Abstract
We examined the production of secreted aspartyl proteinase (Sap), a putative virulence factor of Candida albicans, by a series of 17 isolates representing a single strain obtained from the oral cavity of an AIDS patient before and after the development of clinical and in vitro resistance to fluconazole. Isolates were grown in Sap-inducing yeast carbon base-bovine serum albumin medium containing 0, 0.25, 0.5, or 1 MIC of fluconazole, and cultures were sampled daily for 14 days to determine extracellular Sap activity by enzymatic degradation of bovine serum albumin. Extracellular Sap activity was significantly decreased in a dose-dependent manner for the most fluconazole-susceptible isolate (MIC, 1.0 μg/ml) and significantly increased in a dose-dependent manner for the most fluconazole-resistant isolate (MIC, >64 μg/ml). Enhanced extracellular Sap production could not be attributed to cell death or nonspecific release of Sap, because there was no reduction in the number of CFU and no significant release of enolase, a constitutive enzyme of the glycolytic pathway. Conversely, intracellular Sap concentrations were significantly increased in a dose-dependent manner in the most fluconazole-susceptible isolate and decreased in the most fluconazole-resistant isolate. Enhanced Sap production correlated with the overexpression of a gene encoding a multidrug resistance (MDR1) efflux pump occurring in these isolates. These data indicate that exposure to subinhibitory concentrations of fluconazole can result in enhanced extracellular production of Sap by isolates with the capacity to overexpress MDR1 and imply that patients infected with these isolates and subsequently treated with suboptimal doses of fluconazole may experience enhanced C. albicans virulence in vivo.
Candida albicans is an opportunistic yeast that is a common commensal of human mucosal surfaces. Oral candidiasis has been among the most common and persistent complications associated with human immunodeficiency virus (HIV) infection (6, 36). In severely debilitated or immunocompromised hosts, particularly in those with granulocytopenia, this organism can cause life-threatening infections (20, 26). The increased incidence of serious fungal infections in the immunocompromised patient population (25, 26) and the emergence of azole antifungal drug resistance (10, 27, 34) make investigations to understand the mechanisms of C. albicans pathogenicity and its relationship to drug resistance more important.
Multiple factors have been implicated in the enhancement of C. albicans pathogenicity; these include phospholipase production (4, 9), hyphal formation (4), the expression of drug resistance genes (1), and the production of an extracellularly secreted aspartyl proteinase (Sap) (7, 29). Several lines of evidence indicate that Sap is a pathogenic factor of C. albicans. First, mutations in SAP genes result in attenuated virulence in murine models of disseminated candidiasis (8, 30). Second, Sap is produced in vivo, as demonstrated by indirect fluorescent-antibody staining of tissue sections derived from C. albicans-infected mice (14). Third, Sap-producing C. albicans isolates cause cavitation of newborn mouse skin, which can be blocked by a specific proteinase inhibitor, pepstatin A (22).
Fluconazole is the most commonly prescribed antifungal agent for the prophylaxis and therapy of oral candidiasis and, increasingly more commonly for disseminated candidiasis (27, 40). Fluconazole, a fungistatic azole antifungal agent, inhibits the lanosterol 14α demethylase enzyme required for the biosynthesis of ergosterol, a major functional component of the fungal cell membrane (12, 18, 35). Alterations in the ergosterol biosynthetic pathway or in the structure of ergosterol have been associated with azole drug resistance in C. albicans, as has the overexpression of or presence of point mutations in the ERG11 gene encoding lanosterol 14α demethylase (11, 28, 38, 39, 40). Fluconazole resistance has also been associated with increased levels of mRNAs of the CDR1 and MDR1 genes, which code for corresponding members of the ATP-binding cassette (ABC) transporter and major facilitator families of efflux pumps, respectively, and which have been implicated in the enhanced efflux of fluconazole from Candida cells (31, 32, 40).
A relationship between one of these efflux pumps and the virulence of C. albicans was first suggested in 1995 by Becker et al. (1), who demonstrated that disruption of the MDR1 gene in C. albicans resulted in mutants with reduced virulence in a murine model of disseminated candidiasis. More recently, Graybill et al. (5) demonstrated that a complex relationship exists between fluconazole resistance and C. albicans virulence. Using a murine model of disseminated candidiasis, this group found that among isolates for which the MICs of fluconazole were high, the more virulent strains caused infections which could be successfully treated, whereas the less virulent strains caused infections which were refractory to fluconazole therapy.
Therefore, to better understand the relationship between the development of drug resistance and virulence in C. albicans, we examined the in vitro production of Sap by a series of isolates obtained from a single AIDS patient before and after the development of clinical and in vitro fluconazole resistance (21, 24). Molecular mechanisms of drug resistance for these isolates had previously been characterized (38, 39, 41) and included the development of enhanced expression of both the CDR1 and the MDR1 drug efflux genes during the emergence of drug resistance.
MATERIALS AND METHODS
Microorganisms.
A series of 17 C. albicans isolates for which the MICs of fluconazole ranged from 1.0 μg/ml (isolate 1) to >64 μg/ml (isolate 17) were obtained from a single AIDS patient over a 2-year period (a gift from Theodore White, Seattle Biomedical Research Institute, Seattle, Wash.; originally isolated by Spencer Redding, University of Texas Health Science Center, San Antonio). These isolates had previously been determined to be the same strain by DNA subtype analysis, with only a minor substrain variation occurring between isolates 1 and 2 (21, 24, 41). It was also previously shown that as the patient's clinical response diminished over time, the MIC of fluconazole for these isolates increased (21, 24, 41). This decrease in susceptibility was found to be associated with at least four mechanisms: (i) increased expression of the MDR1 gene between isolates 1 and 2; (ii) a point mutation in the ERG11 gene between isolates 12 and 13; (iii) increased ERG11 gene expression between isolates 12 and 13; and (iv) increased expression of the CDR1 gene between isolates 15 and 17 (38, 39).
Determination of the MIC of fluconazole.
