Abstract
Mannose has recently drawn extensive attention for its substantial anti-cancer activities, but the underlying mechanism remains largely unclear. The aim of this study was to investigate the effects of mannose on experimental colitis-associated colorectal tumorigenesis and underlying mechanisms. Data clearly showed that at plasma concentrations achieved after oral administration, mannose slightly affected malignancy of tumor cells or tumor promoter-induced transformation of pre-neoplastic cells, but substantially suppressed manifestation of the M2-like phenotype of tumor-associated macrophages (TAMs) in a cancer cell and macrophage co-culture model. Mechanistically, mannose might greatly impair the production of tumor cell-derived lactate which has a critical role in the functional polarization of TAMs. Importantly, oral administration of mannose protected mice against colitis-associated colorectal tumorigenesis by normalizing TAM polarization. Collectively, these findings highlight the importance of TAMs in colorectal tumorigenesis, and provide a rationale for introducing mannose supplementation to patients suffering from inflammatory bowel diseases.
Keywords: Colorectal cancer, Mannose, Tumor-associated macrophages, Lactate
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide in 2020, and it is estimated that approximately 1.9 million new cases will occur [1]. Despite modern advances in medicine, the efficacy of CRC therapies remains poor overall. Fortunately, most of CRC cases are sporadic and colorectal carcinogenesis is a multistage process [2]. Analysis of colorectal tumors might help discover molecular targets for CRC chemoprevention. For example, inflammation is linked clinically and epidemiologically to CRC, and COX-2 appears to play a causative role [3,4]. Although selective inhibition of COX-2 has been the gold standard for CRC preventive interventions, its potential cardiovascular side effects have greatly dampened enthusiasm for its long-term intake [5]. Given the high prevalence and poor outcome of CRC, chemoprevention of CRC is a global priority, and identifying novel preventive agents for CRC is a clinical aspiration.
Emerging evidence has indicated that tumor microenvironment (TME) mediates cancer progression. In addition to its pivotal roles in epithelial mesenchymal transition, TME participates in tumor-associated angiogenesis and immune escape [6,7]. The communication between tumor and tumor-associated macrophages (TAMs) has long been implicated in TME, while tumor cell-derived lactate was recently reported to act as an extracellular signaling molecule modulating TAM polarization [8]. Of note, the Warburg effect is a hallmark of cancer, and resulting accumulation of lactate is a common event in tumors as well as premalignant lesions [9]. Collectively, lactate might be a new player in TME.
Mannose is a naturally occurring monosaccharide. It is widely present in various fruits especially in cranberry, peach, and apple [10-12]. In addition to its anti-bacterial, anti-inflammatory and immune-regulatory activities, mannose recently drew extensive attention for its substantial anti-cancer properties [13-18]. Administration alone or in combination with chemotherapy, mannose could suppress tumor growth in xenograft mouse model [19]. Given that mannose is a C-2 epimer of glucose, the compound has been proposed to interfere with glucose metabolism and thereby suppress cancer cell growth. However, one caveat is that the concentration of mannose (25 mM) required to substantially impair cancer cell viability may be much higher than that reached in plasma after oral administration. As such, it pointed to the possibility that other mechanisms may be at play. In the present study, we investigated whether mannose might prevent CRC by targeting tumor cell-derived lactate production as well as its related TAM polarization.
MATERIALS AND METHODS
Materials and chemicals
Primary antibody against hypoxia-inducible factor 1-alpha (HIF-1α) was obtained from Abcam (Cambridge, UK). Primary antibody against F4/80 was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). All other primary antibodies (hexokinase II [HKII], glucose transporters 1 [GLUT1], COX-2, CD206, and arginase-1 [Arg-1]) were obtained from Cell Signaling Technology (Danvers, MA, USA). Other chemicals were purchased from Sigma (St Louis, MO, USA).
Cell culture
Human colon cell lines HCT116, HT29, and SW620, colon cell line CT26, the human monocytic leukemia cell line THP-1, and the mouse JB6 P+ cells were purchased from American Type Culture Collection (Manassas, VA, USA). HCT116, HT29, SW620, CT26, and THP-1 cells were incubated in RPMI1640 medium containing 10% calf bovine serum at 37°C and 5% CO2. JB6 P+ cells were incubated in Eagle’s minimum essential medium (EMEM) medium contained 5% calf bovine serum at 37°C and 5% CO2.
Cell viability
Cell viability was measured by the MTS assay. Colorectal cancer cells (5 × 103) were inoculated in 96-well plates. After mannose treatment for 48 hours, cell viability was measured according to the standard method.
Anchorage-independent growth assay
Malignant transformation was measured by the soft agar assay as described [20,21]. Firstly, agar growth medium was applied over the bottom of a 6-well plate. Next, JB6 P+ cells (8 × 103) were added in a 6-well plate over this layer, and were treated with 10 μg/mL 12-O-tetradecanoylphorbol-13-acetate (TPA) or 10 μg/mL epidermal growth factor (EGF) to induce neoplastic transformation. The number of colonies was analyzed for neoplastic transformation. For unanchored growth of colorectal cancer cells, 3 mL basal modified Eagle’s medium (BME) with 1.25% agar was added to 6-well plates. HCT116 and HT29 cells (8 × 103) were seeded on the upper layer of 6-well plates with 0.5% agar BME medium. After fourteen days of culture, colony formation in soft agar was counted [22].
