Abstract
Introduction
: Few large, randomized trials have evaluated marine n‐3 supplements and cognition in healthy older adults.
Methods
: Healthy community‐dwelling participants aged 60+ years (mean [standard deviation] = 70.9 [5.8] years) in VITAL (randomized trial of n‐3 fats [1 g/day, including 840 mg of eicosapentaenoic acid + docosahexaenoic acid] and vitamin D) were included: 3424 whose cognition was assessed by phone (VITAL‐Cog; eight neuropsychological tests; 2.8 years) and 794 evaluated in person (CTSC‐Cog; nine tests; 2.0 years). The primary outcome was a global score (average of test z‐scores) of change over two assessments. We used multivariable‐adjusted linear mixed models; substudy‐specific results were meta‐analyzed.
Results
: We observed no significant effect of n‐3 supplementation: the mean difference in annual rate of cognitive change for the n‐3 versus placebo group was –0.01 standard units (95% confidence interval [CI]: –0.02, 0.003) in VITAL‐Cog and –0.002 (95% CI: –0.04, 0.03) in CTSC‐Cog; the pooled difference was –0.01 (95% CI: –0.02, 0.003; P = .15).
Discussion
: Marine n‐3 supplementation (1 g/day) did not confer cognitive benefits over 2 to 3 years in community‐dwelling older adults.
Keywords: aging, cognitive function, marine omega‐3 fatty acids, trial
1. BACKGROUND
Marine long‐chain omega‐3 (n‐3) fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been associated with lower risk of cognitive decline. N‐3 fatty acids may help to maintain neuronal tissue integrity 1 and enhance synaptic plasticity; 2 may have direct neuroprotective effects, antioxidant and anti‐inflammatory properties, 3 and may reduce amyloid beta accumulation, 4 important in Alzheimer's disease pathology. Marine n‐3 fats may reduce the incidence of hypertension, or dyslipidemia, 5 which in turn are associated with cognitive decline. 6 , 7 A meta‐analysis of 21 prospective observational studies reported that greater fish and marine n‐3 intakes were associated with lower cognitive impairment risk. 8
RESEARCH IN CONTEXT
Systematic Review: The authors conducted a PubMed search to review the literature and have cited the relevant works, including meta‐analyses of randomized clinical trials of marine n‐3 fatty acids and cognitive function. Previous clinical trials have been small, of short duration, and few were in general community‐dwelling healthy older adults.
Interpretation: Our null findings for marine n‐3 fats and cognition in the VITAL trial cognitive ancillary study contribute to the existing literature in that it was a large randomized clinical trial of n‐3 fatty acids of 2 to 3 years duration among 4218 relatively healthy community‐dwelling older adults, including close to 20% who were Black.
Future Directions: The article proposes future avenues for additional studies further understanding of the effect of marine n‐3 fatty acids on cognitive decline in community‐dwelling older adults: (a) longer term trials; and (b) evaluation of the relation in those with low n‐3 fatty acid status.
Randomized clinical trials (RCTs) of marine n‐3 supplements versus placebo on cognitive decline in healthy community‐dwelling older people have been contradictory, and inconclusive, although most have shown no effect. 9 , 10 Most RCTs had modest sample sizes (n = 21 to 867), with relatively short duration (2.5 to 24 months), making it challenging to draw firm conclusions. In two recent larger and longer studies, 11 , 12 Andrieu et al. 11 followed 1525 participants for 3 years and Chew et al. 12 followed 3073 Age‐Related Eye Disease Study 2 (AREDS2) participants for 5 years; both studies observed no cognitive benefits of marine n‐3 supplements. As these studies included participants who had distinct characteristics (e.g., subjective memory complaints, limitation in instrumental activities of daily living [IADL] 11 or age‐related macular degeneration [AMD]), 12 and had few non‐White participants, additional larger and longer RCTs from a general healthy population are needed.
Thus, we evaluated whether marine n‐3 supplementation (1 g/day, including EPA [460 mg] + DHA [380 mg]) versus placebo (olive oil with negligible amounts of marine n‐3 fats) may provide cognitive benefits over a 2‐ to 3‐year period among 4218 community‐dwelling participants aged 60+ years in the VITAL study (Vitamin D and Omega‐3 Trial; NCT 01169259). 13 , 14 , 15
2. METHODS
2.1. Study design and population
2.1.1. VITAL trial
VITAL 13 , 14 , 15 was a randomized, double‐blind, placebo‐controlled, 2 × 2 factorial clinical trial of marine n‐3 fatty acids (one daily capsule containing 1 g, including EPA [460 mg] and DHA [380 mg]; Omacor fish oil donated by Pronova BioPharma and BASF) and vitamin D3 (vitamin D3 [cholecalciferol], 2000IU/day, donated by Pharmavite) supplements in the primary prevention of cardiovascular disease and cancer. Both agents have undergone extensive quality control testing for nutrient content stability at various temperatures and humidity levels. 13 , 14 , 15 Placebo capsules (olive oil) lacked any significant marine n‐3 fats, and contained an amount of olive oil that was insignificant for health effects. Participants (n = 25,871 US men aged ≥50 and women aged ≥55 years) free of cardiovascular disease and cancer (except non‐melanoma skin cancer) were enrolled and were randomized from 2011 to 2014. They were required to limit out‐of‐study use of supplemental vitamin D3 to ≤800IU/day and supplemental calcium to ≤1200 mg/day and to avoid using out‐of‐study n‐3 supplements. To assess compliance, participants received follow‐up questionnaires at 6 months and 1 year after randomization and annually thereafter; 14 , 15 compliance was defined as self‐reported taking of at least two‐thirds of assigned capsules. In the parent trial, in the intervention group, n‐3 supplementation for 1 year led to a 54.7% increase (from 2.7% to 4.1%; n = 1583) in the plasma n‐3 index compared to < 2% increase in the placebo group, 14 and the plasma n‐3 index remained elevated at a similar level at each of the following years until the study end. The parent VITAL trial main findings have been published. 14 , 15 Because this trial was designed as a 2 × 2 factorial trial, with primary aims focused on the main effects of each agent separately, and because there was no interaction between the two active treatments, we present here the results of the n‐3 fatty acids intervention (with analyses all adjusted for the vitamin D intervention). 16 This substudy protocol was approved by the institutional review board of the Brigham and Women's Hospital.
We used data from two non‐overlapping subsets of VITAL participants. One subset (VITAL‐Cog; n = 3424) were administered telephone cognitive assessments at randomization and again 2.8 years later. Another subset (CTSC‐Cog; n = 794; all enrolled in an ancillary study of depression prevention [VITAL‐DEP; NCT01696435 17 , 18 ]) completed in‐person cognitive assessments at randomization and again 2.0 years later. The interval of 2 to 3 years was chosen to allow us to complete the follow‐up assessments within the parent trial's intervention period.
2.1.2. VITAL‐Cog study population
In VITAL‐Cog (NCT 01669915), eligible participants were ≥60 years old, and in the VITAL screening questionnaire, indicated a willingness to participate in a cognitive function study. From September 2011 through April 2014, participants completed the baseline cognitive interview, an average 1 month prior to randomization (only 1.31% were > 1 month after randomization; range of 1.2 years before to 0.5 years after randomization; Figure 1A). Among the 3658 eligible participants that we tried to contact as of April 2014, 241 (7%) were unreachable; of the 3417 contacted, 3271 (96%) participated. For the analysis, we further excluded 262 participants who were also in the CTSC‐Cog, leaving 3009 participants (2984, including 317 Blacks, with complete scores on all tests and 25 with scores missing on some tests). For the second cognitive assessment (February 2013 to June 2016), of the 3009 who participated in the first assessment, 58 had died (2%) and 322 were unreachable (11%). Of the 2629 contacted, 100 (4%) refused, and 2529 (96%) participated (2501 with complete scores on all tests and 28 with scores missing on some tests).
