Abstract
Treatment of glaucoma by intraocular pressure (IOP) reduction is typically accomplished through the administration of eye drops, the difficult and frequent nature of which contributes to extremely low adherence rates. Poor adherence to topical treatment regimens in glaucoma patients can lead to irreversible vision loss and increased treatment costs. Currently there are no approved treatments for glaucoma that address the inherent inefficiencies in drug delivery and patient adherence. Brimonidine tartrate (BT), a common glaucoma medication, requires dosing every 8–12 h, with up to 97% of patients not taking it as prescribed. This study provides proof-of-principle testing of a controlled release BT formulation. BT was encapsulated in poly(lactic-co-glycolic) acid microspheres and drug release was quantified using UV–Vis spectroscopy. For in vivo studies, rabbits were randomized to receive a single subconjunctival injection of blank (no drug) or BT-loaded microspheres or twice daily topical 0.2% BT drops. The microspheres released an average of 2.1 ± 0.37 μg BT/mg microspheres/day in vitro. In vivo, the percent decrease in IOP from baseline was significantly greater in the treated eye for both topical drug and drug-loaded microspheres versus blank microspheres throughout the 4-week study, with no evidence of migration or foreign body response. IOP measurements in the contralateral, untreated eyes also suggested a highly localized effect from the experimental treatment. A treatment designed using the release systems described in this study would represent a vast improvement over the current clinical standard of 56–84 topical doses over 28 days.
Keywords: glaucoma, microsphere, controlled release, brimonidine, rabbit, intraocular pressure, polymer
1. Introduction
Reducing intraocular pressure (IOP) in glaucoma patients is commonly accomplished by administration of medicated eye drops multiple times daily (Servat and Bernardino, 2011). The frequent dosing regimens, along with the difficulty of correctly administering eye drops, contribute to patient non-adherence rates ranging from 5 to 80% for ocular hypotensive treatments (Olthoff et al., 2005). A recent electronic monitoring study with twice or three times daily brimonidine tartrate (BT) showed that 97% of patients (65/67) had at least one >24 h interval between doses over 4 weeks; 46% had at least one >48 h interval(Hermann et al., 2011a). Poor response to medical glaucoma treatment, whether as a result of non-adherence or lack of efficacy, typically leads to more invasive interventions such as laser, filtering, or glaucoma drainage tube surgery (Mermoud et al., 1993). When partial vision loss or blindness results from complications due to glaucoma, the health care costs of treating the disease increase by at least 46% (Bramley et al., 2008).
Another significant issue with topical administration of glaucoma medication is the limited penetration and uptake into the affected areas of the eye, with only 1–7% of the entire administered dose reaching the aqueous humor (Ghate and Edelhauser, 2008). As a result, the amount given in each eye drop is significantly higher than the amount actually required for IOP reduction. In fact, up to 50% of the given dose is absorbed systemically by the lacrimal drainage system, nasal mucosa, and pharynx, causing systemic side effects and significantly affecting overall tolerability of the drug (Jarvinen et al., 1995). An ideal solution would efficiently deliver the medication directly to the ocular tissues, thereby reducing the concentration of drug needed per dose and, consequently, the degree of local and systemic side effects.
To increase compliance, select glaucoma medications have been explored in the context of sustained release formulations that would ideally aim to decrease the dosing frequency to less than once per day. One such medication is brimonidine tartrate, which in its aqueous form requires dosing as often as every 8 h and has been associated with an average 64% adherence rate (Hermann et al., 2011b). One group developed an intravitreal implant loaded with BT that was used to achieve statistically significant IOP reduction for one month in normotensive rabbits (Deokule et al., 2012). Natu et al. (2011) have also demonstrated over one month of IOP reduction in vivo using a 4 mm diameter by 1 mm thick dorzolamide-loaded subconjunctival implant (Natu et al., 2011). As an alternative to relatively large implants, Giarmoukakis et al. (2013) have developed biodegradable nanoparticles containing latanoprost for minimally invasive subconjunctival injection, achieving statistically significant hypotensive effects for up to 8 days in vivo(Giarmoukakis et al., 2013). This particular route is promising, as a recent study has shown that over 74% of patients surveyed would prefer this route over the rigorous demands of a topical eye drop regimen (Chong et al., 2013). Another advantage of the subconjunctival route is the avoidance of adverse effects associated with intravitreal implants, including cataract formation, retinal detachment, vitreous hemorrhage, and endophthalmitis (Messenger et al., 2013; Moisseiev et al., 2013).
Here we describe the fabrication and testing of a controlled release BT formulation intended for subconjunctival injection using a small gauge needle to avoid the potential complications of a larger implant while still achieving extended drug release. This subconjunctivally delivered microsphere formulation is capable of delivering sufficient levels of brimonidine to achieve IOP reduction steadily over 28 days using biocompatible, biodegradable poly(lactic-co-glycolic) acid (PLGA) microspheres (MS). PLGA has a formidable track record of FDA approval for a wide variety of indications including ocular delivery (Hunter and Lobo, 2011). In our pilot in vivo study, the BTMS showed no evidence of inflammation in the eyes due to the presence of the MS and achieved comparable IOP reduction to the current clinical standard BT eye drops versus the blank microspheres. BTMS administration also demonstrated significantly lower systemic uptake compared with topical BT drops, as indicated by a lack of response in the contralateral eye. The technology represented by the BTMS described here could provide the basis for a minimally invasive treatment system that provides therapeutic levels of BT for 28 days, reducing dosing frequency up to 84 fold.
2. Methods
All chemicals and reagents were of analytical grade and obtained from Fisher Scientific unless otherwise specified. Animal studies were carried out in accordance with the regulations of the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh and the guidelines of the Association for Research in Vision and Ophthalmology (ARVO).
2.1. Microsphere fabrication and characterization
Microspheres (MS) were fabricated using a standard double emulsion procedure (Sanchez et al., 1993; Zweers et al., 2006). Briefly, 200 mg of poly(lactic-co-glycolic) acid (MW 24–38 kDa, viscosity 0.32–0.44 dl/g; Sigma, St. Louis, MO) was mixed with 4 ml of dichloromethane (DCM) and 12.5 mg of an aqueous brimonidine tartrate (BT) solution (Santa Cruz Biotechnologies, Santa Cruz, CA). The drug/polymer solution was sonicated for 10 s (Sonics Vibra-Cell™) before homogenization in 60 ml 2% poly(vinyl alcohol) (PVA—MW ~25,000 Da, 98% hydrolyzed, Polysciences) for 1 min at approximately 7000 RPM (Silverson L4RT-A homogenizer). This double emulsion was then added to 80 ml of 1% PVA and allowed to mix for 3 h to evaporate any remaining DCM. MS were then washed four times by centrifuging for 5 min at 1000 RPM. The MS were resuspended in DI water and placed in a lyophilizer (Virtis Benchtop K freeze dryer, Gardiner, NY) operating at 70 m Torr for 48 h before being stored at −20 °C.
