Abstract
Stroke, including hemorrhagic and ischemic stroke, refers to the blood supply disorder in the local brain tissue for various reasons (aneurysm, occlusion, etc.). It leads to regional brain circulation imbalance, neurological complications, limb motor dysfunction, aphasia, and depression. As the second-leading cause of death worldwide, stroke poses a significant threat to human life characterized by high mortality, disability, and recurrence. Therefore, the clinician has to care about the symptoms of stroke patients in the acute stage and formulate an effective postoperative rehabilitation plan to facilitate the recovery in patients. We summarize a novel application and update of the rehabilitation therapy in limb motor rehabilitation of stroke patients to provide a potential future stroke rehabilitation strategy.
Keywords: stroke, rehabilitation, limb motor function, mirror therapy, robot-assist therapy, motor imagery, music therapy, visual reality
Introduction
Stroke is an acute cerebrovascular disease with high morbidity, mortality, and disability. It is the second leading cause of death worldwide, accounting for 11.6% of deaths. According to the Global Burden of Disease report, an estimated 12.2 million strokes are there worldwide, resulting in 143 million disability-adjusted life years (DALYs) and 6.55 million deaths (GBD 2019 Stroke Collaborators, 2021). China has the highest number of stroke cases globally. The number of patients belonging to the low-income and youth groups is rapidly increasing, with significant gender and regional differences. According to the WHO, in 2019, stroke was the leading cause of death and DALYs in China (World Health Organization [WHO], 2020). Stroke results in lasting sensory, cognitive and visual impairment, impaired limb motor function, and eventually reduce various bodily functions (Katzan et al., 2018a,b). Motor dysfunction is the most common complication of stroke, followed by hemiplegia in about 80% of patients. Half of these symptoms will accompany patients for life and seriously affect their day-to-day activities (Kim et al., 2020). Studies have shown that hemiplegia is the leading cause of long-term disability in stroke patients from the United States, Japan, and France [(Leys et al., 2008; Ovbiagele and Nguyen-Huynh, 2011; Iso, 2021)]. The fatality rate is significantly lower than before with the progress and development of stroke treatment. However, 80% of the survivors have severe sequelae, and the disability rate is about 75% (Langhorne et al., 2018). Effective rehabilitation training can alleviate functional disability, restore the motor function in hemiplegic limbs, and accelerate the rehabilitation process in post-stroke patients (Laver et al., 2020). At present, patient rehabilitation with limb movement disorders after stroke primarily emphasizes early intervention, somehow ignoring the intervention received during the recovery and the sequelae period. There is a decline in the quality-of-life of patients and aggravation of disease conditions. Therefore, improving limb motor function of stroke patients through rehabilitation is essential. Traditional rehabilitation therapy, including massage, acupuncture, physiotherapy, and electrical stimulation, has been widely employed in the clinical practice (McCrimmon et al., 2015; Yang et al., 2016; Cabanas-Valdés et al., 2021). With the progress of science and technology, several potential neurological rehabilitations are being developed using new technologies to restore movement in the stroke patients. In this review, we summarize the novel methods and applications to restore limb motor dysfunction in stroke rehabilitation, which could provide a potential therapeutic strategy against stroke in the future.
Robot-Assisted Therapy
Robotic rehabilitation opens up a new way in stroke rehabilitation (Hesse et al., 2003; Lambercy et al., 2011; Rodgers et al., 2020). RAT uses robot equipment to treat neurological injury and assist in post-stroke rehabilitation. Therefore, it has become a research hotspot in the rehabilitation field. Based on the neuroplasticity principle and the functional remodeling mechanism, multidisciplinary therapeutics such as mechanics, rehabilitation medicine, sensing technology, and control engineering are fully integrated with providing rehabilitation training to the patients through an automated rehabilitation treatment process (Masiero et al., 2014; Morone et al., 2017, 2020). As a result, the RAT can provide high-intensity task-oriented training to stroke patients, reduce the burden on clinical staff, save medical care costs, and improve rehabilitation efficiency. For motor function recovery, robotic devices are mainly divided into exoskeleton robots and end-effector robots. The skeletal robot consists of an external electromechanical system associated with the segments and joints of stroke patients. Therefore, it can move parallel to the bones of patients and directly control individual joints, thereby reducing the abnormal posture (Chang and Kim, 2013; Calabrò et al., 2021; Moggio et al., 2021). On the other hand, end-effector robots apply mechanical forces to the distal extremities for providing force support. However, it could result in abnormal motion patterns because of the limited control over the proximal extremities.
Lamberti et al. (2021) conducted a clinical study consisting of 236 stroke patients (145 males, 91 females) admitted to a rehabilitation program with robot-assisted gait training. After accomplishing a rehabilitation program, the results have shown that the patients exhibited significant improvements in Functional Independence Measure and Functional Ambulatory Category, with a substantial recovery in women, indicating that using robotics for female stroke patients may favor a selective selection functional effect of recovery. A randomized clinical trial with 51 participants compared the effect of RAT and constraint-induced movement therapy to investigate motor recovery in stroke. Wolf Motor Function Test and Fugl-Meyer Assessment Upper Limb were designated as the primary outcome. Upper limb function and motor recovery improved in both the groups, indicating the significant potential of these new methods during stroke rehabilitation (Terranova et al., 2021). Furthermore, previous studies have observed RAT-reduced muscle tone in patients with upper limb spasms after stroke. It suggests that RAT can attenuate limb spasms caused by upper motor neuron disease, associated with repetitive motion and drafting (Veerbeek et al., 2017; Cho et al., 2018; Cho and Song, 2019). In addition, Song et al. (2021) confirmed that RAT could improve motor function and stimulate cortical activation, revealing the other mechanism involved in RAT. A multicentric, randomized controlled trial conducted in Britain compared RAT effectiveness, enhanced upper limb therapy, and usual care. A total of 770 subjects included were randomly assigned to each group. The primary outcome of successful upper limb function (Action Research Arm Test) at three months post rehabilitation did not show any advantages of RAT compared with the standard care of patients having moderate or severe upper limb dysfunction. The results indicated that RAT was not ready for routine clinical practice at the current stage (Rodgers et al., 2019).
At present, RAT has some limitations for further application. Because of the different designs and groups, there are deviations in the results of published reports. The clinical studies have significant heterogeneity in choosing different types of robotic equipment, enrolled patients, intervention time, and intervention intensity. In addition, it raises the issue of compliance among patients actively participating in the training and ensuring rehabilitation post-stroke efficiency (Yoxon et al., 2022).
