Abstract
Background
Enuresis (bed‐wetting) is a socially disruptive and stressful condition which affects from 15% to 20% of five year olds, and up to 2% of young adults.
Objectives
To assess the effects of desmopressin on nocturnal enuresis in children, and to compare desmopressin with other interventions.
Search methods
We searched the Cochrane Incontinence Group Specialised Trials Register (searched 10 May 2006). The reference list of the original version of this review was also searched.
Selection criteria
All randomised controlled trials of desmopressin for nocturnal enuresis in children were included in the review. Trials focused solely on daytime wetting were excluded.
Data collection and analysis
Two reviewers independently assessed the quality of the eligible trials and extracted data.
Main results
Forty seven randomised controlled trials involving 3448 children (of whom 2210 received desmopressin) met the inclusion criteria. The quality of many of the trials was poor.
Desmopressin was effective in reducing bed‐wetting during treatment, compared with placebo (e.g. 20 µg: 1.34 fewer wet nights per week; 95% confidence interval (CI) 1.11 to 1.57), and children were more likely to become dry (e.g. 118/146, 81% versus 140/142, 98% still wet; relative risk (RR) for failure to achieve 14 dry nights with 20 µg was 0.84; 95% CI 0.79 to 0.91). However, there was no difference between the two patient groups after treatment was finished. There was no clear dose‐related effect of desmopressin, but the evidence was limited. Data which compared oral and nasal administration were too few to be conclusive.
In four small trials, there were no significant differences between desmopressin and alarms during treatment when these were used separately, but the chance of failure or relapse after treatment stopped was lower after an alarm in two small trials (40/62, 65% versus 26/57, 46%; RR 1.42, 95% CI 1.05 to 1.91).
Although children had fewer wet nights during treatment when they used desmopressin combined with alarm treatment compared with alarms alone (WMD ‐0.83, 95% CI ‐1.11 to ‐0.55), there were no significant differences either in failure rates during treatment (RR 0.88; 95% CI 0.73 to 1.05) or for relapse after treatment stopped (105/213, 49% versus 118/214, 55%: RR 0.91, 95% CI 0.76 to 1.08).
Comparison with some tricyclic drugs (e.g. amitriptyline) suggested that they might be as effective as desmopressin, although in two trials children were less likely to achieve 14 dry nights with imipramine than desmopressin (RR 0.44, 95% CI 0.27 to 0.73) but there was not enough information about subsequent relapse. There were more side effects with the tricyclics. Desmopressin may be better than diclofenac or indomethacin.
There was not enough information to evaluate the relative effects of behavioural or complementary treatments against desmopressin.
Authors' conclusions
Desmopressin rapidly reduced the number of wet nights per week experienced by children, but the limited evidence available suggested that this was not sustained after treatment stopped. Comparison with alternative treatments suggested that desmopressin and tricyclics had similar clinical effects during treatment, but that alarms may produce more sustained benefits. However, based on the available limited evidence, these conclusions are only tentative. Children should be advised not to drink more than 240 ml (8 ounces) of fluid during the evening before desmopressin treatment in order to avoid the possible risk of water intoxication.
Plain language summary
Desmopressin for bedwetting in children
Bedwetting is a distressing and stressful condition for children and their families. Some children take longer than others to stop bedwetting. Up to 20% still wet at the age of five years, but by the age of 16 only 2% or less do so. Desmopressin is a drug which reduces bedwetting by reducing the amount of urine produced at night. It is taken before bedtime, and the children are also advised not to drink more than 240 ml (8 ounces) of fluid in the evening. However, it only works on the nights when it is used, so does not cure the problem in the long term.
When desmopressin is used, most of the children have fewer wet nights (one night less on average per week) and more become dry (19% compared with only 2% using dummy treatment in five trials involving 288 children). However, many children start wetting again when treatment stops. On the other hand, more children remain dry when alarm treatment is finished (54% after alarm compared with 35% after desmopressin in two trials involving 119 children). Adding desmopressin to alarm treatment did not result in better cure rates after the end of treatment (51% remained dry after combination treatment compared with 45% after alarm alone).
Those using desmopressin (or their parents) should be warned that over‐drinking before bedtime should be avoided as this may lead to serious, but rare, adverse effects. Drugs called tricyclic antidepressants have a similar effect to desmopressin and are cheaper, but have more adverse effects. There are few adverse effects with alarms, other than short‐term disruption for the family. In summary, alarms take longer to reduce bed‐wetting, but their effect may persist longer than desmopressin.
Background
This is one of seven reviews of interventions for bed‐wetting, or non‐organic nocturnal enuresis. The others focus on: tricyclics and related drugs (Glazener 2004e); other drugs (Glazener 2004c); alarms (Glazener 2004a); simple behavioural interventions (Glazener 2004d); complex behavioural interventions (Glazener 2004b); and miscellaneous and complementary therapy (in preparation). All seven reviews were based on the work of Lister‐Sharp and her colleagues at the Centre for Reviews and Dissemination at the University of York, United Kingdom (Lister‐Sharp 1997).
Nocturnal enuresis is the involuntary loss of urine at night, in the absence of organic disease, at an age when a child could reasonably be expected to be dry (by consensus, at a developmental age of five years) (APA 1980; WHO 1992). Although bed‐wetting is pathologically benign and has a high rate of spontaneous remission, it may bring social and emotional stigma, stress and inconvenience to both the children with enuresis and their families (Fitzwater 1992). Children who wet the bed may experience parental disapproval, sibling teasing and repeated treatment failure, which may lower self‐esteem (Warzak 1993). The children may also be at increased risk of emotional and physical abuse (Warzak 1993). Consequently, it is important that enuresis is properly managed on 'humane grounds' (Moffatt 1994).
Although daytime wetting is a significant problem, and is often associated with bed‐wetting, it is usually considered separately. It has been suggested that there are different aetiologies underlying the two conditions (Jarvelin 1989). If daytime symptoms are present, investigations to identify physical causes such as urinary tract dysfunction, congenital malformation and neurogenic disorders are usually necessary (Djurhuus 1992). An organic cause is more often found in children with daytime wetting: more structural abnormalities and functional disorders of the urinary tract were found in daytime wetters than controls (Jarvelin 1990).
Prevalence and causes
Nocturnal enuresis is a complaint that affects many families. Estimating the prevalence of nocturnal enuresis is difficult, however, because there is variation in methods of diagnosis and definitions (de Jonge 1973; Krantz 1994). In the United Kingdom, the generally quoted prevalence rates are that 15% to 20% of five year olds, 7% of seven year olds, 5% of ten year olds, 2% to 3% of 12 to 14 year olds and 1% to 2% of those aged 15 and over wet the bed twice a week on average (Blackwell 1989; Rutter 1973). The incidence of nocturnal enuresis is particularly high amongst children in residential care (Morgan 1970). About 1% of adults remain enuretic. Without treatment, about 15% of bed‐wetting children become dry each year (Forsythe 1974).
The causes of nocturnal enuresis are unclear (Lister‐Sharp 1997). Genetic (APA 1980 1980; Bakwin 1971; Bakwin 1973; Eiberg 1995), physiological (Djurhuus 1992; Norgaard 1993) and psychological (Devlin 1991; Moffatt 1989; Rutter 1973; Shaffer 1977) factors, as well as delay in maturation of the mechanism for bladder control (Jarvelin 1989; Koff 1995), have been suggested. Other factors which may contribute to bed‐wetting include: constipation, sleep apnoea, upper airway obstructive symptoms (Maizels 1993), diet and intake of mild caffeine drinks with diuretic effects (e.g. cola) (Blackwell 1989).
Interventions
Pharmacological, psychological/behavioural and a variety of 'unconventional' interventions are commonly used for people who wet the bed.
Pharmacological interventions include desmopressin, tricyclic drugs (amitriptyline, dothiepin, doxepin, trimipramine, clomipramine, desipramine, imipramine, lofepramine, nortriptyline and protriptyline) (Glazener 2004e); drugs related to the tricyclics (viloxazine, desipramine, mianserin and maprotiline) (Glazener 2004e); and amphetamine, diazepam and oxybutynin (Glazener 2004c). However, some of these drugs are now contraindicated. Simple behavioural interventions include reward systems (such as star charts), lifting, scheduled wakening (Glazener 2004d), and alarms and over‐learning (after successful alarm treatment) (Glazener 2004a). Complex behavioural interventions include multidimensional behavioural treatment such as dry bed training or full spectrum home training (Glazener 2004b). Less common interventions include: psychotherapy, retention control training, surgery, fluid deprivation and complementary therapies.
This review is restricted to pharmacological treatment with desmopressin, or to any other intervention that is compared with, or used in combination with, desmopressin.
Desmopressin
Desmopressin is an analogue of the human pituitary hormone arginine vasopressin. Its antidiuretic effect results from increased reabsorption of water from the kidney, leading to a reduced volume of more concentrated urine entering the bladder (Djurhuus 1992). In 1972, desmopressin was introduced in a dropper bottle allowing drops to be placed into the nose. It has also become available as a measured dose spray giving doses in multiples of 10 µg; a single dose pipette giving doses in multiples of 20 µg; and 0.2 mg oral tablets. Generally, 20 µg to 40 µg is given intranasally at bedtime irrespective of age and body weight (Harris 1989). Although initially prescribed for short‐term treatment, longer‐term treatment may be considered appropriate for some children. It has been recommended that after three months, treatment should be withdrawn for at least one week pending reassessment (BNF 2002).
About 10% of intranasal desmopressin is absorbed from the nasal mucosa. The maximum plasma concentration of desmopressin is reached within an hour, and the biological effect lasts for 10 to 12 hours (Harris 1989).
A review of the adverse effects of desmopressin for nocturnal enuresis noted that 22 adverse experiences, most commonly nasal irritation and nose bleeds, were reported in seven published studies (Hjalmas 1993). Twelve additional published studies reported no adverse effects. Although 21 cases of water intoxication were spontaneously reported by physicians and patients prior to 1993, the authors of the review concluded that desmopressin produces few, mostly mild, adverse effects in children treated for nocturnal enuresis (Hjalmas 1993).
Water intoxication is potentially the most serious complication. It is associated with over‐drinking at bedtime, and its symptoms include headache, nausea, hyponatraemia, cerebral oedema and convulsions. Current guidelines recommend that not more than 240 ml (8 ounces) of fluid should be consumed on any night when desmopressin is used (Bernstein 1997; Robson 1994; Robson 1996).
Objectives
To determine the effects of desmopressin for the treatment of children with nocturnal enuresis.
The following comparisons were made: (1) desmopressin versus no active treatment / placebo; (2) lower versus higher doses of desmopressin; (3) oral versus nasal administration of desmopressin; (4) desmopressin versus other drugs, alone or in combination; (5) desmopressin alone versus alarm treatment alone; (6) desmopressin alone versus desmopressin supplemented by alarm treatment; (7) desmopressin supplemented by alarm treatment versus alarm treatment alone; (8) desmopressin versus behavioural methods, alone or in combination; (9) desmopressin versus complementary treatment .
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials of desmopressin with any comparable control groups for the treatment of nocturnal enuresis.
Types of participants
Children (as defined by trialists, usually as less than 16 years of age) suffering from nocturnal enuresis.
Types of interventions
Any trial that used desmopressin in at least one arm of the study.
Comparisons were made with no active treatment, other types of drugs, and alarm or behavioural interventions, either alone or in combination with desmopressin.
Types of outcome measures
The outcomes considered in this review were:
mean number of wet nights per week during treatment;
number of children failing to attain 14 consecutive dry nights during treatment;
mean number of wet nights per week when children were followed up after treatment ends;
number of children failing during treatment and/or relapsing after treatment ends;
adverse effects.
Search methods for identification of studies
This review has drawn on the search strategy developed for the Incontinence Review Group. Relevant trials were identified from the Group's Specialised Register of controlled trials which is described, along with the group search strategy, under the Incontinence Group's details in The Cochrane Library (For more details please see the ‘Specialized Register’ section of the Group’s module in The Cochrane Library). The register contains trials identified from MEDLINE, CINAHL, the Cochrane Central Register of Controlled Trials (CENTRAL) and hand searching of journals and conference proceedings. The Incontinence Group Specialised Trials Register was searched using the Group's own keyword system. The search terms used were:
({design.cct*} OR {design.rct*}) AND {topic.enuresis*} (All searches were of the keyword field of Reference Manager 9.5N, ISI ResearchSoft.) Date of the most recent search of the register for this review: 10 May 2006.
The trials in the Incontinence Group's trials register are also contained in CENTRAL.
The reference list of a previous systematic review of enuresis treatments was also searched (Lister‐Sharp 1997).
No language restriction or other limits were imposed on the searches.
Data collection and analysis
Identification of primary studies
The titles, and where possible, abstracts of all studies located by the searches were checked to identify any potentially relevant studies. Full papers were then obtained and assessed to identify those which met the inclusion criteria.
Quality assessment
A range of both general and more specific quality issues were noted, including:
level of concealment of random allocation in the trials;
whether data to assess the comparability of groups at baseline were given, including baseline levels of wetting;
use of a 'washout' period if a crossover design was employed;
intention‐to‐treat analysis;
whether outcomes were clearly defined;
blinding;
a follow up of at least three months;
the use of appropriate statistical techniques;
whether useful data (e.g. means and standard deviations) were presented;
whether children with daytime wetting were specifically excluded;
whether children who had physical (organic) causes for their enuresis were specifically excluded.
Data extraction
The data were extracted using a standard form. Included data were processed as described in the Cochrane Reviewers' Handbook (Clarke 2003). Where appropriate, the results were converted to the mean and standard deviation of the number of wet nights per week; the number of children failing to achieve cure during treatment, defined as 14 consecutive dry nights; or the number of children who were not cured during treatment plus those who relapsed after stopping active treatment (to allow for possible differences in initial 'success' rates). When a mean value was reported with no standard deviation, we entered the data into 'Other Data Tables'.
Data analysis
We calculated, where possible, weighted mean differences (WMD) and relative risks (RR) plus 95% confidence intervals (CI). A fixed effect model was used to calculate the pooled estimates and the 95% CIs (Berlin 1989). The WMDs were weighted by the inverse of the variance and reported as differences in the number of wet nights per week. Negative values indicated fewer wet nights in the intervention group at the left‐hand side of the tables. Differences between trials were further investigated when statistically significant heterogeneity was apparent either at the 10% probability level, using the chi squared test or assessment of the I‐squared statistic (Higgins 2003), or from visual inspection of the results. If there was no obvious reason for the heterogeneity, or it persisted despite the removal of outlying trials, a random effects model could have been used.
Crossover trials were indicated by the symbol # after the trial ID. These were analysed as if they were parallel groups, but a sensitivity analysis was carried out by excluding these trials to determine if their inclusion biased the findings.
Results
Description of studies
Of 75 studies identified as potentially relevant, 28 were excluded: 21 were not randomised controlled trials (RCTs); five included some adults with detrusor overactivity or daytime wetting, or both; one did not use desmopressin therapeutically (Bogaert 2005); and one switched children between treatment groups. In this minor update (Issue 3 2006), 2 of the 28 were new excluded studies (see Characteristics of Excluded Studies). Of the remaining included trials, 18 (Bradbury 1995; Faraj 1999; Folwell 1997#; Hamano 2000; Kahan 1998; Leebeek 2001; Longstaffe 2000; Muller 2001#; Natochin 2000; Neveus 1999#; Radmayr 2001; Rodriguez 2001; Schulman 2001a; Schulman 2001b; Sener 1998; Skoog 1997; Vertucci 1997; Yap 1998#) were RCTs added in the previous update (Glazener 2002). Two others (Dimson 1986#; Stenberg 1994#) had been excluded in the first published version of this review (Glazener 2000), and one more was added in the second update (Gibb 2004). In the third update (Issue 3 2006), a further five RCTs have been added (Fera 2004; Hoashi 1995; Lee 2005; Ng 2005; Uygur 1997#).
The 47 included trials were described in 44 reports (three reports described two separate trials each). Seventeen trials used a crossover design (indicated by symbol #). All the trials, bar two (Rodriguez 2001; Hoashi 1995), gave measures of baseline wetting, and all but three specifically excluded children with organic causes of enuresis (Fera 2004; Radmayr 2001; Yap 1998#). However, all three of these trials included only children with monosymptomatic nocturnal enuresis, and excluded children with daytime wetting (Fera 2004; Radmayr 2001; Yap 1998#). In general, sample sizes were small, ranging from 10 to 182 with an average of about 73 children. Out of a total of 3448 children, 2210 received treatment with desmopressin.
Participants
Two studies included some older children or adults (Janknegt 1997; Rittig 1988#), while another focused on adolescents who had failed previous treatment (Stenberg 1994#). Only seven of the trials excluded children who had previously received treatment for their enuresis (Faraj 1999; Fera 2004; Kahan 1998; Muller 2001#; Ng 2005; Radmayr 2001; Sener 1998). The remainder either failed to report this factor (9 trials) or included some children who had failed previous drug or behavioural treatments (31 trials). Of the latter, all the children in one trial had previously failed to improve with desmopressin (Gibb 2004). In another trial, a baseline trial of desmopressin was used to select out those children who failed to respond to desmopressin: only responders were enrolled in the crossover RCT (Uygur 1997#).
Dosage of desmopressin
Some trials used dose titration until dry nights were achieved (Birkasova 1978#; Faraj 1999; Fera 2004; Hamano 2000; Lee 2005; Radmayr 2001; Rittig 1988#; Rodriguez 2001; Rushton 1995; Schulman 2001b; Stenberg 1994#; Terho 1991#; Uygur 1997#), or compared a variety of doses of active drug (Kjoller 1984; Janknegt 1990#; Janknegt 1997; Miller 1990a; Miller 1990b; Neveus 1999#; Schulman 2001a; Skoog 1997). One reduced the dose from 40 µg to 20 µg for the second three weeks of the trial (Leebeek 2001).
Route of administration and comparators
Intranasal administration was used in all but ten of the trials: oral tablets were specified in ten (Fera 2004; Janknegt 1997; Lee 2005;Neveus 1999#; Ng 2005; Schulman 2001a; Schulman 2001b; Skoog 1997; Stenberg 1994#; Yap 1998#), and two different routes of administration were compared in one (Fjellestad 1987#). Twenty trials included other interventions:
other drugs (Burke 1995; Hoashi 1995; Holt 1986; Lee 2005; Natochin 2000; Sener 1998; Vertucci 1997);
alarms (Bradbury 1995; Faraj 1999; Gibb 2004; Leebeek 2001; Longstaffe 2000; Ng 2005; Rodriguez 2001; Sukhai 1989#; Wille 1986);
other behavioural treatment (Fera 2004; Hamano 2000; Kahan 1998); and
complementary treatment (laser acupuncture) (Radmayr 2001).
One ongoing trial has yet to be completed (Hjalmas 2001).
Risk of bias in included studies
Of the 47 identified RCTs which included desmopressin in at least one arm, only 15 described a secure randomised method of allocation (e.g. by computer allocation or use of sealed opaque envelopes) (Birkasova 1978#; Bradbury 1995; Burke 1995; Dimson 1986#; Folwell 1997#; Leebeek 2001; Longstaffe 2000; Neveus 1999#; Ng 2005; Schulman 2001a; Schulman 2001b; Stenberg 1994#; Sukhai 1989#; Uygur 1997#; Yap 1998#). A further 31 trials did not provide adequate details for this to be assessed (although some used double‐blind placebo controls and others were crossover trials), and one trial used a quasi‐randomised method (Natochin 2000).
There were 17 crossover trials, identified by the symbol # (Birkasova 1978#; Dimson 1986#; Fjellestad 1987#; Folwell 1997#; Janknegt 1990#; Muller 2001#; Neveus 1999#; Post 1983a#; Post 1983b#; Rittig 1988#; Stenberg 1994#; Sukhai 1989#; Terho 1984#; Terho 1991#; Tuvemo 1978#; Uygur 1997#; Yap 1998#). One other crossover trial compared desmopressin with imipramine, but only results from the first arm of the trial were used, which in effect formed parallel groups (Vertucci 1997). Because of their crossover design, none of the crossover trials could address longer‐term post‐treatment effects. A further 21 trials used double‐blind placebos to ensure blinding to treatment (Aladjem 1982; Burke 1995; Gibb 2004; Hoashi 1995; Holt 1986; Janknegt 1997; Kahan 1998; Kjoller 1984; Leebeek 2001; Longstaffe 2000; Martin 1993; Miller 1990a; Miller 1990b; Natochin 2000; Rushton 1995; Schulman 2001a; Schulman 2001b; Segni 1982; Sener 1998; Skoog 1997; Uygur 1997#). In the remainder of the studies, blinding to treatment allocation was not possible because the treatments were too dissimilar.
Two trials failed to report systematic baseline measures of wetting (Hoashi 1995; Rodriguez 2001), and three failed to exclude children with organic (physical) causes for their bed‐wetting (Fera 2004; Radmayr 2001; Yap 1998#), but all these trials excluded children with daytime wetting. Three trials included some children with daytime wetting but excluded children with organic (physical) causes (Bradbury 1995; Gibb 2004; Martin 1993). Daytime wetting was specifically excluded or reported separately in 27 trials, and not mentioned in another 17.
Only three trials reported using a washout period between the two arms of a crossover trial (Fjellestad 1987#; Sukhai 1989#; Yap 1998#), while another interposed a 'placebo' arm between the first and second active arms (Janknegt 1990#). Children who did not respond in one trial (Schulman 2001a) received placebo treatment during a two‐week washout phase before being randomly assigned again in a second trial (Schulman 2001b).
Ten trials reported continuous data without a measure of dispersion such as a standard deviation (Dimson 1986#; Fjellestad 1987#; Leebeek 2001; Miller 1990a; Miller 1990b; Muller 2001#; Rittig 1988#; Terho 1991#; Uygur 1997#; Vertucci 1997). Further data are being sought from the authors of some trials. Only two of the included trials provided a power calculation of the sample size, which helps to define the minimum sample size required to detect a true difference between the treatment groups (Janknegt 1990#; Leebeek 2001).