The shape and morphology of the MS was examined using a scanning electron microscope (SEM). Images were taken on the lyophilized blank and drug-loaded MS (BTMS) following gold sputter-coating using a JEOL 6335F Field Emission SEM (JEOL, Peabody, MA). Average microsphere diameter for a minimum of 10,000 MS was determined using volume impedance measurements on a Multisizer 3 Coulter Counter (Beckman Coulter, Indianapolis, IN).
To assess the in vitro drug release kinetics, known masses of lyophilized MS were suspended in phosphate buffered saline (PBS) and incubated at 37 °C. MS suspensions were centrifuged for 10 min at 1000 RPM after predetermined intervals of time and the supernatant was removed for analysis. Brimonidine concentration in PBS samples was measured via UV/Vis absorption using a SoftMax Pro 5 microplate reader (Molecular Devices, Sunnyvale, CA) at 320 nm. The MS aliquots were then resuspended in fresh PBS. The results for the BTMS are reported as the average of three release studies and their standard deviation. Any background signal obtained from the blank MS was subtracted from each measurement.
The maximum and minimum reported brimonidine concentrations reported in rabbit aqueous humor was used as a basis for comparison for in vitro release from the BTMS (Acheampong et al., 2002). Daily brimonidine release values were adjusted to match the mass of particles administered to rabbits in our in vivo studies and plotted against these values, along with the corresponding standard deviation.
2.2. In vivo studies
New Zealand white rabbits, approximately 3 months old, were randomized to receive either blank MS (no drug), BTMS, or 0.2% BT ophthalmic solution (Bausch & Lomb, Tampa, FL) prior to beginning the study, with five animals in each group. Three baseline IOP measurements were taken one day apart between 8am and 10am daily using the TonoVet tonometer (Friedman et al., 2004). (Icare, Finland; small animal setting was used) for one week prior to administering treatment.
On day 0, the right eye of rabbits in the blank or drug-loaded MS groups received a superior subconjunctival injection of 7.5 mg of MS suspended in 0.15 cc sterile saline on a 28G syringe. Rabbits in the BT drops group received a single drop of 0.2% BT solution in one eye twice a day for every day of the study between 8 and 9 am and again between 5 and 6 pm. The dosing volume was that of a standard drop, approximately 50 μl, administered by the same individual each time. The left eye remained untreated in all animals throughout the study.
IOP was measured on days 1, 7, 14, 21, and 28 again between 8 and 10 am using the TonoVet. For animals in the positive control group (topical drops), IOP was measured between a minimum of 30 min and a maximum of 60 min after drop instillation. Eyes were regularly checked for signs of infection or inflammation by instilling sodium fluorescein drops and examining with a portable slit lamp containing a cobalt blue light (Reichert Technologies, Depew, NY).
Animals were sacrificed on Day 28, and both treated and untreated eyes were enucleated for histological analysis. The eyes were fixed in formalin and embedded in paraffin prior to sectioning and staining with hematoxylin and eosin, periodic acid-Schiff (PAS), or Masson’s trichrome stain. All slides were analyzed for any evidence of intra- or extra- ocular abnormalities by a masked examiner.
2.3. Statistical analysis
One-way analysis of variance (ANOVA) was performed on baseline IOP measurements to determine statistically significant differences, if any, in starting IOP values for subsequent comparisons. We then calculated the percent change in IOP at each time point relative to the average baseline IOP for that group. One-way analysis of variance (ANOVA) was used to compare IOP data followed by post hoc testing using the Tukey–Kramer method to correct for multiple testing. Statistically significant differences were designated by a significance criterion at or below p < 0.05.
3. Results
3.1. Microspheres
A preliminary in vitro characterization of the MS was performed to confirm their suitability for use in a subconjunctival injection model prior to beginning assays of drug release. Although a formulation’s in vitro release behavior is not ipso facto analogous to how release would proceed in vivo, it can indeed be indicative of local or topical release scenarios and is, regardless, an important part of the overall characterization of a new, prototype formulation.
Fig. 1 shows scanning electron microscope (SEM) images of the brimonidine tartrate-loaded MS (BTMS). These images confirm that a smooth surface and uniform shape were achieved according to our design specifications. These images also agree with volume impedance measurements, which determined the volume average diameter of the BTMS to be 7.46 ± 2.86 μm. This size distribution is as expected for the conditions used to fabricate the BTMS. Ultimately, these MS are small enough to be easily injected with a 30-gauge needle while still being large enough to avoid phagocytic removal or migration from the site of injection (Shanbhag et al., 1994).
Fig. 1.
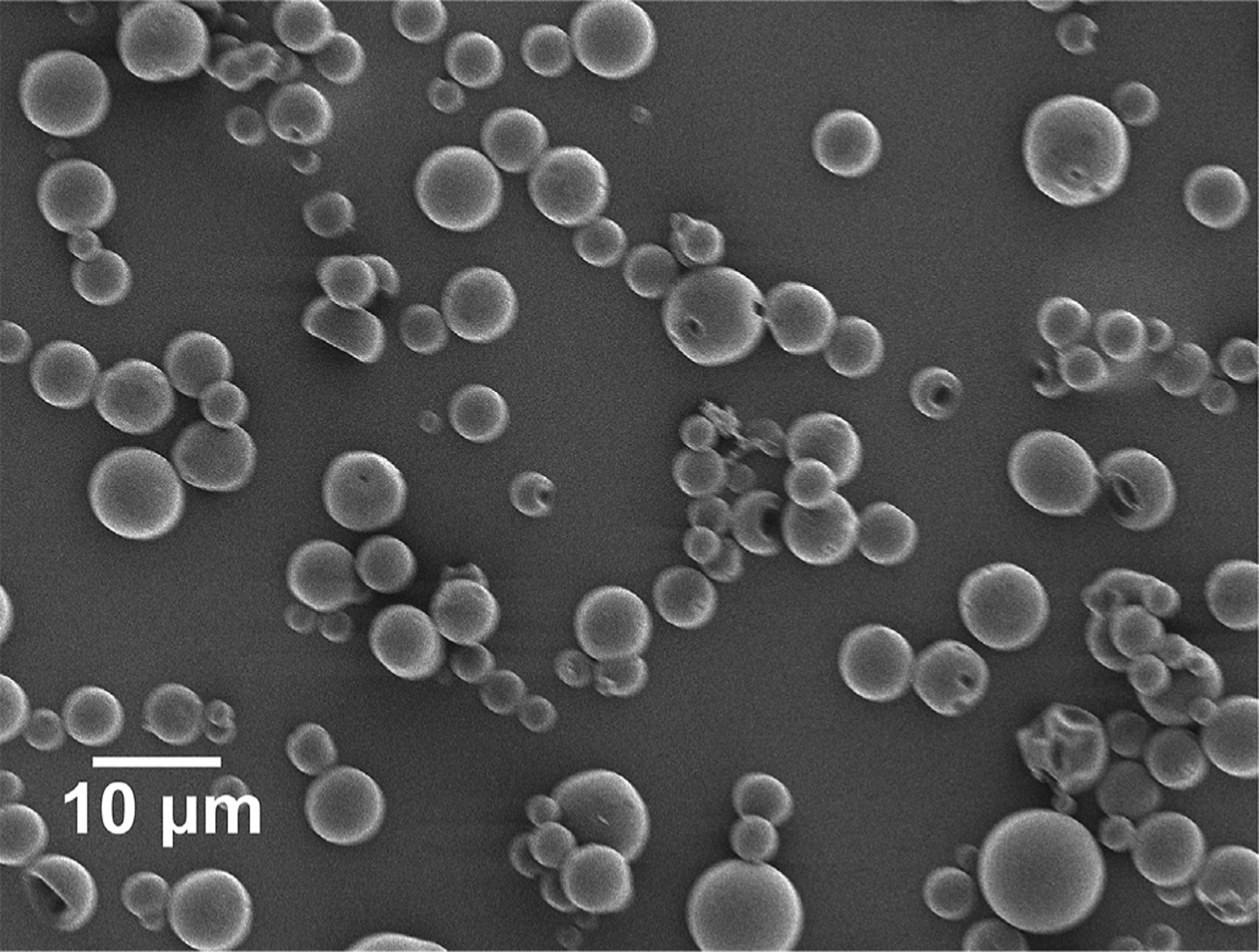
Scanning electron micrograph (SEM) images of brimonidine tartrate-loaded poly (lactic-co-glycolic acid) (PLGA) microspheres (BTMS). These images confirm the desired size and morphology of the BTMS, consistent with volume impedance measurements (average volume diameter = 7.46 ± 2.86 μm).
