Abstract
A new selective high-performance liquid chromatography (HPLC) method with UV detection for the determination of the investigational triazole voriconazole in human plasma by using acetonitrile precipitation followed by reverse-phase HPLC on a C18 column was compared with a simple agar well diffusion bioassay method with Candida kefyr ATCC 46764 as the assay organism. Pooled plasma was used to prepare standard and control samples for both methods. The results of analyses with spiked serum samples (run as unknowns) were concordant by the bioassay and HPLC methods, with expected values being obtained. HPLC demonstrated an improved precision (3.47 versus 12.12%) and accuracy (0.81 versus 1.28%) compared to those of the bioassay method. The range of linearity obtained by both methods (from 0.2 to 10 μg/ml for HPLC and from 0.25 to 20 μg/ml for the bioassay) includes the range of concentrations of voriconazole (from 1.2 to 4.7 μg/ml) which are considered clinically relevant. Although either methodology could be used for the monitoring of patient therapy, the smaller variability observed with HPLC compared to that observed with the bioassay favors the use of HPLC for pharmacokinetic studies.
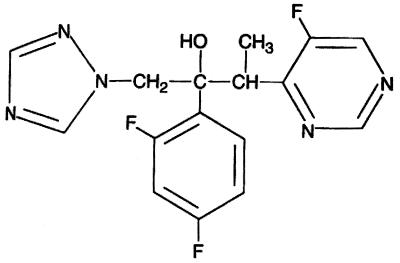
Voriconazole (UK-109,496) is a novel broad-spectrum triazole antifungal agent that has a structure and a spectrum of action similar to those of fluconazole and itraconazole, respectively (1; K. Richardson, A. S. Bell, R. P. Dickinson, S. Naryanaswami, and S. J. Hay, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F69, p. 125, 1995). The chemical structure of voriconazole was synthesized from that of fluconazole by replacement of one triazole moiety by a fluoropyrimidine group and alpha methylation (Fig. 1). This modification resulted in an enhanced spectrum of antifungal activity and increased in vitro potency. Voriconazole binds to the cytochrome P-450 enzyme lanosterol 14-alpha-demethylase, which prevents the conversion of lanosterol to ergosterol (P. F. Troke, A. S. Bell, R. P. Dickinson, C. A. Hitchcock, S. Jezequel, S. Narayanaswami, S. J. Ray, and K. Richardson, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F70, p. 125, 1995). This effect alters the cell membrane function and permeability, resulting in cell dysfunction and growth arrest. This agent has been demonstrated to have substantial preclinical activity in both in vitro (2, 9, 11, 14–16, 21; F. Barchiesi, M. Restrepo, D. A. McGough, and M. G. Rinaldi, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F71, p. 125, 1995; M. R. McGinnis, L. Pasarell, and C. R. Cooper, Jr. Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E76, p. 99, 1995) and in vivo (6, 7, 10; C. A. Hitchcock, R. J. Andrews, B. G. H. Lewis, and P. F. Troke, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F74, p. 125, 1995; C. A. Hitchcock, R. J. Andrews, B. G. H. Lewis, and P. F. Troke, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F75, p. 125, 1995) models against a variety of opportunistic filamentous (5–7; B. Dupont, D. Denning, H. Lode, S. Yonren, P. Troke, and N. Sarantis, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F81, p. 127, 1995; voriconazole investigator's brochure, Pfizer, Inc., New York, N.Y.) and dimorphic fungi and yeasts. In addition, voriconazole displays activity against Candida isolates resistant to fluconazole (3, 17). Early clinical studies have suggested that voriconazole may be effective in the treatment of oropharyngeal candidiasis and acute or chronic pulmonary aspergillosis (4, 13, 18; D. Denning, A. de Favero, E. Gluckman, D. Norfolk, M. Ruhnke, S. Yonren, P. Troke, and N. Sarantis, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F80, p. 126, 1995; Dupont et al., 95th ICAAC; P. F. Troke, K. W. Brammer, C. A. Hitchocock, S. Yonren, and N. Sarantis, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F73, p. 125, 1995).
