Abstract
Maternal nutrition is found to be the key factor that determines fetal health in utero and metabolic health during adulthood. Metabolic diseases have been primarily attributed to impaired maternal nutrition during pregnancy, and impaired nutrition has been an immense issue across the globe. In recent years, type 2 diabetes (T2D) has reached epidemic proportion and is a severe public health problem in many countries. Although plenty of research has already been conducted to tackle T2D which is associated with obesity, little is known regarding the etiology and pathophysiology of lean T2D, a variant of T2D. Recent studies have focused on the effects of epigenetic variation on the contribution of in utero origins of lean T2D, although other mechanisms might also contribute to the pathology. Observational studies in humans and experiments in animals strongly suggest an association between maternal low protein diet and lean T2D phenotype. In addition, clear sex-specific disease prevalence was observed in different studies. Consequently, more research is essential for the understanding of the etiology and pathophysiology of lean T2D, which might help to develop better disease prevention and treatment strategies. This review examines the role of protein insufficiency in the maternal diet as the central driver of the developmental programming of lean T2D.
Keywords: Type 2 diabetes, Maternal low protein diet, Fetal programming, Lean diabetes, Developmental origin of health and disease
Core Tip: This is to review the role of maternal low protein diet and its metabolic impact on the offspring leading to lean type 2 diabetes.
INTRODUCTION
Type 2 diabetes (T2D) is a metabolic disease, which is rapidly increasing among the human population both in developed as well as in developing countries. Diabetes is divided into four major categories: type1, type2, gestational, and other specific diabetes mellitus[1]. As per the national diabetes statistics reports in 2020, 10.5 % (34.2 million) of the United States population are diagnosed with some form of diabetes and 34.5% (88 million) of adults have pre-diabetes. T2D constitutes 90%-95% of all diabetes cases in the United States[2]. The hallmarks of T2D are insulin resistance and insulin deficiency. In most cases of T2D, the etiology of insulin resistance and insulin deficiency can be traced back to obesity and lifestyle aspects.
Scientists from all over the world have spent a great deal of time and effort to understand the causes and consequences of obesity-induced T2D. However, the number of non-obese/lean T2D cases has also dramatically increased globally and especially in Asia and other developing countries. Recent estimates show that 10%-20% of T2D patients are not obese[3]. Although the etiology is not clearly understood, lean T2D is clustered under the umbrella of T2D for patient care. Interestingly, clues from various studies indicate that lean T2D is often observed in the populations where the fetus is exposed to malnutrition during the intrauterine period or early childhood[3-7]. Adequate birth weight and size of the newborn are often considered as indicators of appropriate fetal growth rate and optimal in utero environment[8-10]. In contrast, deprivation of nutrition during fetal development is often marked by low birth weight and it is linked with adult-onset of metabolic diseases such as T2D[11].
Historically, observational studies from the different parts of the world firmly indicate that the individuals exposed to in utero malnutrition due to famine were more prone to the hyperglycemic condition in adult life compared to those who are not born during a famine[12]. For instance, the cohort exposed to Dutch famine (1944-45) in utero were more prone to glucose intolerance than the non-exposure cohort[13]. Similarly, data associated with the Ukrainian famine of 1932–33 have exhibited a higher incidence of T2D among the people born in the famine-affected region than in the regions where no famine was reported[14]. The link between Australian famines and T2D was studied and analysis showed a positive correlation with three years of famine and an increased number of T2D among those who were born during the famine years[12]. Further, Li et al[15] reported that people who were exposed to the Chinese famine in the fetal stage during 1959-1961 were more prone to hyperglycemia and T2D in their adult life, compared to those who were born after this period.
Recent studies indicate that adverse in utero nutrition could cause lean T2D later in life among certain ethnic and socio-economical groups, and people with certain lifestyles. Studies from India[16] (up to 26%) and Caribbean islands[17,18] (5%) report the predominance of lean T2D population. A study on American minorities showed that 13% of T2D patients are lean[4,16] with a fivefold higher incidence in Asians[4]. These observations clearly show that not all diabetics are obese and obesity does not necessarily cause T2D[3,16,19]. With > 42 million Americans experiencing food insecurity, it is a major problem even in the United States especially among the economically disadvantaged[20]. WHO estimates that 1.1 million children had ≤ 2 standard deviations for weight for height ratio (an index of protein-energy malnutrition) in the United States[21]. A recent German study shows that 38% of pregnant women did not consume enough protein[22]. With vegetarian and vegan diets gaining popularity worldwide, low protein intake is more prevalent as these diets are often low in protein[23,24]. Vegetarian mothers consume low protein diet[25,26] and give birth to children with lower birth weights, thus making them susceptible to T2D[26-28].
This atypical diabetic phenotype is known by various names such as Jamaica type diabetes, metabolically obese normal weight (MONW) diabetes, malnutrition-related diabetes mellitus, phasic insulin-dependent diabetes, tropical diabetes, mixed onset type diabetes, J, Z, M or type 3 diabetes, and ketosis resistant growth onset type diabetes[3,17,18,29-33]. This concept of MONW individuals was first proposed in 1981[29] with subsequent validations in animal and human studies[34-37] and the existence of lean T2D has been observed for decades but the etiology and pathophysiology of lean T2D are poorly understood.
The most accepted and validated hypothesis that explains the link between early nutrition and metabolic diseases in adulthood was proposed by David Barker is called, ‘thrifty phenotype hypothesis’[11,38]. This hypothesis explains how impaired in utero nutrition availability results in compromised fetal growth and programs subcellular and metabolic effects in the developing fetus. Further, the hypothesis suggests that the metabolic fate of an individual predisposed to T2D is decided at the early developmental stage and thus attempts to explain why a sub-population of individuals born with low birth weight are more prone to lean T2D compare to normal birth weight individuals[11]. Various subsequent studies have confirmed the reproducibility and epidemiological evidence for the 'thrifty phenotype hypothesis'[39].
In addition, studies based on this hypothesis showed the importance of a sufficient amount of protein in the maternal diet for the development of the fetus and the risk of diseases in adulthood[40]. Although overall well-balanced nutrition is essential for a developing fetus and a healthy offspring, the role of protein is vital in the developmental programming paradigm[41]. A low protein diet is well known to cause various programming effects leading to metabolic disorders in adulthood. Low protein or vegetarian diets are consumed due to various reasons such as poverty, famine, lack of availability, cultural, religious, or moral reasons, personal preference, etc. Although these are common in the developing world, the recent popularity of vegetarian and vegan diets in the developed world is also an important paradigm to be considered. The emerging popularity of vegetarian and vegan diets among the maternal population might compromise growing fetuses, as the amount and bio-availability of proteins are found to be inadequate from plant sources[23,42]. A low protein diet during gestation is often connected with compromised renal function and impaired glucose metabolism[7]. However, the mechanistic basis and the exact patient phenotype of the maternal low protein associated with lean T2D are not well understood. Therefore, a clear understanding of the epidemiological and clinical features of lean T2D is essential to the prevention or treatment strategies.
PATHOPHYSIOLOGY OF LEAN T2D
T2D is a complex metabolic disease with a spectrum of presentations. It is therefore essential to understand the pathophysiology of the disease to offer appropriate prevention and treatment strategies. The pathophysiology of lean T2D is not well defined, although we and others have considered them as a separate subset of T2D[3,43,44] body mass index (BMI) is widely used as a tool to classify T2D patients. Patients with a BMI greater than 25 are considered to have obese T2D[45]. In contrast, the majority of lean T2D patients have a BMI of less than 25 but they have several metabolic characteristics associated with obesity[3]. Observational studies in humans and experiments in rodents suggested that the various environmental and genetic factors could contribute towards the lean T2D phenotype[3,46]. Poor in utero nutrition during fetal development is considered to be the main driver of lean T2D onset[44,46]. We have shown using a novel rat model that the maternal low protein diet is one of the critical causes of the lean T2D phenotype[43].
Although the genetic factors may vary among the different populations, genetic predisposition to fragile beta cells was found to be common in lean T2D patients. The rapid beta-cell apoptosis is the major pathophysiological characteristic of lean T2D compared to elevated insulin resistance in obese T2D[47]. Another interesting aspect that is noticed in this population is the prevalence of truncal obesity. Insulin sensitivity and insulin response are varied among the different ethnic groups (African, Caucasian, and East Asian), and East Asians have more vulnerable beta cells which make them more prone to T2D[48]. Several studies on the South East Asian population have shown that lean T2D patients have central obesity or elevated visceral fat deposits[49]. Even though lean T2D patients have lesser hyperglycemic values, their hemoglobin A1c levels are significantly higher than their obese counterparts[5,44]. Further, the onset of lean T2D is reported at an early age than the obesity-associated T2D[3]. Lean T2D patients showed a significantly lower incidence of hypertension and cardiovascular diseases compare to obese T2D patients but are more susceptible to peripheral neuropathy[3,5]. Apart from environmental and hereditary factors, socioeconomic background is found to be an important aspect of lean T2D prevalence[10,50]. Several studies have reported an inverse relationship between T2D and socioeconomic status[51]. The National Health and Nutrition Examination Survey data indicated this relationship of poverty and higher incidence of T2D among African and Mexican origins in the United States[52]. The Chicago cohort study further showed the prevalence of lean T2D among this minority community[4].
The two crucial characteristic features of developing countries, which make them more vulnerable to lean T2D, are a rapid shift in lifestyle and impaired nutrition. Studies from India have reported the escalating number of lean T2D cases across the country, especially, the urban population of India[53-55]. Similarly, Alemu and the group reported the increased number of lean T2D like cases in the urban population of sub-Saharan Africa[56].
GESTATIONAL LOW PROTEIN PROGRAMMING AND SEX DIFFERENCES IN LEAN T2D
Sex differences in fetal development can be observed as early as the pre-implantation phase[57]. There are major in utero differences between the sexes in growth and metabolic parameters leading to a faster fetal growth in males when compared to females. These differences are attributed to the genes expressed by sex-chromosomes and the actions of sex hormones[57-59]. In addition, differences in the incidence of T2D can also be attributed to the differences in the leptin and insulin sensitivity between sexes[59,60]. These metabolic hormones are influenced by the in utero nutritional environment[61]. Many studies have found a link between T2D and maternal low protein diet[43,59,62,63]. Further, as the nutritional environment often regulates the epigenetic machinery, any change in utero nutritional status may cause permanent alterations in the fetal gene expressions[64].
Our research using a lean T2D rat model indicates a clear sex difference in glucose homeostasis with females developing glucose intolerance earlier in life with faster disease progression than males[43,65]. These animals also showed differential regulation of gluconeogenesis and glycogenolysis as a result of gene expression changes in key genes involved in glucose metabolism[65-68]. Sex difference in hepatic genes associated with glucose homeostasis such as phosphoenolpyruvate carboxykinase (PEPCK) and 11β-hydroxysteroid dehydrogenase type 1 were observed even in low protein programmed fetuses[69]. Similarly, low protein programmed mice offspring were found to have lower birth weight with more glucose intolerant than the controls[70]. In this study, maternal low protein diet activated the visceral adipose tissue neuropeptide Y-Y2 receptor system in female offspring but not in male offspring, which increased abdominal adiposity and insulin resistance in female offspring[70]. This study indicates the importance of neuropeptide Y-Y2 receptor as a potential sex-specific marker and mediator of metabolic programming[70].
