Abstract
Objective
To quantify the risk of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19.
Design
Self-controlled case series and matched cohort study.
Setting
National registries in Sweden.
Participants
1 057 174 people who tested positive for SARS-CoV-2 between 1 February 2020 and 25 May 2021 in Sweden, matched on age, sex, and county of residence to 4 076 342 control participants.
Main outcomes measures
Self-controlled case series and conditional Poisson regression were used to determine the incidence rate ratio and risk ratio with corresponding 95% confidence intervals for a first deep vein thrombosis, pulmonary embolism, or bleeding event. In the self-controlled case series, the incidence rate ratios for first time outcomes after covid-19 were determined using set time intervals and the spline model. The risk ratios for first time and all events were determined during days 1-30 after covid-19 or index date using the matched cohort study, and adjusting for potential confounders (comorbidities, cancer, surgery, long term anticoagulation treatment, previous venous thromboembolism, or previous bleeding event).
Results
Compared with the control period, incidence rate ratios were significantly increased 70 days after covid-19 for deep vein thrombosis, 110 days for pulmonary embolism, and 60 days for bleeding. In particular, incidence rate ratios for a first pulmonary embolism were 36.17 (95% confidence interval 31.55 to 41.47) during the first week after covid-19 and 46.40 (40.61 to 53.02) during the second week. Incidence rate ratios during days 1-30 after covid-19 were 5.90 (5.12 to 6.80) for deep vein thrombosis, 31.59 (27.99 to 35.63) for pulmonary embolism, and 2.48 (2.30 to 2.68) for bleeding. Similarly, the risk ratios during days 1-30 after covid-19 were 4.98 (4.96 to 5.01) for deep vein thrombosis, 33.05 (32.8 to 33.3) for pulmonary embolism, and 1.88 (1.71 to 2.07) for bleeding, after adjusting for the effect of potential confounders. The rate ratios were highest in patients with critical covid-19 and highest during the first pandemic wave in Sweden compared with the second and third waves. In the same period, the absolute risk among patients with covid-19 was 0.039% (401 events) for deep vein thrombosis, 0.17% (1761 events) for pulmonary embolism, and 0.101% (1002 events) for bleeding.
Conclusions
The findings of this study suggest that covid-19 is a risk factor for deep vein thrombosis, pulmonary embolism, and bleeding. These results could impact recommendations on diagnostic and prophylactic strategies against venous thromboembolism after covid-19.
Introduction
Covid-19 has led to a health crisis, with millions of deaths globally. Symptoms range from mild to critical, with the most common severe manifestation being pneumonia with acute respiratory distress syndrome.1 Recently, reports of cardiovascular complications have been increasing,2 and we previously identified covid-19 as a risk factor for myocardial infarction and stroke.3
Previous studies on the risk of venous thromboembolism after covid-19 have shown conflicting results. Although a meta-analysis reported an incidence of venous thromboembolism of around 13%,4 the study included mainly patients with severe covid-19 during the first wave of the pandemic. Another report, including studies with a control group design, did not show an increased rate of venous thromboembolism.5 With such conflicting data, large nationwide studies are needed to better determine the risks of venous thromboembolism after covid-19. Furthermore, thromboprophylaxis raises concerns about bleeding complications. From information obtained on all people who tested positive for SARS-CoV-2 in Sweden, regardless of disease severity, we determined the risk of deep vein thrombosis and pulmonary embolism as well as bleeding after covid-19 using self-controlled case series and matched cohort study methods.
Methods
Data source
The personal identification numbers of people who tested positive for SARS-CoV-2 between 1 February 2020 and 25 May 2021 were sent from the communicable disease surveillance system, SmiNet (Public Health Agency of Sweden), to Statistics Sweden. We set the covid-19 date as the earliest from the date of disease onset, sample date, diagnosis date, or date of report to SmiNet (see supplementary table 1). Only first infections were included. Statistics Sweden identified four people who tested negative (controls) for each participant who tested positive for SARS-CoV-2, matched on age, sex, and county of residence. The index date for control participants was the corresponding date for participants who tested positive for SARS-CoV-2. The personal identification numbers for people who tested positive or negative for SARS-CoV-2 were cross linked with the Inpatient Registry (covid-19 cases: 1987-2021, controls: 1997-2021), Outpatient Registry (1997-2021), Cause of Death Registry (2020-21), Intensive Care Registry (2020-21), the Prescribed Pharmaceutical Registry from the Swedish National Board of Health and Welfare, and the Swedish Intensive Care Registry. We calculated the weighted Charlson comorbidity index for each participant.6 7
The sample size needed to identify a clinically relevant acute effect (incidence rate ratio or risk ratio of 2) with 90% power within a risk period of 30 days was 181 events in the self-controlled case series study and 112 events in the matched cohort study. The sample size needed to identify a clinically significant effect (incidence rate ratio or risk ratio of 1.5) with 90% power in the overall 180 day risk period was 258 events in the self-controlled case series study and 354 events in the matched cohort study. Sample size calculations were performed a priori.
No data were missing in our analysis.