MICs were determined by a broth microdilution modification of National Committee for Clinical Laboratory Standards (NCCLS) method M27-A with RPMI 1640 medium (17). MICs were also determined with the Sap-inducing medium, yeast carbon base-bovine serum albumin (YCB-BSA) broth (Difco, Detroit, Mich.) containing vitamins (0.1 μl/ml; IsoVitaleX enrichment; BBL, Cockeysville, Md.) and 0.2% (wt/vol) each glucose and BSA (fraction V; Sigma Chemical Co., St. Louis, Mo.) and adjusted to pH 5.6. End points were read visually after 48 h of incubation at 35°C for cells grown in RPMI 1640 medium and after 90 h of incubation at 25°C for cells grown in YCB-BSA medium. A 90-h MIC end point was used for isolates tested in YCB-BSA medium because cell growth was slower in this medium than in the nutritionally richer RPMI 1640 medium, and a 25°C incubation temperature was used to parallel conditions used for Sap production assays. Although the MIC endpoint for isolates 16 and 17 in both RPMI 1640 medium and YCB-BSA medium was >64 μg/ml, for ease of presentation, an MIC end point of 64 μg/ml was defined as 1 MIC; designations for 1/4 or 1/2 MIC were based on this definition. MICs of fluconazole for all other isolates represented actual end points determined in YCB-BSA medium.
Determination of extracellular Sap activity.
C. albicans isolates were grown in 300 ml of YCB-BSA medium (final concentration, 107 blastoconidia per ml) containing 0, 1/4, 1/2 or 1 MIC of fluconazole (a gift from Pfizer, Inc., Groton, Conn.) in 1-liter Erlenmeyer flasks rotating at 140 rpm for 14 days at 25°C. Five-milliliter aliquots were removed daily, and Sap activity was determined spectrophotometrically by measuring the sample absorbance at 280 nm following the degradation of the substrate (BSA) as previously described (3, 15). Sap activity was normalized for the number of CFU present in each sample by dividing the absorbance value of the sample by the number of CFU per milliliter for a given day.
Recovery of intra- and extracellular Sap and enolase for use in EIA.
Supernatants from the cultures described above were used to determine extracellular Sap and enolase concentrations by enzyme immunoassays (EIA), and cell pellets were used to determine intracellular Sap and enolase concentrations at the time of peak Sap production (day 9). Reagents from a Puregene isolation kit (Gentra Systems, Minneapolis, Minn.) were used according to the manufacturer's instructions to isolate proteins from C. albicans blastoconidia for the determination of intracellular Sap and enolase concentrations by the EIA described below.
Determination of intra- and extracellular Sap concentrations by EIA.
A double-antibody sandwich EIA was developed to determine the intracellular Sap concentration relative to its extracellular counterpart because intracellular Sap could not be detected by the Sap activity assay described above (unpublished data). Antibodies were raised against purified Sap in New Zealand White female rabbits and were column purified and labeled with horseradish peroxidase (HRPO) as previously described (8a, 16). Purified, unlabeled rabbit anti-Sap antibody was used to coat wells of a 96-well microtiter plate (Immulon II; Dynatech Laboratories, Chantilly, Va.). Intracellular Sap for the EIA was prepared as described above. Extracellular Sap for the EIA was obtained by boiling 300 μl of culture supernatant in a 1.5-ml Eppendorf tube for 5 min, followed by cooling on ice. One hundred microliters of intra- or extracellular Sap was then added to each well of the antibody-coated microtiter plate and incubated at room temperature for 30 min. After three washes with 0.01 M phosphate-buffered saline (PBS) (pH 7.2) containing 0.05% Tween 20 (Sigma), 100 μl of HRPO-conjugated rabbit anti-Sap antibody was added and incubated at room temperature for 30 min. Colorimetric substrate (3′,3′-tetramethylbenzidine) and H2O2 solution (Kirkegaard & Perry, Gaithersburg, Md.) were added, and plates were read immediately with a kinetic microtiter plate reader (SpectraMax; Molecular Devices, Sunnyvale, Calif.) at an absorbance of 650 nm. For intracellular Sap, because a constant cell number (108) was used to recover Sap, 1 U was defined as the amount of Sap giving an absorbance value at 650 nm equivalent to that determined for 1 ng of purified Sap per ml from a standard curve. The extracellular Sap concentration was normalized for cell growth by dividing the total nanograms of Sap recovered per milliliter by the number of CFU per milliliter on a given day.
Determination of intra- and extracellular enolase concentrations by EIA.
Rabbit anti-enolase antibody and Escherichia coli containing the glutathione transferase (GST)-enolase gene construct were gifts from Paula Sundstrom (Ohio State University, Columbus). Recombinant enolase was expressed in E. coli and purified by affinity chromatography (33). Intra- and extracellular enolase concentrations were determined by an indirect inhibition EIA. Intracellular enolase for the EIA was prepared as described above. Samples (200 μl) from 108 disrupted C. albicans blastoconidia were mixed with 200 μl of rabbit anti-enolase antibody and incubated at 25°C for 30 min. One hundred microliters was placed into each of three wells of a 96-well microtiter plate (Immulon II) previously coated with 0.625 μg of recombinant GST-enolase per ml in 0.01 MPBS without Tween 20. After incubation for 30 min at 25°C, the plates were washed with PBS containing 0.05% Tween 20, and the quantity of bound antibody was determined colorimetrically with a specific goat anti-rabbit antibody–HRPO conjugate (Bio-Rad Laboratories, Richmond, Calif.) and tetramethylbenzidine-hydrogen peroxide reagent (Kirkegaard & Perry). Plates were read immediately with a kinetic microtiter plate reader (Molecular Devices). For the determination of intracellular enolase content, because a constant cell number (108) was used to recover enolase, 1 U was defined as the amount of enolase giving an absorbance value equivalent to that determined for 1 ng of purified enolase per ml from a standard curve. The extracellular enolase concentration was normalized for cell growth by dividing the total nanograms of enolase recovered per milliliter by the number of CFU per milliliter.
Determination of cell growth and viability.
To evaluate cell growth and viability, aliquots were removed daily from the culture medium, serially diluted with 0.01 M PBS, and plated on Sabouraud's dextrose agar. Cell viability was determined by assessment of CFU produced after 48 h of incubation at 25°C.
Statistical analysis.
The Student t test was used to determine differences between means; P values of <0.05 were considered statistically significant.
RESULTS
Comparison of the MIC end points of fluconazole with RPMI 1640 medium versus YCB-BSA medium as the growth medium.
