Abstract
Background
Diabetic renal disease (diabetic nephropathy) is a leading cause of end‐stage renal failure. Once the process has started, it cannot be reversed by glycaemic control, but progression might be slowed by control of blood pressure and protein restriction.
Objectives
To assess the effects of dietary protein restriction on the progression of diabetic nephropathy in patients with diabetes.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, ISI Proceedings, Science Citation Index Expanded and bibliographies of included studies.
Selection criteria
Randomised controlled trials (RCTs) and before and after studies of the effects of a modified or restricted protein diet on diabetic renal function in people with type 1 or type 2 diabetes following diet for at least four months were considered.
Data collection and analysis
Two reviewers performed data extraction and evaluation of quality independently. Pooling of results was done by means of random‐effects model.
Main results
Twelve studies were included, nine RCTs and three before and after studies. Only one study explored all‐cause mortality and end‐stage renal disease (ESRD) as endpoints. The relative risk (RR) of ESRD or death was 0.23 (95% confidence interval (CI) 0.07 to 0.72) for patients assigned to a low protein diet (LPD). Pooling of the seven RCTs in patients with type 1 diabetes resulted in a non‐significant reduction in the decline of glomerular filtration rate (GFR) of 0.1 ml/min/month (95% CI ‐0.1 to 0.3) in the LPD group. For type 2 diabetes, one trial showed a small insignificant improvement in the rate of decline of GFR in the protein‐restricted group and a second found a similar decline in both the intervention and control groups. Actual protein intake in the intervention groups ranged from 0.7 to 1.1 g/kg/day. One study noted malnutrition in the LPD group. We found no data on the effects of LPDs on health‐related quality of life and costs.
Authors' conclusions
The results show that reducing protein intake appears to slightly slow progression to renal failure but not statistically significantly so. However, questions concerning the level of protein intake and compliance remain. Further longer‐term research on large representative groups of patients with both type 1 and type 2 diabetes mellitus is necessary. Because of the variability amongst patients, there might perhaps be a six month therapeutic trial of protein restriction in all individuals, with continuation only in those who responded best. Trials are required of different types of protein.
Plain language summary
Protein restriction for diabetic renal disease
Based on 12 studies, including from eight to 160 people with type 1 and type 2 diabetes for at least an average four‐month period, restricted protein intake appeared to slow progression of diabetic kidney disease, but not by much on average. However, individual variation existed, therefore a low‐protein diet may benefit some individuals. A low‐protein diet can be difficult to adhere to, especially over the long term. Reducing the amount of animal protein is the usual method but some evidence suggests that a shift from red meat to white meat and fish or vegetables may give similar results. We found no data on the effects of low‐protein diet on health‐related quality of life and costs.
Background
Description of the condition
Diabetes mellitus
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (i.e. elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group on the Cochrane Library (see 'About the Cochrane Collaboration', 'Collaborative Review Groups'). For an explanation of methodology terms see the main Glossary on the Cochrane Library.
Diabetic Nephropathy
Diabetic nephropathy is one of the most devastating complications in patients with diabetes. In diabetic nephropathy, damage to the kidneys occurs as a consequence of hyperglycaemia, which induces damage of blood vessels leading to several phenomena, including impaired blood flow. Features include increased excretion of protein in the urine, increased blood pressure and declining kidney function. Severe diabetic nephropathy can lead to kidney failure and end‐stage renal disease (ESRD), when individuals must rely on haemo‐dialysis, peritoneal dialysis or kidney transplantation to survive. The natural history of diabetic nephropathy includes several stages, starting with apparent normality in the first few years after diagnosis, followed by incipient nephropathy (characterised by the presence of small amounts of protein in the urine, known as microalbuminuria), then by overt clinical nephropathy leading to progressive renal failure (Gross 2005). Kidney function is measured as the glomerular filtration rate (GFR), which is a measure of the rate at which blood is filtered by the kidneys. Creatinine clearance measurements are often used as a surrogate for GFR. Once overt nephropathy develops, there is a progressive decline in GFR that can be assessed as an absolute decline in ml/min per year. In most patients with diabetic nephropathy, the decrease in GFR approaches linearity and is of the order of 9 to 14 ml/min/year (Mogensen 1976; Parving 1981; Viberti 1983).
With regard to progression to ESRD, the prognosis of type 1 diabetes has improved during the past four decades (Finne 2005). Initial studies in the 1980s demonstrated that approximately. 80% of microalbuminuric type 1 diabetic patients progressed to proteinuria over a period of 6 to 14 years. In more recent studies, only 30% to 45% of microalbuminuric patients have been reported to progress to proteinuria over 10 years (Gross 2005). However, diabetes is still the most important cause of ESRD in industrialised countries (Finne 2005).
Description of the intervention
Various nutritional guidelines for the treatment of diabetes have been published over the years (Connor 2003). The current UK recommendations suggest the amount of protein consumed should not exceed 1 g/kg/day (Connor 2003). The EURODIAB IDDM Complications Study notes that the average protein intake was 1.5 +/‐0.5 g/kg/day (Toeller 1996). In patients with diabetes and nephropathy, dietary recommendations will change depending on the stage of the disease and treatment modality.
Traditionally low‐protein diets (LPD) were rigid and restrictive and prescribed using an exchange system to include both high and low biological value protein. Prescribable low protein products were available to replace foods such as breads, milk and biscuits. Many patients described these products as unpalatable and therefore unhelpful. The energy, sodium and potassium intake would also be carefully assessed and monitored for each individual. Compliance with these regimes was difficult and would have required close dietetic monitoring in order to avoid protein energy malnutrition (PEM). PEM is known to adversely affect outcomes of patients treated by renal replacement therapy (Ikizler 1995). The cause of PEM is often multifactorial and may include a reduced dietary intake and increased nutritional losses. In the early 1980s, a number of studies suggested that restriction of dietary protein slowed progression to renal failure (Brenner 1982; El Nahas 1984; Maschio 1982; Rosman 1984; Sitprija 1983). Most of these studies were on groups of patients with renal failure due to a variety of causes, and results in the few diabetic patients were not reported separately. More recently there have been studies only in patients with diabetes. However protein restriction appears to be little used in routine diabetes care. This may be due to pessimism about compliance; people with diabetes already receive much dietary advice with which compliance is often poor. The lack of use may appear to be justified by the apparently disappointing results from the largest ever trial of protein restriction in renal disease; the Modification of Diet in Renal Disease (MDRD) study, in which a low‐protein diet did not appear to affect the decline in renal function at three years (Klahr 1994). However, the MDRD study was again of a mixed group (glomerulonephritis and polycystic disease made up about half), and there were some promising features. Compliance to the LPD was shown to be possible, and a longer follow‐up was more promising (Levey 1994). Given the variation in response according to the aetiology of the renal impairment, recommendation on the use of LPDs in diabetes should be based on trials in patients with diabetes.
Why it is important to do this review
We are aware of one previous meta‐analyses having been carried out by Pedrini et al. (Pedrini 1996). In five studies of patients with type 1 diabetes mellitus, a LPD significantly slowed the increase in urinary albumin level or the decline GFR or creatinine clearance (relative risk (RR) 0.56, 95% confidence interval (CI) 0.40 to 0.77). The authors concluded that a LPD effectively slows the rate of progression of both diabetic and non‐diabetic renal disease. However, there were only 108 patients included which may be too small to make firm conclusions (Pedrini 1996). To our knowledge, the only other published review is an updated version of the original Cochrane review published as a book chapter in 2003 (Waugh 2003). The present review updates the original Cochrane review to include new evidence from studies which have been published since then.