FIG. 1.
Chemical structure of voriconazole.
The pharmacological characteristics of voriconazole have been studied with healthy volunteers. Preliminary results of those studies suggest that voriconazole has an optimal toxicological profile and is adequately tolerated in humans following oral and parenteral administration. Pharmacokinetic studies indicate that the agent has a substantial level of oral absorption with a high degree of bioavailability. Consistent with its liposolubility and moderate level of binding to plasma proteins (58%), the drug has a high volume of distribution and diffuses widely throughout body fluids and tissues. Similar to other azoles, voriconazole undergoes extensive hepatic metabolism, which results in three major and five minor metabolites, with less than 5% of the drug being excreted unchanged in the urine and feces (voriconazole investigator's brochure, Pfizer, Inc.). The kinetics of voriconazole are nonlinear in humans due to saturation of its first-pass metabolism and systemic clearance (B. E. Patterson and P. E. Coates, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F78, p. 125, 1995; B. E. Patterson, S. Roffey, S. G. Jezequel, and B. Jones, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F79, p. 125, 1995). On the basis of its promising antifungal properties, adequate tolerability, and optimal pharmacological profile, this agent is undergoing evaluation in clinical trials with patients with invasive mycoses.
The quantification of plasma voriconazole levels in clinical trials is an important objective since it will permit a comprehensive characterization of its pharmacological features and monitoring of its levels in clinical plasma samples. For this reason, there has been interest in the development of adequate analytical methods, and a high-performance liquid chromatographic (HPLC) method for the detection of voriconazole in plasma has been described and validated (20). This method involves direct injection of plasma onto a Sephadex column and does not require protein precipitation. Although the results regarding accuracy, precision, and lower limit of quantification are adequate, this method is laborious and technically difficult.
This study describes and validates a new HPLC method that uses precipitation of proteins with acetonitrile by use of a reverse-phase column with UV spectrophotometric detection as well as a bioanalytical method for the detection of voriconazole in plasma. In addition, this report compares the accuracies and precisions of the two assays for the determination of therapeutic concentrations of voriconazole in human plasma.
(This work was partially presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998.)
MATERIALS AND METHODS
Standards and controls for both methods.
Voriconazole (UK-109,496) was kindly supplied by Pfizer Central Research (Sandwich, United Kingdom). A stock standard of 1,000 μg/ml was prepared by placing 10 mg of voriconazole into a 10-ml volumetric flask and diluted in methanol to a total volume of 10 ml. A working standard of 100 μg/ml was prepared by transferring appropriate amounts of stock voriconazole in a 5-ml volumetric flask and diluted in ammonium phosphate buffer to a total volume of 5 ml. Both stock and working voriconazole solutions were stored at −10°C. Standards were prepared with serum from human donors which had been screened to ensure the absence of antifungal agents. Voriconazole standards with concentrations in the range of 0.2, 0.6, 1, 2, 3, 4, and 5 μg/ml and controls with concentrations of 0.8, 1.6, and 3.6 μg/ml were prepared by accurately transferring appropriate volumes of working voriconazole solution to a 50-ml volumetric flask and QS with pooled plasma (Table 1).
TABLE 1.
Preparation of voriconazole standards
| Component | Vol to achieve the following concn (μg/ml):
|
||||||
|---|---|---|---|---|---|---|---|
| 0.2 | 0.6 | 1 | 2 | 3 | 4 | 5 | |
| Voriconazole vol (μl) | 10 | 30 | 50 | 100 | 150 | 200 | 250 |
| Plasma vol (ml) | 49.75 | 49.75 | 49.75 | 49.75 | 49.75 | 49.75 | 49.75 |
| Methanol vol (μl)a | 240 | 220 | 200 | 150 | 100 | 50 | 0 |
Methanol was added to achieve equal amounts in all samples.
HPLC assay of voriconazole.