Effects of interventions
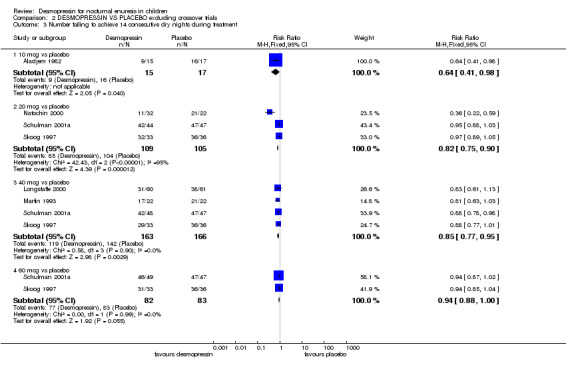
(1) Twenty nine trials compared desmopressin with placebo treatment (Aladjem 1982; Birkasova 1978#; Dimson 1986#; Fjellestad 1987#; Folwell 1997#; Janknegt 1990#; Kjoller 1984; Longstaffe 2000; Martin 1993; Miller 1990a; Miller 1990b; Muller 2001#; Natochin 2000; Neveus 1999#; Post 1983a#; Post 1983b#; Rittig 1988#; Rushton 1995; Schulman 2001a; Schulman 2001b; Segni 1982; Sener 1998; Skoog 1997; Stenberg 1994#; Terho 1984#; Terho 1991#; Tuvemo 1978#; Uygur 1997#; Yap 1998#); (2) Two trials compared oral with nasal administration (Fjellestad 1987#; Faraj 1999); (3) Seven trials compared desmopressin with tricyclic or other drugs (Burke 1995; Hoashi 1995; Holt 1986; Lee 2005; Natochin 2000; Sener 1998; Vertucci 1997); (4) Nine trials compared desmopressin with alarms (Bradbury 1995; Faraj 1999; Gibb 2004; Leebeek 2001; Longstaffe 2000; Ng 2005; Rodriguez 2001; Sukhai 1989#; Wille 1986); (5) Three trials compared desmopressin with behavioural interventions (Fera 2004; Hamano 2000; Kahan 1998); (6) One trial compared desmopressin with laser acupuncture (Radmayr 2001).
1. Desmopressin versus no active treatment / placebo (see Comparisons 01 and 02, Other Data Tables 01)
Desmopressin was better than placebo treatment in achieving fewer wet nights per week during treatment and more children cured during treatment. Desmopressin was better (fewer wet nights per week) at doses of 10 µg in two trials (WMD ‐2.30; 95% CI ‐3.42 to ‐1.18); 20 µg in 12 trials (WMD ‐1.34; 95% CI ‐1.57 to ‐1.11); 40 µg in six trials (WMD ‐1.33; 95% CI ‐1.67 to ‐0.99); and 60 µg in two trials (WMD ‐1.50; 95% CI ‐1.92 to ‐1.08, Comparison 01.01). This held true in a sensitivity analysis when the crossover trials were excluded (e.g. WMD for 20 µg in seven trials ‐1.21; 95% CI ‐1.49 to ‐0.95, Comparison 08.01). Thus, desmopressin significantly reduced bed‐wetting by about one to two nights a week, irrespective of whether the crossover studies were included. There were also fewer wet nights during desmopressin treatment in trials which used variable doses of the drug (Birkasova 1978#; Rushton 1995), or in trials which failed to provide standard deviations (Other Data Tables 01.02).
Ten trials reported the number of children cured (defined as achieving 14 consecutive dry nights) while taking either desmopressin or placebo. Despite differences in desmopressin dose, the trials were consistent, suggesting that desmopressin increased the chances of cure (RR for failure with 20 µg in five trials was 0.84; 95% CI 0.79 to 0.91: RR for 40 µg in six trials was 0.81; 95% CI 0.74 to 0.88: RR for 60 µg in two trials was 0.94; 95% CI 0.89 to 0.99, Comparison 01.03). A sensitivity analysis excluding the crossover trials supported these findings (Comparison 08.03).
However, the data suggest that this effect was not sustained after treatment had finished. In four trials (Aladjem 1982; Kjoller 1984; Miller 1990a; Miller 1990b), there was little difference between the groups in terms of wet nights per week, but the trials were small, the confidence intervals were wide, and two trials did not report standard deviations (Comparison 01.04, Other Data Tables 01.05). Only one small trial reported relapse rates: all the children either failed to respond to treatment or relapsed afterwards (Comparison 01.06) (Dimson 1986#).
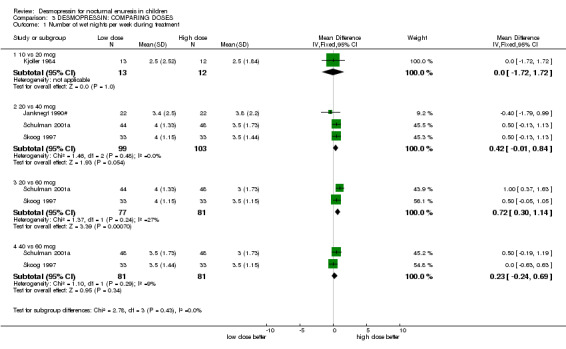
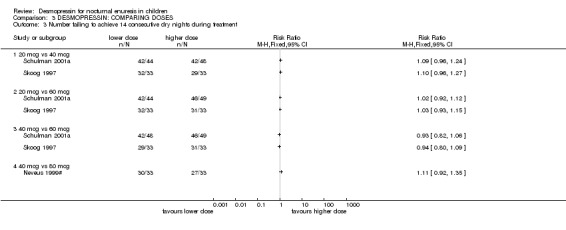
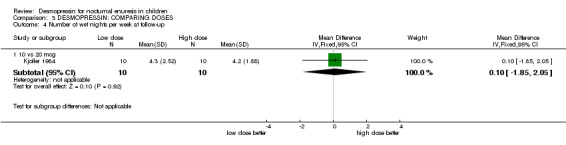
2. Lower versus higher doses of desmopressin (see Comparison 03, Other Data Tables 03)
Eight small trials compared different doses of desmopressin with each other (Janknegt 1990#; Janknegt 1997; Kjoller 1984; Miller 1990a; Miller 1990b; Neveus 1999#; Schulman 2001a; Skoog 1997). Two of these trials were crossovers and two did not report standard deviations. There was some evidence that a higher dose was more likely to reduce the numbers of wet nights than a lower dose (e.g. for 20 µg versus 40 µg the WMD for wet nights during treatment was 0.42; 95% CI ‐0.01 to 0.84: for 20 µg versus 60 µg the WMD was 0.72; 95% CI 0.3 to 0.14, Comparison 02.01, Other Data Tables 02). However, there was no difference in cure rates (Comparison 02.03). One trial reported no difference between two oral doses, but it did not provide any useable data (Janknegt 1997).
3. Oral versus nasal administration of desmopressin (see Comparison 04, Other Data Tables 04)
Only one study compared oral (200 µg) and nasal (20 µg) administration of desmopressin (Fjellestad 1987#). This was a crossover trial which did not provide standard deviations and involved only 20 children. There were insufficient data to judge whether the two routes were equally effective. Nasal discomfort (two children) and epistaxis (three children) were reported with nasal administration. This did not appear to be specifically linked to desmopressin because it was reported equally by patients from both the other groups who received placebo nose drops (Fjellestad 1987#).
4. Desmopressin versus other drugs (see Comparison 05, Other Data Tables 05)
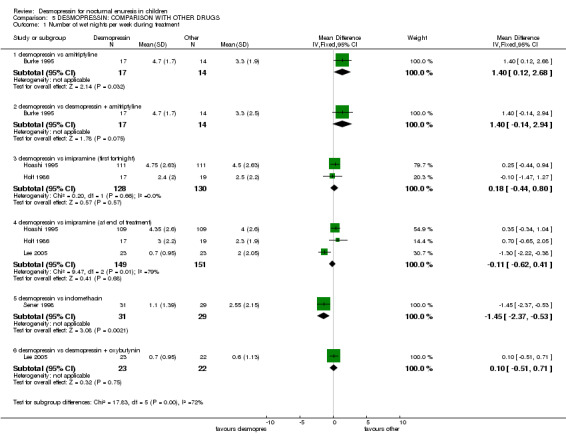
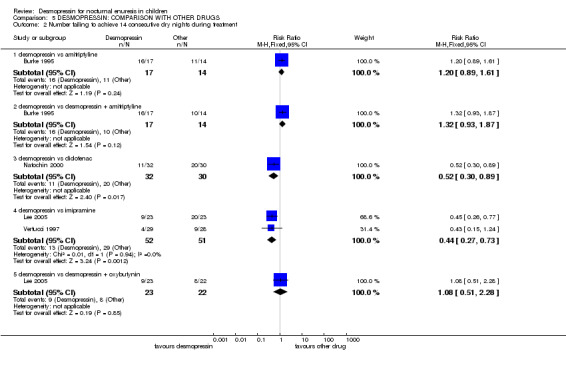
Seven trials compared desmopressin with other drugs: amitriptyline (Burke 1995); imipramine (Hoashi 1995; Holt 1986; Lee 2005; Vertucci 1997); indomethacin (Natochin 2000); and diclofenac (Sener 1998). One trial included an arm which combined amitriptyline with desmopressin (Burke 1995) and another combined desmopressin with oxybutynin (Lee 2005). In two small separate studies, desmopressin performed better than indomethacin (WMD for wet nights per week ‐1.45; 95% CI ‐2.37 to ‐0.53, Comparison 04.01.05) (Sener 1998) and diclofenac (RR for failure to achieve 14 dry nights was 0.52; 95% CI 0.30 to 0.89, Comparison 04.02.03) (Natochin 2000) during treatment, but there was no information about relapse rates after treatment ended. There was not enough evidence to clarify whether desmopressin was better than amitriptyline or oxybutynin added to desmopressin: the sample sizes were too small to address the issue reliably, the follow‐up information was scant, and the confidence intervals were wide. However, more children achieved dry nights with desmopressin than imipramine during treatment (RR for failure to achieve 14 dry nights 0.44, 95%CI 0.27 to 0.73, Comparison 05.02.04) (Lee 2005) but there was no information about subsequent relapse.
5, 6, 7. Desmopressin and alarms
Nine trials compared desmopressin with alarm interventions (Bradbury 1995; Faraj 1999; Gibb 2004; Leebeek 2001; Longstaffe 2000; Ng 2005; Rodriguez 2001; Sukhai 1989#; Wille 1986). In four trials, desmopressin was compared with an alarm alone (Faraj 1999; Longstaffe 2000; Ng 2005; Wille 1986). In three other studies, the children used alarms in both arms of the trials supplemented with either desmopressin or placebo (Gibb 2004; Leebeek 2001; Sukhai 1989#), the latter in a double‐blind crossover design with a two‐week washout period. In a further three trials, children used alarms in both arms of the trial, supplemented by desmopressin in one arm (Bradbury 1995; Ng 2005; Rodriguez 2001).
5. Desmopressin alone versus alarm treatment alone (see Comparison 06)
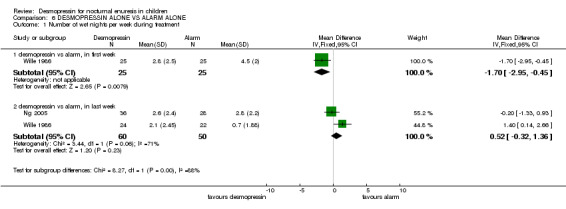
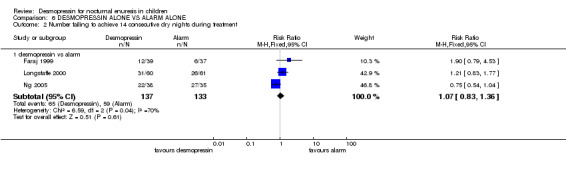
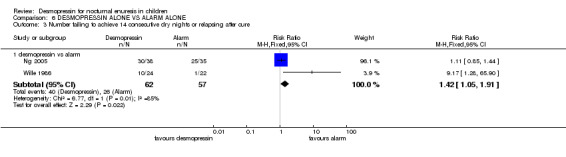
In four trials, desmopressin was compared with an alarm alone (Faraj 1999; Longstaffe 2000; Ng 2005; Wille 1986). One small trial reported that at the end of the first week of treatment, there were 1.7 fewer wet nights per week with desmopressin treatment than with alarm treatment (WMD ‐1.7; 95% CI ‐2.95 to ‐0.45, Comparison 06.01.01) (Wille 1986). In the final week (after three months) there was no significant difference between desmopressin and alarm groups in terms of wet nights per week (WMD 0.52, 95% CI ‐0.32 to 1.36, Comparison 06.01.02) (Ng 2005; Wille 1986) or in numbers remaining wet (RR 1.07, 95%CI 0.83 to 1.36, Comparison 06.02.01) (Faraj 1999; Longstaffe 2000; Ng 2005). However, the relapse rate was significantly less after the end of alarm treatment (40/62, 65% after desmopressin versus 26/57, 46% after alarm, RR 1.42, 95% CI 1.05 to 1.91, Comparison 06.03.01) (Wille 1986; Ng 2005). Where combination of data was possible, the heterogeneity was high.
6. Desmopressin alone versus desmopressin supplemented by alarm treatment (see Comparison 07)
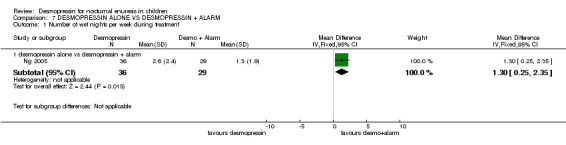
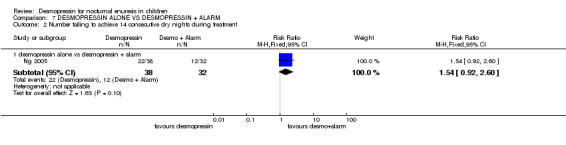
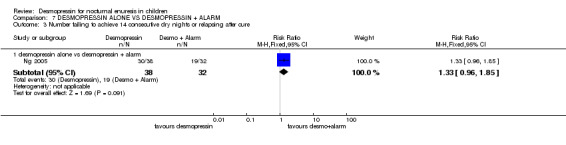
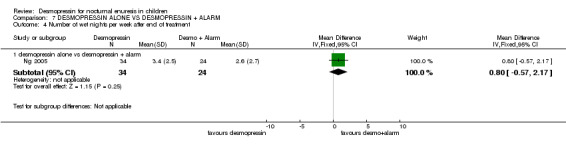
One small trial tested the effect of adding alarm treatment to desmopressin (Ng 2005). The data were too few to draw reliable conclusions.
7. Desmopressin supplemented by alarm treatment versus alarm treatment alone (see Comparison 08)
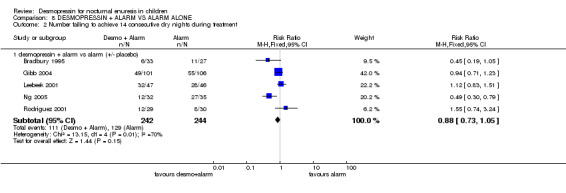
Six trials addressed this comparison (Bradbury 1995; Gibb 2004; Leebeek 2001; Ng 2005; Rodriguez 2001; Sukhai 1989#). Desmopressin combined with alarm treatment was associated with fewer wet nights than alarms alone in four trials (WMD ‐0.83, 95% CI ‐1.11 to ‐0.55, Comparison 08.01.01), whether a placebo was used (Gibb 2004; Sukhai 1989#) or not (Bradbury 1995; Ng 2005). However, this was not reflected in failure rates during treatment: RR for the number of children failing to achieve 14 dry nights was 0.88; 95% CI 0.73 to 1.05, Comparison 08.02.01) with placebo (Gibb 2004; Leebeek 2001) or without placebo (Bradbury 1995; Ng 2005; Rodriguez 2001): the heterogeneity was high.
After treatment stopped, there were no statistically significant differences in the combined failure and relapse rate: RR 0.91, 95% CI 0.76 to 1.08, Comparison 08.03.01) with placebo (Gibb 2004; Leebeek 2001) or without (Bradbury 1995; Ng 2005). However, the trials were small; one was a crossover with a short duration of treatment (Sukhai 1989#); the confidence intervals were wide; and follow‐up results were not available for two trials (Rodriguez 2001; Sukhai 1989#).
8. Desmopressin versus behavioural methods, alone or in combination (see Comparison 09)
Three small trials compared desmopressin alone or in combination with other behavioural methods of managing enuresis (Fera 2004; Hamano 2000; Kahan 1998). The interventions were so dissimilar (retention control training alone in one (Hamano 2000), a complex package of psychological therapy with retention control training in another (Kahan 1998) and a mix of toileting, waking with alarm clock, pelvic floor training and diet and fluid changes in the third (Fera 2004) that data could not be combined. Thus each comparison was addressed by single arms of the trials only.
There was insufficient evidence to compare desmopressin with retention control training alone (Comparisons 09.01.01, 09.02.01, 09.04.01) (Hamano 2000) or with a complex intervention (Comparisons 09.01.04, 09.02.04) (Fera 2004). There was conflicting evidence about the effects of the complex package during treatment (Comparisons 09.01.02 and 03, and 09.02.02 and 03) and afterwards: children recorded fewer wet nights after desmopressin supplemented by the complex package of psychological and behavioural methods, compared to desmopressin alone (WMD ‐2.10; 95% CI ‐2.67 to ‐1.53, Comparison 09.03.02) but this did not reflect in lower failure/relapse rates (RR 0.98; 95% CI 0.91 to 1.06, Comparison 09.04.02) (Kahan 1998).
9. Desmopressin versus complementary treatment (see Comparison 10)
One small trial compared desmopressin with laser acupuncture (Radmayr 2001). However, results were only reported at six months after completing the trial. There was no difference between the groups at follow up, but the numbers were too small to draw reliable conclusions.
Adverse effects
Adverse effects of desmopressin reported amongst 1057 children in 14 trials included (number of events in brackets): anorexia (5), bad taste (2), headache (12), nasal discomfort (20), nosebleeds (6), rash/dermatitis/oedema (6), sight disturbance (1), vomiting (3) and other minor problems (44). Some trials reported that side effects (headaches, stomach ache and nasal symptoms) were equally common with active and placebo treatment (Fjellestad 1987#; Folwell 1997#; Janknegt 1990#), or with desmopressin and tricyclics (Hoashi 1995) or were not due to treatment (Schulman 2001a; Schulman 2001b). Four trials reported that most minor side effects resolved as the trials continued (Schulman 2001a; Schulman 2001b; Skoog 1997; Stenberg 1994#). Side effects were not mentioned in nine trials (Faraj 1999; Fera 2004; Janknegt 1997; Longstaffe 2000; Muller 2001#; Neveus 1999#; Post 1983a#; Post 1983b#; Rodriguez 2001). The remaining 17 trials reported that there were no adverse effects in a further 865 children (Aladjem 1982; Birkasova 1978#; Burke 1995; Kjoller 1984; Leebeek 2001; Natochin 2000; Ng 2005; Radmayr 2001; Rushton 1995; Segni 1982; Sener 1998; Sukhai 1989#; Terho 1984#; Terho 1991#; Tuvemo 1978#; Uygur 1997#; Yap 1998#). One of these trials involved another drug, amitriptyline, with and without desmopressin (Burke 1995). When reported, more adverse effects were associated with tricyclics (83/480, 17.3 per 100 patients (Glazener 2004e)) than with desmopressin (70/1319, 5.3 per 100 patients).
Discussion
This is the third update of the desmopressin review, including 25 new trials identified since the original review was published in 1997 (Lister‐Sharp 1997). The quality of the trials was often poor: small numbers of children were assessed; reporting of data and the method of randomisation were inadequate; and follow up was short or non‐existent. In the crossover trials there was a lack of washout phases and, by their design, no follow up was possible. In particular, little information was available about comparisons with non‐drug interventions. However, all trials focused on children who did not have an organic cause for their enuresis: in the trials which did not specifically exclude organic causes, any children who had daytime wetting were excluded. Only two trials failed to objectively assess baseline wetting. Seven trials selected children who had not had previous enuresis treatment. The majority of the trials (30/46) included at least some children who had failed with previous treatment, and therefore had been referred to specialist clinics; the remainder did not specify past treatment.
Desmopressin compared with placebo
There was clear evidence that desmopressin reduced bed‐wetting by approximately one to two wet nights per week, compared to placebo. In addition, people receiving desmopressin were almost twice as likely as those receiving placebo to achieve at least 14 consecutive dry nights. However, after treatment stopped, the limited evidence available suggested that this improvement was not sustained. Ten crossover trials were analysed as if they were parallel groups. However, a sensitivity analysis showed that their exclusion did not affect the conclusion that desmopressin significantly reduced bed‐wetting by one to two nights a week while on treatment. Crossover trials were unable to contribute follow‐up data after the end of the treatments because the children had received both trial treatments by then.
Dose of desmopressin and route of administration
There were insufficient data to reliably assess whether a higher dose of desmopressin was more effective than a lower dose. To minimise side effects and costs, the lowest dose should be used. In practice, clinicians would increase the dose until the lowest effective dose is achieved. If a higher dose is used with no incremental improvement, the dose should be returned to the lowest effective level. There was not enough evidence to judge whether oral and nasal administration were equally effective, but eight trials used the oral route with no apparent difference in effectiveness. This suggests that equivalent doses may be comparable, irrespective of the route of administration.
Desmopressin compared with other drugs (see also review of tricyclics for enuresis, Glazener 2004e)
The four trials comparing desmopressin with amitriptyline or imipramine were too small to provide definitive results. Desmopressin is more expensive than imipramine, but imipramine has more side‐effects, some of them potentially serious. In two other small trials, desmopressin was better than either indomethacin or diclofenac during treatment, but there was no follow‐up information (Natochin 2000; Sener 1998).
Desmopressin compared with alarms (see also review of alarms for enuresis Glazener 2004a)
Although nine trials including desmopressin and alarm treatments were identified, the findings were difficult to interpret because of the poor quality (small size and dissimilar outcome measures) and variation in interventions (with or without desmopressin or placebo supplementation) of the trials.
In direct comparisons of desmopressin with alarm treatment, the initial advantage of desmopressin (fewer wet nights) was not sustained: children using alarms had fewer wet nights by the end of the trial, and the subsequent relapse rate was lower after alarm treatment (Wille 1986, Ng 2005). While another two small trials demonstrated no significant difference in the failure rate during treatment, follow‐up information was not available (Bradbury 1995; Rodriguez 2001).
