Abstract
Introduction
We evaluated a new Simoa plasma assay for phosphorylated tau (P‐tau) at aa217 enhanced by additional p‐tau sites (p217+tau).
Methods
Plasma p217+tau levels were compared to 18F‐NAV4694 amyloid beta (Aβ) positron emission tomography (PET) and 18F‐MK6240 tau PET in 174 cognitively impaired (CI) and 223 cognitively unimpaired (CU) participants.
Results
Compared to Aβ− CU, the plasma levels of p217+tau increased 2‐fold in Aβ+ CU and 3.5‐fold in Aβ+ CI. In Aβ− the p217+tau levels did not differ significantly between CU and CI. P217+tau correlated with Aβ centiloids P = .67 (CI, P = .64; CU, P = .45) and tau SUVRMT P = .63 (CI, P = .69; CU, P = .34). Area under curve (AUC) for Alzheimer's disease (AD) dementia versus Aβ− CU was 0.94, for AD dementia versus other dementia was 0.93, for Aβ+ versus Aβ− PET was 0.89, and for tau+ versus tau− PET was 0.89.
Discussion
Plasma p217+tau levels elevate early in the AD continuum and correlate well with Aβ and tau PET.
Keywords: Alzheimer's disease, amyloid plaque, Aβ imaging, amyloid imaging, blood biomarkers, blood diagnostic for Alzheimer's disease, paired helical filaments, phosphorylated tau, plasma P‐tau217, positron emission tomography, tau imaging
1. INTRODUCTION
Assessing brain levels of amyloid beta (Aβ) plaque and tau aggregation has a central role in the selection of individuals for clinical trials and assessing the efficacy of therapeutic compounds. It may also aid earlier and more accurate diagnosis of Alzheimer's disease (AD). 1
Despite the extremely low concentration of Aβ and tau in plasma, progress in detection methodologies has recently allowed reliable measurement of plasma Aβ42/Aβ40 and phosphorylated tau (P‐tau). In a recent report, the accuracy of plasma Aβ42/Aβ40 to predict the presence of Aβ in the brain using immunoprecipitation‐mass spectrometry (IMS) assays was equal to 90%. 2 However, despite the promising potential of IMS to provide an Aβ biomarker, it is expensive and difficult to widely deploy. Consequently, measures of plasma P‐tau have recently received much attention. Measurement of plasma tau phosphorylated at threonine 181 (P‐tau181) was the first P‐tau to demonstrate good accuracy. 3 , 4 , 5 P‐tau181 can differentiate AD from cognitively unimpaired (CU) subjects 6 and from other dementias. 7 P‐tau181 was also a good predictor of elevated brain Aβ and tau as measured by positron emission tomography (PET). 8 Subsequently, plasma P‐tau217 and P‐tau231 have also shown strong association with AD pathology with accuracy comparable to cerebrospinal fluid (CSF) and PET measures, 9 , 10 with some evidence of superior performance to plasma P‐tau181. 11 , 12
More recently a high‐sensitivity Simoa assay has been developed using a capture antibody (pT3) that was raised against tau in paired helical filaments (PHFs) of AD brain. 12 , 13 The core requirement for this antibody binding is phosphorylation at aa217 (p217), with enhanced binding when other nearby phosphorylated sites are present, predominantly at aa212. 13 The recognized epitope is thus referred to as “p217+tau” and its measurement in plasma has demonstrated good concordance with CSF markers of AD in a validation cohort of 227 subjects with an area under the curve (AUC) of 0.90 versus CSF Aβ42/40 and 0.95 versus CSF P‐tau181. 14
Plasma p217+tau has not been compared to PET measures. 18F‐NAV4694 and 18F‐MK6240 are PET tracers for imaging Aβ plaques and PHF 3R/4R tau aggregates, respectively. These recent generation tracers have high target‐to‐background ratios, giving a wide dynamic range that may improve sensitivity for detection of low levels of Aβ and tau. 15 , 16
In this study we evaluated the performance of the novel blood‐based biomarker p217+tau against latest generation Aβ and tau PET agents in participants of the Australian Imaging, Biomarkers and Lifestyle study of aging (AIBL) and the Australian Dementia Network (ADNeT) trial screening program. To investigate both the preclinical trial screening and clinical diagnostic performance, we examined CU and cognitively impaired (CI) subjects separately and varied the threshold for Aβ PET from 15 to 50 centiloids (CLs).
2. METHODS
2.1. Participants
Three hundred ninety‐seven participants in the AIBL and ADNeT cohorts had both 18F‐NAV4694 Aβ PET and 18F‐MK6240 tau PET. The AIBL cohort recruitment and evaluation is detailed elsewhere. 17 The ADNeT cohort all had mild cognitive impairment (MCI) or mild dementia and was evaluated as per AIBL. A multi‐disciplinary panel, blind to all imaging and blood results, classified all participants as CU or CI with MCI, AD, or non‐AD dementia. A diagnosis of CU required performance within 1.5 standard devieation (SD) of the published norms for their age group on neuropsychological assessment. A diagnosis of MCI or dementia was assigned according to the criteria of Winblad et al. 18 and Petersen et al. 19 For this study, only individuals with a clinical diagnosis of AD and a positive Aβ PET scan were classified as AD. Those with dementia but a negative Aβ scan were classified as non‐AD dementia.
Institutional ethical review committees approved the AIBL and ADNeT studies, and written informed consent was obtained from all participants.
2.2. Image acquisition
A 20‐minute Aβ PET acquisition was conducted 50 minutes post‐injection of 200 MBq of 18F‐NAV4694. On a separate day, a 20‐minute tau PET acquisition was conducted 90 minutes post‐injection of 185 MBq of 18F‐MK6240.