Objectives
To assess the effects of dietary protein restriction on the progression of diabetic nephropathy in patients with diabetes.
Methods
Criteria for considering studies for this review
Types of studies
Trial Design
Studies were considered eligible if they were randomised controlled trials (RCT) or before and after trials fulfilling the inclusion criteria. This review was not restricted to RCTs, since given the steady progression of nephropathy (Mogensen 1976; Parving 1981; Viberti 1983), it was considered that patients could act as their own controls in a before and after analysis.
Trial Duration
We only included trials with interventions that lasted a minimum of four months as recommended by Zeller 1991 in order to allow serum creatinine levels to reach a steady state .
Exclusion Criteria
Design or analysis flawed, for example if anti‐hypertensive treatment was started or increased at the same time as diet was changed.
Types of participants
Inclusion Criteria
We included trials involving people of any age with either type 1 or type 2 diabetes and nephropathy.
Diagnostic Criteria
Diabetes mellitus
Ideally, the diagnostic criteria for type 1 and type 2 diabetes mellitus should have been described in the trial. To be consistent with changes in classification and diagnostic criteria of diabetes through the years, the diagnosis should have been established using the standard criteria valid at the time of the beginning of the trial.
Diabetic nephropathy
Nephropathy is a clinical diagnosis based upon the finding of proteinuria in a patient with diabetes and in whom there is no evidence of urinary infection. Conventionally, the level of proteinuria for a diagnosis of 'clinical nephropathy' or 'overt nephropathy' is 0.5 g/day, which is roughly equivalent to a urinary albumin excretion rate (UAER) of 300 mg/day. Patients with a UAER between 30 and 300 mg/day (or 20 to 200 mg/L) are defined as having 'microalbuminuria' or 'incipient nephropathy'. Although timed urine collections remain the 'gold standard' for diagnosis, they are cumbersome to use in routine clinical practice and most definitions of clinical or incipient nephropathy depend upon a 'spot' urine sample and thus a test of albumin concentration. Results in excess of 300 mg/day define clinical and incipient nephropathy. Sensitivity and specificity can be improved by using an early morning, first‐voided specimen and correcting the albumin level for creatinine concentration (albumin:creatinine ratio) (Bilous 2005).
Types of interventions
All types of reduced or modified (for example vegetable rather than animal) protein diets lasting a minimum of four months.
Comparisons
Usual (free or unrestricted) protein diet
Exclusion Criteria
Studies were excluded if insufficient details of diet were given or if the intervention was immediately pre‐dialysis. Studies were excluded if results for individuals with diabetes were not given separately.
Types of outcome measures
Primary outcomes
all‐cause mortality;
end‐stage renal disease;
glomerular filtration rate (GFR).
Secondary outcomes
adverse effects (including nutritional status);
measures of compliance with low‐protein diet (LPD);
health‐related quality of life;
costs.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Library (issue 3, 2006);
MEDLINE (until May 2006);
EMBASE (until May 2006);
ISI Proceedings (until July 2006);
Science Citation Index Expanded (until July 2006).
The described search strategy (see for a detailed search strategy Appendix 1) was used for MEDLINE. For use with EMBASE, The Cochrane Library and the other databases this strategy was slightly adapted.
Studies published in any language were included.
Searching other resources
We searched reference lists of relevant trials and reviews.
Data collection and analysis
Selection of studies
All records from each database were imported to the bibliographic package, Reference Manager (Version 10), checked for duplicates and merged into one core database. After reviewing titles and abstracts, full articles were retrieved for further assessment if the information given suggested the study might be relevant. Study selection was independently performed by two reviewers.
Data extraction and management
Data concerning details of study population, intervention and outcomes were extracted independently by two reviewers (LR, AR) using a standard data extraction sheet. Data on participants, interventions and outcomes, as described above, were abstracted. The data extraction sheet included the following items:
general information: published/unpublished, title, authors, reference/source, contact address, country, year of publication,setting;
trial characteristics: design, duration of follow up, method of randomisation, allocation concealment, blinding;
intervention(s): interventions(s), comparison intervention(s);
participants: sampling, exclusion criteria, total number and number in comparison groups, age, type of diabetes mellitus, similarity of groups at baseline, assessment of compliance, withdrawals/losses to follow‐up/drop‐outs;
outcomes: outcomes specified above, how outcomes were assessed, other events, length of follow‐up, quality of reporting of outcomes;
results: for outcomes and times of assessment, intention‐to‐treat analysis.
Assessment of risk of bias in included studies
Assessment of the methodological quality of reporting of RCTs was based largely on the quality criteria specified by Schulz and Jadad (Jadad 1996; Schulz 1995). In particular the following factors were studied:
minimisation of selection bias ‐ a) was the randomisation procedure adequate? b) was the allocation concealment adequate?
minimisation of attrition bias ‐ a) were withdrawals properly described? b) was analysis by intention to treat?
minimisation of detection bias ‐ were outcome assessors blind to the intervention?
Assessment of the quality of reporting of before and after studies was done qualitatively.
Assessment of heterogeneity
In the event of substantial clinical or methodological or statistical heterogeneity, study results were not planned to be combined in a meta‐analysis. Heterogeneity was identified by visual inspection of the forest plots, by using a standard χ2‐test and a significance level of α = 0.1, in view of the low power of such tests. Quantification of heterogeneity was also examined with I2, ranging from 0% to 100% including its 95% confidence interval (Higgins 2002). I2 demonstrates the percentage of total variation across studies due to heterogeneity and was used to judge the consistency of evidence. I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study characteristics and those of subgroups of the main body of evidence.
Assessment of reporting biases
Funnel plots were planned to be used in exploratory data analyses to assess for the potential existence of small study bias. There are a number of explanations for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to study size, poor methodological design of small studies (Sterne 2001) and publication bias.
Data synthesis
Data on changes in glomerular filtration rate (GFR) were summarised in a meta‐analysis for trials with people with type 1 diabetes (only RCTs). Continuous data were expressed as weighted mean differences. Pooled results were analysed using a random‐effects model. It was not possible to do a meta‐analysis on trials with people with type 2 diabetes as insufficient studies were identified. We extracted the baseline and post‐intervention means with standard deviations (SD) for the intervention and control groups. Any standard errors (SE) and confidence intervals (CI) were transformed into SDs where appropriate.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to be only performed if one of the primary outcome parameters demonstrated statistically significant differences between treatment groups. The following subgroup analyses were planned:
gender (female versus male);
age (depending on data but especially older versus younger patients);
type 1 versus type 2 diabetic people.
Subgroup analyses were planned to be mainly used to explore clinical or methodological or statistical heterogeneity.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of study quality, as specified above;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
The robustness of the results was also planned be tested by repeating the analysis using different measures of effects size (risk difference, odds ratio etc.) and different statistical models (fixed and random effects models).
Results
Description of studies
Results of the search
The initial search identified 3632 records (after removal of duplicates), from these, 36 full papers were identified for further examination. The other studies were excluded on the basis of their abstracts because they were not relevant to the question under study. After screening the full text of the selected papers, 12 studies finally met the inclusion criteria.
Included studies
Details of the characteristics of the 12 included studies are shown in the table Characteristics of included studies. In the study by Walker et al., four patients started antihypertensive treatment while on the low‐protein diet (LPD) (Walker 1989). However, as individual patient details were provided, the data for these four patients were excluded from our analysis.
