Abstract
Neuregulin(Nrg)/ErbB and integrin signaling pathways are critical for the normal function of the embryonic and adult heart. Both systems activate several downstream signaling pathways, with different physiological outputs: cell survival, fibrosis, excitation-contraction coupling, myofilament structure, cell-cell and cell-matrix interaction. Activation of ErbB2 by Nrg1β in cardiomycytes or its overexpression in cancer cells induces phosphorylation of FAK (Focal Adhesion Kinase) at specific sites with modulation of survival, invasion and cell-cell contacts. FAK is also a critical mediator of integrin receptors, converting extracellular matrix alterations into intracellular signaling. Systemic FAK deletion is lethal and is associated with left ventricular non-compaction whereas cardiac restriction in adult hearts is well tolerated. Never the less, these hearts are more susceptible to stress conditions like trans-aortic constriction, hypertrophy, and ischemic injury. As FAK is both downstream and specifically activated by integrins and Nrg-1β, here we will explore the role of FAK in the heart as a protective factor and as possible mediator of the crosstalk between the ErbB and Integrin receptors.
Keywords: Neuregulin, Nrg1β, FAK, Integrin, cardiomyocytes, heart
2. Introduction
In 1995 three articles were contemporaneously published in Nature describing the effect of systemic deletion of Neuregulin(Nrg)-1, epidermal growth factor receptors ErbB2 and ErbB4 in mice. These studies demonstrated that Nrg/ErbB signaling is needed for the correct development of heart trabeculae, a structure responsible for the normal function of the embryonic heart[1–3]. Since then our knowledge has greatly increased and it is now clear that this signaling system is also active in the adult heart and is critical for its maintenance under stressed conditions. Specific deletion of ErbB2[4] and ErbB4[5] leads to spontaneous dilated cardiomyopathy associated with higher susceptibility to aortic banding. Both cardiac and cancer research have connected directly and indirectly Nrg-1β/ErbB to several signaling pathway, such as Phosphatidylinositol 3-Kinase (PI3K)/Akt, Mitogen-Activated Protein Kinase (MAPK)/ Extracellular signal-Regulated Kinase (Erk) 1/2, and the non-receptor tyrosine kinase Src/Focal Adhesion Kinase (FAK), and demonstrated its involvement in a wide variety of physiological outputs, including cardiac cell survival, migration, angiogenesis, cytoskeleton, and excitation contraction coupling( for a detailed review on these pathways in the heart see ref. [6]).
The primary role of integrins is to link the extracellular matrix (ECM) to the intracellular signaling. Deletion of β1 subunit, the most common in the heart, suggests that ECM is involved in the differentiation of cardiomyocytes during heart development [7]. Integrins are also critical for the maintenance of the adult heart both under normal and pathological conditions, as their deletion results in a spontaneous increase in fibrosis as well as induction of heart failure [8]. The non-receptor tyrosine kinase FAK is the main effector of integrins, converting changes in the extracellular matrix into intracellular signaling.
As FAK is both downstream and specifically activated by integrins and Nrg-1β, here we will explore the role of FAK in the heart as a protective factor and a possible mediator of the crosstalk between ErbB and Integrin receptors (Fig 1).
Fig 1. FAK activation and role in cardiomyocytes.