In the last decade, various studies have shown the importance of mitochondrial health and its relationship with glucose homeostasis in low protein programming. Zambrano and group have found maternal low protein diet-induced insulin resistance in male Wistar rats; however, females were responsive to glucose[71]. This study, further, suggested that elevated mitochondrial dysfunction in the pancreatic islets of adult male rats might be the mechanism that leads to insulin resistance[71]. Likewise, male offspring of low protein diet-fed mothers showed higher ROS production and impaired electron transport chain function in the mitochondria of the pancreatic islets when compared to female offspring indicating mitochondrial incompetence in males could predispose them to T2D[72]. Similarly, the sex dependent fetal programming in glucose metabolism was also reported in utero low protein programmed piglets. In this study, hepatic gluconeogenesis in newborn male piglets was negatively affected by the maternal low protein diet during pregnancy[73]. Epigenetic changes in the promoter region of the glucose-6-phosphatase gene were sex-specific and resulted in T2D in adult male pigs[73]. In addition, maternal low protein diet diminished liver mtDNA copy number in males and altered the OXPHOS protein expression by the combined binding action of glucocorticoid receptor and methylation of on the hepatic mtDNA promoter, which effect the mtDNA replication and gene expression levels[73,74].
Studies in humans suggest greater prevalence and impact of lean T2D in males than females. Many studies have indicated that women are physiologically inclined to have better insulin sensitivity than men[75-77]. Estrogen has a protective role in insulin sensitivity and glucose homeostasis by the inhibition of Foxo1 though activation of ERα-PI3K-Akt signaling[78]. Another crucial way estrogen protects women from insulin resistance is through mitochondrial biogenesis, as testosterone reduce mitochondrial proliferation[79]. The male preponderance of lean T2D was evident from the studies conducted in India, where more than 60% of lean T2D patients were men. Although the exact causes of sex differences are not clearly understood, it is suggested that the differences observed in this study may be due to predominant male exposure to oxidative stressors such as smoking and alcoholism[3,80].
Another interesting aspect to consider is the role of folate. Folate is routinely given to pregnant women throughout the world to prevent neural tube defect. Recent studies show that excessive folate can also have negative consequences at least in certain populations, ages and ethnicity/genetic background[81-83]. One study in Indian population shows that it can lead to insulin resistance[7]. The authors primarily attribute this to the deficiency of vitamin B12 which is primarily present in animal protein. Rat studies from our group showed that folate offered some protection in low protein programmed offspring by compensatory hyperinsulinemia but make insulin resistance worse in males[84]. Although the mechanism of this sex dependent folate action in insulin resistance is not known and warrant further research, it is important to note how folate may have sex dependent effects and this may also hold a clue in the sex differences that are observed in the human population.
ANIMAL MODELS
Considering the ethical and technical limitations in conducting impaired maternal nutrition and developmental programming studies in humans, various animal models that mimics several aspects of developmental programming have been developed. Due to the shorter lifetime and availability of genetic tools, a substantial amount of research is presently focused on developing clinically reliable rodent models of developmental programming.
To achieve a low protein diet model, the majority of studies followed a diet that has around a 50% reduction of total protein in the diet formulation[43,63,85-87]. However, most of the investigations conserved the isocaloric nature of the diet by manipulating macronutrient proportions by various lipid and carbohydrate ratios[88-93]. Although preferred protein, carbohydrate, and lipid ratio are varied among different research groups; a single research group often stick to one specific diet regimen[67,91,94-97]. The other central deviation apparent among the different low protein models is the timing and duration of the maternal diet management. Majority of the studies have started giving low protein diet from the first day of the pregnancy and continued throughout pregnancy or lactation, although some studies initiated the low protein diet before pregnancy or in some cases in a specific period of in utero growth[65,86,98-102]. The main aim of these refined diet manipulations is to develop a metabolically compromised adult offspring[103]. Moreover, many studies have succeeded in mirroring low birthweight and catch-up growth pattern, which is considered by many as a hallmark of the developmental origin of metabolic disease[104-108]. Pups from maternal low protein mothers weigh less compared to those from control diet-fed mothers. The differences in birth weight disappeared once the mothers were fed with a normal diet or pups were cross-fostered with control mothers. However, the weight differences were permanent, when the maternal low protein diet was continued throughout the weaning[43,100,109]. In addition, due to the variation in macronutrients ratio and the time regime of the diet, the adult metabolic phenotypes reported by various groups are also varied. Insulin resistance, obesity, cardiovascular diseases, and dyslipidemia are the major clinical disorders observed in these models[104,110-113]. A comprehensive list of different low protein programming animal models used are summarized in Table 1.
Table 1.
Summary of key animal models used to investigate the maternal low protein associated insulin resistance and glucose intolerance
|
Animals
|
Diet regimen
|
Age of pups
|
Sex
|
Observations
|
Ref.
|
| Sprague-Dawley rats | 6% protein, -12 to 43 d | 12 wk | Females | Sirt3 dysfunction in skeletal muscle | [138] |
| Sprague-Dawley rats | 10% protein, 2 to 21 d | Newborn | Males | Increased Igf gene expression | [148] |
| Sprague-Dawley rats | 8% protein, 1 to 43 d | 17 wk | Males | Lower fasting insulin and HOMA | [85] |
| Wistar rats | 6% protein, 1 to 21 d | 11 wk | Females | Insulin resistance and glucose Intolerance | [103] |
| Wistar rats | 6% protein, 1 to 43 d | 3 wk | Both | Compromised β-cell structure and function | [163] |
| Wistar rats | 7% protein, 1 to 120 d | 16 wk | Females | Higher glucose tolerance and insulin responsiveness | [98] |
| Wistar rats | 8% protein, 1 to 43 d | 12 wk | Both | Impaired gluconeogenesis, glucose handling and liver structure | [141] |
| Wistar rats | 8% protein, 1 to 43 d | 11 wk | Females | Insulin resistance and glucose Intolerance | [190] |
| Wistar rats | 8% protein, 1 to 21 d | 12 wk | Males | Epigenetic regulation of Hnf4a in islets | [116] |
| Wistar rats | 8% protein, 1 to 21 d | 12 wk | Both | Altered mitochondrial function in islets | [72] |
| Wistar rats | 8% protein, 1 to 21 d | 12 wk | Both | Structural alterations and changes in glucokinase expression in liver | [141] |
| Wistar rats | 8% protein, 1 to 21 d | Fetal Day 21.5 | Both | Altered IGF axis and proliferative capacity of liver | [140] |
| Wistar rats | 9% protein, 1 to 20 d | Fetal Day 20 | Both | Defective hepatic glucose homeostasis | [69] |
| Wistar rats | 10% protein, 1 to 21 d | 4 wk | Both | Impaired hepatic gene expression | [8,122] |
| Wistar rats | 10% protein, 1 to 43 d | 15 wk | Both | Modified glucose metabolism and insulin resistance | [71] |
| C57BL/6J mice | 9% protein, 1 to 39 d | 8 wk | Both | Impaired glucose metabolism, miR-15b up-regulation | [63] |
| C57BL/6J mice | 8% protein, 1 to 21 d | 3 wk | Both | Altered PPAR signaling, insulin resistance and glucose Intolerance | [87] |
| C57BL/6J mice | 8% protein, 1 to 19 d | Newborn | Both | Altered mitochondrial genes expression in liver and skeletal muscle | [89] |
| Mice | 8% protein, 1 to 40 d | 21 wk | Both | Increases abdominal adiposity and glucose intolerance | [70] |
| Pig | 6% protein, -18 to 113 d | Newborn | Both | Affected mitochondrial OXPHOS and glucose-6-phosphatase in liver | [73,74] |
PHYSIOLOGICAL EFFECTS AND MECHANISMS
Many animal models based on a low protein diet have been successful in capturing the phenotypic characteristics of fetal programming of adult metabolic diseases. However, the exact mechanism that leads to these metabolic diseases is not well studied. The dominant hypothesis in the field of developmental programming of adult diseases attribute that the fetal epigenome play a central role. This hypothesis postulates that epigenome is reprogrammed as an adaptation in response to a low protein diet, the associated low birth weight, and the catch-up growth. A recent study in Japanese adults indicates that the reduced beta cell mass in low-birth-weight individuals is directly associated with the future development of T2D[114]. Although the epigenome is prone to modification throughout the lifetime, in utero developmental period was found to be the most vulnerable time to be dysregulated by stressors[115].
Several studies have reported various key genes that are epigenetically modified as a result of developmental programming. For instance, the transcription factor Hnf4a was found to be epigenetically regulated during gestation, and the maternal diet-induced changes in the expression of this gene can cause T2D in adulthood[116]. Similarly, glucose transporter 4 (GLUT4) expression in skeletal is epigenetically controlled by maternal diet during early development and the impaired gene expression often resulted in peripheral insulin resistance[117]. Even though different biological mechanisms might contribute to fetal programming of lean T2D, many recent studies are indicating epigenetic changes as a potential single important driver of the fetal programming effects[118]. Low protein diet exposure during pregnancy in animals exhibited changes in methylation in promoter regions of genes involved in the glucose homeostasis pathway thereby, affecting the gene expression either directly or indirectly[119]. In recent years, many experimental studies in animals and observational studies in humans show that the epigenetic changes associated with gestational low protein are the main regulatory forces mediating the T2D phenotype[118,120]. Changes in the fetal epigenome often mirror the unique in utero environment of the fetus. Epigenetic changes due to gestational low protein arise through the methylation of cytosine in CpG Island present in the promoter region of particular genes, histone protein modification by acetylation, and regulation microRNAs by post-transcriptional modification. The chromatin structure and expression of a specific gene are regulated through DNA methylation in association with histone modifications[121].
A study in pigs found a significant decrease in glucocorticoid receptor binding to the glucose-6-phosphatase (G6PC) promoter which was accompanied by hypomethylation of the G6PC promoter in association with gestational low protein diet[74]. As G6PC is one of the crucial enzymes in glucose homeostasis that catalyzes gluconeogenesis and glycogenolysis, epigenetic changes in the promoter region might contribute to the onset of hyperglycemia[74]. Further, this impaired maternal diet-induced reduction of mtDNA copy number and methylation of mtDNA promoter often leads to changes in OXPHOS gene expression. This may predispose to insulin resistance in adult offspring considering the importance of hepatic mitochondrial OXPHOS activity in glucose homeostasis[73].
Similarly, using maternal low protein programmed rats, Lillycrop et al[8] established that the hepatic PPARα promoter and glucocorticoid receptors were hypomethylated in utero and these epigenetic changes were persistent in adulthood. Further studies demonstrated reduced Dnmt-1 expression and its role in epigenetic changes of glucocorticoid receptors[73,95,96,122]. Moreover, epigenetic changes in the promoter region of PEPCK were found to be the drawing force for impaired glucose homeostasis in animals[123,124]. Anandwardhan and colleagues reported a decreased number of (pro) insulin 2 gene transcripts in the pancreas of low protein in utero programmed rats, due to the histone modification in the promoter region of the insulin 2 gene[125]. Moreover, these epigenetic changes are potentially engaged in the trans-generational transmission of the induced phenotype[122,124,125].
