Abstract
The pharmacokinetics of trovafloxacin following administration of a single intravenous dose of alatrofloxacin, equivalent to 4 mg of trovafloxacin per kg of body weight, were determined in 6 infants (ages 3 to 12 months) and 14 children (ages, 2 to 12 years). There was rapid conversion of alatrofloxacin to trovafloxacin, with an average ± standard deviation (SD) peak trovafloxacin concentration determined at the end of the infusion of 4.3 ± 1.4 μg/ml. The primary pharmacokinetic parameters (average ± SD) analyzed were volume of distribution at steady state (1.6 ± 0.6 liters/kg), clearance (151 ± 82 ml/h/kg), and half-life (9.8 ± 2.9 h). The drug was well tolerated by all children. There were no age-related differences in any of the pharmacokinetic parameters studied. Less than 5% of the administered dose was excreted in the urine over 24 h. On the basis of the mean area under the concentration-time curve of 30.5 ± 10.1 μg · h/ml and the susceptibility (≤0.5 μg/ml) of common pediatric bacterial pathogens to trovafloxacin, dosing of 4 mg/kg/day once or twice daily should be appropriate.
The need for new, effective antimicrobial agents for therapy of infections caused by antibiotic-resistant bacteria has been well documented (5, 6, 9). However, this need has been best illustrated in infants and children by treatment failures of infections caused by antibiotic-resistant strains of Streptococcus pneumoniae, the most common cause of upper and lower respiratory tract bacterial infections, bacteremia, and meningitis in the pediatric age group (5). Clinical isolates with documented resistance to β-lactams, macrolides, sulfonamides, and lincosamides have been noted with increasing frequency from both children and adults (5, 8).
Trovafloxacin, a fluoronaphthyridone antibiotic structurally related to the fluoroquinolones (1), is one of many newer quinolone antibiotics that possess excellent in vitro activity against both penicillin-susceptible and nonsusceptible strains of Streptococcus pneumoniae (11, 17, 19). In vitro activity has also been demonstrated against other gram-positive, gram-negative, and anaerobic pathogens (2, 7, 10, 12) responsible for both community-acquired and nosocomial (3) infections.
Pharmacokinetic data from studies with adults demonstrate a terminal elimination half-life (t1/2) of 10 h for trovafloxacin following administration of a single oral dose (14, 16), with similar values observed following administration of a single intravenous dose of alatrofloxacin, the l-Ala-l-alanine prodrug of trovafloxacin (18). This prodrug is rapidly hydrolyzed to trovafloxacin in the patient's bloodstream.
Trovafloxacin is the first fluoroquinolone-related antibiotic to be investigated in the United States in phase II trials with infants and children. Initial studies of the disposition of trovafloxacin in infants and children demonstrated microbiologically effective concentrations of trovafloxacin in the cerebrospinal fluid (CSF). CSF trovafloxacin concentrations were all ≥0.12 μg/ml, reflecting concentrations between 22 and 30% of the simultaneous concentration in blood when the concentrations were measured 1 to 12 h after administration of an intravenous dose of 180 mg/m2 of body surface area to 37 children and 5 mg/kg of body weight to 11 children (A. Arguedas-Mohs, S. Vargas-Munita, J. Bradley, R. Teng, J. M. Walterspiel, C. Loaiza, R. Riviera, E. M. S. Goodall and J. Vincent, First Int. Joint Pediatr. Infect. Dis. Soc. Eur. Soc. Pediatr. Infect. Dis. Meet. 1995; A. Arguedas-Mohs, S. L. Vargas, C., J. S. Bradley, C. Loaiza, R. Rivera, J. Vincent, R. Teng, and J. N. Walterspiel, 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-105, p. 21, 1997). The concentrations in CSF exceeded the MICs for the primary bacterial pathogens responsible for meningitis in infants and children (10). In the study described here, we have performed an evaluation of the pharmacokinetics of trovafloxacin after the administration of a single intravenous dose of alatrofloxacin in infants and children to determine an optimal dosing regimen for the treatment of invasive bacterial infections.