Designs
Nine RCTs and three before and after studies were included in the review. The mean duration of the interventions ranged from 4.5 months to four years.
Participants
Overall there were 585 participants in the 12 studies. There were 322 individuals with type 1 diabetes and 263 with type 2 diabetes. Eight studies were carried out in people with type 1 diabetes, one study in people with type 2 diabetes and two studies included people with type 1 and type 2 diabetes.
In all but two trials, participants for both groups in RCTs were balanced for baseline characteristics. In one study (Dullart 1993), body mass index was significantly higher in the intervention group. In another study, there were differences in several factors but none significant (Brouhard 1990).
Interventions
The interventions and comparators used in the 12 studies are summarised in Characteristics of included studies. In all studies, the comparison intervention was usual (free or uncontrolled) protein diet (actual protein intake 1 to 2 g/kg/day). In all studies, the intervention was a LPD containing from 0.3 to 0.8 g/kg/day. In two studies the intervention diet was a low‐protein vegetarian diet (Barsotti 1988; Barsotti 1998).
Outcome measures
Primary outcomes
All studies investigated renal function by glomerular filtration rate (GFR) or creatinine clearance as primary endpoints. One study (Hansen 2002) assessed the relative risk (RR) of end‐stage renal disease (ESRD) or death.
Secondary outcomes
All studies assessed compliance with diet with urinary urea. Nine studies reported measures of nutritional status (anthropometric, serum albumin, serum pre‐albumin).
Excluded studies
Twenty four studies were excluded. Reasons for exclusion of studies are given Characteristics of excluded studies. The main reason for exclusion was duration of intervention.
Risk of bias in included studies
Before and after studies
All of the before and after studies adequately reported on withdrawals. None of the studies detailed selection criteria and one stated exclusion criteria (Walker 1989). One study gave actual protein intake in the low‐protein diet group (Walker 1989). The sample size was small (n=8) in one study (Barsotti 1988). Most of the studies were done in selected volunteers and we cannot say how reproducible they would be in a large representative group.
Randomised controlled trials
Of the 12 included studies, nine were RCTs and three had a before and after design.
All RCTs were of parallel design and randomised individuals. The method of randomisation was partially specified in four studies. Four studies reported concealment of allocation. One study reported blinding of outcome assessors (Hansen 2002) and in one study, allocation was not known to the general practitioner or to the laboratory personnel (Pijls 2002). Two studies reported an intention‐to‐treat analysis. All studies adequately reported on withdrawals. Two studies reported details of their sample size calculation (Dullart 1993; Hansen 2002).
Effects of interventions
Primary outcomes
One trial explored all‐cause mortality and end‐stage renal disease (ESRD) as an endpoints (Hansen 2002). ESRD or death occurred in 27% of patients on a usual protein diet as compared with 10% on a low‐protein diet (LPD) (log‐rank test; P=0.042). The relative risk (RR) of ESRD or death was 0.23 (95% CI 0.07 to 0.72) for patients assigned to a LPD, after an adjustment at baseline for the presence of cardiovascular disease (P=0.01).
Change in glomerular filtration rate (GFR)
Type 1 diabetes mellitus
Pooling of the seven RCTs including type 1 diabetic patients by means of random‐effects meta‐analysis resulted in an non‐significant improvement in GFR of 0.1 ml/min/month (95% confidence interval (CI) ‐0.1 to 0.3) in the LPD group. The test for heterogeneity indicated an I2 value of 62%. Two before and after studies were not included in the meta‐analysis. The before and after study by Barsotti et al. showed a significant improvement in GFR of 1.4 ml/min/month (P<0.001) in the intervention group (Barsotti 1988). In another before and after study by Walker and colleagues, GFR improved by 0.04 ml/min/month (P=0.0063) in the intervention group (Walker 1989).
Type 2 diabetes mellitus
Results for studies including type 2 diabetic patients are shown in Appendix 2 . In the trial by Pijls et al. there was a small insignificant improvement in the rate of decline of GFR in the protein‐restricted group (Pijls 2002). The second trial found a similar decline of GFR in both groups (Meloni 2002).
There were two studies which included individuals with type 1 and type 2 diabetes, but did not provide separate data (Barsotti 1998; Meloni 2004). Results for these are shown in Table 2. In the before and after study by Barsotti et al. there was a significant improvement in the rate of decline while on restricted protein diet (0.9 +/‐0.6 versus 0.2 +/‐ 0.2 ml/min/month, P<0.001) (Barsotti 1998). The other study found no statistically significant differences in renal function between the two groups (0.5 +/‐ 0.1 versus 0.5 +/‐ 0.1) (Meloni 2004).
Secondary outcomes
No trial explored health‐related quality of life or costs as an endpoint.
Compliance
The intended protein intake in intervention groups ranged from 0.3 to 0.8 g/kg/day. Actual protein intake ranged from 0.6 to 1.1 g/kg/day, indicating lack of compliance.
Adverse effects, nutritional status
Nine studies assessed nutritional status. One study (Meloni 2002) noted malnutrition in the LPD group on an intended intake of 0.6 g/kg/day, as measured by serum pre‐albumin and serum albumin. No definition of malnutrition was given, but serum pre‐albumin and serum albumin significantly decreased in LPD group.
Discussion
Overall, a restricted protein intake does appear to slow the progression of diabetic nephropathy albeit in a non‐significant way. Progression rate without treatment has been reported to be a decline in glomerular filtration rate (GFR) of 9 to 14 ml/min/year in type 1 diabetes (Mogensen 1976; Parving 1981; Viberti 1983). From the result of our meta‐analysis, this implies that patients who comply with the low‐protein diet can delay dialysis by, on average around one or two months. Variation among patients also needs to be taken into account. Studies did not give sufficient details to quantify this but a small average benefit may conceal larger benefits in some people. To explore the effect of a restricted diet on the progression of diabetic nephropathy further, we looked at the relationship between the difference in protein intake and the difference in the change in GFR between the two groups. Two studies were omitted because of insufficient data. The analysis showed a positive correlation, although not significant (r=0.59, n=10, P=0.07) suggesting improvement in GFR was greater if more restriction was achieved.
Compliance is clearly very important. People with diabetes are already asked to change their diets to conform to healthy eating guidelines. When we presented the results of this review to members of the Scottish Study Group for the Care of Diabetes in the Young, members identified compliance as important, but unlikely. Indeed some members wondered pessimistically if it was worth doing further research into low‐protein diets (LPD) at all. In some of the studies it has been shown that even amongst the volunteer participants, the actual protein intake as indicated by urinary nitrogen output was higher than prescribed. In the Modification of Diet in Renal Disease (MDRD) study (MDRD Study Grp 1989) the lower the prescribed level, the greater the excess taken. It was also noteworthy that the intake indicated by urinary nitrogen excretion was higher than indicated by dietary history. This has implications for monitoring of compliance in future studies.
Cianciaruso et al. noted that compliance was difficult to achieve, "adherence may be difficult, time consuming and unpleasant for the patients" (Cianciaruso 1989). They listed the problems: the use of special low‐protein foods; the high cost; the time for separate cooking of meals; palatability; changes in lifestyle. However, they noted that compliance improved over three years, from about 30% in year one to about 80% by the end of year three. This may have been due to the onset of symptoms relieved by LPDs and fear of approaching dialysis. No data on the proportion with diabetes are given. The difficulty of adherence to low‐protein restriction should not stop us giving people with diabetes the information on the options.