The broth microdilution MICs of fluconazole for the C. albicans isolates used in this study were determined with two growth media: standard NCCLS-recommended RPMI 1640 medium and YCB-BSA medium. YCB-BSA medium was used to induce Sap production by C. albicans isolates (14) and to determine the effect of fluconazole on the extracellular production of Sap. The results shown in Table 1 demonstrate that there were no significant differences in the MICs of fluconazole for any of the isolates tested when either medium was used, with the minor exception of isolates 1 and 2, for which MICs were slightly lower in RPMI 1640 medium. Nonetheless, the MICs of fluconazole for these isolates were within the accepted limits of variation for MICs (i.e., within two twofold serial dilutions).
TABLE 1.
MICs of fluconazole for C. albicans isolates grown in RPMI 1640 or YCB-BSA medium
| Isolate | MIC (μg/ml) of fluconazole in the following medium:
|
|
|---|---|---|
| RPMI 1640a | YCB-BSAb | |
| 1 | 0.25 | 1.0 |
| 2 | 1.0 | 2.0 |
| 3 | 8.0 | 8.0 |
| 12 | 8.0 | 8.0 |
| 13 | 16.0 | 16.0 |
| 14 | 32.0 | 32.0 |
| 15 | 16.0 | 32.0 |
| 16 | >64.0 | >64.0 |
| 17 | >64.0 | >64.0 |
MICs of fluconazole were determined by a broth microdilution modification of NCCLS method M27-A; MIC end points were read visually at 48 h.
MICs of fluconazole were determined by a broth microdilution assay with Sap-inducing YCB-BSA medium instead of RPMI 1640 medium; MIC end points were read visually at 90 h.
Extracellular Sap activity in fluconazole-susceptible and fluconazole-resistant isolates grown in the presence or absence of the drug.
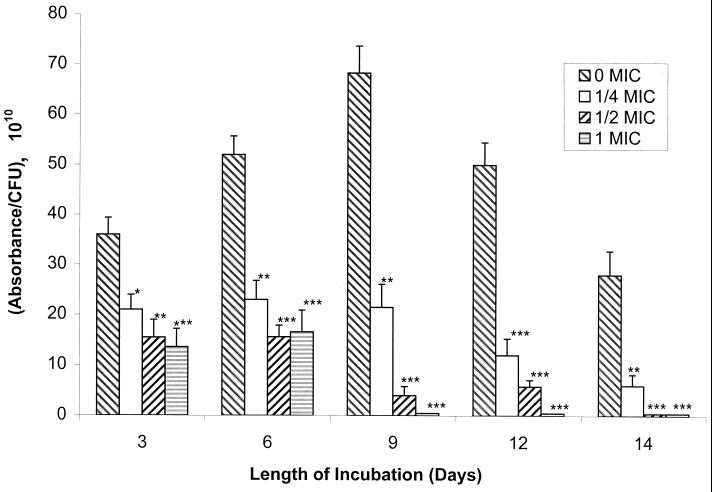
Fluconazole-susceptible and fluconazole-resistant C. albicans isolates were grown in YCB-BSA medium containing 0, 1/4, 1/2, or 1 MIC of fluconazole, and aliquots of culture supernatants obtained daily were tested for extracellular Sap activity. As shown in Fig. 1, Sap activity for the most fluconazole-susceptible isolate (isolate 1) increased and then declined over time in cultures containing no fluconazole (0 MIC), in a manner similar to that previously observed for other strains of C. albicans (15). In contrast, Sap activity declined consistently over time in cultures where fluconazole was present in the growth medium, and this effect was observed at all drug concentrations tested (Fig. 1). In addition, on any given day, a dose-dependent reduction in extracellular Sap activity was observed when the most fluconazole-susceptible isolate was grown in increasing concentrations of drug.
FIG. 1.
Sap activity of fluconazole-susceptible C. albicans isolate 1 in the absence or presence of increasing MICs of fluconazole (0 MIC, 0 μg/ml; 1/4 MIC, 0.25 μg/ml; 1/2 MIC, 0.5 μg/ml; 1 MIC, 1.0 μg/ml) with time in culture. Asterisks denote a significant reduction in Sap activity relative to the activity in control isolates grown without drug (for ∗, ∗∗, and ∗∗∗, P was <0.025, <0.005, and <0.001, respectively). Error bars show standard deviations.
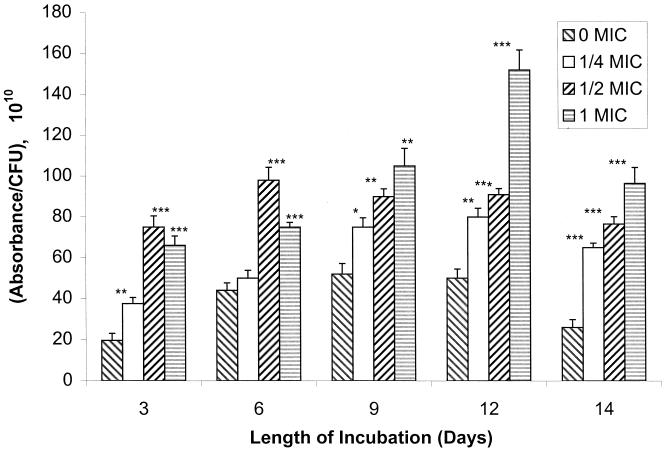
Sap activity for isolate 2, a fluconazole-susceptible isolate for which the MIC was 2.0 μg/ml, also increased and then declined over time in cultures containing no drug (Fig. 2). In the presence of fluconazole, however, Sap activity for isolate 2 consistently increased relative to that in the drug-free control on any given day in a dose-dependent manner, with the exception of samples taken on or before day 6 and exposed to the highest concentration of drug (1 MIC). Although the same absolute concentration of fluconazole was used in some instances for isolates 1 and 2 (i.e., for isolate 1, 1/2 MIC was 0.5 μg/ml and 1 MIC was 1 μg/ml; for isolate 2, 1/4 MIC was 0.5 μg/ml and 1/2 MIC was 1 μg/ml), the effects of the drug on Sap activities were different (decreased Sap activity for isolate 1 and increased Sap activity for isolate 2).
FIG. 2.
Sap activity of fluconazole-susceptible C. albicans isolate 2 in the absence or presence of increasing MICs of fluconazole (0 MIC, 0 μg/ml; 1/4 MIC, 0.5 μg/ml; 1/2 MIC, 1 μg/ml; 1 MIC, 2 μg/ml) with time in culture. Asterisks denote a significant enhancement of Sap activity relative to the activity in control isolates grown without drug (for ∗, ∗∗, and ∗∗∗, P was <0.01, <0.005, and <0.001, respectively). Error bars show standard deviations.
