Abstract
Objective
Variable treatment strategies and protocols have been applied to reduce time durations in the process of acute stroke management. The aim of this study is to investigate the effectiveness of our intra-arterial thrombectomy (IAT) protocol for decreasing door-to-recanalization time duration and improve successful recanalization.
Methods
A systemic and endovascular protocol included door-to-image, image-to-puncture and puncture-to-recanalization. We retrospectively analyzed the patients of pre- (Sep 2012–Apr 2014) and post-IAT protocol (May 2014–Jul 2018). Univariate analysis was used for the statistical significance according to variable factors (age, gender, the location of occluded vessel, successful recanalization TICI 2b-3). Independent t-test was used to compare the time duration.
Results
Among all 267 patients with acute stroke of anterior circulation, there were 50 and 217 patients with pre- and post-IAT protocol. Age, gender, and the location of occluded vessel have no statistical significance (p>0.05). In pre- and post-IAT group, successful recanalization was 39 of 50 (78.0%) and 185/217 (85.3%), respectively (p<0.05). Post-IAT (48.8%, 106/217) group had a higher tendency of good outcome than pre-IAT group (36.0%, 18/50) (p>0.05). Pre- and post-IAT group showed 61.7±21.4 vs. 25±16.0 (p<0.05), 102.0±29.8 vs. 82.7±30.4 (min) (p<0.05), and 79.1±47.5 vs. 58.4±75.3 (p<0.05) in three steps, respectively.
Conclusions
We suggest that the application of systemic and endovascular IAT protocols showed a significant time reduction for faster recanalization in patients with LVO. To build-up the well-designed IAT protocol through puncture-to-recanalization can be needed to decrease time duration and improve clinical outcome in recanalization therapy in acute stroke patients.
Keywords: Acute stroke, Anterior circulation, Intra-arterial mechanical thrombectomy, Protocol
INTRODUCTION
Recent clinical trials have shown that the clinical benefit of endovascular treatment (EVT) in patients with acute ischemic stroke with large vessel occlusion (LVO). It is reported that reducing the time interval from symptom onset to recanalization leads to good outcome. And, these trials have demonstrated the importance of rapid workflow and treatment strategies, and effective teambased care for intra-arterial thrombectomy (IAT) in patient with LVO [8,11,13,25].
Generally, there are several steps from symptom onset to recanalization of occluded vessel in the process of acute stroke treatment. 1) symptom onset–to–emergency room (ER) arrival, 2) ER arrival (door)–to–image, 3) image–to–puncture, 4) puncture–to–recanalization. Pre-hospital notification of stroke patient arrival by emergency medical services paramedics reduces in-hospital delay because it enables earlier activation of stroke team and preparation of imaging modalities before entering ER room, for decreasing onset–to- door time duration [16,22]. Also, in these steps, it is reported that door–to–puncture time can be significantly reduced by a standardized multidisciplinary approach [1,18]. Our study included next three steps of the door–to–recanalization time duration in this study, except symptom onset–to–door duration.
We have made IAT protocol for the rapid and standardized in-hospital workflow from door to recanalization. The aim of this study is to investigate the effectiveness of IAT protocol for decreasing door-to-recanalization time duration and successful recanalization rate in pre- and post-IAT period.
MATERIALS AND METHODS
Patient populations
Data for all patients with acute stroke, who underwent endovascular treatment, was prospectively collected in our stroke data base registry. From this collected data, we retrospectively analyzed clinical and angiographic results, which presented acute ischemic stroke with LVO in anterior circulation including internal carotid (ICA) and middle cerebral artery (MCA) and treated with IAT, retrievable stent or suction thrombectomy with reperfusion catheter from Sep 2012 to Jul 2018.
All patients who visited ER in our institute were evaluated by stroke neurologist and assessed with National Institute of Health Stroke Scale (NIHSS) score. Urgent brain non-contrast CT with CT angiography or stroke MR imaging with Diffusion weighted imaging (DWI), time-of-flight MR angiography was performed in all patients. For IAT, our inclusion criteria were as follows: 1) baseline NIHSS score of >6; 2) evidence of LVO, confirmed on CT or MR angiography; 3) image findings correlated with the clinical symptoms; 4) symptom duration after stroke onset <24 hours. The exclusion criteria were 1) intracerebral hemorrhage on CT or MRI; 2) the presence of established large brainstem or cerebellar infarction on non-contrast CT; 3) previously occluded another intracranial vessel; 4) life expectancy of <3 months. Patient’s informed consent was obtained from the patients or their relatives. Intravenous thrombolysis with tissue-plasminogen activator (tPA) was administered as bridging therapy in patients of no contraindication.
For the rapid and standardized in-hospital process, we have made IAT protocol according to each process from door to recanalization of the occluded vessel; 1) door-to-image, 2) image-to-puncture, 3) puncture-to-recanalization. (Fig. 1) We defined the first two steps as a systemic IAT protocol and the latter as an endovascular IAT protocol, respectively.
Fig. 1.
