Abstract
Introduction
Remote patient monitoring (RPM) has emerged as a potential avenue for optimising the management of symptoms in patients undergoing chemotherapy. However, RPM is a complex, multilevel intervention with technology, workflow, contextual and patient experience components. The purpose of this pilot study is to determine the feasibility of RPM protocol implementation with respect to decentralised recruitment, patient retention, adherence to reporting recommendations, RPM platform usability and patient experience in ambulatory cancer patients at high risk for chemotherapy-related symptoms.
Methods and analysis
This protocol describes a single-arm decentralised feasibility pilot study of technology-enhanced outpatient symptom management system in patients with gastrointestinal and thoracic cancer receiving chemotherapy and cancer care at a single site (MD Anderson Cancer Center, Houston Texas). An anticipated total of 25 patients will be recruited prior to the initiation of chemotherapy and provided with a set of validated questionnaires at enrollment and after our 1-month feasibility pilot trial period. Our intervention entails the self-reporting of symptoms and vital signs via a HIPAA-compliant, secure tablet interface that also enables (1) the provision of self-care materials to patients, (2) generation of threshold alerts to a dedicated call-centre and (3) videoconferencing. Vital sign information (heart rate, blood pressure, pulse, oxygen saturation, weight and temperature) will be captured via Bluetooth-enabled biometric monitoring devices which are integrated with the tablet interface. Protocolised triage and management of symptoms will occur in response to the alerts. Feasibility and acceptability metrics will characterise our recruitment process, protocol adherence, patient retention and usability of the RPM platform. We will also document the perceived effectiveness of our intervention by patients.
Ethics and dissemination
This study has been granted approval by the institutional review board of MD Anderson Cancer Center. We anticipate dissemination of our pilot and subsequent effectiveness trial results via presentations at national conferences and peer-reviewed publications in the relevant medical journals. Our results will also be made available to cancer survivors, their caregivers and hospital administration.
Trial registration number
NCI202107464.
Keywords: CHEMOTHERAPY, Adult oncology, Protocols & guidelines, Health informatics
Strengths and limitations of this study.
The present pilot study will allow researchers to delineate the feasibility of recruitment, acceptability and implementation of a remote patient monitoring platform for the active surveillance of and early intervention for chemotherapy-related symptoms.
The study is limited to patients with gastrointestinal and thoracic cancers, so the results may not be generalisable to other solid organ or haematogenous cancers.
The study is limited to patients at a single, high volume institution, which may limit the generalisability of the outcomes with regard to smaller hospitals and care systems.
The study has been designed to include a diverse patient population, with respect to age, gender and race, which should allow the feasibility results to be generalised to a broad demographic of patients within gastrointestinal and thoracic cancers.
Introduction
The motivation for applying telemedicine and digital health tools in oncology has been evident for several years.1 However, their potential to transform clinical care is now beginning to be realised, partly due to the unparalleled scope of the SARS-CoV2 pandemic and need to maintain continuity of care within the context of quarantine and self-isolation.2 Technologies now in use include remote patient monitoring (RPM), apps, wearables and chatbots among others.3 RPM characterises the real-time acquisition and transmission of health-related data using home-based, sensor-enabled digital monitoring devices and mobile applications to assess vital signs, symptoms and other health-related outcomes.3 RPM has been successfully leveraged for the outpatient management of several chronic conditions including diabetes, heart failure, chronic wounds and chronic obstructive pulmonary disease.4–7 RPM implementation in these clinical settings have been associated with reduced emergency room (ER) utilisation, improved quality-of-life, reduced healthcare spending and better symptom control.8–11