Aβ PET scans were spatially normalized using CapAIBL, 20 and the standard Centiloid (CL) method was applied 21 , 22 using the standard whole cerebellum mask as reference region. A CL value of 25 was selected to determine a positive Aβ scan (Aβ+) 23 , 24 , 25 for the main analysis but Aβ PET thresholds between 15 and 50 CL were also investigated. 18F‐MK6240 tau PET scans were spatially normalized using a CapAIBL principal component analysis (PCA)–based approach 26 and scaled using the cerebellar cortex as the reference region. 18F‐MK6240 tau standardized uptake value ratio (SUVR) values were estimated in two composite regions of interest (ROIs); a mesial temporal ROI (Me) comprising entorhinal cortex, hippocampus, parahippocampus and amygdala; and a meta temporal ROI (MT) composed of the Me ROI plus inferior and middle temporal and fusiform gyri. We also estimated 18F‐MK6240 SUVR in Braak stages IV, V, and VI with ROIs derived from the Freesurfer Desikan‐Killiany Atlas (see Figures S4‐S5). In the CU group, we additionally estimated 18F‐MK6240 SUVR in the entorhinal cortex, amygdala, hippocampus, and inferior temporal cortex separately. These regions were selected as we aimed to assess the early spectrum of Braak stages in CU. The threshold for tau positivity was the 95th percentile of the Aβ− CU group in each composite ROI and individual mesial temporal regions. 27
2.3. Plasma analysis and p217+tau assay design
Fasted blood sampling was performed 3.3 ± 6 months from the time of Aβ PET scan and 1.7 ± 2 months from the tau scan. Plasma from K2‐EDTA tubes (7.5 mL S‐monovette 01.1605.008, Sarstedt) containing pre‐added prostaglandin E1 (33 ng/mL of whole blood, Sapphire Biosciences) to prevent platelet activation, a potential source of peripheral Aβ, was centrifuged at room temperature at 200g for 10 minutes to collect platelet‐rich plasma, and then at 800g for 10 minutes to provide plasma that was snap frozen within 2 hours of collection and stored in vapor phase liquid nitrogen prior to shipping on dry ice from Australia to Janssen R&D (La Jolla, CA, USA). Plasma ptau217+ assay was performed on a SIngle MOlecular Array (Simoa) HD‐X platform blinded to all subject data using the technique described previously. 14 Samples were measured in duplicate, yielding average precision of 12% CV (87% of the samples measured with CV < 20%). Functional assay range (after accounting for sample dilution) was: lower limit of detection (LLOD) = 1 fg/mL, lower limit of quantification (LLOQ) = 10 fg/mL, upper limit of quantification (ULOQ) = 20,000 fg/mL. All samples were >LLOD. Data analysis was then performed by AIBL investigators.
2.4. Statistical analysis
Demographic and clinical characteristics were compared between Aβ status within clinical classifications using standard generalized linear modeling (GLM) for quantitative features and the chi‐square test for categorical comparisons. ROI, vertex, and voxel‐wise correlation between the plasma p217+tau and CL or tau SUVR was performed using Spearman's rank‐based correlation (Spearman's Rho [ρ]). Receiver‐operating characteristic (ROC) AUC analyses were used to explore the discriminative performance of p217+tau. Optimal p217+tau thresholds for detecting Aβ+ and tau+ PET were calculated both using Youden's index and two SD above the mean p217+tau levels in Aβ− CU group. In addition, 95% CIs were shown in square brackets and positive and negative predictive values (PPV, NPV) were not disease prevalence adjusted. Model based performance of p217+tau accounting for age, gender, and APOE ε4 allele status was done using GLM. AUC values between models were compared using DeLong's method. CIs were computed using bootstrap. False discovery rate (FDR) corrected P‐values <.05 were considered statistically significant.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using PubMed, meeting abstracts, and presentations. Plasma phosphorylated tau (P‐tau) measures compare well to positron emission tomography (PET) and post‐mortem across the continuum of AD but accuracy varies across p‐tau target sites and assay methods. There are no reports comparing PET to plasma assays targeting multiple sites of tau phosphorylation as typically found in Alzheimer's disease (AD). The p217+tau assay targets p217, with binding enhanced by phosphorylation at additional sites such as aa212.
Interpretation: Plasma p217+tau elevates early and correlates with both Aβ and tau as measured by PET, indicating that tau phosphorylation is an early event in AD and occurs with Aβ deposition. Plasma p217+tau measurement should assist both selection for trials and diagnosis of AD.
Future directions: Further validation studies, head‐to‐head comparison with other assays, assessing the influence of co‐morbidities, and the ability to measure change in brain Aβ and tau levels are required.
3. RESULTS
3.1. Demographic and clinical characteristics
Three participants had a low CL but high Braak stage V/VI tau on 18F‐MK6240 PET, suggesting that, similar to some PiB case reports, 28 the Aβ PET results were falsely negative. These three subjects all showed clearly elevated plasma p217+tau levels and were kept in the analysis but are displayed with a diamond shape in the scatter plots.
The demographic and clinical characteristic of the cohort are presented in Table 1. Of the 397 participants, 56% were CU, 22% had MCI, and 21% had dementia, of which 82% in the dementia group were Aβ+. Overall, 170 (43%) had Aβ+ PET and 143 (36%) had high tau PET in the meta temporal region (TMT+). Participants with dementia were ≈4 years younger than those in the MCI and CU groups (P < .05).
TABLE 1.