There is a move towards combining behavioural and drug interventions (Howe 1992). The rationale is that the rapid onset of action of drugs will augment the more gradual treatment effect of alarms (Sukhai 1989#). Using low doses of desmopressin as an adjunct to alarm treatment might also be used to ensure that the child only wets the bed once each night, which minimises changes of bedding (Djurhuus 1992). The alternative argument, however, is that by using a drug to reduce the wetting, the child has fewer chances to learn behavioural control with the alarm (Gibb 2004). Although there were indeed fewer wet nights during combination treatment compared with desmopressin alone (Comparison 07.01.01, Ng 2005) and alarm alone (Comparison 08.01.01, Bradbury 1995; Gibb 2004; Ng 2005; Sukhai 1989#), it was not possible to judge whether this was reflected in lower failure or relapse rates (Comparison 07.03.01, Ng 2005; and Comparison 08.03.01, Bradbury 1995; Gibb 2004; Leebeek 2001; Ng 2005). This evidence was limited by small numbers and disparate interventions. The use of desmopressin as an adjunct to alarm treatment may be a good way of easing the initial weeks of alarm treatment or for giving families a break, but it is uncertain whether this helps in the long term. This needs to be evaluated by further research.
Desmopressin compared with other behavioural methods
There was no clear evidence to support the use of a complex package of behavioural methods, delivered either with or without desmopressin, in terms of numbers of children cured. Although desmopressin may have contributed to a reduction in the number of wet nights per week during treatment and afterwards, there was no difference in cure rates. This finding needs to be verified with further research. There was not enough evidence to compare desmopressin with retention control training alone.
Desmopressin compared with complementary treatments
In one small trial (comparing desmopressin with laser acupuncture), there was no difference in the failure rate six months after the end of the trial, but the numbers were too small to reliably compare the groups (Radmayr 2001). However, in view of the lack of effect of desmopressin after treatment stops (see comparison of desmopressin with placebo above, Comparisons 01.04 and 01.06, Other Data Tables 01.05), it was not possible to determine if the apparent cure of three quarters of the children in both groups was an effect of both treatments or due to spontaneous cure with time.
Other considerations
Relevant outcome measures
Most trials reported outcome criteria in terms of number of wet nights per week. The number achieving 14 dry nights was reported less often, and few trials provided follow‐up data or relapse rates. It is likely that parents would prefer a treatment that cured the problem in the long term, rather than simply decreasing the frequency of wet nights during treatment. However, reported outcome measures may reflect different aims of treatment: drugs could be used as a way to reduce the frequency of wetting for a specific purpose such as nights away from home (e.g. on a 'dry for camp' basis, for holidays or staying with friends (Meadow 1989)). Some families may find desmopressin useful over winter to overcome laundry problems.
Daytime wetting / organic causes
Only including trials that definitely excluded all children with daytime wetting would have severely limited the review. Only 26 of the 46 trials specifically excluded children with diurnal wetting; the remainder either failed to mention it (17 trials) or failed to exclude it (3 trials). All but one of the trials specifically excluded children with known organic causes for their enuresis. It is likely that the underlying pathologies of monosymptomatic bed‐wetting and mixed night and day wetting differ. The former group might be expected to respond better to treatment aimed at the symptom of bed‐wetting, whereas the latter might respond better to treatment aimed at the underlying pathology (e.g. urinary tract infection, unstable bladder). Alternative managements for these conditions need to be reviewed.
Settings and previous treatment
It should not be assumed that the interventions that are most effective in the trial situation are always the treatments of choice in the clinical situation. Most of the included trials have recruited children from enuresis clinics or are hospital based. Twenty of the trials included at least some children who had already failed previous treatments and were being referred for further advice. These participating families may be especially motivated to tackle bed‐wetting. In addition, strict inclusion or exclusion criteria were imposed in many of the trials. Consequently, the children involved were not necessarily representative of the wider population of bed‐wetters. Factors that made treatment more likely to be successful included: older age, fewer initial wet nights and larger functional bladder capacity (Rushton 1996).
Adverse effects
The reporting of adverse effects varied. In some trials they were not mentioned, while in others they were reported just as often in placebo groups. Some trials reported no adverse effects in any treatment group. All trials reported that the adverse effects of desmopressin were minor and did not require the treatment to cease. Although there appear to be fewer adverse reactions associated with desmopressin than tricyclic drugs, these are rare but can be serious. The risk of water intoxication should be minimised by restricting evening fluid intake on the nights that desmopressin is used (Bernstein 1997; Robson 1994; Robson 1996). Tricyclics are the most common cause of fatal poisoning in children (Parkin 1972). Overdose with tricyclics may occur accidentally or when children believe that a greater dosage gives a better effect (Wille 1986).
Costs
In the United Kingdom, 16 weeks of drug treatment (the usual time allowed for fourteen consecutive dry nights to be attained using an alarm (Butler 1991)) costs (BNF 2002):
UK£78 for desmopressin nasal spray (20 µg per night) or UK£116 for desmopressin tablets (200 µg);
UK£4 for imipramine hydrochloride (25 mg tablet per night) or UK£14 for imipramine syrup (25 mg);
enuresis alarms (including batteries and sensor) typically cost UK£33.60, although alarms but not sensors (UK£12) may be reused several times.
Although treatment with tricyclic or related drugs is considerably less expensive than alarms or desmopressin, this does not take into account the administrative or human costs involved in using alarms. Alarm systems may not be returned to clinics and have to be followed up. Alarm treatment is accompanied by broken nights for various family members until success is attained. The Guidelines on Minimum Standards of Practice (Morgan 1993) suggest that follow‐up supervisory contacts should occur at least every three weeks, with medication reviewed at least monthly. However, this must be considered in light of the lower likelihood of relapse after completing alarm treatment, compared with after desmopressin, tricyclics or related drugs, and the potential for adverse effects with tricyclics.
Authors' conclusions
Implications for practice.
Desmopressin rapidly reduced the number of wet nights per week, compared with placebo in children with monosymptomatic nocturnal enuresis, but this effect was not sustained after treatment stopped. Only two small RCTs followed up children treated with desmopressin or an enuresis alarm; those treated with the alarm were more likely to achieve long‐term success, but this needs to be confirmed in further research. Children who used alarms supplemented with desmopressin initially had fewer wet nights compared to after alarms alone, but this was not reflected in better cure rates or reduced relapse rates after stopping treatment. Potential difficulties, such as the commitment and time needed to attain success, need to be discussed with families before embarking on alarm treatment.
Treatment with desmopressin is considerably more expensive than with tricyclic drugs, but is associated with fewer adverse effects and less risk of fatal overdose. Although alarm interventions are intermediate in cost and are more disruptive in the short term, they do not have the same risk of side effects.
Patients and their families need to be warned about potential adverse effects associated with desmopressin. In particular, children should be advised not to drink more than 240 ml (8 ounces) of fluid on any night that desmopressin is given in order to avoid the possible risk of water intoxication.
Implications for research.
More trials comparing desmopressin with other methods of management are required, especially with alarms, tricyclic drugs, and other behavioural interventions such as lifting, star charts, reward systems and fluid deprivation. Further trials of desmopressin versus placebo should address the issue of whether the benefits are sustained after stopping treatment. Such trials should focus on children who do not have organic causes of bed‐wetting, and should include adequate assessment of baseline levels of wetting.
The trials should use uniform outcome measures such as: the number of wet nights during treatment and after the end of treatment; the number of children achieving 14 consecutive dry nights (cure rate); adverse effects; acceptability of treatment; compliance; and especially relapse rates.
The trials should include children from a variety of backgrounds and populations, particularly those children who have not already failed previous treatment, in order to increase the generalisability of the results. Important demographic factors that should be considered include previous treatment, age, presence of other organic pathology or daytime wetting and family circumstances, as well as co‐existing psychological, emotional or behavioural problems. It has been suggested that not all interventions are suitable for all children. Therefore, further research is needed to determine which interventions are appropriate for which patient groups and under what circumstances (for example, as a short‐term measure to cover nights away from home), in order to guide choice of treatment.
Children with daytime enuresis are more likely to have specific pathology, such as bladder dysfunction or urinary tract infections. Alternative managements for this condition need to be reviewed.
What's new
| Date | Event | Description |
|---|---|---|
| 16 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 4 October 2006 | New search has been performed | Minor update Issue 3 2006. Includes 5 new trials, and the previous tentative conclusions regarding alarms were strengthened: there is now firmer evidence that alarms are more effective (lower relapse rates) than desmopressin after treatment stops. There is no statistically significant evidence to suggest that combining desmopressin and alarm treatment is more effective than alarm treatment alone. Desmopressin appeared to be better than imipramine during treatment. |
| 20 May 2004 | New search has been performed | Minor update Issue 3, 2004. Six new studies were excluded and one new trial (Gibb 2004) was included. The conclusions were, however, unchanged. |
| 21 May 2002 | New citation required and conclusions have changed | Substantive amendment. The review was updated for Issue 3, 2002. 18 new trials were added, and the data from some old trials recalculated to include relapse rates and failure rates together. |
Acknowledgements
This review was originally written for the NHS Centre for Reviews & Dissemination, University of York, by Deborah Lister‐Sharp, Susan O'Meara, Matthew Bradley and Trevor Sheldon. It is published as: A Systematic Review of the Effectiveness of Interventions for Managing Childhood Nocturnal Enuresis, CRD Report 11, NHS Centre for Reviews and Dissemination, University of York (Lister‐Sharp 1997). We were especially grateful to Deborah Lister‐Sharp for transfer of data, trials and expertise, and were sad to learn of her death.
The York reviewers obtained information from a variety of sources, including organisations, manufacturers and individuals. These are listed in the NHS Centre for Reviews and Dissemination Report, and we acknowledge their contribution to this review.
Extra information was supplied by Dr Yuri Natochin, Professor Christian Radmayr, Dr Seth Schulman and Professor Tack Lee. We are grateful to Penny Dobson (ERIC) for help and encouragement, and for being an external referee. We thank Mr T Matsuoka for a translation from Japanese and Mr HA Glazener for two translations from German. We would also like to thank the Consumer Network at the Australasian Cochrane Centre (ACC) for help with the original synopsis.
Data and analyses
Comparison 1. DESMOPRESSIN VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment | 17 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 10 mcg vs placebo | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐3.42, ‐1.18] |
| 1.2 20 mcg vs placebo | 12 | 813 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐1.57, ‐1.11] |
| 1.3 40 mcg vs placebo | 6 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐1.67, ‐0.99] |
| 1.4 60 mcg vs placebo | 2 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.92, ‐1.08] |
| 1.5 combined dose vs placebo | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐3.4 [‐4.71, ‐2.09] |
| 1.6 dose titration vs placebo | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐2.08, ‐1.09] |
| 2 Number of wet nights per week during treatment (no SDs) | Other data | No numeric data | ||
| 2.1 20 mcg vs placebo | Other data | No numeric data | ||
| 2.2 dose titration vs placebo | Other data | No numeric data | ||
| 3 Number failing to achieve 14 consecutive dry nights during treatment | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 10 mcg vs placebo | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 0.98] |
| 3.2 20 mcg vs placebo | 5 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.78, 0.91] |
| 3.3 40 mcg vs placebo | 6 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.89] |
| 3.4 60 mcg vs placebo | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.00] |
| 3.5 80 mcg vs placebo | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.71, 1.00] |
| 4 Number of wet nights per week at follow‐up | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 10 mcg vs placebo | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐1.10, 1.27] |
| 4.2 20 mcg vs placebo | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐1.15, 2.23] |
| 5 Number of wet nights per week at followup (no SDs) | Other data | No numeric data | ||
| 5.1 20 mcg vs placebo | Other data | No numeric data | ||
| 5.2 40 mcg vs placebo | Other data | No numeric data | ||
| 6 Number failing to achieve 14 consecutive dry nights or relapsing after cure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.2 20 mcg vs placebo | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 DESMOPRESSIN VS PLACEBO, Outcome 1 Number of wet nights per week during treatment.
1.2. Analysis.
Comparison 1 DESMOPRESSIN VS PLACEBO, Outcome 2 Number of wet nights per week during treatment (no SDs).
| Number of wet nights per week during treatment (no SDs) | ||
|---|---|---|
| Study | Desmopressin | Placebo |
| 20 mcg vs placebo | ||
| Dimson 1986# | 3.4 wet nights, n=17 | 5.0 wet nights, n=17 |
| Fjellestad 1987# | 2.9 wet nights, n=19 | 4.5 wet nights, n=19 |
| Miller 1990a | 4.4 wet nights, n=19 | 5.4 wet nights, n=31 |
| Miller 1990b | 5 wet nights, n=27 | 5.3 wet nights, n=26 |
| Muller 2001# | 3.27 wet nights, n=19 | 4.9 wet nights, n=21 |
| dose titration vs placebo | ||
| Rittig 1988# | 0 wet nights, n=34 | 3 wet nights, n=34 |
| Terho 1991# | 2.3 wet nights, n=52 | 4.7 wet nights, n=52 |
| Uygur 1997# | 0.5 wet nights, n=54 | 4.8 wet nights, n=54 |
1.3. Analysis.
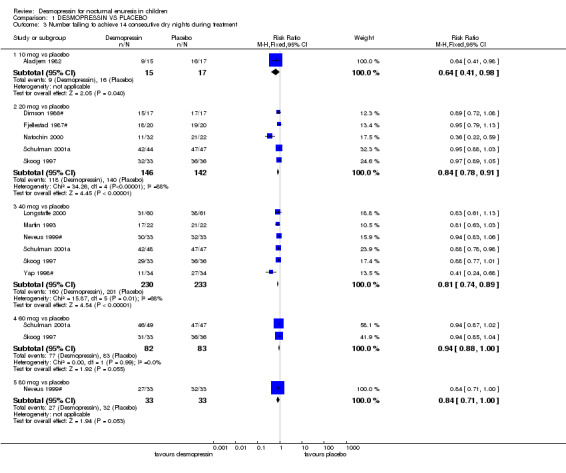
Comparison 1 DESMOPRESSIN VS PLACEBO, Outcome 3 Number failing to achieve 14 consecutive dry nights during treatment.
1.4. Analysis.
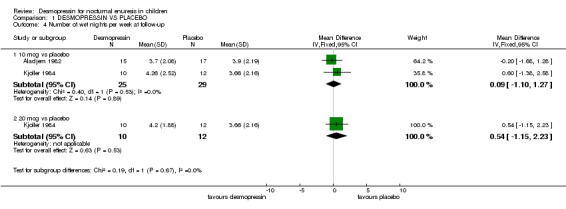
Comparison 1 DESMOPRESSIN VS PLACEBO, Outcome 4 Number of wet nights per week at follow‐up.
1.5. Analysis.
Comparison 1 DESMOPRESSIN VS PLACEBO, Outcome 5 Number of wet nights per week at followup (no SDs).
| Number of wet nights per week at followup (no SDs) | ||
|---|---|---|
| Study | Desmopressin | Placebo |
| 20 mcg vs placebo | ||
| Miller 1990a | 5.6 wet nights, n=19 | 4.9 wet nights, n=16 |
| Miller 1990b | 5.4 wet nights, n=19 | 5.7 wet nights, n=20 |
| 40 mcg vs placebo | ||
| Miller 1990a | 5.5 wet nights, n=26 | 4.9 wet nights, n=16 |
| Miller 1990b | 5.7 wet nights, n=20 | 5.7 wet nights, n=20 |
1.6. Analysis.
Comparison 1 DESMOPRESSIN VS PLACEBO, Outcome 6 Number failing to achieve 14 consecutive dry nights or relapsing after cure.
Comparison 2. DESMOPRESSIN VS PLACEBO excluding crossover trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 10 mcg vs placebo | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐3.42, ‐1.18] |
| 1.2 20 mcg vs placebo | 7 | 523 | Mean Difference (IV, Fixed, 95% CI) | ‐1.22 [‐1.49, ‐0.95] |
| 1.3 40 mcg vs placebo | 3 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐1.28 [‐1.70, ‐0.86] |
| 1.4 60 mcg vs placebo | 2 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.92, ‐1.08] |
| 1.5 combined dose vs placebo | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 dose titration vs placebo | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐2.08, ‐1.09] |
| 2 Number of wet nights per week during treatment (no SDs) | Other data | No numeric data | ||
| 2.1 20 mcg vs placebo | Other data | No numeric data | ||
| 2.2 dose titration vs placebo | Other data | No numeric data | ||
| 3 Number failing to achieve 14 consecutive dry nights during treatment | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 10 mcg vs placebo | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 0.98] |
| 3.2 20 mcg vs placebo | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.75, 0.90] |
| 3.3 40 mcg vs placebo | 4 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.95] |
| 3.4 60 mcg vs placebo | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.00] |
| 4 Number of wet nights per week at follow‐up | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 10 mcg vs placebo | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐1.10, 1.27] |
| 4.2 20 mcg vs placebo | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐1.15, 2.23] |
| 5 Number of wet nights per week at followup (no SDs) | Other data | No numeric data | ||
| 5.1 20 mcg vs placebo | Other data | No numeric data | ||
| 5.2 40 mcg vs placebo | Other data | No numeric data | ||
| 6 Number failing to achieve 14 consecutive dry nights or relapsing after cure | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 10 mcg vs placebo | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 20 mcg vs placebo | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 40 mcg vs placebo | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 60 mcg vs placebo | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 80 mcg vs placebo | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
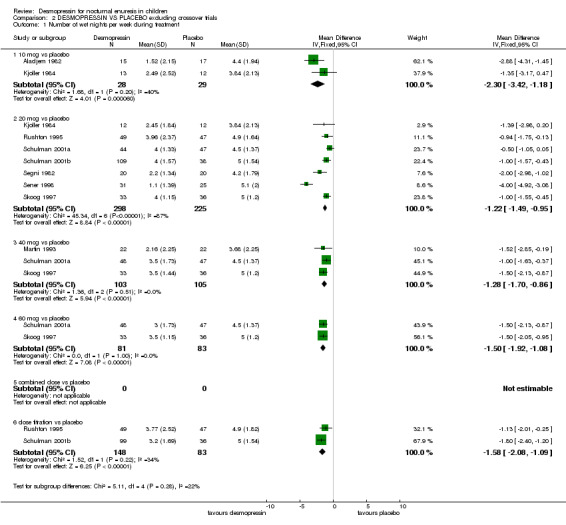
Comparison 2 DESMOPRESSIN VS PLACEBO excluding crossover trials, Outcome 1 Number of wet nights per week during treatment.
2.2. Analysis.
Comparison 2 DESMOPRESSIN VS PLACEBO excluding crossover trials, Outcome 2 Number of wet nights per week during treatment (no SDs).
| Number of wet nights per week during treatment (no SDs) | ||
|---|---|---|
| Study | Desmopressin | Placebo |
| 20 mcg vs placebo | ||
| Miller 1990a | 4.4 wet nights, n=19 | 5.4 wet nights, n=31 |
| Miller 1990b | 5 wet nights, n=27 | 5.3 wet nights, n=26 |
2.3. Analysis.
Comparison 2 DESMOPRESSIN VS PLACEBO excluding crossover trials, Outcome 3 Number failing to achieve 14 consecutive dry nights during treatment.
2.4. Analysis.

Comparison 2 DESMOPRESSIN VS PLACEBO excluding crossover trials, Outcome 4 Number of wet nights per week at follow‐up.
2.5. Analysis.
Comparison 2 DESMOPRESSIN VS PLACEBO excluding crossover trials, Outcome 5 Number of wet nights per week at followup (no SDs).
| Number of wet nights per week at followup (no SDs) | ||
|---|---|---|
| Study | Desmopressin | Placebo |
| 20 mcg vs placebo | ||
| Miller 1990a | 5.6 wet nights, n=19 | 4.9 wet nights, n=16 |
| Miller 1990b | 5.4 wet nights, n=19 | 5.7 wet nights, n=20 |
| 40 mcg vs placebo | ||
| Miller 1990a | 5.5 wet nights, n=26 | 4.9 wet nights, n=16 |
| Miller 1990b | 5.7 wet nights, n=20 | 5.7 wet nights, n=20 |
Comparison 3. DESMOPRESSIN: COMPARING DOSES.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 10 vs 20 mcg | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.72, 1.72] |
| 1.2 20 vs 40 mcg | 3 | 202 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.01, 0.84] |
| 1.3 20 vs 60 mcg | 2 | 158 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [0.30, 1.14] |
| 1.4 40 vs 60 mcg | 2 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.24, 0.69] |
| 2 Number of wet nights per week during treatment (no SDs) | Other data | No numeric data | ||
| 2.1 20 mcg vs 40 mcg | Other data | No numeric data | ||
| 3 Number failing to achieve 14 consecutive dry nights during treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 20 mcg vs 40 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 20 mcg vs 60 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 40 mcg vs 60 mcg | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 40 mcg vs 80 mcg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of wet nights per week at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 10 vs 20 mcg | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.85, 2.05] |
| 5 Number of wet nights per week at followup (no SDs) | Other data | No numeric data | ||
| 5.1 20 mcg vs 40 mcg | Other data | No numeric data |
3.1. Analysis.
Comparison 3 DESMOPRESSIN: COMPARING DOSES, Outcome 1 Number of wet nights per week during treatment.
3.2. Analysis.
Comparison 3 DESMOPRESSIN: COMPARING DOSES, Outcome 2 Number of wet nights per week during treatment (no SDs).
| Number of wet nights per week during treatment (no SDs) | ||
|---|---|---|
| Study | lower dose | higher dose |
| 20 mcg vs 40 mcg | ||
| Miller 1990a | 4.4 wet nights, n=19 | 3.5 wet nights, n=26 |
| Miller 1990b | 5 wet nights, n=27 | 4 wet nights, n=24 |
3.3. Analysis.
Comparison 3 DESMOPRESSIN: COMPARING DOSES, Outcome 3 Number failing to achieve 14 consecutive dry nights during treatment.
3.4. Analysis.
Comparison 3 DESMOPRESSIN: COMPARING DOSES, Outcome 4 Number of wet nights per week at follow‐up.
3.5. Analysis.
Comparison 3 DESMOPRESSIN: COMPARING DOSES, Outcome 5 Number of wet nights per week at followup (no SDs).
| Number of wet nights per week at followup (no SDs) | ||
|---|---|---|
| Study | lower dose | higher dose |
| 20 mcg vs 40 mcg | ||
| Miller 1990a | 5.6 wet nights, n=19 | 5.5 wet nights, n=26 |
| Miller 1990b | 5.4 wet nights, n=19 | 5.7 wet nights, n=20 |
Comparison 4. DESMOPRESSIN: ORAL VS NOSE DROPS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment (no SDs) | Other data | No numeric data | ||
| 2 Number failing to achieve 14 consecutive dry nights during treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.13] |
4.1. Analysis.
Comparison 4 DESMOPRESSIN: ORAL VS NOSE DROPS, Outcome 1 Number of wet nights per week during treatment (no SDs).
| Number of wet nights per week during treatment (no SDs) | ||
|---|---|---|
| Study | oral | nose drops |
| Fjellestad 1987# | 3 wet nights, n=19 | 2.9 wet nights, n=19 |
4.2. Analysis.
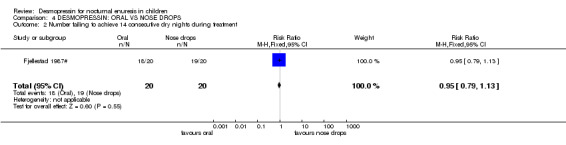
Comparison 4 DESMOPRESSIN: ORAL VS NOSE DROPS, Outcome 2 Number failing to achieve 14 consecutive dry nights during treatment.