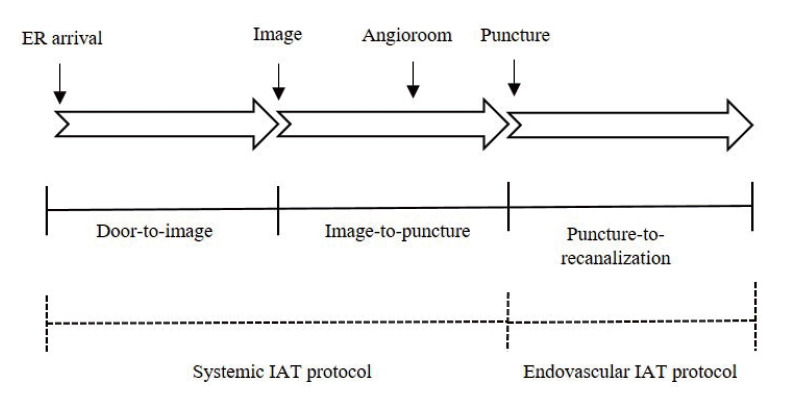
Schematic in-hospital workflow of patients with LVO from door to recanalization of the occluded vessel. LVO, large vessel occlusion.
Systemic IAT protocol
The protocol during door-to-image period
To decrease time duration of door-to-image, when a patient arrives at the ER, “Fast track” alert is to activate the stroke team for rapid neurologic assessment and the decision and acquisition of imaging modalities, and administration of intra-venous tPA to eligible patients. Fig. 2 shows the workflow from door to image and call to the endovascular neurosurgeon, interventional neurologist, and angio-suite technician and nurse. After ER doctor assess neurologic symptom of the patient, they simultaneously do a warm up call (the text message “Stroke Fast Track” is delivered to cell phone) to a neurosurgeon and neurologist, and send the patient to CT room. Before the transportation to CT room to minimize time delay, we prepare the intra-venous line route for tPA or anti-hypertensive drug administration, or for the consciousness sedation (CS) in advance. Usually, we tried to keep the Foley catheterization within 3-5 minutes. If a non-contrast brain CT show intracerebral hemorrhage, the patient will be transferred to neurosurgery. If brain CT show no hemorrhage, the neurologist will perform multi-phase CT Angiography for assessing intra-or extra-cranial artery and collateral status of occluded vessel [19]. At the time of the confirmation of LVO in multi-phase CT angiography, neurologist just called to endovascular neurosurgeon or interventional neurologist together with angioroom technician and nurse.
Fig. 2.
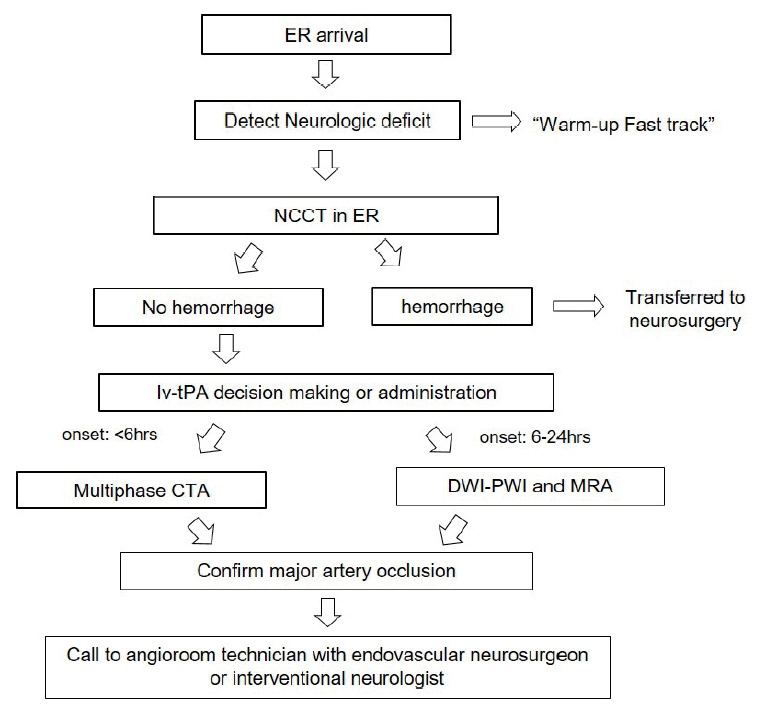
Flowchart of the standardized protocol from door to image to decrease time duration. In the entire protocol, the emphasis is that relevant stroke team (resident, staff, technician, and nurse) do their roles for patient preparation at the same time.
The protocol during image-to-puncture period
To decrease the time duration of image-to-puncture after the acquisition of CT or MR angiography, the next process may be necessary: 1) “no returns” to ER and direct transportation to angioroom, 2) operation the angio-suite in advance before the arrival of the patient, 3) pre-medication of CS to keep the patient from moving, and 4) preparation of sterile IAT kit including basic equipment earlier.
