Abstract
Background
Obesity is pathophysiologically complex in older adults compared to that in young and middle-aged adults. The aim of the present study was to determine the appropriate body mass index (BMI) range based on geriatric evaluation parameters in which complications can be minimized in older adults.
Methods
A total of 1,051 older adult patients who underwent comprehensive geriatric assessment were included. The patients’ demographic characteristics, comorbid diseases, number of drugs, BMI, basic and instrumental activities of daily living (BADL and IADL), Tinetti balance and walking scale, Mini Nutritional Assessment, Geriatric Depression Scale-15, Mini-Mental State Examination, Time Up and Go test, and handgrip strength measurement were extracted from patient records.
Results
Of the patients who took part, 73% were female, and the mean age was 77.22±7.10 years. The most negative results were observed in those with a BMI <25 kg/m2 and in those with a BMI >35 kg/m2. Receiver operating characteristic (ROC) analysis of the optimum BMI cutoff levels to detect the desirable values of geriatric assessment parameters was found to be 31–32 and 27–28 kg/m2 for female and male, respectively.
Conclusion
Older adults with BMI <25 and >35 kg/m2 were at a higher risk of a decrease in functional capacity, and experienced gait and balance problems, fall risk, decrease in muscle strength, and malnutrition. Data from this study suggest that the optimum range of BMI levels for older adults is 31–32 and 27–28 kg/m2 for female and male, respectively.
Keywords: Obesity, Body mass index, Aged, Geriatric assessment
INTRODUCTION
Life expectancy has steadily increased in the recent decades. Moreover, the global prevalence of older adults has increased, owing to a decrease in birth and death rates.1,2) Obesity is an important public health concern that is increasing in the older population and society. In the United States during the 90s, there were approximately 32 million older adults and 26.1% of them had a body mass index (BMI) >30 kg/m2, while in 2008, there were approximately 40 million older adults and 39.5% had a BMI over 30 kg/m2.3) Moreover, according to the Korean National Health Insurance Database, the prevalence of obesity among adults aged 70–79 years has increased from 31.7% in 2006 to 36.6% in 2015. The prevalence of obesity among those over 80 years old was 21.9% in 2006 and increased to 27.5% in 2015.4) In Turkey, the prevalence of obesity in the adult population is >30%. Although the prevalence of obesity is higher in women, its rapid increase in men has also drawn attention in recent years.5)
Obesity among older adults is most likely the result of consuming more calories than expending energy. Decreased basal metabolic rate and physical activity levels in the older adults are important contributors to obesity.6) Often, in older adults, changes in body composition, such as an increase in fat mass and decrease in muscle mass, are observed. Obesity is pathophysiologically complex in older adults compared to that in young and middle-aged adults. This complexity makes it difficult to identify obesity-related comorbidities and creates clinical uncertainty in terms of weight management.1) It should also be noted that some studies in older adults with cardiovascular disease, cancer, and stroke have found that overweight and obese patients have a lower risk of mortality. This situation is known as the obesity paradox.7,8) Therefore, it is not clear which BMI range is most beneficial for older adults in terms of outcomes, such as functionality, risk of falls, nutritional status, and strength. Although some studies have investigated associations between obesity or BMI and geriatric conditions, the present study is the first to examine associations between many geriatric assessment parameters, such as nutritional status, cognitive and functional status, gait and balance, and muscle strength and BMI groups, simultaneously.9)
The aim of the present study was to identify a suitable BMI range that can minimize negative clinical results in geriatric patients, based on geriatric evaluation parameters.
MATERIALS AND METHODS
Participants
Data were utilized from 2,335 older adults who were admitted to a geriatric outpatient clinic in Turkey between January 2017 and November 2020. After obtaining ethics committee and institutional approval of Bezmialem Vakif University (No. 10/29) for the present study, the data were retrospectively analyzed. A total of 1,312 people with either a diagnosis of dementia or history of cerebrovascular disease, and those with missing data were excluded from the study. The final sample included 1,051 patients aged >65 years. Informed consent was obtained from all the participants included in the study.