Motor Imagery Training
Motor imagery (MI), also called mental imagery, executes a particular movement or task without the actual signal output (Hanakawa, 2016; Savaki and Raos, 2019). Although the limb motor function in stroke patients is damaged, the exercise program flowchart stored in the brain is still partially preserved. Therefore, regions within the primary motor cortex, cerebellum, and basal ganglia circuits can be activated during MI (Kraft et al., 2015; Tong et al., 2017). In addition, MI can also induce the functional redistribution and regulation of neural circuits, remodeling the brain neural networks, and improving motor function relearning ability (de Vries and Mulder, 2007; Gowda et al., 2021). Furthermore, repeated training can form a normal motor reflex arc, thus, promoting limb function recovery in the stroke patients (Grabherr et al., 2015; López et al., 2019; Ladda et al., 2021). In the recent years, MIT has gradually been applied in rehabilitation as an active, low-cost, relatively simple, and efficient implementing method, attracting attention in active motor rehabilitation of stroke patients.
Motor imagery training could improve the precision and accuracy of upper limb movements and elevate the movement of hemiplegic limbs (Grabherr et al., 2015). Recently, the application of brain imaging has established the efficacy of MIT in rehabilitating stroke patients (Lioi et al., 2020; Mehler et al., 2020; Colamarino et al., 2022). Early application of MIT in post-stroke hemiplegic patients can enhance sensory information input, promote dormant synapse activation, accelerate the ischemic penumbra reperfusion, and improve cerebral blood supply, thus, enhancing the rehabilitation effect of stroke (Tavazzi et al., 2022). A recently published meta-analysis of ten randomized controlled trials showed that MIT effectively improved upper and lower limb function in stroke patients to complement traditional rehabilitation techniques (Monteiro et al., 2021). The ability to perform motor imagery involves the experience of a particular movement or task and depends on working memory, internally influencing motor representation. Therefore, MIT can improve motor performance by activating neuroplasticity in parietal lobes and related areas (Yoxon and Welsh, 2019, Yoxon and Welsh, 2020). A study applied functional task-oriented MIT in nine individuals and found that MIT could improve upper limbs and motor function of hemiplegic patients and increase visual-motor imagination ability. Page et al. conducted a series of clinical trials on MIT. They observed that compared with the control group, the MIT group (30 min of MIT twice a week for six weeks) developed the ability to perform new activities. It suggested that MI could improve the upper limb motor function and strengthen the learning ability of new skills (Page et al., 2001, 2007, 2009).
Although the efficacy of MIT in improving motor function is evident in stroke patients, many issues require clarification in future studies. First, most studies found that MIT can improve the neurological functions of stroke patients in the short term, but there are a few studies on its long-term effects in rehabilitation. In addition, published reports depicted a variable intervention time of MIT. However, the too long or too short intervention time can lead to unsatisfactory effects or make patients tired, and the choice of proper time duration and intervals are essential. At last, there is no clear standard for undergoing MIT, which may be why MIT has not been recognized and accepted by the patients. A systematic review of 32 articles observed high heterogeneity in the methodological quality and conflicting outcomes from these studies (Guerra et al., 2017). More large-scale randomized controlled trials are needed to determine the most appropriate intervention, density, duration, long-term effects of MIT, the value of MI in stroke rehabilitation, and promoting home-based rehabilitation of stroke patients.
Virtual Reality-Assisted Therapy
Virtual reality (VR) technology uses computer synthesis of 3D environment models to create and experience virtual world technology. It is a multisource information fusion interactive 3D dynamic view. It also provides the physical behavior of the system simulation with scene display, force/tactile sensing device, position tracker, and other equipment. Immersive real feelings can be obtained through visual, auditory, or tactile real-time perception and operation of various objects inside the virtual world (Chang et al., 2020; Xiong et al., 2021). Due to high safety, high interest, timely evaluation, and feedback, VR has been gradually applied in rehabilitation treatment after stroke (Gao et al., 2021; Hao et al., 2021; Xiong et al., 2021).
A randomized controlled trial of 43 participants with stroke showed that the conventional rehabilitative approach combined with VR improves the perceived health-related quality-of-life in stroke patients (Rodríguez-Hernández et al., 2021), confirmed by other clinical studies (Erhardsson et al., 2020; Thielbar et al., 2020; Gueye et al., 2021). A systematic review of 87 studies with 3,540 participants suggested that VR interventions could effectively improve upper- and lower-limb motor function, balance, gait, and daily function of stroke patients without any cognitive benefits (Zhang et al., 2021). Moreover, the underlying mechanism may be associated with the regulation of inflammation, oxidative stress, and neuroplasticity (Huang et al., 2022). VR games have also become popular in the recent years. For example, Nintendo’s VR game comprises a wireless controller, infrared sensor, and a display screen. The sensor in the controller can alter the movement of characters in the game based on the mobility of patients to carry out various virtual games, thereby improving the upper limb movement function in the stroke patients (Hsu et al., 2011; Şimşek and Çekok, 2016; Carregosa et al., 2018; Marques-Sule et al., 2021). Some literature demonstrated no significant difference between VR and traditional training effects (Caglio et al., 2012; Crosbie et al., 2012; Laver et al., 2017). Although VR is not necessarily superior to traditional rehabilitation technology, it can be an effective alternative to rehabilitate stroke patients.
Numerous studies have identified that VR can play a beneficial role in improving limb motor function post-stroke. However, other studies with negative results suggest that more large-scale, multicentric, scientifically designed, and well-conducted clinical randomized controlled trials are needed to clarify VR effects. In addition, some VR rehabilitation equipment can only perform simple human-computer interaction. The patients need to wear complex sensing equipment, and certain virtual scenes do not provide enough immersion. Therefore, a more intelligent VR system should be designed in the future, which could better integrate motor function assessment. It should be a portable and straightforward hardware system with vivid 3D animated characters so that VR technology can improve motor dysfunction after stroke.