The HPLC system consisted of a Beckman 126 solvent module binary pump, a Rheodyne model 7010 sample injection valve with a 100-μl sample loop, a Waters symmetry C18 (5 μm; 3.9 by 20 mm) guard column, a symmetry C18 (5 μm, 4.6 by 250 mm) analytical steel column, and a Beckman module 166 UV programmable detector. Integrations, calculations, and plotting of chromatograms were performed with a Beckman computing integrator (System Gold Software; Beckman Instruments Inc., Houston, Tex.). The reagents used and their suppliers were as follows: methanol (HPLC grade), acetonitrile (HPLC grade), and ammonium hydroxide were purchased from EM Science (Fair Lawn, N.J.), and ammonium dihydrogen phosphate (HPLC grade) was purchased from Fisher Scientific (Gibbstown, N.J.). The mobile phase was prepared by mixing acetonitrile with filtered 0.04 M ammonium dihydrogen phosphate buffer, adjusted to pH 6.0 with ammonia, at 50/50 (vol/vol) proportions. The mobile phase was filtered under vacuum through a 47-mm-diameter, 0.45-μm-pore-size nylon membrane filter. The flow rate was 0.8 ml/min. The UV detector was set at a wavelength of 255 nm. The HPLC apparatus was operated at room temperature. No internal standard was used. All the samples were thawed in a 37°C water bath. A 0.5-ml aliquot of each sample was pipetted into a 2-ml Ene Mate microcentrifuge tube (ISC Bioexpress, Kaysville, Utah), and the tube was vortexed briefly. The samples were then centrifuged at 3,000 rpm for 5 min in a Micro 14 Fisher Scientific microcentrifuge. After centrifugation, the samples were transferred to a 5-ml disposable screw-cap tube (Pyrex, Corning, N.Y.) and 0.8 ml of acetonitrile was added, and then the tube was vortexed and the contents were mixed briefly. After 10 minutes, the samples were centrifuged at 2,500 rpm with a Beckman model TJ-6 centrifuge for 5 min, and 50 μl of the supernatant was injected into the liquid chromatography by using a 100-μl loop. A standard curve was constructed by preparing standards in plasma and using linear regression to correlate the peak height of the voriconazole spike versus voriconazole concentrations.
Bioassay of voriconazole.
Candida kefyr ATCC 46764 was maintained by weekly passage on Sabouraud dextrose agar plates. Prior to use, the organism was inoculated in a solution of yeast nitrogen base (YNB) broth (Difco) with 1% glucose. The broth culture was incubated for 6 h at 35°C, and the turbidity was then adjusted with sterile water to that of a no. 2 McFarland standard (65 to 70% transmission at 590 nm). Agar for the bioassay was prepared by mixing 15 g of BBL agar, 7 g of YNB (Difco), 15 g of Sigma Dextrose glucose, and 7 g of BBL Trypticase peptone, and water was added to give a total volume of 1,000 ml. Previously prepared 45-ml aliquots of the test agar were melted, allowed to cool to 48°C, inoculated with 0.5 ml of adjusted C. kefyr suspension, and then gently mixed by inversion and poured into round plastic disposable petri dishes (150 by 15 mm). The agar was allowed to gel at room temperature for 10 to 15 min. After the solidification of the YNB, 5-mm-diameter wells were bored and with a sterile cork borer and a 16-well template and aspirated. Ten microliters of each standard or control was pipetted into individual wells around the periphery of the plate. The plates were incubated overnight at 37°C. Assay plates were prepared in triplicate, resulting in nine and three measures for each control and standard, respectively. The assay was repeated over 5 days. Zones of inhibition were measured to the nearest 0.1 mm by using a metric caliper micrometer. A computer program was used to calculate the best-fit curve for the standards and the controls. Regression plotting of drug concentration versus bioassay zones and equation derivation was done with JMP computer software (version 3.1; SAS Institute, Cary, N.C.). The equation used was Y = b0 + b1 × X, where Y is the log10 voriconazole concentration (in micrograms per milliliter), X is the zone of inhibition (in millimeters), and b0 and b1 are a constant and the slope of the curve, respectively.
Statistics.