(This work was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 to 30 September 1997.)
MATERIALS AND METHODS
Patient population.
The study was conducted with patients in two pediatric age groups: group 1 comprised children ages 1 to 12 years; group 2 comprised infants ages 3 to 12 months. The children were recruited from Pediatric Pharmacology Research Unit (PPRU) Network sites from June 1996 through February 1997 (San Diego, n = 8; Kansas City, Mo., n = 7; Cleveland, n = 5). All children were receiving antibiotic therapy for treatment of a bacterial infection when they were evaluated for enrollment in the study. The following laboratory tests were performed for all children prior to administration of alatrofloxacin: complete blood count with differential; blood urea nitrogen, serum creatinine, alanine aminotransferase (serum glutamic pyruvic transaminase), aspartate aminotransferase (serum glutamic oxalacetic transaminase), alkaline phosphatase, total bilirubin, and serum albumin concentration determinations; and urinalysis. Serology was obtained for hepatitis B virus infection (hepatitis B virus surface antigen) unless the infant had received an immunization against hepatitis B virus. An analysis of all concurrent medications for possible drug interactions with trovafloxacin was made by one of the investigators (J.V.) prior to patient enrollment. Children were excluded from the study if they had significant underlying renal, hepatic, or hematopoietic dysfunction. Also excluded were infants and children with clinically significant reactive airway disease or central nervous system inflammation. This research protocol was approved by the institutional review board at each study site. Written, informed consent was obtained from parents or legal guardians prior to enrollment in the study, and when appropriate, patient assent was obtained.
Drug administration and sample collection.
Alatrofloxacin (Pfizer Central Research, Groton, Conn.) was administered intravenously as a single dose, equivalent to 4 mg of trovafloxacin per kg, in a 5% dextrose solution through microbore tubing. The study drug, at a concentration of 2 mg/ml, was infused at a constant rate either into a peripheral vein or into a central catheter over 60 min. Several venous blood samples were collected for the determination of trovafloxacin concentrations in serum and were stored at −20°C until they were analyzed. Blood samples were obtained prior to the infusion, at the end of the infusion, and at 1.5, 2, 3, 4, 6, 8, 12, 24, 36, and 48 h from the start of the infusion. Venous blood samples were collected for determination of alatrofloxacin concentrations in plasma and were stored at −20°C prior to assay; these samples were obtained prior to the infusion, at the end of the infusion, and at 1.5 and 2 h from the start of infusion. Urine was collected continuously during the first 24 h after infusion.
The subjects were observed during the infusion for adverse reactions, with vital signs checked prior to infusion, 30 min into the infusion, and at the end of the infusion.
Drug assay methods.
Serum and urine samples were assayed for trovafloxacin concentrations and plasma samples were assayed for alatrofloxacin (l-Ala-l-alanine-trovafloxacin, CP-116,517) and its monoalanine analogue (l-alanine-trovafloxacin, CP-114,009) concentrations by high-performance liquid chromatography with UV detection (15). The linear dynamic range for the trovafloxacin assay was 0.1 to 20 μg/ml for both serum and urine. The intra- and interassay coefficients of variation (CVs) for quality control samples in these assays were <10.4 and <6.83%, respectively. The linear dynamic range for the assay of the alatrofloxacin concentration in plasma extended from 0.025 to 2.50 μg/ml for both the mono- and the dialanine moieties. The CVs for the quality control samples for these compounds ranged from 5.7 to 11.6 and 3.4 to 15.7%, respectively. Since the assay for measurement of the trovafloxacin concentration in serum measures the l-Ala-l-alanine-trovafloxacin, l-alanine-trovafloxacin, and trovafloxacin concentrations, the trovafloxacin concentrations were corrected to unconjugated trovafloxacin concentrations at time points with detectable prodrug concentrations by the following equation: trovafloxacin concentration (corrected) = trovafloxacin concentration (measured) − CP-116,517 concentration − CP-114,009 concentration (concentrations are in molar). Samples with concentrations above the range of the standard curve were diluted in the same biological fluid matrix as that sample.