Having confirmed that the size and surface characteristics of the BTMS were suitable for use in our rabbit model, the next step in the rational design process was to determine the 28-day release profile of drug from the MS. Accordingly, in vitro release of BT from a known mass of these microspheres for 28 days is represented in Fig. 2. As our goal was to release an amount of drug comparable to standard eye drop medication, we report here the amount released as a mass of brimonidine instead of percentage of total amount of drug encapsulated. Also shown in Fig. 2B are the reported minimum and maximum amounts of topical BT solution absorbed into the anterior chamber, as described in the methods section above. As expected, the amount of BT released for the full 28 days was well above the lower limit of absorption, with an average of 2.1 ± 0.37 μg brimonidine/day released over 28 days. This average amount includes days 24–28, at which point release of brimonidine had slowed considerably. Characterization of the microspheres also demonstrated that, in our in vitro setup, particles were completely degraded at or before Day 35. This was determined by a visual lack of microspheres and corresponding exhaustion of drug release.
Fig. 2.
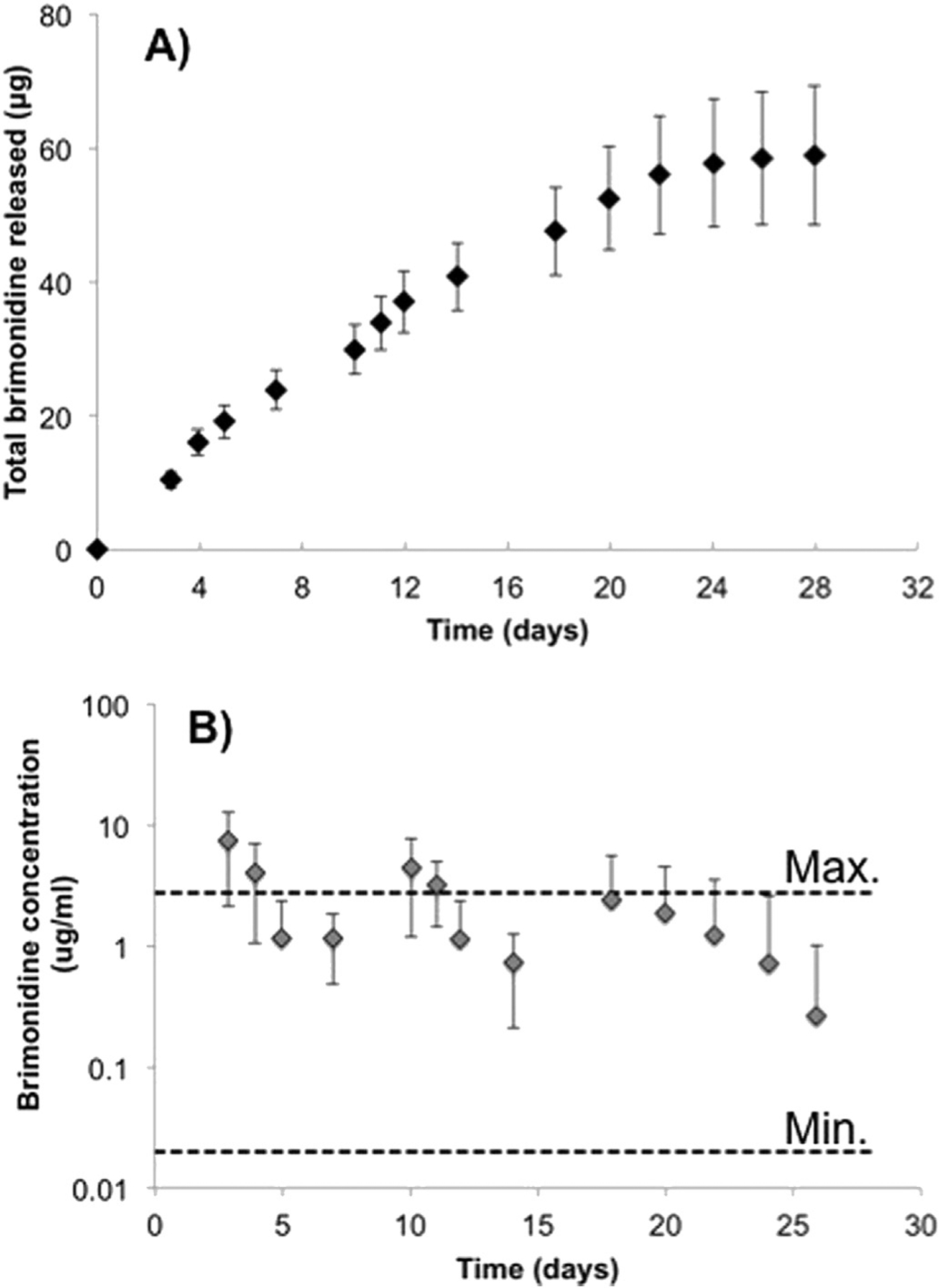
A) Cumulative in vitro release of brimonidine from PLGA microspheres (n = 3). B) Daily release totals relative to maximum and minimum reported concentrations of brimonidine in aqueous humor (Acheampong et al., 2002).
3.2. In vivo studies
Fig. 3 shows an example of the MS bleb in the subconjunctival space on Day 1 of the study, representative of the administration in the right eye only of blank or drug-loaded MS in the 5 experimental treatment and 5 negative control animals. A third set of 5 rabbits received twice-daily topical BT 0.2% drops at the same time each day to serve as the positive control.
Fig. 3.