Recently, Goyal and group demonstrated that the epigenetic modifications by miRNA, small non-coding RNAs consists of 20–22 nucleotides, is one of the molecular mechanisms of maternal low protein-induced T2D[119]. Results from maternal low protein programmed mice found reduced beta-cell mass and insulin levels in the pancreatic islets of the programmed offspring due to the increased expression of miR-15b. As the activities of cyclins are negatively regulated by the presence of miR-15b, the up-regulation of this miRNA may inhibit pancreatic beta-cell proliferation, consequently, stem to T2D phenotype[63]. A microarray study also demonstrated elevated expression of miR-615, miR-124, miR-376b, and decreased expression of miR-708 and miR-879 in maternal low protein programmed mice, which were associated with degenerated metabolic health of the offspring from the weaning age[126].
Apart from the epigenetic changes, maternal malnutrition is the major reason for low birth weight in newborns. Children who are small for gestation age and showed catch-up growth during the early age of development appeared to be more insulin resistant compared with normal-weight children[127]. Moreover, several studies have shown epigenetic changes due to gestational diet-induced fetal programming adult diseases in these offspring[108,128,129].
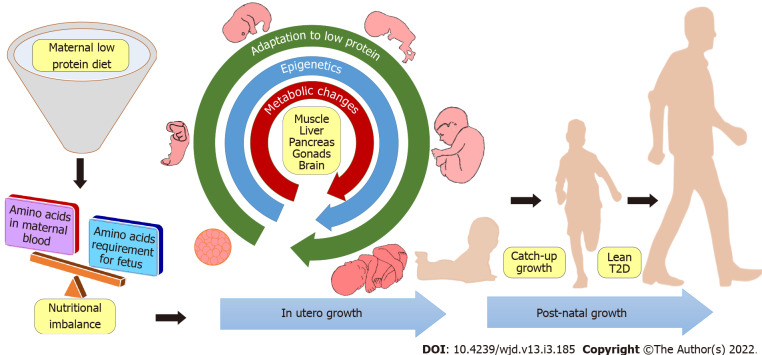
Even though little is known about the mechanism of programming, the secondary effects of fetal programming and their mechanisms are well studied. For example, various organ systems that play vital roles in the metabolism, and how they are affected by the developmental programming of T2D are well characterized. In utero low protein exposure causes long-lasting structural and functional changes in metabolically active organs includes skeletal muscle, liver, pancreas, gonads, and brain.
The in utero environment is crucial in the development of skeletal muscles, and the muscles growth is determined by the number, size, and type of muscle fibers formed during fetal development[130,131]. Maternal undernutrition affects the quality and quantity of skeletal muscles and stem cell activity[132-134]. A maternal low protein diet during gestation affects the normal proliferation and differentiation of bone marrow stem cells and satellite cell function[134,135]. Therefore, imperfections of skeletal muscles development during fetal development are often deleterious to normal muscle functions in adulthood[133]. Studies using maternal low protein diet-based animal models have reported lower expression of GLUT4 and mitochondrial dysfunction in skeletal muscles of low protein offspring[66,67,136-138]. As skeletal muscle functions as one of the main sites for peripheral glucose disposal, functional or structural changes of the myofibrils leads to insulin resistance and glucose intolerance[139].
Similarly, low protein-induced developmental programming caused functional and structural changes in the liver[140,141]. The expression of genes associated with oxidative phosphorylation and glucose metabolism were altered in the liver. Further, in utero low protein exposed rat fetuses showed the altered structure of the liver with decreased proliferation of hepatocytes[142-146]. These animals also had altered hepatic lipid metabolism and hepatic desaturase activities, which may account for fetal growth retardation and insulin resistance[94,147]. In addition, a maternal low protein diet also induces epigenetic changes in methyltransferase machinery resulting in altered epigenetic regulation in the liver[148]. Although further studies are warranted, it is clear from the existing studies that developmental programming induced by a low protein diet affects hepatic structure and function and this may, in turn, make them susceptible to impaired glucose metabolism[141,145,149].
The ability of the pancreatic β-cell to secret insulin is dependent on its structural and functional integrity along with the nutritional availability[150]. Consequently, protein deficiency in the maternal diet is a definite contributor to reduced insulin secretion and decreased β-cell proliferation in low protein programmed animals[151]. The reduced islets area and β cell number are mainly due to the downregulation of genes FoxO1 and Pdx1 genes, or altered expression of Reg1 pathway genes[151-155]. Epigenetic regulation of Hnf4a expression and expression of microRNAs such as miR-15b, miR-199a-3p, and miR 342, and signaling of mTOR in islets of the progeny also found to be associated with low protein-induced beta-cell dysfunction[62,63,156]. Further, a maternal low protein diet demonstrated greater β-cell apoptosis rates and deviates the equilibrium of islet’s apoptosis and replication in the offspring[157-159]. The pancreatic islet cells of these offspring exhibited greater oxidative stress and mitochondrial dysfunction[72,160]. Consequently, lower β-cell reserve, β-cell dysfunction and impaired mitochondrial function in islets may drive towards T2D later in life[62,72,86,155]. With the multiple pathways controlling β-cell functions are modulated by maternal low protein, it is reasonable to hypothesize that the low protein exposure predisposed the offspring to lean T2D[127]. A list of key genes involved in low protein programming is compiled in Figure 1.
Figure 1.
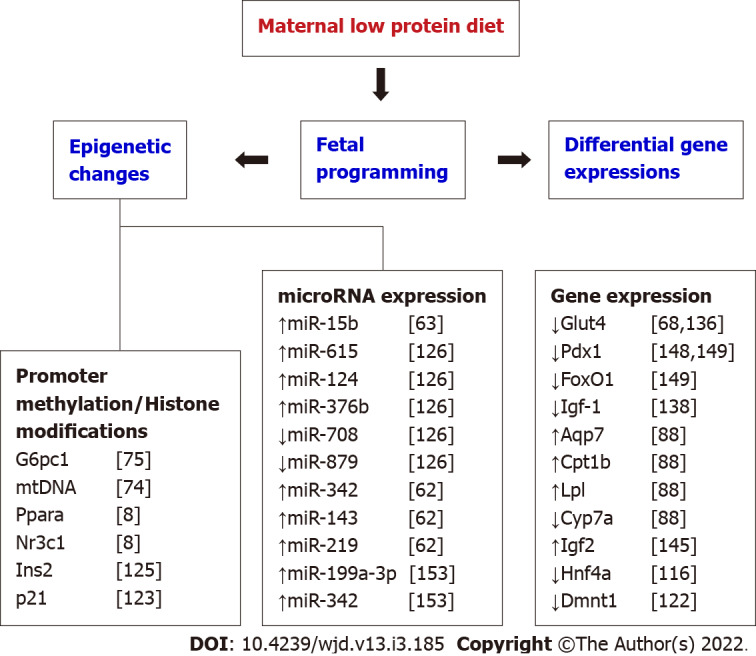
Key gene expression and epigenetic changes observed in different maternal low protein studies.
A balanced in utero nutrition is essential for the normal development of the reproductive system. Epidemiological studies in humans and experimental in studies animals show the low protein/ unbalanced diet in utero severely impacts the development of reproductive organs, sexual maturation, and reproductive function in the offspring[161-164], resulting in decreased testis weight, reduced Sertoli cell numbers, and late-onset of spermatogenesis in males[164-166]. Moreover, the classical male fertility markers, sperm count, and serum testosterone were also diminished in the offspring of low protein exposed mothers[163,164,167]. Similarly, the low protein programmed female offspring were found to be with compromised follicle development and follicle health[168,169]. The numbers of primordial, primary, and secondary follicles were significantly reduced along with abnormal estrous cycle and redox homeostasis[170-172]. The thyroid hormone production and hypothalamic-pituitary-gonadal axis are also found to be affected by maternal low protein diet[173,174]. The impaired reproductive function of offspring may be due to the altered expression of genes associated with steroidogenesis, folliculogenesis, and steroid hormone receptors in gonads[175-178]. In addition, changes in the hypothalamic-pituitary-gonadal axis to low protein may have adverse effects on the normal development and function of gonads[168,179].
The hypothalamus of the brain plays a critical role in glucose homeostasis by controlling hepatic glucose production and peripheral glucose utilization. Therefore, functional, or structural alteration of hypothalamic neurons may often lead to the onset of T2D[180,181]. The low protein programmed progenies have exhibited structural and functional changes in the neuronal centers, hypothalamic nuclei which regulate metabolism and body weight[102,181]. In addition, maternal low protein can also differentially affect the hypothalamic-pituitary-gonadal axis depending on the timing of the impaired nutrition. Early gestational nutrition impairment has been shown to make the pituitary more sensitive to GnRH, resulting in reduced reproductive function[182]. Further, it also alters hypothalamic-pituitary-adrenal axis function by deregulating corticosterone-inducible enzymes and associated enzyme receptors[183]. Other reports also show that brain sparing may not be as effective during in utero low protein exposure leading to compromised brain development in the offspring along with long-lasting deterioration in cognitive and motor functions[184,185].
PREVENTION AND TREATMENT
Insulin resistance and glucose intolerance are the cardinal signs of T2D, and if not prevented, they may lead to severe diabetes complications later in life. Hepatocytes, skeletal muscles, and adipocytes are the major insulin-dependent tissues that participate in the disposal of peripheral glucose. Thus, improving the muscle sensitivity towards insulin and enhancing hepatic glucose homeostasis, along with managing body weight are the central focus of T2D treatment strategies. Among the different drugs that have been prescribed for leanT2D management, metformin is a widely used drug for treating lean T2D along with nutritional and lifestyle modification[186]. Over the past two decades, various randomized control trials conducted in many ethnic groups showed unambiguously that the prevention is feasible by drugs or lifestyle modification[187-190].
Most of the research on treatment or prevention of T2D has been done with obese individuals or animal models, even though 10%-16% of all T2D people have normal BMI. In addition, majority of the studies on the molecular mechanisms of prevention and reversal of T2D were performed in Caucasians. Consequently, it is essential to include other ethnic groups such as Southeast Asian and Chinese populations, which are more prone to diabetes at lower average BMIs or lean T2D compared with white Europeans[191]. Regulating body weight is critical in the management of T2D associated with overweight or obese patients. However, in the case of leanT2D, it seems that leaner patients have severe beta-cell failure than normal-weight patients[4]. Presently, it is not clear that the achievement of lower body weight will help to prevent or reverse the special variants of T2D such as lean T2D[3].