An important question is whether we could achieve almost as much by changing the type of protein in the diet, rather then the amount. Jibani et al. found that albumin excretion rates fell when patients with microalbuminuria were given a predominantly vegetarian diet, although the results were confounded by a sizeable drop in total protein intake, from 1.4 to 1.0 g/kg/day (Jibani 1991). Pecis et al. compared three diets ‐ usual diet with 1.4 g/kg/day, a LPD with 0.5 g/kg/day and a test diet in which chicken and fish replaced red meat (Pecis 1994). They found that the chicken and fish diet had similar effects on GFR to the LPD, but was much more acceptable. However, this was a short‐term study with only three weeks on each diet. They hypothesize that this is due to the much lower levels of glycine, alanine and arginine in chicken and fish compared to red meat, these being the amino acids with greatest effect on GFR. If such diets are as effective as low‐protein ones, then compliance becomes less of an issue. They might also have beneficial affects on cardiovascular risk. Compliance may be improved on a Mediterranean style diet, characterized by abundant plant foods, fresh fruit, olive oil, dairy products, fish and poultry consumed in low to moderate amounts and red meat consumed in low amounts. This review included five studies from Italy, three of which provided data on actual protein intake in the LPD group (Ciavarella 1987; Meloni 2002; Meloni 2004). These studies report good compliance reporting mean protein intakes of 0.7, 0.7 and 0.9 g/kg/day.
In her very useful review, Zeller deals with the methodological problems of studies of protein restriction, such as the heterogeneity of aetiologies, the use of plasma creatinine as an indicator of renal function, the importance of measuring compliance and the need for an adequate duration of follow‐up (Zeller 1991).
A severely restricted protein diet should be followed under the supervision of a specialist as there is a theoretical risk of nutritional deficiencies to occur (Connor 2003). In this review, only one study found evidence of malnutrition on an intended protein intake of 0.6 g/kg/day. It was reported that serum pre‐albumin and serum albumin had significantly decreased in the LPD group (Meloni 2002). In practice, however, the lack of compliance possibly protects against this side effect.
The present body of evidence is limited, and we need studies which are long term and which report important outcomes such as end‐stage renal disease (ESRD) and which examine the balance between efficacy, adverse effects and compliance. We also need more studies in individuals with type 2 diabetes. Although the incidence of nephropathy here is less than in type 1, because type 2 is far commoner, the absolute numbers of patients proceeding to ESRD are similar (Raine 1995).
Authors' conclusions
Implications for practice.
This review has shown that a lower protein intake modestly slows the mean progression of diabetic nephropathy towards renal failure, although in a non‐significant way. However it does not establish what level of protein restriction is most effective in achieving this decline. The optimum level of protein restriction in practice would probably be a compromise between efficacy and compliance. In the document Dietary Recommendations for People with Diabetes (1992), the British Diabetic Association recommend that 15% to 20% of energy intake is from a protein source. A pragmatic approach would be to reduce high protein intake to perhaps a maximum of 1 g/kg/day, or to 0.8 g/kg/day in those patients prepared to comply with that. Changing the type of protein, for example a vegetarian diet or white meat and fish replacing red meat, rather than the amount may achieve the same effects but with greater palatability.
Implications for research.
Further longer‐term research on large representative groups of patients with both type 1 and type 2 diabetes is necessary. Also, taking into account the variability among patients, there might perhaps be a six month trial in all patients, with continuation only in those who responded best. We think the top priority should be for a trial of usual diet (unrestricted protein) versus reduction to say 0.8 g/kg/day (with chicken and fish instead of red meat) versus a vegetarian diet with no restriction in protein versus a vegetarian diet with modest intake (1 g/kg/day) with results both in terms of progression of diabetic nephropathy and compliance. Outcomes should include all of glomerular filtration rate (GFR), quality of life, cost‐effectiveness and cardiovascular risk factors such as lipids. There is a research need in fully informed patients to assess whether dialysis can be postponed for worthwhile periods, even if only in some patients, without undue reduction in quality of life due to dietary restriction.
What's new
| Date | Event | Description |
|---|---|---|
| 6 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 31 May 2006 | New search has been performed | This is an up‐date of the original Cochrane review which appeared in issue 3, 2000. More studies (nine RCTs and three before and after trials) are now included. The conclusions now are more cautious as current data do not indicate a relevant effect of low‐protein diets on important patient outcomes |
Acknowledgements
The first edition of this review was presented in draft form to a meeting of the Scottish Study Group for the Care of the Young Diabetic; we thank them, and in particular Dr James Walker, for useful comments. We also thank Massoud Boroujerdi for statistical advice and the referees, Dr Denis Fouque, Mrs Margaret Hunter and Professor Guiseppe Remuzzi, for their help with the original review. Any remaining mistakes are ours.
Appendices
Appendix 1. Search strategy
| Electronic searches |
| Unless otherwise stated, search terms are free text terms; MesH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent.
DIABETES MELLITUS AND DIABETIC NEPHROPATHY 1. Diabetes Mellitus, Type 1/co, pp, dh 2. Diabetes Mellitus, Type 2/co, pp, dh 3. Diabetic Nephropathies/co, pp, dh, pc, dt 4. Kidney Failure, Chronic/co, pp, pc, dh 5. microalbuminuria.tw. 6. Albuminuria/pc,ur 7. Kidney/pp 8. diabetes.tw. 9. diabetic nephropath$.tw. 10. kidney disease$.tw. 11. renal failure.tw. 12. renal disease$.tw. 13. Glomerular Filtration Rate/de, ph 14. glomerular filtration rate.tw. 15. Creatinine/ 16. creatinine.tw. MODIFIED/RESTRICTED PROTEIN DIET 1. Dietary Proteins/ad, pd, tu 2. protein.tw. 3. diet.tw. 4. Diet, Protein‐Restricted/ 5. Dietary Supplements/ |
Appendix 2. Effect of low protein diet (LPD) on glomerular filtration rate (GFR)
| Study | Type of diabetes | Duration | No. on LPD | UPD (g/kg/day) | LPD (g/kg/day) | UPD: change in GFR | LPD: change in GFR |
| Barsotti 1998 | Type 1 and 2 | 3.7 years | 32 | Not stated | 0.3 or 0.7 | ‐0.9 (0.62) | ‐0.22 (0.21) |
| Barsotti 1988 | Type 1 | 17.4 months | 8 | 1.2‐1.4 | <7.5 g/day <4.5 g/day | ‐1.38 (0.27) | ‐0.03 (0.37) |
| Brouhard 1990 | Type 1 | 12 months | 8 | 1.0 | 0.6 | ‐0.68 (0.4) | ‐0.28 (0.15) |
| Ciavarella 1987 | Type 1 | 4.5 months | 7 | 1.44 | 45 g/day | ‐0.94 (2.19) | 3.33 (6.28) |
| Dullart 1993 | Type 1 | 2 years | 14 | 1.09 | 0.6 | ‐0.41 (0.98) | ‐0.75 (1.23) |
| Hansen 2002 | Type 1 | 4 years | 38 | 1.02 | 0.6 | ‐0.32 (0.3) | ‐0.32 (0.28) |
| Meloni 2002 | Type 1 | 12 months | 15 | 1.39 | 0.6 | ‐0.52 (0.15) | ‐0.51 (0.15) |
| Type 2 | 12 months | 20 | 1.39 | 0.6 | ‐0.52 (0.15) | ‐0.51 (0.13) | |
| Meloni 2004 | Type 1 and 2 | 12 months | 40 | 1.24 | 0.8 | ‐0.5 (0.11) | ‐0.48 (0.13) |
| Pijls 2002 | Type 2 | 12 months | 63 | 1.14 | 0.8 | ‐0.3 (1.17) | ‐0.4 (1.0) |
| Raal 1994 | Type 1 | 6 months | 11 | 1.6 | 0.8 | ‐1.33 (4.5) | 0.5 (3.5) |
| Walker 1989 | Type 1 | 33 months | 15 | 1.13 | 40 g/day | ‐0.53 (0.5) | ‐0.13 (0.39) |
| Zeller 1991 | Type 1 | 37.1 months | 20 | 1.0 | 0.6 | ‐1.06 (1.24) | ‐0.25 (0.4) |
| Footnotes | |||||||
| LPD: low‐protein diet | |||||||
| UPD: usual protein diet | |||||||
| GFR: glomerular filtration rate | |||||||
Data and analyses
Comparison 1. Low protein diet versus usual diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glomerular filtration rate (ml/min) | 7 | 222 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.06, 0.34] |