Comparison 5. DESMOPRESSIN: COMPARISON WITH OTHER DRUGS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 desmopressin vs amitriptyline | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.12, 2.68] |
| 1.2 desmopressin vs desmopressin + amitriptyline | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐0.14, 2.94] |
| 1.3 desmopressin vs imipramine (first fortnight) | 2 | 258 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.44, 0.80] |
| 1.4 desmopressin vs imipramine (at end of treatment) | 3 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.62, 0.41] |
| 1.5 desmopressin vs indomethacin | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐2.37, ‐0.53] |
| 1.6 desmopressin vs desmopressin + oxybutynin | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.51, 0.71] |
| 2 Number failing to achieve 14 consecutive dry nights during treatment | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 desmopressin vs amitriptyline | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.89, 1.61] |
| 2.2 desmopressin vs desmopressin + amitriptyline | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.93, 1.87] |
| 2.3 desmopressin vs diclofenac | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.89] |
| 2.4 desmopressin vs imipramine | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.27, 0.73] |
| 2.5 desmopressin vs desmopressin + oxybutynin | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.51, 2.28] |
| 3 Number of wet nights at followup | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 desmopressin vs imipramine | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.20, 1.60] |
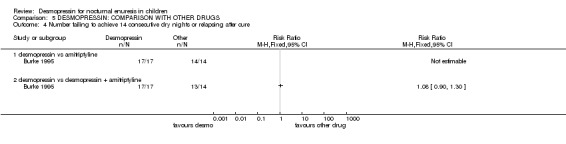
| 4 Number failing to achieve 14 consecutive dry nights or relapsing after cure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 desmopressin vs amitriptyline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 desmopressin vs desmopressin + amitriptyline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of wet nights per week during treatment (no SDs) | Other data | No numeric data | ||
| 5.1 desmopressin vs imipramine (first arm) | Other data | No numeric data |
5.1. Analysis.
Comparison 5 DESMOPRESSIN: COMPARISON WITH OTHER DRUGS, Outcome 1 Number of wet nights per week during treatment.
5.2. Analysis.
Comparison 5 DESMOPRESSIN: COMPARISON WITH OTHER DRUGS, Outcome 2 Number failing to achieve 14 consecutive dry nights during treatment.
5.3. Analysis.

Comparison 5 DESMOPRESSIN: COMPARISON WITH OTHER DRUGS, Outcome 3 Number of wet nights at followup.
5.4. Analysis.
Comparison 5 DESMOPRESSIN: COMPARISON WITH OTHER DRUGS, Outcome 4 Number failing to achieve 14 consecutive dry nights or relapsing after cure.
5.5. Analysis.
Comparison 5 DESMOPRESSIN: COMPARISON WITH OTHER DRUGS, Outcome 5 Number of wet nights per week during treatment (no SDs).
| Number of wet nights per week during treatment (no SDs) | ||
|---|---|---|
| Study | Desmopressin | Alarm |
| desmopressin vs imipramine (first arm) | ||
| Vertucci 1997 | 1 wet night, n=29 | 2.5 wet nights, n=28 |
Comparison 6. DESMOPRESSIN ALONE VS ALARM ALONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 desmopressin vs alarm, in first week | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.95, ‐0.45] |
| 1.2 desmopressin vs alarm, in last week | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.32, 1.36] |
| 2 Number failing to achieve 14 consecutive dry nights during treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 desmopressin vs alarm | 3 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.83, 1.36] |
| 3 Number failing to achieve 14 consecutive dry nights or relapsing after cure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 desmopressin vs alarm | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.05, 1.91] |
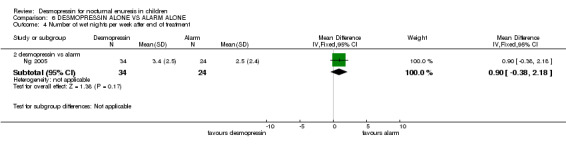
| 4 Number of wet nights per week after end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.2 desmopressin vs alarm | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.38, 2.18] |
6.1. Analysis.
Comparison 6 DESMOPRESSIN ALONE VS ALARM ALONE, Outcome 1 Number of wet nights per week during treatment.
6.2. Analysis.
Comparison 6 DESMOPRESSIN ALONE VS ALARM ALONE, Outcome 2 Number failing to achieve 14 consecutive dry nights during treatment.
6.3. Analysis.
Comparison 6 DESMOPRESSIN ALONE VS ALARM ALONE, Outcome 3 Number failing to achieve 14 consecutive dry nights or relapsing after cure.
6.4. Analysis.
Comparison 6 DESMOPRESSIN ALONE VS ALARM ALONE, Outcome 4 Number of wet nights per week after end of treatment.
Comparison 7. DESMOPRESSIN ALONE VS DESMOPRESSIN + ALARM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 desmopressin alone vs desmopressin + alarm | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.25, 2.35] |
| 2 Number failing to achieve 14 consecutive dry nights during treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 desmopressin alone vs desmopressin + alarm | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.92, 2.60] |
| 3 Number failing to achieve 14 consecutive dry nights or relapsing after cure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 desmopressin alone vs desmopressin + alarm | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.96, 1.85] |
| 4 Number of wet nights per week after end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 desmopressin alone vs desmopressin + alarm | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.57, 2.17] |
7.1. Analysis.
Comparison 7 DESMOPRESSIN ALONE VS DESMOPRESSIN + ALARM, Outcome 1 Number of wet nights per week during treatment.
7.2. Analysis.
Comparison 7 DESMOPRESSIN ALONE VS DESMOPRESSIN + ALARM, Outcome 2 Number failing to achieve 14 consecutive dry nights during treatment.
7.3. Analysis.
Comparison 7 DESMOPRESSIN ALONE VS DESMOPRESSIN + ALARM, Outcome 3 Number failing to achieve 14 consecutive dry nights or relapsing after cure.
7.4. Analysis.
Comparison 7 DESMOPRESSIN ALONE VS DESMOPRESSIN + ALARM, Outcome 4 Number of wet nights per week after end of treatment.
Comparison 8. DESMOPRESSIN + ALARM VS ALARM ALONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 desmopressin + alarm vs alarm (+/‐ placebo) | 4 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.11, ‐0.55] |
| 2 Number failing to achieve 14 consecutive dry nights during treatment | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 desmopressin + alarm vs alarm (+/‐ placebo) | 5 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.05] |
| 3 Number failing to achieve 14 consecutive dry nights or relapsing after cure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 desmopressin + alarm vs alarm (+/‐ placebo) | 4 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.08] |
| 4 Number of wet nights per week after end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
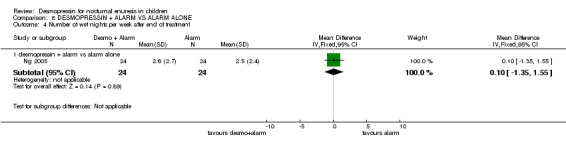
| 4.1 desmopressin + alarm vs alarm alone | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.35, 1.55] |
| 5 Number of wet nights per week during treatment (no SDs) | Other data | No numeric data | ||
| 5.1 desmopressin + alarm vs alarm (+/‐ placebo) | Other data | No numeric data | ||
| 6 Number of wet nights per week after treatment stops (no SDs) | Other data | No numeric data | ||
| 6.1 desmopressin + alarm vs alarm (+/‐ placebo) | Other data | No numeric data |
8.1. Analysis.

Comparison 8 DESMOPRESSIN + ALARM VS ALARM ALONE, Outcome 1 Number of wet nights per week during treatment.
8.2. Analysis.
Comparison 8 DESMOPRESSIN + ALARM VS ALARM ALONE, Outcome 2 Number failing to achieve 14 consecutive dry nights during treatment.
8.3. Analysis.

Comparison 8 DESMOPRESSIN + ALARM VS ALARM ALONE, Outcome 3 Number failing to achieve 14 consecutive dry nights or relapsing after cure.
8.4. Analysis.
Comparison 8 DESMOPRESSIN + ALARM VS ALARM ALONE, Outcome 4 Number of wet nights per week after end of treatment.
8.5. Analysis.
Comparison 8 DESMOPRESSIN + ALARM VS ALARM ALONE, Outcome 5 Number of wet nights per week during treatment (no SDs).
| Number of wet nights per week during treatment (no SDs) | ||
|---|---|---|
| Study | Desmopressin + Alarm | Alarm alone |
| desmopressin + alarm vs alarm (+/‐ placebo) | ||
| Leebeek 2001 | 2.93 wet nights, n=47 | 3.86 wet nights, n=45 |
8.6. Analysis.
Comparison 8 DESMOPRESSIN + ALARM VS ALARM ALONE, Outcome 6 Number of wet nights per week after treatment stops (no SDs).
| Number of wet nights per week after treatment stops (no SDs) | ||
|---|---|---|
| Study | Desmopressin + Alarm | Alarm alone |
| desmopressin + alarm vs alarm (+/‐ placebo) | ||
| Leebeek 2001 | 2.72 wet nights, n=41 | 1.90 wet nights, n=37 |
Comparison 9. DESMOPRESSIN VS BEHAVIOURAL METHODS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of wet nights per week during treatment | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 desmopressin vs retention control training | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.20, 0.40] |
| 1.2 desmopressin + psychology + retention control training vs desmopressin alone | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐2.15, ‐0.85] |
| 1.3 desmopressin + psychology + retention control training vs placebo + psychology + retention control training | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.98, 0.38] |
| 1.4 desmopressin vs toileting, alarm clock, pelvic floor training, dietary and fluid adjustment etc | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.61, 1.03] |
| 2 Number failing to achieve 14 consecutive dry nights during treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 desmopressin vs retention control training | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.62, 1.03] |
| 2.2 desmopressin + psychology + retention control training vs desmopressin alone | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.91, 1.48] |
| 2.3 desmopressin + psychology + retention control training vs placebo + psychology + retention control training | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.98] |
| 2.4 desmopressin vs toileting, alarm clock, pelvic floor training, dietary and fluid adjustment etc | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.66, 37.85] |
| 3 Number of wet nights per week at followup | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.2 desmopressin + psychology + retention control training vs desmopressin alone | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐2.1 [‐2.67, ‐1.53] |
| 3.3 desmopressin + psychology + retention control training vs placebo + psychology + retention control training | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.01, 0.21] |
| 4 Number failing to achieve 14 consecutive dry nights or relapsing after cure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 desmopressin vs retention control training | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| 4.2 desmopressin + psychology + retention control training vs desmopressin alone | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 4.3 desmopressin + psychology + retention control training vs placebo + psychology + retention control training | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.12] |
9.1. Analysis.
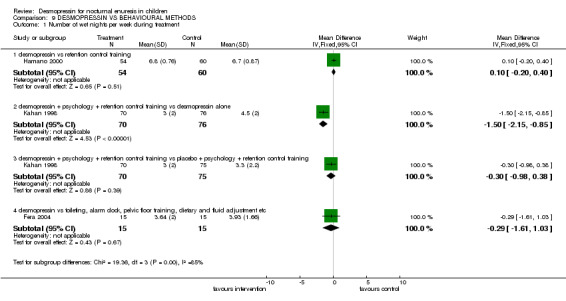
Comparison 9 DESMOPRESSIN VS BEHAVIOURAL METHODS, Outcome 1 Number of wet nights per week during treatment.
9.2. Analysis.
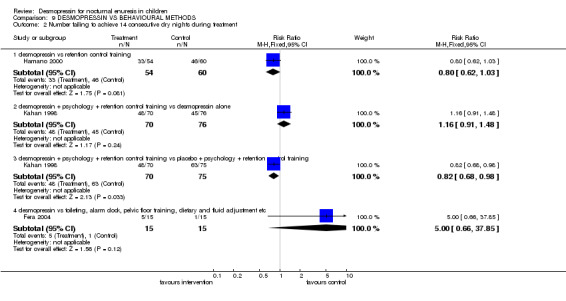
Comparison 9 DESMOPRESSIN VS BEHAVIOURAL METHODS, Outcome 2 Number failing to achieve 14 consecutive dry nights during treatment.
9.3. Analysis.
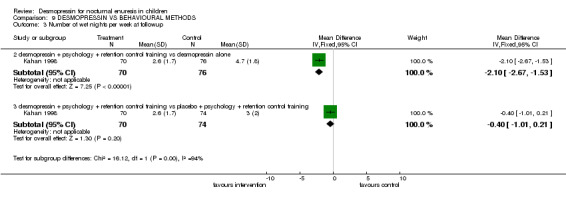
Comparison 9 DESMOPRESSIN VS BEHAVIOURAL METHODS, Outcome 3 Number of wet nights per week at followup.
9.4. Analysis.
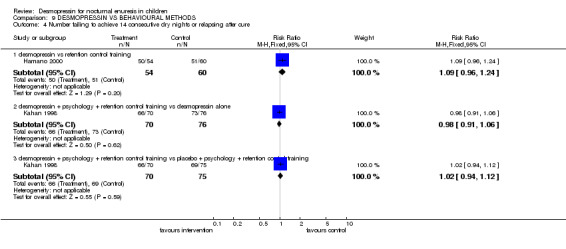
Comparison 9 DESMOPRESSIN VS BEHAVIOURAL METHODS, Outcome 4 Number failing to achieve 14 consecutive dry nights or relapsing after cure.
Comparison 10. DESMOPRESSIN VS COMPLEMENTARY METHODS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Number failing to achieve 14 consecutive dry nights during treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 desmopressin vs laser acupuncture | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.22, 2.01] |
| 4 Number failing to achieve 14 consecutive dry nights or relapsing after cure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 desmopressin vs laser acupuncture | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.88] |
10.2. Analysis.
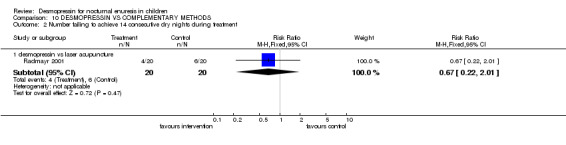
Comparison 10 DESMOPRESSIN VS COMPLEMENTARY METHODS, Outcome 2 Number failing to achieve 14 consecutive dry nights during treatment.
10.4. Analysis.
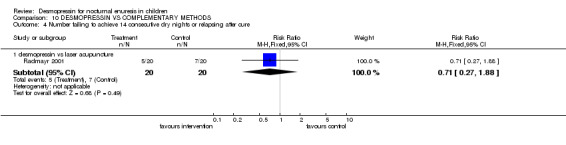
Comparison 10 DESMOPRESSIN VS COMPLEMENTARY METHODS, Outcome 4 Number failing to achieve 14 consecutive dry nights or relapsing after cure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aladjem 1982.
| Methods | RCT (double blind, method not specified) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned Follow up after 30 days | |
| Participants | Number of children: 32 (boys: A:7, B:8) Previous treatment: 5/23 responded to chlorimipramine hydrochloride Mean age (years): A:10.5, B:10.0 (range 7‐15) Baseline wetting: mean (SD) number of wet nights in 30: A:18.7 (6.5), B:21.3 (8.5) No significant difference between groups in urine osmolalities | |
| Interventions | A (15): 10 µg DDAVP intranasally B (17): placebo as above | |
| Outcomes | Mean (SD) number of wet nights out of 30: A:6.5 (9.2), B:18.8 (8.3) Number totally dry: A:6, B:1 Number of wet nights at follow up: A:15.7 (8.9), B:16.9 (9.4) Significant difference in response of children according to age Only those over 10 years became completely dry The only failures (n=3) were less than 10 years old Side effects: none reported Prompt response to DDAVP ‐ as early as 1‐3 days | |
| Notes | Unclear about dropouts Short follow up Age effect suggested Inclusion criteria: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Birkasova 1978#.
| Methods | RCT (double blind crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned Follow up after 4‐6 weeks | |
| Participants | Number of children: 22 (14 boys) Previous treatment: all had failed to respond to psychotherapy and a regimen that included fluid deprivation after 5 pm Some had previously been unsuccessfully treated with imipramine Exclusion criteria: organic causes of enuresis Mean age (years): 6.6 (range 4‐12) Baseline wetting: mean (SD) wet beds per fortnight: 10.6 (4.9) | |
| Interventions | A: 10 µg DDAVP drops intranasally at bedtime B: 40 µg DDAVP drops intranasally at bedtime C: placebo Duration of treatment: 2 weeks in each arm | |
| Outcomes | Mean (SD) number of wet nights per fortnight A+B:4.2 (4.5) C:11 (4.4) Side effects: none reported 5 patients receiving a higher dosage were totally dry 9 continued DDAVP single blind for 4 to 6 weeks then given placebo 7 remained dry without drug 1 wet once monthly and 1 returned to daily wetting 4 who had wet nightly continued on DDAVP for 3 more months by which time they were dry 1 had 1 wet night per fortnight and 1 had 1 wet night in 3 2 patients who were indifferent to wetting showed no response to DDAVP or placebo | |
| Notes | No measure of comparability at baseline No washout period Not clear if intention to treat because no details of dropouts Subjects were very young High and low doses combined in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bradbury 1995.
| Methods | RCT (quota allocation system based on age, baseline wetting, family or housing problems, gender, previous alarm use, daytime wetting and previous dry periods) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: No 6 month follow up | |
| Participants | Number of children: 71 (boys 48) Dropouts A: 3, B: 8 Inclusion criteria: nocturnal enuresis at least 1 night per week (40/71 = severe, >4 times per week) Exclusion criteria: neuropathic bladder, urinary tract abnormalities, cystic fibrosis, allergic rhinitis, deafness/ learning difficulties, UTI Previous treatment: 29 had used alarms | |
| Interventions | A (36): desmopressin 40 µg intranasally and alarm (bell‐and‐pad or Mini Drinite) B (35): alarm alone Duration 6 weeks or until dry | |
| Outcomes | Mean DRY nights per week: A: n=33, mean = 6.1, 95% CI 5.6‐6.7; B: 27, 4.8, 4.0‐5.6 Number not achieving 4 dry nights: A: 6/33; B: 11/27 Number failing or relapsing: A: 10/33; B: 14/27 Side effects: none reported Subgroup analysis in more severe group: A still better than B | |
| Notes | Mini Drinite = body‐worn alarm Relapsing = 2 wet nights in 2 weeks after 4 weeks dry Authors recommended using combined desmopressin and alarm only for children with severe wetting problems | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Burke 1995.
| Methods | RCT (multi‐centre, double blind) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned | |
| Participants | Number of children: 45 (boys: A:11, B:10, C:9) Dropouts: A:0, B:3, C:3 Inclusion criteria: age 6‐17 years; at least 3 wet nights per week for preceding 3 month period and not dry for more than 6 months Exclusion criteria: enuresis treatment in preceding 6 months; nocturnal enuresis of neurogenic origin; urinary tract infection; abnormal urinalysis haematology or blood biochemistry; concomitant medication known to interfere with study medication Age, mean years (SD): A:8.6 (2.4), B:8.9 (2.5), C:8.9 (2.4) (range 6‐14) Baseline wetting: mean (SD) number of wet nights per week: A:5.8 (0.9), B:6.0 (0.9), C:6.3 (0.9) | |
| Interventions | A (14): amitriptyline hydrochloride (25 mg or 50 mg) B (17): desmopressin (20 µg) intranasally C (14): DDAVP and amitriptyline Duration of treatment: 16 weeks Follow up 12 weeks | |
| Outcomes | Mean (SD) number of wet nights per week: A:3.3 (1.9), B:4.7 (1.7), C:3.3 (2.5) Number attaining cure: A:3, B:1, C:5 7 out of 8 children who were cured relapsed. The exception was treated with amitriptyline and DDAVP Follow up: mean (SD) number of wet nights per week: A:(n=10) 3.9 (2.9), B:(n=5) 3.8 (1.9), C:(n=8) 5.1 (3.2) Side effects: none reported Most parents said all the drugs were easy to use | |
| Notes | No significant difference between groups in terms of number, age, height and weight Trial prematurely halted due to one drug ceasing to be available Not stated if intention to treat Not full quota of subjects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dimson 1986#.
| Methods | RCT (double blind crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned | |
| Participants | Number of children: 17 (14 boys) Inclusion criteria: 3 with encopresis Exclusion criteria: UTI, organic disease (clinical or radiological) Previous treatment: failure to respond to tricyclics and alarms Age range 6‐13 years Baseline wetting >50% wet nights in 2‐week observation period | |
| Interventions | A (17): 20 µg desmopressin intranasally B (17): matching placebo Duration of treatment 2 weeks | |
| Outcomes | Mean wet nights per week: A: 117 in 2 weeks in 17 children (= mean 3.4/week); B: 169 in 2 weeks in 17 children (= mean 5.0/ week) Number not achieving 14 dry nights: A: 2 cured, 10 improved, 5 failed; B: 5 improved slightly, 12 failed Number failed or relapsed: A: 17/17; B: 17/17 Side effects: none reported (no overhydration, weight or BP change) | |
| Notes | No washout period All children relapsed after end of trial No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Faraj 1999.
| Methods | RCT (random number tables, details not given) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes | |
| Participants | Number of children: 135 Dropouts: 23 excluded for non‐compliance, and 39 lost to follow up including 12 failed with alarms Inclusion criteria: monosymptomatic nocturnal enuresis, age >5 years Exclusion criteria: previous treatment with DDAVP or alarm, urological pathology, diurnal enuresis, UTI Age, mean years: 11.2 Baseline wetting: A 21% dry nights, B 14% dry nights | |
| Interventions | A (62): desmopressin 20 µg intranasally increasing to 40 µg if response partial B (73): alarm (pad‐and‐bell) Duration of treatment 3 months. If failed at that time, changed to alternative arm | |
| Outcomes | DRY nights at 3 months: A 85%; B: 90% Number not achieving 14 dry nights: A 12/39; B: 6/37 Side effects: not mentioned | |
| Notes | No follow up No mention of adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fera 2004.