Comprehensive Geriatric Assessment
Demographic information (patient age, sex, marital status, people whom they live with), height, weight, BMI, calf circumference measurements, number of drugs used, history of falls in the last year, Barthel basic daily living activities scale (BADL), Lawton instrumental daily living activities scale (IADL), Tinetti balance and gait scale, Mini Nutritional Assessment (MNA) test, Geriatric Depression Scale-15, Mini-Mental State Examination (MMSE), Time Up and Go test (TUG), and handgrip strength (HGS; three measurements were made from the dominant hand and the highest value was taken). According to these parameters, BADL (score ≥91), IADL (score ≥17), Tinetti total (score >19), TUG (< 13.5 s), MNA (score >23.5), GDS (score <5), MMSE (score ≥23), and HGS (female ≥16 kg, male ≥27 kg) were considered healthy.10-17)
Evaluation of Weight Status
BMI was defined as the person’s weight in kilograms divided by the square of the person’s height in meters (kg/m2). The World Health Organization (WHO) categorizes BMI for adults over the age of 20 years as follows: underweight, <18.5 kg/m2; normal weight, 18.5–24.9 kg/m2; pre-obesity, 25–29.9 kg/m2; stage 1 obesity, 30–34.9 kg/m2; stage 2 obesity, 35–39 kg/m2; and stage 3 obesity, >40 kg/m2.18)
Statistical Analysis
IBM SPSS Statistics 22.0 program (IBM, Armonk, NY, USA) was used for statistical analysis. Descriptive statistics were used to assess the central tendency and distribution of the study variables (e.g., mean, standard deviation, median, and frequency). Skewness and kurtosis values were used together with the Shapiro-Wilk test to assess for normal distribution of the data. One-way ANOVA and Kruskal-Wallis tests were used to evaluate more than two normally and non-normally distributed variables, respectively. The chi-squared test was used to evaluate the relationships between the variables. The cutoff scores were assessed using the receiver operating characteristic (ROC) curve. Sensitivity and specificity were calculated for different BMI cutoff scores to detect the desirable cutoff values of BADL, IADL, MNA, Tinetti, TUG, MMSE, GDS, and HGS. After evaluating all BMI cutoff values, the optimum BMI values were determined according to the optimum sensitivity and specificity. The p-values for each area under the curve (AUC) from the ROC were determined. The results were evaluated using a 95% confidence interval, and significance was set at a level of p<0.05.
RESULTS
General Characteristics
A total of 1,051 people, 768 female (73%) and 283 male (27%) were included in the study. The mean age of the participants was 77.22±7.10 years (range, 65–103 years), with the mean age for male and female being 78.41±7.39 and 76.77±6.94 years, respectively. There was a statistically significant difference in the ages of male and female (p=0.002).
The mean BMI of the sample was 30.79±5.77 kg/m2 (range, 18.5–56) with the mean BMI being 28.12±4.37 kg/m2 for male and 31.71±5.92 kg/m2 for female. There was a significant difference in the BMIs of male and female (p<0.001). When female and male were evaluated separately, similar results were obtained. Moreover, when patients aged <80 years were evaluated, BMI was thought to be independent of age. The general characteristics of both sexes are shown in Table 1.
Table 1.
General characteristics
| Characteristic | Female (n=768, 73%) | Male (n=283, 27%) |
|---|---|---|
| Age (y) | 76.77±6.94 | 78.41±7.39 |
| BMI (kg/m2) | 31.71±5.92 | 28.12±4.37 |
| Hypertension (%) | 73 | 58 |
| DM (%) | 36 | 35 |
| CAD (%) | 14 | 27 |
| COPD (%) | 12 | 10 |
| Heart failure (%) | 6 | 12 |
| Barthel | 87.68±15.29 | 87.80±18.41 |
| Lawton | 17.19±5.91 | 17.15±6.01 |
| Tinetti total | 23.77±6.37 | 24.82±6.14 |
| MNA | 23.34±4.11 | 24.36±4.73 |
| GDS | 5.45±4.35 | 3.25±3.89 |
| MMSE | 24.46±4.34 | 25.41±3.90 |
| TUG | 15.00±9.10 | 14.08±10.01 |
| HGS | 18.89±6.64 | 29.41±9.14 |
Values are presented as mean±standard deviation.
BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; Barthel, Barthel basic daily living activities scale; Lawton, Lawton instrumental daily living activities scale; MNA, Mini-Nutritional Assessment; MMSE, Mini-Mental State Examination; HGS, handgrip strength; GDS, Geriatric Depression Scale; TUG, Timed Up and Go test.