Calculation of pharmacokinetic parameters.
The following pharmacokinetic parameters for trovafloxacin were determined: maximum concentration of drug in serum (Cmax; in micrograms per milliliter), time to Cmax (Tmax; in hours), area under the concentration-time curve (AUC) from time zero to infinity (AUC0–∞; in microgram · hour/milliliter) t1/2 (in hours), total clearance (CL; in milliliters per hour per kilogram), and apparent volume of distribution at steady state (VSS; in liters per kilogram). The apparent terminal elimination rate constant (kel) was estimated by least-squares linear regression analysis of the serum trovafloxacin concentration-time data obtained over the terminal log-linear phase. The individual t1/2 was calculated as 0.693/kel. AUC was calculated by the log-linear trapezoidal rule. The area from the last sampling time (t) with a quantifiable concentration to infinity (AUCt–∞) was estimated as Cest (t)/kel, where Cest (t) represents the estimated concentration at time t and kel is based upon the regression analysis. The Cmax of trovafloxacin was obtained directly from the experimental data. Data for AUC, Cmax, and the percentage of trovafloxacin as prodrug are presented as the geometric mean ± standard deviation (SD), and data for the other parameters are presented as the arithmetic mean ± SD. The CL of trovafloxacin from serum was estimated as dose/AUC0–∞, assuming that alatrofloxacin was completely and immediately converted to trovafloxacin. (VSS) was calculated as CL · (AUMC0–∞/AUC0–∞ − t1/2), where AUMC is the area under the first moment curve (calculated by the log-linear trapezoidal method) and t is the infusion duration.
Statistical analysis.
Potential age-related differences in pharmacokinetic parameters were examined by using SAS software (SAS Institute, Inc., Cary, N.C.). The log-transformed AUC0–∞, Cmax, raw Tmax, t1/2, CL, and VSS values were analyzed by using an analysis of variance model by comparing age groups and strength of correlation. The PROC MIXED subroutine was used for these analyses. The LSMEANS subroutine was used to estimate the adjusted mean differences between age groups and their 95% confidence intervals. Statistical significance was achieved in each analysis given a P value of less than 0.05.
RESULTS
Clinical results.
The mean ± SD ages for group 1 and group 2 were 7.2 ± 3.9 years (range, 1.8 to 12.5 years) and 0.6 ± 0.3 years (range, 0.3 to 0.9 years), respectively. The mean ± SD weights for group 1 and group 2 were 28.1 ± 14.8 kg (range, 11.9 to 56.8 kg) and 6.5 kg ± 2.2 (range, 4.0 to 10.0 kg), respectively. Pretherapy laboratory studies for assessment of organ function were within normal limits for all children participating in the study. A complete list of concurrent medications for each child was evaluated, and no child in either group was receiving a medication which was believed to have a possible effect on trovafloxacin distribution, metabolism, or elimination. The infusions were, in general, well tolerated. No child experienced abnormalities of vital signs during or following the infusion. One child in group 1 vomited once during the infusion. One child in each group experienced mild pain at the injection site.
Pharmacokinetic and statistical analyses.