Deep vein thrombosis, pulmonary embolism, and bleeding classification
Outcomes were defined using ICD-9 and ICD-10 (international classification of diseases, ninth and 10th revisions, respectively) diagnosis codes for deep vein thrombosis, pulmonary embolism, and bleeding (see supplementary table 2) as reason for contact in the outpatient or inpatient registries. Depending on the analysis, we chose the first or a recurrent event. A first event was defined as a participant with no previous event between 1 January 1987 and the start of the study period for patients with covid-19, and 1 January 1997 and the start of the study period for control participants. A recurrent event was defined as a participant with a previous event during the period 1987 to day 0 (covid 19) or 1997 to day 0 (index date) for participants with covid-19 and control participants, respectively. In the analysis, we only included the first of the recurrent events occurring within 1-30 days after the covid-19 or index date.
Statistical analysis
We calculated the mean age and standard deviation for the main cohort and for participants with a first or recurrent deep vein thrombosis, pulmonary embolism, or bleeding event. For each analysis we specify the proportion of participants who were female or male. In addition, we show the proportion of participants who had the event among the whole cohort of participants with covid-19 or control participants as the absolute risk over the follow-up period of 30 days.
Self-controlled case series study
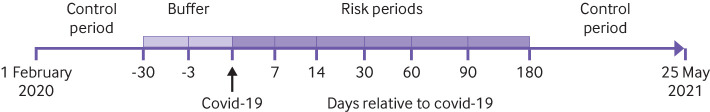
The self-controlled case series method8 was used to determine the incidence rate ratio and corresponding 95% confidence intervals of a first venous thromboembolic or bleeding event during the risk periods 1-7, 8-14, 15-30, 31-60, 61-90, and 91-180 days after covid-19 in the study period from 1 February 2020 to 25 May 2021 (fig 1), using the SCCS package version 1.4 in R version 4.0.2.9 The remaining time was used as the control period, apart from −30 to −4 days and −3 to 0 days before exposure to SARS-CoV-2 relative to the covid-19 date. To avoid selection bias, day 0 was not included in the risk period, as patients presenting with venous thromboembolism or bleeding were more likely to have been tested for SARS-CoV-2.10 We investigated the effect modification of sex, age, pandemic wave (1 February to 31 July 2020 for the first wave; 1 August 2020 to 31 January 2021 for the second wave; and 1 February to 25 May 2021 for the third wave), and severity of covid-19 (mild: not admitted to hospital; severe: admitted to hospital; critical: admitted to intensive care unit). As deaths were found to have little substantive impact on results, as confirmed in sensitivity analyses excluding participants with covid-19 who died during the observation period, we used the standard self-controlled case series method (see supplementary table 3). Further self-controlled case series analyses using splines were undertaken to characterise the risk profiles after covid-19 (see supplementary figures 1-3).
Fig 1.
Overview of self-controlled case series study design, including risk periods, periods before exposure to SARS-CoV-2, and control periods. The covid-19 date was set as the earliest from the date of disease onset, sample date, diagnosis date, or date of report to the communicable disease surveillance system SmiNet (Public Health Agency of Sweden)
Matched cohort study
In the matched cohort study,11 comparing covid-19 cases with four control participants who had not reported SARS-CoV-2 positivity to SmiNet and were matched on age, sex, and county of residence, the risk ratio and 95% confidence interval of venous thromboembolism or bleeding in the first 1-30 days after covid-19 or the index date were determined using unadjusted and adjusted conditional Poisson regression analysis. Several potential confounders were included in the adjusted risk ratio: comorbidities (weighted Charlson comorbidity index), cancer (defined as ICD-10 code C00-C97 in the patient registries within six months before and after covid-19 or the index date), surgery (before venous thromboembolism or bleeding and within 30 days before or during the specified risk period), long term anticoagulation treatment (as defined by at least two filled prescriptions from the pharmacy within 12 months before covid-19 or the index date of pharmaceuticals with anatomical therapeutic chemical codes: B01AA, B01AB, B01AE, and B01AF), and a previous event in the analyses of recurrent events. As a sensitivity analysis to investigate potential residual confounding, we included the individual groups within the Charlson comorbidity index as categorical variables in the analyses (see supplementary tables 18-20).
The risk ratios and 95% confidence intervals of outcomes were determined using the g, package gnm version 1.1-1 in R, version 4.0.2.12 The effect of pandemic waves and severity of covid-19 (mild: not admitted to hospital; severe: admitted to hospital; critical: admitted to intensive care unit) was determined for venous thromboembolism and bleeding using participants who tested negative for SARS-CoV-2 as a baseline. We determined the risk for all venous thromboembolism or bleeding events (first or recurrent event) within 30 days after covid-19 or the index date and stratified across the different disease severity groups using controls as a baseline.
Patient and public involvement
Access to the pseudonymised historical data from our Swedish nationwide registries is restricted by law and is not publicly available. Our research question needed to be clear and focused to enable us to request the data. As our study was related to covid-19 research, there was an urgency for analysis. In addition, the learning curve to extract and analyse the data was steep. This study was conceived from an in-house perspective, and we were not funded for patient or public involvement, nor had we considered the possibility of how to involve patients or the public in other ways such as review of the manuscript or dissemination of findings. We appreciate the role of patients as partners in research and look forward to publishing future research that includes patients and members of the public.