FIGURE 1.
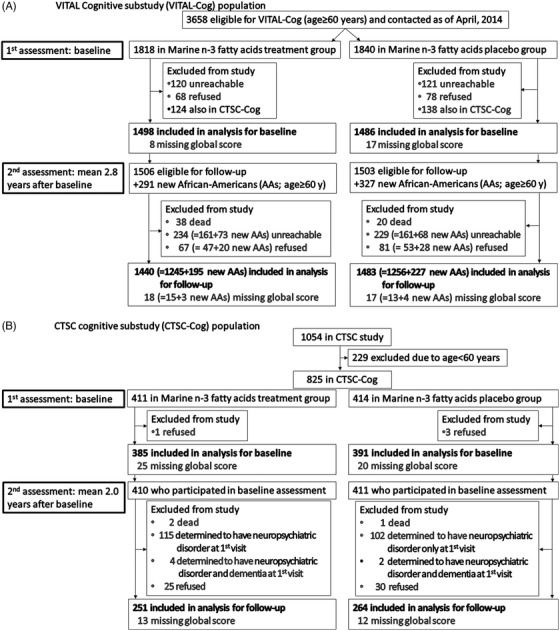
A, VITAL Cognitive substudy (VITAL‐Cog) population. B, CTSC cognitive substudy (CTSC‐Cog) population. CTSC, Clinical and Translational Science Center; VITAL, Vitamin D and Omega‐3 Trial
To allow adequate time within the main VITAL trial period for follow‐up assessments and because we had reached the target of 3000 participants, we stopped administering baseline cognitive assessments in April 2014, even though there were additional eligible participants. However, to increase the number of Blacks, at the initiation of second assessments, we invited 618 additional eligible Blacks (Figure 1A; aged 60+ years at randomization and willing to be part of VITAL‐Cog). Of 618 Blacks, 141 (23%) could not be contacted. Of 477 contacted, 48 refused (10%) and 429 (90%) participated (November 2014 to June 2016; 422 with complete scores on all tests; and 7 with scores missing on some tests). Thus, the total number of unique individuals in the VITAL‐Cog analyses was 3424: of these, 2984 had complete baseline assessments and 2923 ( = 2501+422 new Black participants) had complete second follow‐up assessments.
2.1.3. VITAL‐Cog telephone cognitive function assessment
General cognition was assessed with the Telephone Interview of Cognitive status (TICS; range = 0–41 points), a telephone adaptation of the Mini‐Mental State Examination (MMSE). 19 Verbal memory was evaluated with four tasks: the immediate and delayed recalls of both the East Boston Memory Test (EBMT; range = 0–12) 20 and the TICS 10‐word list (range = 0–10). 19 To assess executive function/attention, a test of category fluency was administered; participants were asked to name as many animals as possible in 1 minute. 21 We also administered an assessment of attention/processing speed, the digit span backward test (range = 0–12 points), which asked participants to repeat backward a series of digits; 22 and the Oral Trail Making Test (TMT), Part A (range = 0–120 seconds), which asked participants to count from 1 to 25 as fast as possible. For assessing executive function, the Oral TMT Part B (range = 0–120 seconds) was given, which asked participants to count to 13 and state the alphabet in alternating order (1‐A, 2‐B, and so on until 13‐M). 23 , 24 For the primary outcome, we evaluated a global composite score with all eight measures (using the baseline distributions in the VITAL‐Cog as the standard). Secondarily, we were interested in the outcomes of TICS, verbal memory, and executive function/attention. We calculated the verbal memory composite score by averaging the z‐scores of four tests: the immediate and delayed recalls of both the EBMT and a 10‐word list. We derived the executive function/attention composite score by averaging the z‐scores of three tests: the animal naming test, and the TMT A and B (square root transformed values for both). At follow‐up, we similarly calculated the composite scores for global, verbal memory, and executive function/attention for the second assessment, by using means and standard deviations (SDs) of the baseline VITAL‐Cog scores.
2.1.4. CTSC‐Cog study population and cognitive function assessment
A subgroup of 1054 VITAL participants received in‐person health assessments, including cognitive assessments as part of VITAL‐DEP, 17 by trained interviewers at the Clinical and Translational Science Center (CTSC) in Boston with randomization (CTSC‐Cog). All participants provided informed consent for the CTSC evaluation. The baseline assessment occurred from January 2012 to March 2014 (mean = 0.5 month before randomization; range of 3.0 months before to within 1 month after randomization). In CTSC‐Cog (Figure 1B), we excluded 229 participants aged < 60 years and 4 people who refused participation, leaving 821 participants (776 with complete scores on all tests and 45 with scores missing on some tests). A 2‐year follow‐up in‐person interview was conducted from January 2014 through April 2016. Of 821 who participated in the baseline assessment, 3 died (0.4%), 217 (26%) were ineligible for VITAL‐DEP follow‐up due to their baseline assessments showing neuropsychiatric disorders, 6 (1%) with baseline neuropsychological testing performance consistent with major neurocognitive disorders and possible dementia, 55 (7%) refused, and 540 participated (515 with complete scores on all tests and 25 with scores missing on some tests). The total number of unique individuals in CTSC‐Cog analyses was 794 (including 44 Blacks), with 776 complete baseline assessments and 515 complete follow‐up assessments.
Cognitive function was assessed in‐person by trained interviewers, using nine cognitive tests assessing general cognition (Modified MMSE [3MS]; range = 0–100), 25 verbal memory and executive function/attention. As per the protocol for VITAL‐DEP (NCT01696435), first, the Hearing Handicap Inventory–Screening Version (HHIE‐S) was administered to all participants. Those who scored at > 50% likelihood of significant hearing impairment were administered only the 3MS (range = 0–100) and not the other cognitive tests. General cognition was assessed by the 3MS. 25 Verbal memory was assessed with the same four tests used in VITAL‐Cog. To assess executive function/attention, two tests of category fluency were also administered (the animal naming test and a test in which participants were asked to name as many vegetables as possible) 21 along with the TMT Part A (range = 0–150 seconds), which asked participants to draw lines to connect the numbers from 1 to 25 as fast as possible, and the TMT Part B (range = 0–300 seconds), which asked participants to draw lines to connect numbers (1–13) and the alphabet (A–L) in alternating order (1‐A, 2‐B, and so on until 13‐M) as fast as possible. 26 , 27 For the primary outcome, we evaluated a global composite score with all nine measures (using the baseline distributions in the CTSC as the standard) and derived similar measures for secondary outcomes as in VITAL‐Cog.
2.1.5. Validation study of the VITAL‐Cog telephone cognitive assessment with the CTSC in‐person assessment
Cognitive assessment by phone has been extensively validated. 28 , 29 In VITAL‐Cog, we validated our telephone cognitive assessment against in‐person assessments among a subset of 181 of the 262 CTSC participants with both assessments who had both within 1 month of each other. We compared the global composite score derived from scores on the eight tests administered by telephone to a similar score derived from the nine tests administered in person. The intraclass correlation between the two modes was 0.64, supporting the validity of our telephone cognitive interview.