1.1. Analysis.
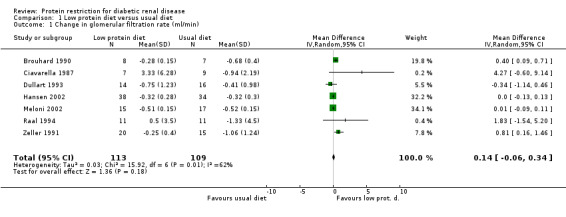
Comparison 1 Low protein diet versus usual diet, Outcome 1 Change in glomerular filtration rate (ml/min).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barsotti 1988.
| Methods | Before and after study. Mean duration on UPD 15.9 months. Mean duration on LPD 17.4 months. | |
| Participants | 8 type 1 patients with severe renal failure. Mean age 44.7. Mean duration of diabetes 20.5 years. | |
| Interventions | LPD was a low protein vegetarian diet. Low phosphorous. Supplemented with EAA and ketoanalogs. 4 patients were permitted to eat normal wheat flour products. 4 used protein‐free substitutes. UPD = 1.2‐1.4 g/kg/day. | |
| Outcomes | Creatinine clearance. Nutritional status assessed by TST & MAMC, serum albumin, serum transferrin and serum complement. Compliance assessed by urinary urea. Actual protein intake in LPD not reported. | |
| Notes | 4 out of the 8 patients changed to maintenance hemodialysis after a period of 11 to 29 months. In 2 patients there was a spontaneous decision to abandon the LPD due to compliance difficulties. In 2, dialysis was started as they were not responding to diuretic therapy. No sign of protein or caloric malnutrition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Barsotti 1998.
| Methods | Before and after study. Mean duration 3.7 years. | |
| Participants | 22 type 1 and 10 type 2 patients with overt diabetic nephropathy. Mean age 44. Duration of diabetes not stated. | |
| Interventions | UPD = Free diet. LPD: Diet A (n=19) 0.3 g/kg/day (patients with creatinine clearance ranging from 19 to 6.5 ml/min) Diet B (n=13) 0.7 g/kg/day (patients with creatinine clearance ranging from 60 to 22 ml/min) Both diets vegetarian. | |
| Outcomes | Creatinine clearance. Malnutrition assessed by anthropometric indices (Wt, TST, MAMC). Compliance assessed by urinary urea. Diet A 45% good, Diet B 69% good. | |
| Notes | No evidence of malnutrition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Brouhard 1990.
| Methods | RCT. 12 months duration. A 3 month trial period to assess compliance. | |
| Participants | 15 type 1 patients with microalbuminuria 30 ug/min. LPD mean age 36. UPD mean age 30. Mean duration of diabetes 19 years. | |
| Interventions | LPD = 0.6 g/kg/day (n=8). UPD = 1.0 g/kg/day (n=7). | |
| Outcomes | GFR. Compliance by uU. Actual protein intake 0.6 g/kg/day. | |
| Notes | After trial period, 1 patient requested normal diet, reinstated. No significant differences in baseline measurements between groups but UPD had worse factors. No mention of malnutrition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ciavarella 1987.
| Methods | RCT. Mean duration 4.5 months. | |
| Participants | 16 type 1 patients with proteinuria > 0.5g/24 hours; creatinine < 1.9mg/dl. Mean age 37. Duration of diabetes 10‐30 years. | |
| Interventions | LPD = 0.71 g/kg/day (n=7). UPD = 1.44 g/kg/day (n=9). | |
| Outcomes | Creatinine clearance. Compliance verified by dietary interview, blood nitrogen and urea nitrogen excretion. Actual protein intake 0.71 g/kg/day. | |
| Notes | Small numbers. No information on number with follow‐up under 4.5 months. No mention of malnutrition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dullart 1993.
| Methods | RCT. Duration 2 years. | |
| Participants | 31 type 1 patients with microalbuminuria (10‐200 ug/min overnight). UPD mean age 39 LPD mean age 43 Duration of diabetes > 5 years. | |
| Interventions | LPD = 0.6 g/kg/day (n=14). UPD = 1.09 g/kg/day (n=16). Diet supplement with methionine if necessary. | |
| Outcomes | GFR. Compliance assessed by uU. Actual protein intake 0.79 g/kg/day. Malnutrition assessed by calorie intake, BMI and serum albumin. | |
| Notes | No evidence of malnutrition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hansen 2002.
| Methods | RCT. Duration 4 years. | |
| Participants | 82 type 1 patients (18‐60 yrs) with diabetic retinopathy, albuminuria >= 300 mg/24 hr. GFR above 20ml/min/1.73 m2 and a pre‐study decline in GFR >=2 ml/min/year (progressive diabetic nephropathy). Mean duration of diabetes LPD 28 years, UPD 27 years. | |
| Interventions | LPD = 0.6 g/kg/day. Supplementation of calcium of 500 mg/day (n=38). UPD = patients' pre‐study diet (n=34). | |
| Outcomes | GFR. RR of ESRD or death. Malnutrition assessed by mid‐arm circumference, serum albumin and body weight. Compliance monitored by dietary interview and uU. Actual protein intake 0.89 g/kg/day. | |
| Notes | Comparable number in both groups received antihypertensives and ACE but +4 at follow‐up on antihypertensive and +4 on ACE. Malnutrition indicators comparable in 2 groups, but data not shown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Meloni 2002.
| Methods | RCT. Duration 12 months. | |
| Participants | 32 type 1 patients (14‐32 yrs), duration of diabetes 20.9 years. 37 type 2 patients (15‐34 yrs), duration of diabetes 24.9 years. Overt nephropathy and hypertension. | |
| Interventions | LPD = 0.6 g/kg/day (n=20). UPD = 1.39 g/kg/day (n=17). | |
| Outcomes | GFR. Malnutrition measured by serum albumin, serum pre‐albumin and anthropometric parameters. Compliance monitored by uU. Actual protein intake 0.68 g/kg/day. | |
| Notes | Malnutrition noted in LPD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Meloni 2004.
| Methods | RCT. Duration 12 months. | |
| Participants | 24 type 1 patients (mean age 47), duration of diabetes 20.9 years. 56 type 2 patients (mean age 63), duration of diabetes 24.9 years. | |
| Interventions | LPD = 0.8 g/kg/day (n=40). UPD = 1.24 g/kg/day (n=40). | |
| Outcomes | GFR. Malnutrition measured by serum albumin and pre‐albumin. Compliance assessed by uU. Actual protein intake 0.86 g/kg/day. | |
| Notes | No sign of malnutrition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pijls 2002.