| Methods | RCT (randomized in 2 groups) Systematic baseline measure of wetting: Yes Organic causes excluded: No Daytime wetting excluded: Yes Setting: Federal University of Sao Paulo | |
| Participants | No. of children (boys): 30 (21) Dropouts: None Inclusion: monosymptomatic nocturnal enuresis, age over 5 years, no daytime wetting Exclusion: none mentioned Previous treatment: none Age, mean years (SD): 9.23 (1.85) Baseline wetting, mean (SD) wet nights in 2 weeks: 9.40 (3.40) | |
| Interventions | A (15): DDAVP (desmopressin), titrated to maximum 0.4 mg at bedtime B (15): behavioural modification (dietary and fluid adjustment, voiding schedules, double voiding, bedtime toileting, alarm clock once at night, pelvic floor training, environmental modifications, changes in parents' attitudes, improvement of self‐esteem, self care) Duration of treatment: 30 days Follow up: none | |
| Outcomes | Wet nights during last 2 weeks of treatment N, mean (SD): A: 15, 7.27 (4), B: 15, 3.93 (3.32) 50% improvement: A: 7/15, B: 8/15 Complete failure: A: 5/15, B: 1/15 Adverse effects: not mentioned | |
| Notes | Groups comparable at baseline on age, gender and baseline wetting No data for children 'cured' (14 consecutive dry nights) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fjellestad 1987#.
| Methods | RCT (double blind, double dummy, cross over) Periods of treatment preceded and followed by one week of observation Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up after 1 week | |
| Participants | Number of children: 30 (20 boys), 1 dropout Exclusion criteria: urinary tract infections; diurnal wetting; faecal soiling; neurological or urological abnormalities; 3+ wet nights a week during baseline Previous treatment: 69% tried one or more other treatment Age, mean (SD): 9.8 (2.5) (range 6‐15) Baseline wetting: mean number of dry nights in week: 2.2 (SD 0.2) | |
| Interventions | A: 200 µg oral desmopressin B: 20 µg intranasal desmopressin C: placebo tablets D: placebo nasal pipette Duration of treatment: 2 weeks placebo then 2 weeks each condition | |
| Outcomes | During treatments mean number of dry nights: A:4, B:4.1, C:2.5 2 patients totally dry while taking tablets; 1 patient totally dry while using intranasal 9 children (31%) remained totally dry Side effects: no significant adverse effects but 2 patients complained of occasional nasal discomfort and 3 of epistaxis but no difference between placebo and active arms | |
| Notes | Not reported if comparable groups Washout one week between each arm Not intention to treat Many results only given graphically Short follow up No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Folwell 1997#.
| Methods | RCT (double‐blind crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes | |
| Participants | Number of children: 31 (22 boys), small number of adults Dropouts/not included: 6 Inclusion criteria: primary monosymptomatic enuresis, age over 6 years Exclusion criteria: diabetes, neurological or renal disease, steroids, diuretics, daytime wetting Previous treatment: failed on alarms, anticholinergics Age, mean years: 11.2 (SD 5.4) | |
| Interventions | A: desmopressin 20 µg intranasal B: placebo 21 days each, then crossed over | |
| Outcomes | Mean wet nights/week during trial: A: 3.24 (SE 0.45); B: 4.86 (0.35) Side effects: A: 1 nausea; B: 1 drowsy and vomiting | |
| Notes | No washout period No follow up Groups reported to be comparable but data not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gibb 2004.
| Methods | RCT (drug dispensed randomly by pharmacist) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: No Setting: Paediatric outpatients, Children's Hospital, Melbourne, Australia |
|
| Participants | Number of children (boys): 207/210 (A:64, B:78) Dropouts: 10 eligible children declined, incomplete data on A:9/101, B:17/106 but dropouts counted as failures for analysis Inclusion criteria: Non‐responders to desmopressin treatment (<50% reduction in wet nights), age 6‐16 years, wetting at least twice per week, some daytime wetting (A:11, B:8) Exclusion criteria: Neuropathic bladder, urinary tract abnormality, cystic fibrosis, allergic rhinitis, UTI in previous 2 weeks, imipramine or diuretics Previous treatment: some had alarm (A:37, B:32) or desmopressin (A:31, B:28) Age: mean 9.4 years (SD 2.08) Baseline wet nights in 28 days: A: 23.9 (SD 5.05), B: 23.7 (5.83) | |
| Interventions | A (84/101): desmopressin (40 µg nasal spray) + alarm (pad and bell)
B (85/106): placebo (nasal spray) + alarm (pad and bell) Duration of treatment: 8 weeks Follow up: 2 months |
|
| Outcomes | Cure = 28 dry nights, relapse = 2 wet nights in 2 weeks Wet nights during treatment (number, mean (SD)): A: 101, 1.8 (1.13), B: 106, 2.4 (1.53) Cure during treatment: A: 52/101, B: 51/106 P=0.63 (failed: A: 49/101, B: 55/106) Relapse after treatment stopped: A: 7, B: 3 Failed or relapsed: A: 56/101, B: 58/106 Adverse effects: A: 1 (headache), B: 1 (nose bleed) Other: compliance same in both groups Cure in daytime wetting: A: 6/11, B: 3/8 | |
| Notes | Intention to treat analysis Groups comparable at baseline on age, wetting, gender, family history, secondary enuresis, daytime wetting and previous treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hamano 2000.
| Methods | RCT (method not given) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up 2 weeks | |
| Participants | Number of children: 114 (88 boys) Dropouts: 18 (not wet or poor compliance) Inclusion criteria: primary monosymptomatic nocturnal enuresis, at least 4 times per week Exclusion criteria: neurological or physical abnormalities, UTI Age: 5‐15 years, A: 9.2; B: 9.4 Baseline wetting (SD): A: 6.8 (0.7); B: 6.7 (0.9) | |
| Interventions | A (54): DDAVP intranasally, 5 µg increasing to 20 if no response, decreasing when strongly responding B (60): retention control training; holding voiding once a day to increase bladder capacity Duration of treatment: 12 weeks | |
| Outcomes | Wet nights per week (N, mean, SD): A: 54, 6.8 (SD 0.76); B: 60, 6.7 (0.87) Number not achieving 14 dry nights A: 33/54; B: 46/60 Relapsing after cure: A 17; B: 5 Failed or relapsing after trial: A: 50/54; B: 51/60 Side effects: adverse events infrequent: A: 2/54 (nasal discomfort); B: 0/60 | |
| Notes | Results presented according to functional bladder capacity but merged here Short follow up Groups comparable at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hoashi 1995.
| Methods | RCT (randomly allocated in blocks of 4) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Setting: Day clinics at drug company | |
| Participants | No. of children (boys): 231 (167) Dropouts: 7 not wet at baseline, 1 moved, 2 daytime wetting, 4 delayed treatment, 2 non‐compliance Inclusion: wet 10/14 nights Exclusion: daytime wetting, organic causes, family disruption Previous treatment: no information Age: at least 6 years Baseline wetting: mean 12.8 wet nights in 2 weeks | |
| Interventions | A (112): desmopressin 10 µg nasal drops + placebo tablet
B (112): imipramine tablet (25 mg) + placebo nasal drops Duration of treatment: 4 weeks Follow up: none |
|
| Outcomes | Wet nights during treatment (N, mean, SE) At 2 weeks: A: 111, 9.5 (SE 0.5), B: 111, 9 (0.5) At 4 weeks: A: 109, 8.7 (SE 0.5), B: 109, 8 (0.5) Adverse effects causing stopping: A: 0, B: 1 Other adverse effects: Oedema, headache, sleepyness, insomnia, sleep disorder, dizziness, appetite loss, nausea, vomiting, diarrhoea, abdominal pain, eyelid swelling, rash, red eyelid, nose symptoms (itchy nose, bleeding nose, blocked nose, runny nose), fever, tiredness, shaking head, thirsty, dry lips Total number of side effects: A: 29/120, B: 29/118 Total number of children with side effects: A: 16/120, B: 16/118 Other outcomes: wetting score, side effect score, satisfaction score, overall score | |
| Notes | Japanese language Data measured from graphs No follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Holt 1986.
| Methods | RCT (method not given, double‐blind) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes | |
| Participants | Number of children: 36 Inclusion criteria: 2 or more wet nights per week; age 8‐12; Exclusion criteria: day time wetting; diabetes, insipidus or other chronic illness where need daily medication; other treatment for bed wetting Mean age (years): A:9.8, B:9.5 (range 8‐12) Baseline wetting: wet bed 2 or more times per week | |
| Interventions | A (19): imipramine 50 mg and placebo nasal spray B (17): intranasal desmopressin 20 µg and placebo tablets Duration of treatment: 4 weeks Follow up: 6 weeks | |
| Outcomes | Results first 2 weeks of treatment: % reduction in wet nights: A:48%, B:45% Results final 2 weeks of treatment: A:54%, B:32% Mean (SD) number of wet nights per first 14 days: A:4.9 (4.3), B:4.8 (4.0) Mean (SD) number of wet nights per last 14 days: A:4.5 (3.7), B:6.0 (4.4) Mean (SD) number of wet nights per 14 days at follow up: A:7.7 (3.9), B:7.3 (4.5) Side effects: B: rash (1) | |
| Notes | Norwegian translation Direct comparison of imipramine and DDAVP Children comparable in terms of sex, age and weight No details of previous treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Janknegt 1990#.
| Methods | RCT (double‐blind randomised crossover of dosages with placebo between) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned Follow up after 4 weeks | |
| Participants | Number of children: 22 (18 boys) No dropouts Inclusion criteria: maximum of 4 dry nights a week during baseline Mean age: 10 years (range 6‐16) Previous treatment: all used imipramine or other medications, or enuresis alarm (8), acupuncture (1), and psychotherapy (1) More than one method used with many patients Baseline wetting: mean (SD) number of dry nights per week: 1.3 (1.3) | |
| Interventions | A: Placebo nasal pipette B: 20 µg desmopressin intranasally C: 40 µg desmopressin intranasally Duration of treatment: 1 month each condition | |
| Outcomes | Mean (SD) number of dry nights per week: A:1.7 (1.8), B:3.6 (2.5), C:3.2 (2.2) At follow up mean (SD) number of dry nights per week: 2.2 (1.8) Morning urine osmolality not significantly different in pre‐treatment or treatment periods Significant increase in body weight No significant changes in blood pressure, haematology or blood chemistry Side effects: most common adverse reactions were headaches (3) and stomach ache (3) (though no different from placebo) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Janknegt 1997.
| Methods | RCT (double blind multi‐centre trial, method not given) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes 12 week open label follow up | |
| Participants | Number of children: 65 (boys: A:18, B:19) 3 dropouts Exclusion criteria: diurnal wetting; other medical conditions; urological causes of wetting Mean age: 19.4 years (range 12‐45) At least 6 wet nights in 2 weeks at baseline | |
| Interventions | A (34): desmopressin tablets 200 µg B (31): desmopressin tablets 400 µg Duration of treatment: 4 weeks | |
| Outcomes | Mean change from baseline wetting (wet nights per week 95% CI) A:‐3.2 (‐2.4, ‐4.1), B:‐3.4 (‐2.7, ‐4.1) Side effects: not mentioned | |
| Notes | Only DDAVP responders Mixed age group (some adults) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kahan 1998.
| Methods | RCT (double‐blind, method not specified) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned Follow up for 2 months | |
| Participants | Number of children: 228 Dropouts: A 6, B 1; due to difficulty complying with schedule of behavioural therapy Inclusion criteria: primary nocturnal enuresis at least 2 times per week, age >7 years Exclusion criteria: previous treatment, other physical disorders, previous traumatic life events, psychiatric disorders, abnormal laboratory findings Baseline differences in wetting between groups: A 5.1 (SD 2.1); B 5.5 (1.8); C 5.8 (1.6): P=0.044 | |
| Interventions | A (70): desmopressin and behaviour therapy B (75): placebo and behaviour therapy C (76): desmopressin alone (20 µg intranasal) Duration 8 weeks | |
| Outcomes | Wet nights at end of trial (N, mean, SD): A: 70, 3.0 (2.0); B: 75, 3.3 (2.2); C: 76, 4.5 (2.0) Number not achieving 14 dry nights: A: 48/70; B: 63/75; C: 45/76 Number failing or relapsing: A: 66/70; B: 69/75; 73/76 Wet nights after end of trial (N, mean, SD): A: 70, 2.6 (1.7); B: 74, 3.0 (2.0); C: 76, 4.7 (1.8) Side effects: nasal itch: A 5, B 1, C 4 7 dropouts due to problems with behavioural treatment schedule | |
| Notes | Power calculation given Behaviour treatment = psychological (teaching self control, taking responsibility), bladder continence exercises, giving information about the mechanisms of wetting Blinding of treatment not possible due to differences in interventions (but placebo and DDAVP blinded) Group A had less wetting at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kjoller 1984.
| Methods | RCT (double‐blind) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned Follow up after 3 months | |
| Participants | Number of children: 37 Dropouts from follow up: A:3, B:2 Inclusion criteria: normal, healthy children who had failed with previous treatment, informed consent, more than 25% wet nights during baseline Previous treatment: all failed treatment with tricyclic antidepressants and/or enuresis alarm Mean age: 11.0 years (range 9‐15) Baseline wetting: mean (SD) number of wet nights per 100: A:56.6 (8.0), B:65.9 (7.5), C:64.7 (7.3) | |
| Interventions | A (13): 10 µg DDAVP intranasally before bedtime B (12): 20 µg DDAVP intranasally before bedtime C (12): placebo Duration of treatment: 1 month | |
| Outcomes | Number of wet nights per 100 (mean, SD): A:35.5 (10), B:35.0 (7.6), C:54.8 (8.8) Follow up: mean (SD) number of wet nights per 100: A:60.9 (11.4), B:60.0 (8.5), C:52.3 (8.9) Side effects: none reported DDAVP ineffective when participants had respiratory tract infections | |
| Notes | Not reported if the groups were comparable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lee 2005.
| Methods | RCT (randomly assigned to 1 of 3 groups) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: 53% also had daytime wetting but results available separately Setting: 2 hospitals, 2003 to 2004 | |
| Participants | Number of children (boys): 145 (100) Monosymptomatic enuresis (Trial 1): 68 Polysymptomatic (+ daytime wetting, Trial 2): 77 Dropouts: 13 (drug side effects or outcome unknown: A: 3, B: 3, C: 7) Inclusion criteria: at least 3 wet nights/week Exclusion criteria: drug treatment in 14 days prior to start of study Age: 7.8 years (SD 2.5) (range 5 to 15) Baseline wetting: 6.36x/week (SD 1.5) | |
| Interventions | Trial 1 (monosymptomatic enuresis) A (22): desmopressin 0.1 or 0.2 mg, oxybutynin 5 mg B (23): desmopressin0.2 mg increased to 0.4 mg if no response C (23): imipramine 25 mg Given orally before bedtime Duration of treatment: 6 months Follow up: none | |
| Outcomes | Trial 1 Wet nights at 6 months, N mean (SD): A: 22, 0.93 (1.35), B: 23, 0.7 (0.95), C: 23, 2.0 (2.05) No. children cured (excellent response = 0‐1 wet night per month): A: 14/22, B: 14/23, C: 3/23 No. children failed (not excellent): A: 8/22, B: 9/23, C: 20/23 Trial 2 Wet nights at 6 months, N mean (SD): A: 26, 1.2 (1.55), B: 26, 1.23 (0.88), C: 25, 2.63 (2) No. children cured (excellent response = 0‐1 wet night per month): A: 9/26, B: 7/26, C: 3/25 No. children failed (not excellent): A: 17/26, B: 19/26, C: 22/25 Adverse effects (both trials, including dropouts): A: 0/51, B: 2/52, C: 22/55 | |
| Notes | Data obtained from author enabling results to be given separately for nocturia and daytime wetting groups Groups comparable at baseline on age, sex, disease type, baseline wetting Higher side effect and drop out rate in imipramine group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Leebeek 2001.
| Methods | RCT (double blind parallel group study) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up at 2 weeks and 6 months after end of trial | |
| Participants | Number of children (boys): 93 (62) Inclusion criteria: at least 6 wet nights per week Exclusion criteria: treatment in previous 2 weeks; daytime wetting/ pollakisuria; urological or psychological disease; poor motivation to use alarm Previous treatment: none in previous 2 weeks Age: 6‐14 years Baseline wetting: mean number of wet nights: A: 6.14, B: 6.12 (NS) | |
| Interventions | A (47): alarm and desmopressin 40 µg intranasal for 3 weeks, then alarm and desmopressin 20 µg for 3 weeks, then alarm alone for 3 weeks B (46): alarm and placebo for 6 weeks, then alarm alone for 3 weeks | |
| Outcomes | Wet nights (number, mean): First 3 weeks: A: 47, 2.93, B: 45, 3.86 (P=0.014) Last 3 weeks, alarm only: A: 43, 2.77, B: 39, 2.21 Number cured 2 weeks after end of trial: A: 15/47, B: 17/46 Number cured 6 months after end of trial: A: 17/47, B: 17/46 i.e. failed at 6 months: A: 20/47, B: 21/46 Wet nights at 6 months (number, mean): A: 41, 2.72, B: 37, 1.90 Adverse events: none in either group | |
| Notes | Power calculation provided Groups comparable for sex and age SDs not given (authors contacted for more information) Study supported by drug company (Ferring Pharmaceuticals A/S Ferring) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Longstaffe 2000.
| Methods | RCT (computer generated randomisation) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Setting: Recruited from hospital clinic and advertising | |
| Participants | Number of children: 182 At 6 months 17 withdrew due to failure (A 8; B 5; C 4) Inclusion criteria: primary monosymptomatic nocturnal enuresis, age >7 years, wet >3 times per week, normal bladder capacity Exclusion criteria: daytime wetting, CNS disorder, developmental delay, current alarm or DDAVP treatment, encopresis, other medical problems | |
| Interventions | A (61): alarm B (60): desmopressin intranasally C (61): placebo Duration 6 months, then failures crossed over to alternative arm for 6 months (not randomised) | |
| Outcomes | Number not achieving 14 dry nights after 6 months: A: 26/61; B: 31/60; C: 38/61 All children improved psychologically, e.g. behaviour and self concept, regardless of outcome or treatment assignment Side effects: not mentioned | |
| Notes | Dose of DDAVP not given No follow up as failures assigned to alternative treatment Blinding to method not possible for alarm group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Martin 1993.
| Methods | RCT (double‐blind randomised placebo controlled) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: No Only 7 DDAVP successes followed up | |
| Participants | Number of children: 44 Boys: A:54%, B:41% Mean age (year): A:8.9, B:9.13 Previous treatment: all children had failed to improve during 1 month treatment with motivational therapy and bladder training Baseline wetting: mean (SD) % of dry nights per month: A:24.45 (18.8), B:19.9 (20) | |
| Interventions | A (22): placebo B (22): 40 µg desmopressin drops 15 minutes before retiring Duration of treatment: 2 months | |
| Outcomes | Mean (SD) % of dry nights per month after 2 months: A:47.4 (32.1), B:69.2 (33.5) Number (%) of children becoming totally dry: A:1 (5), B:5 (27) Side effects: anorexia (1) | |
| Notes | Groups similar at baseline No follow up Very small sample Unclear about dropouts Inclusion/ exclusion criteria not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miller 1990a.
| Methods | RCT (multi‐centre, double‐blind) Trial A = Centre 1 Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned No significant differences between groups except that more older children in group (A) in one centre 4 weeks open label phase then 2 week no treatment Number at 2 week follow up: 1A:19, 1B:26, 1C:16 | |
| Participants | Number of children: 99 (70% boys) 4 dropouts Inclusion criteria: children aged 7‐14 with nocturnal enuresis; informed consent from parents; 10 or more wet nights per fortnight; no organic urologic disorders; no urinary tract infection; no abnormal urine osmolality Previous treatment: 58% taken other drugs; 87% imipramine; 40% tried other measures of these, 76% tried enuresis alarm Age range 7‐14 (46% aged 7‐8) Baseline wetting: mean number of wet nights in 14: 1A:12.3, 1B:11.8, 1C:12.3 | |
| Interventions | 1A (32): 20 µg desmopressin acetate intranasally at bedtime 1B (36): 40 µg desmopressin acetate intranasally at bedtime 1C (31): placebo Duration of treatment: 4 weeks | |
| Outcomes | Mean number of wet nights in final 14 days: 1A:8.7, 1B:7.0, 1C:10.7 Active treatment significantly different to placebo 2 week follow up: mean number of wet nights per 14: 1A:11.1, 1B:11.0, 1C:9.9 Side effects: no serious adverse events (6 minor) | |
| Notes | No intention to treat Short follow up Statistics suggest sample size too small for conclusive findings No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miller 1990b.
| Methods | RCT (multi‐centre, double‐blind) Trial B = Centre 2 Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned No significant differences between groups except that more older children in group (A) in one centre 4 weeks open label phase then 2 week no treatment Number at 2 week follow up: 2A:19, 2B:20, 2C:20 | |
| Participants | Number of children: 77 (70% boys) 4 dropouts Inclusion criteria: children aged 7‐14 with nocturnal enuresis; informed consent from parents; 10 or more wet nights per fortnight; no organic urologic disorders; no urinary tract infection; no abnormal urine osmolality Previous treatment: 58% taken other drugs; 87% imipramine; 40% tried other measures of these, 76% tried enuresis alarm Age range 7‐14 (46% aged 7‐8) Baseline wetting: mean number of wet nights in 14: 2A:12.6, 2B:12.3, 2C:12.4 | |
| Interventions | 2A (27): 20 µg desmopressin acetate intranasally at bedtime 2B (24): 40 µg desmopressin acetate intranasally at bedtime 2C (26): placebo Duration of treatment: 4 weeks | |
| Outcomes | Mean number of wet nights in final 14 days: 2A:10.0, 2B:8.1, 2C:10.5 Active treatment significantly better than placebo except A versus C not significant 2 week follow up: mean number of wet nights per 14: 2A:10.8, 2B:11.4, 2C:11.3 Side effects: no serious adverse events (11 minor) | |
| Notes | No intention to treat Short follow up Statistics suggest sample size too small for conclusive findings No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Muller 2001#.