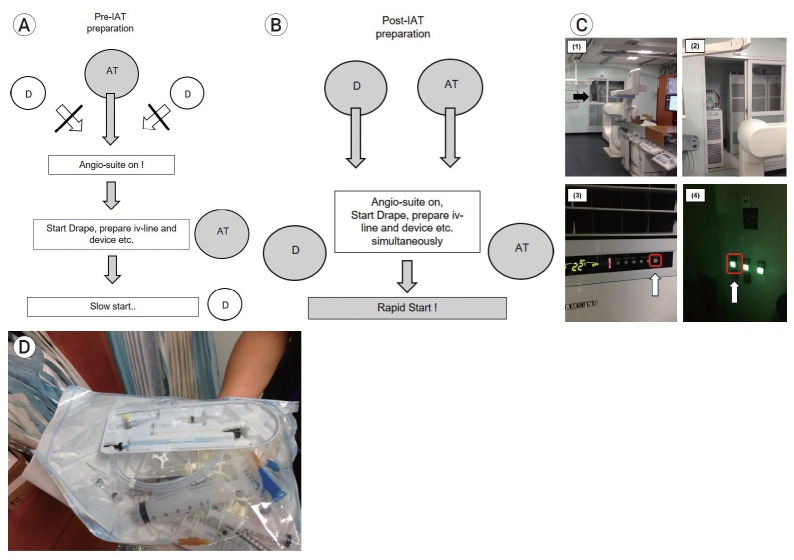
First, for “no returns” to ER, in the patient who is suggestive of embolic LVO, we do not prepare the L-tube in ER. However, in the patient who is suggestive of atherosclerotic intracranial stenosis, we prepare the L-tube for the oral administration of aspirin 300 mg and clopidogrel 325 mg, especially in the patient with low consciousness level. Second, it is important to minimize time delay to operate and prepare the angio-suite machine in advance. In the pre-IAT period, most of physicians cannot operate the angioroom machine, because the technician usually turns it on in advance. However, it is important for rapid procedure for anyone who visits angioroom for the first time to know how to turn on and prepare the angio-machine, because the technician or nurse does not always arrive at the hospital before the physician. And so, Fig. (3A and B) shows that anyone who first visit the angioroom do the same preparation for operating angio-suite and the easy way to turn it on is to make these serial pictures’ file on the desktop computer so that anyone who comes into the angioroom can see it (Fig. 3C). Third, the patient’s severe movement makes the procedure difficult and the time delay. So, we need to prepare dexmedetomidine (Precedex®) as pre-medication for consciousness sedation in ER. To prevent sudden decrease of BP, it does not give loading dose, but gives maintenance dose. And so, it takes approximately 10 minutes for the patient to be sedated, we should begin the CS as soon as the patient arrives in angioroom. Also, for the rapid preparation of all device for endovascular procedure, the angio-room technicians prepare several sterile IAT kit including basic equipment such as syringes, 3-way check valves, flushing lines, 0.035 wire, and flushing lines, it can be especially helpful to decrease time duration in off-duty (Fig. 3D).
Fig. 3.
The flowchart of the preparation of angio-suite on (angio-room door opening, angio-machine switch on etc.) in image-to-puncture; (A) In the pre-IAT period, the arrival of the technician makes the angio-suite on, (B) In the post-IAT period, whether doctor or angio-room technician first visit angioroom, angio-suite on can start, (C) Serial pictures with the order in which the angio-machine is operated (these files are placed on the desktop of angio-room computer, which is easily accessible to anyone who first visit.), (D) Sterile IAT kit including basic equipment for endovascular procedure such as syringes, 3-way check valves, flushing lines, 0.035 wire, and flushing lines, it can be especially helpful to decrease time duration in off-duty. IAT, intra-arterial mechanical thrombectomy.
Endovascular IAT protocol
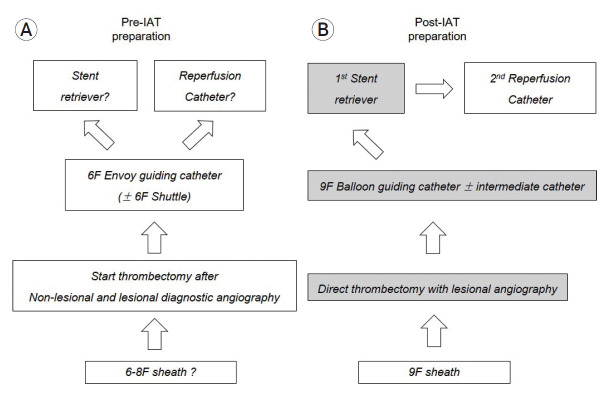
In the puncture-to-recanalization period, there are flowchart of the preparation of all devices for the endovascular procedure (Fig. 4) In the pre-IAT period, from the femoral sheath to thrombectomy devices (stent or reperfusion catheter), no standardized protocol for what instrument to use. We usually performed non-lesional (contralateral side ICA of occluded vessel and vertebral artery) and lesional (the side of occluded vessel) side angiography and then started the procedure for endovascular thrombectomy. And so, after the post-IAT period, standardized protocol is set for which instrument to use (Fig. 4A, B).
Fig. 4.
The flowchart of the preparation of all devices which is necessary for the IAT in puncture-to-recanalization; (A) In the pre-IAT period, from the femoral sheath to devices (stent or reperfusion catheter), no standardized protocol, (B) In the post-IAT period, through the whole procedure, standardized protocol is set for which instrument to use. IAT, intra-arterial mechanical thrombectomy.