Results
Overall, 1 057 174 people with covid-19 (517 434 (49%) males, 539 740 (51%) females) were reported to SmiNet (table 1). Mean age was 40.2 (SD 19) years. The dataset included 42 potential reinfections; five of these had the exact same date, although one year apart, indicating a coding error. In total, 4 076 342 matched control participants were identified.
Table 1.
Characteristics of participants with covid-19 and matched control participants without covid-19 and those with a first venous thromboembolic or bleeding event during days 1-30 after covid-19* or index date. Values are numbers (percentages) unless stated otherwise
| Cohorts | Deep vein thrombosis | Pulmonary embolism | Bleeding | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n=1 057 174) | Controls (n=4 076 342) | Cases (n=401) | Controls (n=267) | Cases (n=1761) | Controls (n=171) | Cases (n=1002) | Controls (n=1292) | ||||
| Mean (SD) age (years) | 40.2 (19) | 40.2 (19.1) | 59.3 (15.5) | 55.8 (17.8) | 62.5 (15.3) | 62.2 (18.7) | 62.7 (18.4) | 54.3 (19.9) | |||
| Sex: | |||||||||||
| Men | 517 434 (48.9) | 2 000 973 (49.1) | 244 (60.8) | 140 (52.4) | 1170 (66.4) | 83 (48.5) | 546 (54.5) | 508 (39.3) | |||
| Women | 539 740 (51.055) | 2 075 369 (50.913) | 157 (39.2) | 127 (47.6) | 591 (33.6) | 88 (51.5) | 456 (45.5) | 784 (60.7) | |||
| Died | 19 073 (1.8) | 13 675 (0.3) | 46 (11.5) | 21 (7.9) | 281 (16) | 22 (12.9) | 195 (19.5) | 83 (6.4) | |||
| Disease severity: | |||||||||||
| Uninfected | - | 4 076 342 (100) | - | 267 (100) | - | 171 (100) | - | 1292 (100) | |||
| Mild | 999 113 (94.5) | - | 155 (38.7) | - | 239 (13.6) | - | 349 (34.8) | - | |||
| Hospital admission | 50 279 (4.8) | - | 163 (40.6) | - | 1037 (58.9) | - | 467 (46.6) | - | |||
| Intensive care unit admission | 7782 (0.7) | - | 83 (20.7) | - | 485 (27.5) | - | 186 (18.6) | - | |||
| Mean (SD) wCCI score | 0.81 (1.9) | 0.73 (1.8) | 1.57 (2.5) | 1.90 (2.4) | 1.48 (2.3) | 2.29 (2.9) | 1.98 (2.68) | 1.54 (2.4) | |||
| Previous venous thromboembolism† | 19 988 (1.9) | 63 895 (1.6) | - | - | - | - | - | - | |||
| Previous bleeding | 61 150 (5.8) | 200 672 (4.9) | - | - | - | - | - | - | |||
wCCI=weighted Charlson comorbidity index.
Earliest date from date of disease onset, sample date, diagnosis date, or date of report to the communicable disease surveillance system SmiNet (Public Health Agency of Sweden).
Event (deep vein thrombosis, pulmonary embolism, pregnancy related thrombosis, or bleeding) occurred between 1987 and day 0 (date of covid-19 or index date) for participants with covid-19 and between 1997 and day 0 for control participants.
Covid-19 as independent risk factor
Deep vein thrombosis
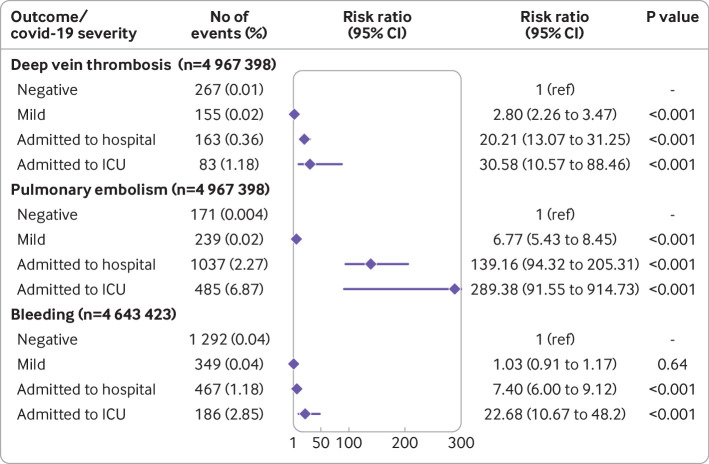
During the self-controlled case series period, 1761 participants had a first deep vein thrombosis event; of these 783 (44.5%) were female participants and 978 (55.5%) male participants. Compared with the control period, the incidence rate ratios of a first deep vein thrombosis were 5.59 (95% confidence interval 4.47 to 6.98) during the first week after covid-19 and 7.44 (6.06 to 9.14) during the second week and significantly increased up to 70 days and also during the period before exposure to SARS-CoV-2 (−3 days to day 0) and in the 30 days before covid-19 (table 2, supplementary figure 1). The incidence rate ratio for deep vein thrombosis during 1-90 days after covid-19 generally increased with age and was highest during the first pandemic wave in Sweden compared with the second and third waves. Sex did not appear to have a significant effect on the association between covid-19 and deep vein thrombosis (see supplementary table 4). The risk appeared to be increased in patients with more severe covid-19 (see supplementary table 7).