| Methods | RCT (double blind crossover, initial assignment of groups 'created at random') Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned | |
| Participants | Number of children (boys): 40 (29) Inclusion criteria: at least 3 wet nights per week, primary nocturnal enuresis Exclusion criteria: anatomical abnormalities, abnormal serum or urine analysis Previous treatment: none Baseline wetting: mean wet nights 5.35 (median 5.5, 95% CI 4.5‐6) Age (mean, median, range): A: 8.7, 8.9 (6‐13); B: 8.6, 8 (6.3‐11.9) Recruited from Children's Hospital, University of Kiel, Germany | |
| Interventions | A (19): 20 µg desmopressin intranasally first 2 weeks B (21): 0.9% saline first 2 weeks Duration of treatment: crossed over after 2 weeks | |
| Outcomes | Number of wet nights during trial, mean (median, 95% CI): A: 3.27 (3, 2 to 4); B: 4.9 (5.25, 4.5 to 6): (P<0.001) Responders to desmopressin (undefined): 27/40 children (67.5%). Data not provided for placebo treatment Reaction time: no difference between the groups Short‐term memory: better on desmopressin, more words remembered, P=0.012 Children slept more deeply on desmopressin (14/18) than placebo (4/18), P=0.03 | |
| Notes | No useable data Groups comparable at baseline on gender, age, weight and height SDs not given (authors contacted for more infomation but not supplied) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Natochin 2000.
| Methods | CCT (randomisation using case record numbers) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Setting: St Petersburg State Medical Academy, Russia | |
| Participants | Number of children: 62 (43 boys) plus 22 (15) on placebo Inclusion criteria: primary nocturnal enuresis Exclusion criteria: other medication, daytime wetting, other disease Age: range 6‐15 years (mean 9.8 +/‐ SD 7) Baseline wetting: at least 6 wet nights per 2 weeks | |
| Interventions | A (32): desmopressin 10.5 ‐ 24.5 µg intranasally B (30): diclofenac tablet 1 mg/kg body mass C (22): placebo (intranasally) for 2 weeks then given desmopressin Duration of treatment: A and B 4 weeks each, C (placebo) 2 weeks only Follow up: none | |
| Outcomes | Number not achieving 14 dry nights: A: 11/32; B: 20/30; C: 21/22 Side effects: none reported | |
| Notes | Extra information supplied by authors (method of randomisation, results confirmed, no side effects) Parallel groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Neveus 1999#.
| Methods | RCT (double blind crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes | |
| Participants | Number of children: 33 (30 boys) Dropouts: 2 extra withdrew Inclusion criteria: primary monosymptomatic nocturnal enuresis Previous treatment: resistant to 0.4 mg desmopressin Age: range 6‐16 years, mean 10.4 Baseline wetting: at least 6 wet nights without treatment, mean 11.4 in 2 weeks | |
| Interventions | A (33): 0.4 mg desmopressin orally B (33): 0.8 mg desmopressin orally C (33): placebo Duration: 5 nights each | |
| Outcomes | Number not achieving 14 dry nights: A: 30/33; B: 27/33; C: 32/33 Side effects: not mentioned | |
| Notes | Washout period of 48 hours between each phase No follow up as all children treated after end of trial with high dose desmopressin or combined desmopressin and anticholinergics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ng 2005.
| Methods | RCT 'randomly allocated' by consecutive sealed envelopes Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes | |
| Participants | Number of children 105 Dropouts: 12 (defaulted from treatment: A7, B2, C3; defaulted from follow up A2, B2, C5) Inclusion: primary nocturnal enuresis Exclusion: UTI in previous 3 months, daytime wetting, polyuric disorders, abnormal urinalysis, renal disease, previous diuretics, unwilling to be randomised Previous treatment: none (excluded if had desmopressin, alarms or tricyclics) Age: range 7‐12 years Baseline wetting: at least 3 wet nights in baseline 2 weeks | |
| Interventions | A (35): alarm only ('Wet‐Stop' alarm) B (38): oral desmopressing 200 µg, increased to 400 µg if > 1 wet night C (32): both treatments Duration of treatment: 12 weeks Follow up: 12 weeks | |
| Outcomes | Wet nights during trial (N, mean (SD)): A: 28, 2.8 (2.2), B: 36, 2.6 (2.4), C: 29, 1.3 (1.9) Not achieving 14 dry nights: A: 27/35, B: 22/38, C: 12/32 (ITT, dropouts = failure) Wet nights after trial (N, mean (SD)): A: 24, 2.5 (2.4), B: 34, 3.4 (2.5), C: 24, 2.6 (2.7) Not achieving 14 dry nights or relapsing after: A: 35/35, B: 30/38, C: 19/32 (ITT, dropouts = failure) Adverse effects: none All children who responded completely to the alarm stayed dry afterwards | |
| Notes | All children had star charts and kept wetting diaries Comparable at baseline on wetting frequency, age, gender, urine osmolality More children failed to comply in Group A (alarm only), these were included as failures in the dry night analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Post 1983a#.
| Methods | RCT (Trial 1 = multi‐centre double‐blind randomised crossover trial) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned Follow up after 1‐3 months | |
| Participants | Trial 1: Number of children: 52 (40 boys) Inclusion criteria: healthy children age 6‐16; history of severe primary or secondary enuresis Exclusion criteria: organic causes Previous treatment: 18 had previous pharmacologic treatment; 3 had undergone urethral dilation procedures and 16 subjects had been involved in an identical study of lower dose (20 µg) of desmopressin Mean age (years): 9.0 Baseline wetting: mean (SEM) number of dry nights: 2.52 (0.28) | |
| Interventions | 1A: 40 µg desmopressin intranasally at bedtime 1B: placebo Drinking prohibited until the next morning Duration of treatment: 2 weeks each arm | |
| Outcomes | Trial 1 Mean (SEM) number of dry nights per 14: A:6.23 (0.65), B:4.00 (0.53) No significant order effects Post treatment results: mean number of dry nights per 14: 3.44 (0.50) Only 4 of 21 responders reported persistent effect During longer term study of 9 patients at Syracuse, the mean number of dry nights while taking desmopressin: 5.11 (1.31) was the same as that during the 2 week period: 5.11 (1.59) Side effects: not mentioned | |
| Notes | Not stated if comparable groups No washout No details of dropouts ‐ unclear if intention to treat Results from 3 centres combined because no significant difference in mean number of wet nights during active treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Post 1983b#.
| Methods | RCT (Trial 2 = multi‐centre double‐blind randomised crossover trial) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned Follow up after 1‐3 months | |
| Participants | Trial 2: Number of children: 20 (15 boys) Inclusion criteria: healthy children age 6‐16; history of severe primary or secondary enuresis Exclusion criteria: organic causes Previous treatment: 18 had previous pharmacologic treatment; 3 had undergone urethral dilation procedures and 16 children had been involved in an identical study of lower dose (20 µg) of desmopressin Mean age: 8.9 years Baseline wetting: mean (SEM) number of dry nights: 1.90 (0.43) | |
| Interventions | 2A: 20 µg desmopressin intranasally at 8 pm each night 2B: placebo Drinking prohibited until the next morning Duration of treatment: 2 weeks each arm | |
| Outcomes | Trial 2 Mean (SEM) number of dry nights per 14: A:4.25 (0.88), B:2.35 (0.51) Post treatment mean (SEM) number of dry nights per 14: 4.00 (0.66) Comparing the results of 16 children who had both low and high dose (Trial 1), they did better on high dose ‐ mean paired increase (SEM): 2.18 (0.90), t=2.44 P=0.05. 6 children had increase of 3 or more dry nights while on higher dose Side effects: not mentioned | |
| Notes | Not stated if comparable groups No washout No details of dropouts ‐ unclear if intention to treat Results from 3 centres combined because no significant difference in mean number of wet nights during active treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Radmayr 2001.
| Methods | RCT (details not given) Systematic baseline measure of wetting: Yes Organic causes excluded: No apart from UTI Daytime wetting excluded: Yes Setting: Recruitment from Department of Urology and Anaesthesiology, Austria Follow up 6 months after end of trial | |
| Participants | Number of children (boys): 40 (31) Inclusion criteria: primary monosymptomatic nocturnal enuresis, high night‐time urine production (polyuria), age >5 years Exclusion criteria: UTI Previous treatment: none Age mean (range): A: 8.6 (5‐16), B: 8 (5‐14) Baseline wetting 5.5 wet nights per week; mean (range): A: 5.5 (2‐7), B: 6 (4‐7) | |
| Interventions | A (20): desmopressin, intranasal, 20 µg increasing to 40 µg if no response (12/20 children) B (20): laser acupuncture, see Notes Duration of treatment: 3 months | |
| Outcomes | Response = decrease of 90% wet nights; partial response = decrease of 50%; failed = less than 50% decrease Non‐response at end of trial: A: 3/20, B: 2/20 Partial response: A: 1/20, B: 4/20 Non‐response at 6 months after end of trial: A: 3/20, B: 5/20 (counting 1 who did not finish acupuncture course as a failure) Partial response at 6 months: A:2/20, B:2/20 Adverse events: none in either group | |
| Notes | Laser acupuncture described and illustrated, soft laser stimulation for 30 of several pre‐defined acupuncture areas, 3 visits per week, minimum 10, maximum 15 visits | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rittig 1988#.
| Methods | RCT (double‐blind randomised crossover) Placebo period of 3 weeks randomly and blindly placed throughout treatment period Only patients who responded in dose titration period entered into randomised trial Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes 6 non‐responders were not entered into crossover trial (all children, 4 girls and 2 boys) | |
| Participants | Number of children: 34 (12 boys, 11 girls, 8 women and 3 men) Previous treatment: all failed previous treatments including alarms and/or tricyclics Age (years): children mean: 13 (range 8‐17); adults mean: 25 (range 18‐45) Baseline wetting: at least 3 wet nights per week | |
| Interventions | Dose titration period then: A: optimum dose of desmopressin intranasally B: placebo (3 weeks blindly inserted into 24 week period) Duration of treatment: 24 weeks | |
| Outcomes | Mean number of dry nights per week: A:7, B:4 Side effects: headache (2); sight disturbance (1) | |
| Notes | Some adults Only patients who responded to desmopressin included Children and adults analysed together Placebo period not equal to active drug period No washout period No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rodriguez 2001.
| Methods | RCT (method not specified) Systematic baseline measure of wetting: No Organic causes excluded: Yes Daytime wetting excluded: Yes | |
| Participants | Number of children: 84 (80% boys) 3 dropouts after 3 months Inclusion criteria: wetting at least 1 time per week, age >7 years Exclusion criteria: diurnal enuresis, encopresis, neurological abnormalities Previous treatment: 38% of children Age range 7‐14 years Hospital clinic, Spain | |
| Interventions | A (30): bed alarm B (29): alarm and desmopressin 20 µg or 40 µg for more frequent wetters (>2 times per week) Duration 4‐6 months | |
| Outcomes | Response: A: 73.3%; B: 58.6% [=number not achieving 14 dry nights: A: 8/30; B: 12/29] All children treated with desmopressin if not cured at 6 months, therefore follow up not possible Side effects: not reported | |
| Notes | Spanish language No follow up possible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rushton 1995.
| Methods | RCT (double‐blind multi‐institutional) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up after 5 months | |
| Participants | Number of children: 96 (71 boys) No dropouts reported Inclusion criteria: confirmed monosymptomatic nocturnal enuresis; wet 6+ nights during 14 day baseline; no organic urological disease; no daytime wetting; no central diabetes insipidus; no urinary tract infection in previous 18 months; no use of any drug that could affect urine concentration; no medical treatment for hyperactivity or attention deficit disorder; no history of acute or perennial rhinitis, rhinorrhoea or nasal polyps; no clinically significant medical disease that may interfere with the study Mean age: 9.7 years (range 7‐14) Severity: mean number of wet nights during 2 week baseline: A:11.16 (2.44), B:10.96 (2.53) No significant difference between the groups in demographics | |
| Interventions | A (49): 20 µg desmopressin spray, dose doubled if not completely dry after 14 days B (47): placebo as above Duration of treatment: 4 weeks | |
| Outcomes | Mean number of wet nights (SD) Period 1 (20 µg): A:7.91 (4.74), B:9.79 (3.28) Period 2 (40 µg): A:7.54 (5.04), B:9.79 (3.63) Side effects: none reported No meaningful differences between responders and non‐responders with regard to demographic variables of age, gender, race or family history | |
| Notes | No follow up results No details of previous treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schulman 2001a.
| Methods | RCT (double‐blind placebo controlled multi‐centre parallel group design) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up: none | |
| Participants | Number of children (boys): 193 (133) Dropouts: 6, due to non‐compliance, consent withdrawn, failure to keep diary Inclusion criteria: at least 3 wet nights per week, informed consent Exclusion criteria: organic urological disease; daytime wetting; diabetes insipidus; UTI; known hypersensitivity to desmopressin; antibiotics, diuretics; hyperactivity Previous treatment: none in previous 30 days Age range 6‐16 years Baseline wetting (mean in 2 weeks, range): A: 11 (5‐14), B: 10 (4‐14), C: 10 (6‐14), D: 10 (6‐14) Multicentre trial in 16 centres in USA | |
| Interventions | A (46): desmopressin 0.2 mg oral B (49): desmopressin 0.4 mg C (50): desmopressin 0.6 mg D (48): matching placebo Treatment for 2 weeks, then 2‐week placebo washout before being randomly assigned to second trial | |
| Outcomes | Mean wet nights during 2 weeks treatment: A: 4 (SD 1.33), B: 3.5 (1.73), C: 3 (1.73), D: 4.5 (1.37) Number not achieving 14 dry nights during trial: A: 42/44, B: 42/48, C: 46/49, D: 47/47 Number improved during trial: A: 4, B: 9, C: 9, D: 3 Adverse events (1 or more per child): 43/143 on desmopressin, 13/48 on placebo. Headache, increased cough, and abdominal pain, all mild (81% desmopressin, 62% placebo) or otherwise moderate, all resolved before end of study and were mostly unrelated to treatment (79% desmopressin, 77% placebo) No child stopped trial because of side effects | |
| Notes | First trial, dose ranging phase Fluid restriction 2 hours before bedtime Groups comparable at baseline Dropouts comparable from all groups No SDs ‐ authors contacted for more information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schulman 2001b.
| Methods | RCT (double‐blind placebo controlled multi‐centre parallel group design) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up: none | |
| Participants | Children from Trial 1 were eligible to be randomised again if they continued to have at least 3 wet nights per week during the placebo washout phase (31 children withdrew at this stage, 1 due to UTI) Dropouts: 11 | |
| Interventions | E (110): desmopressin 0.2 mg increased every 2 weeks if no response to max 0.6 mg F (38): matching placebo, tablets changed every 2 weeks if no response | |
| Outcomes | Number of children requiring maximum increase in dose by 8 weeks: E: 86/99, F: 38/38 Improvement (50% reduction from baseline level of wetting): E: 51/99 [28 at 0.2 mg; 16 at 0.4 mg; 8 at 6 mg]: F: 7/35 First 2 weeks, desmopressin dose = 0.2 mg, mean wet nights in 1 week: E: n=109, mean=4 (SD 1.57), F: 38, 5 (1.54) Last 2 weeks, dose increased up to 0.6 mg: E: 99, 3.2 (1.69), F: 36, 4.5 (1.5) Adverse events (1 or more per child): 43/143 on desmopressin, 13/48 on placebo. Rhinitis, pharyngitis, infection, headache and fever, all mild (79% desmopressin, 92% placebo) or otherwise moderate, all resolved before end of study and were mostly unrelated to treatment (80% desmopressin, 83% placebo) 1 child on desmopressin and 1 on placebo stopped trial because of nervousness | |
| Notes | Second trial, dose titration phase Fluid restriction 2 hours before bedtime Authors contacted for more information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Segni 1982.
| Methods | RCT Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned Dropouts included in analysis Followed up after 1 week | |
| Participants | Number of children: 40 (boys: A:14, B:10) Dropouts: A:0, B:12 Inclusion criteria: age 4‐15 years; constant enuresis; no physical deformity or neurological damage Age 4‐15 years with persistent enuresis, mean = 8.59 years Baseline wetting: A:5.2, B:4.6 | |
| Interventions | A (20): 20 to 30 µg desmopressin intranasally B (20): placebo Duration of treatment: 1 week | |
| Outcomes | From graph: mean number of wet nights per week (SEM): A:2.2 (0.3), B:4.2 (0.4) No cases of total dryness Side effects: none reported | |
| Notes | Italian language Not reported if comparable groups Comparison with placebo for 1 week only Results taken from graph Follow up for 1 week only No details of previous treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sener 1998.
| Methods | RCT (method not specified) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned | |
| Participants | Number of children: 85 Inclusion criteria: primary enuresis, age >5 years Exclusion criteria: enuresis treatment, other disease symptoms Age: mean 8 years, range 6‐15 Baseline wetting at least 3 wet nights/week Dry nights in 2 weeks: A: 1.5 (SE 0.3); B: 1.5 (0.4); C: 1.1 (0.3) | |
| Interventions | A (31): desmopressin 20 µg, intranasally B (29): indomethacin 100 mg/day, suppository C (25): placebo Duration of treatment: 4 weeks Follow up: none | |
| Outcomes | DRY nights/2 weeks (number, mean, SE) on treatment: A: 31, 11.8 (0.5); B: 29, 8.9 (0.8); C: 25, 3.8 (0.8) [= WET nights per week, number, mean, SD: A: 31, 1.1 (SD 1.39); B: 29, 2.55 (2.15); C: 25, 5.1 (2)] Side effects: none reported | |
| Notes | Parallel groups Blinding not possible due to different routes of administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Skoog 1997.
| Methods | RCT (parallel group trial) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes | |
| Participants | Number of children: 147 (112 boys) 6 extra had no outcome data, 12/147 discontinued trial Inclusion criteria: Primary nocturnal enuresis Exclusion criteria: Organic urological disease, daytime wetting, diabetes insipidus, UTI, previous non‐response to desmopressin Age: means 9.1 to 9.5 years, range 5‐17 Baseline wetting at least 3 wet nights/week for 2 weeks 14 centres in USA | |
| Interventions | A (37): 200 µg desmopressin orally B (35): 400 µg desmopressin C (37): 600 µg desmopressin D (38): placebo (double blind) Duration 6 weeks | |
| Outcomes | Wet nights during last 2 weeks of trial (number, mean, SD): A: 33, 4 (SD 1.15), B: 33, 3.5 (1.44), C: 33, 3.5 (1.15), D: 36, 5 (1.2) Number not achieving 14 dry nights: A: 32/33, B: 29/33, C: 31/33, D: 36/36 Side effects: 66 children on desmopressin and 21 on placebo had at least one adverse event (rhinitis, headache, pharyngitis, infection, cough) 92% were mild, 87% were unrelated to treatment, 89% resolved spontaneously. 3 serious events with desmopressin (2 vomiting, 1 atopic dermatitis) required treatment to stop | |
| Notes | Dropouts and reasons clearly described No follow up Parallel group study (not crossover) Number of wet nights calculated from decrease from baseline, using SE at baseline as proxy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Stenberg 1994#.
| Methods | RCT (double blind crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes | |
| Participants | Number of children: 10 Inclusion criteria: adolescents (puberty stage at least 2, at least 12 years) Exclusion criteria: treatment in previous 2 weeks, daytime wetting, UTI, urinary tract abnormalities Previous treatment: failed using alarms, desmopressin, other drugs Age range 11‐21, median 13 years Baseline wetting 4.7 (SD 1.1) wet nights/week Department of Paediatric Surgery, Sweden | |
| Interventions | A : desmopressin orally (dosage based on titration period) B : placebo Duration 4 weeks each | |
| Outcomes | Wet nights during trial (number, mean, SD): A: 10, 1.8 (SD 1.4); B: 10, 4.1 (1.5) Side effects: headache (5); abdominal pain (6); nausea and vertigo (1) All resolved while treatment continued | |
| Notes | No washout period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sukhai 1989#.
| Methods | RCT (double blind randomised crossover with 2 weeks washout) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up: 4 weeks to 6 months | |
| Participants | Number of children: 28 (21 boys) Inclusion criteria: Normal urine concentration capacity of 800 milliosmol/kg or higher; 3 or more wet nights per week during observation period; informed parental consent; no urological or renal disorder; no history of daytime wetting; no chronic urinary tract infection; no neurological or cardiovascular disease Previous treatment: 19 had previous attempts at treatment, including alarm (n=9) and tricyclic antidepressants (n=10) Age: mean 11 years (range 7 to 16) xx Severity at baseline: mean (SEM) number of dry nights per week = 1.4 (0.3) No dropouts | |
| Interventions | A: enuresis alarm and bedtime dose of 20 µg DDAVP intranasally B: enuresis alarm and bedtime dose of placebo Duration of treatment: 2 weeks in each condition | |
| Outcomes | Mean (SE) dry nights during treatment: A: 5.1 (0.4) B: 4.1 (0.4) 6 week follow up: 14 dry, 5 relapsed 4.5 month follow up: 9 remained dry Side effects: none reported Mean urine osmolality significantly increased from baseline Significantly higher urine osmolality with DDAVP than placebo Steady significant increase in body weight | |
| Notes | Very good study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Terho 1984#.
| Methods | RCT (double‐blind, randomised crossover 2 periods of DDAVP and 2 periods on placebo, each period lasting 3 weeks and mutual order of all 4 periods being selected randomly) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up after 4 weeks | |
| Participants | Number of children: 54 but 5 children excluded Exclusion criteria: faecal soiling; voiding difficulties; obvious neurological abnormalities; diurnal wetting Previous treatment: 49 had awakening protocol: 46 had water deprivation; 43 had tricyclic antidepressants; 13 had psychological counselling; 2 had alarm device and 1 had no previous treatment Age range: 7‐16 years Baseline wetting: no details | |
| Interventions | A: 20 µg DDAVP intranasal drops B: placebo Duration of treatment: 2 periods of 3 weeks in each condition | |
| Outcomes | Mean % (SD) number of wet nights during combined periods: A:30.9 (28.7), B:57.5 (26.1) Only 1 child remained dry during follow up period Side effects: none reported | |
| Notes | Not reported if comparable groups No washout Unclear if intention to treat Baseline and follow up results lumped together 5 children excluded because of error in medication Short follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Terho 1991#.