The first trial associated with lifestyle modification and/drug therapy was started in China with a follow-up period of 23 years[192] and many other studies have followed since. Other studies include: the American diabetes prevention program outcome study[193]; the Finnish diabetes prevention study[194]; and the Indian short message service study[195] revealed the influence of lifestyle modification can persist long after the termination of the active phase of the trial. Although lifestyle modifications have been recognized to be very effective, safe, and ideal strategy for prevention, the effectiveness of relative risk reduction through these strategies exhibited some variations among different ethnic populations[192-196]. A study conducted among the impaired insulin tolerant lean Indian population found that lifestyle modification alone prevented the diabetes onset, regardless of relatively low BMI and highly insulin-resistant characteristics of the population[197].
Although the underlying pathophysiology of lean T2D is not completely understood, many studies using the maternal low protein model have shown potential prevention approaches[157]. The most promising approach among them is associated with one-carbon metabolism and the molecules involved in it. Some studies have reported the effectiveness of folic acid supplementation as a preventive treatment against the adverse effects of fetal programming[198-200]. Similarly, Burdge and team reported that the folic acid supplementation reversed the maternal low protein-induced phenotype epigenetically in the offspring when treated during the juvenile-pubertal period[201]. In contrast, our study reported a partial inhibition of gestational low protein-induced glucose intolerance only in female rats, when the maternal low protein diet was supplemented with folic acid from day 4 of the pregnancy until delivery[65]. Similar to our data, Lillycrop and colleagues also reported the inefficiency of folic acid supplementation for the inhibition of gestational low protein-induced change in gene profile, although they found changes in the expression of genes associated with redox homeostasis[8]. An observational study from Pune, India (Pune Maternal Nutrition Study), noticed that when the mother was vitamin B12 deficient, high amount of folic acid intake was not enough to prevent the insulin resistance in the offspring[7]. However, the high protein to carbohydrate ratio in maternal diet was found to be effective in maintaining glucose homeostasis in the offspring[137]. Thus ensuring sufficient protein in the maternal diet is essential to prevent lean T2D.
CONCLUSION
In summary, lean T2D is a discrete subgroup of T2D with a set of specific clinical profiles. Atypical characteristics of leanness associated with insulin resistance needed to be dissected further for a better understanding of the etiology of the disease. As the progression of T2D may take many years in humans, the assessment and prevention studies with human subjects may also warrant many years. Therefore, the development of a well-defined animal model, which mirrors not only the pathophysiology of lean T2D but also the etiology of the disease, might be the most important step in this area of research. Nevertheless, there is a lack of a single animal model that can constitute all pathophysiological and etiological changes similar to humans. In addition, the severity of lean T2D is different between sexes, due to sex hormones and sex dependent expression of genes. Among different molecular mechanisms involved in the onset of lean T2D, the epigenetic underpinning of metabolism appears to be the most promising lead. Although the mechanism of developmental programming is currently not well characterized. With the current literature, it may be summarized that maternal low protein diet leads to diminished essential amino acids levels in the maternal circulation and consequently to the fetus. In such low protein environment, fetus is acclimatized and revises its growth and metabolic set points. This adaptation is thought to be due to the overall alteration of epigenetic and metabolic attributes of fetal energy homeostasis. Although these adaptations may be beneficial for the fetus, a nutritional mismatch with protein abundance in the adulthood often leads to metabolic derangements leading to diseases such as lean T2D. This concept is summarized in Figure 2. A better understanding of the molecular mechanisms of the disease may pave the way for more effective preventive and treatment strategies.
Figure 2.
Proposed mechanism of maternal low protein associated lean type 2 diabetes.
Obesity associated T2D is a serious public health problem throughout the developing and developed countries whereas nutritional deficiency especially protein deficiency is a major concern in under developed and developing countries. With studies showing a link between maternal protein consumption and T2D in offspring, it is essential to probe further and take action to avert a global crisis. Public health measures to alleviate poverty and access to nutritious and protein rich diet during pregnancy is essential to prevent lean T2D. Scientific understanding of the disease to prevent and treat T2D, along with effective health education and public policies can mitigate this growing global epidemic.
Footnotes
Conflict-of-interest statement: Authors have no conflict of interest.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 28, 2021
First decision: December 4, 2021
Article in press: February 10, 2022
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nie XQ, Saisho Y S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
Contributor Information
Vidyadharan Alukkal Vipin, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, TX 77030, United States.
Chellakkan Selvanesan Blesson, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, TX 77030, United States; Family Fertility Center, Texas Children's Hospital, Houston, TX 77030, United States. selvanes@bcm.edu.
Chandra Yallampalli, Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, TX 77030, United States.
References
- 1.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 2. National diabetes statistics report, 2020. [cited 28 Sep 2021] Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html .
- 3.George AM, Jacob AG, Fogelfeld L. Lean diabetes mellitus: An emerging entity in the era of obesity. World J Diabetes . 2015;6:613–620. doi: 10.4239/wjd.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman NJ, Miernik J, Philipson L, Fogelfeld L. Lean versus obese diabetes mellitus patients in the United States minority population. J Diabetes Complications . 2014;28:500–505. doi: 10.1016/j.jdiacomp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Das S, Fonseca V. Low bodyweight type 2 diabetes in India: clinical characteristics and pathophysiology. Diabetes Metab Syndr . 2009;3:60–66. [Google Scholar]
- 6.Kong X, Xing X, Hong J, Zhang X, Yang W. Genetic variants associated with lean and obese type 2 diabetes in a Han Chinese population: A case-control study. Medicine (Baltimore) 2016;95:e3841. doi: 10.1097/MD.0000000000003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV, Joshi N, Lubree HG, Deshpande VU, Rege SS, Fall CH. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia . 2008;51:29–38. doi: 10.1007/s00125-007-0793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillycrop KA, Rodford J, Garratt ES, Slater-Jefferies JL, Godfrey KM, Gluckman PD, Hanson MA, Burdge GC. Maternal protein restriction with or without folic acid supplementation during pregnancy alters the hepatic transcriptome in adult male rats. Br J Nutr . 2010;103:1711–1719. doi: 10.1017/S0007114509993795. [DOI] [PubMed] [Google Scholar]
- 9.Negrato CA, Gomes MB. Low birth weight: causes and consequences. Diabetol Metab Syndr . 2013;5:49. doi: 10.1186/1758-5996-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Thomas N, Grunnet LG, Poulsen P, Christopher S, Spurgeon R, Inbakumari M, Livingstone R, Alex R, Mohan VR, Antonisamy B, Geethanjali FS, Karol R, Vaag A, Bygbjerg IC. Born with low birth weight in rural Southern India: what are the metabolic consequences 20 years later? Eur J Endocrinol . 2012;166:647–655. doi: 10.1530/EJE-11-0870. [DOI] [PubMed] [Google Scholar]
- 11.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia . 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 12.de Rooij SR, Roseboom TJ, Painter RC. Famines in the last 100 years: implications for diabetes. Curr Diab Rep . 2014;14:536. doi: 10.1007/s11892-014-0536-7. [DOI] [PubMed] [Google Scholar]
- 13.Roseboom TJ, Van Der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Perceived health of adults after prenatal exposure to the Dutch famine. Paediatr Perinat Epidemiol. 2003;17:391–397. doi: 10.1046/j.1365-3016.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 14.Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:787–794. doi: 10.1016/S2213-8587(15)00279-X. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, Ma G, Hu FB. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59:2400–2406. doi: 10.2337/db10-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barma PD, Ranabir S, Prasad L, Singh TP. Clinical and biochemical profile of lean type 2 diabetes mellitus. Indian J Endocrinol Metab . 2011;15:S40–S43. doi: 10.4103/2230-8210.83061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugh-Jones P. Diabetes in Jamaica. Lancet . 1955;269:891–897. doi: 10.1016/s0140-6736(55)92530-7. [DOI] [PubMed] [Google Scholar]
- 18.Diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1985;727:1–113. [PubMed] [Google Scholar]
- 19.Perry JR, Voight BF, Yengo L, Amin N, Dupuis J, Ganser M, Grallert H, Navarro P, Li M, Qi L, Steinthorsdottir V, Scott RA, Almgren P, Arking DE, Aulchenko Y, Balkau B, Benediktsson R, Bergman RN, Boerwinkle E, Bonnycastle L, Burtt NP, Campbell H, Charpentier G, Collins FS, Gieger C, Green T, Hadjadj S, Hattersley AT, Herder C, Hofman A, Johnson AD, Kottgen A, Kraft P, Labrune Y, Langenberg C, Manning AK, Mohlke KL, Morris AP, Oostra B, Pankow J, Petersen AK, Pramstaller PP, Prokopenko I, Rathmann W, Rayner W, Roden M, Rudan I, Rybin D, Scott LJ, Sigurdsson G, Sladek R, Thorleifsson G, Thorsteinsdottir U, Tuomilehto J, Uitterlinden AG, Vivequin S, Weedon MN, Wright AF MAGIC; DIAGRAM Consortium; GIANT Consortium, Hu FB, Illig T, Kao L, Meigs JB, Wilson JF, Stefansson K, van Duijn C, Altschuler D, Morris AD, Boehnke M, McCarthy MI, Froguel P, Palmer CN, Wareham NJ, Groop L, Frayling TM, Cauchi S. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet . 2012;8:e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman-Jensen A, Rabbitt M, Gregory C, Singh A. Household food security in the United States, 2015. Economic Research Report . 2016:44. [Google Scholar]
- 21. Nutrition Landscape Information System (NLiS). [cited 28 Sep 2021]. Available from: https://www.who.int/teams/nutrition-and-food-safety/databases/nutrition-landscape-information-system .