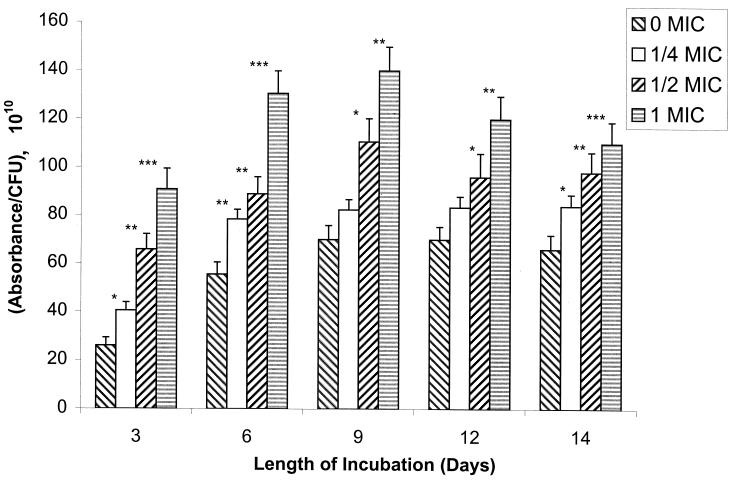
In a manner similar to that for the fluconazole-susceptible isolates (isolates 1 and 2), Sap activity for the most fluconazole-resistant isolate (isolate 17) also increased and then declined over time in cultures containing no drug (Fig. 3). In the absence of drug, all isolates produced similar peak quantities of Sap (peak Sap activity was reached by all isolates by day 9; the range in peak Sap activity [absorbance/CFU per ml · 1010] was 52 to 70; compare Fig. 1, 2, and 3). However, in contrast to the results for the most susceptible isolate (isolate 1), a dose-dependent increase in Sap activity was observed when the most resistant isolate (isolate 17) was grown in increasing concentrations of fluconazole (Fig. 3). Isolates 3 to 17 demonstrated an enhancement of absolute Sap activity relative to isolate 2; this result corresponded to the overexpression of the MDR1 gene by these isolates (Table 2).
FIG. 3.
Sap activity of fluconazole-resistant C. albicans isolate 17 in the absence or presence of increasing MICs of fluconazole (0 MIC, 0 μg/ml; 1/4 MIC, 16 μg/ml; 1/2 MIC, 32 μg/ml; 1 MIC, 64 μg/ml) with time in culture. Asterisks denote a significant enhancement of Sap activity relative to the activity in control isolates grown without drug (for ∗, ∗∗, and ∗∗∗, P was <0.025, <0.005, and <0.001, respectively). Error bars show standard deviations.
TABLE 2.
Effect of fluconazole on Sap activity compared with reported mechanisms of resistance for the same isolatesa
| Isolate | MIC (μg/ml) of fluconazoleb | Sap activity with fluconazolec | MDR1 | ERG point mutationd | ERG11e | CDR1e |
|---|---|---|---|---|---|---|
| 1 | 1 | ↓ 3.2 | 0 | 0 | → | → |
| 2 | 2 | ↑ 1.4 | ↑ 12 | 0 | → | → |
| 3 | 8 | ↑ 2.8 | ↑ 25 | 0 | → | → |
| 12 | 8 | ↑ 2.6 | ↑ 25 | 0 | → | → |
| 13 | 16 | ↑ 2.1 | ↑ 25 | + | ↑ 4.5 | → |
| 15 | 32 | ↑ 2.5 | ↑ 25 | + | ↑ 4.5 | → |
| 16 | >64 | ↑ 2.4 | ↑ 25 | + | ↑ 4.5 | ↑ 5 |
| 17 | >64 | ↑ 2.0 | ↑ 25 | + | ↑ 4.5 | ↑ 5 |
As reported by White (38), progressively enhanced expression of MDR1, ERG11, and CDR1 was observed over time for isolates 1 to 17; 0, no gene expression or no point mutation was observed. ↑ and ↓, fold increase or decrease.
Determined by modified NCCLS method M27-A with YCB-BSA medium.
Fold increase (↑) or decrease (↓) of absolute Sap activity for isolates grown in the presence of 1/2 MIC of fluconazole relative to that for the same isolates grown in the absence of fluconazole on the day of peak Sap activity (day 9). Definition of 1/2 MIC for each isolate: 1, 0.5 μg/ml; 2, 1.0 μg/ml; 3 and 12, 4.0 μg/ml; 13, 8.0 μg/ml; 15, 16.0 μg/ml; and 16 and 17, 32.0 μg/ml.
+, point mutation in the ERG11 gene associated with increased azole resistance between isolates 12 and 13 (38).
→, Constitutive expression (38).
Determination of intra- and extracellular Sap concentrations by EIA.
A double-antibody sandwich EIA was used to compare the intracellular and extracellular concentrations of Sap because no detectable Sap enzyme activity could be detected intracellularly in any isolate tested (unpublished data). This observation is consistent with the hypothesis that Sap is inactive intracellularly and becomes activated upon extracellular secretion (37).
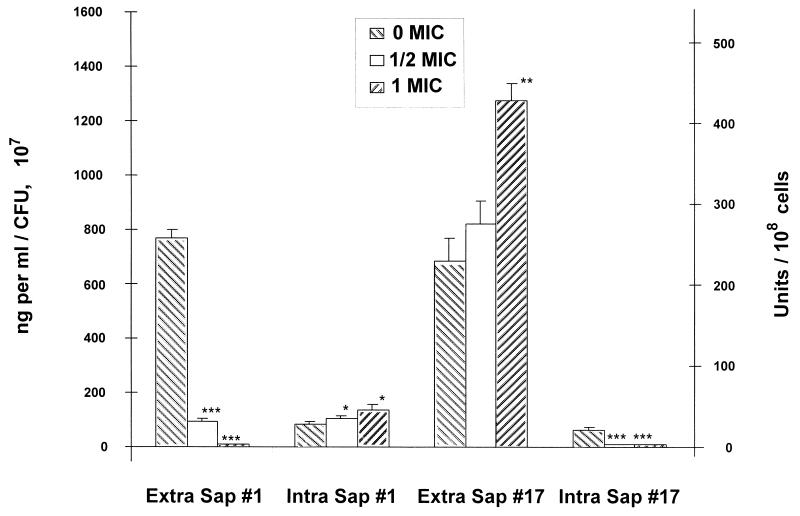
Figure 4 demonstrates that when the most fluconazole-susceptible isolate (isolate 1) was grown in increasing concentrations of the drug, the extracellular Sap concentration declined and the intracellular Sap concentration increased in a dose-dependent manner. In contrast, when the most fluconazole-resistant isolate (isolate 17) was grown in increasing concentrations of the drug, the extracellular concentration of Sap increased and the intracellular Sap concentration declined (Fig. 4). These results indicate that exposure to fluconazole resulted in the intracellular accumulation of Sap by isolate 1 and the transfer of Sap from the intracellular to the extracellular compartment by isolate 17.