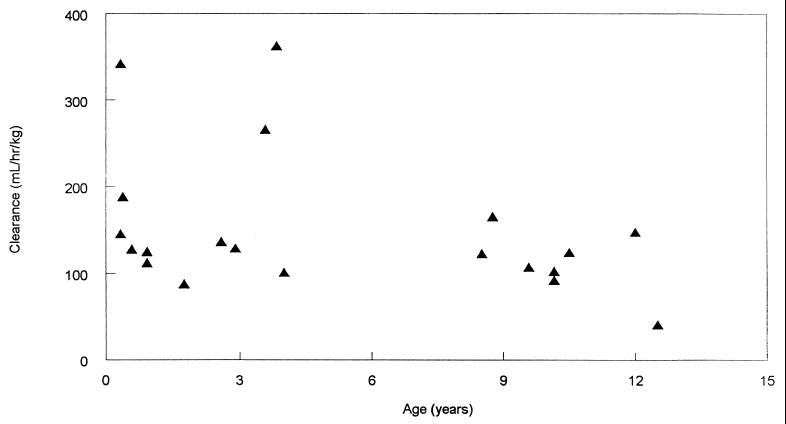
The calculated pharmacokinetic parameters for trovafloxacin for each child, by age group, are illustrated in Table 1. A comparison of the pharmacokinetic parameters for the children in group 1 and the children in group 2 was made, with no statistically significant differences noted between the two groups. An analysis of trends in pharmacokinetic parameters with increasing age over both age groups (3 months to 12 years) did not yield statistically significant associations. Figure 1 documents the mean ± SD serum trovafloxacin concentration-versus-time profiles for both age groups. Figure 2 demonstrates the relationship between CL and age.
TABLE 1.
Pharmacokinetic parameters following administration of alatrofloxacin (equivalent to trovafloxacin at 4 mg/kg)
| Group and subject no. | Age (yr) | AUC (μg · h/ml) | Cmax μ (μg/ml) | tmax (h) | t1/2 (h) | CL (ml/h/kg) | VSS (liters/kg) |
|---|---|---|---|---|---|---|---|
| Group 1 | |||||||
| 1 | 3.6 | 15.03 | 2.95 | 1.50 | 13.00 | 266.20 | 2.86 |
| 2 | 4.0 | 39.37 | 3.35 | 1.50 | 11.40 | 101.60 | 1.61 |
| 3 | 3.8 | 11.10 | 39.70a | 1.00 | 4.90 | 363.30 | 2.14 |
| 4 | 1.75 | 45.57 | 5.63 | 1.00 | 10.80 | 87.80 | 1.25 |
| 5 | 2.58 | 29.99 | 6.39 | 1.00 | 13.50 | 136.50 | 1.76 |
| 6 | 2.9 | 32.07 | 6.06 | 1.00 | 12.10 | 129.00 | 1.86 |
| 7 | 12.5 | 43.22 | 3.41 | 1.00 | 9.50 | 40.70 | 0.50 |
| 8 | 8.5 | 32.51 | 2.94 | 2.00 | 12.60 | 123.00 | 1.77 |
| 9 | 9.58 | 37.16 | 4.24 | 1.00 | 10.90 | 107.60 | 1.41 |
| 10 | 10.16 | 43.22 | 4.60 | 1.00 | 9.40 | 92.60 | 1.10 |
| 11 | 12 | 27.05 | 2.85 | 1.00 | 10.30 | 147.90 | 1.81 |
| 12 | 8.75 | 24.18 | 2.92 | 1.00 | 7.80 | 165.40 | 1.67 |
| 13 | 10.5 | 32.14 | 3.81 | 1.00 | 13.30 | 124.40 | 1.80 |
| 14 | 10.16 | 39.02 | 4.53 | 1.00 | 5.70 | 102.50 | 0.76 |
| Mean | 7.2 | 32.25 | 3.97 | 1.14 | 10.37 | 142.01 | 1.59 |
| SD | 3.86 | 10.32 | 1.25 | 0.22 | 2.09 | 81.21 | 0.58 |
| % CV | 49.7 | 31.99 | 30.16 | 19.64 | 20.15 | 57.18 | 36.58 |
| Group 2 | |||||||
| 1 | 0.58 | 31.20 | 5.00 | 1.00 | 10.00 | 128.00 | 1.56 |
| 2 | 0.33 | 27.50 | 3.05 | 1.00 | 14.10 | 145.50 | 2.45 |
| 3 | 0.92 | 31.73 | 7.59 | 1.00 | 6.20 | 125.20 | 1.02 |
| 4 | 0.375 | 21.29 | 4.48 | 1.00 | 8.40 | 187.90 | 1.64 |
| 5 | 0.92 | 35.69 | 4.40 | 1.00 | 8.30 | 112.10 | 1.13 |
| 6 | 0.33 | 11.68 | 2.96 | 1.00 | 4.60 | 342.40 | 1.93 |
| Mean | 0.58 | 24.96 | 4.35 | 1.00 | 8.60 | 173.52 | 1.62 |
| SD | 0.28 | 10.27 | 1.51 | 0.00 | 2.30 | 61.09 | 0.39 |
| % CV | 48.9 | 41.15 | 34.74 | 0.00 | 26.74 | 35.21 | 23.74 |
| All subjects | |||||||
| Mean | 5.2 | 30.53 | 4.27 | 1.10 | 9.84 | 151.47 | 1.60 |
| SD | 4.5 | 10.01 | 1.37 | 0.26 | 2.92 | 81.95 | 0.55 |
| % CV | 85.6 | 32.79 | 32.03 | 23.78 | 29.68 | 54.10 | 34.52 |
The concentration was considered an outlier and was excluded from the calculations.
FIG. 1.
Mean ± SD serum trovafloxacin concentration versus time by age group: circles, 2 to 12 years; triangles, 3 to 12 months.