Mirror Therapy
Mirror therapy also called mirror visual feedback therapy, is based on visual stimulation and flat mirror imaging. It observes the movement of healthy limbs to create the illusion of normal movement of paralyzed limbs through visual feedback, simulation of reality, and optical illusion. As a cheap, convenient, and straightforward treatment, mirror therapy cannot only for the clinical use but also for training patients at home. Mirror therapy was first proposed in 1996 and effectively reduced pain in patients with amputated arms (Ramachandran and Rogers-Ramachandran, 1996). Subsequently, studies conducted in stroke patients with hemiplegia after ictus showed that mirror therapy could significantly rehabilitate patients with upper limb motor dysfunction (Yavuzer et al., 2008; Nogueira et al., 2021; Zhuang et al., 2021). In addition, the role and impact of mirror therapy have also been explored in lower limb motor rehabilitation (Sütbeyaz et al., 2007; Li et al., 2018; Louie et al., 2019). Compared with the control group, the lower limb motor function and the daily living activities in the mirror group were significantly improved (Gandhi et al., 2020). Furthermore, a randomized controlled trial with 30 patients applied mirror therapy combined with transferable electrical stimulation to treat chronic stroke. The results depicted a significant improvement of muscle strength, Modified Ashworth Scale, Berg Balance Scale, velocity, cadence, step length, and the stride length of gait in the group treated with afferent electrical stimulation through mirror therapy (Lee and Lee, 2019).
Although the neurophysiological mechanism of mirror therapy is not fully elucidated, recent studies have revealed some possible mechanisms. Mirror therapy can reduce asymmetrical activation between the hemispheres, stimulate the ipsilateral, and the contralateral primary motor cortex, extensively activate the mirror neuron system, and induce partial motor neuron pathways on the affected side, facilitating brain function remodeling (Deconinck et al., 2015; Shih et al., 2017; Ding et al., 2019; Jaafar et al., 2021). Furthermore, the mirror neuron system can contribute to the recovery of limb motor function (Rizzolatti et al., 2009; Garrison et al., 2010). In addition, mirror therapy elevates the excitability of the cortical regions through visual feedback and promotes the remodeling of brain function, leading to motor function recovery (Calmels et al., 2006; Nojima et al., 2012).
Mirror therapy is a safe and widely used operable adjunctive therapy with a positive impact. However, the optimal intervention stage and the most effective intensity of mirror therapy have not been determined because of the varying protocols and the small sample size of the current studies. Therefore, it is suggested to standardize the implementation of mirror therapy, refine the efficacy standard, and conduct multicentric, randomized controlled trials with large sample sizes in the future. In addition, the development of more portable devices could provide updated rehabilitation services in stroke patients.
Music Therapy
Music-based interventions have emerged as a promising tool for motor rehabilitation after stroke because they integrate motor training with multimodal stimulation (Altenmüller and Schlaug, 2015). Music therapy is currently divided into passive and active treatment based on patient participation. Passive music therapy means “listening to music,” the melody, rhythm, and other factors that act on the nervous system of the patient during listening to music. Active music therapy is the ability of a patient to imitate percussion rhythms or play musical instruments under the guidance of a music therapist (Sihvonen et al., 2017; Grau-Sánchez et al., 2020; Daniel et al., 2021). The therapeutic effect promotes the recovery of limb motor function through continuous stimulation of the motor cortex within the brain (Rojo et al., 2011; Grau-Sánchez et al., 2013; Ripollés et al., 2016). The impact of music therapy in stroke is primarily manifested through improving the exercise completion quality, strengthening the cognitive function recovery, and reducing depression and other related negative emotions after stroke (Kim et al., 2011; Le Danseur et al., 2019; Haire et al., 2021; Palumbo et al., 2021).
Music therapy can affect the brain structure and function of stroke patients and has a significant effect in treating neurological defects (Särkämö et al., 2008; Huang et al., 2021). Segura et al. (2021) designed a home-based enriched music-supported therapy program for patients recovering from chronic stroke for self-rehabilitation at home. After a ten-week intervention of three sessions per week, the patients improved the upper limb motor function by achieving most motor tests of the Minimal Detectable Change or Minimal Clinically Important Difference. Fujioka et al. (2018) investigated the effects of music-supported therapy in chronic stroke patients on motor, cognitive, and psychosocial functions compared with conventional physical training. The results revealed the beneficial effect of music therapy in all the measured aspects. Moreover, Tong et al. (2015) applied music support therapy to 30 patients with stroke. After four weeks of treatment, it was observed that the time and motion quality completing the Wolf Motor Function Test of patients are significantly better in the music group than those in the mute group. Furthermore, most patients within the music group could independently finish the playing task (Tong et al., 2015). In addition, music could act on the network structure of the brain stem, awaken the cerebral cortex, regulate the peripheral nerves, improve muscle function, and enhance the physical vitality of stroke patients (Fujioka et al., 2012b; Särkämö and Soto, 2012; Barclay et al., 2020). Music can also transmit impulses to the reticular structure of the brain stem and cerebral cortex through auditory pathways, inducing the release of brain-derived neurotrophic factors (Chen et al., 2021). Moreover, music therapy can affect the endocrine function of the hypothalamic-pituitary region through acoustic vibration. It promotes the secretion of pituitary hormones, enzymes, and active substances beneficial to nerve recovery. As a result, blood flow is regulated, and nerve cells are excited, promoting the recovery of limb motor function and improving daily living activities (Yamasaki et al., 2012).
Does the type of music affect the impact of rehabilitation? Stroke patients mainly improve their upper limb and finger function utilizing active music therapy by playing a musical instrument. However, the beneficial effects do not relate to what kind of music is being played. For passive music therapy, most studies used nostalgic music and classical music or chose the favorite music of patients (Fujioka et al., 2012a; Palumbo et al., 2021), which helped relax the patients and made them more willing to participate in the rehabilitation training. Rhythmic auditory stimulation is a neuromusic therapy technique utilizing rhythm to improve motor function. Its primary mechanism could be the synchronous effect between the auditory and motor centers, causing resonance. Fujioka et al. showed that β oscillation was related to prosodic stimulation in the auditory area, motor area, inferior frontal gyrus, and cerebellum (Fujioka et al., 2012a,2015). Therefore, music can cause the generation of β oscillation, which may be the better choice in rehabilitating stroke patients.
Many randomized controlled trials have established that music-based therapy can treat post-stroke motor dysfunction. However, the therapeutic effect of music therapy is still controversial (Schauer and Mauritz, 2003; Särkämö et al., 2008; Tong et al., 2015; Fotakopoulos and Kotlia, 2018). Therefore, more large randomized controlled trials and high-quality meta-analyses are needed to guide clinical practice better.