Although overall mortality from cancer is declining,12 patients with cancer still face debilitating physical and psychosocial treatment side effects including fatigue, myalgias, pain, shortness of breath, sleep disturbance and depression.13 14 Unfortunately, clinicians often fail to recognise the incidence and severity of chemotherapy-induced symptoms, even in instances that mandate the reporting of treatment toxicities.15–17 Poor symptom management is associated with increased healthcare spending, and worse quality of life, clinical outcomes and overall survival.9 18–20 This has led to considerable interest in leveraging symptom self-reporting during ambulatory cancer care.21 Pioneering work by Basch et al 19 identified statistically significant reductions in ER utilisation, chemotherapy disruptions, and gains in quality-adjusted survival.19 A recent phase III randomised controlled trial (RCT) by Absolom et al identified improved physical well-being (6 and 12 weeks) and self-efficacy (18 weeks) among patients with breast, colorectal or gynaecological cancers that were exposed to electronic self-reporting of symptoms during cancer treatment.22 Of note, there were no associated improvements in treatment delays, dose reductions, chemotherapy drug changes or hospital admissions in the treatment group.22
However, despite evidence of RPM effectiveness, systematic approaches for symptom assessment, patient–provider communication and early intervention are still lacking.23 This is largely because the full integration of electronic patient-reported outcomes (ePROs) with a health system’s medical record and clinical informatics platform has not been widespread.24 As a result, clinicians do not have ‘real time’ access to patient generated- health data (eg, vitals signs and ePROs) and the prevailing care model for treatment-toxicities is largely patient-initiated and reactive that is, unable to proactively monitor and mitigate symptom burden before they escalate.25 RPM-enabled digital touch points can reassure patients that their treatment team is connected to them and informed about all aspects of their disease course. Furthermore, the American Society of Oncology has strongly advocated for the increased integration of standardised personal health information (eg, demographics, health status, treatment status, side effects and symptoms) in real time into routine inpatient and outpatient patient care.26 27 Unfortunately, the evidence for RPM efficacy in oncology is limited and most studies have excluded the caregivers’ experiences and involvement, which is not a realistic representation of the utility of remote monitoring in the oncology population.28 29
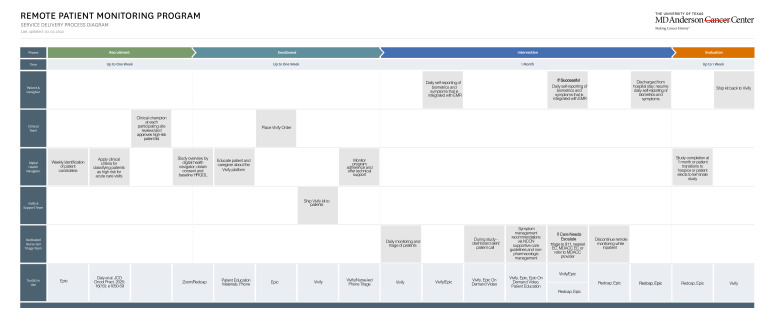
We posit that the implementation of a RPM platform that facilitates ePRO capture and protocolised, point-in-time vital sign measurements has the potential to enhance real-time clinical decision making, improve health-related quality-of-life, lower symptom burden, mitigate treatment delays and engender greater patient engagement. Additionally, moving from an episodic care model to a more continuous care model has the potential of improving patient experience. At MD Anderson Cancer Center (MDACC), we have developed a technology and operational infrastructure for remotely monitoring chemotherapy symptoms in-between clinic visits. It entails the active surveillance of patient-reported biometrics (heart rate, blood pressure, temperature, oxygen saturation and weights) and adverse events (Patient-Reported Outcome-Common Terminology Criteria for Adverse Events (PRO-CTCAE)) by advanced care practitioners (ie, nurse practitioners), guided by backend threshold alerts for both vitals and ePROs. Automated self-care advice for patients and caregivers is also delivered across the platform. In this work, we articulate our framework for the development and pilot-testing of an RPM platform, for the active surveillance of treatment-related symptoms, in patients with gastrointestinal and thoracic cancers who are receiving neoadjuvant or adjuvant chemotherapy in the ambulatory setting.30 Lastly, the evolving COVID-19 pandemic has also catalysed greater awareness and implementation of decentralised clinical trials in the life sciences sector.31 This paradigm shift is in response to pandemic-related regulatory waivers for trial conduct, a need to preserve the availability of personnel protective equipment for hospital staff, and an acknowledgement of the high cost structure and limited patient access in traditional ‘brick and mortar’ trial infrastructure.31–33 We will also use the proposed pilot study to develop and implement a decentralised or virtual workflow for patient recruitment, education about the RPM platform, enrolment, symptom monitoring and study completion (figure 1).34
Figure 1.