In the post-IAT setting, 9 F femoral sheath was placed in the femoral artery, and 9 F balloon-guiding catheter is used for direct access proximal to target occlusion site. 9 F balloon guiding catheter is usually placed on distal common carotid artery (CCA) or proximal internal carotid artery (ICA). A 5 F or 6 F intermediate catheter system (Sofia, Microvention, Tustin, CA, USA) was introduced and placed proximal to target artery. Through the intermediate catheter, retrievable stent (Solitaire FR, ev3, Irvine, CA, USA) was first tried during endovascular thrombectomy. The thrombus was placed within the center of total length of retrievable stent in consideration of no presence of stent strut to distal 5-10 mm of the proximal stent marker, the stent was deployed completely by pulling back the micro-catheter near proximal to the proximal stent marker. And then, angiography was performed to confirm the immediate flow restoration for adequate stent placement. From deployment to pullback of stent, it was left for about 5 minutes to allow the thrombus formation to be embedded within the stent. The Solitaire FR stent and the micro-catheter were pulled back simultaneously after inflating the balloon of balloon-guiding catheter (BGC). And then, regardless of the presence of clot retrieval in the stent strut, aspirations were performed two to three times with a 50 cc syringe to remove the floating thrombus that was cut off from the main thrombus and migrated to distal.
After the failure of recanalization with stent retriever, we tried penumbra reperfusion catheter. we placed the catheter just proximal to the clot occlusion site, and used the negative pressure at the proximal tip by continuous aspiration with a 50 mL syringe. Then, the catheter was carefully pulled back.
Also, we have made endovascular IAT protocol that includes detailed procedures according to the location of the occluded vessel, such as MCA and ICA. First, MCA protocol were as follows (Fig. 5). We divided the occlusion site into non-bifurcation vs. bifurcation type thrombus. Bifurcation type thrombus is usually located in distal M1 site involving the MCA bifurcation. This occlusion site (distal M1 occlusion) is high probability of embolic occlusion, and non-bifurcation occlusion (proximal M1 occlusion) is likely to be Intracranial artery stenosis (ICAS) (Fig. 5). To be suspicious of ICAS, Intra-arterial tirofiban (Aggrastat®) infusion (0.5-1.5 mg) through the micro-catheter was achieved in just proximal portion of the occlusion site. In the patient refractory to tirofiban infusion, for the balloon angioplasty, Gateway® percutaneous trans-luminal angioplasty (PTA) balloon (Stryker Neurovascular, Fremont, CA) was advanced and inflated very carefully through 0.014-inch exchange micro-wire (Transcend [Stryker Neurovascular, Natick, MA, USA]). Balloon was carefully inflated to near 80% of normal distal vessel. In the permanent stent deployment, intra-procedural loading with 300mg of aspirin and 325 mg of clopidogrel was initiated before the placement of rescue stenting.
Fig. 5.
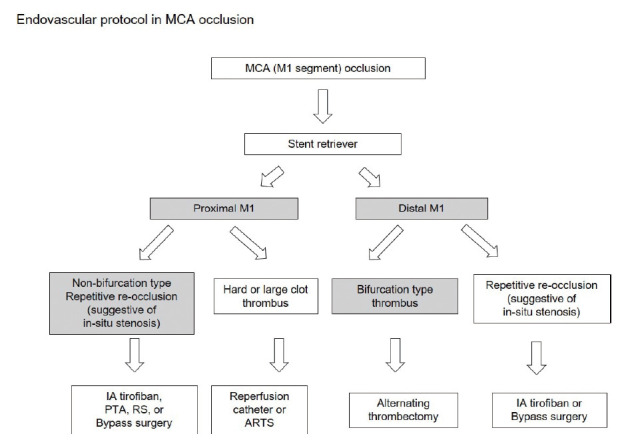
The flowchart of treatment modalities according to the location (proximal or distal) of occluded vessel in MCA occlusion. MCA, middle cerebral artery.
In ICA protocol, the treatment process includes whether carotid artery stenosis (CAS) is present. When it is suggestive of carotid artery stenosis, suction thrombectomy with guiding catheter or intermediate catheter was performed to remove blood clots as possible. Balloon angioplasty is then performed in CAS, irrespective of carotid stenting. Usually, in the patients with ICA occlusion, remote or contact aspiration were first used with a reperfusion catheter for recanalization. And then, in the distal ICA occlusion, a combination of stents and reperfusion catheters was usually performed (Fig. 6).
Fig. 6.
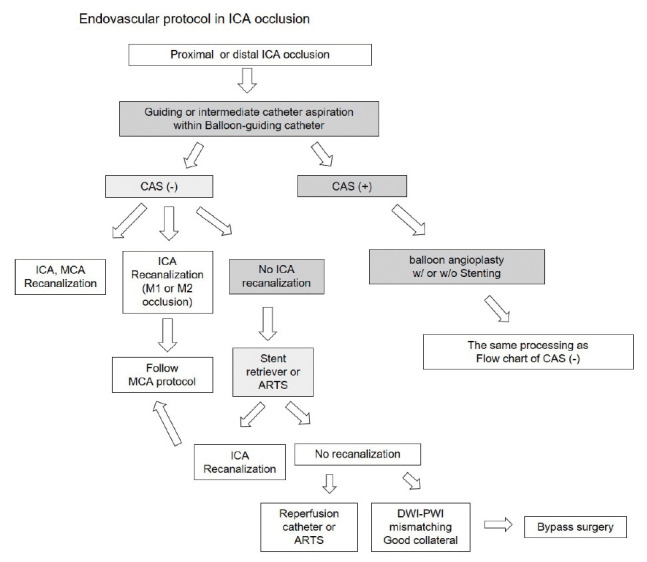
The flowchart of treatment modalities according to the location (proximal or distal) of occluded vessel and the presence of CAS in ICA occlusion. CAS, carotid artery stenosis; ICA, internal carotid artery.