| Methods | RCT. Duration 28 months. Data for 12 months. | |
| Participants | 160 type 2 patients. Duration >5yrs. UPD mean age 65, duration of diabetes 7.2 years. LPD mean age 63, duration of diabetes 6.7 years. | |
| Interventions | LPD = 0.8 g/kg/day (n=63). UPD = 1.14 g/kg/day (n=68). | |
| Outcomes | Estimated GFR measured with cimetidine‐influenced creatinine clearance. Compliance assessed by uU. Actual protein intake 1.1 g/kg/day. | |
| Notes | No mention of malnutrition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Raal 1994.
| Methods | RCT. Mean duration 6 months. | |
| Participants | 26 type 1 patients. UPD mean age 30 years. LPD mean age 29 years. Duration of diabetes >= 10 years. | |
| Interventions | LPD = 0.8 g/kg/day (n=11). UPD = 2 g/kg/day (n=11). | |
| Outcomes | GFR was measured by Cr EDTA. Malnutrition assessed by body weights, serum total protein, and serum albumin. Compliance assessed by dietary history carried out by a dietitian and by measurement of 24 hour uU nitrogen excretion. Actual protein intake 0.87 g/kg/day. | |
| Notes | Body weight, serum total protein, and serum albumin concentrations did not decrease in patients consuming the protein‐restricted diet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Walker 1989.
| Methods | Before and after study. Mean duration on UPD was 29 months. Mean duration on LPD was 33 months. | |
| Participants | 19 type 1 patients with proteinuria and GFR>20ml/min. Mean age 42 years. Mean duration of diabetes 24 years. | |
| Interventions | LPD = 40g/day, half and half animal and vegetable sources. Patients whose urinary protein excretion rate exceeded 3g/24h were allowed an additional 1.6g of dietary protein per extra gram of urinary protein. UPD = patients' normal diet. | |
| Outcomes | GFR was assessed by clearance of Cr‐labelled edetic acid. Malnutrition assessed by MAMC and plasma albumin. Compliance assessed by uU, dietary history and weighed food record. Actual protain intake 0.67 g/kg/day. | |
| Notes | 4 patients were started on ACE inhibitors while on LPD. Data on these patients have been excluded from the analysis. LPD had no untoward nutritional effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Zeller 1991.
| Methods | RCT . Mean duration 35 months , minimum 12 months. | |
| Participants | 47 type 1 patients with proteinuria over 500mg/24 hours , with diabetic retinopathy and no other cause of renal failure . LPD mean age 33. Mean duration of diabetes 21 years. UPD mean age 35. Mean duration of diabetes 22.4 years. | |
| Interventions | LPD = 0.6g / kg / ideal body weight/day (n=20). UPD = at least 1 g/kg/day (n=15). | |
| Outcomes | GFR by iothalamate and creatinine. Malnutrition measured by weight, mid‐arm circumference, and serum albumin. Compliance assessed by uU and dietary history. Actual protein intake 0.72 g/kg/day. | |
| Notes | Malnutrition measures showed no significant change. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
LPD: Low protein diet UPD: Usual protein diet EAA: Essential amino acids TST: Triceps skinfold thickness MAMC: Middle arm muscle circumference GFR: Glomerular filtration rate BMI: Body mass index RR: Relative risk ESRD: End‐stage renal disease ACE: Angiotensin converting enzyme RCT: Randomised controlled trial Wt: Weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Attman 1983 | Late (pre‐dialysis) intervention |
| Azadbakht 2003 | Duration only 7 weeks |
| Bending 1988 | Duration only 3 weeks |
| Brodsky 1992 | Duration only 12 weeks |
| Brouhard 1986 | Control group were non‐diabetic |
| Cianciaruso 1989 | Trial to assess compliance to a low protein diet |
| Cohen 1987 | Duration only 3 weeks |
| Evanoff 1989 | Changes in antihypertensive therapy |
| Facchini 2003 | Comparison diet not usual diet (carbohydrate‐restricted, low‐iron‐available, polyphenol‐enriched diet |
| Gin 1991 | Aim was to assess the effect of the increase in carbohydrate on insulin sensitivity |
| Gross 2002 | Duration only 4 weeks |
| Hansen 1999 | Duration only 8 weeks |
| Jibani 1991 | Duration only 8 weeks |
| Kupin 1987 | Two consecutive dietary periods of one week |
| Levine 1989 | No comparison period, duration only 15 weeks |
| Mollsten 2001 | Case control study |
| Parillo 1988 | Duration only 10 days |
| Pecis 1994 | Duration only 3 weeks |
| Pedersen 1989 | Duration only 4 weeks |
| Rudberg 1988 | Duration only 10 days |
| Stephenson 2005 | Duration only 8 weeks |
| Stojceva‐Taneva 2001 | Observational study |
| Wheeler 2002 | Duration only 6 weeks |
| Wiseman 1987 | Duration only 3 weeks |
Contributions of authors
LYNN ROBERTSON: searching for trials, quality assessment of trials, data extraction, data analysis, development of final review.
NORMAN WAUGH: protocol development, selection of studies, quality assessment of trials, data extraction, data analysis, development of final review.