Demographic and clinical characteristics
| CU (n = 223) | MCI (n = 91) | Dementia (n = 83) | |
|---|---|---|---|
| Age | 75.2 (5.70) | 73.6 (7.99) | 70.7 (7.91)* , + |
| Gender, N male (%) | 101 (45.3%) | 51 (56.0%) | 49 (59.0%) |
| Education (years) | 14 (3.0) | 12.5 (3.1)* | 12 (3.0)* |
| APOE ε4+ (%) | 72 (32.3%) | 46 (50.5%)* | 46 (55.4%)* |
| MMSE, median (IQR) | 29 (2.00) | 27 (4.00)* | 23 (4.00)* , + |
| CDR SoB, median (IQR) | 0.0 (0.00) | 1.0 (1.50)* | 4.0 (1.00)* , + |
| AIBL PACC, mean (SD) | −0.4 (0.84) | −2.4 (1.12)* | −4.3 (2.39)* , + |
| Centiloid, mean (SD) | 19.5 (38.11) | 72.8 (66.16)* | 93.9 (55.58)* , + |
| Aβ+, N (%) | 46 (20.6%) | 56 (61.4%)* | 68 (81.9%)* , + |
| MK6240 SUVRMT, mean (SD) | 1.02 (0.23) | 1.48 (0.61)* | 1.93 (0.77)* , + |
| TauMT+, N (%) | 31 (13.9%) | 48 (52.7%)* | 64 (77.1%)*, + |
| Adjusted HV (SD) | 2.98 (0.26) | 2.78 (0.40)* | 2.57 (0.42)* , + |
| Plasma p217+tau, mean (SD) | 87.8 (67.8) | 183.7 (141.0)* | 229.9 (150.7)* , + |
Abbreviations: CU, cognitively unimpaired; MCI, mild cognitive impairment. the total number of individuals (N), positive Aβ scan (Aβ+), SUVR in the Meta Temporal region (SUVRMT)
P‐value < .05 compared to CU.
P‐value < .05 compared to MCI.
AIBL PACC is the AIBL preclinical Alzheimer's cognitive composite; MMSE is the Mini‐Mental State Examination, CDR‐SoB is the Clinical Dementia Rating Scale Sum of Boxes, TMT+ means positive for tau in the tau PET meta temporal ROI; HV is hippocampal volume.SD and IQR are respectively the standard deviation and the interquartile range.
Stratification by Aβ status within each clinical classification is provided in Table S1.
3.2. Plasma p217+tau levels in clinical groups by Aβ PET status
Figure 1A shows the concentration of plasma p217+tau in the different clinical groups, subdivided into Aβ− and Aβ+. The mean p217+tau concentration in CU Aβ+ participants (162.7 fg/mL [SD: 85.76]) was approximately twice that of the CU Aβ− participants (71.2 fg/mL [SD: 46.63]; P < 10–18). Mean p217+tau concentration in MCI Aβ+ (247.1 fg/mL [SD: 141.23]) was not significantly different from those with Aβ+ dementia (AD) (260.5 fg/mL [SD: 148.43]) but both groups showed significantly higher concentration than in the CU Aβ+ group and the CU Aβ−, MCI Aβ−, and dementia Aβ− groups. Levels of p217+tau in the MCI Aβ− (82.1 fg/mL [SD: 55.81]) and the Aβ− non‐AD dementia (93.4 fg/mL [SD: 54.97]) participants were not significantly higher than CU Aβ− participants.
FIGURE 1.
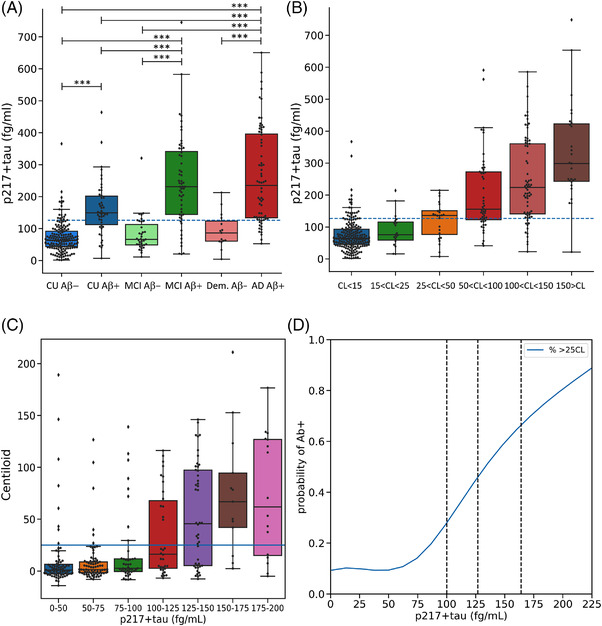
Plasma p217+tau concentrations between (A) clinical classification and amyloid beta (Aβ) PET status and (B) Centiloid (CL) levels of Aβ. The dashed line corresponds to the threshold derived by the Youden index. (C) CL results versus p217+tau level in 25 fg/mL intervals, (D) probability of being Aβ PET positive versus p217+tau level. Vertical lines in (D) are Youden index derived from cognitively unimpaired (CU) (100.3 fg/mL), Youden index derived from total cohort (126.7 fg/mL) and +2.0 SD of the Aβ‐ CU (164 fg/mL). *** P value < 0.0005
When subdividing the CL scale into five levels (Figure 1B), the ptau217+tau concentration was progressively higher as CL values rose from 25 CL. This progressive increase remained in those who were tau PET negative (see Figure S1). Figure 1C shows the CL level with increasing p217+tau level and Figure 1D shows the probability of having a positive Aβ scan versus p217+tau level.
3.3. Correlations of plasma p217+tau with Aβ PET and tau PET
Vertex‐based analysis (Figure 2 and Figures S7‐S11) and scatter plots (Figures 3, 4, 5) demonstrate moderate to strong correlations between plasma p217+tau and Aβ and tau PET. The correlation in all subjects between p217+tau and Aβ PET CL gave a Spearman's ρ of 0.67 (P < 10‐53). The correlation was significantly stronger in CI than in CU (CI: ρ = 0.64, P < 10‐21; CU: ρ = 0.45, P < 10‐12; Z‐score difference 2.6).
FIGURE 2.