Real-time qPCR assay
RNA was isolated from colonic tissues and cellular samples by Trizol. For qPCR analysis, total RNA (1 μg) was reverse transcribed into cDNAs. cDNAs were used for qPCR analysis according to the manufacturer’s protocols [23]. All primers used are listed in Table S1. The mRNA levels were presented as relative values compared with β-actin.
Western blot analysis
Protein sample was isolated from colonic tissues and cellular fragments by radio-immunoprecipitation assay (RIPA) buffer. The western blot assay was performed on the basis of previous protocols [24]. The protein blots were detected by an enhanced electrochemiluminescence (ECL) chemiluminescence reaction reagent.
Animal study
The protocols for all animal experiments were approved by the Institutional Animal Ethics Committee of Jiangnan University (JN. No 20201230c04601317 [387]). Seven-week-oldmale C57BL/6N mice (Beijing Vital River Laboratory Animal Technology Co., Ltd., China, Beijing) were raised at the barrier facility in the experimental animal center of Jiangnan University. The conditions of the barrier facility are photological cycle (12/12 hours dark/light period), constant humidity (50 ± 5%), and stable temperature (20 ± 2°C).
Animal model of AOM/DSS-induced colorectal cancer
After adaptation for 1 week, all mice were divided into five groups: Control group; azoxymethane (AOM)/dextran sodium sulfate (DSS) group; AOM/DSS + L Mannose group (1,000 mg/kg); AOM/DSS + H Mannose group (2,000 mg/kg); Mannose alone group (2,000 mg/kg). Mice received 10 mg/kg AOM by i.p. injection. After 7 days, 3% DSS was provided in the water bottle for 1 week. Then mice were switched to normal water for 2 weeks [25]. The above period was repeated three times. Meanwhile, mannose groups were treated with 1,000 or 2,000 mg/kg mannose via gavage once per day. The body weight and the survival rate of all groups were recorded weekly during the experimental period. At week 13, mice were sacrificed, and colorectal tumors were counted. After gross examination, the sectional tumors were fixed.
Animal models of DSS-induced colitis
As the same as CRC model, mice were divided into five groups: Control group; DSS group; DSS + L Mannose group (1,000 mg/kg); DSS + H Mannose group (2,000 mg/kg); Mannose control group (2,000 mg/kg). Mannose groups received 1,000 mg/kg and 2,000 mg/kg mannose by gavage once daily for 2 weeks. From the 8th day, 3% DSS was provided for 7 days and the body weight was recorded, whilst the disease activity index (DAI) was calculated according to the method of Liu et al. [26]. At the 14th day, mice were eutanized, and a series of apparent data was collected.
Histology and immunohistochemistry assays
Pathologic analysis was performed by hematoxylin-eosin (H&E) staining. The fixed colons were embedded and cut into thin sections followed by H&E staining. Immunohistochemical staining for F4/80 and CD206 was performed by using an immunohistochemistry kit (D601037-0020; Sangon Biotech, Shanghai, China) on the basis of the manufacturer’s protocols.
Statistical analysis