| Methods | RCT (double‐blind randomised crossover) Children allocated to 2 periods of desmopressin and 2 periods of placebo. Each period lasted for 3 weeks and mutual order of all 4 periods selected at random. Closed by 3 week observation period Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Follow up after 3 weeks | |
| Participants | Number of children: 52 (35 boys), no dropouts Inclusion criteria: lifelong nocturnal enuresis; no diurnal wetting; no soiling; no urological or renal pathological conditions Previous treatment: 52 had night awakening; 52 had fluid restriction; 29 had used tricyclic antidepressants; 25 had used enuresis alarms Age range: 5‐13 years Baseline wetting: mean (SD) number of dry nights per week: 0.6 (0.2) | |
| Interventions | A: intranasal desmopressin (20 µg) at bedtime rising to 40 µg if no response B: placebo Duration of treatment: 2 periods of 3 weeks in each condition | |
| Outcomes | Mean (SD) number of dry nights per week: Period 1: A:4.4, B:2.1 Period 2: A:4.6, B:2.5 15 children became totally dry during desmopressin treatment. 5 children remained dry after treatment. 47 patients relapsed after treatment Side effects: none reported | |
| Notes | Not reported if comparable groups No washout reported Short follow up No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tuvemo 1978#.
| Methods | RCT (double‐blind randomised crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned No follow up | |
| Participants | Number of children: 18 Inclusion criteria: age at least 6 years Age: range 6‐12 years Previous treatment: children had not responded satisfactorily to previous treatment with imipramine or amitriptyline Baseline wetting: mean (SEM) number of dry nights out of 28: 7.5 (2.98) | |
| Interventions | A: 20 µg intranasal DDAVP (Minerin) just before bedtime after emptying bladder B: identical placebo as above Duration of treatment: 28 days in each condition | |
| Outcomes | Mean (SE) number of dry nights out of 28: A:21.7 (1.72), B:12.1 (2.07) Side effects: none reported (no physical or subjective side effects observed) Number of children whose results were said to be excellent: 8; relatively good: 8; unsatisfactory: 2 Follow up after 6 months | |
| Notes | Not stated if comparable groups No washout No details of dropouts: unclear if intention to treat No follow up Active and placebo results combined so cannot see any order or carryover effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Uygur 1997#.
| Methods | RCT (double‐blind randomised crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned | |
| Participants | 65 children but 11 excluded before RCT because they did not respond to trial of desmopressin Dropouts: 1 (UTI); 3 (found no longer to respond to desmopressin during 6 month open treatment) Inclusion criteria: primary nocturnal enuresis Exclusion criteria: urological disease, non‐response to 2 week trial of desmopressin Age: 7‐17 years Baseline wetting: '3 or more wet nights / week' | |
| Interventions | A (54): desmopressin spray, 20 mcg or 40 mcg if no response B (54): placebo spray Duration of treatment: 2 weeks each arm Follow up: 6 months open desmopressin treatment | |
| Outcomes | Wet nights in 2 weeks: A: 1, B: 9.6 (no SDs) | |
| Notes | No useable data Population selected on the basis of all initially responding to desmopressin No SDs Baseline comparability of groups not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vertucci 1997.
| Methods | RCT (double blind crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Not mentioned | |
| Participants | Number of children: 57 Dropouts: 5 Inclusion criteria: primary nocturnal enuresis, age > 5 years, baseline wetting at least 3 nights/week, Previous treatment: not mentioned Age range 6‐15 6 Child Neuropsychiatry Clinics, Italy | |
| Interventions | A (29): desmopressin 30 µg intranasal then imipramine B (28): imipramine 0.9 mg/kg then desmopressin Duration of treatment: 3 weeks each Follow up: 2 weeks | |
| Outcomes | Mean wet nights during first arm of trial: A: n=29, 1; B: 28, 2.5 Number not achieving 14 dry nights: A: 4/29; B: 9/28 Mean wet nights after both drugs: A: 29, 3.5; B: 28, 2.8 Side effects: desmopressin: 1 back pain, 1 inflamed nasal mucosa; imipramine: 1 pallor, restlessness and cold extremities | |
| Notes | Data estimated from graphs Data only given from first arm of trial here No washout SDs not available Blinding not possible due to different routes of administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wille 1986.
| Methods | RCT Systematic baseline measure of wetting: Yes Organic causes excluded: Yes Daytime wetting excluded: Yes Distribution of social class of parents in 2 groups was similar Dropouts not included in analysis | |
| Participants | Number of children: 50 (boys and girls) Inclusion criteria: age over 6 years; not dry for more than 6 months; at least 3 wet nights per week at baseline; written informed parental consent; no treatment for enuresis during previous year; no daytime wetting; no cardiovascular disease; no renal disorder; no neurological disease; no urinary tract infection Age: over 6 years Baseline wetting: mean number of dry nights per week: A:2.1, B:1.9 Number completing treatment: A:24, B:22 | |
| Interventions | A (25): intranasal desmopressin (20 µg) B (25): enuresis alarm Duration of treatment: 3 months | |
| Outcomes | Mean (SEM) number of dry nights per week in first week: A:4.2 (0.5), B:2.5 (0.4) In last week of treatment: A:4.9 (0.5), B:6.3 (0.4) A:10 relapses given 3 months more treatment. Successful for 7/10 but 4/7 relapsed immediately and 1/7 after 2 months. B: 1 relapsed and further treatment unsuccessful. Side effects: A: nasal discomfort (5); bad taste in throat (2); B:false alarms (21); alarm did not go off (5); alarm did not wake child (15); other family members woken (15); child frightened by alarm (1). Lab tests: urine osmolality and density higher during treatment with desmopressin and urine osmolality in alarm group lower during treatment than before | |
| Notes | Direct comparison of desmopressin and alarm Not intention to treat analysis Results taken from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yap 1998#.
| Methods | RCT (double blind crossover) Systematic baseline measure of wetting: Yes Organic causes excluded: No Daytime wetting excluded: Yes | |
| Participants | Number of children: 37 (22 boys) 3 excluded because data incomplete Inclusion criteria: Primary monosymptomatic nocturnal enuresis Exclusion criteria: No current enuresis treatment Hospital clinic, Singapore | |
| Interventions | A: (34) DDAVP 400 mg oral B (34) Placebo Duration of treatment 5 weeks, 2 week washout | |
| Outcomes | Wet nights during trial (number, mean, SD): A: 34, 2.5 (2.7); B: 34, 4.5 (2.1) Number not achieving 14 dry nights: A: 11/34; B: 27/34 Wet nights after trial (number, mean, SD): 34, 4.5 (2.1) Side effects: None reported (looked at body weight, BP, serum sodium and osmolality, urine osmolality, water retention or intoxication) | |
| Notes | Groups comparable at baseline (same children) Follow up data unusable as all had had both treatments | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
# = crossover trial; BP = blood pressure; CCT = controlled clinical trial; CI = confidence interval; CNS = central nervous system; DDAVP = 'brand name' for desmopressin; ITT = intention to treat; kg = kilograms; mg = milligrams; NS = not significant; RCT = randomised controlled trial; SD = standard deviation; SE = standard error; SEM = standard error of the mean; µg = micrograms; UTI = urinary tract infection; wt = weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bernasconi 1982 | RCT: No Interventions: Desmopressin 5 µg, 10 µg rising to 40 µg |
| Bogaert 2005 | RCT: Yes, of different doses of desmopressin Excluded as not actually used to treat enuresis (was a dose‐response study for one day only) Intervention: single dose oral lyophilisate desmopressin: 30, 60, 120, 240, 360, 480 mg, placebo |
| Butler 2001 | RCT: No Comparison group: No Intervention: Withdrawal from desmopressin or imipramine treatment, use of alarms optional |
| Caione 1994 | RCT: No mention of method of allocation to groups Organic causes excluded: Yes Systematic baseline measures of wetting: Yes Interventions: Desmopressin, acupuncture |
| Caione 1995 | RCT: Yes Participants all had daytime wetting as well as bedwetting; some adults were included Interventions: Desmopressin, oxybutynin |
| Capozza 1991 | RCT: No Comparison group: Yes Organic causes excluded: Yes Systematic baseline measures of wetting: Yes Interventions: Desmopressin, acupuncture |
| Chiozza 1999 | RCT: No (open multicentre trial) Comparison groups: Yes Interventions: desmopressin, 20, 30, 40 µg per day |
| Eckford 1994 | RCT: Yes Comparison group: Yes Organic causes excluded: No Systematic baseline: No Systematic outcome measures: No Intervention: Desmopressin in adults with multiple sclerosis |
| Evans 1992 | RCT: No Comparison group: Yes Organic causes excluded: Yes Systematic baseline measures of wetting: Yes Intervention: Intranasal desmopressin |
| Ferrie 1984 | RCT: No Comparison group: crossover trial Organic causes excluded: Yes Systematic baseline measures of wetting: Yes Intervention: Intranasal desmopressin |
| Jones 1959 | RCT: No Comparison group: Yes Organic causes excluded: No Systematic baseline measurement of wetting: No Systematic outcome measures: Yes Interventions: Pituitary snuff and propantheline |
| Key 1992 | RCT: No Comparison group: No Organic causes excluded: Yes Systematic baseline: No Systematic outcome measures: Yes Intervention: Desmopressin (DDAVP) |
| Kim 2001 | RCT: Yes Comparison group: Yes Organic causes excluded: No Systematic baseline measurement of wetting: Yes Systematic outcome measures: Yes (but improvement rates only) Interventions: Imipramine, desmopressin 2 children switched from imipramine to desmopressin due to side effects but data not given according to allocated groups therefore excluded as not intention to treat |
| Knudsen 1989 | RCT: No Participants included adults (mean age 15.8 years) Intervention: Desmopressin |
| Marson 1955 | RCT: Unclear Participants: 4 Intervention: pituitrin snuff, placebo snuff |
| Matthieson 1994 | RCT: No Comparison group: No Organic causes excluded: Yes Systematic baseline: Yes Systematic outcome measures: Yes Intervention: Desmopressin |
| Miller 1988 | RCT: No Comparison group: No Organic causes excluded: Yes Systematic baseline: No Systematic outcome measures: Yes Intervention: Desmopressin |
| Miller 1989 | RCT: No Comparison group: No Organic causes excluded: Yes Systematic baseline: Yes Systematic outcome measures: Yes Intervention: Desmopressin |
| Monda 1995 | RCT: No Comparison group: Yes Organic causes excluded: Yes Systematic baseline: No Systematic outcome measures: Yes Intervention: Desmopressin, imipramine, alarms |
| Petersen 1996 | RCT: Yes Design: Randomized placebo‐controlled, double blind, crossover study Participants were adults with multiple sclerosis, urinary frequency and incontinence Intervention: Desmopressin |
| Ramsden 1982 | RCT: No Comparison group: Yes Organic causes excluded: Yes Systematic baseline: No Systematic outcome measures: Yes Intervention: Desmopressin (DDAVP) |
| Robinson 2002 | RCT: Yes (double‐blind randomised placebo controlled trial) Intervention: Desmopressin Excluded because participants were adult women with severe daytime incontinence |
| Snajderova 2001 | RCT: No Intervention: Desmopressin |
| Steffens 1993 | RCT: No Comparison group: No Organic causes excluded: Yes Systematic baseline: No Systematic outcome measures: No Intervention: Desmopressin (vasopressin) |
| Stenberg 1993 | RCT: No Comparison group: No Organic causes excluded: No Systematic baseline: Yes Systematic outcome measures: Yes Intervention: Desmopressin |
| Stenberg 1995 | RCT: No Comparison group: No Organic causes excluded: No Systematic baseline: Yes Systematic outcome measures: Yes Intervention: Desmopressin |
| Taylor 1981 | RCT: Yes, crossover Participants: Adults with detrusor instability Intervention: Desmopressin (DDAVP) |
| Wood 1994 | RCT: No Comparison group: Yes Organic causes excluded: No Systematic baseline: Yes Systematic outcome measures: Yes Intervention: Desmopressin |
DDAVP = 'brand name' for desmopressin; µg = microgram; RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Hjalmas 2001.
| Trial name or title | Cessation of desmopressin treatment after response |
| Methods | |
| Participants | 77 responders to the SWEET trial |
| Interventions | Abrupt or tapered cessation of treatment after response to desmopressin |
| Outcomes | Relapse |
| Starting date | |
| Contact information | See references |
| Notes |
SWEET (Swedish Enuresis Trial)
Contributions of authors
CMAG (the contact reviewer) originally based this review on work done at the NHS Centre for Reviews and Dissemination, University of York, UK (see acknowledgements). CMAG used the data extracted by the York reviewers, converted them into Cochrane Review format, and separated them into seven component intervention reviews (of which this is one). This review includes trials from the York review plus extra ones identified since then. JHCE performed double data abstraction for the new trials, edited the text and provided a clinical perspective and interpretation.
Sources of support
Internal sources
Chief Scientist Office, Sottish Executive Health Department, UK.
External sources
National Health Service Research and Development Programme, UK.
Declarations of interest
JHCE has received reimbursement for attending a conference, fees for lecturing and a consultancy fee which was paid into a research fund from Ferring Pharmaceuticals, manufacturers of desmopressin.
Edited (no change to conclusions)
References
References to studies included in this review
Aladjem 1982 {published data only}
- Aladjem M, Wohl R, Boichis H, Orda S, Lotan D, Freedman S. Desmopressin in nocturnal enuresis. Archives of Disease in Childhood 1982;57(2):137‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Birkasova 1978# {published data only}
- Birkasova M, Birkas O, Flynn MJ, Cort JH. Desmopressin in the management of nocturnal enuresis in children: a double‐blind study. Pediatrics 1978;62(6):970‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Bradbury 1995 {published data only}
- Bradbury M. Combination therapy for nocturnal enuresis with desmopressin and an alarm device. Scandinavian Journal of Urology & Nephrology 1997;183:61‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Bradbury MG, Meadow SR. Combined treatment with enuresis alarm and desmopressin for nocturnal enuresis. Acta Paediatrica 1995;84(9):1014‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burke 1995 {published data only}
- Burke JR, Mizusawa Y, Chan A, Webb KL. A comparison of amitriptyline, vasopressin, and amitriptyline with vasopressin in nocturnal enuresis. Pediatric Nephrology 1995;9(4):438‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dimson 1986# {published data only}
- Dimson SB. DDAVP and urine osmolality in refractory enuresis. Archives of Disease in Childhood 1986;61(11):1104‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faraj 1999 {published data only}
- Faraj G, Cochat P, Cavailles ML, Chevallier C. [Treatment of isolated nocturnal enuresis: alarm or desmopressin?] [French]. Archives de Pediatrie 1999;6(3):271‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fera 2004 {published data only}
- Fera P, Glashan R, Lelis MA, Gonzales SR, Nogueira MDP, Almeida F, Srougi M, Bruschini H. Immediate outcome of DDAVP versus behavioural modification for treatment of monosymptomatic nocturnal enuresis: a prospective randomized study of 30 patients (Abstract). Neurourology & Urodynamics 2004;23(5/6):555‐6. [Google Scholar]
Fjellestad 1987# {published data only}
- Fjellestad‐Paulsen A, Wille S, Harris AS. Comparison of intranasal and oral desmopressin for nocturnal enuresis. Archives of Disease in Childhood 1987;62(7):674‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Folwell 1997# {published data only}
- Folwell AJ, Macdiarmid SA, Crowder HJ, Lord AD, Arnold EP. Desmopressin for nocturnal enuresis: urinary osmolality and response. British Journal of Urology 1997;80(3):480‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gibb 2004 {published data only}
- Gibb S, Nolan T, South M, Noad L, Bates G, Vidmar S. Evidence against a synergistic effect of desmopressin with conditioning in the treatment of nocturnal enuresis. Journal of Pediatrics 2004;144(3):351‐7. [DOI] [PubMed] [Google Scholar]
Hamano 2000 {published data only}
- Hamano S, Yamanishi T, Igarashi T, Ito H, Murakami S. Functional bladder capacity as predictor of response to desmopressin and retention control training in monosymptomatic nocturnal enuresis. European Urology 2000;37(6):718‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hoashi 1995 {published data only}
- Hoashi E, Hibi I, Maekawa K, Yoshida S, Ikoma F, Akashi S, et al. Clinical evaluation of desmopressin acetate (kw‐8008) in patients with primary nocturnal enuresis: double‐blind comparative study in comparison with imipramine hydrochloride. Kiso to rinsho 1995;29(16):4219‐57. [Google Scholar]
Holt 1986 {published data only}
- Holt J, Borresen B. Enuresis nocturna in school children in Bodo. A therapeutic trial with a vasopressin analog: desmopressin and imipramine. Tidsskrift for den Norske Laegeforening 1986;106(8):651‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Janknegt 1990# {published data only}
- Janknegt RA, Smans AJ. Treatment with desmopressin in severe nocturnal enuresis in childhood. British Journal of Urology 1990;66(5):535‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Janknegt 1997 {published data only}
- Janknegt RA, Zweers HHM, Namdar Z. Evaluation of oral desmopressin in the management of primary nocturnal enuresis in adolescents and adults (multi‐centre, randomized, double‐blind). Neurourology & Urodynamics 1995; Vol. 14, issue 5:422‐3.