FIG. 2.
Relationship between trovafloxacin CL and age.
The mean Cmax values for the older and younger age groups were similar, 3.97 and 4.35 μg/ml, respectively. This analysis excludes a single determination for one subject in the older cohort with a Cmax value of 39.7 μg/ml. Since this concentration is more than six times the Cmax for all other subjects, it was believed to be in error and was excluded from the analyses. The trovafloxacin concentrations 24 h postdosing averaged 0.32 ± 0.12 and 0.23 ± 0.11 μg/ml for the older and younger groups, respectively.
Only 10 subjects had any prodrug detectable at the end of the infusion. In these subjects, the trovafloxacin prodrug(s) concentration averaged only 1.5% of the total measured trovafloxacin concentration.
Complete urine collections were available and were analyzed for seven of the older subjects (mean age, 8.3 ± 3.0 years). The mean renal CL for these subjects was 6.2 ± 4.7 ml/h/kg. The total amount of trovafloxacin collected in the urine during the 24-h period following drug administration averaged less than 5% of the administered dose.
DISCUSSION
Trovafloxacin, the first fluoroquinolone-related antibiotic to be prospectively studied in children, was evaluated in phase I and II trials in preparation for studies of invasive pediatric infections. As trovafloxacin demonstrates a broad spectrum of activity against gram-positive and gram-negative aerobes and anaerobes (2, 7, 10, 12, 19), treatment of a wide range of serious pediatric infections is possible. In particular, the in vitro activity of trovafloxacin against S. pneumoniae is excellent, with the drug having similar activity against both penicillin-susceptible and nonsusceptible strains (11, 17, 19).
Following the infusion of alatrofloxacin, rapid conversion to trovafloxacin occurred, as half of the children had no detectable concentrations of any prodrug on completion of the 60-min intravenous infusion. In the subjects with detectable prodrug, the concentrations were low, representing only 1.5% of the total trovafloxacin concentration. Trovafloxacin is eliminated primarily by nonrenal mechanisms. In monkeys urinary excretion accounts for less than 5% of the total dose, and in rats 99% of an administered dose was found in the feces as trovafloxacin and its glucuronide metabolite (13). This suggests a high degree of biliary excretion. In adult volunteers, intravenously administered trovafloxacin (given as alatrofloxacin) demonstrates dose proportionality between 30 and 300 mg, with a 300-mg dose resulting in an average Cmax of 4.3 μg/ml, a CL of 97 ml/h/kg, a t1/2 of 10.8 h, a VSS of 1.38 liters/kg, and an AUC of 43.4 μg · h/ml (16, 18). The urinary excretion of unchanged drug accounted for about 5% of the total dose. Our data from studies with children confirm the findings of studies with adults of negligible renal CL.