Least-squares linear regression was performed by using standard techniques. Both procedures were repeated over 5 days, and each control was run five (HPLC) and nine (bioassay) times. The within- and between-run variabilities of the assays was estimated by computing the coefficient of variation (CV). The agreement between both analytical methods has been evaluated by computing the mean square error and bias (19). For these calculations, HPLC was considered the reference method.
RESULTS
HPLC.
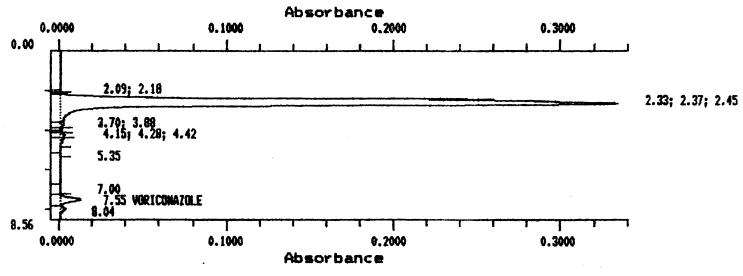
Under the chromatographic conditions described above, voriconazole was found to have a retention time of 7.5 min. Voriconazole was well separated from endogenous background peaks in serum. A chromatogram for a serum sample spiked with 5 μg of voriconazole per ml is shown in Fig. 2. Standard curves were constructed by plotting the peak heights against the concentrations of the standards. Peak height values were used instead of peak area values, as it has been demonstrated that this gives improved precision and accuracy for low concentrations of voriconazole (12). In practice, a linear relationship was found over a range of drug concentrations from 0.2 to 10 μg/ml. A voriconazole concentration of 0.2 μg/ml was the lowest that could be reproducibly determined. HPLC results demonstrated a linear relationship between peak height ratios and voriconazole concentrations, giving standard curves with r2 values of >0.99. To determine the accuracy of the HPLC assay, the same spiked controls used with the bioassay were assayed on five different occasions, and the values obtained were averaged and correlated with the expected values. There was an excellent agreement between the measured concentrations and the spiked concentrations (average accuracy, 2.82). Similarly, the method was highly precise, as demonstrated by the relatively small CVs at each concentration (average CV, <2.5%). The interday variability of the HPLC, estimated as the average CV for the assays with the spiked controls on 5 different days, was <4%.
FIG. 2.
Chromatogram of a serum sample spiked with 5 μg of voriconazole per ml.
Bioassay.
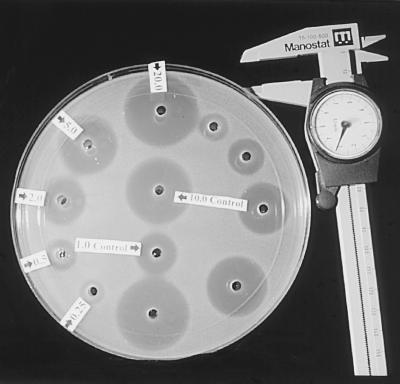
The zone margins of inhibition were sharp and clearly delineated, as shown in Fig. 3. To determine the accuracy of the bioassay, spiked controls containing 0.8, 1.6, and 3.6 μg/ml were assayed five times on five separate occasions. The values obtained were averaged and correlated with the expected values. The concentrations used in the bioassay standard curve were 0.6, 1, 2, 3, 4, and 5 μg/ml. Standard curves were linear, with r2 always being >0.99. The intraday variability of the bioassay was estimated by computing the CV for the measurements of each of the spiked unknown samples containing 0.8, 1.6, and 3.6 μg/ml that averaged <3%. The interday variability of the bioassay, estimated as the average CV for the assays of the spiked controls run on 5 different days, was <13%. The bioassay was sensitive down to 0.25 μg/ml. Below this value the zones were poorly defined and too close to the well for accurate measurement.
FIG. 3.
Bioassay plate for voriconazole. Wells contain standards of 0.25, 0.5, 1.0, 2.0, 5.0, and 10.0 μg/ml.
Correlation of HPLC and bioassay results.