FIG. 4.
Comparison of extracellular (Extra Sap; left axis) and intracellular (Intra Sap; right axis) Sap concentrations of fluconazole-susceptible (isolate 1) and fluconazole-resistant (isolate 17) C. albicans isolates by EIA on the day of peak Sap production (day 9). Fluconazole concentrations tested for isolate 1: 0 MIC, 0 μg/ml; 1/2 MIC, 0.5 μg/ml; 1 MIC, 1.0 μg/ml. Fluconazole concentrations tested for isolate 17: 0 MIC, 0 μg/ml; 1/2 MIC, 32 μg/ml; 1 MIC, 64 μg/ml. Asterisks denote a significant reduction or enhancement of Sap concentration relative to the concentration for control isolates grown without drug (for ∗, ∗∗, and ∗∗∗, P was <0.05, <0.005, and <0.001, respectively). Error bars show standard deviations.
Determination of intra- and extracellular enolase concentrations by EIA.
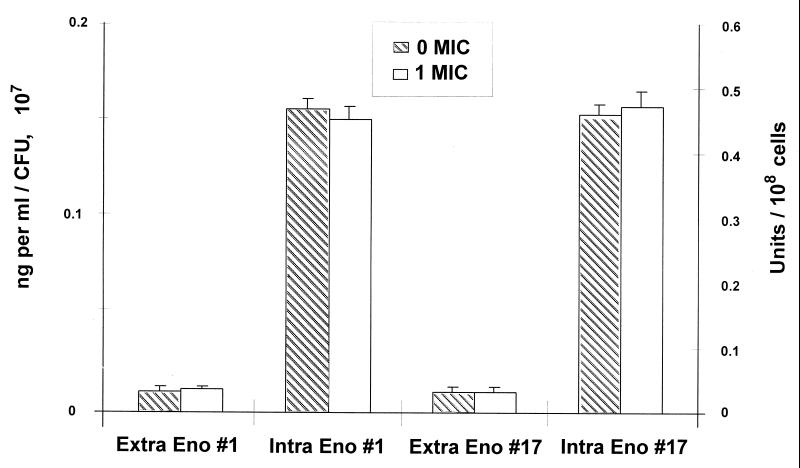
To determine whether the enhanced extracellular production of Sap by the most resistant isolate (isolate 17) following fluconazole exposure occurred by an active or a passive mechanism, the intra- and extracellular concentrations of enolase, a constitutive, intracellular “housekeeping” enzyme of the glycolytic pathway (molecular mass, 48 kDa, similar to the molecular mass of Sap, 43 kDa), were measured in isolates 1 and 17 by EIA. No differences in intra- or extracellular enolase concentrations were observed between the two isolates (Fig. 5). Therefore, the enhanced production of extracellular Sap by isolates grown in the presence of fluconazole could not be attributed to passive leakage of intracellular contents.
FIG. 5.
Comparison of extracellular (Extra Eno; left axis) and intracellular (Intra Eno; right axis) enolase concentrations of fluconazole-susceptible (isolate 1) and fluconazole-resistant (isolate 17) C. albicans isolates by EIA on the day of peak Sap production (day 9). Fluconazole concentrations tested for isolate 1: 0 MIC, 0 μg/ml; 1 MIC, 1.0 μg/ml. Fluconazole concentrations tested for isolate 17: 0 MIC, 0 μg/ml; 1 MIC, 64 μg/ml. No significant differences in extracellular or intracellular enolase concentrations were observed between isolates grown in the presence or absence of fluconazole. Error bars show standard deviations.
Determination of cell growth and viability in the presence or absence of fluconazole.
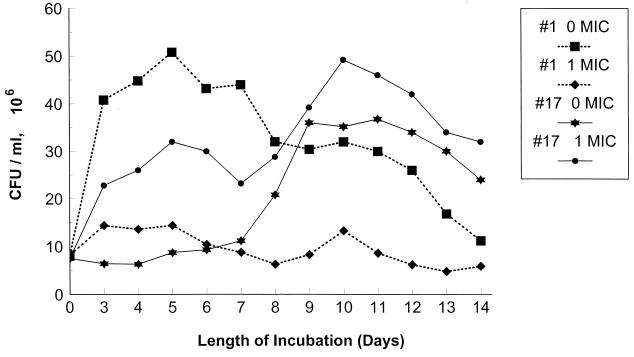
The enhanced production of extracellular Sap by the most fluconazole-resistant isolate (isolate 17) grown in the presence of fluconazole could not be attributed to stasis of cell growth or to cell death. Indeed, the resistant isolate (isolate 17) grew as well or better in the presence of the drug than in its absence. Figure 6 depicts the recovery of CFU from cultures of the most fluconazole-susceptible isolate (isolate 1) and the most fluconazole-resistant isolate (isolate 17) grown in the absence and presence of fluconazole. Whereas the growth of isolate 1 in fluconazole resulted in stasis of growth (but no cell death), growth in the presence of the drug did not inhibit the growth or decrease the viability of isolate 17 (Fig. 6; mean percent decrease in cell growth on days 3 to 14 for isolate 1, −23.9 ± 9.4 [n = 12]; mean percent increase in cell growth on days 3 to 14 for isolate 17, +12.2 ± 6.6 [n = 12]; P < 0.001 compared to isolate 1). Growth of isolate 2 in the presence of fluconazole was similar to that of isolate 17 (data not shown; mean percent increase in cell growth on days 3 to 14 for isolate 2, +11.9 ± 5.3 [n = 12]; P > 0.05 compared to isolate 17 and <0.001 compared to isolate 1).
FIG. 6.