Although the majority of trovafloxacin is excreted largely unchanged in the feces, glucuronidation does represent a significant metabolic pathway. From pooled samples of serum obtained over the first 120 h after the administration of a single radiolabeled dose, 22% of the trovafloxacin metabolite activity was found as the trovafloxacin glucuronide metabolite, while 52% remained as unchanged trovafloxacin (4).
In another adult study, 200 mg of trovafloxacin was administered orally to 12 volunteers, resulting in a Cmax of 2.2 μg/ml, an AUC of 30.4 mg · h/liter, and a t1/2 of 11.3 h. In the same study, 300 mg was administered to 12 additional adult volunteers in either a fasted or a fed state. For those fasted volunteers, the AUC was similar to that for the fed volunteers (39.5 versus 38.2 mg · h/liter); the measured Cmax (2.6 versus 2.3 μg/ml) and Tmax (1.4 versus 3.6 h) were statistically similar (18). The bioavailability in adults was 87.6% (16).
In the present study the Cmax of 4.27 μg/ml after the administration of alatrofloxacin at a concentration equivalent to 4 mg of trovafloxacin per kg was similar to the Cmax of 4.3 μg/ml found in studies with adults with administration of an intravenous dose of alatrofloxacin equivalent to 300 mg of trovafloxacin (18). However, the mean AUC for adults receiving this dose (43.4 mg · h/liter) was higher than that for either group of children (32.25 mg · h/liter for the older children and 24.96 mg · h/liter for the infants) receiving the equivalent of 4 mg of trovafloxacin per kg. The t1/2 in the current study was nearly identical to that observed in adults. The mean CL and VSS for these infants and children (151 ml/h/kg and 1.6 liters/kg, respectively) were slightly greater than those previously reported for adults (97 ml/h/kg and 1.38 liters/kg, respectively) when normalized to body weight. However the magnitude of these differences is probably of little clinical importance. We did not detect any age-related differences in pharmacokinetics; however, the evaluation was limited by the high variability seen in CL (CV, >50%; Table 1), particularly for children under 4 years of age.
This study suggests that infants and children between 3 months and 13 years of age can receive similar weight-adjusted doses and will achieve concentration-in-serum profiles that support once- or twice-daily dosing for most common pediatric infections.
ACKNOWLEDGMENTS
Support for this study was provided in part by Pfizer Central Research and by grants 5U10HD31318, 1U10HD31313-05, and HD31323-05 from the National Institute of Child Health and Human Development to the PPRU Network.