The patients were divided into groups according to WHO BMI criteria—Group 1 (BMI 18.5–24.9), Group 2 (BMI 25–29.9), Group 3 (BMI 30–34.9), Group 4 (BMI 35–39.9), and Group 5 (BMI ≥40). A total of 181 (17.2%) patients were living alone, 544 (51.8%) with their spouses, 173 (16.4%) with their children, and 153 (14.6%) with someone else (caregiver, relative, etc.).
The mean number of drugs used was 5.08±3.11 (range, 0–15), with that being 4.9±3.39 for male and 5.15 ± 3.00 for female, and there was no statistically significant difference between female and male (p=0.261). The number of drugs (5.51±3.13) used by those who stated that they had fallen in the past year was significantly higher than that used by those who had no falls (4.86±3.06) (p=0.003).
Relationships between Geriatric Assessment Parameters and BMI Groups
In the evaluation of the correlation between geriatric assessment parameters and BMI, a significant negative correlation was found between the Tinetti balance test and BMI. A significant positive correlation was observed between Tinetti walking, MNA, calf circumference, MMSE score, number of drugs used by individuals, and BMI (p<0.05). Fig. 1 and Table 2 show the associations between BMI groups and geriatric assessment parameters.
Fig. 1.
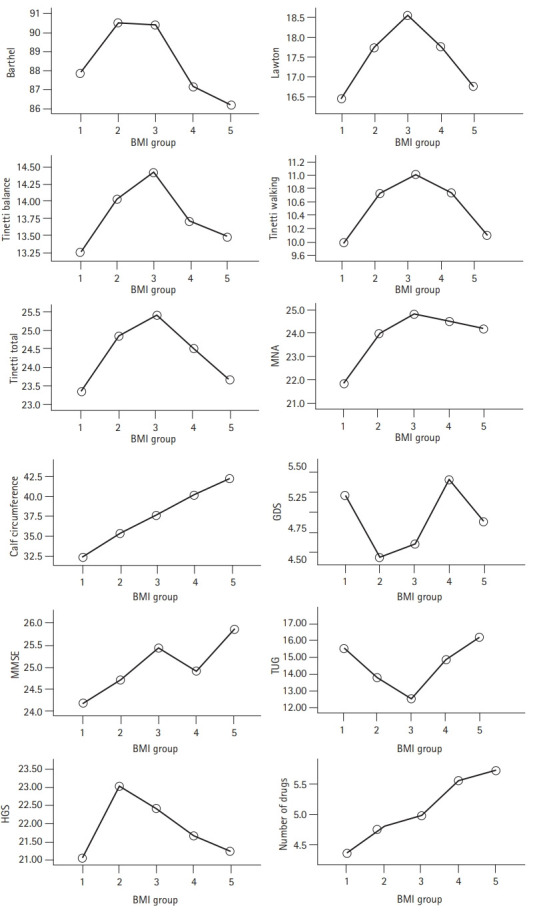
Evaluation of the relationship between geriatric assessment parameters and BMI groups. BMI, body mass index; Barthel scale, Barthel basic daily living activities scale; Lawton scale, Lawton instrumental daily living activities scale; MNA, Mini-Nutritional Assessment; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; TUG, Timed Up and Go test; HGS, handgrip strength. Group 1 (BMI 18.5–24.9), Group 2 (BMI 25–29.9), Group 3 (BMI 30–34.9), Group 4 (BMI 35–39.9), and Group 5 (BMI ≥40).
Table 2.