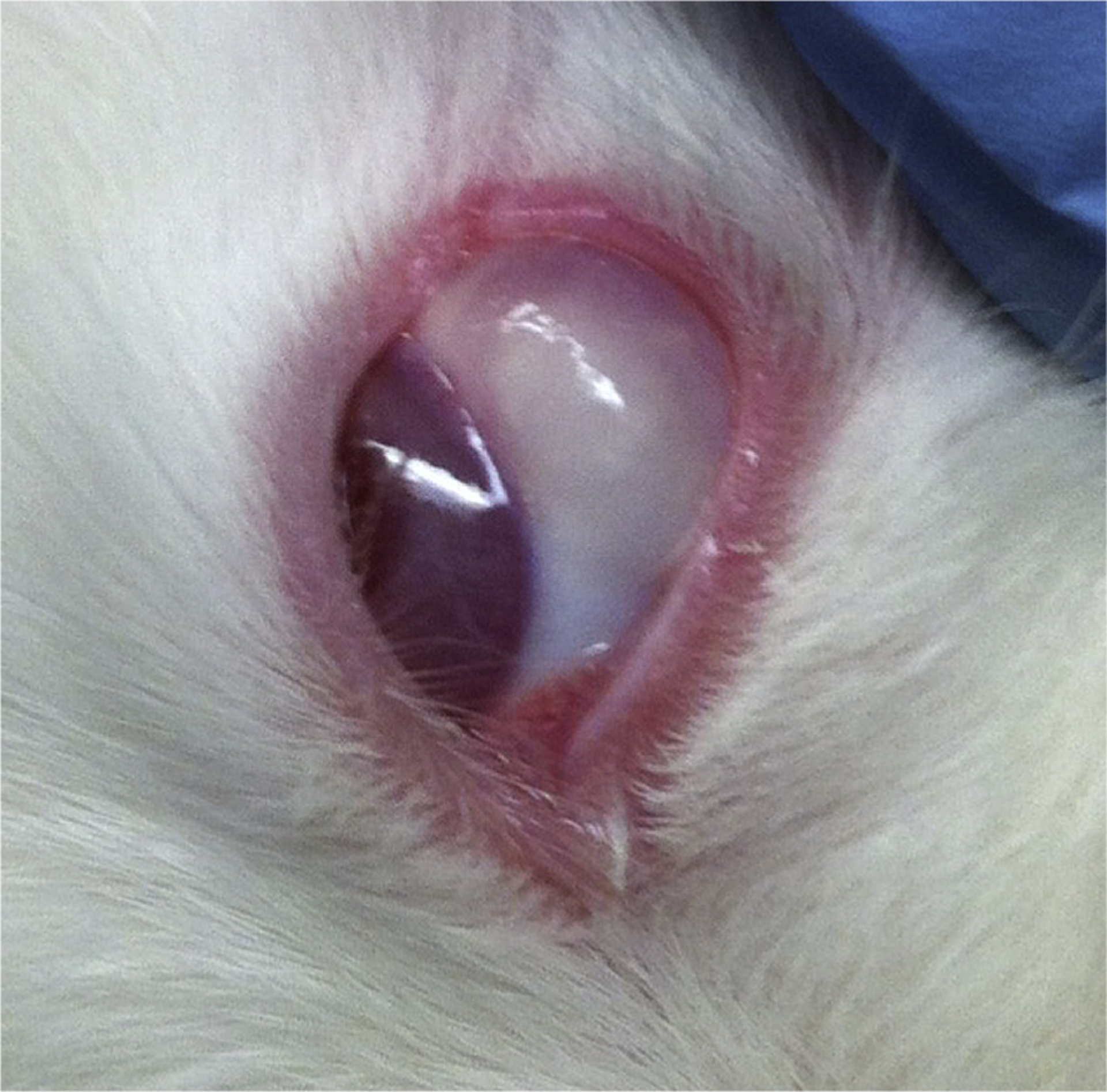
Brimonidine tartrate-loaded microsphere (BTMS) bleb in subconjunctival space of New Zealand white rabbit on Day 0 of study.
IOP was measured over 28 days by the same individual at approximately the same time of morning for each measurement. Initially, several baseline IOP measurements were taken on each rabbit before beginning treatment. Baseline IOP values were not significantly different in either eye between groups. Following administration of drug or MS (blank or BT-loaded), IOP measurements were taken at each time point in the study, between 30 and 60 min after eye drops were administered to the positive control group. Fig. 4A and B demonstrate the actual IOP values recorded at each time point for all three groups in the treated (right) eye and control (left) eye, respectively. IOP values are reported as the average IOP and standard deviation for the five animals in each group.
Fig. 4.
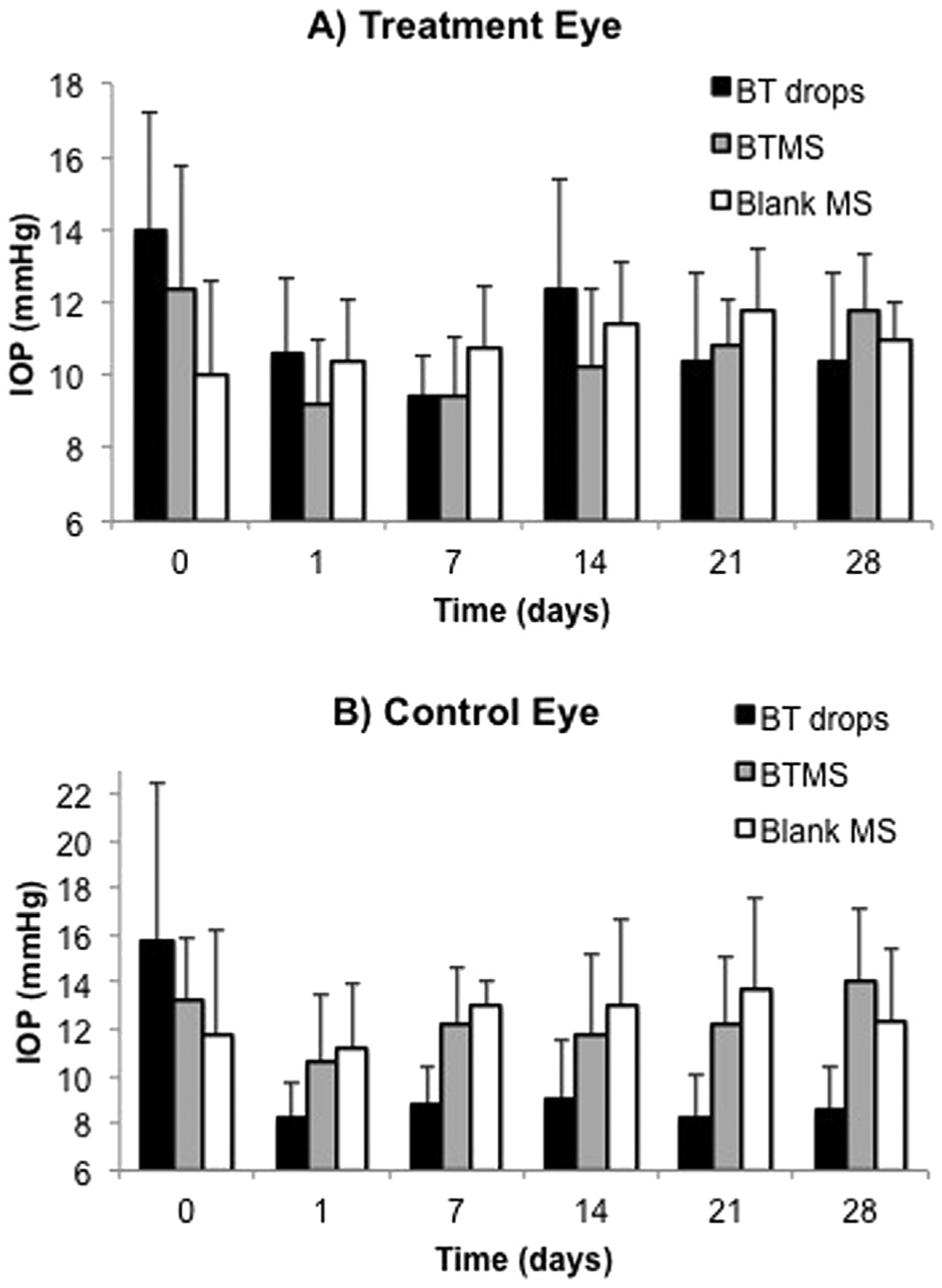
Actual intraocular pressure (IOP) measurements in each of the three groups taken from A) the right eye (treated eye) and B) the left eye (untreated eye). N = 5 for all groups, with average ± standard deviation shown.