2.1.6. Plasma omega‐3 index
Baseline blood samples were obtained among willing participants and assayed for the plasma n‐3 index (EPA plus DHA as a percentage of total fatty acids) 30 by Quest Diagnostics with the use of liquid chromatography–tandem mass spectrometry. Results were based on peak areas, with each individual phospholipid fatty acid divided by the total phospholipid fatty acid (19 total) areas (the coefficient of variation for EPA was 10.4% and DHA was 10.9%, with a high correlation among blinded duplicates [r = 0.79]). 14
2.1.7. Statistical analyses
We compared characteristics at randomization by treatment group using Wilcoxon's rank‐sum tests for continuous variables and Chi‐square tests for proportions. Primary analyses were conducted using the intention‐to‐treat principle. For each substudy, we examined mean performance at each cognitive assessment in the treatment versus placebo groups using linear mixed models with random intercepts. 31 We treated mean scores at each assessment as repeated continuous outcomes and modeled the treatment effect with a time by treatment interaction; time was modeled as a continuous variable representing years between randomization and each cognitive assessment. We fitted models by maximum likelihood, incorporating the longitudinal correlation within participants; for statistical testing, we used Wald tests. We calculated multivariable‐adjusted mean differences in annual rate of cognitive change and 95% confidence intervals (CIs); information on covariates at pre‐randomization were collected by questionnaires. We used two models: model 1 included just the treatment group, while model 2 was additionally adjusted for age at randomization (years), sex, highest attained education, race, vitamin D3 (the other randomized agent), and depression history.
In secondary analyses, we evaluated potential effect modification by baseline blood n‐3 levels, which was prespecified given that supplementation may have stronger effects on subgroups with relatively lower blood n‐3 levels. We evaluated effect modification by testing the three‐way interaction terms in multivariable‐adjusted linear mixed models for possible risk factors of cognitive decline (assessed pre‐randomization): age, self‐reported race, sex, vitamin D3 assignment, education, depression, body mass index (BMI kg/m2), diabetes, hypertension, high cholesterol, multiple cardiovascular disease risk factors, baseline seafood intake, and baseline cognitive score.
We first evaluated associations separately by substudy and then pooled the substudy‐specific results using the Dersimonian and Laird meta‐analytic approach incorporating fixed‐effects; 32 when the P for heterogeneity of results was < 0.05, we incorporated random effects. Because the TICS and 3MS had different scales, for pooling, we multiplied the 3MS scores by 0.41 to generate the same scale as the TICS. For the n‐3 supplementation effect on the global composite score and the prespecified effect modification by baseline omega‐3 index evaluation, significance tests were two‐sided with α = 0.05 for the pooled main effect and interaction terms. For the effect of n‐3 supplementation on the three secondary outcomes, we used a Bonferroni corrected‐significance threshold of 0.0167 ( = 0.05/3); for the post hoc evaluation of the pooled effect modification by the additional non‐pre‐specified 14 factors for the primary outcome, we used a Bonferroni corrected significance threshold of 0.00357 ( = 0.05/14); for the post hoc evaluation of the pooled effect modification for the three secondary outcomes, we used a Bonferroni corrected significance threshold of 0.0011 ( = 0.05/45 [for 3 outcomes x 15 modifiers]). We used SAS (release 9.4; SAS Institute Inc.).
3. RESULTS
VITAL‐Cog participants (n = 3424) were aged 60 to 91 years (mean [SD] = 71.9 [5.4]) at the first cognitive assessment (Table 1); 58.9% were women, 22.2% were Black, and 49.8% had some years of post‐college studies. CTSC‐Cog participants (n = 794) were aged 60 to 87 years (mean [SD] = 67.1 [5.3]) at the first cognitive assessment; 50.4% were women, 5.7% were Black, and 55.5% had some years of post‐college studies.
TABLE 1.
Baseline characteristics of participants aged 60+ years in the VITAL cognitive substudy by N‐3 supplement assignment for VITAL‐Cog (n = 3424) and CTSC‐Cog (n = 794)*
| VITAL‐Cog (n = 3424) | CTSC‐Cog (n = 794) | |||
|---|---|---|---|---|
| N‐3 group (n = 1699) | Placebo group (n = 1725) | N‐3 group (n = 396) | Placebo group (n = 398) | |
| mean (SD) | ||||
|
Age at first interview, years * (n = 2984 in VITAL‐Cog; n = 776 in CTSC‐Cog) |
71.9 (5.6) (n = 1498) |
71.8 (5.3) (n = 1486) |
67.1 (5.3) (n = 385) |
67.1 (5.3) (n = 391) |
|
Age at second interview, years * (n = 2923 in VITAL‐Cog; n = 515 in CTSC‐Cog) |
73.4 (5.7) (n = 1440) |
73.3 (5.7) (n = 1483) |
69.4 (5.4) (n = 251) |
69.7 (5.3) (n = 264) |
| Cognitive test scores at first interview | ||||
| VITAL‐Cog only tests | ||||
| TICS | 33.9 (2.8) | 33.9 (2.8) | – | – |
| OTMT‐Part A, seconds | 10.4 (3.5) | 10.5 (3.8) | – | – |
| OTMT‐Part B, seconds | 38.5 (24.7) | 37.4 (23.5) | – | – |
| Digit span backward | 6.8 (2.3) | 6.8 (2.4) | – | – |
| CTSC‐Cog only tests | ||||
| 3MS | – | – | 94.5 (4.8) | 95.2 (4.4) |
| TMT‐Part A, seconds | – | – | 29.9 (10.6) | 29.2 (10.4) |
| TMT‐Part B, seconds | – | – | 82.1 (44.8) | 80.5 (42.3) |
| Vegetable naming test | – | – | 15.4 (4.5) | 15.6 (4.5) |
| Common tests across VITAL‐Cog and CTSC‐Cog | ||||
| TICS 10‐word list recall‐immediate | 4.7 (1.7) | 4.7 (1.7) | 4.7 (1.4) | 4.8 (1.3) |
| TICS 10‐word list recall‐delayed | 2.7 (1.9) | 2.8 (1.9) | 1.9 (1.7) | 2.0 (1.8) |
| EBMT‐immediate | 9.6 (1.7) | 9.6 (1.7) | 9.7 (1.7) | 9.8 (1.6) |
| EBMT‐delayed | 9.3 (1.8) | 9.2 (1.8) | 9.3 (1.9) | 9.4 (1.6) |
| Animal naming test | 19.5 (5.6) | 19.6 (5.6) | 20.9 (5.8) | 20.5 (6.2) |
| Global composite score | –0.003 (0.6) | 0.003 (0.6) | –0.03 (0.6) | 0.03 (0.6) |
| Baseline omega‐3 (EPA+DHA) Index (%) † | 2.7 (0.9) | 2.7 (0.9) | 3.0 (0.9) | 3.0 (1.0) |
| Body mass index (kg/m2) | 27.4 (5.4) | 27.5 (5.3) | 28.5 (5.5) | 27.7 (5.1) |
| n (%) | ||||
| Vitamin D3 assignment | ||||
| Active group | 844 (49.7) | 866 (50.2) | 198 (50.0) | 198 (49.8) |
| Placebo group | 855 (50.3) | 859 (49.8) | 198 (50.0) | 200 (50.3) |
| Sex | ||||
| Female | 987 (58.1) | 1029 (59.7) | 202 (51.0) | 198 (49.8) |
| Male | 712 (41.9) | 696 (40.4) | 194 (49.0) | 200 (50.3) |
| Self‐reported race/ethnicity | ||||
| Non‐Hispanic White | 1219 (73.4) | 1210 (71.8) | 338 (88.0) | 348 (89.2) |
| Black | 359 (21.6) | 384 (22.8) | 21 (5.5) | 23 (5.9) |
| Other | 83 (5.0) | 92 (5.5) | 25 (6.5) | 19 (4.9) |
| Highest attained education | ||||
| High school or under | 187 (11.0) | 185 (10.7) | 31 (7.8) | 32 (8.1) |
| College | 648 (38.2) | 694 (40.3) | 147 (37.1) | 143 (36.0) |
| Graduate school | 860 (50.7) | 843 (49.0) | 218 (55.1) | 222 (55.9) |
| Depression ‡ | ||||
| No | 1365 (82.7) | 1381 (82.9) | 313 (80.3) | 320 (83.3) |
| Yes | 286 (17.3) | 284 (17.1) | 77 (19.7) | 64 (16.7) |
Abbreviations: 3MS, Modified Mini‐Mental State Examination (range = 0–100); 25 CTSC, Clinical and Translational Science Center for VITAL in Boston, MA; DHA, docosahexaenoic acid; EBMT, East Boston Memory Test (range = 0–12); 20 EPA, eicosapentaenoic acid; OTMT, Oral Trail Making Test (range = 0–120 seconds) 23 , 24 ; SD, standard deviation; TICS, Telephone Interview for Cognitive Status (range = 0–41); 19 TMT, Trail Making Test (range= 0–150 seconds for part A and range = 0–300 seconds for part B); 26 , 27 VITAL, Vitamin D and Omega‐3 Trial.
