1016/S1470-2045(18)30608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salem J-E, Allenbach Y, Vozy A, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med 2019;380:2377–9. 10.1056/NEJMc1901677 [DOI] [PubMed] [Google Scholar]
- 4.Wei SC, Meijers WC, Axelrod ML, et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov 2021;11:614–25. 10.1158/2159-8290.CD-20-0856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latek R, Fleener C, Lamian V, et al. Assessment of belatacept-mediated costimulation blockade through evaluation of CD80/86-receptor saturation. Transplantation 2009;87:926–33. 10.1097/TP.0b013e31819b5a58 [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Shen J, Hong Y, et al. Time-varying belatacept exposure and its relationship to efficacy/safety responses in kidney-transplant recipients. Clin Pharmacol Ther 2012;92:251–7. 10.1038/clpt.2012.84 [DOI] [PubMed] [Google Scholar]
- 7.Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for Glucocorticoid-Refractory acute graft-versus-host disease. N Engl J Med 2020;382:1800–10. 10.1056/NEJMoa1917635 [DOI] [PubMed] [Google Scholar]
- 8.Pitoiset F, Cassard L, El Soufi K, et al. Deep phenotyping of immune cell populations by optimized and standardized flow cytometry analyses. Cytometry A 2018;93:793–802. 10.1002/cyto.a.23570 [DOI] [PubMed] [Google Scholar]
- 9.Chen C-Y, Chiu C-F, Bai L-Y. Treatment of pembrolizumab-induced cutaneous lesions with ruxolitinib. Eur J Cancer 2019;113:69–71. 10.1016/j.ejca.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 10.Power JR, Alexandre J, Choudhary A, et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation 2021;144:1521–3. 10.1161/CIRCULATIONAHA.121.055816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-004699supp001.pdf (4.1MB, pdf)