- Janknegt RA, Zweers HM, Delaere KP, Kloet AG, Khoe SG, Arendsen HJ. Oral desmopressin as a new treatment modality for primary nocturnal enuresis in adolescents and adults: a double‐blind randomized, multicenter study. Dutch Enuresis Study Group. Journal of Urology 1997;157(2):513‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Kahan 1998 {published data only}
- Kahan E, Morel D, Amir J, Zelcer C. A controlled trial of desmopressin and behavioral therapy for nocturnal enuresis. Medicine 1998;77(6):384‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Kjoller 1984 {published data only}
- Kjoller SS, Hejl M, Pedersen PS. Enuresis treated with minurin (DDAVP). A controlled clinical study. Ugeskrift for Laeger 1984;146(43):3281‐2. [MEDLINE: ] [PubMed] [Google Scholar]
- Pedersen PS, Hejl M, Kjoller SS. Desamino‐D‐arginine vasopressin in childhood nocturnal enuresis. Journal of Urology 1985;133(1):65‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2005 {published and unpublished data}
- Lee T, Suh HJ, Lee HJ, Lee JE. Comparison of effects of treatment of primary nocturnal enuresis with oxybutynin plus desmopressin, desmopressin alone or imipramine alone: a randomized controlled clinical trial. Journal of Urology 2005;174(3):1084‐7. [DOI] [PubMed] [Google Scholar]
Leebeek 2001 {published data only}
- Leebeek‐Groenewegen ANJA, Blom J, Sukhai R, Heijden B. Efficacy of desmopressin combined with alarm therapy for monosymptomatic nocturnal enuresis. Journal of Urology 2001;166(6):2456‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Longstaffe 2000 {published data only}
- Longstaffe S, Moffatt ME, Whalen JC. Behavioral and self‐concept changes after six months of enuresis treatment: a randomized, controlled trial. Pediatrics 2000;105(4:Pt 2):935‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Martin 1993 {published data only}
- Martin Hernandez E, Fernandez Ovies JM, Guzman‐Guzman A, Alvarez Aldean J, Barrio Corrales F. Clinical effectiveness and safety of desmopressin in the treatment of infantile enuresis. Revista Espanola de Pediatria 1993;49(294):497‐501. [Google Scholar]
Miller 1990a {published data only}
- Miller K, Klauber GT. Desmopressin acetate in children with severe primary nocturnal enuresis. Clinical Therapeutics 1990;12(4):357‐66. [MEDLINE: ] [PubMed] [Google Scholar]
Miller 1990b {published data only}
- Miller K, Klauber GT. Desmopressin acetate in children with severe primary nocturnal enuresis. Clinical Therapeutics 1990;12(4):357‐66. [MEDLINE: ] [PubMed] [Google Scholar]
Muller 2001# {published data only}
- Eggert P, Fritz A, Stecker B, ller D. Desmopressin has an influence on the arousability of children with primary nocturnal enuresis. Journal of Urology 2004;171(6 Pt 2):2586‐8. [DOI] [PubMed] [Google Scholar]
- Flokowski H, Cavez‐Kattau K, Carlsson G, Muller D, Eggert P. Central nervous effect of dDAVP in children with nocturnal enuresis: Effect or side‐effect (Abstract). Monatsschrift fur Kinderheilkunde 1999;147(Suppl 2):62. [Google Scholar]
- Fritz A, Chavez‐Kattau K, Eggert P. Influence of DDAVP on the ability to wake children with primary Enuresis Nocturna. Klinische Padiatrie 2000;212(5):S108. [Google Scholar]
- Muller D, Florkowski H, Chavez‐Kattau K, Carlsson G, Eggert P. The effect of desmopressin on short‐term memory in children with primary nocturnal enuresis. Journal of Urology 2001;166(6):2432‐4. [PubMed] [Google Scholar]
Natochin 2000 {published and unpublished data}
- Natochin YV, Kuznetsova AA. Nocturnal enuresis: correction of renal function by desmopressin and diclofenac. Pediatric Nephrology 2000;14(1):42‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neveus 1999# {published data only}
- Neveus T, Lackgren G, Tuvemo T, Olsson U, Stenberg A. Desmopressin resistant enuresis: pathogenetic and therapeutic considerations. Journal of Urology 1999;162(6):2136‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ng 2005 {published data only}
- Ng CF‐N, Wong SN, Hong Kong Childhood Enuresis Study Group. Comparing alarms, desmopressin, and combined treatment in Chinese enuretic children. Pediatric Nephrology 2005;20(2):163‐9. [DOI] [PubMed] [Google Scholar]
Post 1983a# {published data only}
- Post EM, Richman RA, Blackett PR, Duncan KP, Miller K. Desmopressin response of enuretic children. Effects of age and frequency of enuresis. American Journal of Diseases of Children 1983;137(10):962‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Post 1983b# {published data only}
- Post EM, Richman RA, Blackett PR, Duncan KP, Miller K. Desmopressin response of enuretic children. Effects of age and frequency of enuresis. American Journal of Diseases of Children 1983;137(10):962‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Radmayr 2001 {published and unpublished data}
- Radmayr C, Schlager. Prospective randomized trial using laser acupuncture versus desmopressin in the treatment of nocturnal enuresis. European Urology 2001;40(2):201‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rittig 1988# {published data only}
- Rittig S, Knudsen UB, Sorensen S, Djurhuus JC, Norgaard JP. Long range double‐blind crossover study of DDAVP intranasal spray in the management of nocturnal enuresis (Abstract). Neurourology & Urodynamics 1988;7(3):184‐6. [Google Scholar]
- Rittig S, Knudsen UB, Sorensen S, Djurhuus JC, Norgaard JP. Long term double‐blind cross over study of desmopressin intranasal spray in the management of nocturnal enuresis. In: Meadow SR editor(s). Desmopressin in Enuresis. Proceedings of an International Symposium held at the Royal College of Physicians. London: Horus Medical Publications, 1988:43‐55. [Google Scholar]
Rodriguez 2001 {published data only}
- Rodriguez do Forno A, Ariceta Iraola G. Results of a therapeutic strategy against monosymptomatic nocturnal enuresis background. Anales Espanoles de Pediatria 2001;54(1):38‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Rushton 1995 {published data only}
- Rushton HG, Belman AB, Skoog S, Zaontz MR, Sihelnik S. Predictors of response to desmopressin in children and adolescents with monosymptomatic nocturnal enuresis. Scandinavian Journal of Urology & Nephrology Supplementum 1995;173:109‐11. [MEDLINE: ] [PubMed] [Google Scholar]
- Rushton HG, Belman AB, Zaontz M, Skoog SJ, Sihelnik S. Response to desmopressin as a function of urine osmolality in the treatment of monosymptomatic nocturnal enuresis: a double‐blind prospective study. Journal of Urology 1995;154(2 Pt 2):749‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rushton HG, Belman AB, Zaontz MR, Skoog SJ, Sihelnik S. The influence of small functional bladder capacity and other predictors on the response to desmopressin in the management of monosymptomatic nocturnal enuresis. Journal of Urology 1996;156(2 Pt 2):651‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulman 2001a {published and unpublished data}
- Schulman SL, Stokes A, Salzman PM. The efficacy and safety of oral desmopressin in children with primary nocturnal enuresis. Journal of Urology 2001;166(6):2427‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Schulman 2001b {published and unpublished data}
- Schulman SL, Stokes A, Salzman PM. The efficacy and safety of oral desmopressin in children with primary nocturnal enuresis. Journal of Urology 2001;166(6):2472‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Segni 1982 {published data only}
- Segni G, Salvaggio E, Parenti D, Cataldi L, Auriti C, Rossodivita A, et al. New therapeutic prospect in enuresis: desmopressin (DDAVP). Minerva Pediatrica 1982;34(1‐2):45‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Sener 1998 {published data only}
- Sener F, Hasanoglu E, Soylemezoglu O. Desmopressin versus indomethacin treatment in primary nocturnal enuresis and the role of prostaglandins. Urology 1998;52(5):878‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Skoog 1997 {published data only}
- Skoog SJ, Stokes A, Turner KL. Oral desmopressin: a randomized double‐blind placebo controlled study of effectiveness in children with primary nocturnal enuresis. Journal of Urology 1997;158(3 Pt 2):1035‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Stenberg 1994# {published data only}
- Stenberg A, Lackgren G. Desmopressin tablets in the treatment of severe nocturnal enuresis in adolescents. Pediatrics 1994;94(6 Pt 1):841‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Sukhai 1989# {published data only}
- Sukhai RN, Mol J, Harris AS. Combined therapy of enuresis alarm and desmopressin in the treatment of nocturnal enuresis. European Journal of Pediatrics 1989;148(5):465‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Terho 1984# {published data only}
- Terho P, Kekomaki M. Management of nocturnal enuresis with a vasopressin analogue. Journal of Urology 1984;131(5):925‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Terho 1991# {published data only}
- Terho P. Desmopressin in nocturnal enuresis. Journal of Urology 1991;145(4):818‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tuvemo 1978# {published data only}
- Tuvemo T. DDAVP in childhood nocturnal enuresis. Acta Paediatrica Scandinavica 1978;67(6):753‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uygur 1997# {published data only}
- Uygur MC, Ozgu IH, Ozen H, Ozen S, Toklu C, Ergen A, Tekgul S, Remzi D. Long‐term treatment of nocturnal enuresis with desmopressin intranasal spray. Clinical Pediatrics 1997;36(8):455‐9. [DOI] [PubMed] [Google Scholar]
Vertucci 1997 {published data only}
- Vertucci P, Lanzi C, Capece G, Fano M, Gallai V, Margari L, et al. Desmopressin and imipramine in the management of nocturnal enuresis: a multicentre study. British Journal of Clinical Practice 1997;51(1):27‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Wille 1986 {published data only}
- Wille S. Comparison of desmopressin and enuresis alarm for nocturnal enuresis. Archives of Disease in Childhood 1986;61(1):30‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille S. Primary nocturnal enuresis in children. Background and treatment. Scandinavian Journal of Urology & Nephrology Supplementum 1994;156:1‐48. [PubMed] [Google Scholar]
Yap 1998# {published data only}
- Yap HK, Chao SM, Tan AY, Murugasu B, Ong EK, Low EH. Efficacy and safety of oral desmopressin in the treatment of primary nocturnal enuresis in Asian children. Journal of Paediatrics & Child Health 1998;34(2):151‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bernasconi 1982 {published data only}
- Bernasconi S, Monti G. [The use of DDAVP (desmopressin) in primary nocturnal enuresis] [Italian]. Minerva Pediatrica 1982;34(3):131‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Bogaert 2005 {published data only}
- Bogaert G, Vande Walle J, Mattson S, Schurmans T, Hoebeke P, Deboe V, Norgaard J. A pharmacodynamic study in children with primary nocturnal enuresis using a new 'melt' orodispersible formulation of desmopressin (Abstract). Neurourology & Urodynamics 2005;24(5/6):578‐9. [Google Scholar]
Butler 2001 {published data only}
- Butler RJ, Holland P, Robinson J. Examination of the structured withdrawal program to prevent relapse of nocturnal enuresis. Journal of Urology 2001;166(6):2463‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Caione 1994 {published data only}
- Caione P, Nappo S, Capozza N, Minni B, Ferro F. [Primary enuresis in children. Which treatment today?] [Italian]. Minerva Pediatrica 1994;46(10):437‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Caione 1995 {published data only}
- Caione P, Arena F, Biraghi M, Cigna RM, Chendi D, Chiozza ML, et al. Nocturnal enuresis and daytime wetting: a multicentric trial with oxybutynin and desmopressin. European Urology 1997;31(4):459‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Caione P, Giorgi PL, Passerini‐Glazel G, Chiozza ML, Artibani W, Gado R, et al. Desmopressin (DDAVP) and oxybutynin in nocturnal enuresis: results of a multicentre trial. Proceedings of the International Children's Continence Society, Sydney, Australia. 1995:77‐81.
Capozza 1991 {published data only}
- Capozza N, Creti G, Gennaro M, Minni B, Caione P. The treatment of nocturnal enuresis. A comparative study between desmopressin and acupuncture used alone or in combination. Minerva Pediatrica 1991;43(9):577‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Chiozza 1999 {published data only}
- Chiozza ML, Gado R, Toro R, Ferrara P, Fois A, Giorgi P, et al. Italian multicentre open trial on DDAVP spray in nocturnal enuresis. Scandinavian Journal of Urology and Nephrology 1999;33(1):42‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eckford 1994 {published data only}
- Eckford SD, Swami KS, Jackson SR, Abrams PH. Desmopressin in the treatment of nocturia and enuresis in patients with multiple sclerosis. British Journal of Urology 1994;74(6):733‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Evans 1992 {published data only}
- Evans JH, Meadow SR. Desmopressin for bed wetting; length of treatment, vasopressin secretion and response. Archives of Disease in Childhood 1992;67(2):184‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrie 1984 {published data only}
- Ferrie BG, MacFarlane J, Glen ES. DDAVP in young enuretic patients: a double‐blind trial. British Journal of Urology 1984;56(4):376‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jones 1959 {published data only}
- Jones K, Tibbetts RW. Pituitary snuff, propantheline and placebos in the treatment of enuresis. Journal of Mental Science 1959;105:371‐81. [7727] [DOI] [PubMed] [Google Scholar]
Key 1992 {published data only}
- Key DW, Bloom DA, Sanvordenker J. Low‐dose DDAVP in nocturnal enuresis. Clinical Pediatrics 1992;31(5):299‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2001 {published data only}
- Kim KM, Kang J, Ha IS, Chung HI. Therapeutic effects of imipramine and DDAVP in the children with primary monosymptomatic nocturnal enuresis: a randomized prospective study. Neurourology & Urodynamics 2001;20(4):379‐80. [Google Scholar]
Knudsen 1989 {published data only}
- Knudsen UB, Rittig S, Pedersen JB, Norgaard JP, Djurhuus JC. Long‐term treatment of nocturnal enuresis with desmopressin ‐ influence on urinary output and haematological parameters. Neurourology & Urodynamics 1989; Vol. 8, issue 4:348‐9.
Marson 1955 {published data only}
- Marson FGW. Posterior pituitary snuff treatment of nocturnal enuresis. Br Med J 1955;1:1194‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthieson 1994 {published data only}
- Matthiesen TB, Rittig S, Djurhuus JC, Norgaard JP. A dose titration and an open 6‐week efficacy and safety study of desmopressin tablets in the management of nocturnal enuresis. Journal of Urology 1994;151(2):460‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 1988 {published data only}
- Miller K, Goldberg S, Atkin B. Nocturnal enuresis: experience with long term use of intranasally administered desmopressin in enuresis.. In: Meadow SR editor(s). Proceedings of an International Symposium held at the Royal College of Physicians. London: Horus Medical Publications, 1989:56‐62. [DOI] [PubMed] [Google Scholar]
Miller 1989 {published data only}
- Miller K, Goldberg S, Atkin B. Nocturnal enuresis: experience with long‐term use of intranasally administered desmopressin. Journal of Pediatrics 1989;114(4 Pt 2):723‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Monda 1995 {published data only}
- Monda JM, Husmann DA. Primary nocturnal enuresis: a comparison among observation, imipramine, desmopressin acetate and bed‐wetting alarm systems. Journal of Urology 1995;154(2 Pt 2):745‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Petersen 1996 {published data only}
- Petersen T, Bang R, Norgard JP, Djurhuus JC. Desmopressin in the treatment of urinary frequency and incontinence in patients with MS. A randomized, placebo‐controlled, double‐blind, cross‐over study. European Journal of Neurology 1996;3 Suppl 2:17‐8. [Google Scholar]
Ramsden 1982 {published data only}
- Ramsden PD, Hindmarsh JR, Bowditch JDP, Price DA, Yeates WK. DDAVP for adult enuresis ‐ a preliminary report. Proceedings of the International Continence Society (ICS), 9th Annual Meeting 1979:91, 249‐52.
- Ramsden PD, Hindmarsh JR, Price DA, Yeates WK, Bowditch JD. DDAVP for adult enuresis ‐ a preliminary report. British Journal of Urology 1982;54(3):256‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robinson 2002 {published data only}
- Robinson D, Dixon A, Cardozo L, Anders K. Does being in control make life better? The use of desmopressin in daytime urinary incontinence (Abstract). Proceedings of the International Continence Society (ICS), 32nd Annual Meeting, Heidelberg, Germany 28‐30 August. 2002:270‐1. [14525]
Snajderova 2001 {published data only}
- Snajderova M, Lehotska V, Kernova T, Kocnarova N, Archmanova E, Janda P, et al. Desmopressin in a long‐term treatment of children with primary nocturnal enuresis‐‐a symptomatic therapy?. European Journal of Pediatrics 2001;160(3):197‐8. [12138] [DOI] [PubMed] [Google Scholar]
Steffens 1993 {published data only}
- Steffens J, Netzer M, Isenberg E, Alloussi S, Ziegler M. Vasopressin deficiency in primary nocturnal enuresis. Results of a controlled prospective study. European Urology 1993;24(3):366‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stenberg 1993 {published data only}
- Stenberg A, Lackgren G. Treatment with oral desmopressin in adolescents with primary nocturnal enuresis. Efficacy and long‐term effect. Clinical Pediatrics 1993;Spec No(July):25‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stenberg 1995 {published data only}
- Stenberg A, Lackgren G. Desmopressin tablet treatment in nocturnal enuresis. Scandinavian Journal of Urology & Nephrology Supplementum 1995;173:95‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Taylor 1981 {published data only}
- Taylor MC, Bates CP. DDAVP in the management of nocturnal frequency and enuresis due to bladder instability. Proceedings of the International Continence Society (ICS), 11th Annual Meeting 1981:144‐5.
Wood 1994 {published data only}
- Wood CM, Butler RJ, Penney MD, Holland PC. Pulsatile release of arginine vasopressin (AVP) and its effect on response to desmopressin in enuresis. Scandinavian Journal of Urology & Nephrology Supplementum 1994;163:93‐101. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
Hjalmas 2001 {published data only}
- Hjalmas K. SWEET, the Swedish Enuresis Trial. Scandinavian Journal of Urology & Nephrology Supplementum 1995;173:89‐93. [MEDLINE: ] [PubMed] [Google Scholar]
- Hjalmas K, Hanson E, Hellstrom AL, Kruse S, Sillen U. Long‐term treatment with desmopressin in children with primary monosymptomatic nocturnal enuresis: an open multicentre study. Swedish Enuresis Trial (SWEET) Group. British Journal of Urology 1998;82(5):704‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
APA 1980
- American Psychiatric Association. Functional enuresis. In: American Psychiatric Association, editor(s). Diagnostic and Statistical Manual of Mental Disorders (DSM). 3rd Edition. Arlington (VA): American Psychiatric Publishing Inc, 1980. [Google Scholar]
Bakwin 1971
- Bakwin H. Enuresis in twins. American Journal of Diseases of Children 1971;121(3):222‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakwin 1973
- Bakwin H. The genetics of enuresis. In: Kolvin I, MacKeith RC, Meadow SR editor(s). Bladder control and enuresis. London: William Heinemann Medical Books, 1973. [Google Scholar]
Berlin 1989
- Berlin JA, Laird NM, Sacks HS, Chalmers TC. A comparison of statistical methods for combining event rates from clinical trials. Statistics in Medicine 1989;8(2):141‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernstein 1997
- Bernstein SA, Williford SL. Intranasal desmopressin‐associated hyponatremia: a case report and literature review. Journal of Family Practice 1997;44(2):203‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Blackwell 1989
- Blackwell C. A guide to enuresis: A guide to treatment of enuresis for professionals. Bristol: ERIC, 1989:1‐86. [Google Scholar]
BNF 2002
- British National Formulary. British National Formulary. Vol. 43, London: British Medical Association and the Royal Pharmaceutical Society of Great Britain, March 2002. [ISBN: 0 85369 510 5 ISBN: 0 7279 1613 0] [Google Scholar]
Butler 1991
- Butler RJ. Establishment of working definitions in nocturnal enuresis. Archives of Diseases in Childhood 1991;66(2):267‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD, editors. Optimal search strategy for RCTs. Appendix 5c.2. Cochrane Reviewers Handbook 4.1.6 (updated January 2003). The Cochrane Library, Issue 1. Oxford: Update Software, 2003. [Google Scholar]
de Jonge 1973
- Jonge GA. Epidemiology of enuresis: A survey of the literature. In: Kolvin I, MacKeith RC, Meadow SR editor(s). Bladder control and enuresis. London: William Heinemann Medical Books, 1973. [Google Scholar]
Devlin 1991
- Devlin JB. Prevalence and risk factors for childhood nocturnal enuresis. Irish Medical Journal 1991;84(4):118‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Djurhuus 1992
- Djurhuus JC, Norgaard JP, Rittig S. Monosymptomatic bedwetting. Scandinavian Journal of Urology & Nephrology Supplementum. 141 1992;141:7‐19. [MEDLINE: ] [PubMed] [Google Scholar]
Eiberg 1995
- Eiberg H, Berendt I, Mohr J. Assignment of dominant inherited nocturnal enuresis (ENUR1) to chromosome 13q. Nature Genetics 1995;10(3):354‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fitzwater 1992
- Fitzwater D, Macknin ML. Risk/benefit ratio in enuresis therapy. Clinical Pediatrics 1992;31(5):308‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Forsythe 1974
- Forsythe WI, Redmond A. Enuresis and spontaneous cure rate. A study of 1129 enuretics. Archives of Disease in Childhood 1974;49(4):259‐63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glazener 2004a
- Glazener CMA, Evans JH, Peto RE. Alarm interventions for nocturnal enuresis in children. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD002911. DOI: 10.1002/14651858.CD002911.pub2] [DOI] [PubMed] [Google Scholar]
Glazener 2004b
- Glazener CMA, Evans JHC, Peto RE. Complex behavioural and educational interventions for nocturnal enuresis in children. Cochrane Database of Systematic Reviews 2004, Issue 1. [Art. No.: CD004668. DOI: 10.1002/14651858.CD004668] [DOI] [PubMed] [Google Scholar]
Glazener 2004c
- Glazener CMA, Evans JHC, Peto RE. Drugs for nocturnal enuresis in children (other than desmopressin and tricyclics). Cochrane Database of Systematic Reviews 2003, Issue 4. [Art. No.: CD002238. DOI: 10.1002/14651858.CD002238] [DOI] [PubMed] [Google Scholar]
Glazener 2004d
- Glazener CMA, Evans JE. Simple behavioural interventions for nocturnal enuresis in children. Cochrane Database of Systematic Reviews 2004, Issue 2. [Art. No.: CD003637. DOI: 10.1002/14651858.CD003637.pub2] [DOI] [PubMed] [Google Scholar]
Glazener 2004e
- Glazener CMA, Evans JH, Peto RE. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD002117. DOI: 10.1002/14651858.CD002117] [DOI] [PubMed] [Google Scholar]
Harris 1989
- Harris AS. Clinical experience with desmopressin: efficacy and safety in central diabetes insipidus and other conditions. Journal of Paediatrics 1989;114(4 pt 2):711‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hjalmas 1993
- Hjalmas K, Bengtsson B. Efficacy, safety, and dosing of desmopressin for nocturnal enuresis in Europe. Clinical Pediatrics 1993;Spec No:19‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Howe 1992
- Howe AC, Walker CE. Behavioural management of toilet training, enuresis and encopresis. Pediatric Clinics of North America 1992;39(3):413‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jarvelin 1989
- Jarvelin MR. Developmental history and neurological findings in enuretic children. Developmental Medicine & Child Neurology 1989;31(6):728‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jarvelin 1990
- Jarvelin MR, Huttunen NP, Seppanen J, Seppanen U, Moilanen I. Screening of urinary tract abnormalities among day and nightwetting children. Scandinavian Journal of Urology & Nephrology 1990;24(3):181‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koff 1995
- Koff SA. Why is desmopressin sometimes ineffective at curing bedwetting?. Scandinavian Journal of Urology & Nephrology 1995;173(Suppl):103‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Krantz 1994
- Krantz I, Jylkas E, Ahlberg BM, Wedel H. On the epidemiology of nocturnal enuresis‐a critical review of methods used in descriptive epidemiological studies on nocturnal enuresis. Scandinavian Journal of Urology & Nephrology 1994;163(Suppl):75‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Maizels 1993
- Maizels M, Gandhi K, Keating B, Rosenbaum D. Diagnosis and treatment for children who cannot control urination. Current Problems in Pediatrics 1993;23(10):402‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meadow 1989
- Meadow SR, Evans JH. Desmopressin for enuresis. British Medical Journal 1989;298(6688):1596‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moffatt 1989
- Moffatt ME. Nocturnal enuresis: psychologic implications of treatment and nontreatment. Journal of Pediatrics 1989;114(4 Pt 2):697‐704. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moffatt 1994
- Moffatt ME. Nocturnal enuresis‐is there a rationale for treatment?. Scandinavian Journal of Urology & Nephrology 1994;163:55‐66. [MEDLINE: ] [PubMed] [Google Scholar]
Morgan 1970
- Morgan R. The treatment of enuresis amongst child clients of a social services department. British Journal of Social Work 1970;9(2):217‐31. [Google Scholar]
Morgan 1993
- Morgan R. Guidelines in the Minimum Standards of Practice in the Treatment of Enuresis. Bristol: Enuresis Resource and Information Centre, 1993. [Google Scholar]
Norgaard 1993
- Norgaard JP, Djurhuus JC. The pathophysiology of enuresis in children and young adults. Clinical Pediatrics 1993;Spec No:5‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parkin 1972
- Parkin JM, Fraser MS. Poisoning as a complication of enuresis. Developmental Medicine & Child Neurology 1972;14(6):727‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robson 1994
- Robson WL, Leung AK. Side effects and complications of treatment with desmopressin for enuresis [Review]. Journal of the National Medical Association 1994;86(10):775‐8. [PMC free article] [PubMed] [Google Scholar]
Robson 1996
- Robson WL, Norgaard JP, Leung AK. Hyponatremia in patients with nocturnal enuresis treated with DDAVP. European Journal of Pediatrics 1996;155(11):959‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rushton 1996
- Rushton HG, Belman AB, Zaontz MR, Skoog SJ, Sihelnik S. The influence of small functional bladder capacity and other predictors on the response to desmopressin in the management of monosymptomatic nocturnal enuresis. Journal of Urology 1996;156(2 Pt 2):651‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rutter 1973
- Rutter M, Yule W, Graham P. Enuresis and behavioural deviance. Some epidemiological considerations. In: Kolvin I, MacKeith RC, Meadow SR editor(s). Bladder control and enuresis. London: William Heinemann Medical Books, 1973:137‐50. [Google Scholar]
Shaffer 1977
- Shaffer D. Enuresis. In: Rutter M editor(s). Child psychiatry: Modern approaches. Oxford: Blackwell Scientific Publications, 1977. [Google Scholar]
Warzak 1993
- Warzak WJ. Psychosocial implications of nocturnal enuresis. Clinical Pediatrics 1993;Spec No:38‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organisation. Nonorganic enuresis. The ICD‐10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: WHO, 1992. [Google Scholar]
References to other published versions of this review
Glazener 2000
- Glazener CMA, Evans JHC. Desmopression for nocturnal enuresis in children. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI] [PubMed] [Google Scholar]
Glazener 2002
- Glazener CMA, Evans JHC. Desmopressin for nocturnal enuresis in children. Cochrane Database of Systematic Reviews 2002, Issue 3. [Art. No.: CD002112. DOI: 10.1002/14651858.CD002112] [DOI] [PubMed] [Google Scholar]
Lister‐Sharp 1997
- Lister‐Sharp D, O'Meara S, Bradley M, Sheldon TA. A Systematic Review of the Effectiveness of Interventions for Managing Childhood Nocturnal Enuresis. Vol. CRD Report 11, York: NHS Centre for Reviews and Dissemination, University of York. York Publishing Services Ltd, 1997. [Google Scholar]