To better understand the changes in IOP over course of the study, the relative differences in IOP compared to each of the baseline values was calculated. Fig. 5A and B depict the percent change in IOP at each time point relative to day 0 for all three groups, again in the right eye and left eye, respectively. In the treated eye, there were no significant differences in percent IOP change between the BTMS (experimental treatment) and clinical standard BT drops (positive control). Percent change in IOP relative to baseline was significantly lower (indicating a net drop over time) compared to the blank MS for both the BT drops (day 1, p < 0.05; days 7 and 28, p < 0.01) and BTMS (days 1, 7, 14, and 28, p < 0.05) in the treated eye for the majority of the study.
Fig. 5.
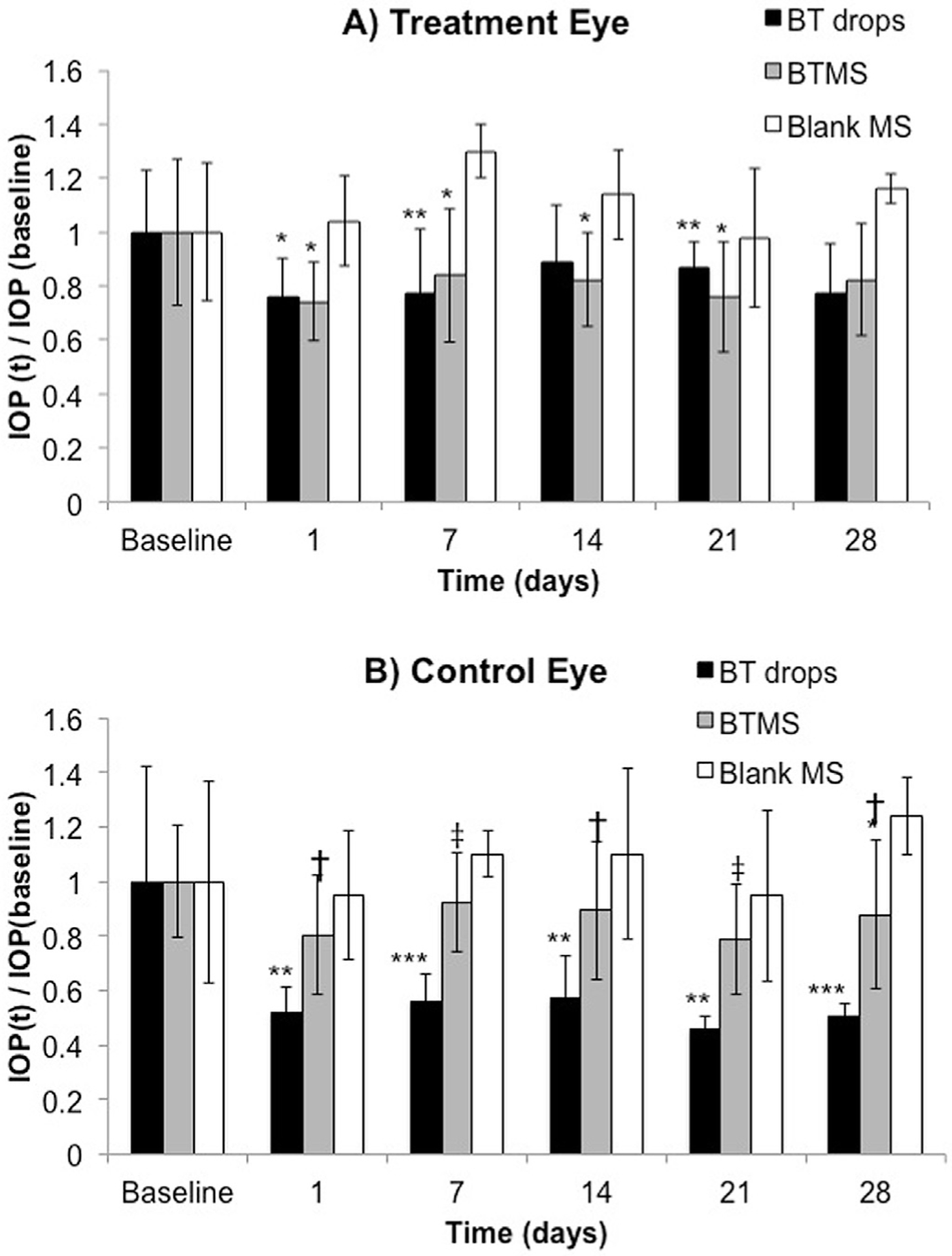
Percent change in IOP relative to baseline for each of the three groups in A) the right eye (treated eye) and B) the left eye (untreated eye). N = 5 for all groups, with average ± standard deviation shown (*p < 0.05, **p < 0.01 compared to blank MS group; †p < 0.05, ‡p < 0.01 compared to BT drops).
Percent change in IOP in the untreated eye showed a markedly different effect from that of the treated eye, however. The drop in IOP in the untreated eye was significantly greater in animals receiving the topical BT drops in the contralateral eye compared to IOP of the untreated eye in those animals receiving BTMS treatment in the contralateral eye (days 1, 14, 28, p < 0.05; day 21, p < 0.01; day 28, p < 0.005). Animals receiving topical BT drops in the treated eye also exhibited significantly greater drop in IOP throughout the study in the untreated eye compared to IOP in the untreated eye of animals receiving blank MS treatment (days 1, 14, 21, p < 0.01; days 7 and 28, p ≪ 0.001) Notably, decreases in IOP were not significantly different between the BTMS and blank MS group except for the measurement day 28 (p < 0.05).
In addition to determining the efficacy of the BTMS in vivo, we also sought to investigate the safety and compatibility of the PLGA MS in the local environment throughout the study. The cornea, conjunctiva, anterior chamber, and periocular tissues were inspected using a portable slit lamp throughout the study for adverse side effects including inflammation. Eyes were enucleated and stained using H&E, PAS, and Masson’s trichrome for histological analysis following sacrifice of the rabbits on Day 28. Histological examination revealed minimal amounts of fibrous tissue surrounding the area of injection (1–2 cell layers thick). No acute or chronic inflammation suggestive of a foreign body response or infection was present. Additionally, there was no evidence of microsphere migration from the original injection site. The partially degraded MS in the subconjunctival space can be seen in Fig. 6. Similar images for the remaining rabbits that received either blank or drug-loaded MS showed that the tissue surrounding the MS appeared normal.