Outcome measurements
The extent of recanalization was classified according to the Thrombolysis in cerebral infarction (TICI) grading scale. TICI grade 2b and 3 were successful recanalization, whereas TICI grades 0 to 2a were unsuccessful recanalization. 3-month follow-up scores were obtained by telephone follow-up or an outpatient visit by nursing practitioner. Baseline characteristics included age, gender, the location of occluded vessel in anterior circulation (M1, M2, and ICA). The outcomes were measured according to post-procedural TICI grade, the modified Rankin Score (mRS) scores at 3 months. The good and poor outcome of mRS score at 3 months were <2 and 3-6, respectively. And, we divided into two groups: prevs. post-IAT group. To assess the effectiveness of IAT protocol, we checked the time duration (min) in each three steps: 1) door-to-image, 2) image-to-puncture, and 3) puncture-to-recanalization, respectively.
Statistical analysis
Statistical analysis was performed with commercial software (SPSS, version 15.0, SPSS Inc., De, USA). The descriptive analysis for each data is presented as means±standard deviation. The Chi-square’s test was used for the statistical significance of the independent variables (Gender, age, the location of occluded vessel, successful recanalization TICI 2b-3, and good outcome mRS 0-2 at 3-month) between pre- and post-IAT groups. Also, independent t-test was used for the statistical significance to compare the time duration including 1) door-to-image, 2) image-to-puncture, and 3) puncture-to-recanalization between two groups. A p-value of less than 0.05 was considered significant.
RESULTS
All 267 patients who underwent IAT in acute stroke of anterior circulation were included in this study. There are 50 patients with pre-IAT protocol from Sep 2012 to Apr 2014 and 217 patients with post-IAT protocol from May 2014 to Jul 2018), respectively. Baseline characteristics such as age, gender, the location of occluded vessel, and the presence of successful recanalization is shown in Table 1.
Table 1.
Baseline characteristics of the patients treated with preand post-IAT protocol
| Variables | Pre-IAT (N=50) | Post-IAT (N=217) | p value |
|---|---|---|---|
| Gender (M:F) | 24:26 | 115:102 | >0.05 |
| Age | 65.4±17.5 | 69.8±15.3 | >0.05 |
| Location of occluded vessel | >0.05 | ||
| MCA M1 | 28 (56%) | 102 (47%) | |
| MCA M2 | 7 (14%) | 13 (6%) | |
| ICA | 15 (30%) | 102 (47%) | |
| Successful recanalization (TICI 2b, 3) | 39/50 (78%) | 185/217 (85.3%) | <0.05 |
| Good outcome (mRS 0-2) | 18/50 (36.0%) | 106/217 (48.8%) | >0.05 |
MCA, middle cerebral artery; ICA, internal carotid artery; TICI, thrombolysis in cerebral infarction; mRS, the modified Rankin Score.
In pre- and post-IAT group, the number of patients was 24 (47.1%) of 50 and 115 (52.9%) of 217 in male. Mean age of pre- and post-IAT group were 65.4±17.5 and 69.8±15.3, respectively. According to the location of occluded artery, there were as follows: 28 (56.0%) MCA M1, 7 (14.0%) MCA M2, and 15 (30.0%) ICA in pre-IAT group; 102 (47.0%) MCA M1, 13 (6.0%) MCA M2, and 102 (47.0%) ICA in post-IAT group, respectively. Age, gender, and the location of the occluded vessel have no statistical significance between two groups (p>0.05). Successful recanalization was achieved in 39/50 (78.0%) vs. 185/217 (85.3%) in pre- and post-IAT group.
Successful recanalization showed the statistical significance between two groups (p<0.05). There was no statistical significance according to good outcome (mRS 0-2) (p>0.05) between two groups. The mean percentage of pre- and post-IAT group had 36.0% (18/50) and 48.8% (106/217), respectively. Post-IAT group had a higher tendency of good outcome than pre-IAT group.
Additionally, time duration of door-to-image, image-to-puncture, and puncture-to-recanalization were as follows; 61.7±21.4 vs. 25±16.0 (p=0.000), 102.0±29.8 vs. 82.7±30.4 (p=0.001), and 79.1±47.5 vs. 58.4±75.3 (p=0.034), respectively. All three steps after IAT protocol had statistically significant time reductions than those before IAT protocol (p<0.05) (Table 2).
Table 2.
The comparison of time interval in door–to–image, image–to–puncture, and puncture–to–recanalization period between pre-and post-IAT group
| Pre-IAT (N=50) | Post-IAT (N=217) | p value | |
|---|---|---|---|
| Door-to-Image (min) | 61.7±21.4 | 25±16.0 | 0.000 |
| Image-to-puncture (min) | 102.0±29.8 | 82.7±30.4 | 0.001 |
| Puncture-to-recanalization (min) | 79.1±47.5 | 58.4±75.3 | 0.034 |
IAT, intra-arterial mechanical thrombectomy
DISCUSSION
The protocol during door-to-image period
The time duration from symptom onset to recanalization of occluded artery result in the clinical outcomes of patient with major arterial occlusion. Recent several trials have shown that median time duration from symptom onset to groin puncture was 212, 330, and 245 minutes in IMS III, MR RESCUE, and Synthesis expansion, respectively [3,5,12]. This mainly resulted from the use of older devices and delayed time duration from stroke onset to initiation of endovascular treatment. And so, endovascular thrombectomy showed the lower recanalization rate in acute stroke patients, that is suggested to be inferior to IV thrombolysis.