Statistical analysis used GraphPad Prism 8.0 Software (GraphPad Software, Inc., San Diego, CA, USA). All results were presented as mean ± standard deviation (SD). Data were analyzed by one-way ANOVA. Statistical significance was described as *P < 0.05, **P < 0.01, and ***P < 0.001 compared with controls and models.
RESULTS
Mannose attenuated colitis-associated colorectal tumorigenesis in mice
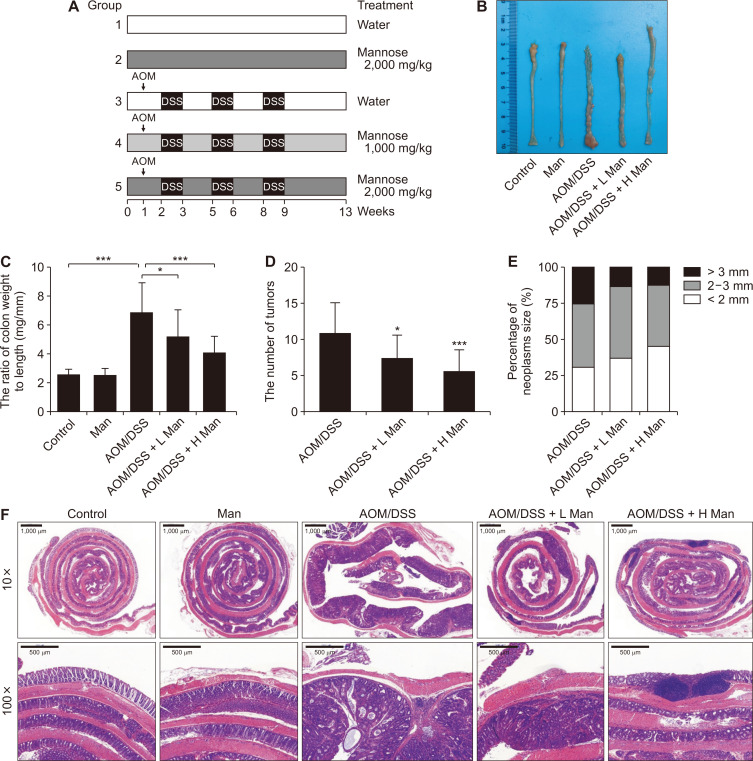
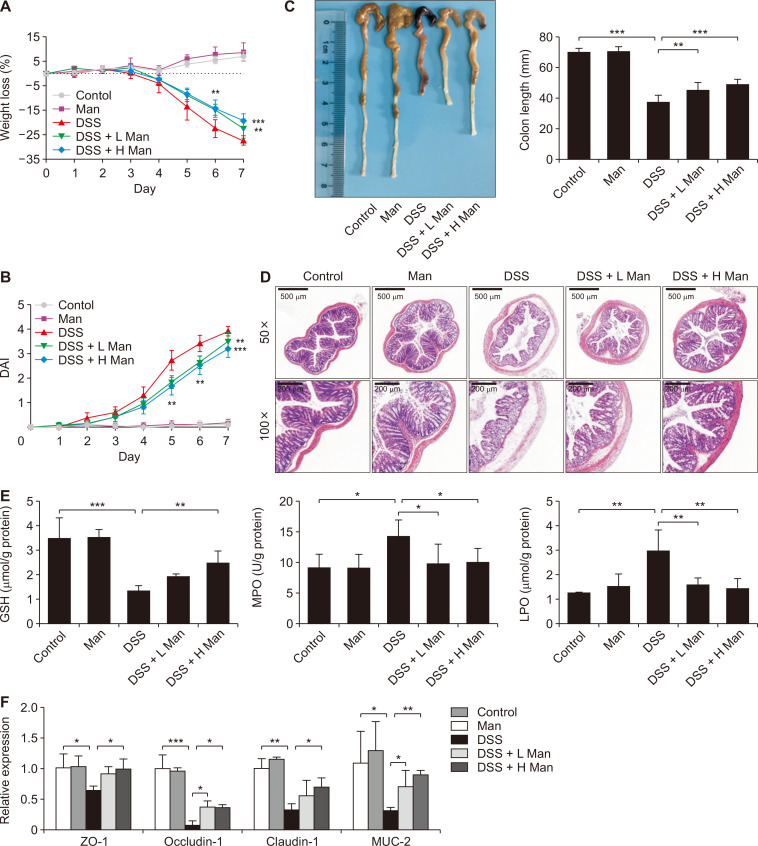
To assess the potential efficacy of mannose in colorectal cancer chemoprevention, a well-established colitis-associated carcinogenesis mouse model was adopted (Fig. 1A). Data clearly showed that oral administration of mannose for 14 weeks greatly reduced colonic mucosal thickening, the number and the size of tumors (Fig. 1B-1E). Pathological analysis showed that the thickness of the colon increased due to the tumor burden. There were many areas of inflammatory cell infiltration. However, these symptoms were lessened after mannose treatment (Fig. 1F). Inflammatory bowel disease is well-known as a risk factor for CRC. We found that mannose treatment greatly improved the symptoms of experimentally induced ulcerative colitis including body weight loss, colon shortening, and DAI (Fig. 2A-2C). Pathological analysis showed that crypt structures were destroyed and goblet cells decreased in colitis mice. These symptoms were lessened after mannose treatment (Fig. 2D). In addition, mannose ameliorated oxidative stress injury and intestinal barrier damage (Fig. 2E and 2F). Collectively, mannose protected against colitis-associated colorectal tumorigenesis in mice.
Figure 1. Mannose attenuated colitis-associated tumorigenesis.
(A) Experimental protocol for CRC animal model. Normal mice (Control); mice were treated with 2,000 mg/kg mannose (Man); mice treated with AOM plus DSS (AOM/DSS); mice treated with AOM plus DSS and 1,000 mg/kg mannose (AOM/DSS + L Man; mice treated with AOM plus DSS and 2,000 mg/kg mannose (AOM/DSS + H Man). (B) Representative photos of the colons. (C) Effect of mannose on the ratio of colon weight to colon length. (D) Effect of mannose on the number of tumors. (E) Effect of mannose on the percentage of different neoplasms sizes. (F) Representative H&E staining of the colon from each group of mice. Data represent the mean ± standard deviation (n = 10). CRC, colorectal cancer; AOM, azoxymethane; DSS, dextran sodium sulfate. *P < 0.05 and ***P < 0.001 compared with the AOM/DSS group.
Figure 2. Mannose alleviated DSS-induced colitis.
(A) Effect of mannose on weight loss rate. Normal mice (Control); Mice treated with 2,000 mg/kg mannose (Man); Mice treated with DSS (DSS); Mice treated with DSS and 1,000 mg/kg mannose (DSS + L Man); Mice treated with DSS and 2,000 mg/kg mannose (DSS + H Man). (B) Effect of mannose on DAI. (C) Representative photos and the length of colon. (D) Representative H&E staining of the colon from each group of mice. (E) Effect of mannose on oxidative stress (GSH, MPO, and LPO). (F) Effect of mannose on the intestinal barrier. Data represent the mean ± standard deviation (n = 10). DSS, dextran sodium sulfate; DAI, disease activity index; GSH, reduced glutathione; MPO, myeloperoxidase; LPO, lipid peroxide. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the DSS group.