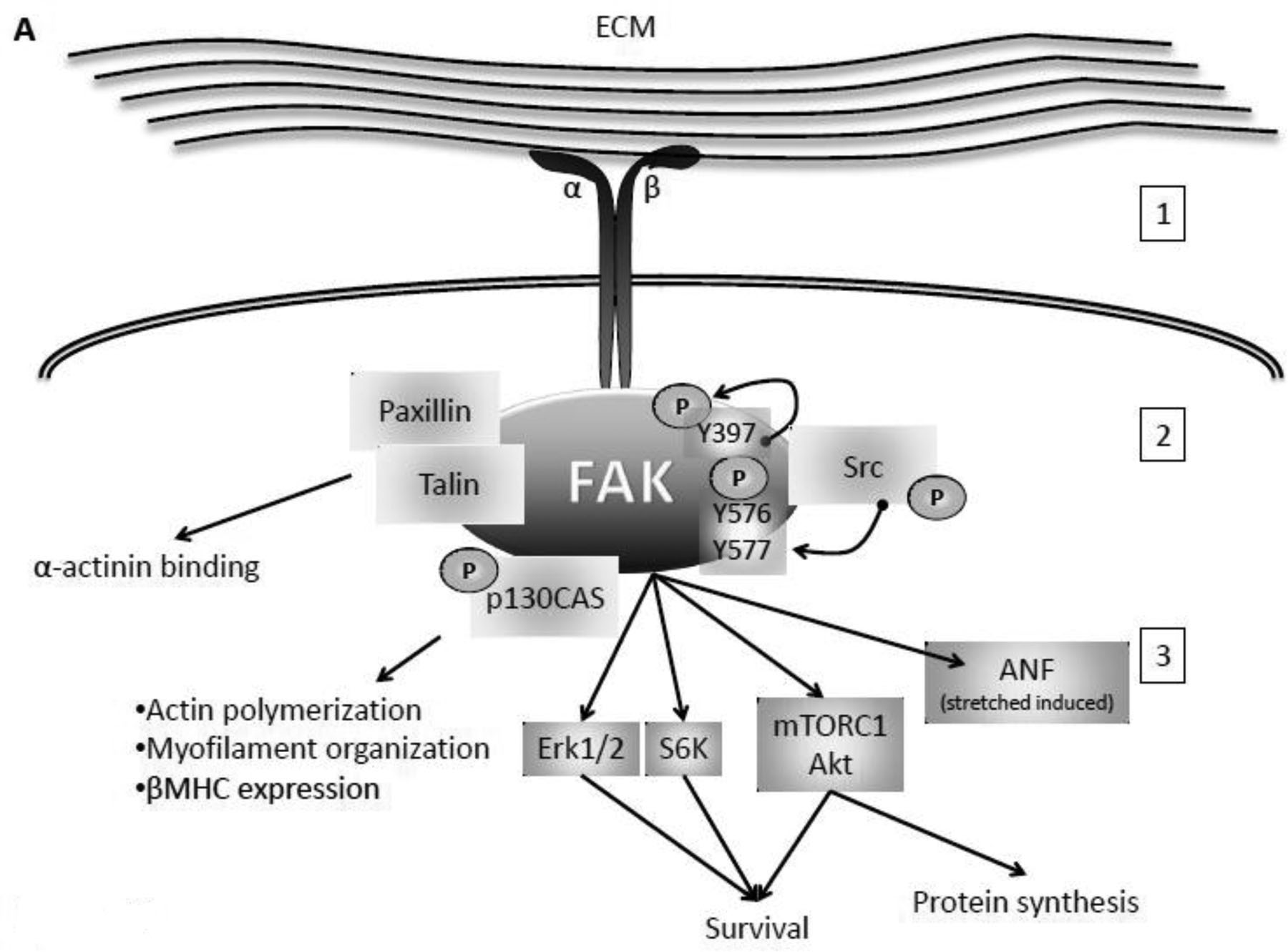
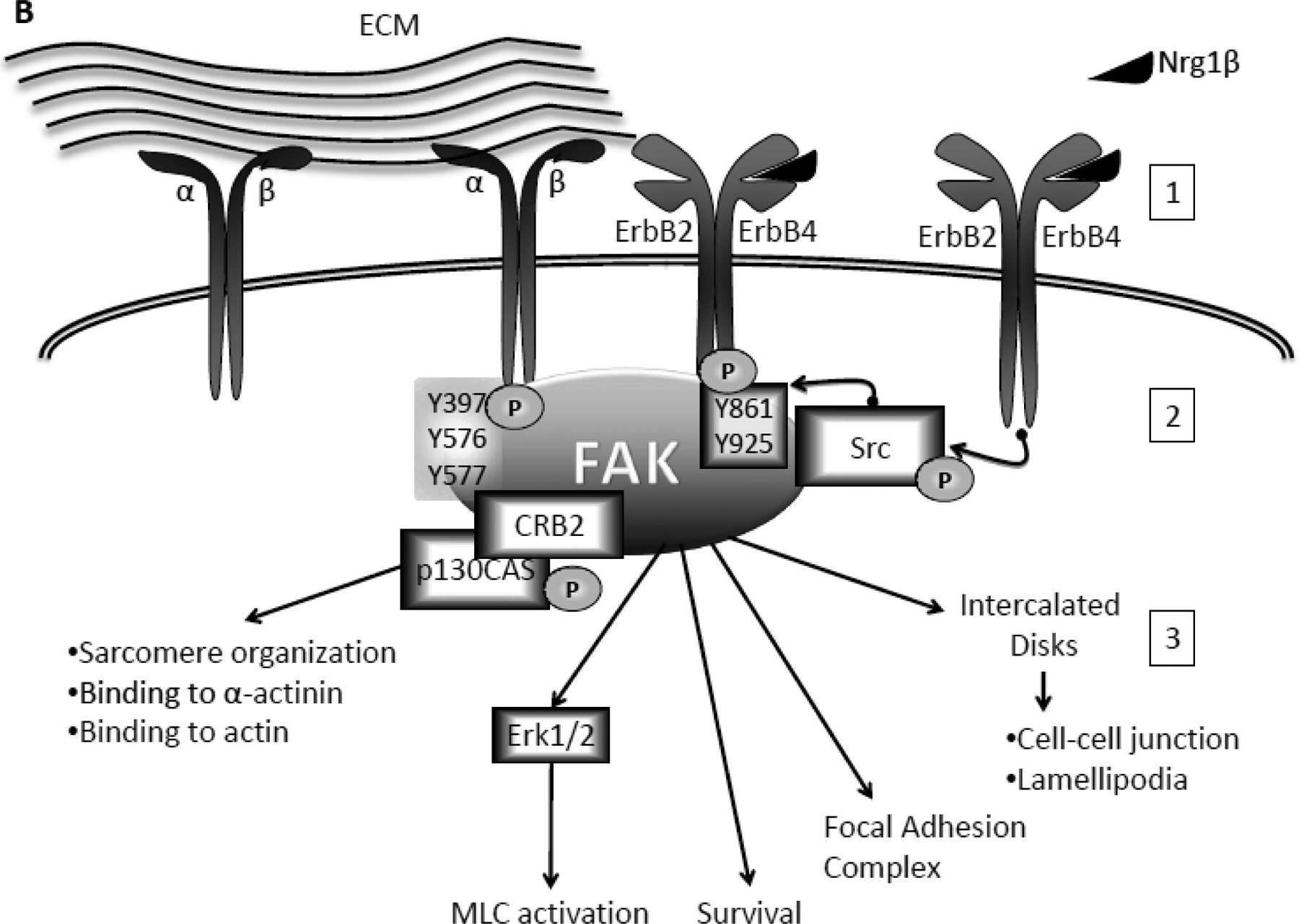
A. Integrin-dependent activation of FAK. Binding to the extracellular matrix (ECM) and mechanical stretch activates integrin receptors at the cell surface (1). To initiate intracellular signaling integrin dimer induces conformational changes in FAK and auto-phosphorylation on tyrosine (Y) 397. Src can then phosphorylate FAK at Y576 and Y577 in the activation loop (2). Activated FAK can then: interact with Paxillin and Talin to bind α-actinin; induce actin polymerization, myofilament organization, and expression of Myosine Heavy Chain (MHC) via p130CAS; and promote survival via Erk1/2, S6K, mTORC1, and Akt, protein synthesis via mTORC1 and Akt, and stretch induced expression of ANF (3). B. Nrg1β-specific phosphorylation of FAK and its role in cardiomyocytes. Upon binding to Nrg1β (1), the ErbB2/ErbB4 heterodimer induces phosphorylation of FAK at Y861 and Y925 via Src (2). Phosphorylated FAK: is involved in sarcomere organization and binding to actin and α-actinin via interaction with p130CAS and CRB adaptor proteins; induces activation of Myosin Light Chain (MLC) via Erk1/2; promotes myocyte survival and focal adhesion complex formation; and migrates to the intercalated disks where it promotes cell-cell interaction and lamellipodia formation (3).