Process map of workflow for decentralised remote patient monitoring pilot study.
Aims
Primary aims
The primary aim of this external pilot study is to investigate the feasibility of protocol implementation (ie, recruitment process, evaluation of eligibility criteria, assessment of usability of technology platform) prior to a non-blinded, RCT of the effectiveness of technology-enhanced (ie, RPM with ePRO capture) outpatient management of treatment-related symptoms. Our a priori specified feasibility objectives are as follows:
Patient eligibility and recruitment—Defined as an approach-to-consent rate of >60% among eligible patients (ie, enrolment rate). Two patients per month, on average, should be consented into the programme.
Adherence—Defined as >70% adherence with PRO-CTCAE surveys 4 days per week and >80% adherence to biometrics reporting 4 days per week.
Implementation outcomes—The feasibility, acceptability and appropriateness of our intervention will be assessed via the following validated four-item psychometric tools: FIM (‘Feasibility of Intervention Measure’), AIM (‘Acceptability of Intervention Measure’) and IAM (‘Intervention Appropriateness Measure’).35 Each item is scored on a 5-point Likert scale (completely disagree to completely agree) and our pilot will be considered successful if the calculated mean scores for each of the implementation measures is >3.35
Feasibility outcomes—We will monitor the generation of an alert (red or yellow) after an abnormal vital sign or self-reporting of a severe symptom burden. We will also record the number of adverse events related to the use of the biometric devices that is, blood pressure cuff, weight scale, thermometer, etc. Lastly, we will track the clinical action that is associated with an alert generation, for example, phone consultation, video visit, care escalation to the ER.
Secondary aims
Following completion of the 1 month pilot reporting period, participants will also be invited to share their experiences with our RPM platform through the use of questionnaires.
Perceived effectiveness—Participating patients will be asked to respond to the following two questions: ‘I found the remote monitoring system helped me manage my symptoms’ and ‘I found that this remote monitoring system helped me better communicate with my care team’.17 Responses will be graded according to a Likert scale (1–5) based on these responses: ‘strongly disagree’, ‘disagree’, ‘neither agree nor disagree’, ‘agree’, ‘strongly agree’.
Usability—The validated Symptom Usability Scale (SUS) will be provided to patients to assess usability of the RPM platform.36 The scale is a reliable tool that consists of 10 questions regarding the usability of an electronic or technology system. Responses are on a 5-point Likert scale (1–5) and our RPM platform will be considered usable if the mean score is greater than 68, concordant with published work.
Methods and analysis
Setting, Patient population and eligibility criteria
This will be a single-arm, single-institution pilot study in the gastrointestinal and thoracic medical oncology clinic at MDACC. Patient enrolment began on 1 July 2021 and is anticipated to conclude by 25 March 2022. We will approach English-fluent adults (≥18 years) with gastrointestinal (stomach, liver, gallbladder, bile duct, pancreas, small bowel, appendix, colon, rectum and anal) and thoracic (oesophageal and lung) cancers who are scheduled to initiate or continue outpatient chemotherapy at The University of Texas MD Anderson Cancer Center in Houston, Texas. There will be no restrictions or exclusions applied based on underlying tumour histology. Patients on combination chemotherapy and immunotherapy or combination chemotherapy and biologics will also be eligible for inclusion. We plan to recruit a diverse sample of patients (n=25), reflecting at least three patients of age >65 years, at least three patients from racial/ethnic minority groups and a balanced gender distribution.17 Patients will be invited by their oncologist providers to participate in the study based on the following published clinical criteria: (1) baseline comorbidities that increase risk of chemotherapy adverse events, (2) provider-identified social barriers to care, (3) inability to tolerate oral intake or ailment sufficient, (4) high tumour burden, (5) high levels of psychosocial distress or multiple symptomatic complaints, (6) recent ER visits or hospitalisations, defined as within the preceding 6 months, (7) recent dose reduction with initial antineoplastic treatment and (8) combined modality therapy for example, chemoradiation.37
Exclusions
Patients receiving investigational new drug treatments (ie, not yet approved by the Food and Drug Administration) or concurrently enrolled in a phase 1 clinical trials will be excluded due to the associated structured reporting and regulatory requirements. Patients with a requirement for inpatient infusion (ie, CAR-T cell therapy), living in institutional settings (ie, prison), with a history of dementia, physical disability or neurological deficits that prohibit their ability to report symptom burden will also be excluded. These disabilities include but are not limited to severe visual, hearing or cognitive impairments which prevent a patient from using the tablets and biometric devices, and an inability to stand that would prevent them from using the weight scale. Patients may participate if they do not have a caregiver or if their caregiver declines participation. Caregivers will participate only with consent of the patient.