Effects of mannose on malignant transformation of epithelial cells
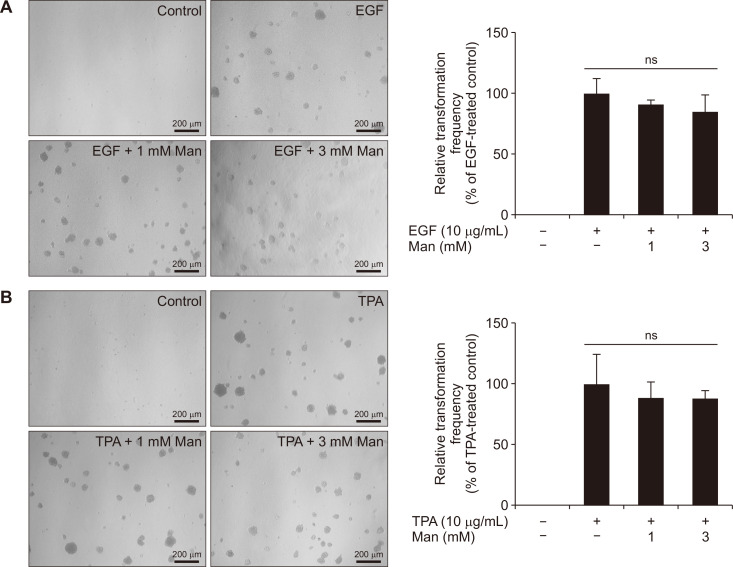
Malignant transformation of epithelial cells is a key factor for tumor development. To investigate the effect of mannose on malignant transformation in the early stage of CRC, we used JB6 P+ cells. For EGF-induced malignant transformation, mannose did not inhibit the colony formation (Fig. 3A). Likewise, mannose did not inhibit the TPA-induced malignant transformation (Fig. 3B). In general, our results showed that mannose did not affect malignant transformation of the colonic epithelial cells.
Figure 3. The effect of mannose on the neoplastic cell transformation.
(A) Mannose (Man) did not prevent the TPA-induced transformation of JB6 P+ cells (×100). (B) Mannose did not prevent the EGF-induced transformation of JB6 P+ cells (×100). Data represent the mean ± standard deviation. EGF, epidermal growth factor; TPA, 12-O-tetradecanoylphorbol-13-acetate; ns, not significant compared with the TPA or EGF groups.
Effects of mannose on colon cancer cell viability and anchorage-independent growth
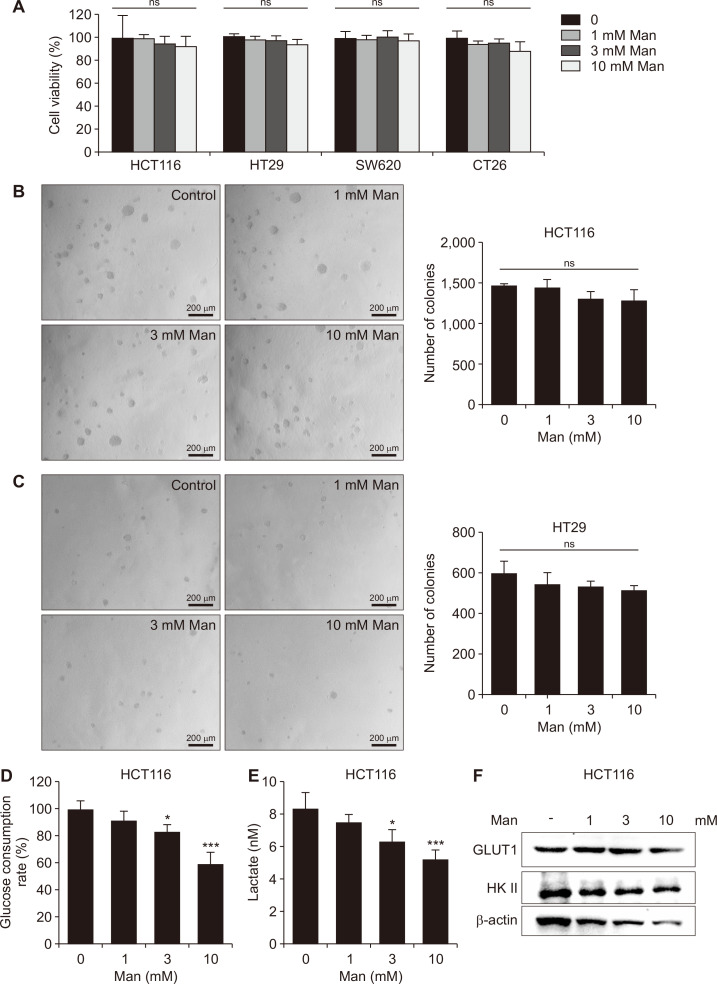
Limitless replicative potential and unanchored growth are two important malignant features of tumor cells. To elucidate the mechanism by which mannose exerted its cancer chemopreventive activity, we examined the effects of mannose on the viability and anchorage-independent growth of colon cancer cells. Unexpectedly, at concentrations reached in plasma or colon after oral administration of mannose, it failed to affect either cell viability or anchorage-independent growth ability in a panel of colorectal cancer cell lines (Fig. 4A-4C).