AILEEN ROBERTSON: data extraction, development of final review.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Barsotti 1988 {published data only}
- Barsotti G, Ciardella F, Morelli E, Cupisti A, Mantovanelli A, Giovannetti S. Nutritional treatment of renal failure in type 1 diabetic nephropathy. Clinical Nephrology 1988;29:280‐287. [PubMed] [Google Scholar]
- Barsotti G, Navalesi R, Giampietro O, Cioardella F, Morelli E, Cupisti A, Mantovanelli A, Giovanetti S. Effects of a vegetarian, supplemented diet on renal function, proteinuria and glucose metabolism in patients with 'overt' diabetic nephropathy and renal insufficency. Contributions to Nephrology 1988;65:87‐94. [DOI] [PubMed] [Google Scholar]
Barsotti 1998 {published data only}
- Barsotti G, Cupisti A, Barsotti M, Sposini S, Palmieri D, Meola M, Lenti C, Morelli E. Dietary treatment of diabetic nephropathy with chronic renal failure. Nephrology Dialysis Transplantation 1998;13 (Suppl 8):49‐52. [DOI] [PubMed] [Google Scholar]
Brouhard 1990 {published data only}
- Brouhard BH, LaGrone L. Effect of dietary protein restriction on renal function reserve in diabetic nephropathy. The American Journal of Medicine 1990;89:427‐31. [DOI] [PubMed] [Google Scholar]
Ciavarella 1987 {published data only}
- Ciavarella A, Gianfranco MI, Stefoni S, Borgnino LC, Bannini P. Reduced albuminuria after dietary protein restrictions in insulin dependent diabetic patients with clinical nephropathy. Diabetes Care 1987;10:407‐13. [DOI] [PubMed] [Google Scholar]
Dullart 1993 {published data only}
- Dullart RP, Beusenkamp BJ, Meijer S, Doormaal JJ, Sluiter WJ. Long term effects of protein‐restricted diet on albuminuria and renal function in IDDM patients without clinical nephropathy and hypertension. Diabetes Care 1993;16:483‐92. [DOI] [PubMed] [Google Scholar]
Hansen 2002 {published data only}
- Hansen HP, Tauber‐Lassen E, Jensen BR, Parving H‐H. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney International 2002;62:220‐228. [DOI] [PubMed] [Google Scholar]
Meloni 2002 {published data only}
- Meloni C, Morosetti M, Suraci C, Pennafina MG, Tozzo C, Taccone‐Gallucci M, Casciani CU. Severe dietary protein restriction in overt diabetic nephropathy: benefits or risks?. Journal of Renal Nutrition 2002;12:96‐101. [DOI] [PubMed] [Google Scholar]
Meloni 2004 {published data only}
- Meloni C, Tatangelo P, Cipriani S, Rossi V, Suraci C, Tozzo C, Rossini B, Cecilia A, Franco D, Straccialano E, Casciani CU. Adequate protein dietary restriction in diabetic and nondiabetic patients with chronic renal failure. Journal of Renal Nutrition 2004;14:208‐13. [PubMed] [Google Scholar]
Pijls 2002 {published data only}
- Pijls LTJ, Vries H, Donker AJM, Eijk T. The effect of protein restriction on albuminuria in patients with type 2 diabetes mellitus: a randomized trial. Nephrology Dialysis Transplantation 1999;14:1445‐1453. [DOI] [PubMed] [Google Scholar]
- Pijls LTJ, Vries H, Eijk JT, Donker AJM. Protein restriction, glomerular filtration rate and albuminuria in patients with type 2 diabetes mellitus: A randomized trial. European Journal of Clinical Nutrition 2002;56:1200‐1207. [DOI] [PubMed] [Google Scholar]
Raal 1994 {published data only}
- Raal FJ, Kalk WJ, Lawson M, Esser JD, Buys R, Fourie L. Effect of moderate dietary protein restriction on the progression of overt diabetic nephropathy: a 6‐month prospective study. American Journal of Clinical Nutrition 1994;60:579‐85. [DOI] [PubMed] [Google Scholar]
Walker 1989 {published data only}
- Walker JF, Bending JJ, Dodds RA, Mattock MB, Murrells TJ, Keen H. Restriction of dietary protein and progression of renal failure in diabetic nephropathy. Lancet 1989;December:1411‐5. [DOI] [PubMed] [Google Scholar]
Zeller 1991 {published data only}
- Zeller K, Whittaker E, Sullivan L, Raskin P, Jacobson HR. Effect of restricting dietary protein on the progression of renal failure in patients with insulin‐dependent diabetes mellitus. New England Journal of Medicine 1991;324:78‐84. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Attman 1983 {published data only}
- Attman PO, Bucht H, Larsson O, Uddebom G. Protein restricted diet in diabetic renal failure. Clinical Nephrology 1983;19:217‐20. [PubMed] [Google Scholar]
Azadbakht 2003 {published data only}
- Azadbakht L, Shakerhosseini R, Atabak S, Jamshidian M, Mehrabi Y, Esmaill‐Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. European Journal of Clinical Nutrition 2003;57:1292‐4. [DOI] [PubMed] [Google Scholar]
Bending 1988 {published data only}
- Bending JJ, Dodds RA, Keen H, Viberti G. Renal response to restricted protein intake in diabetic nephropathy. Diabetes 1988;27:1641‐6. [DOI] [PubMed] [Google Scholar]
Brodsky 1992 {published data only}
- Brodsky IG, Robbins DC, Hiser E, Fuller SP, Fillyaw M, Devlin JT. Effects of low protein diets on protein metabolism in insulin‐dependent diabetes mellitus patients with early nephropathy. Journal of Clinical Endocrinologgy Metabolism 1992;75:351‐7. [DOI] [PubMed] [Google Scholar]
Brouhard 1986 {published data only}
- Brouhard BH, LaGrone LF, Richards GE, Travis LB. Short‐term protein loading in diabetics with a ten‐year duration of disease. American Journal of Disease in Childhood 1986;140:473‐6. [DOI] [PubMed] [Google Scholar]
Cianciaruso 1989 {published data only}
- Cianciaruso B, Capuano A, D' Amaro E, Ferrara N, Nastasi A, Conte G, Bellizzi V, Andreucci VE. Dietary compliance to a low protein and phosphate diet in patients with chronic renal failure. Kidney International 1989;36:173‐6. [PubMed] [Google Scholar]
Cohen 1987 {published data only}
- Cohen D, Dodds R, Viberti G. Effect of protein restriction in insulin dependent diabetics at risk of nephropathy. British Medical Journal 1987;294:795‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evanoff 1989 {published data only}
- Evanoff G, Thompson C, Brown J, Weinman E. Prolonged dietary protein restriction in diabetic nephrology. Archives of Internal Medicine 1989;149:1129‐33. [PubMed] [Google Scholar]
- Evanoff GV, Thompson CS, Brown J, Weinman EJ. The Effect of Dietary Protein Restriction on the progression of Diabetic Nephropathy. Archives of Internal Medicine 1987;147:492‐495. [PubMed] [Google Scholar]
Facchini 2003 {published data only}
- Facchini FS, Saylor KL. A low‐iron‐available, polyphenol‐enriched, carbohydrate‐restricted diet to slow progression of diabetic nephropathy.. Diabetes 2003;52:1204‐9. [DOI] [PubMed] [Google Scholar]
Gin 1991 {published data only}
- Gin H, Aparicio M, Potaux L, Merville P, Combe Ch, Precigout V, Bouchet J‐L, Aubertin J. Low ‐protein, low‐phosphorous diet and tissue insulin sensitivity in insulin‐dependent diabetic patients with chronic renal failure. Nephron 1991;57:411‐5. [DOI] [PubMed] [Google Scholar]
Gross 2002 {published data only}
- Gross JL, Zelmanovitz T, Moulin CC, De M, V, Perassolo M, Leitao C, Hoefel A, Paggi A, Azevedo MJ. Effect of a chicken‐based diet on renal function and lipid profile in patients with type 2 diabetes: a randomized crossover trial. Diabetes Care 2002;25:645‐51. [DOI] [PubMed] [Google Scholar]
Hansen 1999 {published data only}
- Hansen HP, Christensen PK, Tauber‐Lassen E, Klausen A, Jensen BR, Parving H‐H. Low‐protein diet and kidney function in insulin‐dependent diabetic patients with diabetic nephropathy. Kidney International 1999;55:621‐628. [DOI] [PubMed] [Google Scholar]
Jibani 1991 {published data only}
- Jibani MM, Bloodworth LL, Foden E, Griffiths KD, Galpin OP. Predominantly vegetarian diet in patinets with incipient and early clinical diabetic nephropathy: effects on albumin excretion rate and nutritional status. Diabetic Medicine 1991;8:949‐53. [DOI] [PubMed] [Google Scholar]