RPM intervention
Eligible patients will be provided with an orientation on the Vivify platform for RPM programme (VivifyHealth, Plano, TX) that outlines programme goals, proper use of the equipment, and technical instructions for self-reporting. The process for identifying of eligible patients, patient and caregiver education, study consent, and RPM training materials will be implemented in a contactless fashion via electronic medical record (EMR)-enabled Zoom videoconferencing (figure 1). The ‘teach-back’ method will be used to ensure patient understanding.38 The Vivify platform is HIPAA-compliant, FDA-registered as a Class 1 Medical Device Data System that is commercially available and allows for transmission of biometric data to MDACC’s patient call centre (askMDAnderson), staffed by a dedicated team of oncology trained nurses and advanced care practitioners (ie, nurse practitioners and physician assistants). These clinicians have received additional training in the assessment and management of advanced cancer symptoms as well as the Vivify platform used in this study.
The Vivify platform includes a set of wireless, Bluetooth-connected biometric devices that are provided to patients, including a scale, blood pressure cuff, pulse oximeter, thermometer and tablet computer loaded with the Vivify mobile application and a measure of the frequency and severity of twelve treatment-related symptoms (ie, appetite loss, nausea, vomiting, cough, constipation, hot flashes, diarrhoea, dyspnoea, pain, neuropathy, fatigue and dysuria).19 39 40 Furthermore, if necessary, an internet hot-spot also will be provided. Consistent with our decentralised study design, these devices will be delivered directly to patients by mail from Vivify.34 Vivify will also be responsible for troubleshooting technical problems. The Vivify RPM biometric devices and tablet are comparable to commercially available devices that can be purchased by consumers. At the time of study completion, the kits will be picked up from the patient’s home by Vivify. Patients will be asked to take one reading per day (Monday through Friday) with each device, and to complete one symptom assessment per day (Monday through Friday) via the tablet. Weight assessments will be performed weekly. Study staff will monitor completion of daily device usage and ePRO completion via the Vivify dashboard and will contact patients to address potential technical issues if 3 or more days of data are missing. In our previous studies, this ‘digital navigation’ approach has resulted in early resolution of technical problems and improved data collection.41
As part of the informed consent (online supplemental file 1) and study onboarding process, patients will be asked to follow instructions for proactively seeking medical care that have been provided as part of patient education and by their healthcare team, and not to rely on any feedback received as a result of data submitted through the Vivify platform.
bmjopen-2021-057693supp001.pdf (105.5KB, pdf)
The Vivify platform supports the creation of algorithms to detect vital signs or symptom PROs that exceed predetermined threshold values. Biometric or PRO data that exceed pre-specified threshold values will generated an alert email to the askMDAnderson staff (table 1).19 Our self-reporting system for symptom burden will be adapted from the National Cancer Institute’s CTCAE pertaining to the following common symptoms encountered during chemotherapy: constipation, diarrhoea, fatigue, dry mouth, decreased appetite, difficulty swallowing, nausea, pain, vomiting. These symptoms are graded on a 0 (non-present) to 4 (very severe) and the corresponding alert will be triggered by absolute values of greater than or equal to 2 (table 1).
Table 1.
Threshold values for alerts on RPM platform
| Biometric variable | Medium trigger | High trigger |
| BP systolic (hypertension) | 155–179 | ≥180 |
| Systolic (hypotension) | 90–99 | ≤89 |
| Diastolic (hypertension) | 101–109 | >110 |
| Diastolic (hypotension) | None | None |
| Oxy sat | <94% | <90% |
| HR | ||
| Bradycardia | None | <55 |
| Tachycardia | None | >110 |
| Finger stick | <70,>120 | <55, >150 |
| Temp | >100.5, | >102 |
| Weight | None | Loss of 10 pounds |
| PRO measure for symptom burden | Medium trigger | High trigger |
| PRO-CTCAE value | 2 | >3 or increase by more than 2 points from prior value |
BP, blood pressure; CTCAE, Common Terminology Criteria for Adverse Events; HR, heart rate; PRO, patient-reported outcome; RPM, remote patient monitoring.