And, time duration of door to puncture and image to puncture mainly affect successful recanalization rate. It is reported that every additional hour from door to puncture and from imaging to puncture has the association with a 22% and 26% decrease in the odds of TICIJ 2b/3 successful recanalization,2) respectively. The most likely explanation is as follows: 1) thrombus modification (higher fibrin contents and more organized configuration of old thrombus compared to fresh thrombus), 2) strong adhesion of the thrombus within the vessel wall over time which makes entrapment and retrieval of the thrombus more difficult [6,14].
As mentioned above, when the suspected stroke patient arrived to ER, neurologist, interventional neurologist, and endovascular neurosurgeon receive warm up call message, which is called as “Stroke Fast Track”. This notification induces stroke team members to wait and prepare for possible EVT. According to a survey of stroke centers in the US, initial contact with a stroke team physician was made prior to image acquisition at 86% (25/29) of centers [10].
After the detection of no intracranial hemorrhage, rapid and non-invasive CT angiography (CTA) supply the important information on occluded vessel of the stenosis degree of extra- and intra-cranial vasculature. Currently, stroke guideline recommends CT/CTA to be an initial assessment of possible acute stroke patient [4,23]. We usually perform non contrast CT/Multiphase CT angiography within 6 hours and we check Brain MRI with MRA in the patient with symptom onset 6-24 hours. Although several imaging modalities is applied to suspected acute stroke patient, these imaging modalities should not delay intra-venous tPA infusion or endovascular thrombectomy [4,9]. Intra-venous tPA can be administered in the CT room immediately after making decision by stroke physician. We included several system in IAT protocol to decrease time duration in this door-to-image period, 1) rapid notification of suspected stroke patient arrived to ER to stroke team, 2) warm up call message, which is called as “Stroke Fast Track”, waiting and preparing for EVT, 3) Direct transportation to CT room, 4) rapid acquisition and administration of intra-venous line route for intra-venous tPA, consciousness sedation, and anti-hypertensive medication.
The protocol during image-to-puncture period
After the acquisition of brain imaging, the “no turn back” approach to ER is an important option to decrease door-to-puncture and image-to-puncture time eligible for EVT. To prevent no return from CT room to ER, we should get intra-venous line route in CT room. We should not return to ER for unnecessary L-tube insertion. If the L-tube insertion for aspirin plus clopidogrel medication is necessary for the process of EVT, we perform this procedure after the arrival in angioroom. And, in the aspect of iv-tPA administration, the patient who is eligible for EVT should be directly transported to the angio-suite without returning to the emergency room [1,24].
When door-to-image process is in progress from ER to CT room, it begins to prepare in angioroom simultaneously for the image-to-angioroom and angioroom-to-puncture. As the essential members of stroke team, there are angio-room technician and nurse, and they prepare the angiography suite for the EVT in advance [7]. This team-based approach resulted in a marked reduction in the treatment time duration and increase in the number of patients successfully treated in the American Heart Association’s Best Practice Strategies [15].
As shown in Fig. 3(D), a standardized IAT kit can be made and equipped in the angiography suite. This kit includes basic equipment such as variable sized syringes, trays, 3-way check valves, flushing lines, 9 F femoral sheath, 5 F angiography catheter and 0.035-inch guidewire, rotating hemostatic valves. This kit is helpful to decrease the time duration in angiography suite, especially in off-duty time. Previous paper reported that a standardized stroke kit, so called as BRISK (brisk recanalization ischemic stroke kit), was used for decreasing time duration in all processes [7].
In most cases of our stroke patient, we perform consciousness anesthesia (CS) to prevent the patient from moving in advance. And for CS, we use Precedex®, which of loading dose can cause the blood pressure to drop suddenly, so we give it maintenance dose. Several studies reported that the use of CS has more benefits, such as in-hospital mortality, hospital costs, rate of pneumonia, and length of hospital stay, compared to general anesthesia (GA) [8,17]. However, recent randomized controlled studies shown that there was no difference between the use of CS and GA in neurologic outcomes 90 days after stroke [26]. In our opinion, CS has the following advantages: 1) It takes a short time to prepare, 2) neurological symptoms can be identified during the procedure, 3) anesthesia machine is not required. Whether it is consciousness or general anesthesia, it is better option to prepare rapidly according to the protocol made by each hospital.
We summarize several contents on workflow of this image-to-puncture period to decrease time duration, 1) not wait for the response of tPA, 2) the activation of stroke team eligible for EVT, 3) Rapid direct transportation from CT room to angiography-suite, 4) consciousness sedation rather than general anesthesia during the procedure, and 5) In-advance preparation of angiography-suite including the switch on of angio-machine, basic equipment, and all devices for EVT.
The protocol during puncture-to-recanalization period
Several factors can affect the time duration from puncture to final recanalization. They include the patient condition, the status of respiration, sudden vomiting, severe movement by agitation, sudden headache by the stretch of vessel associated with stent retriever, and the sudden reduction of heart rate (HR) or blood pressure (BP), especially in PTA or stenting in CAS. In addition to this, a life-saving protocol and cardio-pulmonary resuscitation kit are required for managing the patient in sudden cardiac or respiratory arrest.