Table 2.
Incidence rate ratios for a first deep vein thrombosis, pulmonary embolism, and bleeding after covid-19 using self-controlled case series (SCCS)
| Period (days) | Deep vein thrombosis | Pulmonary embolism | Bleeding | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of events | Incidence rate ratio (95% CI) | P value | No of events | Incidence rate ratio (95% CI) | P value | No of events | Incidence rate ratio (95% CI) | P value | |||
| Control period | 826 | 1 (ref) | - | 603 | 1 (ref) | - | 4499 | 1 (ref) | - | ||
| Days: | |||||||||||
| −30 to −4 | 98 | 1.39 (1.12 to 1.73) | 0.003 | 157 | 2.76 (2.29 to 3.33) | <0.001 | 646 | 1.58 (1.44 to 1.72) | <0.001 | ||
| −3 to 0 | 89 | 8.71 (6.92 to 10.97) | <0.001 | 336 | 39.04 (33.59 to 45.37) | <0.001 | 305 | 5.06 (4.48 to 5.71) | <0.001 | ||
| 1 to 7 | 97 | 5.59 (4.47 to 6.98) | <0.001 | 530 | 36.17 (31.55 to 41.47) | <0.001 | 357 | 3.46 (3.09 to 3.87) | <0.001 | ||
| 8 to 14 | 122 | 7.44 (6.06 to 9.14) | <0.001 | 641 | 46.40 (40.61 to 53.02) | <0.001 | 267 | 2.75 (2.42 to 3.13) | <0.001 | ||
| 15 to 30 | 182 | 5.31 (4.44 to 6.36) | <0.001 | 590 | 20.24 (17.63 to 23.23) | <0.001 | 378 | 1.85 (1.65 to 2.06) | <0.001 | ||
| 31 to 60 | 146 | 2.59 (2.12 to 3.15) | <0.001 | 190 | 4.14 (3.44 to 4.99) | <0.001 | 501 | 1.49 (1.35 to 1.65) | <0.001 | ||
| 61 to 90 | 70 | 1.42 (1.09 to 1.85) | 0.009 | 96 | 2.48 (1.95 to 3.15) | <0.001 | 306 | 1.06 (0.94 to 1.2) | 0.36 | ||
| 91 to 180 | 131 | 1.13 (0.91 to 1.41) | 0.27 | 124 | 1.40 (1.11 to 1.77) | 0.005 | 668 | 1.07 (0.97 to 1.18) | 0.20 | ||
| Method (days 1 to 30): | |||||||||||
| SCCS | 401 | 5.90 (5.12 to 6.80) | <0.001 | 1761 | 31.59 (27.99 to 35.63)* | <0.001 | 1002 | 2.48 (2.30 to 2.68)* | <0.001 | ||
| Matched cohort study | 401 | 4.98 (4.96 to 5.01) | <0.001 | 1761 | 33.05 (32.8 to 33.3)† | <0.001 | 1002 | 1.88 (1.71 to 2.07)† | <0.001 | ||
CI=confidence interval.
Relative incidence (95% CI).
Risk ratio (95% CI). 30 day relative risk of same events calculated using adjusted conditional Poisson regression analysis in matched cohort study is shown for comparison.
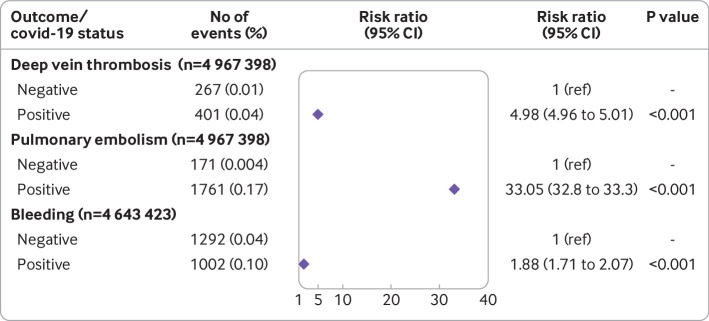
In the matched cohort study, a first deep vein thrombosis occurred in 401 patients with covid-19 (absolute risk 0.039%) and 267 control participants (absolute risk 0.007%) during the follow-up period (30 days) (table 1, supplementary table 10). The risk ratio of a first deep vein thrombosis was 4.98 (4.96 to 5.01) during the 1-30 days after covid-19 (fig 2, supplementary table 10). This finding is comparable with an incidence rate ratio of 5.90 (5.12 to 6.80) for days 1-30 using the self-controlled case series method (table 2). This risk was further increased in patients with more severe covid-19 (fig 3, supplementary table 11) and was higher during the first pandemic wave in Sweden compared with the second and third waves (see supplementary table 12). For the matched cohort study of all deep vein thrombosis events (first and recurrent) during days 1 to 30 after covid-19 or the index date, 594 occurred among patients with covid-19 (absolute risk 0.056) and 579 among control participants (absolute risk 0.014; see supplementary table 15). The adjusted risk ratio for all deep vein thrombosis events after covid-19 was 4.5 (3.84 to 5.28; see supplementary table 15).