The alerts, based on biometric and/or ePRO data, will also be transmitted in near real-time a secure, HIPAA-compliant Web dashboard that is accessible to askMDAnderson staff. On receipt of an alert email, the askMDAnderson staff will review the data via the Vivify dashboard and will provide follow-up and referral as clinically appropriate, using National Comprehensive Cancer Network symptom management guidelines as a point of reference for non-pharmacological recommendations.42 43 The Vivify platform is fully integrated with MDACC’s EMR (Epic, Madison, WI), allowing the primary clinical team to also have access to the patient-reported data. Lastly, the end-user license agreement associated with the Vivify device and study consent documents will reinforce that patients should not substitute the RPM programme with the need to notify their care team if they are experiencing concerning symptoms. Following the initiation of a severe symptom alert, this message will appear on the Vivify platform: ‘The Chemo Remote Monitoring Program hours are 8 a.m. to 8 p.m., Monday through Friday. If you are concerned, need assistance after hours, or feel that the symptoms are worsening, please contact your primary oncologist office or go to your local emergency room. In case of an emergency, call 911’.
Data collection
Using a combination of data abstraction from the EMR by the study team and patient feedback via surveys, we hope to collect the following information:
Patient demographics—age, race, sex, presence of caregiver and marital status.
Clinical information—cancer histology, stage, location, chemotherapy regimen, line of chemotherapy, Eastern Cooperative Oncology Group status
Implementation and feasibility measures as outlined above that is, consent rate, AIM, FIM, IAM, perceived effectiveness rating and SUS score.
All data points will be collated and stored on a REDCap (Research Electronic Data CAPture) database. REDCap is a secure web application that is widely used in health services research. It is able to support the administration of surveys, data export from external sources such as an EMR, and provides a user-friendly interface for data entry.44
Data analysis plan
Descriptive statistics (eg, means, medians, numbers, percentages, ranges and SD) for demographic and clinical characteristics of participants will be reported. Response rates (ie, completion of required assessments), most frequent symptoms, proportion of actioned alerts will also be described. Graphical methods (eg, boxplots and histograms) will also be employed to examine the distributions of outcome measures. By design, the present pilot study is not intended or powered to determine the efficacy of RPM in the outpatient management of treatment-related symptoms. This important scientific question will be addressed with our planned RCT. Our anticipated sample size of 25 will allow us to be relatively precise in our conclusions with respect to continuous implementation outcomes (FIM, AIM, IAM) and facilitate preliminary estimates for our larger trial. All data analysis will be carried out with SAS, version 9.4 statistical software (SAS Institute).
Patient and public involvement
The research team has engaged with the Patient and Family Advisory Council (PFAC) during the conceptualisation and design phase of our pilot. Specifically, they provided timely guidance on the patient-centeredness of our research methods and the ways in which our anticipated pilot study results were meaningful. PFAC members also provided information on the acceptability and feasibility of our schedule for patient self-reporting of PROs and biometric data that is, daily Monday through Friday. PFAC is a unique programme composed of patients, survivors and caregivers, it serves as the patients’ ‘voice’ for institutional committees, operational projects, and department-level initiatives. The PFAC will be retained in an advisory capacity for the duration of both the pilot and subsequent RCT. PFAC members were not directly involved in study conduct or patient recruitment. Given our implementation focus, we do not intend to distribute pilot study results to participants. The study leadership team (Drs Offodile and Peterson) plan to meet with the PFAC two to three times a year to review and iterate study plans and seek feedback with respect long-term sustainability and implementation of RPM at MDACC.
Experience design
We used a human-centred design thinking approach to anticipate the needs of our patients and created end-to-end experiential touchpoints that would enable a seamless experience on the programme.