Evaluation of the relationship between geriatric assessment parameters and BMI groups
| Group 1 (BMI <24.9) | Group 2 (BMI 25–29.9) | Group 3 (BMI 30–34.9) | Group 4 (BMI 35–39.9) | Group 5 (BMI >40) | p-value | |
|---|---|---|---|---|---|---|
| Number of subjects | 163 | 298 | 320 | 182 | 88 | |
| Age (y) | 79.12±7.26 | 78.27±6.75 | 75.26±6.70 | 75.22±6.59 | 74.20±6.89 | 0.003 |
| Sex, female (%) | 61% | 65% | 80% | 91% | 95% | 0.001 |
| BMI (kg/m2) | 22.81±1.85 | 27.63±1.39 | 32.32±1.36 | 36.77±1.29 | 43.45±4.17 | 0.001 |
| Barthel | 87.91±18.47 | 90.52±11.94 | 90.40±12.61 | 87.14±13.41 | 86.20±12.73 | 0.005 |
| Lawton | 16.45±6.53 | 17.73±5.20 | 18.55±5.03 | 17.76±5.38 | 16.72±5.62 | 0.009 |
| Tinetti balance | 13.25±4.27 | 14.03±3.30 | 14.43±3.09 | 13.71±3.62 | 13.48±3.76 | 0.016 |
| Tinetti walking | 9.99±3.25 | 10.73±2.46 | 11.01±2.38 | 10.73±2.28 | 10.09±2.84 | 0.002 |
| Tinetti total | 23.34±6.85 | 24.85±5.23 | 25.43±5.11 | 24.52±5.43 | 23.65±6.20 | 0.004 |
| MNA | 21.82±5.17 | 23.91±3.72 | 24.83±3.53 | 24.50±3.15 | 24.16±2.95 | 0.001 |
| Calf circumference (cm) | 32.37±2.86 | 35.33±3.10 | 37.66±3.57 | 40.20±3.72 | 42.28±3.32 | 0.001 |
| GDS | 5.22±4.61 | 4.44±4.28 | 4.60±4.14 | 5.41±4.23 | 4.88±4.22 | 0.156 |
| MMSE | 24.17±4.08 | 24.69±4.01 | 25.45±3.23 | 24.91±4.23 | 25.89±3.32 | 0.005 |
| TUG | 15.55±10.66 | 13.78±7.37 | 12.52±6.58 | 14.79±8.96 | 16.18±10.98 | 0.001 |
| HGS | 21.03±9.25 | 23.03±8.91 | 22.42±8.66 | 21.67±8.53 | 22.41±8.66 | 0.229 |
| Number of drugs | 4.35±3.12 | 4.81±3.22 | 4.98±2.94 | 5.56±2.86 | 5.73±3.25 | 0.005 |
Values are presented as mean±standard deviation.
BMI, body mass index; Barthel scale, Barthel basic daily living activities scale; Lawton scale, Lawton instrumental daily living activities scale; MNA, Mini-Nutritional Assessment; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; TUG, Timed Up and Go test; HGS, handgrip strength.
Determination of Optimum Cutoff Points
ROC analysis for the optimum BMI in older women and men who were healthier according to the cutoff values of BADL, IADL, MNA, Tinetti, TUG, MMSE, GDS, and HGS are shown in Table 3. ROC analysis of the optimum BMI cutoff levels (Table 3) to detect the desirable values of these geriatric assessment parameters is shown in Fig. 2. Table 3 and Fig. 2 complement each other in this regard. The optimum BMI values were 31–32 kg/m2 for female and 27–28 kg/m2 for male (Table 3).
Table 3.
Evaluation of cutoff values of BMI with ROC analysis
| Female group |
Male group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95%) | BMI Cutoff | p-value | Sens (%) | Spec (%) | AUC (95%) | BMI Cutoff | p-value | Sens (%) | Spec (%) | |
| Barthel (score ≥ 91) | 0.576 (0.533–0.619) | 31.56 | 0.002 | 0.564 | 0.562 | 0.540 (0.461–0.619) | 27.81 | 0.305 | 0.545 | 0.540 |
| Lawton (score ≥ 17) | 0.552 (0.503–0.601) | 31.24 | 0.020 | 0.554 | 0.555 | 0.533 (0.450–0.616) | 27.45 | 0.431 | 0.521 | 0.522 |
| Tinetti total (score >19) | 0.560 (0.497–0.622) | 31.20 | 0.035 | 0.553 | 0.543 | 0.635 (0.502–0.769) | 27.10 | 0.030 | 0.594 | 0.604 |
| MNA (score >23.5) | 0.648 (0.604–0.693) | 31.21 | 0.001 | 0.615 | 0.616 | 0.646 (0.561–0.731) | 27.12 | 0.002 | 0.585 | 0.586 |
| GDS (score <5) | 0.488 (0.444–0.532) | 31.56 | 0.588 | 0.478 | 0.476 | 0.556 (0.471–0.642) | 27.37 | 0.186 | 0.540 | 0.547 |
| MMSE (score ≥23) | 0.589 (0.536–0.643) | 31.21 | 0.002 | 0.549 | 0.544 | 0.613 (0.518–0.707) | 27.10 | 0.030 | 0.593 | 0.595 |
| TUG (<13.5 s) | 0.525 (0.479–0.572) | 31.32 | 0.274 | 0.533 | 0.529 | 0.522 (0.439–0.605) | 27.45 | 0.596 | 0.524 | 0.529 |
| HGS (female, ≥16 kg) | 0.610 (0.563–0.657) | 31.20 | 0.001 | 0.575 | 0.527 | 0.579 (0.664–0.787) | 27.37 | 0.040 | 0.575 | 0.587 |
BMI, body mass index; ROC, receiver operating characteristic; AUC, area under the curve; Sens, sensitivity; Spec, specificity; Barthel, Barthel basic daily living activities scale; Lawton, Lawton instrumental daily living activities scale; MNA, Mini-Nutritional Assessment; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; TUG, Timed Up and Go test; HGS, handgrip strength.