Conclusion
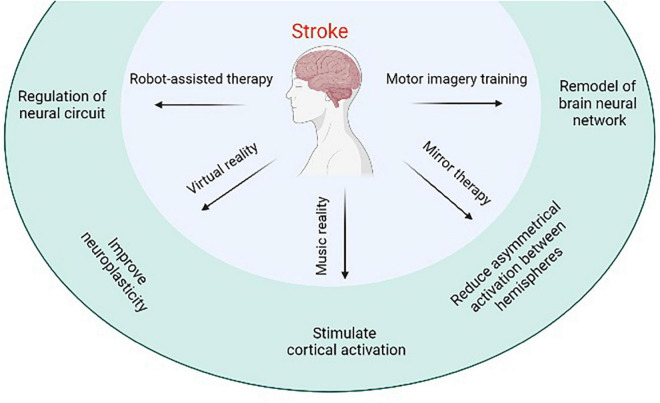
Stroke-induced neurological injury significantly reduces limb motor function in patients and leads to a decline in the quality of life. RAI, MIT, music therapy, and other emerging modern rehabilitation methods have a particular effect on improving limb motor function of stroke patients, making up for the deficiency of traditional rehabilitation measures, saving human and material resources, and becoming the hot spots of rehabilitation research (Figure 1). Several high-quality, large-sample, multicentric randomized controlled studies are needed in the future to promote positive development in stroke rehabilitation research. The current research results are neutral, and the intervention and control groups have a similar effect on movement recovery. To improve the design and implementation method of stroke rehabilitation research, we expanded the inclusion criteria to improve the inclusion rate and the universality of results, ensure the implementation of allocation hiding, and characterize the leading indicators of follow-up measures on time. The focus of future research may include but is not limited to the molecular level of the mechanism underlying rehabilitation, artificial intelligence in rehabilitation technology, and medical big data analysis, trying to achieve the best rehabilitation effect on stroke patients.
FIGURE 1.
Schematic illustration of emerging the modern rehabilitation methods.
Author Contributions
PL and FX designed and wrote the manuscript. XL provided constructive advice on the structure of this manuscript. JX, XB, JH, BZ, and FL gave constructive advice and participated in proofreading of this article. All the authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by the Hangzhou Medical and Health Science and Technology Project (Grant No. B20210533) to FX and the Hangzhou Medical and Health Science and Technology Project (Grant No. B20200395) to PL.
References
- Altenmüller E., Schlaug G. (2015). Apollo’s gift: new aspects of neurologic music therapy. Prog. Brain Res. 217 237–252. 10.1016/bs.pbr.2014.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay R. E., Stevenson T. J., Poluha W., Semenko B., Schubert J. (2020). Mental practice for treating upper extremity deficits in individuals with hemiparesis after stroke. Cochrane Database Syst. Rev. 5:Cd005950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanas-Valdés R., Calvo-Sanz J., Serra-Llobet P., Alcoba-Kait J., González-Rueda V., Rodríguez-Rubio P. R. (2021). The Effectiveness of Massage Therapy for Improving Sequelae in Post-Stroke Survivors. A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 18:4424. 10.3390/ijerph18094424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglio M., Latini-Corazzini L., D’Agata F., Cauda F., Sacco K., Monteverdi S., et al. (2012). Virtual navigation for memory rehabilitation in a traumatic brain injured patient. Neurocase 18 123–131. 10.1080/13554794.2011.568499 [DOI] [PubMed] [Google Scholar]
- Calabrò R. S., Morone G., Naro A., Gandolfi M., Liotti V., D’Aurizio C., et al. (2021). Robot-Assisted Training for Upper Limb in Stroke (ROBOTAS): An Observational, Multicenter Study to Identify Determinants of Efficacy.. J Clin. Med. 10:5245. 10.3390/jcm10225245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmels C., Holmes P., Jarry G., Hars M., Lopez E., Paillard A., et al. (2006). Variability of EEG synchronization prior to and during observation and execution of a sequential finger movement. Hum. Brain Mapp. 27 251–266. 10.1002/hbm.20181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carregosa A. A., Aguiar Dos Santos L. R., Masruha M. R., Coêlho M., Machado T. C., Souza D. C. B., et al. (2018). Virtual Rehabilitation through Nintendo Wii in Poststroke Patients: Follow-Up. J. Stroke Cerebrovasc. Dis. 27 494–498. 10.1016/j.jstrokecerebrovasdis.2017.09.029 [DOI] [PubMed] [Google Scholar]
- Chang C., Bang K., Wetzstein G., Lee B., Gao L. (2020). Toward the next-generation VR/AR optics: a review of holographic near-eye displays from a human-centric perspective. Optica 7 1563–1578. 10.1364/OPTICA.406004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. H., Kim Y. H. (2013). Robot-assisted Therapy in Stroke Rehabilitation. J. Stroke 15 174–181. 10.5853/jos.2013.15.3.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zheng J., Shen G., Ji X., Sun L., Li X., et al. (2021). Music Therapy Alleviates Motor Dysfunction in Rats With Focal Cerebral Ischemia-Reperfusion Injury by Regulating BDNF Expression. Front. Neurol. 12:666311. 10.3389/fneur.2021.666311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. H., Song W. K. (2019). Robot-Assisted Reach Training With an Active Assistant Protocol for Long-Term Upper Extremity Impairment Poststroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 100 213–219. 10.1016/j.apmr.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Cho K. H., Hong M. R., Song W. K. (2018). Upper limb robotic rehabilitation for chronic stroke survivors: a single-group preliminary study. J. Phys. Ther. Sci. 30 580–583. 10.1589/jpts.30.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamarino E., Pichiorri F., Toppi J., Mattia D., Cincotti F. (2022). Automatic Selection of Control Features for Electroencephalography-Based Brain-Computer Interface Assisted Motor Rehabilitation: The GUIDER Algorithm. Brain Topogr. 35 182–190. 10.1007/s10548-021-00883-9 [DOI] [PubMed] [Google Scholar]
- Crosbie J. H., Lennon S., McGoldrick M. C., McNeill M. D., McDonough S. M. (2012). Virtual reality in the rehabilitation of the arm after hemiplegic stroke: a randomized controlled pilot study. Clin. Rehabil. 26 798–806. 10.1177/0269215511434575 [DOI] [PubMed] [Google Scholar]
- Daniel A., Koumans H., Ganti L. (2021). Impact of Music Therapy on Gait After Stroke. Cureus 13:e18441. 10.7759/cureus.18441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries S., Mulder T. (2007). Motor imagery and stroke rehabilitation: a critical discussion. J. Rehabil. Med. 39 5–13. 10.2340/16501977-0020 [DOI] [PubMed] [Google Scholar]