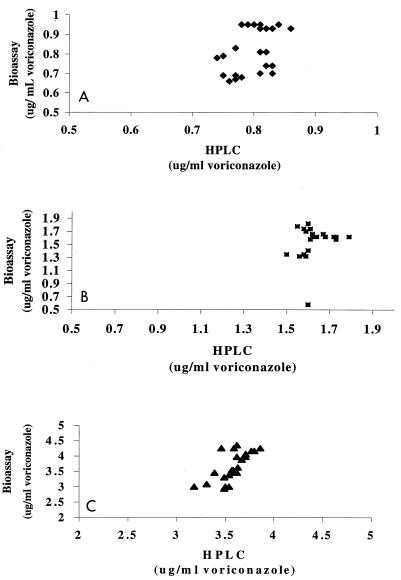
The scatterplots in Fig. 4 depict the agreement between the HPLC and bioassay when used to assay the spiked controls at concentration of 0.8, 1.6, and 3.6 μg/ml. The correlation between both methods has been assessed by calculating the mean square error and bias (Table 2), and the results demonstrate an excellent correlation between the two techniques (r2 > 0.99), although the bioanalytical method has a greater degree of variability.
FIG. 4.
Scatterplot depicting the agreement between the HPLC method (x axis) and the microbiological method (y axis) for determination of voriconazole levels when the drug was used at concentrations of 0.8 μg/ml (⧫; A), 1.6 μg/ml (■; B), and 3.6 μg/ml (▴; C).
TABLE 2.
Correlation between HPLC and microbiological method for assay of voriconazole in spiked plasma samples
| Amt (μg/ml) of voriconazole added to sample | Mean squared prediction error (95% CI) | Bias (95% CI) |
|---|---|---|
| 0.8 | 0.010 (0.007–0.014) | 0.018 (−0.025–0.060) |
| 1.6 | 0.063 (−0.022–0.147) | −0.065 (−0.0166–0.037) |
| 3.6 | 0.139 (0.064–0.212) | 0.074 (0.078–0.229) |
DISCUSSION
This study compares and validates an HPLC method and a bioassay method for the determination of voriconazole levels in human plasma. The HPLC method with UV detection involves a single-step protein precipitation with acetonitrile followed by reverse-phase HPLC on a C18 column and does not require the use of an internal standard. The bioanalytical method consists of a simple agar well-based microbiological method that determines voriconazole concentrations by measuring graded responses of C. kefyr and comparing unknown concentrations of voriconazole in serum against a standard curve. The results obtained in this study demonstrate an excellent correlation between the two procedures, which had similar lower limits of quantitation:: 0.2 μg/ml for HPLC and 0.25 μg/ml for the bioassay. The HPLC method has improved precision (3.47 versus 12.12%) and accuracy (0.81 versus 1.28%) compared to those for the bioanalytical method.
Voriconazole represents a novel, broad-spectrum antifungal agent with interesting and promising features with potential clinical activity. In order to use this agent properly as treatment for patients with fungal disease, it is required that we have adequate knowledge of its pharmacological properties. The implication of pharamacokinetic studies in the clinical development of a new antifungal agent is patently illustrated by considering the data available for other relatively new triazoles including fluconazole and itraconazole. Pharmacokinetic studies have been pivotal in defining such critical issues as the necessity to monitor plasma itraconazole levels, given the erratic oral absorption and dose-dependent pharmacokinetics of itraconazole, and the requirement to modify fluconazole doses on the basis of renal function (1).
Another HPLC analytical method for the determination of voriconazole concentrations in plasma has been described previously (20). In contrast to the method described in this report, that method uses as the initial procedure injection of the plasma sample directly onto a Sephadex size-exclusion column without protein precipitation and requires the use of an internal standard. Although that method has an extremely low limit of quantitation, in the nanogram range, technical difficulties impair its widespread use for pharmacokinetic studies and monitoring of patients. The major disadvantage involves the requirement of a precolumn with a maximum pressure limit of about 180 lb/in2. This results in frequent blockage of the system after 100 injections and, consequently, repeated replacement of the precolumn. This problem is avoided by precipitation of plasma proteins, as used for the method described here.