Figure 4. Mannose disturbed the glucose metabolism in colon cancer cells without affecting their growth.
(A) Effect of mannose (Man) on cell viability (HCT116, HT29, SW620, and CT26). (B, C) Effect of mannose on the anchorage-independent growth of colon cancer cells (×100). (D) Effect of mannose on the consumption of glucose in HCT116 cells. (E) Effect of mannose on production of lactate in HCT116 cells. (F) The protein levels of GLUT1 and HK II in HCT116 cells. The cells were treated with 1, 3, and 10 mmol/L mannose for 24 hours. Data represent the mean ± standard deviation. HK II, hexokinase II; GLUT1, glucose transporters 1; ns, not significant. *P < 0.05 and ***P < 0.001 compared with the control group.
Mannose inhibited production of lactate in colon cancer cells
Given that mannose might not directly affect malignant characteristics of cancer cells, we hypothesized that mannose prevented colorectal cancer via some indirect effects. Interestingly, at concentrations reached in plasma after oral administration of mannose, it could inhibit the consumption of glucose and the production of lactate in HCT116 cells (Fig. 4D and 4E). Then we tried to explain how mannose regulated the glucose metabolism in tumor cells. We found that the expression of HK II and GLUT1 decreased after mannose treatment in HCT116 cells (Fig. 4F). Generally speaking, our results suggested that mannose might inhibit the production of lactate via HK II.
Mannose attenuated colitis-associated tumorigenesis by targeting TAM polarization
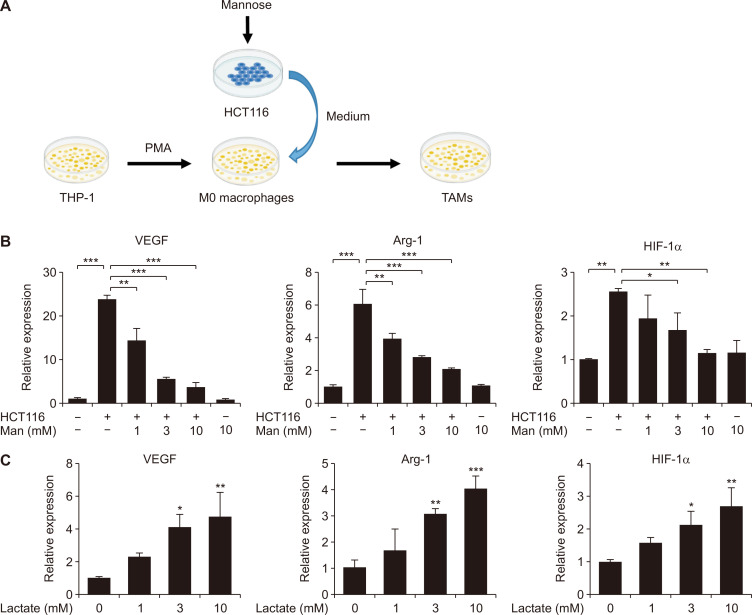
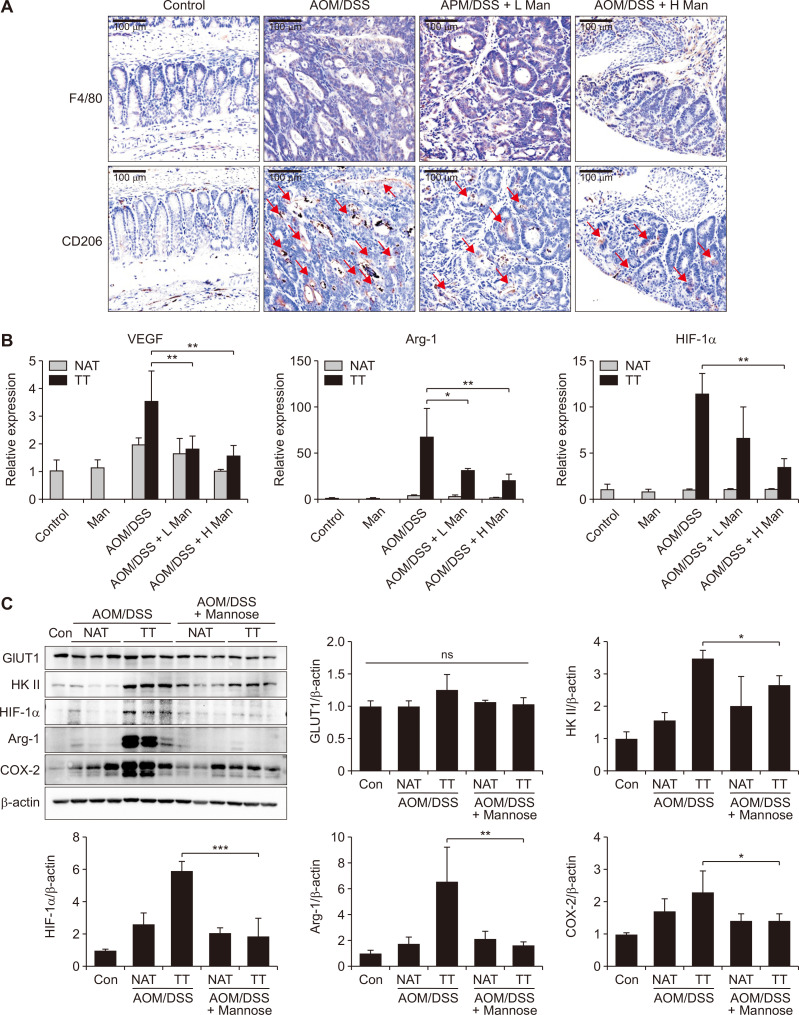
According to the previous reports, tumor-derived lactate could induce polarization of TAMs. Our results also confirmed that lactate could induce TAM polarization through upregulation of HIF-1α (Fig. 5C). TAMs possessed some important homeostatic actions that supported tumor maintenance and growth [27]. So we proposed that mannose attenuated colitis-associated tumorigenesis by targeting TAM polarization. Firstly, we incubated M0 macrophages with the medium of colon cancer cells to induce TAMs in vitro (Fig. 5A). Our results showed that mannose could inhibit TAM polarization in vitro. The expressions of TAM marker proteins including VEGF, Arg-1, and HIF-1α decreased after mannose treatment (Fig. 5B). Next, immunohistochemical analysis suggested that all CRC groups expressed high levels of the macrophage marker (F4/80). However, the expression of the TAM marker (CD206) decreased after mannose treatment (Fig. 6A). Moreover, consistent with the above observation, the expression levels of VEGF, Arg-1, and HIF-1α decreased in the tumor tissues of each group after mannose treatment (Fig. 6B and 6C). Lastly, we confirmed that mannose inhibited the expression HK II. At the same time, the expression levels of Arg-1, HIF-1α, COX-2, and HK II were significantly increased in tumor tissues compared with the normal adjacent tissues (Fig. 6C). Taken together our results suggested that mannose alleviated colitis-associated tumorigenesis by targeting TAMs.
Figure 5. Mannose regulated TAM polarization by lactate in vitro.
(A) Schematic diagram of method for construction of TAMs. (B) The mRNA levels of TAM markers (VEGF, Arg-1, and HIF-1α) in TAMs derived from THP-1 cells subjected to differentiation by PMA and subsequent stimulation with HCT116 cells. (C) Effect of lactate on the mRNA expression of VEGF, Arg-1, and HIF-1α during TAM polarization. THP-1 cells were differentiated into M0 macrophages by incubation with PMA for 24 hours. Then 1, 3, and 10 mmol/L lactate was treated to M0 THP-1 cells. Data represent the mean ± standard deviation. TAMs, tumor-associated macrophages; Arg-1, arginase-1; HIF-1α, hypoxia-inducible factor 1-alpha; PMA, phorbol 12-myristate 13-acetate. *P < 0.05, **P < 0.01 and ***P < 0.001.