- 22.Diemert A, Lezius S, Pagenkemper M, Hansen G, Drozdowska A, Hecher K, Arck P, Zyriax BC. Maternal nutrition, inadequate gestational weight gain and birth weight: results from a prospective birth cohort. BMC Pregnancy Childbirth . 2016;16:224. doi: 10.1186/s12884-016-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: a review. Public Health Nutr . 2012;15:2287–2294. doi: 10.1017/S1368980012000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarys P, Deliens T, Huybrechts I, Deriemaeker P, Vanaelst B, De Keyzer W, Hebbelinck M, Mullie P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients . 2014;6:1318–1332. doi: 10.3390/nu6031318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward RJ, Abraham R, McFadyen IR, Haines AD, North WR, Patel M, Bhatt RV. Assessment of trace metal intake and status in a Gujerati pregnant Asian population and their influence on the outcome of pregnancy. Br J Obstet Gynaecol. 1988;95:676–682. doi: 10.1111/j.1471-0528.1988.tb06529.x. [DOI] [PubMed] [Google Scholar]
- 26.Piccoli GB, Clari R, Vigotti FN, Leone F, Attini R, Cabiddu G, Mauro G, Castelluccia N, Colombi N, Capizzi I, Pani A, Todros T, Avagnina P. Vegan-vegetarian diets in pregnancy: danger or panacea? BJOG . 2015;122:623–633. doi: 10.1111/1471-0528.13280. [DOI] [PubMed] [Google Scholar]
- 27.Reddy S, Sanders TA, Obeid O. The influence of maternal vegetarian diet on essential fatty acid status of the newborn. Eur J Clin Nutr . 1994;48:358–368. [PubMed] [Google Scholar]
- 28.Campbell-Brown M, Ward RJ, Haines AP, North WR, Abraham R, McFadyen IR, Turnlund JR, King JC. Zinc and copper in Asian pregnancies--is there evidence for a nutritional deficiency? Br J Obstet Gynaecol. 1985;92:875–885. doi: 10.1111/j.1471-0528.1985.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 29.Ruderman NB, Schneider SH, Berchtold P. The "metabolically-obese," normal-weight individual. Am J Clin Nutr . 1981;34:1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramanyam A, Yajnik CS, Tandon N. Non-Traditional Forms of Diabetes Worldwide: Implications for Translational Investigation. In: Robertson RP. Translational Endocrinology & Metabolism: Type 2 Diabetes Update. Washington D.C.: The Endocrine Society; 2011, 43-68. [Google Scholar]
- 31.Morrison EY, Ragoobirsingh D, Thompson H, Fletcher C, Smith-Richardson S, McFarlane S, Pascoe K, DasGupta T, Fray JC. Phasic insulin dependent diabetes mellitus: manifestations and cellular mechanisms. J Clin Endocrinol Metab . 1995;80:1996–2001. doi: 10.1210/jcem.80.7.7608247. [DOI] [PubMed] [Google Scholar]
- 32.Cuisinier-Raynal JC, Ducorps M, Grandpierre G. Le diabète sucré tropical, un nouvel indicateur nutritionnel? Med Trop (Mars) 1985;45:179–184. [PubMed] [Google Scholar]
- 33.Hoet JJ, Tripathy BB. Report of the International Workshop on types of Diabetes Peculiar to the Tropics. Diabetes Care. 1996;19:1014. [PubMed] [Google Scholar]
- 34.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes . 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 35.St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care . 2004;27:2222–2228. doi: 10.2337/diacare.27.9.2222. [DOI] [PubMed] [Google Scholar]
- 36.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA . 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Indulekha K, Surendar J, Anjana RM, Geetha L, Gokulakrishnan K, Pradeepa R, Mohan V. Metabolic obesity, adipocytokines, and inflammatory markers in Asian Indians--CURES-124. Diabetes Technol Ther. 2015;17:134–141. doi: 10.1089/dia.2014.0202. [DOI] [PubMed] [Google Scholar]
- 38.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol . 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull . 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 40.Vaughan OR, Rosario FJ, Powell TL, Jansson T. Regulation of Placental Amino Acid Transport and Fetal Growth. Prog Mol Biol Transl Sci. 2017;145:217–251. doi: 10.1016/bs.pmbts.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Chmurzynska A. Fetal programming: link between early nutrition, DNA methylation, and complex diseases. Nutr Rev . 2010;68:87–98. doi: 10.1111/j.1753-4887.2009.00265.x. [DOI] [PubMed] [Google Scholar]
- 42.Shaikh MG, Anderson JM, Hall SK, Jackson MA. Transient neonatal hypothyroidism due to a maternal vegan diet. J Pediatr Endocrinol Metab . 2003;16:111–113. doi: 10.1515/jpem.2003.16.1.111. [DOI] [PubMed] [Google Scholar]
- 43.Blesson CS, Schutt AK, Balakrishnan MP, Pautler RG, Pedersen SE, Sarkar P, Gonzales D, Zhu G, Marini JC, Chacko SK, Yallampalli U, Yallampalli C. Novel lean type 2 diabetic rat model using gestational low-protein programming. Am J Obstet Gynecol . 2016;214:540.e1–540.e7. doi: 10.1016/j.ajog.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr . 2004;134:205–210. doi: 10.1093/jn/134.1.205. [DOI] [PubMed] [Google Scholar]
- 45.Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Gil MJ, Valentí V, Rotellar F, Ramírez B, Salvador J, Frühbeck G. Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI. Obesity (Silver Spring) . 2011;19:1439–1444. doi: 10.1038/oby.2011.36. [DOI] [PubMed] [Google Scholar]
- 46.Olaogun I, Farag M, Hamid P. The Pathophysiology of Type 2 Diabetes Mellitus in Non-obese Individuals: An Overview of the Current Understanding. Cureus . 2020;12:e7614. doi: 10.7759/cureus.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho YS, Chen CH, Hu C, Long J, Ong RT, Sim X, Takeuchi F, Wu Y, Go MJ, Yamauchi T, Chang YC, Kwak SH, Ma RC, Yamamoto K, Adair LS, Aung T, Cai Q, Chang LC, Chen YT, Gao Y, Hu FB, Kim HL, Kim S, Kim YJ, Lee JJ, Lee NR, Li Y, Liu JJ, Lu W, Nakamura J, Nakashima E, Ng DP, Tay WT, Tsai FJ, Wong TY, Yokota M, Zheng W, Zhang R, Wang C, So WY, Ohnaka K, Ikegami H, Hara K, Cho YM, Cho NH, Chang TJ, Bao Y, Hedman ÅK, Morris AP, McCarthy MI DIAGRAM Consortium; MuTHER Consortium, Takayanagi R, Park KS, Jia W, Chuang LM, Chan JC, Maeda S, Kadowaki T, Lee JY, Wu JY, Teo YY, Tai ES, Shu XO, Mohlke KL, Kato N, Han BG, Seielstad M. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet . 2011;44:67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care . 2013;36:1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohan V, Vijayaprabha R, Rema M, Premalatha G, Poongothai S, Deepa R, Bhatia E, Mackay IR, Zimmet P. Clinical profile of lean NIDDM in South India. Diabetes Res Clin Pract . 1997;38:101–108. doi: 10.1016/s0168-8227(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 50.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA . 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 51.Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 2002;53:891–895. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 52.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health . 2001;91:76–83. doi: 10.2105/ajph.91.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramachandran A, Snehalatha C. Current scenario of diabetes in India. J Diabetes . 2009;1:18–28. doi: 10.1111/j.1753-0407.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 54.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, Rao PV, Yajnik CS, Prasanna Kumar KM, Nair JD Diabetes Epidemiology Study Group in India (DESI) High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia . 2001;44:1094–1101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 55.Ramachandran A, Snehalatha C, Viswanathan V, Viswanathan M, Haffner SM. Risk of noninsulin dependent diabetes mellitus conferred by obesity and central adiposity in different ethnic groups: a comparative analysis between Asian Indians, Mexican Americans and Whites. Diabetes Res Clin Pract . 1997;36:121–125. doi: 10.1016/s0168-8227(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 56.Alemu S, Dessie A, Seid E, Bard E, Lee PT, Trimble ER, Phillips DI, Parry EH. Insulin-requiring diabetes in rural Ethiopia: should we reopen the case for malnutrition-related diabetes? Diabetologia . 2009;52:1842–1845. doi: 10.1007/s00125-009-1433-5. [DOI] [PubMed] [Google Scholar]
- 57.Gutiérrez-Adán A, Perez-Crespo M, Fernandez-Gonzalez R, Ramirez MA, Moreira P, Pintado B, Lonergan P, Rizos D. Developmental consequences of sexual dimorphism during pre-implantation embryonic development. Reprod Domest Anim . 2006;41 Suppl 2:54–62. doi: 10.1111/j.1439-0531.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, McClusky R, Itoh Y, Reue K, Arnold AP. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology . 2013;154:1092–1104. doi: 10.1210/en.2012-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dearden L, Bouret SG, Ozanne SE. Sex and gender differences in developmental programming of metabolism. Mol Metab . 2018;15:8–19. doi: 10.1016/j.molmet.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes . 2012;61:2833–2841. doi: 10.2337/db11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction . 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 62.Alejandro EU, Jo S, Akhaphong B, Llacer PR, Gianchandani M, Gregg B, Parlee SD, MacDougald OA, Bernal-Mizrachi E. Maternal low-protein diet on the last week of pregnancy contributes to insulin resistance and β-cell dysfunction in the mouse offspring. Am J Physiol Regul Integr Comp Physiol . 2020;319:R485–R496. doi: 10.1152/ajpregu.00284.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su Y, Jiang X, Li Y, Li F, Cheng Y, Peng Y, Song D, Hong J, Ning G, Cao Y, Wang W. Maternal Low Protein Isocaloric Diet Suppresses Pancreatic β-Cell Proliferation in Mouse Offspring via miR-15b. Endocrinology . 2016;157:4782–4793. doi: 10.1210/en.2016-1167. [DOI] [PubMed] [Google Scholar]
- 64.Edelmann MN, Auger AP. Epigenetic impact of simulated maternal grooming on estrogen receptor alpha within the developing amygdala. Brain Behav Immun . 2011;25:1299–1304. doi: 10.1016/j.bbi.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blesson CS, Schutt A, Chacko S, Marini JC, Mathew PR, Tanchico D, Balakrishnan M, Yallampalli C. Sex Dependent Dysregulation of Hepatic Glucose Production in Lean Type 2 Diabetic Rats. Front Endocrinol (Lausanne) . 2019;10:538. doi: 10.3389/fendo.2019.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blesson CS, Chinnathambi V, Kumar S, Yallampalli C. Gestational Protein Restriction Impairs Glucose Disposal in the Gastrocnemius Muscles of Female Rats. Endocrinology . 2017;158:756–767. doi: 10.1210/en.2016-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blesson CS, Sathishkumar K, Chinnathambi V, Yallampalli C. Gestational protein restriction impairs insulin-regulated glucose transport mechanisms in gastrocnemius muscles of adult male offspring. Endocrinology . 2014;155:3036–3046. doi: 10.1210/en.2014-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blesson CS, Schutt AK, Vipin VA, Tanchico DT, Mathew PR, Balakrishnan M, Betancourt A, Yallampalli C. In utero low-protein-diet-programmed type 2 diabetes in adult offspring is mediated by sex hormones in rats†. Biol Reprod . 2020;103:1110–1120. doi: 10.1093/biolre/ioaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwong WY, Miller DJ, Wilkins AP, Dear MS, Wright JN, Osmond C, Zhang J, Fleming TP. Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol Reprod Dev . 2007;74:48–56. doi: 10.1002/mrd.20606. [DOI] [PubMed] [Google Scholar]