- Deconinck F. J., Smorenburg A. R., Benham A., Ledebt A., Feltham M. G., Savelsbergh G. J. (2015). Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabil. Neural. Repair 29 349–361. 10.1177/1545968314546134 [DOI] [PubMed] [Google Scholar]
- Ding L., Wang X., Guo X., Chen S., Wang H., Cui X., et al. (2019). Effects of camera-based mirror visual feedback therapy for patients who had a stroke and the neural mechanisms involved: protocol of a multicentre randomised control study. BMJ Open 9:e022828. 10.1136/bmjopen-2018-022828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardsson M., Alt Murphy M., Sunnerhagen K. S. (2020). Commercial head-mounted display virtual reality for upper extremity rehabilitation in chronic stroke: a single-case design study. J. Neuroeng. Rehabil. 17:154. 10.1186/s12984-020-00788-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotakopoulos G., Kotlia P. (2018). The Value of Exercise Rehabilitation Program Accompanied by Experiential Music for Recovery of Cognitive and Motor Skills in Stroke Patients. J. Stroke Cerebrovasc. Dis. 27 2932–2939. 10.1016/j.jstrokecerebrovasdis.2018.06.025 [DOI] [PubMed] [Google Scholar]
- Fujioka T., Dawson D. R., Wright R., Honjo K., Chen J. L., Chen J. J., et al. (2018). The effects of music-supported therapy on motor, cognitive, and psychosocial functions in chronic stroke. Ann. N. Y. Acad. Sci. Epub online ahead of print. 10.1111/nyas.13706 [DOI] [PubMed] [Google Scholar]
- Fujioka T., Ross B., Trainor L. J. (2015). Beta-Band Oscillations Represent Auditory Beat and Its Metrical Hierarchy in Perception and Imagery. J. Neurosci. 35 15187–15198. 10.1523/jneurosci.2397-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T., Trainor L. J., Large E. W., Ross B. (2012a). Internalized timing of isochronous sounds is represented in neuromagnetic β oscillations. J. Neurosci. 32 1791–1802. 10.1523/JNEUROSCI.4107-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T., Ween J. E., Jamali S., Stuss D. T., Ross B. (2012b). Changes in neuromagnetic beta-band oscillation after music-supported stroke rehabilitation. Ann. N. Y. Acad. Sci. 1252 294–304. 10.1111/j.1749-6632.2011.06436.x [DOI] [PubMed] [Google Scholar]
- Gandhi D. B., Sterba A., Khatter H., Pandian J. D. (2020). Mirror Therapy in Stroke Rehabilitation: Current Perspectives. Ther. Clin. Risk Manag. 16 75–85. 10.2147/TCRM.S206883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Ma L., Lin C., Zhu S., Yao L., Fan H., et al. (2021). Effects of Virtual Reality-Based Intervention on Cognition, Motor Function, Mood, and Activities of Daily Living in Patients With Chronic Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Aging Neurosci. 13:766525. 10.3389/fnagi.2021.766525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison K. A., Winstein C. J., Aziz-Zadeh L. (2010). The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil. Neural. Repair 24 404–412. 10.1177/1545968309354536 [DOI] [PubMed] [Google Scholar]
- GBD 2019 Stroke Collaborators (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20 795–820. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda A. S., Memon A. N., Bidika E., Salib M., Rallabhandi B., Fayyaz H. (2021). Investigating the Viability of Motor Imagery as a Physical Rehabilitation Treatment for Patients With Stroke-Induced Motor Cortical Damage. Cureus 13:e14001. 10.7759/cureus.14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr L., Jola C., Berra G., Theiler R., Mast F. W. (2015). Motor imagery training improves precision of an upper limb movement in patients with hemiparesis. NeuroRehabilitation 36 157–166. 10.3233/NRE-151203 [DOI] [PubMed] [Google Scholar]
- Grau-Sánchez J., Amengual J. L., Rojo N., Veciana de Las Heras M., Montero J., Rubio F., et al. (2013). Plasticity in the sensorimotor cortex induced by Music-supported therapy in stroke patients: a TMS study. Front. Hum. Neurosci. 7:494. 10.3389/fnhum.2013.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau-Sánchez J., Münte T. F., Altenmüller E., Duarte E., Rodríguez-Fornells A. (2020). Potential benefits of music playing in stroke upper limb motor rehabilitation. Neurosci. Biobehav. Rev. 112 585–599. 10.1016/j.neubiorev.2020.02.027 [DOI] [PubMed] [Google Scholar]
- Guerra Z. F., Lucchetti A. L. G., Lucchetti G. (2017). Motor Imagery Training After Stroke: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Neurol. Phys. Ther. 41 205–214. 10.1097/NPT.0000000000000200 [DOI] [PubMed] [Google Scholar]
- Gueye T., Dedkova M., Rogalewicz V., Grunerova-Lippertova M., Angerova Y. (2021). Early post-stroke rehabilitation for upper limb motor function using virtual reality and exoskeleton: equally efficient in older patients. Neurol. Neurochir. Pol. 55 91–96. 10.5603/PJNNS.a2020.0096 [DOI] [PubMed] [Google Scholar]
- Haire C. M., Vuong V., Tremblay L., Patterson K. K., Chen J. L., Thaut M. H. (2021). Effects of therapeutic instrumental music performance and motor imagery on chronic post-stroke cognition and affect: A randomized controlled trial. NeuroRehabilitation 48 195–208. 10.3233/NRE-208014 [DOI] [PubMed] [Google Scholar]
- Hanakawa T. (2016). Organizing motor imageries. Neurosci. Res. 104 56–63. 10.1016/j.neures.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Hao J., Xie H., Harp K., Chen Z., Siu K. C. (2021). Effects of Virtual Reality Intervention on Neural Plasticity in Stroke Rehabilitation: A Systematic Review. Arch. Phys. Med. Rehabil. S0003–9993, 01305–8. 10.1016/j.apmr.2021.06.024 [DOI] [PubMed] [Google Scholar]
- Hesse S., Schulte-Tigges G., Konrad M., Bardeleben A., Werner C. (2003). Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch. Phys. Med. Rehabil. 84 915–920. 10.1016/s0003-9993(02)04954-7 [DOI] [PubMed] [Google Scholar]
- Hsu J. K., Thibodeau R., Wong S. J., Zukiwsky D., Cecile S., Walton D. M. (2011). A “Wii” bit of fun: the effects of adding Nintendo Wii(®) Bowling to a standard exercise regimen for residents of long-term care with upper extremity dysfunction. Physiother. Theory Pract. 27 185–193. 10.3109/09593985.2010.483267 [DOI] [PubMed] [Google Scholar]