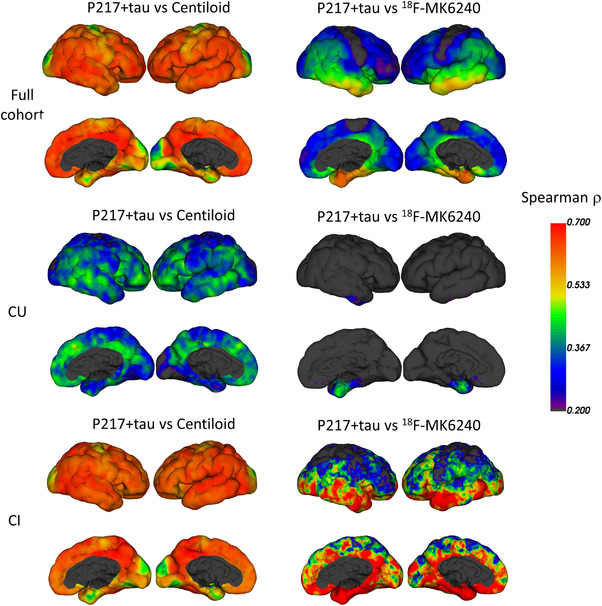
Vertex‐based analysis of regional Spearman correlation between plasma p217+tau and Centiloid (left column) and 18F‐MK6240 SUVR (right column). CU is cognitively unimpaired, CI is cognitively impaired
FIGURE 3.
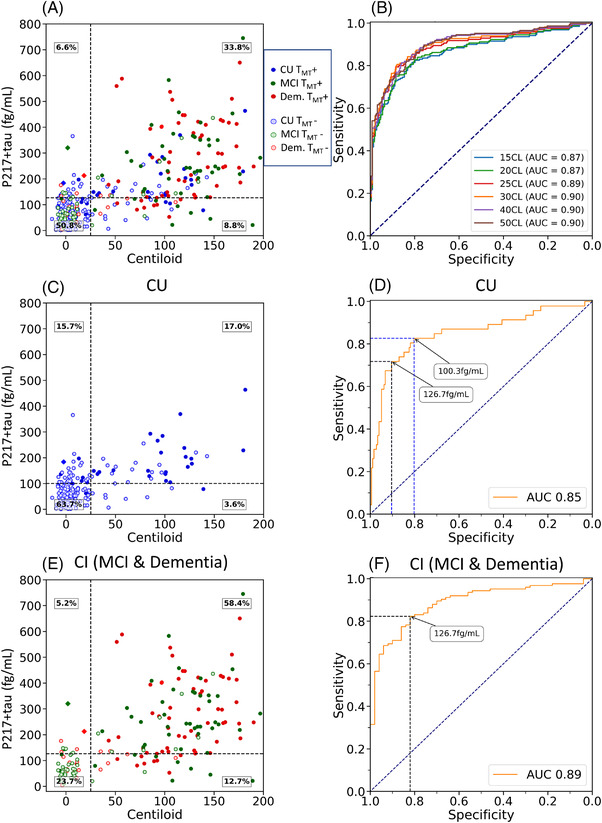
Plasma p217+tau versus Centiloid measures of amyloid beta (Aβ). Scatter plot and receiver‐operating characteristic (ROC) curve (A & B), with ROC curves using different CL thresholds to define Aβ+ PET; full cohort (A & B), cognitively unimpaired sub‐cohort (C & D) and cognitively impaired sub‐cohort (E & F). Clinical groups are color‐coded with red for dementia, green for mild cognitive impairment (MCI), and blue for cognitively unimpaired (CU). Solid circles are tau PET positive (TMT+). The black dashed horizontal line corresponds to the p217+tau threshold derived from the Youden index. In (C) and (D) the CU specific threshold is shown. The cognitively impaired (CI) group threshold was the same as the whole cohort threshold. The diamond shapes are the three Aβ−/TMT+ subjects
FIGURE 4.
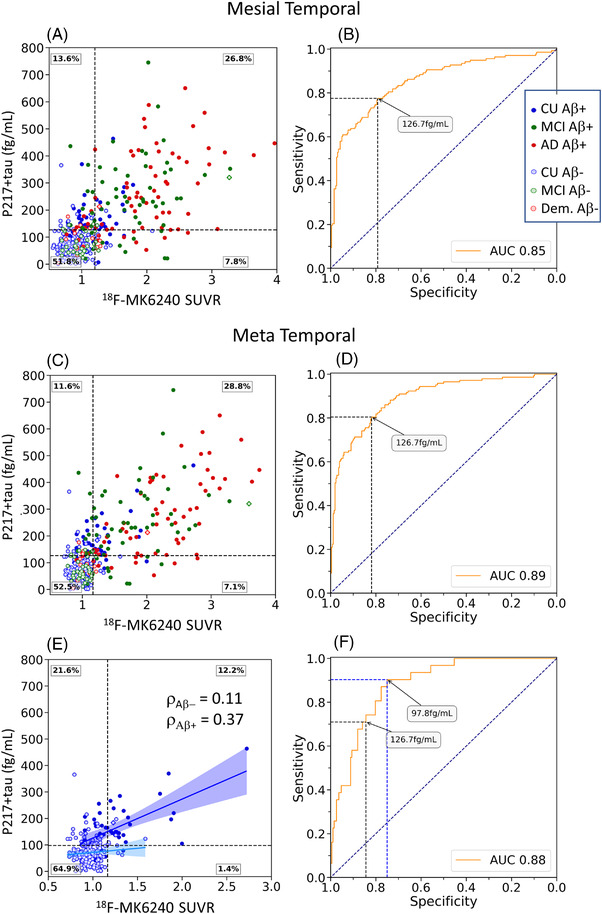
Plasma p217+tau versus PET SUVR measures of tau. Scatter plot and ROC curve for mesial temporal regions of interest (ROI) (A & B) and meta temporal ROI (C & D) and in cognitively unimpaired (CU) alone (E & F). Clinical groups are color‐coded with red for dementia, green for mild cognitive impairment (MCI), and blue for CU. Solid circles are Aβ PET positive. The black dashed horizontal line corresponds to the p217+tau threshold derived from the Youden index. Linear correlation and Spearman co‐efficient are shown for the Aβ+ (dark blue) and Aβ− CU (light blue) in part E
FIGURE 5.