Fig. 2.
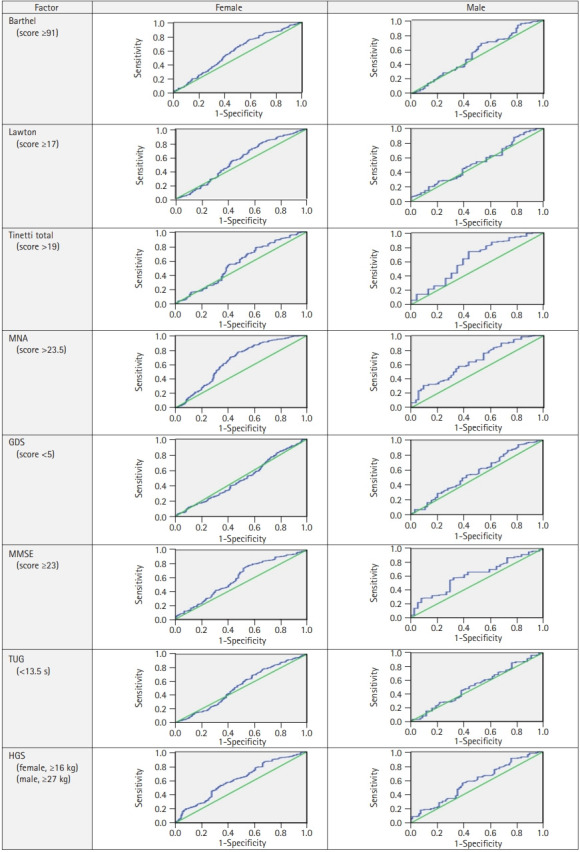
ROC analysis of BMI cutoff levels to detect the desirable values of geriatric assessment parameters. ROC, receiver operating characteristic; BMI, body mass index; Barthel, Barthel basic daily living activities scale; Lawton, Lawton instrumental daily living activities scale; MNA, Mini-Nutritional Assessment; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; TUG, Timed Up and Go test; HGS, handgrip strength.
When the relationship between chronic diseases and BMI groups was evaluated, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease (COPD) were more prevalent in patients with a BMI ≥30 kg/m2 than in those with a BMI <30 kg/m2 (p<0.05) (Supplementary Table S1).
DISCUSSION
Obesity is an important public health issue that is increasing among the older adult population as well as in the society. In the present study, 503 patients (54.6%) had a BMI >30 kg/m2. Several studies have shown that the prevalence of hypertension, metabolic syndrome, coronary heart disease, obstructive sleep apnea syndrome, and osteoarthritis increases with increasing obesity in geriatric patients and that patients require more surgical intervention.19-21) A significant difference was found between BMI groups in terms of hypertension, diabetes mellitus, and COPD prevalence in the present study as was seen in previous studies. It was observed that the frequency of these diseases increased significantly, especially when BMI was >35 kg/m2. There were no significant differences between the groups in terms of the frequency of coronary artery disease and heart failure.
Galanos et al.22) observed a J-shaped relationship between BMI and decreased muscle strength in older individuals aged 65–85 years. Weakness was found to increase in both low and high BMI values for both sexes. Obesity is a potential risk factor for undesirable surgical outcomes in older adults. Surgical complications associated with obesity include poor wound healing, risk of infection, increased operative time, and breathing difficulties.23) However, some studies have shown that 30-day postoperative mortality reduced or long-term survival improved in those who are generally overweight or have milder levels of obesity, thus supporting the obesity paradox hypothesis.8)
In the present study, a significant positive correlation was found between the number of drugs used and BMI. Moreover, a significant difference was observed between the BMI groups in terms of the number of drugs used. It has previously been found that those individuals with a BMI >35 kg/m2 consume the highest number of drugs. This may be because hypertension, diabetes mellitus, and COPD are more common in those with BMI >30 kg/m2, and the accumulation of drugs used to treat each individual’s condition increases the total number of drugs taken.