- Huang C. Y., Chiang W. C., Yeh Y. C., Fan S. C., Yang W. H., Kuo H. C., et al. (2022). Effects of virtual reality-based motor control training on inflammation, oxidative stress, neuroplasticity and upper limb motor function in patients with chronic stroke: a randomized controlled trial. BMC Neurol. 22:21. 10.1186/s12883-021-02547-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. H., Dou Z. L., Jin H. M., Cui Y., Li X., Zeng Q. (2021). The Effectiveness of Music Therapy on Hand Function in Patients With Stroke: A Systematic Review of Randomized Controlled Trials. Front. Neurol. 12:641023. 10.3389/fneur.2021.641023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso H. (2021). Cardiovascular disease, a major global burden: Epidemiology of stroke and ischemic heart disease in Japan. Glob. Health Med. 3 358–364. 10.35772/ghm.2020.01113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafar N., Che Daud A. Z., Ahmad Roslan N. F., Mansor W. (2021). Mirror Therapy Rehabilitation in Stroke: A Scoping Review of Upper Limb Recovery and Brain Activities. Rehabil. Res. Pract. 2021:9487319. 10.1155/2021/9487319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzan I. L., Schuster A., Newey C., Uchino K., Lapin B. (2018a). Patient-reported outcomes across cerebrovascular event types: More similar than different. Neurology 91 e2182–e2191. [DOI] [PubMed] [Google Scholar]
- Katzan I. L., Thompson N. R., Uchino K., Lapin B. (2018b). The most affected health domains after ischemic stroke. Neurology 90 e1364–e1371. 10.1212/WNL.0000000000005327 [DOI] [PubMed] [Google Scholar]
- Kim D. S., Park Y. G., Choi J. H., Im S. H., Jung K. J., Cha Y. A., et al. (2011). Effects of music therapy on mood in stroke patients. Yonsei Med. J. 52 977–981. 10.3349/ymj.2011.52.6.977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Thayabaranathan T., Donnan G. A., Howard G., Howard V. J., Rothwell P. M., et al. (2020). Global Stroke Statistics 2019. Int. J. Stroke 15 819–838. 10.1177/1747493020909545 [DOI] [PubMed] [Google Scholar]
- Kraft E., Schaal M. C., Lule D., König E., Scheidtmann K. (2015). The functional anatomy of motor imagery after sub-acute stroke. NeuroRehabilitation 36 329–337. 10.3233/NRE-151221 [DOI] [PubMed] [Google Scholar]
- Ladda A. M., Lebon F., Lotze M. (2021). Using motor imagery practice for improving motor performance - A review. Brain Cogn. 150:105705. 10.1016/j.bandc.2021.105705 [DOI] [PubMed] [Google Scholar]
- Lambercy O., Dovat L., Yun H., Wee S. K., Kuah C. W., Chua K. S., et al. (2011). Effects of a robot-assisted training of grasp and pronation/supination in chronic stroke: a pilot study. J. Neuroeng. Rehabil. 8:63. 10.1186/1743-0003-8-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti N., Manfredini F., Lissom L. O., Lavezzi S., Basaglia N., Straudi S. (2021). Beneficial Effects of Robot-Assisted Gait Training on Functional Recovery in Women after Stroke: A Cohort Study. Medicina 57:1200. 10.3390/medicina57111200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne P., Collier J. M., Bate P. J., Thuy M. N., Bernhardt J. (2018). Very early versus delayed mobilisation after stroke. Cochrane Database Syst. Rev. 10:Cd006187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver K. E., dey-Wakeling Z. A., Crotty M., Lannin N. A., George S., Sherrington C. (2020). Telerehabilitation services for stroke. Cochrane Database Syst. Rev. 1:Cd010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver K. E., Lange B., George S., Deutsch J. E., Saposnik G., Crotty M. (2017). Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 11:Cd008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Danseur M., Crow A. D., Stutzman S. E., Villarreal M. D., Olson D. M. (2019). Music as a Therapy to Alleviate Anxiety During Inpatient Rehabilitation for Stroke. Rehabil. Nurs. 44 29–34. 10.1097/rnj.0000000000000102 [DOI] [PubMed] [Google Scholar]
- Lee D., Lee G. (2019). Effect of afferent electrical stimulation with mirror therapy on motor function, balance, and gait in chronic stroke survivors: a randomized controlled trial. Eur. J. Phys. Rehabil. Med. 55 442–449. 10.23736/S1973-9087.19.05334-6 [DOI] [PubMed] [Google Scholar]
- Leys D., Béjot Y., Debette S., Giroud M. (2008). Burden of stroke in France. Int. J. Stroke 3 117–119. 10.1111/j.1747-4949.2008.00188.x [DOI] [PubMed] [Google Scholar]
- Li Y., Wei Q., Gou W., He C. (2018). Effects of mirror therapy on walking ability, balance and lower limb motor recovery after stroke: a systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 32 1007–1021. 10.1177/0269215518766642 [DOI] [PubMed] [Google Scholar]
- Lioi G., Butet S., Fleury M., Bannier E., Lécuyer A., Bonan I., et al. (2020). A Multi-Target Motor Imagery Training Using Bimodal EEG-fMRI Neurofeedback: A Pilot Study in Chronic Stroke Patients. Front. Hum. Neurosci. 14:37. 10.3389/fnhum.2020.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López N. D., Monge Pereira E., Centeno E. J., Miangolarra Page J. C. (2019). Motor imagery as a complementary technique for functional recovery after stroke: a systematic review. Top. Stroke Rehabil. 26 576–587. 10.1080/10749357.2019.1640000 [DOI] [PubMed] [Google Scholar]
- Louie D. R., Lim S. B., Eng J. J. (2019). The Efficacy of Lower Extremity Mirror Therapy for Improving Balance, Gait, and Motor Function Poststroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 28 107–120. 10.1016/j.jstrokecerebrovasdis.2018.09.017 [DOI] [PubMed] [Google Scholar]
- Marques-Sule E., Arnal-Gómez A., Buitrago-Jiménez G., Suso-Martí L., Cuenca-Martínez F., Espí-López G. V. (2021). Effectiveness of Nintendo Wii and Physical Therapy in Functionality, Balance, and Daily Activities in Chronic Stroke Patients. J. Am. Med. Dir. Assoc. 22 1073–1080. 10.1016/j.jamda.2021.01.076 [DOI] [PubMed] [Google Scholar]
- Masiero S., Poli P., Rosati G., Zanotto D., Iosa M., Paolucci S., et al. (2014). The value of robotic systems in stroke rehabilitation. Expert Rev. Med. Devices 11 187–198. 10.1586/17434440.2014.882766 [DOI] [PubMed] [Google Scholar]