Kupin 1987 {published data only}
- Kupin WL, Cortes P, Dumler F, Feldkamp CS, Kilates MC, Levin NW. Effect on renal function of change from high to moderate protein intake in type 1 diabetic patients. Diabetes 1987;36:73‐9. [DOI] [PubMed] [Google Scholar]
Levine 1989 {published data only}
- Levine SE, S'Elia JA, Bistrian B, Smith‐Ossman S, Gleason R, Mitche WE, Miller GG. Protein restricted diets in diabetic nephropathy. Nephron 1989;52:55‐61. [DOI] [PubMed] [Google Scholar]
Mollsten 2001 {published data only}
- Mollsten AV, Dahlquist GG, Stattin EL, Rudberg S. Higher intakes of fish protein are related to a lower risk of microalbuminuria in young Swedish type 1 diabetic patients. Diabetes Care 2001;24:805‐10. [DOI] [PubMed] [Google Scholar]
Parillo 1988 {published data only}
- Parrilo M, Riccardi G, Pacioni D, Iovine C, Contaldo F, Isernia C, Marco F, Perrotti N, Rivellese A. Metabolic consequences of feeding a high carbohydrate, high fibre diet to diabetic patients with chronic kidney failure. American Journal of Clinical Nutrition 1988;48:255‐9. [DOI] [PubMed] [Google Scholar]
Pecis 1994 {published data only}
- Pecis M, Azevedo MJ, Gross JL. Chicken and fish diet reduced glomerular hyperfiltration in IDDM patients. Diabetes Care 1994;17:665‐72. [DOI] [PubMed] [Google Scholar]
Pedersen 1989 {published data only}
- Pedersen M, Mogensen CE, Schonau JF, Moller B, Lykke G, Pedersen O. Renal effects from limitation of high dietary protein in normoallbuminuric diabetic patients. Kidney International 1989;36:115‐21. [PubMed] [Google Scholar]
Rudberg 1988 {published data only}
- Rudberg S, Dahlquist G, Aperia A, Persson B. Reduction of protein intake decreases glomerular filtration rate in young Type 1 (insulin‐dependent) diabetic patients mainly in hyperfiltering patients. Diabetologia 1988;31:878‐83. [DOI] [PubMed] [Google Scholar]
Stephenson 2005 {published data only}
- Stephenson TJ, Setchell KD, Kendall CW, Jenkins DJ, Anderson JW, Fanti P. Effect of soy protein‐rich diet on renal function in young adults with insulin‐dependent diabetes mellitus. Clinical Nephrology 2005;64:1‐11. [DOI] [PubMed] [Google Scholar]
Stojceva‐Taneva 2001 {published data only}
- Stojceva‐Taneva O, Polenakovic M, Grozdanovski R, Sikole A. Lipids, protein intake, and progression of diabetic nephropathy. Nephrology Dialysis Transplantation 2001;16 (Suppl. 6):90‐91. [DOI] [PubMed] [Google Scholar]
Wheeler 2002 {published data only}
- Wheeler ML, Fineberg SE, Fineberg NS, Gibson RG, Hackward LL. Animal versus plant protein meals in individuals with type 2 diabetes and microalbuminuria: effects on renal, glycemic, and lipid parameters.[see comment]. Diabetes Care 2002;25:1277‐82. [DOI] [PubMed] [Google Scholar]
Wiseman 1987 {published data only}
- Wiseman MJ, Bognetti E, Dodds R, Keen H, Viberti G. Changes in renal function in response to protein restricted diet in type 1 (insulin‐dependent) diabetic patients. Diabetologia 1987;30:154‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Bilous 2005
- Bilous RW. The kidney in systemic disease. In: Warrell DA, Cox TM, Firth JD, Benz EJ editor(s). Oxford Textbook of Medicine. 4th Edition. Vol. 3, Oxford: Oxford University Press, 2005. [Google Scholar]
Brenner 1982
- Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease. New England Journal of Medicine 1982;307:652‐9. [DOI] [PubMed] [Google Scholar]
Connor 2003
- Connor H, Annan F, Bunn E, Frost G, McGough N, Sarwar T, Thomas B. The implementation of nutritional advice for people with diabetes. Diabetic Medicine 2003;20:786‐807. [DOI] [PubMed] [Google Scholar]
El Nahas 1984
- Nahas AM, Masters‐Thomas A, Brady SA. Selective effect of low protein diets in chronic renal diseases. British Medical Journal 1984;289:1337‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finne 2005
- Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen‐Riska C. Incidence of end‐stage renal disease in patients with type 1 diabetes. JAMA 2005;295:1782‐1787. [DOI] [PubMed] [Google Scholar]
Gross 2005
- Gross JL, Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic Nephropathy: Diagnosis, Prevention, and Treatment. Diabetes Care 2005;28:164‐176. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ikizler 1995
- Ikizler TA, Greene JH, Wingard RL. Spontaneous dietary protein intake during progression of chronic renal failure. Journal of the American Society of Nephrology 1995;6:1386‐1391. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Klahr 1994
- Klahr S, Levey AS, Beck GJ. The effects of dietary protein restriction and blood pressure control on the progression of chronic renal diseases. New England Journal of Medicine 1994;330:884. [DOI] [PubMed] [Google Scholar]
Levey 1994
- Levey AS, Beck GJ, Caggiula AW. Trends towards a beneficial effect of a low protein diet during additional follow‐up in the Modification of Diet in Renal Disease Study. Journal of the American Society of Nephrology 1994;5:336A. [Google Scholar]
Maschio 1982
- Maschio G, Oldrizzi L, Tessitore N, D'Angelo A, Valvo E, Lupo A, et al. Effects of dietary protein and phosphorus restriction on the progression of early renal failure. Kidney International 1982;22:371‐6. [DOI] [PubMed] [Google Scholar]
MDRD Study Grp 1989
- Modification of Diet in Renal Disease (MDRD) Study Group. Nutritional status of patients with different levels of chronic renal insufficiency. Kidney International 1989;36(Supp 27):S184‐94. [PubMed] [Google Scholar]
Mogensen 1976
- Mogensen CE. Progression of nephropathy in long‐term diabetes with proteinuria and effect of initial anti‐hypertensive therapy. Scandinavian Journal of Clinical Investigations 1976;36:383‐8. [DOI] [PubMed] [Google Scholar]
Parving 1981
- Parving H‐H, Smidt UM, Friisberg B, Bonnevie‐Nielsen V, Andersen AR. A prospective study of glomerular filtration rate and arterial blood pressure in insulin‐dependent diabetics with diabetic nephropathy. Diabetologia 1981;20:457‐461. [DOI] [PubMed] [Google Scholar]
Pedrini 1996
- Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. The Effect of Dietary Protein Restriction on the Progression of Diabetic and Nondiabetic Renal diseases: A Meta‐analysis. Annals of Internal Medicine 1996;124(7):627‐632. [DOI] [PubMed] [Google Scholar]
Raine 1995
- Raine AEG. The rising tide of diabetic nephropathy ‐ the warning before the flood?. Nephrology Dialysis and Transplantation 1995;10:460‐1. [DOI] [PubMed] [Google Scholar]
Rosman 1984
- Rosman JB, Meijer S, Sluiter WJ, Wee PMT, Piers‐Becht TPM, Donker AJM. Prospective randomised trial of early protein restriction in chronic renal failure. Lancet 1984;December:1291‐6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Sitprija 1983
- Sitprija V, Suvanpha R. Low protein diet and chronic renal failure in Buddist monks. British Medical Journal 1983;287:469‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Toeller 1996
- Toeller M, Klischan A, Heitkamp G, Schumacher W, Milne R, Buyken A, Karamanos B, Gries FA. Nutrutional intake of 2868 IDDM patients from 30 centres in Europe. Diabetologia 1996;39:929‐939. [DOI] [PubMed] [Google Scholar]
Viberti 1983
- Viberti GC, Bilous RW, Mackintosh D, Keen H. Monitoring Glomerular Function in Diabetic Nephropathy. The American Journal of Medicine 1983;74:256‐64. [DOI] [PubMed] [Google Scholar]
Waugh 2003
- Waugh N, Robertson A. Treatment of diabetic nephropathy: low protein diet. Management of Diabetic Nephropathy. London: Martin Dunitz Ltd, 2003. [Google Scholar]