Ethics and dissemination
All study documents (ie, protocol, consent, educational materials) have been approved by the MDACC Institutional Review Board. Patients will be informed that their participation is completely voluntary and that there will receive no compensation. They will also be assured that they will receive standard-of-care and shall be exposed to no negative consequences as a result of either their participation or refusal. All patients will be able to opt out of the study at any time and for any reason. We anticipate dissemination of our pilot and subsequent effectiveness trial results via presentations at national conferences and peer-reviewed publications in the relevant medical journals. No patient identifying information will be used in the publication of findings.
Protection against risks to patient safety
The present pilot study is of minimal risk to patients. We will use validated survey questionnaires, the content of which are not sensitive in nature. It is possible that our remote monitoring devices may incite anxiety, annoyance or distress in patients. However, we believe that the possibility of such adverse events is minimal. To reduce this risk of distress, during the onboarding and consent process, patients will be instructed to (1) not substitute routine medical care with the device readings and (2) contact their primary oncology care team if at any point they feel concern or worry. Furthermore, all patients will receive standard chemotherapy education, which includes explicit guidance as to when to seek urgent medical care. The Vivify devices are FDA approved, HIPAA-compliant, commercially available and meet the highest standards of protecting patient privacy. All data transmission will leverage standard encryption and security protocols. Vivify will not participate in the study design, patient recruitment, data interpretation, analysis, or dissemination of results. Patients and the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
All information extracted from the medical record will be entered onto coded data sheets which will be maintained on stored in approved locations. Each patient will be assigned a unique study identification number by MDACC research staff which will be programmed into the Vivify tablet and devices. Electronic data will be strictly stored only on password-protected Institutional computers, accessible only to the PI and collaborators. Only the PI and the collaborators will be participating in the collection and analysis of data. No patient identifying information will be used in presentation or publication of this material. On study termination, all data, questionnaires and remaining identifiers will be banked indefinitely in REDCap according to institutional policy for future use only in IRB-approved research settings. Lastly, all study personnel will undergo the requisite human subjects research training which includes procedures for maintaining patient confidentiality.
Trial status
Pilot study is now open and recruiting patients.
Supplementary Material
Acknowledgments
The authors are very grateful to all the clinicians and advance practice providers in the gastrointestinal and thoracic medical oncology clinics who have been critical to this pilot study (patient recruitment, providing feedback on protocol elements, assisting with patient education). We also thank members of the PFAC at MDACC for their dedicated support to this programme.
Footnotes
Twitter: @anaeze_offodile
Contributors: AO, DAD, SJ, MJO and SP developed the initial pilot study concept. AO, SRD, SJ, JPF, DAD, MJO and SP assisted with the study design. CJM and ED contributed to drafting the protocol. SS provided oversight of the analytical plan. All authors made critical revisions to the manuscript. AO is the principal Investigator and assumes final responsibility for all aspects of trial design, the protocol, and the trial conduct. All authors have read and approved this manuscript. There is agreement on accountability for all aspects of the work including questions related to the accuracy and integrity of the work.
Funding: Support provided, in part, by the Assessment, Intervention and Measurement (AIM) Shared Resource through a Cancer Center Support Grant (CA16672, PI: P. Pisters, MD Anderson Cancer Center), from the National Cancer Institute, National Institutes of Health.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Sirintrapun SJ, Lopez AM. Telemedicine in cancer care. Am Soc Clin Oncol Educ Book 2018;13:540–5. 10.1200/EDBK_200141 [DOI] [PubMed] [Google Scholar]
- 2. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med Overseas Ed 2020;382:1679–81. 10.1056/NEJMp2003539 [DOI] [PubMed] [Google Scholar]
- 3. Andreu-Perez J, Leff DR, Ip HMD, et al. From Wearable Sensors to Smart Implants--Toward Pervasive and Personalized Healthcare. IEEE Trans Biomed Eng 2015;62:2750–62. 10.1109/TBME.2015.2422751 [DOI] [PubMed] [Google Scholar]