Although the lower limit of quantitation of these two methods (on the order of micrograms) is relatively high compared to that of the other reported method, the linearity range obtained by both methods effectively covers what is currently believed to be the clinically relevant range for voriconazole concentrations in plasma. In humans, the range of concentration of voriconazole in plasma following the administration of multiple oral and intravenous doses ranging from 4 to 6 mg/kg of body weight varies from 1.2 to 4.7 μg/ml, which are within the limits in which both analytical methods were linear (0.2 and 10 μg/ml for HPLC and 0.25 and 20 μg/ml for bioassay; r2 > 0.99).
The major advantage of the bioassay over the HPLC method is its relative simplicity. The HPLC method is moderately laborious and requires expensive equipment. For instance, the processing of 15 samples requires about 5 h for extraction and analysis by HPLC, which is twofold the time required to process the same number of samples by the bioassay (plus an overnight incubation to read the results). In addition, the bioassay is easy to perform and does not require special equipment. The major advantages of the HPLC method compared to the bioassay are its enhanced accuracy (0.81 versus 1.28%) and precision (3.47 versus 12.12%). A second difference between both methods pertains to their ability to measure active metabolites. This potential problem has been elegantly demonstrated with itraconazole. Itraconazole is metabolized through the cytochrome P-450 detoxification system to yield several metabolites, with one of them, an hydroxylate metabolite, being a very active antifungal agent. While the HPLC method detects only the parent compound with a predefined chemical structure and not the different chemical species that result from metabolic reactions, the bioanalytical method will detect any active substances, irrespective of their chemical characteristics. This factor explains the discrepancies observed between the two methods when analyzing clinical plasma samples for their itraconazole concentrations (8). These considerations are not applicable in the case of voriconazole, since the metabolism of this agent results in substances which do not possess antifungal activity.
Many questions about the measurement of voriconazole levels and the clinical relevance of those levels remain to be addressed. As additional data from ongoing and future studies are collected and analyzed, clearer concepts as to the utility of both analytic and bioassays may emerge. As with the azoles developed prior to the development of voriconazole, there will undoubtedly arise issues involving perceived and/or genuine resistance, drug-drug interactions, absorption difficulties, and metabolism nuances in specific patients or patient groups and the need for knowledge about the pharmacokinetics and pharmacodynamics of the drug. It is hoped that assays such as those described here will be of value and assistance in gathering such data and providing information for the optimal clinical use of a new and potentially significant weapon in the treatment of invasive mycotic infections.
In conclusion, the results of this study indicate that both analytical methods are adequate for analysis of voriconazole and are suitable for use in clinical laboratories. The HPLC method, although more laborious, is more accurate and precise, being more appropriate for pharmacokinetic studies. The microbiological method, however, is relatively simple and has sufficient precision and accuracy to be used to monitor drug levels in patients for routine clinical work-ups.
ACKNOWLEDGMENT
S.P. is partially supported by the SEIMC-SmithKline Beecham grant 1997 (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica).