- McCrimmon C. M., King C. E., Wang P. T., Cramer S. C., Nenadic Z., Do A. H. (2015). Brain-controlled functional electrical stimulation therapy for gait rehabilitation after stroke: a safety study. J. Neuroeng. Rehabil. 12:57. 10.1186/s12984-015-0050-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler D. M. A., Williams A. N., Whittaker J. R., Krause F., Lührs M., Kunas S., et al. (2020). Graded fMRI Neurofeedback Training of Motor Imagery in Middle Cerebral Artery Stroke Patients: A Preregistered Proof-of-Concept Study. Front. Hum. Neurosci. 14:226. 10.3389/fnhum.2020.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moggio L., de Sire A., Marotta N., Demeco A., Ammendolia A. (2021). Exoskeleton versus end-effector robot-assisted therapy for finger-hand motor recovery in stroke survivors: systematic review and meta-analysis. Top. Stroke Rehabil. 1–12. Epub online ahead of print. 10.1080/10749357.2021.1967657 [DOI] [PubMed] [Google Scholar]
- Monteiro K. B., Cardoso M. D. S., Cabral V., Santos A., Silva P. S. D., Castro J. B. P., et al. (2021). Effects of Motor Imagery as a Complementary Resource on the Rehabilitation of Stroke Patients: A Meta-Analysis of Randomized Trials. J. Stroke Cerebrovasc Dis. 30:105876. 10.1016/j.jstrokecerebrovasdis.2021.105876 [DOI] [PubMed] [Google Scholar]
- Morone G., Cocchi I., Paolucci S., Iosa M. (2020). Robot-assisted therapy for arm recovery for stroke patients: state of the art and clinical implication. Expert Rev. Med. Devices 17 223–233. 10.1080/17434440.2020.1733408 [DOI] [PubMed] [Google Scholar]
- Morone G., Paolucci S., Cherubini A., De Angelis D., Venturiero V., Coiro P., et al. (2017). Robot-assisted gait training for stroke patients: current state of the art and perspectives of robotics. Neuropsychiatr. Dis. Treat 13 1303–1311. 10.2147/NDT.S114102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Parma J. O., Leão S., Sales I. S., Macedo L. C., Galvão A., et al. (2021). Mirror therapy in upper limb motor recovery and activities of daily living, and its neural correlates in stroke individuals: A systematic review and meta-analysis. Brain Res. Bull. 177 217–238. 10.1016/j.brainresbull.2021.10.003 [DOI] [PubMed] [Google Scholar]
- Nojima I., Mima T., Koganemaru S., Thabit M. N., Fukuyama H., Kawamata T. (2012). Human motor plasticity induced by mirror visual feedback. J. Neurosci. 32 1293–1300. 10.1523/JNEUROSCI.5364-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovbiagele B., Nguyen-Huynh M. N. (2011). Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics 8 319–329. 10.1007/s13311-011-0053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. J., Levine P., Leonard A. (2007). Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke 38 1293–1297. 10.1161/01.STR.0000260205.67348.2b [DOI] [PubMed] [Google Scholar]
- Page S. J., Levine P., Sisto S. A., Johnston M. V. (2001). Mental practice combined with physical practice for upper-limb motor deficit in subacute stroke. Phys. Ther. 81 1455–1462. 10.1093/ptj/81.8.1455 [DOI] [PubMed] [Google Scholar]
- Page S. J., Szaflarski J. P., Eliassen J. C., Pan H., Cramer S. C. (2009). Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil. Neural. Repair 23 382–388. 10.1177/1545968308326427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A., Aluru V., Battaglia J., Geller D., Turry A., Ross M., et al. (2021). Music Upper Limb Therapy-Integrated (MULT-I) Provides a Feasible Enriched Environment and Reduces Post Stroke Depression: A Pilot Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. Epub online ahead of print. 10.1097/PHM.0000000000001938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V. S., Rogers-Ramachandran D. (1996). Synaesthesia in phantom limbs induced with mirrors. Proc. Biol. Sci. 263 377–386. 10.1098/rspb.1996.0058 [DOI] [PubMed] [Google Scholar]
- Ripollés P., Rojo N., Grau-Sánchez J., Amengual J. L., Càmara E., Marco-Pallarés J., et al. (2016). Music supported therapy promotes motor plasticity in individuals with chronic stroke. Brain Imaging Behav. 10 1289–1307. 10.1007/s11682-015-9498-x [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fabbri-Destro M., Cattaneo L. (2009). Mirror neurons and their clinical relevance. Nat. Clin. Pract. Neurol. 5 24–34. 10.1038/ncpneuro0990 [DOI] [PubMed] [Google Scholar]
- Rodgers H., Bosomworth H., Krebs H. I., van Wijck F., Howel D., Wilson N., et al. (2020). Robot-assisted training compared with an enhanced upper limb therapy programme and with usual care for upper limb functional limitation after stroke: the RATULS three-group RCT. Health Technol. Assess. 24 1–232. 10.3310/hta24540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers H., Bosomworth H., Krebs H. I., van Wijck F., Howel D., Wilson N., et al. (2019). Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet 394 51–62. 10.1016/S0140-6736(19)31055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Hernández M., Criado-Álvarez J. J., Corregidor-Sánchez A. I., Martín-Conty J. L., Mohedano-Moriano A., Polonio-López B. (2021). Effects of Virtual Reality-Based Therapy on Quality of Life of Patients with Subacute Stroke: A Three-Month Follow-Up Randomized Controlled Trial. Int. J. Environ. Res. Public Health 18:2810. 10.3390/ijerph18062810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo N., Amengual J., Juncadella M., Rubio F., Camara E., Marco-Pallares J., et al. (2011). Music-supported therapy induces plasticity in the sensorimotor cortex in chronic stroke: a single-case study using multimodal imaging (fMRI-TMS). Brain INJ 25 787–793. 10.3109/02699052.2011.576305 [DOI] [PubMed] [Google Scholar]
- Särkämö T., Soto D. (2012). Music listening after stroke: beneficial effects and potential neural mechanisms. Ann. N. Y. Acad. Sci. 1252 266–281. 10.1111/j.1749-6632.2011.06405.x [DOI] [PubMed] [Google Scholar]
- Särkämö T., Tervaniemi M., Laitinen S., Forsblom A., Soinila S., Mikkonen M., et al. (2008). Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 131 866–876. 10.1093/brain/awn013 [DOI] [PubMed] [Google Scholar]