- 4. Barnett TE, Chumbler NR, Vogel WB, et al. The effectiveness of a care coordination home telehealth program for veterans with diabetes mellitus: a 2-year follow-up. Am J Manag Care 2006;12:467–74. [PubMed] [Google Scholar]
- 5. Louis AA, Turner T, Gretton M, et al. A systematic review of telemonitoring for the management of heart failure. Eur J Heart Fail 2003;5:583–90. 10.1016/S1388-9842(03)00160-0 [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen LM, Phanareth K, Nolte H, et al. Internet-based monitoring of asthma: a long-term, randomized clinical study of 300 asthmatic subjects. J Allergy Clin Immunol 2005;115:1137–42. 10.1016/j.jaci.2005.03.030 [DOI] [PubMed] [Google Scholar]
- 7. Bartoli L, Zanaboni P, Masella C, et al. Systematic review of telemedicine services for patients affected by chronic obstructive pulmonary disease (COPD). Telemed e-Health 2009;15:877–83. 10.1089/tmj.2009.0044 [DOI] [PubMed] [Google Scholar]
- 8. Cleland JGF, Louis AA, Rigby AS, et al. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care management system (TEN-HMS) study. J Am Coll Cardiol 2005;45:1654–64. 10.1016/j.jacc.2005.01.050 [DOI] [PubMed] [Google Scholar]
- 9. Noel HC, Vogel DC, Erdos JJ, et al. Home telehealth reduces healthcare costs. Telemed e-Health 2004;10:170–83. 10.1089/tmj.2004.10.170 [DOI] [PubMed] [Google Scholar]
- 10. Santamaria N, Carville K, Ellis I. The effectiveness of digital imaging and remote expert wound consultation on healing rates in chronic lower leg ulcers in the Kimberley region of Western Australia. Prim Intent Aust J Wound Manag 2004;12:62–70. [Google Scholar]
- 11. Bartoli G, Cipolletti R, Di Antonio G, et al. A convergent approach to (R)-Tiagabine by a regio- and stereocontrolled hydroiodination of alkynes. Org Biomol Chem 2010;8:3509–17. 10.1039/c005042c [DOI] [PubMed] [Google Scholar]
- 12. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA A Cancer J Clin 2021;71:7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 13. Rhondali W, Yennurajalingam S, Chisholm G, et al. Predictors of response to palliative care intervention for chronic nausea in advanced cancer outpatients. Support Care Cancer 2013;21:2427–35. 10.1007/s00520-013-1805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tookman AJ, Jones CL, DeWitte M, et al. Fatigue in patients with advanced cancer: a pilot study of an intervention with infliximab. Support Care Cancer 2008;16:1131–40. 10.1007/s00520-008-0429-x [DOI] [PubMed] [Google Scholar]
- 15. Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J Clin Oncol 2004;22:3485–90. 10.1200/JCO.2004.03.025 [DOI] [PubMed] [Google Scholar]
- 16. Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol 2015;33:910–5. 10.1200/JCO.2014.57.9334 [DOI] [PubMed] [Google Scholar]
- 17. Wright AA, Raman N, Staples P, et al. The hope pilot study: harnessing patient-reported outcomes and biometric data to enhance cancer care. JCO Clin Cancer Inform 2018;14:1–12. 10.1200/CCI.17.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirkova J, Walsh D, Rybicki L, et al. Symptom severity and distress in advanced cancer. Palliat Med 2010;24:330–9. 10.1177/0269216309356380 [DOI] [PubMed] [Google Scholar]
- 19. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016;34:557–65. 10.1200/JCO.2015.63.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–8. 10.1001/jama.2017.7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Judson TJ, Bennett AV, Rogak LJ, et al. Feasibility of long-term patient self-reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol 2013;31:2580–5. 10.1200/JCO.2012.47.6804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Absolom K, Warrington L, Hudson E, et al. Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol 2021;39:734–47. 10.1200/JCO.20.02015 [DOI] [PubMed] [Google Scholar]
- 23. Maguire R, Fox PA, McCann L, et al. The eSMART study protocol: a randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer. BMJ Open 2017;7:e015016. 10.1136/bmjopen-2016-015016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basch E, Bennett A, Pietanza MC. Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst 2011;103:1808–10. 10.1093/jnci/djr493 [DOI] [PubMed] [Google Scholar]
- 25. Richards HS, Blazeby JM, Portal A, et al. A real-time electronic symptom monitoring system for patients after discharge following surgery: a pilot study in cancer-related surgery. BMC Cancer 2020;20:543. 10.1186/s12885-020-07027-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meropol NJ, Kris MG, Winer EP. The American Society of Clinical Oncology’s blueprint for transforming clinical and translational cancer research. J Clin Oncol 2012;30:690–1. 10.1200/JCO.2011.40.1125 [DOI] [PubMed] [Google Scholar]
- 27. American Society of Clinical Oncology . ASCO’s Blueprint for Transforming Clinical and Translational Cancer Research. Available: https://www.asco.org/sites/new-www.asco.org/files/content-files/research-and-progress/documents/2011-blueprint-accelerating-progress-against-cancer.pdf [Accessed 02 Aug 2021].