Characteristics as of randomization unless noted otherwise; for categorical variables, the percentages do not add to 100% due to rounding errors and numbers do not add to the total due to missing values, which were taken out of descriptive statistical analyses. In the VITAL‐Cog, 501 completed only the baseline, 440 completed only the second assessment and 2483 completed both assessments. In the CTSC‐Cog, 498 completed both assessments, 279 completed only the baseline and 17 completed only the second assessment.
The plasma Omega‐3 Index is a measure of the amount of EPA and DHA relative to other fatty acids in the plasma.
Depression is defined as a lifetime history of a depression diagnosis or of treatment for depression; current use of antidepressants; experiencing 2 or more weeks of depression in the past 2 years; or scoring 10 points or higher on the Patient Health Questionnaire‐8.
In VITAL‐Cog, n‐3 supplementation had no effect on the global composite score at the end of follow‐up (mean = 2.8 years [range = 1.4–4.3 years]; Table 2): the least squares mean was –0.28 standard units (standard error [SE] = 0.02) for the n‐3 group and –0.26 (SE = 0.01) for the placebo group (mean difference = –0.02, 95% CI: –0.07, 0.02).
TABLE 2.
Cognitive function at two assessments by N‐3 supplement assignment, for VITAL‐Cog participants aged 60+ years, (n = 3424) assessed by telephone and for CTSC‐Cog participants aged 60+ years, (n = 794) assessed in person*
| VITAL‐COG (n = 3424; telephone assessments) | CTSC‐COG (n = 794; in‐person assessments) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N‐3 group | Placebo group | Difference in score at each timepoint (N3 group – placebo group;95% CI) ‡ | N‐3 group | Placebo group | Difference in score at each timepoint (N3 group – placebo group; 95% CI) ‡ | ||||||
| N | Mean (SE) ‡ | N | Mean (SE) ‡ | N | Mean (SE) ‡ | N | Mean (SE) ‡ | ||||
|
Primary outcome: Global composite score † |
Difference in score ‡ |
Primary outcome: Global composite score † |
Difference in score ‡ | ||||||||
| First assessment score | 14980 | –0.03 (0.01) | 1486 |
–0.02 (0.01) |
–0.01 (–0.05, 0.03) | First assessment score ‡ | 385 | –0.03 (0.03) | 391 |
0.02 (0.03) |
–0.05 (–0.13, 0.03) |
| Second assessment score | 1440 | –0.28 (0.02) | 1483 |
–0.26 (0.01) |
–0.02 (–0.07, 0.02) | Second assessment score ‡ | 251 | 0.06 (0.03) | 264 |
0.11 (0.03) |
–0.05 (–0.14, 0.04) |
| Secondary outcomes | Secondary outcomes | ||||||||||
| Verbal composite score † | Difference in score ‡ | Verbal composite score † | Difference in score ‡ | ||||||||
| First assessment score | 14980 | –0.01 (0.02) | 1486 |
–0.01 (0.02) |
0.0003 (–0.05, 0.05) | First assessment score ‡ | 385 | –0.02 (0.04) | 391 |
0.02 (0.03) |
–0.04 (–0.14, 0.05) |
| Second assessment score | 1440 | –0.05 (0.02) | 1483 |
0.01 (0.02) |
–0.05 (–0.11, 0.002) | Second assessment score ‡ | 251 | 0.12 (0.04) | 264 |
0.16 (0.04) |
–0.05 (–0.17, 0.07) |
|
Executive function/ |
Difference in score ‡ |
Executive function/ |
Difference in score ‡ | ||||||||
| First assessment score | 14980 | –0.03 (0.02) | 1486 |
–0.01 (0.02) |
–0.01 (–0.06, 0.03) | First assessment score ‡ | 385 | –0.02 (0.04) | 391 |
0.01 (0.04) |
–0.03 (–0.13, 0.07) |
| Second assessment score | 1440 | –0.50 (0.02) | 1483 |
–0.52 (0.02) |
0.01 (–0.03, 0.06) | Second assessment score ‡ | 251 | –0.02 (0.04) | 264 |
0.02 (0.04) |
–0.04 (–0.14, 0.06) |
| TICS | Difference in score ‡ | 3MS | Difference in score ‡ | ||||||||
| First assessment score | 14980 | 33.85 (0.07) | 1486 |
33.88 (0.07) |
–0.03 (–0.23, 0.17) | First assessment score ‡ | 385 | 94.45 (0.24) | 391 |
95.21 (0.22) |
–0.76 (–1.41, –0.11) |
| Second assessment score | 1440 | 33.82 (0.08) | 1483 |
34.07 (0.07) |
–0.25 (–0.46, ‐0.05) | Second assessment score ‡ | 251 | 95.34 (0.25) | 264 |
95.87 (0.22) |
–0.53 (–1.19, 0.12) |
Abbreviations: 3MS, Modified Mini‐Mental State Examination (range = 0–100); CI, confidence interval; CTSC, Clinical and Translational Science Center for VITAL in Boston, MA; SE, standard error; TICS, Telephone Interview of Cognitive Status (range = 0–41); 19 VITAL, Vitamin D and Omega‐3 Trial.
In the VITAL‐Cog, 2483 completed both assessments, 501 completed only the baseline, 440 completed only the second assessment. In the CTSC‐Cog, 497 completed both assessments, 279 completed only the baseline, and 18 completed only the second assessment.