- Savaki H. E., Raos V. (2019). Action perception and motor imagery: Mental practice of action. Prog. Neurobiol. 175 107–125. 10.1016/j.pneurobio.2019.01.007 [DOI] [PubMed] [Google Scholar]
- Schauer M., Mauritz K. H. (2003). Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clin. Rehabil. 17 713–722. 10.1191/0269215503cr668oa [DOI] [PubMed] [Google Scholar]
- Segura E., Grau-Sánchez J., Sanchez-Pinsach D., De la Cruz M., Duarte E., Arcos J. L., et al. (2021). Designing an app for home-based enriched Music-supported Therapy in the rehabilitation of patients with chronic stroke: a pilot feasibility study. Brain INJ 35 1585–1597. 10.1080/02699052.2021.1975819 [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Wu C. Y., Lin K. C., Cheng C. H., Hsieh Y. W., Chen C. L., et al. (2017). Effects of action observation therapy and mirror therapy after stroke on rehabilitation outcomes and neural mechanisms by MEG: study protocol for a randomized controlled trial. Trials 18:459. 10.1186/s13063-017-2205-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvonen A. J., Särkämö T., Leo V., Tervaniemi M., Altenmüller E., Soinila S. (2017). Music-based interventions in neurological rehabilitation. Lancet Neurol. 16 648–660. 10.1016/S1474-4422(17)30168-0 [DOI] [PubMed] [Google Scholar]
- Şimşek T. T., Çekok K. (2016). The effects of Nintendo Wii(TM)-based balance and upper extremity training on activities of daily living and quality of life in patients with sub-acute stroke: a randomized controlled study. Int. J. Neurosci. 126 1061–1070. 10.3109/00207454.2015.1115993 [DOI] [PubMed] [Google Scholar]
- Song K. J., Chun M. H., Lee J., Lee C. (2021). The effect of robot-assisted gait training on cortical activation in stroke patients: A functional near-infrared spectroscopy study. NeuroRehabilitation 49 65–73. 10.3233/NRE-210034 [DOI] [PubMed] [Google Scholar]
- Sütbeyaz S., Yavuzer G., Sezer N., Koseoglu B. F. (2007). Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 88 555–559. 10.1016/j.apmr.2007.02.034 [DOI] [PubMed] [Google Scholar]
- Tavazzi E., Bergsland N., Pirastru A., Cazzoli M., Blasi V., Baglio F. (2022). MRI markers of functional connectivity and tissue microstructure in stroke-related motor rehabilitation: A systematic review. Neuroimage Clin. 33:102931. 10.1016/j.nicl.2021.102931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova T. T., Simis M., Santos A. C. A., Alfieri F. M., Imamura M., Fregni F., et al. (2021). Robot-Assisted Therapy and Constraint-Induced Movement Therapy for Motor Recovery in Stroke: Results From a Randomized Clinical Trial. Front. Neurorobot. 15:684019. 10.3389/fnbot.2021.684019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielbar K. O., Triandafilou K. M., Barry A. J., Yuan N., Nishimoto A., Johnson J., et al. (2020). Home-based Upper Extremity Stroke Therapy Using a Multiuser Virtual Reality Environment: A Randomized Trial. Arch. Phys. Med. Rehabil. 101 196–203. 10.1016/j.apmr.2019.10.182 [DOI] [PubMed] [Google Scholar]
- Tong Y., Forreider B., Sun X., Geng X., Zhang W., Du H., et al. (2015). Music-supported therapy (MST) in improving post-stroke patients’ upper-limb motor function: a randomised controlled pilot study. Neurol. Res. 37 434–440. 10.1179/1743132815Y.0000000034 [DOI] [PubMed] [Google Scholar]
- Tong Y., Pendy J. T., Jr., Li W. A., Du H., Zhang T., Geng X., et al. (2017). Motor Imagery-Based Rehabilitation: Potential Neural Correlates and Clinical Application for Functional Recovery of Motor Deficits after Stroke. Aging Dis. 8 364–371. 10.14336/AD.2016.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerbeek J. M., Langbroek-Amersfoort A. C., van Wegen E. E., Meskers C. G., Kwakkel G. (2017). Effects of Robot-Assisted Therapy for the Upper Limb After Stroke. Neurorehabil. Neural. Repair. 31 107–121. [DOI] [PubMed] [Google Scholar]
- World Health Organization [WHO] (2020). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2019. Geneva: WHO. [Google Scholar]
- Xiong J., Hsiang E. L., He Z., Zhan T., Wu S. T. (2021). Augmented reality and virtual reality displays: emerging technologies and future perspectives. Light Sci. Appl. 10:216. 10.1038/s41377-021-00658-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki A., Booker A., Kapur V., Tilt A., Niess H., Lillemoe K. D., et al. (2012). The impact of music on metabolism. Nutrition 28 1075–1080. 10.1016/j.nut.2012.01.020 [DOI] [PubMed] [Google Scholar]
- Yang A., Wu H. M., Tang J. L., Xu L., Yang M., Liu G. J. (2016). Acupuncture for stroke rehabilitation. Cochrane Database Syst. Rev. 2016:Cd004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuzer G., Selles R., Sezer N., Sütbeyaz S., Bussmann J. B., Köseoğlu F., et al. (2008). Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 89 393–398. 10.1016/j.apmr.2007.08.162 [DOI] [PubMed] [Google Scholar]
- Yoxon E., Welsh T. N. (2019). Rapid motor cortical plasticity can be induced by motor imagery training. Neuropsychologia 134:107206. [DOI] [PubMed] [Google Scholar]
- Yoxon E., Welsh T. N. (2020). Motor system activation during motor imagery is positively related to the magnitude of cortical plastic changes following motor imagery training. Behav. Brain Res. 390:112685. [DOI] [PubMed] [Google Scholar]
- Yoxon E., Brillinger M., Welsh T. N. (2022). Behavioural indexes of movement imagery ability are associated with the magnitude of corticospinal adaptation following movement imagery training. Brain Res. 1777:147764. [DOI] [PubMed] [Google Scholar]
- Zhang B., Li D., Liu Y., Wang J., Xiao Q. (2021). Virtual reality for limb motor function, balance, gait, cognition and daily function of stroke patients: A systematic review and meta-analysis. J. Adv. Nurs. 77 3255–3273. 10.1111/jan.14800 [DOI] [PubMed] [Google Scholar]
- Zhuang J. Y., Ding L., Shu B. B., Chen D., Jia J. (2021). Associated Mirror Therapy Enhances Motor Recovery of the Upper Extremity and Daily Function after Stroke: A Randomized Control Study. Neural Plast. 2021:7266263. 10.1155/2021/7266263 [DOI] [PMC free article] [PubMed] [Google Scholar]