Fig. 6.
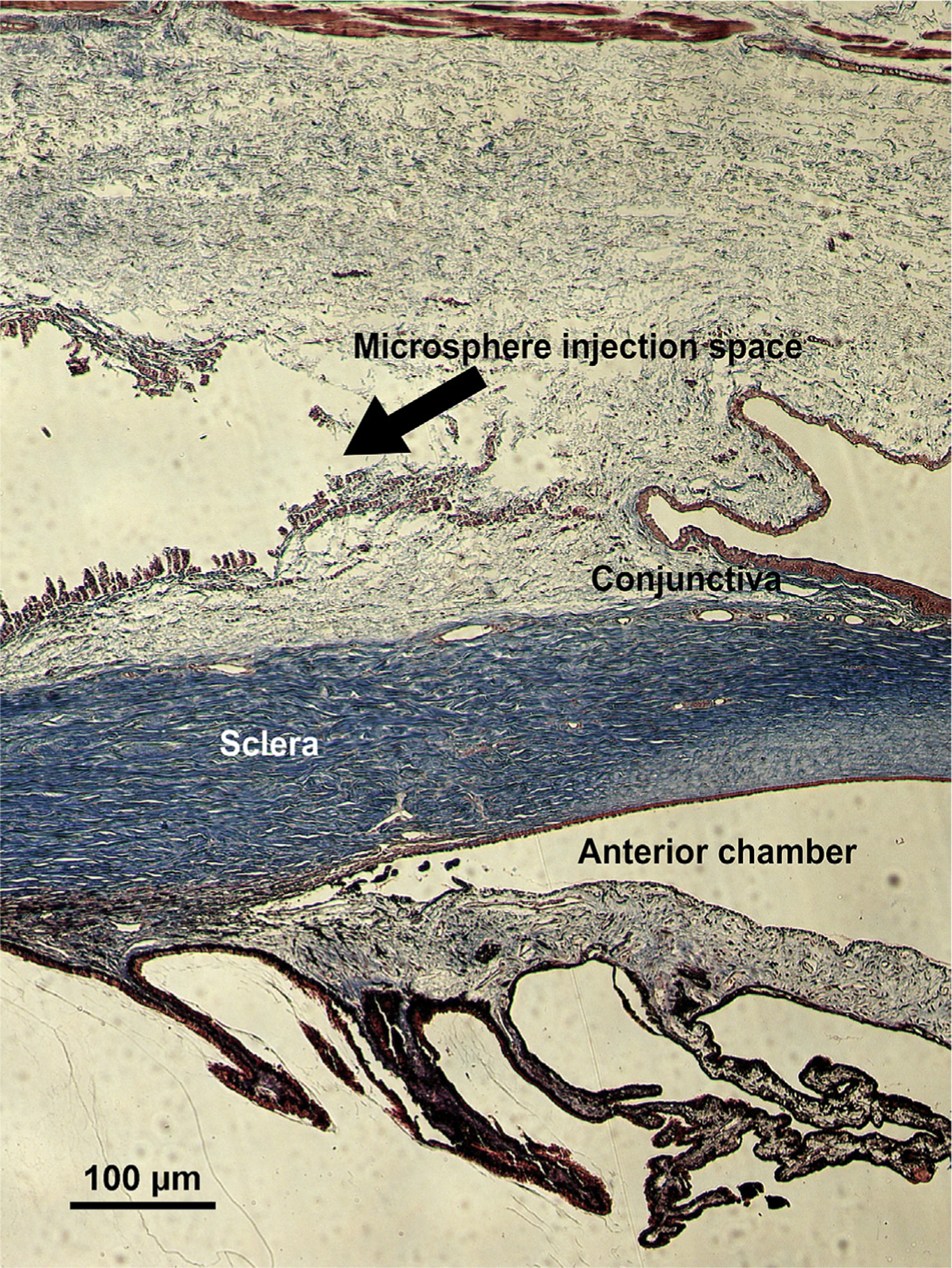
Partially degraded BTMS in the subconjunctival space (stained with Masson’s trichrome) following sacrifice on Day 28 of the study. Arrows and labels indicate also the sclera, conjunctiva, and anterior chamber.
4. Discussion
It is estimated that nearly 7.32 million adults in the United States will be diagnosed with primary open angle glaucoma by the year 2050, up from 2.71 million in 2011 (Vajaranant et al., 2012). The majority of IOP-reducing drugs to treat glaucoma are eye drops, which must be administered 1–4 times per day by the patient to reduce the risk of irreversible vision loss. The rigorous dosing schedule, initial lack of symptoms, and difficulty of drop administration lead to notoriously unsatisfactory patient compliance rates (Hermann et al., 2011a). Additionally, eye drop administration requires high concentrations of drug to overcome the many absorption barriers in the eye (Ghate and Edelhauser, 2008). For these reasons, alternative delivery methods for anti-glaucoma medications are being explored.
The use of biocompatible, biodegradable PLGA microspheres (MS) to encapsulate drugs, such as brimonidine tartrate, provides a platform for controlled drug release for tunable and potentially long periods of time (Kimura and Ogura, 2001). This is shown in Fig. 2A, as the MS developed for this study released what we hypothesize to be therapeutically relevant concentrations of brimonidine in vitro for 28 days. The MS are also ideal for administration through injections since the size can be designed to be small enough to permit injection through a small needle, but large enough to stay located at the site of injection. Indeed, Amrite and Kompella (2005) demonstrated that particles larger than 200 nm were retained for over two months in the administration site following subconjunctival injection (Amrite and Kompella, 2005). Thus, the size distribution of the BT-loaded MS (BTMS) demonstrated in Fig. 1 was deemed to be appropriate for subconjunctival injection, which a majority of glaucoma patients surveyed indicate that they would prefer over topical drops (Chong et al., 2013).
The data represented in Fig. 5A suggest that a single injection of the BTMS formulation produces an IOP lowering effect comparable to twice daily BT eye drops for 28 days. Both groups achieved statistically significant decreases in IOP compared to the negative control group. Notably, the only difference between the BTMS and blank microsphere treated groups is the release of BT, as confirmed by in vitro release assays. This strongly suggests that release of BT is both present and effective in vivo. In terms of other methods to quantify release in vivo, direct measurement of therapeutic drug concentration in vivo is particularly challenging for this type of system because of the low concentrations of drug present. Several sensitive assay methods are available for BT biodistribution and in vivo pharmacokinetic data (Acheampong and Tang-Liu, 1995; Jiang et al., 2009; Karamanos et al., 1999), and will be prioritized for investigation in future studies. For the purposes of exploring the viability of the formulation in this pilot study, we relied upon IOP outcomes as a measure of the effectiveness of the treatment over time.