Fig 2.
Adjusted relative risks with 95% confidence intervals of a first deep vein thrombosis, pulmonary embolism, and bleeding event within 30 days after covid-19 in matched cohort study adjusted for weighted Charlson comorbidity index score, cancer, surgery, and long term anticoagulation treatment. Total cohort consists of individuals who tested positive for SARS-CoV-2 and those who were not reported to the Public Health Agency of Sweden (test negative) as baseline
Fig 3.
Adjusted relative risks with 95% confidence intervals of a first deep vein thrombosis, pulmonary embolism, and bleeding event within 30 days after covid-19 in matched cohort study adjusted for weighted Charlson comorbidity index score, cancer, surgery, and long term anticoagulation treatment, and stratified according to disease severity using control participants who were not reported to the Public Health Agency of Sweden (test negative) as baseline. ICU=intensive care unit
All results remained significant even after adjusting for the effect of comorbidities (weighted Charlson comorbidity index and individual comorbidity groups; see supplementary tables 10 and 18), cancer diagnosis, surgery, and long term anticoagulation treatment. Long term anticoagulation treatment did not appear to protect against deep vein thrombosis (see supplementary table 10).
Pulmonary embolism
During the self-controlled case series period, 3267 participants had a first pulmonary embolism. The incidence rate ratio of a first pulmonary embolism was 36.17 (31.55 to 41.47) during the first week after covid-19 and 46.40 (40.61 to 53.02) during the second week compared with the control period and significantly increased up to 110 days and also during the period before exposure to SARS-CoV-2 (−3 days to day 0) and the 30 days before covid-19 (table 1, supplementary figure 2). Significant effect modification by sex, age, and pandemic wave was found (see supplementary table 5). Specifically, the incidence rate ratios were higher in male participants than in female participants during the first three months after covid-19, and highest in the age group 50-70 years. The incidence rate ratios for pulmonary embolism were highest during the first wave in Sweden compared with the later ones in the first two weeks after covid-19; however, after the acute phase the incidence rate ratios were higher in wave 3 compared with wave 1 (see supplementary table 5). The risk was observed to be increased in participants with more severe covid-19 (see supplementary table 8).
During days 1 to 30 after covid-19 or the index date, a first pulmonary embolism event occurred in 1761 patients with covid-19 (absolute risk 0.17%) and 171 control participants (absolute risk 0.004%) (fig 2 and fig 3). The risk ratio of a first pulmonary embolism event was 33.05 (32.80 to 33.30) (fig 2, supplementary table 10) in the first month after covid-19, comparable to an incidence rate ratio of 31.59 (27.99 to 35.63) using the self-controlled case series method for the same period (table 2). This risk was further increased in patients with more severe covid-19 (fig 3, supplementary table 11) and was substantially increased in patients admitted to hospital and those admitted to an intensive care unit (increase of about 140-fold and 290-fold, respectively). The risk of a first pulmonary embolism event was highest during the first pandemic wave (increase of 54-fold) compared with the second wave (increase of 25-fold) and third wave (increase of 44-fold) (see supplementary table 13). For the matched cohort study of all events, 444 pulmonary embolism events occurred in control participants (absolute risk 0.01) and 2226 in patients with covid-19 (absolute risk 0.21). The adjusted risk ratio was 34.68 (30.05 to 40.02) within 30 days after covid-19 (see supplementary table 16).
All results remained significant, even after adjusting for the effect of comorbidities (weighted Charlson comorbidity index and individual comorbidity groups; see supplementary tables 10 and 19), cancer diagnosis, surgery, and long term anticoagulation treatment. Long term anticoagulation treatment was found to be protective against pulmonary embolism (risk ratio 0.3 (0.29 to 0.31); see supplementary table 10).
Bleeding
During the self-controlled case series period, 7927 participants had a first bleeding event. The incidence rate ratio of a first bleeding event was 3.46 (3.09 to 3.87) during the first week after covid-19 and 2.75 (2.42 to 3.13) during the second week compared with the control period, and significantly increased up to 60 days and also during the period before exposure to SARS-CoV-2 (−3 days to day 0) and the month before covid-19 (table 2, supplementary figure 3). Moreover, sex, age, and pandemic wave were observed to be significant effect modifiers (see supplementary table 6). Specifically, the incidence rate ratios were higher in male participants than in female participants during the first two months after covid-19, higher with increasing age, and higher during the first pandemic wave compared with the second and third waves (see supplementary table 6). No increased risk of bleeding was found in mild cases, but a noticeable increase was observed in more severe cases (see supplementary table 9).