First, for the preparation of all device including sheath, angio-catheter, intermediate catheter, micro-catheter, and stent or balloon, it is helpful for the operator to determine all devices in advance. We made a flowchart on the preparation of all devices in the EVT in our institute. (Fig. 4A and B) In the pre-IAT period, there are no standardized setting what instrument to use from the femoral sheath to thrombectomy devices (the order of stent retriever or reperfusion catheter etc.) For assessing the Willisian collateral status, inessential process such as non-lesional angiography was performed before lesional angiography. In the post-IAT period, through the whole procedure, non-lesional angiography was not done for decreasing unnecessary time interval (Fig. 4A). And, standardized protocol is set for which instrument to use, such as 9 Fr femoral sheath, 9 Fr balloon-guiding catheter, 5 Fr diagnostic angiography (lesional direct angiography), 5 or 6 Fr intermediate catheter, micro-catheter, the first choice of stent retriever, reperfusion catheter after the failure of stent retriever.
Before the start of procedure, we are likely to overlook the CT angiography but have to review it once. Head and neck CT angiography inform the many helpful information in the planning of EVT. This contains the difficulties approaching the target vessel, the selection of intermediate catheter to overcome the tortuous vessel, the prediction of the failure of catheter advance because of severely tortuous vessel, and the preparation of the possibilities of carotid puncture etc. Similarly, it is reported that CTA includes these considerations, such as selection of an appropriate catheter to overcome tortuosity, optimal location for balloon guide catheter placement, and deployment zones for retrieval stent or an aspiration catheter [20].
And, from the operator’s point of view, to make decision on the next procedure and prepare the next device after the failure of the thrombectomy can interfere with rapid progress. In our opinion, when the vessel is not recanalized, it is important to predict the next procedure in advance and prepare necessary devices rather than to determine what to do at that time. Then, we made the MCA and ICA protocol to predict the next step of the procedure. For example, in the endovascular protocol of MCA occlusion, we can predict and prepare the device according to the location of occluded vessel. (Fig. 5) And if the distal M1 occlusion happen, stent retriever only may be necessary which is suggestive of embolic occlusion. But, in the proximal M1 occlusion, we can consider and prepare another device and medication. When severe intracranial stenosis (ICAS) is suggested in the location of occluded vessel, we prepare aspirin 300 mg and clopidogrel 325 mg loading dose (L-tube preparation in low consciousness level patient), the Intra-arterial tirofiban (Aggrastat®) infusion (0.5-1.5 mg) through the micro-catheter, Gateway® PTA balloon (Stryker Neurovascular, Fremont, CA), with or without Rescue stenting (Solitaire AB stent). Also, in the endovascular protocol of ICA occlusion, we can prepare the carotid PTA balloon and stenting in advance (Fig. 6).
In last step, we summarize several contents on flowchart of this puncture-to-recanalization period to decrease time duration, 1) confirm important information of proximal vessel tortuosity or target vessel location etc. in CT angiography, 2) share the changed situation with the neurologist, technician and nurse in angioroom, 3) predict and prepare systemic anti-platelet agent and intra-arterial anti-thrombolytic agent through the micro-catheter in advance, 4) standardization of thrombectomy procedure.
Finally, there are several literatures that they compared time duration or clinical outcomes before and after the protocol from door to recanalization [1,7,21]. It was reported that the implementation of a standardized protocol resulted in a significant reduction in time to recanalization for patients with an ischemic stroke [7]. Another papers demonstrated that the protocol through door-to-recanalization leads to time reduction in whole process and better clinical outcome at 3 months before and after the protocol [1,21]. Our study, like other papers, had similar result in that it reduced the time interval by creating IAT protocols and produced good clinical outcomes. However, there was no statistical significance according to good outcome (mRS 0-2) (p>0.05) between two groups. Post-IAT (48.8%, 106/217) group had a higher tendency of good outcome than pre-IAT group (36.0%, 18/50). Also, additional endovascular procedure itself protocol according to the failed thrombectomy can be implemented in predicting and preparing the next process without delay that has its merits.
This study has several limitations due to retrospective analysis, and small number of patients. These data come from a single comprehensive stroke center and are not generalized to all stroke centers. There was a big difference in the number and data available for the pre-IAT period patients compared to post-IAT patients. There was no significant difference in 3-month good outcome, which is most likely due to small sample size and confounding baseline characteristics of the patients. This study was conducted mainly for process improvement purposes.
CONCLUSIONS
We suggest that the application of systemic and endovascular IAT protocols showed a significant time reduction for faster recanalization in patients with acute major arterial occlusion. Post-IAT (48.8%, 106/217) group had a higher tendency of good outcome than pre-IAT group (36.0%, 18/50). To build-up the well-designed IAT protocol according to each step of door-to-image, image-to-puncture, and puncture-to-recanalization can be needed to decrease time duration and improve clinical outcome in recanalization therapy in the patients with acute major arterial occlusion.