The present study showed that there was a significant difference between the BMI groups in terms of Tinetti balance, gait scale (total), and TUG test and the worst results were seen in the BMI range of 25 and >35 kg/m2, for both tests. Although there was no difference between the BMI groups in terms of HGS, the worst results were in this BMI range, similar to the Tinetti total score and TUG duration, which are predictive of fall risk and sarcopenia.24) Falsarella et al.25) evaluated the effect of muscle mass on the functionality of 99 older women and observed that decreased muscle mass was associated with walking speed and poor physical performance on TUG tests. Leyk et al.26) found that HGS is highly correlated with lean body tissue. Studies have shown that BMI is weakly correlated with HGS. The weakness of this relationship is that BMI is not an indicator of lean body tissues in individuals.27) As a result of the decrease in HGS, recovery after illness or surgery is delayed and physical function loss occurs. Indeed, there are publications showing a relationship between muscle strength and acute and chronic diseases.28,29) In our study, similar parameters (TUG, Tinetti, muscle strength) increased the fall risk among individuals with a BMI <25 kg/m2, which is considered a normal BMI. This suggests that ideal BMI values in older individuals may differ from those in the normal population. However, the risk of sarcopenic obesity increases with BMI values >35 kg/m2 and especially >40 kg/m2, and the risk of falling increases and functional capacity decreases in this group of older individuals. Thirty percent of people aged 65 years and over fall each year, and this rate increases to approximately 40% for people aged 85 years and over.30) Various degrees of injuries occur in 12%–40% of older individuals who experience a fall and 20% require medical assistance.31) In the present study, the relationship between the BMI groups and the Barthel scale, which shows functional capacity, and the Lawton scale, was evaluated. A significant difference was found between the BMI groups in terms of the Barthel and Lawton scale scores. In both scales, it was determined that the scale scores for BMI <25 kg/m2 and BMI >35 kg/m2 were lower, thus their functional capacities were lower. Therefore, it seems ideal for the older adults to have a BMI of 25–35 kg/m2 to maintain their functionality and reduce the risk of falling.
When we evaluated BMI with respect to MNA, a significant difference was found between the groups. When we examined the BMI groups, the best MNA results were in the range of BMI 30–35 kg/m2, and the MNA score tended to decrease gradually in individuals with a BMI >35 kg/m2. Our results showed that malnutrition, which is one of the most important causes of mortality and morbidity in the older, should be considered not only in individuals with low BMI but also in those with obesity.32,33)
Additionally, a strong positive correlation was found between calf circumference and BMI. Studies have found that calf circumference is correlated with other nutritional anthropometric measurements, including BMI, free fat mass, and mobility.34) The linear correlation curve showing the relationship between BMI and calf circumference, and especially the tendency of TUG to be increased for BMI of 30 kg/m2, indicates that sarcopenic obesity should not be overlooked when using a 31-cm cutoff for calf circumference screening for sarcopenia.34)
The present study also showed a significant positive correlation between BMI and MMSE score. The MMSE is a useful and standardized test that is frequently used in clinical practice for the detection of cognitive disorders, monitoring the stage of dementia and response to treatment, and epidemiological studies of dementia.35) In a meta-analysis by Beydoun et al.,36) the existence of a U-shaped relationship between BMI and dementia was demonstrated, and both obesity and underweight were associated with an increased risk of dementia. The reason for the difference in the present study may be exclusion of patients with dementia and previous cerebrovascular disease.
The findings of this study must be interpreted considering these limitations. One limitation is the possible selection bias due to the retrospective design of the study. Another limitation is the cross-sectional study design; thus, causal relationships could not be determined. Next, the metabolic syndrome was not evaluated, and only BMI measurements were used to investigate its relationship with geriatric conditions. Finally, there are no mortality data to determine the optimum cutoff BMI values in older patients; therefore, this study investigated the relationship between current geriatric assessment parameters and BMI. The strengths of the present study include large sample size and simultaneous evaluation of multiple geriatric parameters.