In the VITAL‐Cog: global score is a composite score representing the mean of the z‐scores of eight tests: TICS (range 0–41), immediate and delayed recalls of the East Boston Memory Test, category fluency (animal naming test), delayed recall of the TICS 10‐word list, Oral Trail Making Test A, Oral Trail Making Test B, and digit span backward. Verbal memory score is a composite score representing the mean of the z‐scores of four tests: the immediate and delayed recalls of both the TICS 10‐word list and the East Boston Memory Test. Executive function/attention score is a composite score representing the mean of the z‐scores of four tests: Trail Making Test A and B, category fluency tests (naming animals), and digit‐span backward. In the CTSC‐Cog: the global score is a composite score representing the mean of the z‐scores of nine tests: 3MS, immediate and delayed recalls of the East Boston Memory Test, category fluency tests (naming animals and vegetables), the immediate and delayed recalls of a 10‐word list and Trail Making Tests A and B. Verbal memory score was defined the same way as in VITAL‐Cog. Executive function/attention score is a composite score representing the mean of the z‐scores of four tests: Trail Making Tests A and B, category fluency tests (naming animals and vegetables).
Least squares means and standard errors and differences of least squares means and standard errors were derived from univariate models.
Similarly, in CTSC‐Cog, at the end of follow‐up (Table 2; mean 2.0 years [range = 1.0–3.1]), the least squares mean for the global score was 0.06 (SE = 0.03) for the n‐3 group and 0.11 (SE = 0.03) for the placebo group (mean difference = –0.05, 95% CI: –0.14, 0.04). The least squares means for the individual tests are provided in Table 3.
TABLE 3.
Cognitive function at two assessments by N‐3 supplement assignment, for VITAL‐Cog participants aged 60+ years, (n = 3424) assessed by telephone and for CTSC‐Cog participants aged 60+ years, (n = 794) assessed in person: Individual cognitive tests*
| VITAL‐COG (n = 3424; telephone assessments) | CTSC‐COG (n = 794; in‐person assessments) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N‐3 group | Placebo group | N‐3 group | Placebo group | ||||||||
| N | Mean (SE) ‡ | N | Mean (SE) ‡ | Difference in score at each timepoint (N3 group – placebo group;95% CI) ‡ | N | Mean (SE) ‡ | N | Mean (SE) ‡ | Difference in score at each timepoint (N3 group – placebo group; 95% CI) ‡ | ||
| Cognitive tests common to the two substudies † | Cognitive tests common to the two substudies † | ||||||||||
| TICS 10 words ‐immediate recall | Difference in score ‡ |
TICS 10 words immediate recall |
Difference in score ‡ | ||||||||
| First assessment score | 14980 | 4.65 (0.04) | 1486 | 4.66 (0.04) | –0.01 (–0.13, 0.11) | First assessment score ‡ | 385 | 4.69 (0.07) | 391 | 4.78 (0.06) | –0.09 (–0.28, 0.09) |
| Second assessment score | 1440 | 4.66 (0.04) | 1483 | 4.76 (0.04) | –0.10 (–0.22, 0.03) | Second assessment score ‡ | 251 | 4.88 (0.09) | 264 | 4.93 (0.08) | –0.05 (–0.28, 0.19) |
|
TICS 10 words ‐ delayed recall |
Difference in score ‡ |
TICS 10 words ‐ delayed recall |
Difference in score ‡ | ||||||||
| First assessment score | 14980 | 2.67 (0.05) | 1486 | 2.73 (0.05) | –0.06 (–0.19, 0.08) | First assessment score ‡ | 385 | 1.92 (0.09) | 391 | 2.01 (0.09) | –0.09 (–0.33, 0.15) |
| Second assessment score | 1440 | 2.66 (0.05) | 1483 |
2.78 (0.05) |
–0.12 (–0.26, 0.03) | Second assessment score ‡ | 251 | 2.13 (0.10) | 264 | 2.31 (0.11) | –0.18 (–0.48, 0.11) |
|
EBMT – immediate recall |
Difference in score ‡ |
EBMT – immediate recall |
Difference in score ‡ | ||||||||
| First assessment score | 14980 | 9.60 (0.04) | 1486 | 9.57 (0.04) | 0.03 (–0.09, 0.15) | First assessment score ‡ | 385 | 9.71 (0.09) | 391 | 9.77 (0.08) | –0.06 (–0.29, 0.17) |
| Second assessment score | 1440 | 9.48 (0.05) | 1483 | 9.60 (0.05) | –0.12 (–0.25, 0.01) | Second assessment score ‡ | 251 | 9.96 (0.10) | 264 | 10.03 (0.10) | –0.07 (–0.34, 0.20) |
|
EBMT – delayed recall |
Difference in score ‡ |
EBMT – delayed recall |
Difference in score ‡ | ||||||||
| First assessment score | 14980 | 9.25 (0.05) | 1486 | 9.21 (0.05) | 0.04 (–0.09, 0.17) | First assessment score ‡ | 385 | 9.31 (0.09) | 391 | 9.35 (0.08) | –0.04 (–0.28, 0.20) |
| 2nd assessment score | 1440 | 9.13 (0.05) | 1483 | 9.18 (0.05) | –0.05 (–0.19, 0.10) | Second assessment score ‡ | 251 | 9.55 (0.10) | 264 | 9.57 (0.10) | –0.01 (–0.30, 0.27) |
| Animal naming test | Difference in score ‡ | Animal naming test | Difference in score ‡ | ||||||||
| First assessment score | 14980 | 19.34 (0.14) | 1486 | 19.47 (0.14) | –0.13 (–0.53, 0.26) | First assessment score ‡ | 385 | 20.81 (0.29) | 391 | 20.49 (0.31) | 0.32 (–0.52, 1.15) |
| Second assessment score | 1440 | 19.14 (0.16) | 1483 | 19.30 (0.15) | –0.16 (–0.59, 0.27) | Second assessment score‡ | 251 | 20.09 (0.32) | 264 | 20.84 (0.33) | –0.76 (–1.66, 0.14) |
| Cognitive tests unique to each substudy † | Cognitive tests unique to each substudy † | ||||||||||
| Digit span backward | Difference in score ‡ | Vegetable naming test | Difference in score ‡ | ||||||||
| First assessment score | 14980 | 6.75 (0.06) | 1486 | 6.81 (0.06) | –0.05 (–0.22, 0.12) | First assessment score ‡ | 385 | 15.31 (0.23) | 391 | 15.60 (0.23) | –0.29 (–0.93, 0.35) |
| Second assessment score | 1440 | 2.07 (0.08) | 1483 |
1.94 (0.07) |
0.13 (–0.08, 0.33) | Second assessment score ‡ | 251 | 15.10 (0.26) | 264 | 15.23 (0.24) | –0.13 (–0.84, 0.58) |
|
Oral Trail Making Test – Part A |
Difference in score ‡ |
Trail Making Test – Part A |
Difference in score ‡ | ||||||||
| First assessment score | 14980 | 10.41 (0.09) | 1486 | 10.52 (0.10) | –0.11 (–0.36, 0.15) | First assessment score ‡ | 385 | 29.89 (0.53) | 391 |
29.26 (0.53) |
0.63 (–0.84, 2.10) |
| Second assessment score | 1440 | 10.19 (0.14) | 1483 | 10.30 (0.14) | –0.11 (–0.50, 0.28) | Second assessment score ‡ | 251 | 29.67 (0.56) | 264 |
29.35 (0.51) |
0.32 (–1.16, 1.81) |
|
Oral Trail Making Test – Part B |
Difference in score ‡ |
Trail Making Test – Part B |
Difference in score ‡ | ||||||||
| First assessment score | 14980 | 39.16 (0.63) | 1486 | 38.27 (0.61) | 0.88 (–0.84, 2.60) | First assessment score ‡ | 385 | 82.24 (2.27) | 391 | 80.73 (2.13) | 1.51 (–4.60, 7.61) |
| Second assessment score | 1440 | 38.96 (0.62) | 1483 | 38.94 (0.62) | 0.02 (–1.70, 1.75) | Second assessment score ‡ | 251 | 72.56 (1.76) | 264 | 73.63 (2.08) | –1.07 (–6.41, 4.27) |
Abbreviations: 3MS, Modified Mini‐Mental State Examination (range = 0–100); 25 CI, confidence interval; CTSC, Clinical and Translational Science Center for VITAL in Boston, MA; SE, standard error; TICS, Telephone Interview of Cognitive Status (range = 0–41); 19 VITAL, Vitamin D and Omega‐3 Trial.