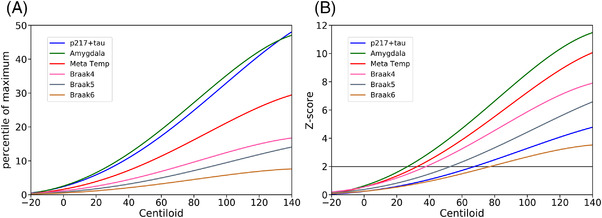
Modeling of 18F‐MK6240 quantification and p217+tau as a function of CL using polynomial curves; (A) normalized by linear transform of p217+tau levels and each tau PET, regions of interest (ROIs), SUVR to a scale of zero to 100 where zero is the mean of the results for each marker in Aβ− CU (<15 CL) and 100 is the mean of the results for each marker from the 30 individuals with the highest values for each marker. (B) Normalized by Z‐score using the results from Aβ to cognitively unimpaired (CU) to define the normal range for p217+tau level and each tau PET, ROI, SUVR. The horizontal line is +2 standard deviations (SD) (A) suggests that plasma p217+tau increases early, similar to amygdala tau at low levels of Aβ, whereas (B) shows that the wide normal range for p217+tau delays reaching a two SD threshold for significance
The correlation between p217+tau and tau SUVR was moderate to strong in the meta temporal ROI (Spearman ρ = 0.63, P < 10–46) and the mesial temporal ROI (Me) (Spearman ρ = 0.60, P < 10–40), but was progressively lower when the ROI was restricted to higher Braak stage regions. In CI subjects, the correlation was again strongest in the meta temporal ROI (ρ = 0.69). In CU the correlation was lower; meta temporal ROI (ρ = 0.34); mesial temporal ROI (ρ = 0.33), whereas Figure 2 shows that the regional correlation matches the early areas of tau accumulation as seen in the CU and the predominant regions of tau accumulation as shown in the CI. The correlations persisted between ptau217+ and tau SUVRMT when analysis included only the Aβ+ participants (ρ = 0.52 in CI; ρ = 0.37 in CU). There was no correlation between p217+tau and tau PET in Aβ− individuals (P > .05). Surface projection of the Pearson correlation is also available in Figure S7.
3.4. Performance of plasma p217+tau versus Aβ PET
For discriminating between the diagnostic groups, the AUC of p217+tau for Aβ+ AD versus Aβ− CU was 0.94 [0.90 to 0.97] and for Aβ+ AD versus Aβ+ CU was 0.67 [0.57 to 0.77]. For Aβ+ AD versus all CU was 0.88 [0.84 to 0.92] and for Aβ+ AD versus Aβ− dementia the AUC was 0.93 [0.90 to 0.96].
For discriminating Aβ+ PET from Aβ− PET in the entire cohort, the AUC was 0.89 [0.86 to 0.93], or 0.90 if the three Aβ−/tau+ PET individuals (all three had elevated plasma p217+tau) were removed. The Youden index provided a threshold concentration of 126.7 fg/mL [100.0 to 134.4 fg/mL] that yielded an accuracy of 0.85, sensitivity 0.79 [0.75 to 0.89], specificity 0.89 [0.80 to 0.92], PPV 0.84, and NPV 0.85. P217+tau accurately identified Aβ+ individuals among CI participants (AUC = 0.89, [0.84 to 0.93]) and among CU (AUC = 0.84 [0.76 to 0.91]). The Youden threshold was also 126.7 fg/mL [97.4 to 177.2] for the CI (Figure 3), giving a sensitivity of 0.82 [0.66 to 0.92] and a specificity of 0.82 [0.75 to 0.98], PPV 0.92, and NPV 0.65. Youden index provided an optimal threshold of 100.3 fg/mL [100.3 to 137.3] for CU participants (Figure 3), with a sensitivity of 0.80 [0.78 to 0.95], specificity of 0.83 [0.67 to 0.90], PPV 0.49, and NPV 0.95. Applying the whole cohort p217+tau threshold of 126.7 fg/mL to CU gave a sensitivity of 0.72 [0.6 to 0.83], specificity of 0.90 [0.86 to 0.94], PPV 0.66, and NPV 0.92.
The Z‐score threshold for p217+tau derived from CU with <15 CL on Aβ PET was higher at 164 fg/mL, resulting in lower sensitivity of 0.60, higher specificity of 0.96, and PPV 0.91, and NPV 0.76 for all subjects to detect positive Aβ PET. In CI, the higher threshold gave sensitivity 0.66, specificity 0.94, PPV 0.96, and NPV 0.53. In CU it gave sensitivity 0.43, specificity 0.96, PPV 0.74, and NPV 0.87.
Altering the CL threshold for Aβ+ PET between 25 to 50 CL had no effect on AUC. However, at 15 and 20 CL thresholds, the AUC decreased due to more p217+tau false‐negative results (Figure 3).
3.5. Performance of plasma p217+tau versus 18F‐MK6240 tau PET
Plasma p217+tau accurately discriminated individuals with elevated tau in all examined ROIs, with the highest AUC in the meta temporal ROI (AUC = 0.89 [0.86 to 0.92]). The scatter plots and ROC curves are shown in Figure 4A and C for the mesial temporal and meta temporal ROI, respectively. The Youden index thresholds were 126.7 fg/mL (Figure 4 B and D), the same as for Aβ PET, and provided an accuracy of 0.81, sensitivity of 0.80 [0.71 to 0.93], and specificity of 0.82 [0.71 to 0.93]).