- 70.Han R, Li A, Li L, Kitlinska JB, Zukowska Z. Maternal low-protein diet up-regulates the neuropeptide Y system in visceral fat and leads to abdominal obesity and glucose intolerance in a sex- and time-specific manner. FASEB J. 2012;26:3528–3536. doi: 10.1096/fj.12-203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zambrano E, Bautista CJ, Deás M, Martínez-Samayoa PM, González-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol . 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theys N, Bouckenooghe T, Ahn MT, Remacle C, Reusens B. Maternal low-protein diet alters pancreatic islet mitochondrial function in a sex-specific manner in the adult rat. Am J Physiol Regul Integr Comp Physiol . 2009;297:R1516–1525. doi: 10.1152/ajpregu.00280.2009. [DOI] [PubMed] [Google Scholar]
- 73.Jia Y, Li R, Cong R, Yang X, Sun Q, Parvizi N, Zhao R. Maternal low-protein diet affects epigenetic regulation of hepatic mitochondrial DNA transcription in a sex-specific manner in newborn piglets associated with GR binding to its promoter. PLoS One. 2013;8:e63855. doi: 10.1371/journal.pone.0063855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jia Y, Cong R, Li R, Yang X, Sun Q, Parvizi N, Zhao R. Maternal low-protein diet induces gender-dependent changes in epigenetic regulation of the glucose-6-phosphatase gene in newborn piglet liver. J Nutr. 2012;142:1659–1665. doi: 10.3945/jn.112.160341. [DOI] [PubMed] [Google Scholar]
- 75.Karakelides H, Irving BA, Short KR, O'Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes . 2010;59:89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Høeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol (1985) 2009;107:824–831. doi: 10.1152/japplphysiol.91382.2008. [DOI] [PubMed] [Google Scholar]
- 77.Vistisen B, Hellgren LI, Vadset T, Scheede-Bergdahl C, Helge JW, Dela F, Stallknecht B. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur J Endocrinol . 2008;158:61–68. doi: 10.1530/EJE-07-0493. [DOI] [PubMed] [Google Scholar]
- 78.Yan H, Yang W, Zhou F, Li X, Pan Q, Shen Z, Han G, Newell-Fugate A, Tian Y, Majeti R, Liu W, Xu Y, Wu C, Allred K, Allred C, Sun Y, Guo S. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes . 2019;68:291–304. doi: 10.2337/db18-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Capllonch-Amer G, Lladó I, Proenza AM, García-Palmer FJ, Gianotti M. Opposite effects of 17-β estradiol and testosterone on mitochondrial biogenesis and adiponectin synthesis in white adipocytes. J Mol Endocrinol. 2014;52:203–214. doi: 10.1530/JME-13-0201. [DOI] [PubMed] [Google Scholar]
- 80.Shrivastav M, Kharkwal N, Tiwari A, Gupta KK. A Cross Sectional Study of Type 2 Diabetes Mellitus Comparing Different Factors between Lean Body Weight, Non Obese and Obese Patients in Western Uttar Pradesh. Curr Med Res Opin . 2020;3:405–409. [Google Scholar]
- 81.Selhub J, Rosenberg IH. Excessive folic acid intake and relation to adverse health outcome. Biochimie . 2016;126:71–78. doi: 10.1016/j.biochi.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 82.Li Z, Gueant-Rodriguez RM, Quilliot D, Sirveaux MA, Meyre D, Gueant JL, Brunaud L. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin Nutr . 2018;37:1700–1706. doi: 10.1016/j.clnu.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Maruvada P, Stover PJ, Mason JB, Bailey RL, Davis CD, Field MS, Finnell RH, Garza C, Green R, Gueant JL, Jacques PF, Klurfeld DM, Lamers Y, MacFarlane AJ, Miller JW, Molloy AM, O'Connor DL, Pfeiffer CM, Potischman NA, Rodricks JV, Rosenberg IH, Ross SA, Shane B, Selhub J, Stabler SP, Trasler J, Yamini S, Zappalà G. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr . 2020;112:1390–1403. doi: 10.1093/ajcn/nqaa259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blesson CS, Schutt A, Mathew PR, Tanchico D, Balakrishnan M, Yallampalli U, Yallampalli C. Folate treatment partially reverses gestational low-protein diet-induced glucose intolerance and the magnitude of reversal is age and sex dependent. Nutrition . 2018;49:81–89. doi: 10.1016/j.nut.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arentson-Lantz EJ, Zou M, Teegarden D, Buhman KK, Donkin SS. Maternal high fructose and low protein consumption during pregnancy and lactation share some but not all effects on early-life growth and metabolic programming of rat offspring. Nutr Res. 2016;36:937–946. doi: 10.1016/j.nutres.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 86.Rodríguez-Trejo A, Ortiz-López MG, Zambrano E, Granados-Silvestre Mde L, Méndez C, Blondeau B, Bréant B, Nathanielsz PW, Menjivar M. Developmental programming of neonatal pancreatic β-cells by a maternal low-protein diet in rats involves a switch from proliferation to differentiation. Am J Physiol Endocrinol Metab . 2012;302:E1431–E1439. doi: 10.1152/ajpendo.00619.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Wang Z. Maternal protein restriction induces early-onset glucose intolerance and alters hepatic genes expression in the peroxisome proliferator-activated receptor pathway in offspring. J Diabetes Investig . 2015;6:269–279. doi: 10.1111/jdi.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mesquita FF, Gontijo JA, Boer PA. Expression of renin-angiotensin system signalling compounds in maternal protein-restricted rats: effect on renal sodium excretion and blood pressure. Nephrol Dial Transplant . 2010;25:380–388. doi: 10.1093/ndt/gfp505. [DOI] [PubMed] [Google Scholar]
- 89.Mortensen OH, Olsen HL, Frandsen L, Nielsen PE, Nielsen FC, Grunnet N, Quistorff B. A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J Biomed Sci. 2010;17 Suppl 1:S38. doi: 10.1186/1423-0127-17-S1-S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ostreicher I, Almeida JR, Campean V, Rauh M, Plank C, Amann K, Dötsch J. Changes in 11beta-hydroxysteroid dehydrogenase type 2 expression in a low-protein rat model of intrauterine growth restriction. Nephrol Dial Transplant. 2010;25:3195–3203. doi: 10.1093/ndt/gfq354. [DOI] [PubMed] [Google Scholar]
- 91.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension in adult female rat offspring--influence of oestradiol. Br J Nutr . 2012;107:665–673. doi: 10.1017/S0007114511003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate . 1990;57:107–118. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- 93.Zhou D, Pan YX. Gestational low protein diet selectively induces the amino acid response pathway target genes in the liver of offspring rats through transcription factor binding and histone modifications. Biochim Biophys Acta . 2011;1809:549–556. doi: 10.1016/j.bbagrm.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 94.Ozanne SE, Martensz ND, Petry CJ, Loizou CL, Hales CN. Maternal low protein diet in rats programmes fatty acid desaturase activities in the offspring. Diabetologia . 1998;41:1337–1342. doi: 10.1007/s001250051074. [DOI] [PubMed] [Google Scholar]
- 95.Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr . 2008;100:278–282. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr . 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 97.Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res . 2001;2:139–143. doi: 10.1155/EDR.2001.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berleze KJ, Müller AP, Schweigert ID, Longoni A, Sordi F, de Assis AM, Rotta LN, de Souza DO, Perry ML. Gestational and postnatal low protein diet alters insulin sensitivity in female rats. Exp Biol Med (Maywood) . 2009;234:1437–1444. doi: 10.3181/0903-RM-111. [DOI] [PubMed] [Google Scholar]
- 99.Chen JH, Martin-Gronert MS, Tarry-Adkins J, Ozanne SE. Maternal protein restriction affects postnatal growth and the expression of key proteins involved in lifespan regulation in mice. PLoS One . 2009;4:e4950. doi: 10.1371/journal.pone.0004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qasem RJ, Cherala G, D'mello AP. Maternal protein restriction during pregnancy and lactation in rats imprints long-term reduction in hepatic lipid content selectively in the male offspring. Nutr Res . 2010;30:410–417. doi: 10.1016/j.nutres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Zheng S, Rollet M, Yang K, Pan YX. A gestational low-protein diet represses p21(WAF1/Cip1) expression in the mammary gland of offspring rats through promoter histone modifications. Br J Nutr . 2012;108:998–1007. doi: 10.1017/S0007114511006222. [DOI] [PubMed] [Google Scholar]
- 102.Crossland RF, Balasa A, Ramakrishnan R, Mahadevan SK, Fiorotto ML, Van den Veyver IB. Correction: Chronic Maternal Low-Protein Diet in Mice Affects Anxiety, Night-Time Energy Expenditure and Sleep Patterns, but Not Circadian Rhythm in Male Offspring. PLoS One . 2018;13:e0201079. doi: 10.1371/journal.pone.0201079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes . 1991;40 Suppl 2:115–120. doi: 10.2337/diab.40.2.s115. [DOI] [PubMed] [Google Scholar]
- 104.Aroutiounova N, Fandrich R, Kardami E, Tappia PS. Prenatal exposure to maternal low protein diet suppresses replicative potential of myocardial cells. Nutr Metab Cardiovasc Dis . 2009;19:707–712. doi: 10.1016/j.numecd.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 105.Alwasel SH, Kaleem I, Sahajpal V, Ashton N. Maternal protein restriction reduces angiotensin II AT(1) and AT(2) receptor expression in the fetal rat kidney. Kidney Blood Press Res . 2010;33:251–259. doi: 10.1159/000317739. [DOI] [PubMed] [Google Scholar]
- 106.Coupé B, Grit I, Darmaun D, Parnet P. The timing of "catch-up growth" affects metabolism and appetite regulation in male rats born with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol . 2009;297:R813–R824. doi: 10.1152/ajpregu.00201.2009. [DOI] [PubMed] [Google Scholar]
- 107.Cianfarani S, Germani D, Branca F. Low birthweight and adult insulin resistance: the "catch-up growth" hypothesis. Arch Dis Child Fetal Neonatal Ed . 1999;81:F71–F73. doi: 10.1136/fn.81.1.f71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J Clin Endocrinol Metab . 2002;87:4657–4661. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 109.Desai M, Crowther NJ, Lucas A, Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr . 1996;76:591–603. doi: 10.1079/bjn19960065. [DOI] [PubMed] [Google Scholar]
- 110.Gao H, Yallampalli U, Yallampalli C. Protein restriction to pregnant rats increases the plasma levels of angiotensin II and expression of angiotensin II receptors in uterine arteries. Biol Reprod . 2012;86:68. doi: 10.1095/biolreprod.111.095844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Augustyniak RA, Singh K, Zeldes D, Singh M, Rossi NF. Maternal protein restriction leads to hyperresponsiveness to stress and salt-sensitive hypertension in male offspring. Am J Physiol Regul Integr Comp Physiol . 2010;298:R1375–R1382. doi: 10.1152/ajpregu.00848.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Erhuma A, McMullen S, Langley-Evans SC, Bennett AJ. Feeding pregnant rats a low-protein diet alters the hepatic expression of SREBP-1c in their offspring via a glucocorticoid-related mechanism. Endocrine. 2009;36:333–338. doi: 10.1007/s12020-009-9225-8. [DOI] [PubMed] [Google Scholar]