Growth of fluconazole-susceptible (isolate 1) and fluconazole-resistant (isolate 17) C. albicans isolates in YCB-BSA medium with or without fluconazole added. Fluconazole concentrations tested: isolate 1, 0 MIC, 0 μg/ml, and 1 MIC, 1 μg/ml; isolate 17, 0 MIC, 0 μg/ml, and 1 MIC, 64 μg/ml. Fluconazole exposure induced stasis of cell growth for isolate 1, but no fluconazole-induced stasis of cell growth was observed for isolate 17.
Effect of fluconazole on Sap activity compared with reported mechanisms of fluconazole resistance.
Table 2 summarizes the effect of fluconazole on Sap production and the reported mechanisms of drug resistance for isolates used in this study. For ease of presentation, data for the effect of fluconazole on absolute Sap activity are represented as the fold increase or decrease of activity in the presence of 1/2 MIC of the drug relative to Sap activity in the absence of fluconazole on the day of peak Sap activity (day 9). Enhanced clinical and in vitro resistance in this series of isolates developed gradually and occurred as a result of several stepwise genetic alterations (21, 24, 38, 40). The increase in resistance was found to be associated with several mechanisms, including increased expression of the MDR1 gene between isolates 1 and 2, a point mutation in the ERG11 gene between isolates 12 and 13, an increase in ERG11 gene expression between isolates 12 and 13, and increased expression of the CDR1 gene between isolates 15 and 17 (Table 2) (38, 39). Sap production for the most susceptible isolate (isolate 1) was decreased by 3.2-fold in the presence of fluconazole compared to that in its absence. In contrast, the Sap activity of isolate 2 was increased by 1.4-fold and that of isolate 3 was increased by 2.8-fold when grown in the presence of fluconazole (Table 2). Isolate 1 was previously reported to show little discernible expression of MDR1 mRNA by Northern blot analysis (38). In contrast, isolate 2 demonstrated a 12-fold increase in expression of the MDR1 gene and isolates 3 to 17 showed a 25-fold increase in expression (38). Isolates recovered after isolate 3 did not demonstrate any additional enhancement of Sap activity (mean fold increase in Sap activity for isolates 3 to 17, 2.4 ± 0.3 [n = 18]) despite the occurrence of a point mutation in the ERG11 gene and overexpression of the ERG11 and CDR1 genes (Table 2).
DISCUSSION
Investigations to understand the mechanisms of C. albicans pathogenicity and their relationship to drug resistance have become more important in recent years as a result of the increased incidence of serious fungal infections in the immunocompromised patient population (26) and the emergence of azole antifungal drug resistance (10, 19, 27). We therefore sought to determine if a relationship existed between the production of Sap, a virulence factor of C. albicans, and the development of azole resistance.
The earliest suggestion that a relationship between drug resistance and C. albicans virulence existed was in work done by Becker et al. (1), who demonstrated that disruption of the MDR1 gene in C. albicans resulted in mutants with reduced virulence in a murine model of disseminated candidiasis. However, the same group later reported that the “ura-blaster” technique used to produce the gene disruption may itself have reduced the virulence of the microorganism (13). Recently, Graybill et al. (5), using serial isolates obtained from patients who developed clinical and in vitro fluconazole resistance and a murine model of disseminated candidiasis, found that among isolates for which fluconazole MICs were high, the more virulent isolates caused infections which could be successfully treated, whereas the less virulent isolates caused infections which were refractory to fluconazole therapy. Clearly, the relationship between virulence and azole drug resistance is a complex one.
We also used serial isolates from a single patient who developed clinical and in vitro resistance to fluconazole. Preliminary work with these isolates in a murine model of disseminated candidiasis suggested that the most resistant isolate was innately more virulent than the most susceptible isolate from this same patient (T. Wu, K. Wright, S. F. Hurst, and C. J. Morrison, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. F-81, p. 311, 1999). In addition, we found that unlike that of the most susceptible isolate, growth of the most resistant isolate in increasing concentrations of fluconazole resulted in a dose-dependent increase in the in vitro production of Sap (T. Wu, K. Wright, S. F. Hurst, and C. J. Morrison, Abstr. 5th ASM Candida Candidiasis Conf., abstr. A19, p. 28, 1999), correlating with increased virulence in vivo (Wu et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). Furthermore, preliminary studies suggested that this phenomenon is not unique because similar results have been observed for a series of isolates which were obtained from a woman with vulvovaginal candidiasis and which demonstrated increased resistance to azole drugs over time (Wu et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). In addition, unlike mice infected with susceptible isolates, mice infected with resistant isolates and treated with clinical doses of fluconazole demonstrated increased mortality and organ burden relative to untreated controls (Wu et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). Only when mice were treated with the highest dose of fluconazole (10 mg/kg of body weight, equivalent to 800 mg in humans) was survival improved (Wu et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). These results imply that patients systemically infected with a fluconazole-resistant C. albicans isolate and treated with clinical doses of fluconazole below 800 mg per day may be adversely affected due to the enhancement of C. albicans Sap production and virulence. Coincidentally, others have reported that oral isolates obtained from HIV-positive patients after antifungal drug therapy demonstrated significantly increased Sap production compared to those obtained either before or during an episode of thrush (K. Vargas and D. R. Soll, Abstr. 5th ASM Candida Candidiasis Conf., abstr. A21, p. 28, 1999). In addition, high-frequency phenotype switching was greatly increased in HIV-positive patient isolates compared to isolates obtained from control subjects, and variability in Sap activity was found in different switched phenotypes (Vargas and Soll, Abstr. 5th ASM Candida Candidiasis Conf.). Clearly, additional work examining these important phenomena is required before a complete understanding of the relationship between azole drug resistance and virulence of C. albicans can be achieved.
We found that enhanced Sap production by isolates grown in subinhibitory concentrations of fluconazole corresponded to the development of increased drug resistance. A dose-dependent enhancement of Sap production resulting from growth in fluconazole was observed to occur as early as isolate 2, an isolate which still remained susceptible to the drug but which appeared to adapt to its presence in the growth medium by initiating enhanced Sap production at a later time than the truly resistant isolates. Increased expression of the MDR1 gene was shown by others to occur between isolates 1 and 2 and to be most pronounced in isolates 3 to 17 (38). Enhanced Sap production by isolate 2 compared to isolate 1 after growth in fluconazole corresponded to the occurrence of overexpression of the MDR1 gene in this series of isolates (38). Additional enhancement of Sap production occurred at isolate 3 and was maintained until isolate 17. In contrast, no additional enhancement of Sap production was observed when a point mutation in the ERG11 gene or overexpression of ERG11 or CDR1 occurred.