Figure 6. Mannose attenuated colitis-associated tumorigenesis by targeting TAMs in mice.
(A) F4/80 and CD206 in colon tissues were detected by immunohistochemical analysis. (B) The mRNA levels of TAMs markers (VEGF, Arg-1, and HIF-1α) in colon tissues of each group mice. (C) The protein levels of GLUT1, HK II, HIF-1α, Arg-1, and COX-2 in the colon tissues. Data represent the mean ± standard deviation. TAMs, tumor-associated macrophages; HK II, hexokinase II; GLUT1, glucose transporters 1; Arg-1, arginase-1; NAT, normal adjacent tissue; TT, tumor tissue; ns, not significant; AOM, azoxymethane; DSS, dextran sodium sulfate; IHC, immunohistochemical. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the AOM/DSS group.
DISCUSSION
CRC is a serious malignancy associated with colonic mucosa inflammation [28]. Dietary factors are thought to modulate the risk of colorectal cancer [29]. For example, fruit and vegetable intake might reduce the risk of colorectal cancer [30,31]whereas red meat consumption would increase the risk of colorectal cancer [32]. So it is particularly important to select proper dietary factors for colorectal cancer prevention. Mannose is a natural monosaccharide present in lots of plants and fruits, such as cranberry and peach. Previous reports showed that mannose possessed numerous biological activities and a health-beneficial function [16,19]. However, its potential in colitis-associated colorectal cancer chemoprevention remains unclear.
In this study, we demonstrated that mannose inhibited colitis and colitis-associated colorectal cancer. In line with our results, revious studies suggested that the whole component of cranberry could be used as a dietary factor for colitis-related carcinogenesis [33,34]. It is noticeable that, fructose is also an isomer of glucose and has a high content in fruits. But within the tumors, fructose is converted to fructose-1-phosphate, resulting in activation of glycolysis and accelerating synthesis of fatty acids which promote tumor growth [35]. Our study provided a valuable suggestion for replacing fructose with mannose in food industry.
Furthermore, we found that mannose attenuated colitis and colitis-associated colorectal cancer by inhibiting inflammation and inducing TAM polarization. At the beginning of CRC, the inflammation has the most critical role in the pathogenesis [28]. Considering the fact that mannose has anti-inflammatory activity, its chemopreventive function against CRC might partly be attributable to the anti-inflammatory actions. Nevertheless, mannose inhibited lactate production in colon cancer cells through inhibition of HK II. As a result, TAM polarization induced by tumot-derived lactate was regulated. After mannose treatment, the expression of TAM markers (Arg-1 and VEGF) decreased. TAMs represent an important part of the TME. TAMs have some important homeostatic actions that contribute to tumor maintenance and growth [36]. The role of VEGF in promoting blood vessel formation and supporting tumor growth has been widely reported [37]. Arg-1 is involved in polyamines metabolism. Polyamines are essential metabolites for cell division and promote tumor cell proliferation in vitro [38]. These would be beneficial to the growth of tumor cells.