In CU, the highest AUC (0.88 [0.83 to 0.92]) was observed in the meta temporal region with a Youden index threshold of 97.8 fg/mL (Figure 4F), giving a sensitivity of 0.9 [0.77 to 0.97] and specificity of 0.75 [0.71 to 0.88]. Applying the 126.7fg/mL threshold to the CU group gave a sensitivity of 0.71 [0.51 to 0.84] and specificity of 0.85 [0.80 to 0.89]. Correlation between p217+tau and tau PET was only significant (P < .05) in the CU subjects who were Aβ+ and was strongest in the meta temporal ROI (P = .37) (Figure 4E Aβ+ [dark blue] and Aβ− CU [light blue]).
In CI, the highest AUC were observed in the meta temporal and the inferior temporal regions, with AUCs of 0.86 [0.81 to 0.91] (Figure S3). The mesial temporal AUC was lower at 0.81 [0.75 to 0.86]. The Youden index threshold for the meta temporal ROI provided a threshold of 148.4 fg/mL, with a sensitivity of 0.73 [0.65 to 0.83] and specificity of 0.90 [0.82 to 0.97]. Applying the threshold of 126.7 fg/mL to the CI cohort gave a sensitivity of 0.82 [0.77 to 0.89] and a specificity of 0.71 [0.6 to 0.79].
3.5.1. p217+tau versus tau PET in subregions of the mesial temporal in CU
The AUC for p217+tau to detect regional tau+ PET in the CU was higher for the meta temporal region at 0.88 [0.83 to 0.92] than the amygdala 0.81 [0.74 to 0.88] (P = .2), hippocampus 0.81 [0.74 to 0.88], parahippocampus 0.86 [0.77 to 0.93] (P = .2), or entorhinal cortex 0.78 [0.71 to 0.83] (P = .01). Plotting of the data points (see Figure S13) shows that discordance was due predominantly to p217+tau+/tau PET− subjects with a disproportionately high prevalence of Aβ+ in this category compared to p217+tau−/tau PET−.
3.6. p217+tau predicts Aβ and tau pathology independent of confounders
AUC values from base models incorporating age, sex, and APOE ε4 allele status to predict Aβ+ and meta temporal tau+ PET were 0.72 [0.67 to 0.77] and 0.69 [0.64 to 0.76], respectively. Adding p217+tau to the base model significantly improved prediction (P‐value via DeLong's ROC test <.0001) compared to the base model alone, and produced an AUC of 0.91 [0.86 to 0.93] for Aβ+ PET and an AUC of 0.89 [0.86 to 0.92] for meta temporal tau+ PET. The combined model, however, did not perform significantly better than p217+tau alone that gave an AUC of 0.89 [0.86 to 0.93] to predict Aβ+ and 0.89 [0.86 to 0.92] for meta temporal tau+ PET.
3.7. Modeling of 18F‐MK6240 SUVR and p217+tau as a function of CL
To unveil the chronological order of the emergence of elevation in biomarkers, we normalized 18F‐MK6240 SUVR and p217+tau concentration to the same scale, using two different methods and mapped against CL. Aβ PET level were used as a surrogate for duration of disease development, 35 since abnormal accumulation of Aβ as measured by PET is the first hallmark pathology of AD and continues at a relatively slow and constant rate over decades. First, we linearly normalized the range of biomarkers from zero to 100, with zero being the mean of the CU Aβ− individuals with <15 CL to increase certainty of Aβ negativity, 24 and 100 being the mean of the 30 individuals with the highest biomarker value. When normalizing the range of values between 0 and 100, (Figure 5A), the fitted polynomial curves of 18F‐MK6240 in amygdala and plasma p217+tau were nearly identical and rose very early when Aβ level was quite low and before other tau PET regions began to rise. Second, we normalized each biomarker to a Z‐score, again using those CU with <15 CL to provide the normal range. Figure 5B shows that although we have demonstrated in Figure 5A that plasma p217+tau rises early, it takes until 70 CL to exceed 2 SD of the normal range.
4. DISCUSSION
We determined the performance of a plasma P‐tau assay that utilizes an antibody raised against the PHFs of AD. Human PHF tau is phosphorylated at multiple sites. 29 Plasma p217+tau measures tau that is phosphorylated at aa217 with binding enhanced by the simultaneous presence of phosphorylation at aa212, so to may better reflect the tau of AD than single‐binding site p‐tau assays. 13 To evaluate this assay, we employed PET tracers, which may give greater sensitivity than those previously used for comparison to plasma P‐tau. 8 , 22 , 30
Plasma p217+tau displayed high accuracy in detecting Aβ+ individuals across the clinical spectrum. Compared to Aβ− PET CU, plasma p217+tau concentration was 2‐fold higher in Aβ+ CU and 3.5‐fold higher in Aβ+ MCI and dementia. The AUC for Aβ+AD versus Aβ− CU was 0.94, for Aβ+AD versus Aβ− dementia was 0.93, while the AUCs for all Aβ+ versus Aβ− and for tau+ versus tau− were equal at 0.89. In the CU group, the AUC was 0.84 for Aβ+ versus Aβ− PET and 0.88 for tau+ versus tau− PET. Adding age, sex, and APOEε4 did not significantly improve the prediction of Aβ+ PET (AUC 0.89 vs 0.91) or tau+ PET (unchanged at 0.89). These results compare favorably to other plasma P‐tau assays, although the use of different assay methods, different anti‐tau antibodies, and cohort variation prevents direct comparison. The range of AUC reported for Aβ+ versus Aβ− PET for plasma P‐tau measures has ranged from 0.76 to 0.92. 3 , 4 , 8 , 9 , 10 , 31 , 32 Our results with p217+tau are at the high end of reported plasma P‐tau assay performance and used Simoa technology that is kit based, fully automated, and has a large installation base, and is therefore well suited to widespread deployment.