- 28. Peterson SK, Shinn EH, Basen-Engquist K, et al. Identifying early dehydration risk with home-based sensors during radiation treatment: a feasibility study on patients with head and neck cancer. J Natl Cancer Inst - Monogr 2013;2013:162–8. 10.1093/jncimonographs/lgt016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamdar M, Centi AJ, Agboola S, et al. A randomized controlled trial of a novel artificial intelligence-based smartphone application to optimize the management of cancer-related pain. J Clin Oncol 2019;37:11514. 10.1200/JCO.2019.37.15_suppl.11514 [DOI] [Google Scholar]
- 30. Moore CG, Carter RE, Nietert PJ, et al. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci 2011;4:332–7. 10.1111/j.1752-8062.2011.00347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Offodile AC, Aloia T. Oncology clinical transformation in response to the COVID-19 pandemic. JAMA Health Forum 2020;1:e201126. 10.1001/jamahealthforum.2020.1126 [DOI] [PubMed] [Google Scholar]
- 32. Kadakia KT, Asaad M, Adlakha E. Virtual clinical trials in Oncology—Overview, challenges, policy considerations, and future directions. JCO Clin Cancer Informatics 2021;5:421–5. [DOI] [PubMed] [Google Scholar]
- 33. Unger JM, Vaidya R, Hershman DL, et al. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst 2019;111:245–55. 10.1093/jnci/djy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kadakia KT, Halperin DM, Offodile AC. Operationalizing virtual trials in Oncology—From aspiration to action. JCO Clin Cancer Informatics 2021;5:953–7. [DOI] [PubMed] [Google Scholar]
- 35. Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci 2017;12:108. 10.1186/s13012-017-0635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Hum Comput Interact 2008;24:574–94. 10.1080/10447310802205776 [DOI] [Google Scholar]
- 37. Daly B, Kuperman G, Zervoudakis A, et al. Insight care pilot program: redefining seeing a patient. JCO Oncol Pract 2020;16:e1050–9. 10.1200/OP.20.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. White M, Garbez R, Carroll M, et al. Is “teach-back” Associated with knowledge retention and hospital readmission in hospitalized heart failure patients? J Cardiovasc Nurs 2013;28:137–46. 10.1097/JCN.0b013e31824987bd [DOI] [PubMed] [Google Scholar]
- 39. Daly B, Gorenshteyn D, Nicholas KJ, et al. Building a clinically relevant risk model: predicting risk of a potentially preventable acute care visit for patients starting antineoplastic treatment. JCO Clin Cancer Inform 2020;4:275–89. 10.1200/CCI.19.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Daly B, Nicholas K, Gorenshteyn D, et al. Misery Loves company: presenting symptom clusters to urgent care by patients receiving antineoplastic therapy. J Oncol Pract 2018;14:e484–95. 10.1200/JOP.18.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Offodile AC, Seitz AJ, Peterson SK. Digital health navigation: an enabling infrastructure for optimizing and integrating virtual care into oncology practice. JCO Clin Cancer Inform 2021;5:1151–4. 10.1200/CCI.21.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vidall C, Dielenseger P, Farrell C, et al. Evidence-based management of chemotherapy-induced nausea and vomiting: a position statement from a European cancer nursing forum. Ecancermedicalscience 2011;5:211. 10.3332/ecancer.2011.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. NCCN . National comprehensive cancer network (NCCN) guidelines for supportive care. Available: https://www.nccn.org/professionals/physician_gls/default.aspx#supportive [Accessed 01 Apr 2021].
- 44. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057693supp001.pdf (105.5KB, pdf)