REFERENCES
- 1.Bailey E M, Krakovsky D J, Rybak M J. The triazole antifungal agents: a review of itraconazole and fluconazole. Pharmacotherapy. 1990;10:146–153. [PubMed] [Google Scholar]
- 2.Barry A L, Brown S D. In vitro studies of two triazole antifungal agents (voriconazole [UK-109,496] and fluconazole) against Candida species. Antimicrob Agents Chemother. 1996;40:1948–1949. doi: 10.1128/aac.40.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belanger P, Nast C C, Fratti R, Sanati H, Ghannoum M. Voriconazole (UK-109,496) inhibits the growth and alters the morphology of fluconazole-susceptible and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:1840–1842. doi: 10.1128/aac.41.8.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caillot D, Casanovas O, Bernard A, Couaillier J F, Durand C, Cuisenier B, Solary E, Piard F, Petrella T, Bonnin A, Couillault G, Dumas M, Guy H. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139–147. doi: 10.1200/JCO.1997.15.1.139. [DOI] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198–202. doi: 10.1128/jcm.36.1.198-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George D, Miniter P, Andriole V T. Efficacy of UK-109,496, a new azole antifungal agent, in an experimental model of invasive aspergillosis. Antimicrob Agents Chemother. 1996;40:86–91. doi: 10.1128/aac.40.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girmenia C, Luzi G, Monaco M, Martino P. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J Clin Microbiol. 1998;36:1436–1438. doi: 10.1128/jcm.36.5.1436-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hostetler J S, Heykants J, Clemons K V, Woestenborghs R, Hanson L H, Stevens D A. Discrepancies in bioassay and chromatography determinations explained by metabolism of itraconazole to hydroxyitraconazole: studies of interpatient variations in concentrations. Antimicrob Agents Chemother. 1993;37:2224–2227. doi: 10.1128/aac.37.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marco F, Pfaller M A, Messer S, Jones R N. In vitro activities of voriconazole (UK-109,496) and four other antifungal agents against 394 clinical isolates of Candida spp. Antimicrob Agents Chemother. 1998;42:161–163. doi: 10.1128/aac.42.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin M V, Yates J, Hitchcock C A. Comparison of voriconazole (UK-109,496) and itraconazole in prevention and treatment of Aspergillus fumigatus endocarditis in guinea pigs. Antimicrob Agents Chemother. 1997;41:13–16. doi: 10.1128/aac.41.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGinnis M R, Pasarell L, Sutton D A, Fothergill A W, Cooper C R, Rinaldi M G. In vitro evaluation of voriconazole against some clinically important fungi. Antimicrob Agents Chemother. 1997;41:1832–1834. doi: 10.1128/aac.41.8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller H W, Eitel J. Quality control in the determination of cortisol in plasma/serum by using, on every sample, two different three-step separation methods including ultrafiltration, restricted-access high-performance liquid chromatography and reverse-phase high-performance liquid chromatography and contrasting results to immunoassays. J Chromatogr B. 1996;678:137–150. doi: 10.1016/0378-4347(95)00479-3. [DOI] [PubMed] [Google Scholar]
- 13.Murphy M, Bernard E M, Ishimaru T, Armstrong D. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 1997;41:696–698. doi: 10.1128/aac.41.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen M H, Yu C Y. In vitro comparative efficacy of voriconazole and itraconazole against fluconazole-susceptible and -resistant Cryptococcus neoformans isolates. Antimicrob Agents Chemother. 1998;42:471–472. doi: 10.1128/aac.42.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen M H, Yu C Y. Voriconazole against fluconazole-susceptible and resistant Candida isolates: in-vitro efficacy compared with that of itraconazole and ketoconazole. J Antimicrob Chemother. 1998;42:253–256. doi: 10.1093/jac/42.2.253. [DOI] [PubMed] [Google Scholar]
- 16.Radford S A, Johnson E M, Warnock D W. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogen. Antimicrob Agents Chemother. 1997;41:841–843. doi: 10.1128/aac.41.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruhnke M, Schmidt-Westhaussen A, Trautmann M. In vitro activities of voriconazole (UK-109,496) against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infections. Antimicrob Agents Chemother. 1997;41:575–577. doi: 10.1128/aac.41.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz S, Milatovic D, Thiel E. Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute-leukemia. Br J Haematol. 1997;97:663–665. doi: 10.1046/j.1365-2141.1997.972911.x. [DOI] [PubMed] [Google Scholar]
- 19.Sheiner L B, Beal S L. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 20.Stopher D A, Gage R. Determination of a new antifungal agent, voriconazole, by multidimensional high-performance liquid chromatography with direct plasma injection onto a size-exclusion column. J Chromatogr B. 1997;691:441–448. doi: 10.1016/s0378-4347(96)00408-2. [DOI] [PubMed] [Google Scholar]
- 21.Wildfeuer A, Seidl H P, Paule I, Haberreiter A. In vitro activity of voriconazole against yeasts, moulds and dermatophytes in comparison with fluconazole, amphotericin B and griseofulvin. Arzneimittel-Forschung. 1997;47:1257–1263. [PubMed] [Google Scholar]