Our data (Figures 3, 4, 5) suggest that plasma p217+tau begins to rise (1) soon after brain Aβ levels begin to trend up as assessed by PET, (2) concordant with the rise in tau in the amygdala region on PET, and (3) prior to the rise in meta temporal tau PET. This is supported by the vertex‐based analysis of correlation in CU, that p217+tau and Aβ are highly associated in brain regions where early Aβ deposition occurs and with tau in the anteromedial temporal lobe (Figure 2). However, the plasma p217+tau level does not reach +2 SD above the mean found in Aβ− CU until Aβ reaches moderate levels (≈70 CL). These findings are consistent with reports for plasma P‐tau measures of p181, p217, and p231. 7 , 10 , 33 , 34 Wide intersubject variability in plasma P‐tau in persons without evidence of AD pathology on amyloid PET remains a challenge and warrants further investigation.
Our data also suggest that tau aggregates in the amygdala region and plasma p217+tau increase together and very early in the development of AD when Aβ load is beginning to rise (Figure 5A). The amygdala performed best in our comparison of p217+tau with tau PET mesial temporal subregions. This may be due to a reduced partial volume effect compared to smaller structures and less spill‐over effect from off‐target binding in the meninges giving a more robust signal. However, tau SUVR in the entorhinal did not correlate as strongly as tau in the amygdala. This may be due to the effect of primary age‐related tauopathy (PART), where tau aggregates are present without Aβ plaques 35 and predominantly in the entorhinal cortex. The ability of tau PET and plasma P‐tau measures to detect PART is unclear and requires further investigation.
Only longitudinal studies will determine if what appear to be false positive p217+tau results in CU compared to PET are due to variation and limitation in the plasma measure or are detecting very early stage AD prior to PET becoming significantly positive, and this work is in progress under the AIBL study umbrella. In contrast to the CU, the CI show more “false‐negative” p217+tau results when compared to both Aβ and tau PET. Many of these false‐negative p217+tau results were A+/T+ on PET, so this requires further investigation.
Reflecting the impact of disease prevalence in our study population (71% Aβ+ in CI vs 21% in CU), the p217+tau positive and negative predictive values of p217+tau for Aβ+ PET were very different for the CI and CU cohorts. The PPVs were high in the CI group for predicting Aβ (0.94) and tau (0.93), suggesting that p217+tau may be a good test to confirm AD in patients with objective cognitive impairment. However, the NPV were relatively low at 0.66 and 0.65, suggesting that a negative p217+tau do not exclude AD. Lowering the p217+tau threshold to 100 fg/mL in the CI group only improved the NPV to 0.73, whereas lowering the PPV to 0.88 and at 75 fg/mL, the NPV was 0.78 and PPV 0.84. P217+tau has potential as a diagnostic tool and in combination with other biomarkers, as discussed by Schindler et al, 36 may increase accuracy and provide an affordable and accurate diagnostic tool. Conversely, in the CU group the NPV were high (>0.94), suggesting that a negative p217+tau test can largely exclude AD pathology in persons with normal cognitive test results. However, the associated lower PPV (0.50) in CU indicates that positive results would need confirmation by PET or CSF analysis. These findings show that triage with p217+tau may substantially reduce the number of screening Aβ PET studies needed to identify Aβ+ PET participants for preclinical AD trials, resulting in considerable recruitment cost savings.
This study has several limitations. First the results need validation in other cohorts and more diverse populations. Second, this is a cross‐sectional study, and longitudinal analysis is required to confirm the predictive value of p217+tau as a marker of disease progression and to clearly determine when abnormal levels of p217+tau can be measured along the disease trajectory.
In conclusion, an elevated level of plasma p217+tau is associated with both elevated Aβ and tau across the clinical spectrum of AD. Elevated p217+tau strongly supports a diagnosis of AD in persons with MCI or dementia, whereas a low level in CU persons is strong evidence against preclinical AD.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE/PUBLICATION
This study was approved by the Austin Health Human Research Ethics Committee (HREC/18/Austin/201). All participants gave written consent for publication of de‐identified data.
CONFLICTS OF INTEREST
Christopher C. Rowe has received research grants from NHMRC, Enigma Australia, Biogen, Eisai, and Abbvie. He is on the scientific advisory board for Cerveau Technologies and consulted for Prothena, Eisai, Roche, and Biogen Australia. Victor Villemagne is and has been a consultant or paid speaker at sponsored conference sessions for Eli Lilly, Life Molecular Imaging, GE Healthcare, Abbvie, Lundbeck, Shanghai Green Valley Pharmaceutical Co Ltd, and Hoffmann La Roche. Ashley Bush is a shareholder in Alterity Ltd, Cogstate Ltd, and Mesoblast Ltd. He is a paid consultant for, and has a profit share interest in, Collaborative Medicinal Development Pty Ltd. He has received lecture fees from Biogen and Merck Sharp & Dohme P/L. Ziad Saad, Gallen Triana‐Baltzer, Randy Slemmon, and Hartmuth Kolb are employees of Janssen R&D. The other authors did not report any conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Some of the data used in the preparation of this article were obtained from the Australian Imaging Biomarkers and Lifestyle flagship study of aging (AIBL), funded by the Commonwealth Scientific and Industrial Research Organization (CSIRO), National Health and Medical Research Council (NHMRC), and participating institutions. AIBL researchers are listed at www.aibl.csiro.au. The authors thank all participants who took part in the study, as well as their families. The research was supported by the Australian Federal Government through NHMRC grants APP1132604, APP1140853, and APP1152623 and by a grant from Enigma Australia. Janssen Pharmaceuticals paid a commercial data access fee to the AIBL study of aging.