In the VITAL‐Cog, 2483 completed both assessments, 501 completed only the baseline, 440 completed only the second assessment. In the CTSC‐Cog, 497 completed both assessments, 279 completed only the baseline, and 18 completed only the second assessment.
For a description of the tests, see previous section on VITAL‐Cog telephone cognitive function assessment section.
Least squares means and standard errors and differences of least squares means and standard errors were derived from univariate models.
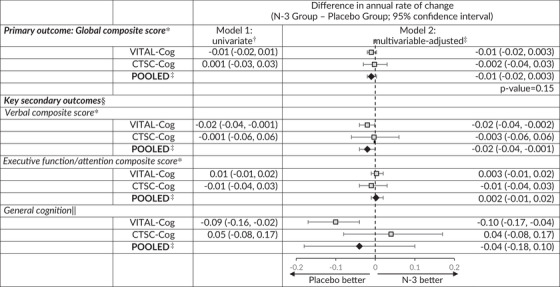
There were no significant effects of n‐3 supplementation on the primary outcome of change over time in the global composite score (Table 4). The pooled mean differences in the annual rate of decline were –0.01 (95% CI: –0.02, 0.003; P = .15) for the global score.
TABLE 4.
Meta‐analysis of the mean differences (95% CI) in change over time among VITAL‐Cog participants (n = 3424) and CTSC‐Cog participants (n = 794), by N‐3 supplement assignment
|
Abbreviations: CI, confidence interval; 3MS, Modified Mini‐Mental State Examination (range = 0–100); CI, confidence interval; CTSC, Clinical and Translational Science Center for VITAL in Boston, MA; TICS, Telephone Interview of Cognitive Status (range = 0‐41); 19 VITAL, Vitamin D and Omega‐3 Trial.
For definitions of the global scores and the key secondary outcomes for the two populations, see footnotes for Tables 2.
From linear mixed models of cognitive performance: model 1 includes time since randomization modeled as a continuous variable, omega‐3 assignment, and their interaction.
From linear mixed models of cognitive performance: model 2 is model 1 with adjustment for vitamin D assignment (yes/no), sex (male/female), age at randomization (years), race/ethnicity (non‐Hispanic White, Black, other), education (high school or under, college, graduate school), history of depression (yes/no; see footnote in Table 1 for definition). Pooled using Dersimonian and Laird 32 fixed‐effects method for meta‐analysis except for general cognition where the P for heterogeneity across the two substudies was 0.04 and results were meta‐analyzed with random effects.
For secondary outcomes, none of the differences in the annual rate of change were significant at the significance threshold of 0.0167 (raw P‐values ≥.04; based on Bonferroni correction of doing three simultaneous tests for the three secondary outcomes: 0.05/3 = 0.0167).
Due to the differences in scale between the TICS (0–41) used in VITAL‐Cog and 3MS (range 0–100) used in CTSC‐Cog, for pooling purposes, the 3MS scores were multiplied by 0.41 for conversion to the same scale as the TICS scores. As the P for heterogeneity across the two substudies was 0.04, the results were meta‐analyzed with the Dersimonian and Laird 32 method incorporating random effects.
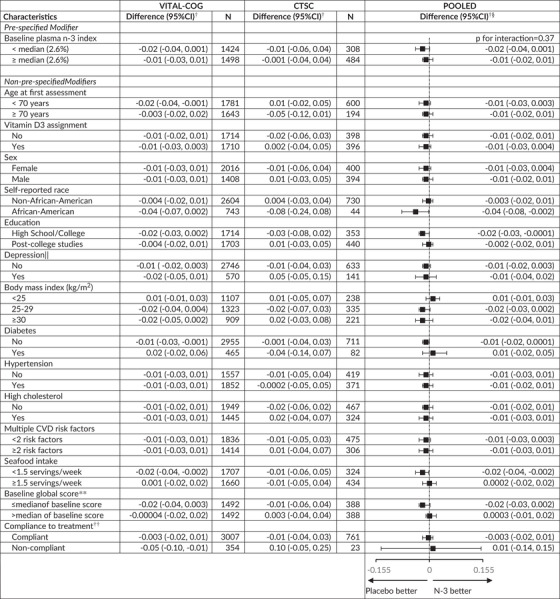
For prespecified interaction analysis (Table 5), we observed no statistically significant effect modification by baseline plasma n‐3 index for the global score (pooled p‐interaction = 0.37). For the global score, we observed no significant interactions for the other 14 effect modifiers evaluated (Table 5 footnote 3).
TABLE 5.
Mean difference (95% CI) in rate of change in global score* between the N‐3 and placebo groups: Effect modification by risk factors for cognitive decline
|
Abbreviations: CI, confidence interval; CTSC‐Cog, subset that received in‐person interviews at the Harvard Clinical and Translational Science Center for VITAL in Boston, MA; CVD, cardiovascular disease; VITAL‐Cog, subset that received telephone cognitive interviews in VITAL.
For definitions of the global scores for the two populations, see footnote for Table 2.
Mean difference in annual rate of decline of n‐3 – placebo groups from multivariable‐adjusted linear mixed models: see footnotes for Tables 2 and 3. The stratified analyses were done among those with non‐missing data on the effect modifier.
None of the interaction terms were significant at the Bonferroni‐corrected significance threshold of P = .0036 (= 0.05/14 other modifiers): p‐interaction≥0.04
Stratum‐specific estimates and interaction terms were pooled using Dersimonian and Laird fixed‐effects method for meta‐analysis 32 except for where the P for heterogeneity across the two substudies for the interaction term for age was < 0.05 (P = .03) and results were meta‐analyzed with random‐effects.
See footnote in Table 1 for definition of depression.
Median for the global score was 0.05 in both the VITAL‐Cog and the CTSC‐Cog.
Compliance is defined as self‐reported taking of ≥two‐thirds of pills on all the follow‐up questionnaires between the first and the second cognitive assessment and not initiating out‐of‐study fish oil supplementation.
Similarly, we observed no effect of n‐3 supplementation on the secondary outcomes, after correction for multiple testing (Table 4): –0.02 (95% CI: –0.04, –0.001) for the verbal composite score, 0.002 (95% CI: –0.01, 0.02) for the executive function/attention composite score, and –0.04 (95% CI: –0.18, 0.10) for general cognition (TICS/3MS).