In previous studies, 25 mM mannose could inhibit tumor cell growth by interfering with glucose metabolism. But, it is worth noting that the plasma concentration of mannose is approximately 1.5 mM after oral administration (Fig. S1). It is much less than the concentration of mannose inhibiting tumor cell growth. Our results demonstrated that mannose did not directly affect the malignant characteristics of colorectal cancer cells at concentrations reached in plasma after oral administration. It attenuated colorectal cancer by targeting TAMs. In this context, our study provides a novel mechanism responsible for the anti-tumor effect of mannose.
Although our studies are promising, there are several weaknesses. For example, our results were mainly derived from animal and cell studies, thus the most significant issue is whether our results are applicable for CRC patients. To confirm whether mannose-rich diet intake is propitious to colorectal cancer patients, we need to carry out well-designed clinical studies. Moreover, our study is based on colitis-associated colorectal tumorigenesis. Therefore, pathogenic factors and pathological process would be distinguished from those of the sporadic colorectal cancer.
Gut microbiome plays an important role in the pathogenesis of CRC and is also a link between obesity and CRC [39,40]. Of note, mannose could alter gut microbiome, prevent diet-induced obesity, and improve host metabolism [41]. Therefore, the future investigation would be focused on determining whether mannose could improve colorectal cancer by regulating gut microbiota.
In conclusion, our investigation demonstrated that mannose effectively prevented CRC and might be worth further being investigated as a CRC chemopreventive dietary factor. Importantly, we explained for the first time that mannose might alleviate CRC in terms of regulating TAM polarization.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.15430/JCP.2022.27.1.31.
Footnotes
FUNDING
This work was supported by the National Natural Science Foundation of China (81773064 and 31972973), National Youth 1000 Talents Plan, the Jiangsu Specially-Appointed Professor Program, Jiangsu Province Recruitment Plan for High-level, Innovative and Entrepreneurial Talents (Innovative Research Team), and Collaborative innovation center of food safety and quality control in Jiangsu Province.
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 3.Cao L. Assessment of thyroid cancer risk in more than 334,000 patients with inflammatory bowel disease: a case-control study and a meta-analysis. World J Surg Oncol. 2018;16:182. doi: 10.1186/s12957-018-1485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 5.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 6.Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78:1019–33. doi: 10.1016/j.molcel.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z, Lu ZR. Molecular imaging of the tumor microenvironment. Adv Drug Deliv Rev. 2017;113:24–48. doi: 10.1016/j.addr.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37. doi: 10.1038/nrc3038. Erratum in: Nat Rev Cancer 2011; 11:618. [DOI] [PubMed] [Google Scholar]
- 10.Davis JA, Freeze HH. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta. 2001;1528:116–26. doi: 10.1016/S0304-4165(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D, Chia C, Jiao X, Jin W, Kasagi S, Wu R, et al. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med. 2017;23:1036–45. doi: 10.1038/nm.4375. [DOI] [PubMed] [Google Scholar]
- 12.Wei Z, Huang L, Cui L, Zhu X. Mannose: good player and assister in pharmacotherapy. Biomed Pharmacother. 2020;129:110420. doi: 10.1016/j.biopha.2020.110420. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Gu R, Zhu Y, Lian X, Wang S, Liu X, et al. D-mannose attenuates bone loss in mice via Treg cell proliferation and gut microbiota-dependent anti-inflammatory effects. Ther Adv Chronic Dis. 2020;11:2040622320912661. doi: 10.1177/2040622320912661.29ec727375984ef3b7769ee52f8b1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z, Miao J, Zhang T, He M, Zhou X, Zhang H, et al. d-Mannose suppresses osteoarthritis development in vivo and delays IL-1β-induced degeneration in vitro by enhancing autophagy activated via the AMPK pathway. Biomed Pharmacother. 2021;135:111199. doi: 10.1016/j.biopha.2020.111199. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Xie Q, Shen Y, Lu G, Yao H, Chen Y, et al. Involvement of mannose receptor in the preventive effects of mannose in lipopolysaccharide-induced acute lung injury. Eur J Pharmacol. 2010;641:229–37. doi: 10.1016/j.ejphar.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Torretta S, Scagliola A, Ricci L, Mainini F, Di Marco S, Cuccovillo I, et al. D-mannose suppresses macrophage IL-1β production. Nat Commun. 2020;11:6343. doi: 10.1038/s41467-020-20164-6.33a11b42ec3643e18f2c5585a702657e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sha J, Cao D, Cui R, Xia L, Hua X, Lu Y, et al. Mannose impairs lung adenocarcinoma growth and enhances the sensitivity of A549 cells to carboplatin. Cancer Manag Res. 2020;12:11077–83. doi: 10.2147/CMAR.S278673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Jalali Motlagh N, Wang C, Wojtkiewicz GR, Schmidt S, Chau C, et al. d-mannose suppresses oxidative response and blocks phagocytosis in experimental neuroinflammation. Proc Natl Acad Sci USA. 2021;118:e2107663118. doi: 10.1073/pnas.2107663118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez PS, O'Prey J, Cardaci S, Barthet VJA, Sakamaki JI, Beaumatin F, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature. 2018;563:719–23. doi: 10.1038/s41586-018-0729-3. [DOI] [PubMed] [Google Scholar]