In conclusion, the present study suggests that the ideal BMI ranges for young and middle-aged individuals are not ideal for older patients; especially, older individuals with BMI values <25 and >35 kg/m2 have a higher risk of decreased functional capacity, balance, walking, mobilization disorders, fall risk, reduction in muscle strength, and malnutrition. Therefore, a BMI between 25 and 35 kg/m2 may be optimal for health in the older population. Data from this study suggest that the optimum BMI range is 31–32 kg/m2 for female and 27–28 kg/m2 for male. With broader studies on this subject, the ideal BMI range for older people can be determined.
Footnotes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
None.
AUTHOR CONTRIBUTION
Conceptualization, PS, MK; Data curation, PS, MK; Methodology, MK; Supervision, LS, EC; Writing-original draft, MK; Writing-review & editing, PS, LS, EC, MZ.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4235/agmr.22.0012.
Evaluation of the relationship between chronic diseases and BMI
REFERENCES
- 1.Kim TN. Elderly obesity: is it harmful or beneficial? J Obes Metab Syndr. 2018;27:84–92. doi: 10.7570/jomes.2018.27.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolubasi S. SSosyal yardima başvuran yaşlilarin sosyoekonomik ve sosyokültürel özellikleri: karşilaştirmali bir çalişma. tezli yüksek lisans [Socioeconomic and sociocultural characteristics of elderly people applying for social assistance: a comparative study] [thesis] Izmir, Turkey: Ege University; 2018. [Google Scholar]
- 3.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 4.Seo MH, Kim YH, Han K, Jung JH, Park YG, Lee SS, et al. Prevalence of obesity and incidence of obesity-related comorbidities in Koreans based on National Health Insurance Service Health Checkup Data 2006-2015. J Obes Metab Syndr. 2018;27:46–52. doi: 10.7570/jomes.2018.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turkish Endocrine and Metabolism Society . Obezite tanı ve tedavi kılavuzu [Obesity diagnosis and treatment guide] Ankara, Turkey: Turkish Endocrine and Metabolism Society; 2014. [Google Scholar]
- 6.Bolubasi S. Yaşlı Obezlerde Egzersiz Yaklaşımı ve Etkileri [Exercise approach and its effects in elderly obese people] Turk J Diab Obes. 2020;1:54–9. [Google Scholar]
- 7.Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag. 2019;15:89–100. doi: 10.2147/VHRM.S168946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quante M, Dietrich A, ElKhal A, Tullius SG. Obesity-related immune responses and their impact on surgical outcomes. Int J Obes (Lond) 2015;39:877–83. doi: 10.1038/ijo.2015.21. [DOI] [PubMed] [Google Scholar]
- 9.Naruishi K, Yumoto H, Kido JI. Clinical effects of low body mass index on geriatric status in elderly patients. Exp Gerontol. 2018;110:86–91. doi: 10.1016/j.exger.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Soysal P, Ates Bulut E, Yavuz I, Isik AT. Decreased basal metabolic rate can be an objective marker for sarcopenia and frailty in older males. J Am Med Dir Assoc. 2019;20:58–63. doi: 10.1016/j.jamda.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Unutmaz GD, Soysal P, Tuven B, Isik AT. Costs of medication in older patients: before and after comprehensive geriatric assessment. Clin Interv Aging. 2018;13:607–13. doi: 10.2147/CIA.S159966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucukdeveci AA, Yavuzer G, Tennant A, Suldur N, Sonel B, Arasil T. Adaptation of the modified Barthel Index for use in physical medicine and rehabilitation in Turkey. Scand J Rehabil Med. 2000;32:87–92. [PubMed] [Google Scholar]
- 13.Shelkey M, Wallace M. Katz index of independence in activities of daily living. J Gerontol Nurs. 1999;25:8–9. doi: 10.3928/0098-9134-19990301-05. [DOI] [PubMed] [Google Scholar]
- 14.Dokuzlar O, Koc Okudur S, Soysal P, Kocyigit SE, Yavuz I, Smith L, et al. Factors that increase risk of falling in older men according to four different clinical methods. Exp Aging Res. 2020;46:83–92. doi: 10.1080/0361073X.2019.1669284. [DOI] [PubMed] [Google Scholar]