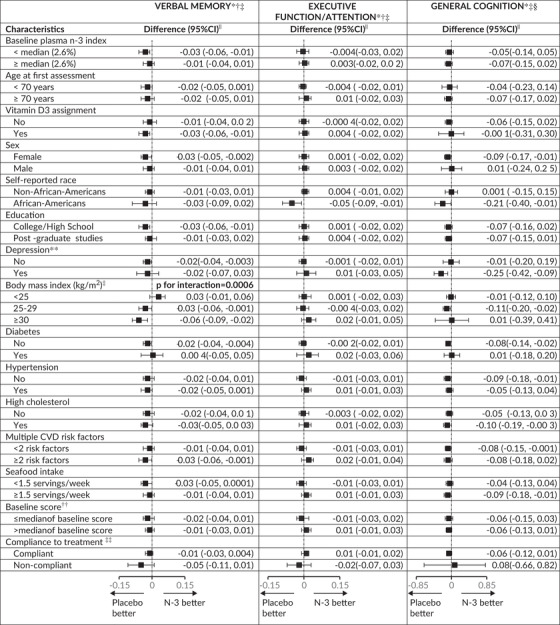
Also, no statistically significant pooled effect modification was observed for the executive function/attention score and TICS/3MS score (Table 6). However, for the verbal memory score, we observed a significant interaction by BMI (pooled p‐interaction = 0.0006), where in overweight (25≤BMI < 30 kg/m2) and obese participants (BMI≥30 kg/m2), n‐3 supplementation was associated with worse decline (pooled mean difference in annual rate of decline = –0.03 [95% CI: –0.06, –0.001] and –0.06 [95% CI: –0.09, –0.02], respectively) but not in those with BMI < 25 kg/m2.
TABLE 6.
Pooled* results across VITAL‐Cog and CTSC‐Cog for mean difference in annual rate for the secondary outcomes for N3 group versus placebo group: Effect modification by risk factors for cognitive decline
| |||
Abbreviations: 3MS, Modified Mini‐Mental State Examination; CI, confidence interval; CTSC‐Cog, subset that received in‐person interviews at the Harvard Clinical and Translational Science Center for VITAL in Boston, MA; CVD, cardiovascular disease; TICS, Telephone Interview of Cognitive Status; VITAL‐Cog, subset that received telephone interviews in VITAL.
Stratum‐specific estimates and interaction terms were pooled using Dersimonian and Laird fixed‐effects method for meta‐analysis 32 except for where the P for heterogeneity across the two substudies was < 0.05; for these, the results were meta‐analyzed with random‐effects.
For definitions of the verbal memory and executive function scores for the two populations, see footnotes for Tables 2.
For these secondary analyses, none of the tests of the pooled interaction terms were significant at the Bonferroni‐corrected significance threshold of P = .0011 (= 0.05/45 [3 outcomes, 15 modifiers[; raw p for interactions≥0.05) except for the interaction by body mass index for verbal memory score: P for interaction = 0.0006.
Due to the differences in scale between the TICS (0–41) and 3MS (range 0–100), for pooling purposes, the 3MS scores were multiplied by 0.41 for conversion to the same scale as the TICS scores.
From multivariable‐adjusted linear mixed models of cognitive performance: multivariable‐adjusted analysis with adjustment for vitamin D3 assignment (yes/no), sex (male/female), age at randomization (years), race/ethnicity (non‐Hispanic White, Black, other), education (high school or under, college, graduate school), history of depression (yes/no), except when a particular factor was being used for stratified analyses or tested for interaction.
For the definition of depression, see footnote in Table 1.
For the verbal memory score, the median was –0.02 standard units in VITAL‐Cog and 0.02 in the CTSC‐Cog; for the executive memory/attention score, the median was 0.04 in VITAL‐Cog and 0.02 in the CTSC‐Cog; for TICS, the median was 34 in VITAL‐Cog and for the 3MS in CTSC‐Cog, the median was 96 (equivalent to 39 on the transformed variable to have the same range as the TICS).
Compliance is defined as self‐reported taking of ≥two‐thirds of pills on all the follow‐up questionnaires between the first and the second cognitive assessment and not initiating out‐of‐study fish oil supplementation.
In sensitivity analyses in which we restricted the analyses to those who reported no hearing impairment (68% in VITAL‐Cog; 86% in CTSC‐Cog) or restricted the analyses in CTSC‐Cog to those who did not have baseline neuropsychiatric disorders/possible dementia (72%) or restricted the analyses to those who were in the top 90% of performance in each outcome or, in VITAL‐Cog, restricted the analyses to those enrolled from the first assessment, results were similar to the main results.
4. DISCUSSION
In this randomized trial among 4218 generally healthy older participants followed for 2 to 3 years, marine n‐3 supplementation (1 g/day, including 840 mg of EPA + DHA) versus placebo (olive oil with negligible amount of marine n‐3 fats) was not associated with cognitive decline. A subgroup analysis by baseline plasma levels of n‐3 fatty acids showed no interaction for any of the outcomes, despite n‐3 supplementation increasing plasma n‐3 levels. 14 A significant effect modification was observed by BMI for the secondary outcome of a verbal memory score, with adverse effects of n‐3 fats found in those who were overweight or obese but not in those with normal weights; however, this was unexpected. Although existing literature 33 supports a possible beneficial effect of n‐3 fatty acids in older adults with early stages of dementia, overall, these results do not provide support for n‐3 fatty acids conferring cognitive benefits over 2 to 3 years for healthy older adults.
Our null results are consistent with a meta‐analysis 10 that evaluated 36 RCTs of marine n‐3 fatty acids (with ≥6 month duration) that observed little or no effect of supplementation on cognitive outcomes. In the AREDS2 trial 12 among 3073 participants (aged 50–85 years) with intermediate AMD, an n‐3 supplement with 650 mg EPA and 350 mg DHA was administered for 5 years; there was no significant difference in yearly change of the global score (–0.03 [99% CI: –0.20, 0.13], P = .63) or the TICS score (–0.10 [99% CI: –0.24, 0.04], P = .07) between participants receiving n‐3 supplements versus placebo. In the 3‐year Multidomain Alzheimer Preventive Trial among 1525 community‐dwelling older persons aged ≥70 years without dementia but with subjective memory complaints or limitation in IADL or slow gait, 11 n‐3 supplements (225 mg EPA and 800 mg DHA) were part of a multipronged intervention that also involved cognitive training and physical activity; the investigators observed no overall difference ( P = .72) in cognitive decline between the n‐3 supplemented versus placebo groups. Thus, our results were consistent with those from prior studies but were unique because they included 2 to 3 years follow‐up from 4218 healthy older participants free of cardiovascular disease and cancer at baseline, of whom ≈20% were Black.
We observed suggestive adverse effects of n‐3 supplementation for the secondary outcome of verbal memory score, particularly among those with BMI > 25 kg/m2 (67% of the population; interaction by BMI = 0.0006). The mechanisms underlying these unexpected results are unclear, and these results may have chance findings, especially because n‐3 fatty acids may favorably influence cardiometabolic risk factors. 5 Further study of these associations is warranted given the immense global popularity of n‐3 supplements 34 and the projected global increases in obesity. 35
Our study had several limitations. In VITAL‐Cog, we used telephone cognitive assessments; however, our telephone assessment was found to be valid, and the main results were similar in VITAL‐Cog and CTSC‐Cog. Our trial focused on well‐educated individuals (> 50% had post‐graduate studies) free of cancer and cardiovascular disease at enrollment and who consumed a median of 1.5 servings of fish per week; this likely led to modest observed cognitive decline with few participants being low in n‐3 fatty acid intake. Both factors may have limited our ability to detect modest effects of n‐3 supplements on cognition. Also, the 2‐ to 3‐year follow‐up period may have been too short to allow for detecting effects of n‐3 supplementation, particularly in a population at relatively low risk for cognitive decline. Given our and other studies’ results, 11 , 12 marine n‐3 fats are not supported for prevention of cognitive decline in healthy older adults; however, future trials of marine n‐3 fats and cognitive decline in healthy older adults may benefit from durations of > 5 years, with possibly greater doses of EPA (460 mg/day) and DHA (380 mg/day) than used here (e.g., 4950 mg/day 36 ) and should consider targeting recruitment to those with low n‐3 fatty acid status.