- 20.Dong Z, Cmarik JL. Harvesting cells under anchorage-independent cell transformation conditions for biochemical analyses. Sci STKE. 2002;2002:pl7. doi: 10.1126/stke.2002.130.pl7. [DOI] [PubMed] [Google Scholar]
- 21.Kim JE, Son JE, Jeong H, Kim DJ, Seo SG, Lee E, et al. A novel cinnamon-related natural product with Pim-1 inhibitory activity inhibits leukemia and skin cancer. Cancer Res. 2015;75:2716–28. doi: 10.1158/0008-5472.CAN-14-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun G, Zheng Z, Lee MH, Xu Y, Kang S, Dong Z, et al. Chemoprevention of colorectal cancer by artocarpin, a dietary phytochemical from Artocarpus heterophyllus. J Agric Food Chem. 2017;65:3474–80. doi: 10.1021/acs.jafc.7b00278. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Zhang C, Zhang H, Li H. Xanthine oxidoreductase promotes the progression of colitis-associated colorectal cancer by causing DNA damage and mediating macrophage M1 polarization. Eur J Pharmacol. 2021;906:174270. doi: 10.1016/j.ejphar.2021.174270. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Wang W, Wang M, Liu X, Lee MH, Wang M, et al. High salt intake attenuates breast cancer metastasis to lung. J Agric Food Chem. 2018;66:3386–92. doi: 10.1021/acs.jafc.7b05923. [DOI] [PubMed] [Google Scholar]
- 25.Zhao R, Coker OO, Wu J, Zhou Y, Zhao L, Nakatsu G, et al. Aspirin reduces colorectal tumor development in mice and gut microbes reduce its bioavailability and chemopreventive effects. Gastroenterology. 2020;159:969–83.e4. doi: 10.1053/j.gastro.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Ding J, Zhang H, Shen J, Hao Y, Zhang X, et al. Lactobacillus casei LH23 modulates the immune response and ameliorates DSS-induced colitis via suppressing JNK/p-38 signal pathways and enhancing histone H3K9 acetylation. Food Funct. 2020;11:5473–85. doi: 10.1039/D0FO00546K. [DOI] [PubMed] [Google Scholar]
- 27.Shan K, Feng N, Cui J, Wang S, Qu H, Fu G, et al. Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J Cell Mol Med. 2020;24:8045–56. doi: 10.1111/jcmm.15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai B, Wu F, Ying K, Xu Y, Shan L, Lv Y, et al. Therapeutic effects of dihydroartemisinin in multiple stages of colitis-associated colorectal cancer. Theranostics. 2021;11:6225–39. doi: 10.7150/thno.55939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244–60.e16. doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song M, Lan Y, Wu X, Han Y, Wang M, Zheng J, et al. The chemopreventive effect of 5-demethylnobiletin, a unique citrus flavonoid, on colitis-driven colorectal carcinogenesis in mice is associated with its colonic metabolites. Food Funct. 2020;11:4940–52. doi: 10.1039/D0FO00616E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolk A. Potential health hazards of eating red meat. J Intern Med. 2017;281:106–22. doi: 10.1111/joim.12543. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Song M, Cai X, Neto C, Tata A, Han Y, et al. Chemopreventive effects of whole cranberry (Vaccinium macrocarpon) on colitis-associated colon tumorigenesis. Mol Nutr Food Res. 2018;62:e1800942. doi: 10.1002/mnfr.201800942. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Xue L, Tata A, Song M, Neto CC, Xiao H. Bioactive components of polyphenol-rich and non-polyphenol-rich cranberry fruit extracts and their chemopreventive effects on colitis-associated colon cancer. J Agric Food Chem. 2020;68:6845–53. doi: 10.1021/acs.jafc.0c02604. [DOI] [PubMed] [Google Scholar]
- 35.Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science. 2019;363:1345–9. doi: 10.1126/science.aat8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology. 2019;8:1596004. doi: 10.1080/2162402X.2019.1596004.9f95c976455e4abe9c185050ab0b1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 38.Chang CI, Liao JC, Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001;61:1100–6. [PubMed] [Google Scholar]
- 39.Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018;15:671–82. doi: 10.1038/s41575-018-0025-6. [DOI] [PubMed] [Google Scholar]
- 40.Peng M, Lee SH, Rahaman SO, Biswas D. Dietary probiotic and metabolites improve intestinal homeostasis and prevent colorectal cancer. Food Funct. 2020;11:10724–35. doi: 10.1039/D0FO02652B. [DOI] [PubMed] [Google Scholar]
- 41.Sharma V, Smolin J, Nayak J, Ayala JE, Scott DA, Peterson SN, et al. Mannose alters gut microbiome, prevents diet-induced obesity, and improves host metabolism. Cell Rep. 2018;24:3087–98. doi: 10.1016/j.celrep.2018.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.