REFERENCES
- 1.Brighty K E, Gootz T D. The chemistry and biological profile of trovafloxacin. J Antimicrob Chemother. 1997;39:1–14. doi: 10.1093/jac/39.suppl_2.1. [DOI] [PubMed] [Google Scholar]
- 2.Citron D M, Appleman M D. Comparative in vitro activities of trovafloxacin (CP-99,219) against 221 aerobic and 217 anaerobic bacteria isolated from patients with intra-abdominal infections. Antimicrob Agents Chemother. 1997;41:2312–2316. doi: 10.1128/aac.41.10.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunha B A, Qadri S M, Yeno Y, Walters E A, Domenico P. Antibacterial activity of trovafloxacin against nosocomial gram-positive and gram-negative isolates. J Antimicrob Chemother. 1997;39:29–34. doi: 10.1093/jac/39.suppl_2.29. [DOI] [PubMed] [Google Scholar]
- 4.Dalvie D K, Khosla N, Vincent J. Excretion and metabolism of trovafloxacin in humans. Drug Metab Dispos. 1997;25:423–427. [PubMed] [Google Scholar]
- 5.Friedland I R, McCracken G H. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 6.Gold H S, Moellering R C. Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 7.Gooding B B, Jones R N. In vitro antimicrobial activity of CP-99,219, a azabicyclo-naphthyridone. Antimicrob Agents Chemother. 1993;37:349–353. doi: 10.1128/aac.37.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofman J, Cetron M S, Farley M M, Baughman W S, Facklan R R, Elliott J A, Peaver K A, Breiman R F. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 9.Neu H C. The crisis in antibiotic resistance. Science. 1992;527:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 10.Neu H C, Chin N X. In vitro activity of the new fluoroquinolone CP-99,219. Antimicrob Agents Chemother. 1994;38:2615–2622. doi: 10.1128/aac.38.11.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson-Liljequist B, Hoffman B M, Hedlund J. Activity of trovafloxacin against blood isolates of Streptococcus pneumoniae in Sweden. Eur J Clin Microbiol Infect Dis. 1996;15:671–675. doi: 10.1007/BF01691157. [DOI] [PubMed] [Google Scholar]
- 12.Spangler S K, Jacobs M R, Appelbaum P C. Activity of CP 99, 219 compared with those of ciprofloxacin, grepafloxacin, metronidazole, cefoxitin, piperacillin, and piperacilin-tazobactam against 489 anaerobes. Antimicrob Agents Chemother. 1994;38:2471–2476. doi: 10.1128/aac.38.10.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teng R, Girard D, Gootz T D, Foulds G, Liston T E. Pharmacokinetics of trovafloxacin (CP-99,219), a new quinolone, in rats, dogs, and monkeys. Antimicrob Agents Chemother. 1996;40:561–566. doi: 10.1128/aac.40.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng R, Harris S C, Nix D E, Schentag J J, Foulds G, Liston T E. Pharmacokinetics and safety of trovafloxacin (CP 99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J Antimicrob Chemother. 1995;36:385–394. doi: 10.1093/jac/36.2.385. [DOI] [PubMed] [Google Scholar]
- 15.Teng R, Tensfeldt T G, Liston T E, Foulds G. Determination of trovafloxacin, a new quinolone antibiotic, in biological samples by reversed-phase high-performance liquid chromatography. J Chromatogr. 1996;675:53–59. doi: 10.1016/0378-4347(95)00340-1. [DOI] [PubMed] [Google Scholar]
- 16.Teng R, Dogolo L C, Willavize S A, Friedman H L, Vincent J. Oral bioavailability of trovafloxacin with and without food in healthy volunteers. J Antimicrob Chemother. 1997;39:87–92. doi: 10.1093/jac/39.suppl_2.87. [DOI] [PubMed] [Google Scholar]
- 17.Thomson K S, Chartrand S A, Sanders C C, Block S L. Trovafloxacin, a new fluoroquinolone with potent activity against Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:478–480. doi: 10.1128/aac.41.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent J, Venitz J, Teng R, Baris B A, Willavize S A, Polzer R J, Friedman H L. Pharmacokinetics and safety of trovafloxacin in healthy male volunteers following administration of single intravenous doses of the prodrug, alatrofloxacin. J Antimicrob Chemother. 1997;39:75–80. doi: 10.1093/jac/39.suppl_2.75. [DOI] [PubMed] [Google Scholar]
- 19.Visalli M A, Jacobs M R, Appelbaum P C. Activity of CP 99,219 (trovafloxacin) compared with ciprofloxacin sparfloxacin, clinafloxacin, lomefloxacin and cefuroxime against ten penicillin-susceptible and penicillin-resistant pneumococci by time-kill methodology. J Antimicrob Chemother. 1996;37:77–84. doi: 10.1093/jac/37.1.77. [DOI] [PubMed] [Google Scholar]