Our study had several strengths. This was a randomized trial with > 3500 participants, and high rates of follow‐up and adherence to assigned treatment group. Second, we were able to investigate the effect of n‐3 supplements on multiple cognitive domains. Finally, our study population was diverse and included a relatively high proportion of Blacks (≈20%). 37 , 38 , 39 , 40
In conclusion, among generally well‐educated healthy adults aged 60+ years, marine n‐3 supplementation (1 g/day) versus placebo (olive oil with negligible amount of marine n‐3 fats) did not slow cognitive decline over 2 to 3 years.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
VITAL (NCT 01169259), VITAL‐DEP (NCT01696435), and VITAL‐Cog (NCT 01669915) are registered with ClinicalTrials.gov. VITAL‐Cog was supported by R01 AG036755; VITAL‐DEP was supported by R01 MH091448; and VITAL was supported by grants U01 CA138962 and R01 CA138962, including support from the National Cancer Institute, National Heart, Lung and Blood Institute, Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple institutes, including the National Heart, Lung and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Mental Health; and others. The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Kang JH, Vyas CM, Okereke OI, et al. Marine n‐3 fatty acids and cognitive change among older adults in the VITAL randomized trial. Alzheimer's Dement. 2022;8:e12288. 10.1002/trc2.12288
REFERENCES
- 1. Cansev M, Wurtman RJ, Sakamoto T. Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimers Dement. 2008;4:S153‐S168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Su HM. Mechanisms of n‐3 fatty acid‐mediated development and maintenance of learning memory performance. J Nutr Biochem. 2010;21:364‐373. [DOI] [PubMed] [Google Scholar]
- 3. Molfino A, Gioia G, Rossi Fanelli F, Muscaritoli M. The role for dietary omega‐3 fatty acids supplementation in older adults. Nutrients. 2014;6:4058‐4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cole GM, Ma QL, Frautschy SA. Omega‐3 fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids. 2009;81:213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sokola‐Wysoczanska E, Wysoczanski T, Wagner J, et al. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—a review. Nutrients. 2018;10:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fillit H, Nash DT, Rundek T, Zuckerman A. Cardiovascular risk factors and dementia. Am J Geriatr Pharmacother. 2008;6:100‐118. [DOI] [PubMed] [Google Scholar]
- 7. Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R. A systematic review of meta‐analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis. 2019;70:S165‐S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild‐to‐severe cognitive impairment risks: a dose‐response meta‐analysis of 21 cohort studies. Am J Clin Nutr. 2016;103:330‐340. [DOI] [PubMed] [Google Scholar]
- 9. Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012;(6):CD005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brainard JS, Jimoh OF, Deane KHO, et al. Omega‐3, omega‐6, and polyunsaturated fat for cognition: systematic review and meta‐analysis of randomized trials. J Am Med Dir Assoc. 2020;21:1439‐1450.e21. [DOI] [PubMed] [Google Scholar]
- 11. Andrieu S, Guyonnet S, Coley N, et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurol. 2017;16:377‐389. [DOI] [PubMed] [Google Scholar]
- 12. Chew EY, Clemons TE, Agron E, et al. Effect of omega‐3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA. 2015;314:791‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA‐3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega‐3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manson JE, Cook NR, Lee IM, et al. Marine n‐3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang JH, Vyas CM, Okereke OI, et al. Effect of vitamin D on cognitive decline: results from two ancillary studies of the VITAL randomized trial. Sci Rep. 2021;11:23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okereke OI, Reynolds CF 3rd, Mischoulon D, et al. Effect of long‐term vitamin d3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okereke OI, Vyas CM, Mischoulon D, et al. Effect of long‐term supplementation with marine Omega‐3 fatty acids vs Placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2021;326:2385‐2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 20. Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57:167‐178. [DOI] [PubMed] [Google Scholar]
- 21. Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253‐1258. [DOI] [PubMed] [Google Scholar]
- 22. Wechsler D, WMS‐R: Wechsler Memory Scale‐Revised: manual. San Antonio: Psychological Corporation; 1987.
- 23. Abraham E, Axelrod BN, Ricker JH. Application of the Oral Trail Making Test to a mixed clinical sample. Arch Clin Neuropsychology. 1996;11:697‐701. [Google Scholar]
- 24. Bastug G, Ozel‐Kizil ET, Sakarya A, Altintas O, Kirici S, Altunoz U. Oral trail making task as a discriminative tool for different levels of cognitive impairment and normal aging. Arch Clin Neuropsychol. 2013;28:411‐417. [DOI] [PubMed] [Google Scholar]
- 25. Teng EL, Chui HC. The Modified Mini‐Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314‐318. [PubMed] [Google Scholar]
- 26. Army Individual Test Battery . Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 27. Reitan RM, Wolfson D. The Halstead–Reitan Neuropsycholgical Test Battery: Therapy and Clinical Interpretation. Tuczon, AZ: Neuropsychological Press; 1985. [Google Scholar]
- 28. Evans DA, Grodstein F, Loewenstein D, Kaye J, Weintraub S. Reducing case ascertainment costs in U.S. population studies of Alzheimer's disease, dementia, and cognitive impairment‐Part 2. Alzheimers Dement. 2011;7:110‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rapp SR, Legault C, Espeland MA, et al. Validation of a cognitive assessment battery administered over the telephone. J Am Geriatr Soc. 2012;60:1616‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris WS, Von Schacky C. The Omega‐3 Index: a new risk factor for death from coronary heart disease?. Preventive medicine. 2004;39:212‐220. [DOI] [PubMed] [Google Scholar]
- 31. Fitzmaurice GM, Laird NM, Ware JH. Modelling the Mean: Analyzing Response Profiles. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, Inc; 2004:103‐139. [Google Scholar]
- 32. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 33. Canhada S, Castro K, Perry IS, Luft VC. Omega‐3 fatty acids' supplementation in Alzheimer's disease: a systematic review. Nutr Neurosci. 2018;21:529‐538. [DOI] [PubMed] [Google Scholar]
- 34. https://www.marketwatch.com/press‐release/marine‐omega‐3‐market‐2020‐top‐countries‐data‐market‐size‐with‐global‐demand‐analysis‐and‐business‐opportunities‐outlook‐2024‐2020‐08‐13?mod=mw_quote_news&tesla=y. Accessed August 23, 2020.
- 35. Popkin BM. The nutrition transition and its health implications in lower‐income countries. Public Health Nutr. 1998;1:5‐21. [DOI] [PubMed] [Google Scholar]
- 36. Stavrinou PS, Andreou E, Aphamis G, et al. The effects of a 6‐Month high dose Omega‐3 and Omega‐6 polyunsaturated fatty acids and antioxidant vitamins supplementation on cognitive function and functional capacity in older adults with mild cognitive impairment. Nutrients. 2020:12:(2):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Powe CE, Evans MK, Wenger J, et al. Vitamin D‐binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478‐2485. [DOI] [PubMed] [Google Scholar]
- 39. Holick MF. Bioavailability of vitamin D and its metabolites in black and white adults. N Engl J Med. 2013;369:2047‐2048. [DOI] [PubMed] [Google Scholar]
- 40. Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126‐1129. [DOI] [PubMed] [Google Scholar]