- 15.Soysal P, Isik AT, Arik F, Kalan U, Eyvaz A, Veronese N. Validity of the Mini-Nutritional Assessment Scale for evaluating frailty status in older adults. J Am Med Dir Assoc. 2019;20:183–7. doi: 10.1016/j.jamda.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Durmaz B, Soysal P, Ellidokuz H, Isik AT. Validity and reliability of geriatric depression scale-15 (short form) in Turkish older adults. North Clin Istanb. 2018;5:216–20. doi: 10.14744/nci.2017.85047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harith S, Tan SL. Translation and validation of the Malay version of Comprehensive Geriatric Assessment Questionnaire for older adults in Malaysia. Ann Geriatr Med Res. 2020;24:115–24. doi: 10.4235/agmr.20.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . Geneva, Switzerland: World Health Organization; 2021. Obesity and overweight (WHO fact sheet No. 311) [Internet] [cited 2022 Mar 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 19.Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25:622–7. doi: 10.1038/sj.ijo.0801585. [DOI] [PubMed] [Google Scholar]
- 20.Carmelli D, Swan GE, Bliwise DL. Relationship of 30-year changes in obesity to sleep-disordered breathing in the Western Collaborative Group Study. Obes Res. 2000;8:632–7. doi: 10.1038/oby.2000.81. [DOI] [PubMed] [Google Scholar]
- 21.Nagamine M, Jiang H, Merrill C. Rockville, MD: Healthcare Cost and Utilization Project; 2006. Trends in elderly hospitalizations, 1997–2004 [Internet] [cited 2022 Mar 25]. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb14.pdf. [PubMed] [Google Scholar]
- 22.Galanos AN, Pieper CF, Cornoni-Huntley JC, Bales CW, Fillenbaum GG. Nutrition and function: is there a relationship between body mass index and the functional capabilities of community-dwelling elderly? J Am Geriatr Soc. 1994;42:368–73. doi: 10.1111/j.1532-5415.1994.tb07483.x. [DOI] [PubMed] [Google Scholar]
- 23.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–77. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Kopke S, Meyer G. The Tinetti test: Babylon in geriatric assessment. Z Gerontol Geriatr. 2006;39:288–91. doi: 10.1007/s00391-006-0398-y. [DOI] [PubMed] [Google Scholar]
- 25.Falsarella GR, Coimbra IB, Barcelos CC, Iartelli I, Montedori KT, Santos MN, et al. Influence of muscle mass and bone mass on the mobility of elderly women: an observational study. BMC Geriatr. 2014;14:13. doi: 10.1186/1471-2318-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leyk D, Gorges W, Ridder D, Wunderlich M, Ruther T, Sievert A, et al. Hand-grip strength of young men, women and highly trained female athletes. Eur J Appl Physiol. 2007;99:415–21. doi: 10.1007/s00421-006-0351-1. [DOI] [PubMed] [Google Scholar]
- 27.Martin S, Neale G, Elia M. Factors affecting maximal momentary grip strength. Hum Nutr Clin Nutr. 1985;39:137–47. [PubMed] [Google Scholar]
- 28.Norman K, Stobaus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30:135–42. doi: 10.1016/j.clnu.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–66. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 30.Akyol AD. Falls in the elderly: what can be done? Int Nurs Rev. 2007;54:191–6. doi: 10.1111/j.1466-7657.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz M, Bozdemir MN, Kilicaslan I, Atescelik M, Gurbuz S, Mutlu B, et al. Elderly trauma: the two years experience of a university-affiliated emergency department. Eur Rev Med Pharmacol Sci. 2012;16 Suppl 1:62–7. [PubMed] [Google Scholar]
- 32.Secher M, Soto ME, Villars H, van Kan GA, Vellas B. The Mini Nutritional Assessment (MNA) after 20 years of research and clinical practice. Rev Clin Gerontol. 2007;17:293–310. [Google Scholar]
- 33.Lee LC, Tsai AC, Wang JY, Hurng BS, Hsu HC, Tsai HJ. Need-based intervention is an effective strategy for improving the nutritional status of older people living in a nursing home: a randomized controlled trial. Int J Nurs Stud. 2013;50:1580–8. doi: 10.1016/j.ijnurstu.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Rolland Y, Lauwers-Cances V, Cournot M, Nourhashemi F, Reynish W, Riviere D, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51:1120–4. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- 35.Van der Weele GM, Gussekloo J, De Waal MW, De Craen AJ, Van der Mast RC. Co-occurrence of depression and anxiety in elderly subjects aged 90 years and its relationship with functional status, quality of life and mortality. Int J Geriatr Psychiatry. 2009;24:595–601. doi: 10.1002/gps.2162. [DOI] [PubMed] [Google Scholar]
- 36.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–18. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of the relationship between chronic diseases and BMI