- 113.Sohi G, Marchand K, Revesz A, Arany E, Hardy DB. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7alpha-hydroxylase promoter. Mol Endocrinol. 2011;25:785–798. doi: 10.1210/me.2010-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sasaki H, Saisho Y, Inaishi J, Watanabe Y, Tsuchiya T, Makio M, Sato M, Kitago M, Yamada T, Itoh H. Associations of birthweight and history of childhood obesity with beta cell mass in Japanese adults. Diabetologia . 2020;63:1199–1210. doi: 10.1007/s00125-020-05127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect . 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sandovici I, Smith NH, Nitert MD, Ackers-Johnson M, Uribe-Lewis S, Ito Y, Jones RH, Marquez VE, Cairns W, Tadayyon M, O'Neill LP, Murrell A, Ling C, Constância M, Ozanne SE. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci U S A . 2011;108:5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J Biol Chem . 2008;283:13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stevenson K, Lillycrop KA, Silver MJ. Fetal programming and epigenetics. Curr Opin Endocr Metab Res . 2020;13:1–6. [Google Scholar]
- 119.Goyal D, Limesand SW, Goyal R. Epigenetic responses and the developmental origins of health and disease. J Endocrinol. 2019;242:T105–T119. doi: 10.1530/JOE-19-0009. [DOI] [PubMed] [Google Scholar]
- 120.Vickers MH. Developmental programming of the metabolic syndrome - critical windows for intervention. World J Diabetes . 2011;2:137–148. doi: 10.4239/wjd.v2.i9.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med . 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr . 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol . 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 124.Hoile SP, Lillycrop KA, Thomas NA, Hanson MA, Burdge GC. Dietary protein restriction during F0 pregnancy in rats induces transgenerational changes in the hepatic transcriptome in female offspring. PLoS One . 2011;6:e21668. doi: 10.1371/journal.pone.0021668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hardikar AA, Satoor SN, Karandikar MS, Joglekar MV, Puranik AS, Wong W, Kumar S, Limaye A, Bhat DS, Januszewski AS, Umrani MR, Ranjan AK, Apte K, Yajnik P, Bhonde RR, Galande S, Keech AC, Jenkins AJ, Yajnik CS. Multigenerational Undernutrition Increases Susceptibility to Obesity and Diabetes that Is Not Reversed after Dietary Recuperation. Cell Metab. 2015;22:312–319. doi: 10.1016/j.cmet.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 126.Zheng J, Xiao X, Zhang Q, Wang T, Yu M, Xu J. Maternal Low-Protein Diet Modulates Glucose Metabolism and Hepatic MicroRNAs Expression in the Early Life of Offspring †. Nutrients . 2017;9 doi: 10.3390/nu9030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mericq V, Martinez-Aguayo A, Uauy R, Iñiguez G, Van der Steen M, Hokken-Koelega A. Long-term metabolic risk among children born premature or small for gestational age. Nat Rev Endocrinol. 2017;13:50–62. doi: 10.1038/nrendo.2016.127. [DOI] [PubMed] [Google Scholar]
- 128.Soto N, Bazaes RA, Peña V, Salazar T, Avila A, Iñiguez G, Ong KK, Dunger DB, Mericq MV. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab. 2003;88:3645–3650. doi: 10.1210/jc.2002-030031. [DOI] [PubMed] [Google Scholar]
- 129.Deng HZ, Li YH, Su Z, Ma HM, Huang YF, Chen HS, Du ML. Association between height and weight catch-up growth with insulin resistance in pre-pubertal Chinese children born small for gestational age at two different ages. Eur J Pediatr . 2011;170:75–80. doi: 10.1007/s00431-010-1274-8. [DOI] [PubMed] [Google Scholar]
- 130.Wigmore PM, Stickland NC. Muscle development in large and small pig fetuses. J Anat . 1983;137 (Pt 2):235–245. [PMC free article] [PubMed] [Google Scholar]
- 131.Patel HP, Jameson KA, Syddall HE, Martin HJ, Stewart CE, Cooper C, Sayer AA. Developmental influences, muscle morphology, and sarcopenia in community-dwelling older men. J Gerontol A Biol Sci Med Sci. 2012;67:82–87. doi: 10.1093/gerona/glr020. [DOI] [PubMed] [Google Scholar]
- 132.Confortim HD, Jerônimo LC, Centenaro LA, Felipe Pinheiro PF, Brancalhão RM, Michelin Matheus SM, Torrejais MM. Effects of aging and maternal protein restriction on the muscle fibers morphology and neuromuscular junctions of rats after nutritional recovery. Micron . 2015;71:7–13. doi: 10.1016/j.micron.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 133.Jousse C, Muranishi Y, Parry L, Montaurier C, Even P, Launay JM, Carraro V, Maurin AC, Averous J, Chaveroux C, Bruhat A, Mallet J, Morio B, Fafournoux P. Perinatal protein malnutrition affects mitochondrial function in adult and results in a resistance to high fat diet-induced obesity. PLoS One . 2014;9:e104896. doi: 10.1371/journal.pone.0104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oreffo RO, Lashbrooke B, Roach HI, Clarke NM, Cooper C. Maternal protein deficiency affects mesenchymal stem cell activity in the developing offspring. Bone . 2003;33:100–107. doi: 10.1016/s8756-3282(03)00166-2. [DOI] [PubMed] [Google Scholar]
- 135.Raja JS, Hoffman ML, Govoni KE, Zinn SA, Reed SA. Restricted maternal nutrition alters myogenic regulatory factor expression in satellite cells of ovine offspring. Animal . 2016;10:1200–1203. doi: 10.1017/S1751731116000070. [DOI] [PubMed] [Google Scholar]
- 136.Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol . 2006;575:241–250. doi: 10.1113/jphysiol.2006.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Metges CC, Görs S, Lang IS, Hammon HM, Brüssow KP, Weitzel JM, Nürnberg G, Rehfeldt C, Otten W. Low and high dietary protein:carbohydrate ratios during pregnancy affect materno-fetal glucose metabolism in pigs. J Nutr . 2014;144:155–163. doi: 10.3945/jn.113.182691. [DOI] [PubMed] [Google Scholar]
- 138.Claycombe KJ, Roemmich JN, Johnson L, Vomhof-DeKrey EE, Johnson WT. Skeletal muscle Sirt3 expression and mitochondrial respiration are regulated by a prenatal low-protein diet. J Nutr Biochem . 2015;26:184–189. doi: 10.1016/j.jnutbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 139.Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med . 2000;6:924–928. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]
- 140.El-Khattabi I, Grégoire F, Remacle C, Reusens B. Isocaloric maternal low-protein diet alters IGF-I, IGFBPs, and hepatocyte proliferation in the fetal rat. Am J Physiol Endocrinol Metab . 2003;285:E991–E1000. doi: 10.1152/ajpendo.00037.2003. [DOI] [PubMed] [Google Scholar]
- 141.Burns SP, Desai M, Cohen RD, Hales CN, Iles RA, Germain JP, Going TC, Bailey RA. Gluconeogenesis, glucose handling, and structural changes in livers of the adult offspring of rats partially deprived of protein during pregnancy and lactation. J Clin Invest . 1997;100:1768–1774. doi: 10.1172/JCI119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martin LJ, Meng Q, Blencowe M, Lagarrigue S, Xiao S, Pan C, Wier J, Temple WC, Devaskar SU, Lusis AJ, Yang X. Maternal High-Protein and Low-Protein Diets Perturb Hypothalamus and Liver Transcriptome and Metabolic Homeostasis in Adult Mouse Offspring. Front Genet . 2018;9:642. doi: 10.3389/fgene.2018.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ozanne SE, Smith GD, Tikerpae J, Hales CN. Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am J Physiol . 1996;270:E559–E564. doi: 10.1152/ajpendo.1996.270.4.E559. [DOI] [PubMed] [Google Scholar]
- 144.Ramadan WS, Alshiraihi I, Al-karim S. Effect of maternal low protein diet during pregnancy on the fetal liver of rats. Ann Anat . 2013;195:68–76. doi: 10.1016/j.aanat.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 145.Blesson CS, Schutt A, Chacko S, Marini J, Balakrishanan M, Yallampalli C. Sex Dependent Dysregulation of Hepatic Glucose Production in Gestational Low Protein Programmed Insulin Resistant Rat Offspring. Front Endocrinol. 2019 doi: 10.3389/fendo.2019.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mortensen OH, Olsen HL, Frandsen L, Nielsen PE, Nielsen FC, Grunnet N, Quistorff B. A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J Biomed Sci. 2010;17 Suppl 1:S38. doi: 10.1186/1423-0127-17-S1-S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Döring F, Lüersen K, Schmelzer C, Hennig S, Lang IS, Görs S, Rehfeldt C, Otten W, Metges CC. Influence of maternal low protein diet during pregnancy on hepatic gene expression signature in juvenile female porcine offspring. Mol Nutr Food Res . 2013;57:277–290. doi: 10.1002/mnfr.201200315. [DOI] [PubMed] [Google Scholar]
- 148.Gong L, Pan YX, Chen H. Gestational low protein diet in the rat mediates Igf2 gene expression in male offspring via altered hepatic DNA methylation. Epigenetics . 2010;5:619–626. doi: 10.4161/epi.5.7.12882. [DOI] [PubMed] [Google Scholar]
- 149.Rees WD, Hay SM, Cruickshank M, Reusens B, Remacle C, Antipatis C, Grant G. Maternal protein intake in the pregnant rat programs the insulin axis and body composition in the offspring. Metabolism . 2006;55:642–649. doi: 10.1016/j.metabol.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 150.Malaisse WJ, Sener A, Herchuelz A, Hutton JC. Insulin release: the fuel hypothesis. Metabolism . 1979;28:373–386. doi: 10.1016/0026-0495(79)90111-2. [DOI] [PubMed] [Google Scholar]
- 151.Dumortier O, Blondeau B, Duvillié B, Reusens B, Bréant B, Remacle C. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia. 2007;50:2495–2503. doi: 10.1007/s00125-007-0811-0. [DOI] [PubMed] [Google Scholar]
- 152.Zhang L, Chen W, Dai Y, Zhu Z, Liu Q. Detection of expressional changes induced by intrauterine growth restriction in the developing rat pancreas. Exp Biol Med (Maywood) . 2016;241:1446–1456. doi: 10.1177/1535370216638771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cox AR, Beamish CA, Carter DE, Arany EJ, Hill DJ. Cellular mechanisms underlying failed beta cell regeneration in offspring of protein-restricted pregnant mice. Exp Biol Med (Maywood) 2013;238:1147–1159. doi: 10.1177/1535370213493715. [DOI] [PubMed] [Google Scholar]
- 154.Sparre T, Reusens B, Cherif H, Larsen MR, Roepstorff P, Fey SJ, Mose Larsen P, Remacle C, Nerup J. Intrauterine programming of fetal islet gene expression in rats--effects of maternal protein restriction during gestation revealed by proteome analysis. Diabetologia . 2003;46:1497–1511. doi: 10.1007/s00125-003-1208-3. [DOI] [PubMed] [Google Scholar]
- 155.Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol . 2005;288:R368–R373. doi: 10.1152/ajpregu.00206.2004. [DOI] [PubMed] [Google Scholar]
- 156.Alejandro EU, Gregg B, Wallen T, Kumusoglu D, Meister D, Chen A, Merrins MJ, Satin LS, Liu M, Arvan P, Bernal-Mizrachi E. Maternal diet-induced microRNAs and mTOR underlie β cell dysfunction in offspring. J Clin Invest . 2014;124:4395–4410. doi: 10.1172/JCI74237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Merezak S, Hardikar AA, Yajnik CS, Remacle C, Reusens B. Intrauterine low protein diet increases fetal beta-cell sensitivity to NO and IL-1 beta: the protective role of taurine. J Endocrinol. 2001;171:299–308. doi: 10.1677/joe.0.1710299. [DOI] [PubMed] [Google Scholar]