IOP measurements in the control eye demonstrate one of the potential key advantages to controlled release technology, namely an apparent reduction in systemic uptake due to more efficient, localized delivery and lower drug concentrations (Yasukawa et al., 2001). In Figs. 4B and 5B, the contralateral eye in animals receiving the topical drops, which itself did not receive any form of treatment, experienced a significant hypotensive effect that was not observed in either group receiving the microspheres (blank or BT-loaded). While the rapid and substantial drop in IOP in the untreated eye may be counterintuitive, the phenomenon of asymmetric, bilateral IOP reduction has in fact been noted previously in normotensive rabbits and monkeys treated with topical BT drops (Burke et al., 1995; Yuksel et al., 2002). We hypothesize that because of the BTMS administration route, concentration of released drug, or some combination of those two factors, there is a marked decrease in systemic uptake of the drug when compared to topical eye drops (though IOP values suggest such cross-talk is still non-negligible for the BTMS). In support of this hypothesis, similar studies using controlled release systems in the eye have confirmed similar findings (Diebold and Calonge, 2010). In future studies we will seek to further investigate this phenomenon with biodistribution studies tracking local and distal drug concentration over time systemically and in both eyes. These studies will require higher resolution of time points following drop administration to fully understand the extent of asymmetry, if any, between the treated and fellow eyes.
Topical eye drops can result in systemic toxicity effects due to the concentration of drug that must be given by this route (Jarvinen et al., 1995). Drugs administered subconjunctivally, as in this study, are subject to vascular and to a lesser extent lymphatic clearance, which prevents distribution into the anterior chamber by eliminating the drug into systemic circulation (Kim et al., 2008). Although this pathway could produce systemic toxicity, the risk is minimized using controlled-release technology because direct administration to ocular tissues requires less administered drug overall. The entire BT payload of the drug-loaded microspheres tested in this study, for example, is equivalent to less than 3 drops of topical BT 0.2% solution (whose concentration is 2 mg/ml, and assuming a 50 μl drop vs. 0.25 mg of total BT in each BTMS injection). Notably, Fig. 2B shows that the amount released from the depot is still within the range reported in aqueous humor for eye drops (Acheampong et al., 2002) (though it must be noted that not all released drug can be expected to absorb through the cornea/sclera (Conrad and Robinson, 1980)). This underscores the efficiency of the controlled release system and possible applications for using a pharmacologic agent, especially in a patient with systemic contraindications for a certain drug. Just as the systemic absorption for topical administration of eye drops is orders of magnitude lower than that of oral drug delivery, local controlled release has the potential for even lower systemic delivery and therefore toxicity. The PLGA microspheres themselves are also safe to use in the eye because they degrade into lactic and glycolic acid, natural byproducts of metabolism. Other studies testing of PLGA formulations in the eye have confirmed that material degradation shows no evidence of ocular toxicity (Chang et al., 2011; Souza et al., 2014). As expected, neither the blank nor the BTMS resulted in evidence of a foreign body response, infection, or irritation in the conjunctiva, retina, or optic nerve (partially demonstrated in Fig. 6; images of the posterior segment not shown). Similarly, untreated eyes appeared normal following histological analysis.
Similar in vivo studies have demonstrated the safety of subconjunctival injections of PLGA microspheres or analogous implants (Amrite et al., 2006; Peng et al., 2011). It is conceivable that variability in IOP could occur due to material degradation or the physical presence of the subconjunctival MS bleb (Fig. 3) and any resulting inflammation due to the MS injection. However, the data in Fig. 4 demonstrate that the presence of the blank MS was not responsible for any significant change in IOP over the 28-day study. The inherent fluctuations in IOP, such as the uncharacteristic mean IOP increase on Day 14 for the BT drops group due to a single animal with unusually high IOP, suggest that differences relative to the baseline value may be a better indicator of efficacy over the course of the study.
The types of controlled release systems discussed here represent not only a potential improvement over current treatment options for glaucoma but also many other ocular diseases. This technology can be readily adapted to other anti-glaucoma medications (including combination therapies), or molecules for treating other conditions. Any such formulation has a high potential to address issues of compliance associated with these treatments as well as the insufficient absorption and retention inherent to traditional topical treatment modalities. This study serves to demonstrate that controlled release of BT is feasible in animals, and, if proven safe in humans, this type of drug treatment could revolutionize glaucoma therapy. A more thorough examination of in vivo drug release is needed to confirm that the effectiveness of the BTMS is in fact due to released drug.
5. Conclusion
The BTMS presented in this study are capable of releasing a presumed therapeutically relevant amount of a common glaucoma medication for 28 days in vitro. Additionally, in this pilot study of healthy rabbits, subconjunctivally injected BTMS produced an IOP reduction comparable to twice daily topical BT over a 28-day course with significantly less systemic uptake. Subconjunctival injections of the MS resulted in neither foreign body response nor infection after the full 28-day experimental period, though the effect of repeat injections warrants further consideration. Future studies will further investigate the pharmacokinetics (primarily through aqueous humor levels of drug) and safety of this treatment method, which has been recently reported by patients to be preferable to conventional eye drop medication (Chong et al., 2013). We will also explore the potential for adapting this formulation to a topically administered system (Mealy et al., 2014) to maximize potential benefits to patients, as well as investigating the use of other drugs such as prostaglandin analogs or even combined therapeutics.
Financial support
This work was funded by the Ocular Tissue Engineering and Regenerative Ophthalmology (OTERO) fellowship through the Louis J. Fox Center for Vision Restoration of UPMC and University of Pittsburgh, Pittsburgh, PA and an unrestricted grant from Research to Prevent Blindness, NY.