Without further substantive evidence, it is currently only speculation that there is a specific relationship between the up-regulation of genes encoding efflux pumps associated with azole drug resistance and the enhancement of Sap activity. Indeed, previous studies have examined the up-regulation of efflux pump genes in cells grown in the absence of fluconazole rather than in its presence (37). However, the data suggest that there is a link between enhanced in vitro Sap activity induced by fluconazole and overexpression of one drug efflux pump gene, MDR1. Indeed, Sap activity has been shown by others to be phase specific in some strains of C. albicans (23), and preliminary data suggest that a number of phase-specific genes, including a gene for another drug efflux pump, CDR3, are regulated by the same trans-acting factors through a MADS-box binding site (S. R. Lockhart, M. Nguyen, and D. R. Soll, Abstr. 5th ASM Candida Candidiasis Conf., abstr. B39, p. 45–46, 1999). It is therefore plausible that the up-regulation of one gene may result in the up-regulation of other phase-specific genes. CDR3, however, has not yet been directly linked to azole drug resistance. This is not to suggest that enhanced production of Sap by resistant isolates grown in the presence of fluconazole is nonspecific. Enhanced Sap production in the presence of fluconazole occurred by an active and selective mechanism rather than a passive one, as demonstrated by the lack of nonspecific release of the constitutive housekeeping enzyme, enolase. Nor could enhanced release of Sap be attributed to passive release upon cell death. Indeed, isolates 2 and 17 grew better in the presence of fluconazole, perhaps because the release of enhanced levels of Sap caused enhanced degradation of the substrate BSA, the sole nitrogen source in YCB-BSA medium, thereby supporting enhanced growth. In addition, all results were expressed as Sap activity divided by CFU per milliliter for each isolate, so that any effects of fluconazole on cell growth did not interfere with an accurate assessment of the effect of fluconazole on Sap activity.
Efflux mechanisms have been suggested for the transport of Sap into membrane-bound vesicles (2), and up-regulation of such transport mechanisms, whether directly or indirectly, may explain the enhancement of Sap activity observed in resistant isolates grown in the presence of fluconazole. Indeed, Cdr1p, an ABC transporter of C. albicans which confers resistance to azoles and a wide range of functionally and structurally diverse compounds, has been shown to translocate phosphatidylethanolamine from inner to outer leaflets of the plasma membrane in a manner analogous to the floppase function of human multidrug resistance (MDR) proteins (S. Dogra, S. Krishnamurthy, and R. Prasad, Abstr. 5th ASM Candida Candidiasis Conf., abstr. C18, p. 53, 1999). At least one ABC transporter protein, encoded by the gene CDR4 in C. albicans, has also been suggested to be involved in phospholipid translocation (D. Sanglard, F. Ischer, M. Monod, S. Dogra, R. Prasad, and J. Bille, Abstr. 5th ASM Candida Candidiasis Conf., abstr. C27, p. 56, 1999).
In summary, we have observed enhanced production of Sap by C. albicans isolates for which MICs were as low as 2 μg/ml and as high as >64 μg/ml when these isolates were grown in the presence of subinhibitory concentrations of fluconazole. This observation is especially important in view of the potential in vivo up-regulation of this virulence factor by fluconazole upon treatment of patients. Such enhancement of Sap activity in vivo has been suggested in preliminary animal model studies (Wu et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.) and in studies of HIV-positive patients after azole treatment for thrush (Vargas and Soll, Abstr. 5th ASM Candida Candidiasis Conf.). Improved understanding of this phenomenon is needed in order to circumvent possible negative side effects of fluconazole therapy. Future studies with wild-type strains and their counterparts containing disrupted resistance genes will be conducted, as will an examination of the effects of other azole antifungal drugs, such as ketoconazole and itraconazole, on C. albicans Sap production and virulence.
ACKNOWLEDGMENTS
We thank Theodore White of the Seattle Biomedical Research Institute, Seattle, Wash., and Spencer Redding of the University of Texas Health Science Center, San Antonio, for providing us with clinical isolates for this study. We also thank Paula Sundstrom of Ohio State University, Columbus, for providing clones producing recombinant enolase and for providing anti-enolase antibodies and David Warnock and Theodore White for helpful comments.
REFERENCES
- 1.Becker J M, Henry L K, Jiang W D, Koltin Y. Reduced virulence of Candida albicans mutants affected in multidrug resistance. Infect Immun. 1995;63:1253–1257. doi: 10.1128/iai.63.11.4515-4518.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon R D, Fischer F J, Niimi K, Niimi M, Arisawa M. Drug pumping mechanisms in Candida albicans. Jpn J Med Mycol. 1998;39:73–78. doi: 10.3314/jjmm.39.73. [DOI] [PubMed] [Google Scholar]
- 3.Crandall M, Edwards J E. Segregation of proteinase-negative mutants from heterozygous Candida albicans. J Gen Microbiol. 1987;133:2817–2824. doi: 10.1099/00221287-133-10-2817. [DOI] [PubMed] [Google Scholar]
- 4.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 5.Graybill J R, Montalbo E, Kirkpatrick W, Luther M F, Revankar S G, Patterson T F. Fluconazole versus Candida albicans: a complex relationship. Infect Immun. 1998;42:2938–2942. doi: 10.1128/aac.42.11.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenspan D, Greenspan J, Schiodt M, Pindborg J. AIDS and the mouth. Copenhagen, Denmark: Munksgaard; 1990. pp. 91–102. [Google Scholar]
- 7.Hube B. Candida albicans secreted aspartyl proteinases. Curr Top Med Mycol. 1996;7:55. [PubMed] [Google Scholar]
- 8.Hube B, Sanglard D, Odds F C, Hess D, Monod M, Schafer W, Brown A J P, Gow N A R. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect Immun. 1997;65:3529–3538. doi: 10.1128/iai.65.9.3529-3538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Hurst, S. F., G. H. Reyes, D. W. McLaughlin, E. Reiss, and C. Morrison. Comparison of commercial latex agglutination and sandwich enzyme immunoassays with a competitive binding inhibition enzyme immunoassay for detection of antigenemia and antigenuria in a rabbit model of invasive aspergillosis. Clin. Diagn. Lab. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 9.Ibrahim A S, Mirbod F, Filler S G, Banno Y, Cole G T, Kitajima Y, Edwards J E, Nozawa Y, Ghannoum M A. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun. 1995;63:1993–1998. doi: 10.1128/iai.63.5.1993-1998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson E M, Warnock D W, Luker J, Porter S R, Scully C. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother. 1995;35:103–114. doi: 10.1093/jac/35.1.103. [DOI] [PubMed] [Google Scholar]