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
REFERENCES
- 1.Aghaebrahim A, Streib C, Rangaraju S, Kenmuir CL, Giurgiutiu DV, Horev A, et al. Streamlining door to recanalization processes in endovascular stroke therapy. J Neurointerv Surg. 2017 Apr;9(4):340–5. doi: 10.1136/neurintsurg-2016-012324. [DOI] [PubMed] [Google Scholar]
- 2.Bourcier R, Goyal M, Liebeskind DS, Muir KW, Desal H, Siddiqui AH, et al. Association of time from stroke onset to groin puncture with quality of reperfusion after mechanical thrombectomy: a meta-analysis of individual patient data from 7 randomized clinical trials. JAMA Neurol. 2019 Apr;76(4):405–11. doi: 10.1001/jamaneurol.2018.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013 Mar;368(10):893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casaubon LK, Boulanger JM, Blacquiere D, Boucher S, Brown K, Goddard T, et al. Canadian stroke best practice recommendations: hyperacute stroke care guidelines, update 2015. Int J Stroke. 2015 Aug;10(6):924–40. doi: 10.1111/ijs.12551. [DOI] [PubMed] [Google Scholar]
- 5.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013 Mar;368(10):904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fennell VS, Setlur Nagesh SV, Meess KM, Gutierrez L, James RH, Springer ME, et al. What to do about fibrin rich ‘tough clots’? Comparing the Solitaire stent retriever with a novel geometric clot extractor in an in vitro stroke model. J Neurointerv Surg. 2018 Sep;10(9):907–10. doi: 10.1136/neurintsurg-2017-013507. [DOI] [PubMed] [Google Scholar]
- 7.Frei D, McGraw C, McCarthy K, Whaley M, Bellon RJ, Loy D, et al. A standardized neurointerventional thrombectomy protocol leads to faster recanalization times. J Neurointerv Surg. 2017 Nov;9(11):1035–40. doi: 10.1136/neurintsurg-2016-012716. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015 Mar;372(11):1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 9.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 Mar;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 10.Kansagra AP, Meyers GC, Kruzich MS, Cross DT, 3rd, Moran CJ. Wide variability in prethrombectomy workflow practices in the United States: a multicenter survey. AJNR Am J Neuroradiol. 2017 Dec;38(12):2238–42. doi: 10.3174/ajnr.A5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khatri P, Yeatts SD, Mazighi M, Broderick JP, Liebeskind DS, Demchuk AM, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014 Jun;13(6):567–74. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013 Mar 7;368(10):914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DH, Kim B, Jung C, Nam HS, Lee JS, Kim JW, et al. Consensus statements by Korean Society of Interventional Neuroradiology and Korean Stroke Society: hyperacute endovascular treatment workflow to reduce door-to-reperfusion time. Korean J Radiol. 2018 Sep-Oct;19(5):838–48. doi: 10.3348/kjr.2018.19.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017 Aug;82(2):223–32. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 15.Leifer D, Bravata DM, Connors JJ, 3rd, Hinchey JA, Jauch EC, Johnston SC, et al. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow-up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Mar;42(3):849–77. doi: 10.1161/STR.0b013e318208eb99. [DOI] [PubMed] [Google Scholar]
- 16.Lin CB, Peterson ED, Smith EE, Saver JL, Liang L, Xian Y, et al. Emergency medical service hospital prenotification is associated with improved evaluation and treatment of acute ischemic stroke. Circ Cardiovasc Qual Outcomes. 2012 Jul;5(4):514–22. doi: 10.1161/CIRCOUTCOMES.112.965210. [DOI] [PubMed] [Google Scholar]
- 17.McDonald JS, Brinjikji W, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anaesthesia during mechanical thrombectomy for stroke: a propensity score analysis. J Neurointerv Surg. 2015 Nov;7(11):789–94. doi: 10.1136/neurintsurg-2014-011373. [DOI] [PubMed] [Google Scholar]
- 18.Mehta BP, Leslie-Mazwi TM, Chandra RV, Bell DL, Sun CH, Hirsch JA, et al. Reducing door-to-puncture times for intra-arterial stroke therapy: a pilot quality improvement project. J Am Heart Assoc. 2014 Nov;3(6):e000963. doi: 10.1161/JAHA.114.000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015 May;275(2):510–20. doi: 10.1148/radiol.15142256. [DOI] [PubMed] [Google Scholar]
- 20.Menon BK, Demchuk AM. Computed tomography angiography in the assessment of patients with stroke/TIA. Neurohospitalist. 2011 Oct;1(4):187–99. doi: 10.1177/1941874411418523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panezai S, Meghpara S, Kulhari A, Brar J, Suhan L, Singh A, et al. Institution of code neurointervention and its impact on reaction and treatment times. J Vasc Interv Neurol. 2020 Jan;11(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Patel MD, Rose KM, O’Brien EC, Rosamond WD. Prehospital notification by emergency medical services reduces delays in stroke evaluation: findings from the North Carolina stroke care collaborative. Stroke. 2011 Aug;42(8):2263–8. doi: 10.1161/STROKEAHA.110.605857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015 Oct;46(10):3020–35. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi AI, Egila H, Adil MM, Siddiqi H, Mian N, Hassan AE, et al. “No turn back approach” to reduce treatment time for endovascular treatment of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014 May-Jun;23(5):e317–23. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016 Sep;316(12):1279–88. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 26.Schregel K, Behme D, Tsogkas I, Knauth M, Maier I, Karch A, et al. Effects of workflow optimization in endovascularly treated stroke patients - a pre-post effectiveness study. PLoS One. 2016 Dec;11(12):e0169192. doi: 10.1371/journal.pone.0169192. [DOI] [PMC free article] [PubMed] [Google Scholar]