During days 1 to 30 after covid-19 or the index date, a first bleeding event occurred in 1002 patients with covid-19 (absolute risk 0.10%) and 1292 control participants (absolute risk 0.04%). The risk ratio of a first bleeding event was 1.88 (1.71 to 2.07), comparable to an incidence rate ratio of 2.48 (2.30 to 2.68) using the self-controlled case series method for the same period (fig 2, table 2, supplementary table 10). In contrast with venous thromboembolic events, but in accordance with the SCCS analyses, the risk of a first bleeding event was observed not to be increased in patients with mild covid-19 but was further increased in patients with more severe covid-19 (fig 3, supplementary table 11). In the matched cohort study of all bleeding events, 2370 control participants (absolute risk 0.06%) and 1677 patients with covid-19 (absolute risk 0.16%) had a first or recurrent bleeding event during days 1 to 30 after covid-19 or the index date. The adjusted risk ratio was 1.9 (1.76 to 2.05) (see supplementary table 17).
All results remained significant even after adjusting for the effect of comorbidities (weighted Charlson comorbidity index and individual comorbidity groups; see supplementary tables 10 and 19), cancer diagnosis, surgery, and long term anticoagulation treatment. Long term anticoagulation treatment was associated with an increased risk of bleeding (risk ratio 2.37 (1.79 to 3.14); see supplementary table 10).
Discussion
This study found an increased risk of a first deep vein thrombosis up to three months after covid-19, pulmonary embolism up to six months, and a bleeding event up to two months, with the risk of pulmonary embolism in the acute phase being especially high. This study used a nationwide cohort consisting of all people who tested positive for SARS-CoV-2 regardless of disease severity. The risks were observed to be consistently increased regardless of whether a self-controlled case series or matched cohort study statistical method was used.
Strengths and limitations of this study
The incidence of risk during the period before exposure to SARS-CoV-2 (days −3 to day 0) were found to be high for all three outcomes, driven by an increased number of events at day 0. Some of the patients with these high risks were likely infected with SARS-CoV-2 before the event. However, because including day 0 in the risk period would introduce test bias,10 we excluded this day and incorporated it in the pre-exposure period. In addition, risks were observed to be increased during the buffer period (−30 to −4 days), probably because of a delay in testing or documentation of covid-19 diagnosis, or due to reverse causality (ie, nosocomial covid-19).
Furthermore, we compared the risk of outcomes among the different pandemic waves and found the incidences or risk to be higher in the first wave, especially during the acute phase of covid-19. This could be explained by improvements in the treatment arsenal against covid-19, especially the widespread use of thromboprophylaxis after the first wave. Additionally, we found an excess risk of outcomes in patients with more severe covid-19 admitted to hospital, but especially in those admitted to an intensive care unit. The rates for venous thromboembolism in this patient group have been shown to be high despite thromboprophylaxis.13
We acknowledge limitations to our study. Firstly, registry based information is at risk of containing incomplete or inaccurate data. Secondly, venous thromboembolism may have been underdiagnosed in patients with covid-19. Critically ill patients may be too unstable for diagnostic evaluation of venous thromboembolism, or the evaluation might be delayed owing to contagiousness issues. Furthermore, as registry data for control participants was limited to 1997, events might have more often been falsely classified as first events in the control participants, possibly resulting in underestimation of the risks. However, overestimation of the risks might also exist owing to limited testing for covid-19, especially during the first pandemic wave. In addition, vaccine data were not available for our study. By week 17 (30 days before the end of our data), 30.3% of the population aged 12 years or older had received their first vaccine dose (Swedish Public Health Agency data). The vaccines were prioritised for elderly people primarily, therefore the distribution of vaccines was increased in elderly age groups compared with younger age groups. As we found that older people have an increased risk of deep vein thrombosis, pulmonary embolism, and bleeding, it is possible that vaccine coverage provides a protective effect in age groups older than 50 years. Potentially this could partially explain why the incidence rate ratios were lower (at least in the acute phase) during the third pandemic wave. Moreover, we acknowledge residual confounding as a limitation to our study. However, the similarity of the results between the matched cohort study and self-controlled case series analysis, which controls for all confounders that might be regarded as fixed during the study period (thus including body mass index and smoking) suggests that these are not major confounders. Furthermore, the results remained unchanged when adjusting for specific comorbidities from the Charlson comorbidity index.