Doré V, Doecke JD, Saad ZS, et al. Plasma p217+tau versus NAV4694 amyloid and MK6240 tau PET across the Alzheimer's continuum. Alzheimer's Dement. 2022;14:e12307. 10.1002/dad2.12307
Vincent Doré and James Doecke contributed equally to this study.
REFERENCES
- 1. Sevigny J, Suhy J, Chiao P, et al. Amyloid PET screening for enrichment of early‐stage Alzheimer disease clinical trials: experience in a phase 1b clinical trial. Alzheimer Dis Assoc Disord. 2016;30:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid‐beta biomarkers for Alzheimer's disease. Nature. 2018;554:249–254. [DOI] [PubMed] [Google Scholar]
- 3. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–433. [DOI] [PubMed] [Google Scholar]
- 4. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lantero Rodriguez J, Karikari TK, Suárez‐Calvet M, et al. Plasma p‐tau181 accurately predicts Alzheimer's disease pathology at least 8 years prior to post‐mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang C‐C, Chiu M‐J, Chen T‐F, Chang H‐L, Liu B‐H, Yang S‐Y. Assay of plasma phosphorylated tau protein (Threonine 181) and total tau protein in early‐stage Alzheimer's disease. J Alzheimer's Dis. 2018;61:1323–1332. [DOI] [PubMed] [Google Scholar]
- 7. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26:379–386. [DOI] [PubMed] [Google Scholar]
- 8. Mielke MM, Hagen CE, Xu J, et al. Plasma phospho‐tau181 increases with Alzheimer's disease clinical severity and is associated with tau‐ and amyloid‐positron emission tomography. Alzheimer's Dement. 2018;14:989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative Accuracy of plasma phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p‐tau231: a new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol. 2021;141(5):709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20:739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mielke MM, Frank RD, Dage JL, et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 2021;78:1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Kolen K, Malia TJ, Theunis C, et al. Discovery and functional characterization of hPT3, a humanized anti‐phospho tau selective monoclonal antibody. J Alzheimers Dis. 2020;77:1397–1416. [DOI] [PubMed] [Google Scholar]
- 14. Triana‐Baltzer G, Moughadam S, Slemmon R, et al. Development and validation of a high‐sensitivity assay for measuring p217+tau in plasma. Alzheimers Dement (Amst). 2021;13:e12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rowe CC, Pejoska S, Mulligan RS, et al. Head‐to‐Head comparison of 11C‐PiB and 18F‐AZD4694 (NAV4694) for β‐amyloid imaging in aging and dementia. J Nucl Med. 2013;54:880–886. [DOI] [PubMed] [Google Scholar]
- 16. Pascoal TA, Shin M, Kang MS, et al. In vivo quantification of neurofibrillary tangles with [(18)F]MK‐6240. Alzheimers Res Ther. 2018;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellis KA, Bush AI, Darby D, et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a Longitudinal Study of Alzheimer's disease. Int Psychogeriatr. 2009;21:672–687. [DOI] [PubMed] [Google Scholar]
- 18. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. [DOI] [PubMed] [Google Scholar]
- 19. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bourgeat P, Doré V, Fripp J, et al. Implementing the centiloid transformation for 11C‐PiB and β‐amyloid 18F‐PET tracers using CapAIBL. Neuroimage. 2018;183:387–93. [DOI] [PubMed] [Google Scholar]
- 21. Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11:1–15.e1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rowe CC, Jones G, Dore V, et al. Standardized expression of 18F‐NAV4694 and 11C‐PiB β‐amyloid PET results with the Centiloid Scale. J Nucl Med. 2016;57:1233–1237. [DOI] [PubMed] [Google Scholar]
- 23. Amadoru S, Doré V, McLean CA, et al. Comparison of amyloid PET measured in Centiloid units with neuropathological findings in Alzheimer's disease. Alzheimer's Res Ther. 2020;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doré V, Bullich S, Rowe CC, et al. Comparison of (18)F‐florbetaben quantification results using the standard Centiloid, MR‐based, and MR‐less CapAIBL(®) approaches: validation against histopathology. Alzheimers Dement. 2019;15:807–816. [DOI] [PubMed] [Google Scholar]
- 25. La Joie R, Ayakta N, Seeley WW, et al. Multisite study of the relationships between antemortem [(11)C]PIB‐PET Centiloid values and postmortem measures of Alzheimer's disease neuropathology. Alzheimers Dement. 2019;15:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dore V, Bourgeat P, Burnham SC, et al. Ic‐P‐167: automated reporting of tau pet quantification on brain surface. Alzheimer's Dement. 2019;15:P131–P132. [Google Scholar]
- 27. Jack CR, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimer's Dement. 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cairns NJ, Ikonomovic MD, Benzinger T, et al. Absence of Pittsburgh compound b detection of cerebral amyloid β in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol. 2009;66:1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament‐tau) from Alzheimer's disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. [DOI] [PubMed] [Google Scholar]
- 30. Navitsky M, Joshi AD, Kennedy I, et al. Standardization of amyloid quantitation with florbetapir standardized uptake value ratios to the Centiloid scale. Alzheimer's Dement. 2018;14:1565–1571. [DOI] [PubMed] [Google Scholar]
- 31. Brickman AM, Manly JJ, Honig LS, et al. Plasma p‐tau181, p‐tau217, and other blood‐based Alzheimer's disease biomarkers in a multi‐ethnic, community study. Alzheimer's Dement. 2021;17:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barthélemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated‐tau isoforms track CNS change in Alzheimer's disease. J Exp Med. 2020;217:e20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moscoso A, Grothe MJ, Ashton NJ, et al. Time course of phosphorylated‐tau181 in blood across the Alzheimer's disease spectrum. Brain. 2021;144:325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Mol Med. 2019;11:e11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age‐related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schindler SE, Bateman RJ. Combining blood‐based biomarkers to predict risk for Alzheimer's disease dementia. Nature Aging. 2021;1:26–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