References
- Acheampong A, Tang-Liu DD, 1995. Measurement of brimonidine concentrations in human plasma by a highly sensitive gas chromatography/mass spectrometric assay. J. Pharm. Biomed. Anal 13, 995–1002. [DOI] [PubMed] [Google Scholar]
- Acheampong AA, Small D, Baumgarten V, Welty D, Tang-Liu D, 2002. Formulation effects on ocular absorption of brimonidine in rabbit eyes. J. Ocul. Pharmacol. Ther 18, 325–337. [DOI] [PubMed] [Google Scholar]
- Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB, 2006. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Investig Ophthalmol. Vis. Sci 47, 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrite AC, Kompella UB, 2005. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J. Pharm. Pharmacol 57, 1555–1563. [DOI] [PubMed] [Google Scholar]
- Bramley T, Peeples P, Walt JG, Juhasz M, Hansen JE, 2008. Impact of vision loss on costs and outcomes in medicare beneficiaries with glaucoma. Arch. Ophthalmol 126, 849–856. [DOI] [PubMed] [Google Scholar]
- Burke J, Kharlamb A, Shan T, Runde E, Padillo E, Manlapaz C, Wheeler L, 1995. Adrenergic and imidazoline receptor-mediated responses to UK-14,304–18 (brimonidine) in rabbits and monkeys. A species difference. Ann. N. Y. Acad. Sci 763, 78–95. [DOI] [PubMed] [Google Scholar]
- Chang E, McClellan AJ, Farley WJ, Li DQ, Pflugfelder SC, De Paiva CS, 2011. Biodegradable PLGA-based drug delivery systems for modulating ocular surface disease under experimental murine dry eye. J. Clin. Exp. Ophthalmol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RS, Su DH, Tsai A, Jiang Y, Htoon HM, Lamoureux EL, Aung T, Wong TT, 2013. Patient acceptance and attitude toward an alternative method of subconjunctival injection for the medical treatment of glaucoma. J. Glaucoma 22, 190–194. [DOI] [PubMed] [Google Scholar]
- Conrad JM, Robinson JR, 1980. Mechanisms of anterior segment absorption of pilocarpine following subconjunctival injection in albino rabbits. J. Pharm. Sci 69, 875–884. [DOI] [PubMed] [Google Scholar]
- Deokule SP, Baffi JZ, Guo H, Nazzaro M, Kaneko H, 2012. Evaluation of extended release brimonidine intravitreal device in normotensive rabbit eyes. Acta Ophthalmol 90, e344–348. [DOI] [PubMed] [Google Scholar]
- Diebold Y, Calonge M, 2010. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res 29, 596–609. [DOI] [PubMed] [Google Scholar]
- Friedman DS, Wolfs RCW, O’Colmain BJ, Klein BE, Hugh RT, West S, Leske MC, Mitchell P, Congdon N, Kempen J, Tielsch J, 2004. Prevalence of open-angle glaucoma among adults in the United States. Arch. Ophthalmol 122, 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghate D, Edelhauser HF, 2008. Barriers to glaucoma drug delivery. J. Glaucoma 17, 147–156. [DOI] [PubMed] [Google Scholar]
- Giarmoukakis A, Labiris G, Sideroudi H, Tsimali Z, Koutsospyrou N, Avgoustakis K, Kozobolis V, 2013. Biodegradable nanoparticles for controlled subconjunctival delivery of latanoprost acid: in vitro and in vivo evaluation. Preliminary results. Exp. Eye Res 112, 29–36. [DOI] [PubMed] [Google Scholar]
- Hermann MM, Bron AM, Creuzot-Garcher CP, Diestelhorst M, 2011a. Measurement of adherence to brimonidine therapy for glaucoma using electronic monitoring. J. Glaucoma 20, 502–508. [DOI] [PubMed] [Google Scholar]
- Hermann MM, Papaconstantinou D, Muether PS, Georgopoulos G, Diestelhorst M, 2011b. Adherence with brimonidine in patients with glaucoma aware and not aware of electronic monitoring. Acta Ophthalmol 89, e300–305. [DOI] [PubMed] [Google Scholar]
- Hunter RS, Lobo AM, 2011. Dexamethasone intravitreal implant for the treatment of noninfectious uveitis. Clin. Ophthalmol 5, 1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen K, Jarvinen T, Urtti A, 1995. Ocular absorption following topical delivery. Adv. Drug Deliv. Rev 16, 3–19. [Google Scholar]
- Jiang S, Chappa AK, Proksch JW, 2009. A rapid and sensitive LC/MS/MS assay for the quantitation of brimonidine in ocular fluids and tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 877, 107–114. [DOI] [PubMed] [Google Scholar]
- Karamanos NK, Lamari F, Katsimpris J, Gartaganis S, 1999. Development of an HPLC method for determining the alpha 2-adrenergic receptor agonist brimonidine in blood serum and aqueous humor of the eye. Biomed. Chromatogr 13, 86–88. [DOI] [PubMed] [Google Scholar]
- Kim SH, Csaky KG, Wang NS, Lutz RJ, 2008. Drug elimination kinetics following subconjunctival injection using dynamic contrast-enhanced magnetic resonance imaging. Pharm. Res 25, 512–520. [DOI] [PubMed] [Google Scholar]
- Kimura H, Ogura Y, 2001. Biodegradable polymers for ocular drug delivery. Ophthalmologica 215, 143–155. [DOI] [PubMed] [Google Scholar]
- Mealy JE, Fedorchak MV, Little SR, 2014. In vitro characterization of a controlled-release ocular insert for delivery of brimonidine tartrate. Acta Biomater 10, 87–93. [DOI] [PubMed] [Google Scholar]
- Mermoud A, Salmon JF, Barron A, Straker C, Murray AD, 1993. Surgical management of post-traumatic angle recession glaucoma. Ophthalmology 100, 634–642. [DOI] [PubMed] [Google Scholar]
- Messenger WB, Beardsley RM, Flaxel CJ, 2013. Fluocinolone acetonide intravitreal implant for the treatment of diabetic macular edema. Drug Des. Dev. Ther 7, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisseiev E, Goldstein M, Waisbourd M, Barak A, Loewenstein A, 2013. Long-term evaluation of patients treated with dexamethasone intravitreal implant for macular edema due to retinal vein occlusion. Eye (Lond) 27, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu MV, Gaspar MN, Fontes Ribeiro CA, Cabrita AM, de Sousa HC, Gil MH, 2011. In vitro and in vivo evaluation of an intraocular implant for glaucoma treatment. Int. J. Pharm 415, 73–82. [DOI] [PubMed] [Google Scholar]
- Olthoff CM, Schouten JS, van de Borne BW, Webers CA, 2005. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology 112, 953–961. [DOI] [PubMed] [Google Scholar]
- Peng Y, Ang M, Foo S, Lee WS, Ma Z, Venkatraman SS, Wong TT, 2011. Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS One 6, e22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Vila-Jato JL, Alonso MJ, 1993. Development of biodegradable microspheres and nanospheres for the controlled release of cyclosporin A. Int. J. Pharm 99, 263–273. [Google Scholar]
- Servat JJ, Bernardino CR, 2011. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging 28, 267–282. [DOI] [PubMed] [Google Scholar]
- Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT, 1994. Macrophage/particle interactions: effect of size, composition and surface area. J. Biomed. Mater. Res 28, 81–90. [DOI] [PubMed] [Google Scholar]
- Souza MC, Fialho SL, Souza PA, Fulgencio GO, Da Silva GR, Silva-Cunha A, 2014. Tacrolimus-loaded PLGA implants: in vivo release and ocular toxicity. Curr. Eye Res 39, 99–102. [DOI] [PubMed] [Google Scholar]
- Vajaranant TS, Wu S, Torres M, Varma R, 2012. The changing face of primary open-angle glaucoma in the United States: demographic and geographic changes from 2011 to 2050. Am. J. Ophthalmol 154, 303–314 e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa T, Kimura H, Tabata Y, Ogura Y, 2001. Biodegradable scleral plugs for vitreoretinal drug delivery. Adv. Drug Deliv. Rev 52, 25–36. [DOI] [PubMed] [Google Scholar]
- Yuksel N, Karabas L, Altintas O, Yildirim Y, Caglar Y, 2002. A comparison of the short-term hypotensive effects and side effects of unilateral brimonidine and apraclonidine in patients with elevated intraocular pressure. Ophthalmologica 216, 45–49. [DOI] [PubMed] [Google Scholar]
- Zweers ML, Engbers GH, Grijpma DW, Feijen J, 2006. Release of anti-restenosis drugs from poly(ethylene oxide)-poly(DL-lactic-co-glycolic acid) nanoparticles. J. Control. Release 114, 317–324. [DOI] [PubMed] [Google Scholar]