Comparison with other studies
Previous studies have shown an association between thrombosis and infections.14 15 The risk of events in our study was, however, observed to be much higher. This could be explained by several pathophysiological alterations in covid-19, such as a direct effect of the virus on endothelial cells,16 an exaggerated inflammatory response, down regulation of angiotensin converting enzyme 2 receptors, and activation of the coagulation system.17 Although deep vein thrombosis and pulmonary embolism traditionally belong in the venous thromboembolism spectrum of disease, the relative incidence of pulmonary embolism was much higher, which could be due to immunothrombosis (thrombosis in the pulmonary vessels from local inflammation).18 The increased risk of bleeding could be related to endothelial dysfunction,16 coagulopathy,17 or disseminated intravascular coagulation.19
Our results are in line with those of similar studies that used the self-controlled case series method to determine the association between covid-19 and thromboembolic events. A large study from England using electronic health records found that 4671 patients were admitted to hospital due to venous thromboembolism within 28 days of testing positive for SARS-CoV-2, and the incidence rate ratio for venous thromboembolism was about 14-fold increased during the first two weeks, decreasing to an increase of about eightfold during the third week and threefold during the fourth week.20 Because the authors did not discriminate between first or recurrent events, nor did they differentiate between deep vein thrombosis and pulmonary embolism, it is difficult to compare the incidence rate ratios between their and our study. Furthermore, the English study only included people who were vaccinated against covid-19. The only other comparable nationwide self-controlled case series study to ours is from Scotland, where all people who tested positive for SARS-CoV-2 from 1 March to 5 October 2020 were included.21 Those authors identified 179 people with deep vein thrombosis and 417 with pulmonary embolism during the study period, with incidence rate ratios increased by about 12-fold and 17-fold during the first week after covid-19, respectively. Day 0 was included in the first risk period, thereby the incidence rate ratios may be inflated as a result of test bias.10 Although the Scottish study found an increased relative incidence of deep vein thrombosis (1.77) during the period 28 to 56 days after covid-19 that is comparable to the finding in our study (2.59) in similar periods, it was not statistically significant. Currently, no studies have used the self-controlled case series method to determine the association between covid-19 and bleeding. However, a Danish study that compared the incidence of major bleeding between participants who tested positive for SARS-CoV-2 and those who tested negative, did not find a higher incidence for those who tested positive.22 The Danish study did not use matched controls but instead included people who probably displayed symptoms similar to those of covid-19 but who did not test positive for SARS-CoV-2. Therefore, in the Danish study it is difficult to determine if infection with SARS-CoV-2 increases the risk of bleeding compared with the background population.
Conclusions and policy implications
The present findings have major policy implications. Our findings arguably support thromboprophylaxis to avoid thrombotic events, especially for high risk patients,23 and strengthens the importance of vaccination against covid-19. It remains to be established whether SARS-CoV-2 infection increases the risk of venous thromboembolism or bleeding more than it does for respiratory infections, such as influenza, but also whether the period of thromboprophylaxis after covid-19 should be extended. Future clinical research would be beneficial in this context.
What is already known on this topic
It is well known that covid-19 increases the risk of venous thromboembolism
Less evidence exists on the length of time this risk is increased, if risk changed during the pandemic waves, and whether covid-19 also increases the risk of bleeding
What this study adds
The findings of this study suggest that covid-19 is an independent risk factor for deep vein thrombosis, pulmonary embolism, and bleeding, and that the risk of these outcomes is increased for three, six, and two months after covid-19, respectively
This study also found a higher risk of events in patients with comorbidities, patients with more severe covid-19, and during the first pandemic wave compared with the second and third waves
Web extra.
Extra material supplied by authors
Supplementary information: Additional tables 1-20 and figures 1-3
Contributors: IK and OFR contributed equally to this project and are therefore co-first authors. IK did the study design, methodology, investigation, funding acquisition, literature review, data interpretation, visualisation, and writing of the original draft. OFR did the study design, data curation, formal analysis, investigation, methodology, software, visualisation, and writing of the original draft. PF contributed to the study design, formal analysis, investigation, methodology, software, visualisation, data validation, and writing, reviewing, and editing of the report. HJ did the study design, investigation, data interpretation, and writing, reviewing, and editing of the report. MS did the study design, methodology, data interpretation, and writing, reviewing, and editing of the report. EL did the study design, data curation, software, data presentation and visualisation, data validation, data interpretation, and writing, reviewing, and editing of the report. KL did the study design, supervision, data interpretation, and writing, reviewing, and editing of the report. AMFC conceptualised the study; did the data collection, data curation, study design, investigation, funding acquisition, and methodology; supervised the study; and did the project administration, literature review, data interpretation, and writing, reviewing, and editing of the report. OFR, AMFC, PF, and EL have accessed and verified the underlying data. IK, OFR, EL, and AMFC had full access to all the data; PF had accessed to anonymised data (owing to policies about data sharing from the national Swedish authorities). All authors had final responsibility for the decision to submit for publication. AMFC had final responsibility for submission and is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by the Region Västerbotten (central ALF (Agreement for Medical Education and Research) funding RV-836351 and base unit ALF funding RV-939769 to AMFC; and central ALF funding RV-941902 to KL), Umeå University (strategic funding during 2020 from the Department of Clinical Microbiology to AMFC), the Laboratory for Molecular Infection Medicine Sweden (MIMS to AMFC), Stroke Research in Northern Sweden (to AMFC), the Swedish Kidney Foundation (F2020-0025 to AMFC), the Scandinavian Research Foundation for venous diseases (to AMFC), and the Heart Foundation in Northern Sweden, Arnerska Research Foundation, and Kempes Foundation (to IK). The funding agencies had no role in the study design, data collection, analysis and interpretation of data, writing of the report, or decision to submit the article for publication. The views and conclusions in this document are those of the authors and not influenced by the funding agencies.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.”