- 11.Kelly S L, Lamb D C, Corran A J, Baldwin B C, Kelly D E. Mode of action and resistance to azole antifungals associated with the formation of 14α-methylergosta-8,24(28)-dien-3β,6α-diol. Biochem Biophys Res Commun. 1995;207:910–915. doi: 10.1006/bbrc.1995.1272. [DOI] [PubMed] [Google Scholar]
- 12.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea and Febiger; 1992. pp. 81–104. [Google Scholar]
- 13.Lay J, Henry L K, Clifford J, Koltin Y, Bulawa C E, Becker J M. Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect Immun. 1998;66:5301–5306. doi: 10.1128/iai.66.11.5301-5306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald F, Odds F C. Inducible proteinase of Candida albicans in diagnostic serology and pathogenesis of systemic candidiasis. J Med Microbiol. 1980;13:431–438. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- 15.Morrison C J, Hurst S F, Bragg S L, Kuykendall R J, Diaz H, McLaughlin D W, Reiss E. Purification and characterization of the extracellular aspartyl proteinase of Candida albicans: removal of extraneous proteins and cell wall mannoprotein and evidence for lack of glycosylation. J Gen Microbiol. 1993;139:1177–1186. doi: 10.1099/00221287-139-6-1177. [DOI] [PubMed] [Google Scholar]
- 16.Morrison C J, Hurst S F, Bragg S L, Kuykendall R J, Diaz H, Pohl J, Reiss E. Heterogeneity of the purified extracellular aspartyl proteinase from Candida albicans: characterization with monoclonal antibodies and N-terminal amino acid sequence analysis. Infect Immun. 1993;61:2030–2036. doi: 10.1128/iai.61.5.2030-2036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. 17. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 18.Odds F C. Candida and candidosis: a review and bibliography. London, United Kingdom: Bailliere Tindall; 1988. [Google Scholar]
- 19.Odds F C. Resistance of clinically important yeasts to antifungal agents. Int J Antimicrob Agents. 1996;6:145–147. doi: 10.1016/0924-8579(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller M A. Epidemiology of fungal infection. J Hosp Infect. 1995;30(Suppl.):329–338. doi: 10.1016/0195-6701(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller M A, Rhine C J, Redding S W, Smith J, Farinacci G, Fothergill A W, Rinaldi M G. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from patients with AIDS and oral candidiasis. J Clin Microbiol. 1994;32:59–64. doi: 10.1128/jcm.32.1.59-64.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray T L, Payne C D. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: a role for Candida acid proteinase. Infect Immun. 1988;56:1942–1949. doi: 10.1128/iai.56.8.1942-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray T L, Payne C D, Soll D R. Variable expression of Candida acid proteinase by “switch-phenotypes” of individual Candida albicans strains. Clin Res. 1988;36:687A. [Google Scholar]
- 24.Redding S, Smith J, Farinacci G, Rinaldi M, Fothergill A, Rhine C J, Pfaller M. Resistance of Candida albicans to fluconazole during treatment of oropharyngeal candidiasis in a patient with AIDS: documentation by in vitro susceptibility testing and DNA subtype analysis. Clin Infect Dis. 1994;18:240–242. doi: 10.1093/clinids/18.2.240. [DOI] [PubMed] [Google Scholar]
- 25.Reef S E, Mayer K H. Opportunistic candidal infections in patients infected with human immunodeficiency virus: prevention issues and priorities. Clin Infect Dis. 1995;21(Suppl. 1):S99–S102. doi: 10.1093/clinids/21.supplement_1.s99. [DOI] [PubMed] [Google Scholar]
- 26.Rees J R, Pinner R W, Hajjeh R A, Brant M E, Reingold A L. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27:1138–1147. [PubMed] [Google Scholar]
- 27.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez R J, Low C, Bottema C D K, Parks L W. Multiple functions for sterols in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1985;112:47–54. doi: 10.1016/0005-2760(85)90057-8. [DOI] [PubMed] [Google Scholar]
- 29.Ruchel R, De Bernardis F, Ray T L, Sullivan P A, Cole G T. Candida acid proteinases. J Med Vet Mycol. 1992;30(Suppl. 1):123–132. [PubMed] [Google Scholar]
- 30.Sanglard D, Hube B, Monod M, Odds F, Gow N A R. A triple deletion of secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanglard D, Isher F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundstrom P, Aliaga G R. Molecular cloning of cDNA and analysis of protein secondary structure of Candida albicans enolase, an abundant, immunodominant glycolytic enzyme. J Bacteriol. 1992;174:6789–6799. doi: 10.1128/jb.174.21.6789-6799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanden Bossche H, Marichal P, Odds F C. Molecular mechanisms of drug resistance in fungi. Trends Microbiol. 1994;2:393–400. doi: 10.1016/0966-842x(94)90618-1. [DOI] [PubMed] [Google Scholar]
- 35.Vanden Bossche H V, Warnock D W, Dupont B, Kerridge D, Sengupta S, Improvisi L, Marichal P, Odds F C, Provost F, Ronin O. Mechanisms and clinical impact of antifungal drug resistance. J Med Vet Mycol. 1994;32(Suppl. 1):189–202. doi: 10.1080/02681219480000821. [DOI] [PubMed] [Google Scholar]
- 36.Vuffray A, Durussel C, Boerlin P, Boerlin-Petzold F, Bille J, Glauser M P, Chave J P. Oropharyngeal candidiasis resistant to single-dose therapy with fluconazole in HIV-infected patients. AIDS. 1994;8:708–709. doi: 10.1097/00002030-199405000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Ward M, Kodama K H. Introduction to fungal proteinases and expression in fungal systems. Adv Exp Med Biol. 1991;306:149–160. doi: 10.1007/978-1-4684-6012-4_20. [DOI] [PubMed] [Google Scholar]
- 38.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increase in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White T C, Pfaller M A, Rinaldi M G, Smith J, Redding S. Stable azole drug resistance associated with a sub-strain of Candida albicans from an HIV-infected patient. Oral Dis. 1997;3(Suppl. 1):S102–S109. doi: 10.1111/j.1601-0825.1997.tb00336.x. [DOI] [PubMed] [Google Scholar]