- 158.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology . 1999;140:4861–4873. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- 159.Mateus Gonçalves L, Vettorazzi JF, Vanzela EC, Figueiredo MS, Batista TM, Zoppi CC, Boschero AC, Carneiro EM. Amino acid restriction increases β-cell death under challenging conditions. J Cell Physiol . 2019;234:16679–16684. doi: 10.1002/jcp.28389. [DOI] [PubMed] [Google Scholar]
- 160.Theys N, Clippe A, Bouckenooghe T, Reusens B, Remacle C. Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction. PLoS One . 2009;4:e6110. doi: 10.1371/journal.pone.0006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ajuogu PK, Al-Aqbi MAK, Hart RA, McFarlane JR, Smart NA. A low protein maternal diet during gestation has negative effects on male fertility markers in rats - A Systematic Review and Meta-analysis. J Anim Physiol Anim Nutr (Berl) . 2021;105:157–166. doi: 10.1111/jpn.13411. [DOI] [PubMed] [Google Scholar]
- 162.Fabozzi G, Iussig B, Cimadomo D, Vaiarelli A, Maggiulli R, Ubaldi N, Ubaldi FM, Rienzi L. The Impact of Unbalanced Maternal Nutritional Intakes on Oocyte Mitochondrial Activity: Implications for Reproductive Function. Antioxidants (Basel) . 2021;10 doi: 10.3390/antiox10010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rodríguez-González GL, Vigueras-Villaseñor RM, Millán S, Moran N, Trejo R, Nathanielsz PW, Larrea F, Zambrano E. Maternal protein restriction in pregnancy and/or lactation affects seminiferous tubule organization in male rat offspring. J Dev Orig Health Dis . 2012;3:321–326. doi: 10.1017/S2040174412000360. [DOI] [PubMed] [Google Scholar]
- 164.Zambrano E, Rodríguez-González GL, Guzmán C, García-Becerra R, Boeck L, Díaz L, Menjivar M, Larrea F, Nathanielsz PW. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol . 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kotsampasi B, Balaskas C, Papadomichelakis G, Chadio SE. Reduced Sertoli cell number and altered pituitary responsiveness in male lambs undernourished in utero. Anim Reprod Sci . 2009;114:135–147. doi: 10.1016/j.anireprosci.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 166.Oliveira JSd, Silva AADN, Magalhães CP, Souza SLd, Silva Junior VAd, Melo ENd. Protein restriction during intrauterine and lactation periods: effects on testicular development in pre-puberty rats. Acta Scientiarum Biological Sciences . 2015:37. [Google Scholar]
- 167.Oliveira JSd, Silva AAdN, Souza SLd, Morais RNd, Neves de Melo E, Maia FCL, Júnior VA. Histomorphometric evaluation of the testicular parenchyma of rats submittedto protein restriction during intrauterine and postnatal life. Turkish Journal of Biology 2017; 41: 428-438. [Google Scholar]
- 168.Guzmán C, Cabrera R, Cárdenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol . 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Winship AL, Gazzard SE, Cullen-McEwen LA, Bertram JF, Hutt KJ. Maternal low-protein diet programmes low ovarian reserve in offspring. Reproduction. 2018;156:299–311. doi: 10.1530/REP-18-0247. [DOI] [PubMed] [Google Scholar]
- 170.Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One . 2010;5:e15558. doi: 10.1371/journal.pone.0015558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Côrtes LS, Silveira HS, Lupi LA, de Mello Santos T, Cavariani MM, Domeniconi RF, Gaiotte LB, de Morais Oliveira DA, Justulin LA, de Almeida Chuffa LG. Maternal protein restriction impairs nutrition and ovarian histomorphometry without changing p38MAPK and PI3K-AKT-mTOR signaling in adult rat ovaries. Life Sci . 2021;264:118693. doi: 10.1016/j.lfs.2020.118693. [DOI] [PubMed] [Google Scholar]
- 172.Chan KA, Jazwiec PA, Gohir W, Petrik JJ, Sloboda DM. Maternal nutrient restriction impairs young adult offspring ovarian signaling resulting in reproductive dysfunction and follicle loss. Biol Reprod . 2018;98:664–682. doi: 10.1093/biolre/ioy008. [DOI] [PubMed] [Google Scholar]
- 173.Lisboa PC, Oliveira E, Fagundes AT, Santos-Silva AP, Conceição EP, Passos MC, Moura EG. Postnatal low protein diet programs leptin signaling in the hypothalamic-pituitary-thyroid axis and pituitary TSH response to leptin in adult male rats. Horm Metab Res . 2012;44:114–122. doi: 10.1055/s-0031-1299747. [DOI] [PubMed] [Google Scholar]
- 174.Guzmán C, García-Becerra R, Aguilar-Medina MA, Méndez I, Merchant-Larios H, Zambrano E. Maternal protein restriction during pregnancy and/or lactation negatively affects follicular ovarian development and steroidogenesis in the prepubertal rat offspring. Arch Med Res . 2014;45:294–300. doi: 10.1016/j.arcmed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 175.da Silva Faria T, de Bittencourt Brasil F, Sampaio FJ, da Fonte Ramos C. Effects of maternal undernutrition during lactation on estrogen and androgen receptor expressions in rat ovary at puberty. Nutrition . 2010;26:993–999. doi: 10.1016/j.nut.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 176.Barsky M, Merkison J, Hosseinzadeh P, Yang L, Bruno-Gaston J, Dunn J, Gibbons W, Blesson CS. Fetal programming of polycystic ovary syndrome: Effects of androgen exposure on prenatal ovarian development. J Steroid Biochem Mol Biol . 2021;207:105830. doi: 10.1016/j.jsbmb.2021.105830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Teixeira CV, Silandre D, de Souza Santos AM, Delalande C, Sampaio FJ, Carreau S, da Fonte Ramos C. Effects of maternal undernutrition during lactation on aromatase, estrogen, and androgen receptors expression in rat testis at weaning. J Endocrinol . 2007;192:301–311. doi: 10.1677/joe.1.06712. [DOI] [PubMed] [Google Scholar]
- 178.Sui S, Jia Y, He B, Li R, Li X, Cai D, Song H, Zhang R, Zhao R. Maternal Low-protein Diet Alters Ovarian Expression of Folliculogenic and Steroidogenic Genes and Their Regulatory MicroRNAs in Neonatal Piglets. Asian-Australas J Anim Sci . 2014;27:1695–1704. doi: 10.5713/ajas.2014.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Khorram O, Keen-Rinehart E, Chuang TD, Ross MG, Desai M. Maternal undernutrition induces premature reproductive senescence in adult female rat offspring. Fertil Steril . 2015;103:291–8.e2. doi: 10.1016/j.fertnstert.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shimazu T, Minokoshi Y. Systemic Glucoregulation by Glucose-Sensing Neurons in the Ventromedial Hypothalamic Nucleus (VMH) J Endocr Soc . 2017;1:449–459. doi: 10.1210/js.2016-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Plagemann A, Harder T, Rake A, Melchior K, Rohde W, Dörner G. Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams. J Nutr. 2000;130:2582–2589. doi: 10.1093/jn/130.10.2582. [DOI] [PubMed] [Google Scholar]
- 182.Kotsampasi B, Chadio S, Papadomichelakis G, Deligeorgis S, Kalogiannis D, Menegatos I, Zervas G. Effects of maternal undernutrition on the hypothalamic-pituitary-gonadal axis function in female sheep offspring. Reprod Domest Anim . 2009;44:677–684. doi: 10.1111/j.1439-0531.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 183.Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr . 1996;126:1578–1585. doi: 10.1093/jn/126.6.1578. [DOI] [PubMed] [Google Scholar]
- 184.Marwarha G, Claycombe-Larson K, Schommer J, Ghribi O. Maternal low-protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring. J Nutr Biochem . 2017;45:54–66. doi: 10.1016/j.jnutbio.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rushmore RJ, McGaughy JA, Mokler DJ, Rosene DL. The enduring effect of prenatal protein malnutrition on brain anatomy, physiology and behavior. Nutr Neurosci . 2020:1–8. doi: 10.1080/1028415X.2020.1859730. [DOI] [PubMed] [Google Scholar]
- 186.DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab . 1991;73:1294–1301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- 187.Backholer K, Peeters A, Herman WH, Shaw JE, Liew D, Ademi Z, Magliano DJ. Diabetes prevention and treatment strategies: are we doing enough? Diabetes Care . 2013;36:2714–2719. doi: 10.2337/dc12-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cefalu WT, Buse JB, Tuomilehto J, Fleming GA, Ferrannini E, Gerstein HC, Bennett PH, Ramachandran A, Raz I, Rosenstock J, Kahn SE. Update and Next Steps for Real-World Translation of Interventions for Type 2 Diabetes Prevention: Reflections From a Diabetes Care Editors' Expert Forum. Diabetes Care . 2016;39:1186–1201. doi: 10.2337/dc16-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ramachandran A, Snehalatha C. Diabetes prevention programs. Med Clin North Am . 2011;95:353–372, viii. doi: 10.1016/j.mcna.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 190.Merezak S, Reusens B, Renard A, Goosse K, Kalbe L, Ahn MT, Tamarit-Rodriguez J, Remacle C. Effect of maternal low-protein diet and taurine on the vulnerability of adult Wistar rat islets to cytokines. Diabetologia . 2004;47:669–675. doi: 10.1007/s00125-004-1357-z. [DOI] [PubMed] [Google Scholar]
- 191.Taylor R, Al-Mrabeh A, Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol . 2019;7:726–736. doi: 10.1016/S2213-8587(19)30076-2. [DOI] [PubMed] [Google Scholar]
- 192.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 193.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lindström J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, Uusitupa M, Tuomilehto J Finnish Diabetes Prevention Study (DPS) Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologia . 2013;56:284–293. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- 195.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 196.Nanditha A, Snehalatha C, Raghavan A, Vinitha R, Satheesh K, Susairaj P, Simon M, Selvam S, Ram J, Naveen Kumar AP, Godsland IF, Oliver N, Johnston DG, Ramachandran A. The post-trial analysis of the Indian SMS diabetes prevention study shows persistent beneficial effects of lifestyle intervention. Diabetes Res Clin Pract . 2018;142:213–221. doi: 10.1016/j.diabres.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 197.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia . 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 198.Chmurzynska A, Stachowiak M, Pruszynska-Oszmalek E. Maternal protein and folic acid intake during gestation does not program leptin transcription or serum concentration in rat progeny. Genes Nutr. 2012;7:217–222. doi: 10.1007/s12263-011-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Engeham SF, Haase A, Langley-Evans SC. Supplementation of a maternal low-protein diet in rat pregnancy with folic acid ameliorates programming effects upon feeding behaviour in the absence of disturbances to the methionine-homocysteine cycle. Br J Nutr. 2010;103:996–1007. doi: 10.1017/S0007114509992662. [DOI] [PubMed] [Google Scholar]
- 200.Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, Poston L, Hanson MA. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension . 2006;47:982–987. doi: 10.1161/01.HYP.0000215580.43711.d1. [DOI] [PubMed] [Google Scholar]
- 201.Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr . 2009;139:1054–1060. doi: 10.3945/jn.109.104653. [DOI] [PubMed] [Google Scholar]