The corresponding author (AMFC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results of the study will be disseminated to academia, stakeholders and wider audiences using different platforms, such as conferences, journals, websites, and traditional media as well as social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the Swedish Ethical Review Authority (DNR 2020-02150).
Data availability statement
The study protocol (R script) for the SCCS and matched cohort study are available on request from the corresponding author. The study used secondary registry data, which is regulated by the Public Access to Information and Secrecy Act (2009:400) and is protected by strict confidentiality. However, for the purpose of research, after formal application to access personal data, the responsible authority can grant access to data, although this is contingent on vetting by the Ethical Review Authority of Sweden, according to the Act (2003:460) concerning the Ethical Review of Research Involving Humans. Synthetic (ie, depersonalised and jittered) data can be provided on request from the corresponding author.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for Covid-19 . Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thakkar S, Arora S, Kumar A, et al. A Systematic Review of the Cardiovascular Manifestations and Outcomes in the Setting of Coronavirus-19 Disease. Clin Med Insights Cardiol 2020;14:1179546820977196. 10.1177/1179546820977196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet 2021;398:599-607. 10.1016/S0140-6736(21)00896-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mansory EM, Srigunapalan S, Lazo-Langner A. Venous Thromboembolism in Hospitalized Critical and Noncritical COVID-19 Patients: A Systematic Review and Meta-analysis. TH Open 2021;5:e286-94. 10.1055/s-0041-1730967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mai V, Tan BK, Mainbourg S, et al. Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: A systematic review with meta-analysis. Vascul Pharmacol 2021;139:106882. 10.1016/j.vph.2021.106882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 7. Ludvigsson JF, Appelros P, Askling J, et al. Adaptation of the Charlson Comorbidity Index for Register-Based Research in Sweden. Clin Epidemiol 2021;13:21-41. 10.2147/CLEP.S282475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006;25:1768-97. 10.1002/sim.2302 [DOI] [PubMed] [Google Scholar]
- 9.Ghebremichael-Weldeselassie Y, Whitaker H, Farrington P. SCCS: The Self-Controlled Case Series Method. R package version 1.4., 2021.
- 10. Fonseca-Rodríguez O, Fors Connolly AM, Katsoularis I, Lindmark K, Farrington P. Avoiding bias in self-controlled case series studies of coronavirus disease 2019. Stat Med 2021;40:6197-208. 10.1002/sim.9179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cummings P, McKnight B, Greenland S. Matched cohort methods for injury research. Epidemiol Rev 2003;25:43-50. 10.1093/epirev/mxg002 [DOI] [PubMed] [Google Scholar]
- 12.Turner H, Firth D. Generalized nonlinear models in R: An overview of the gnm package. R package version 1.1-1, 2020.
- 13. Helms J, Tacquard C, Severac F, et al. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) . High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089-98. 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt M, Horvath-Puho E, Thomsen RW, Smeeth L, Sørensen HT. Acute infections and venous thromboembolism. J Intern Med 2012;271:608-18. 10.1111/j.1365-2796.2011.02473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Connolly-Andersen AM, Whitaker H, Klingström J, Ahlm C. Risk of Venous Thromboembolism Following Hemorrhagic Fever With Renal Syndrome: A Self-controlled Case Series Study. Clin Infect Dis 2018;66:268-73. 10.1093/cid/cix777 [DOI] [PubMed] [Google Scholar]
- 16. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-8. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magadum A, Kishore R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020;9:E2508. 10.3390/cells9112508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020;2:e437-45. 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou X, Cheng Z, Luo L, et al. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb Res 2021;201:23-9. 10.1016/j.thromres.2021.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 2021;374:n1931. 10.1136/bmj.n1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ho FK, Man KKC, Toshner M, et al. Thromboembolic Risk in Hospitalized and Nonhospitalized COVID-19 Patients: A Self-Controlled Case Series Analysis of a Nationwide Cohort. Mayo Clin Proc 2021;96:2587-97. 10.1016/j.mayocp.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dalager-Pedersen M, Lund LC, Mariager T, et al. Venous Thromboembolism and Major Bleeding in Patients With Coronavirus Disease 2019 (COVID-19): A Nationwide, Population-Based Cohort Study. Clin Infect Dis 2021;73:2283-93. 10.1093/cid/ciab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spyropoulos AC, Goldin M, Giannis D, et al. HEP-COVID Investigators . Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern Med 2021;181:1612-20. 10.1001/jamainternmed.2021.6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional tables 1-20 and figures 1-3
Data Availability Statement
The study protocol (R script) for the SCCS and matched cohort study are available on request from the corresponding author. The study used secondary registry data, which is regulated by the Public Access to Information and Secrecy Act (2009:400) and is protected by strict confidentiality. However, for the purpose of research, after formal application to access personal data, the responsible authority can grant access to data, although this is contingent on vetting by the Ethical Review Authority of Sweden, according to the Act (2003:460) concerning the Ethical Review of Research Involving Humans. Synthetic (ie, depersonalised and jittered) data can be provided on request from the corresponding author.