3. Nrg-1β/ErbB2/ErbB4 signaling
3.1. Nrg-1β/ErbB dependent Akt and Erk1/2 signaling and their role in the heart.
Both Erk1/2 and Akt signaling pathways have been extensively studied in the heart and we will just briefly summarize these studies here (for a detailed review on these pathways as NRG-1β downstream effectors please refers to Pentassuglia and Sawyer, 2009, Experimental Cell Research: The role of Neuregulin-1β/ErbB signaling in the heart[6]). Several studies conducted so far demonstrate that both Erk1/2 and Akt mediate Nrg1β-dependent cell survival, metabolism, and growth in the heart under normal and stressed conditions. Postnatal cardiac-specific deletion of ErbB2 leads to spontaneous dilated cardio-myopathy and a higher susceptibility to stress stimuli [4]. Cardiac-specific deletion of both Grb2-associated binder (GAB) [9, 10] 1 and 2, scaffolding adaptor proteins that mediates Nrg1β/ErbB signaling, abolishes Nrg-1β induced phosphorylation of both Erk1/2 and Akt. Concomitantly these hearts show profound dilated features associated with deposition of both collagen and elastic fibers, and alterations at the cardiac vessels [9].
There is growing evidence that the Nrg-1β/ErbB2 signaling plays a critical role in conditions of stress. Ex-vivo ischemia reperfusion of isolated hearts in the Langendorff system induces Nrg-1β cleavage, activation and phosphorylation of the ErbB4 receptor and downstream signaling pathways. These data suggests that the ErbB receptors are possibly involved in cardiac recovery[11]. Nrg-1β preconditioning attenuates apoptotic cell death during ischemic injury as shown by a decrease in cleaved caspase-3 and an increase in the phosphorylation levels of Akt. Concomitant inhibition of PI3K signaling was able to block Nrg-1β-dependent cardioprotection [12]. In isolated adult myocytes pretreatment with Nrg-1β prevents doxorubicin-induced cell death. Akt inhibition blocks this effect, whereas a constitutively active form of Akt exerts a function similar to Nrg-1β itself [13]. Akt also mediates Nrg-1β/ErbB protection against reactive oxygen species (ROS). Nrg-1β pretreatment significantly decreases ROS in cultured myocytes treated with hydrogen peroxide, while inhibition of Akt abolishes this effect [14]. In mice treated with doxorubicin, Nrg-1β promotes survival and preservation of cTnI and cTnC from degradation in the heart via Akt signaling [15], further proving a critical role for Akt in Nrg-1β/ErbB-dependent survival.
Erk1/2 signaling activated by Nrg-1β has been implicated in the promotion of cardiomyocyte differentiation from embryonic stem cells. During the development of embryonic bodies there is a distinctive pattern of ErbB receptor expression. All cells of the embryonic body express ErbB2 but only the myocyte fraction expresses ErbB4, which is essential for their development and survival[16]. ErbB induced cardiomyocyte development requires the activation of Erk1/2, as the expression of either wild type or constitutively active MEK1 is sufficient to increase the number of cells expressing myosin heavy chain [17]. In both neonatal and adult myocytes, Erk1/2 mediates Nrg-1β-dependent hypertrophy, protein expression, and sarcomere structure [18–20]. The inhibition of ErbB2 or Erk1/2 leads to myofilament disarray both in adult and neonatal myocytes[18, 21]. These data support a role for the Nrg-1β/ErbB2/Erk1/2 signaling axis in the assembly and maintenance of the contractile apparatus in the heart.
3.2. Nrg-1β dependent FAK activation
FAK, a component of the Focal Adhesion Complex (FAC), interacts and regulates several structural and signaling proteins, including the Nrg-1β signaling pathway in the heart. FAK has three distinct domains: the N-teminal FERM (F for 4.1 protein, E for ezrin, R for radixin and M for moesin), which has autoinhibitory function[10, 22], a central kinase domain[23, 24], and a C-terminal Focal Adhesion Targeting (FAT) domain[25, 26]. The first step of FAK activation requires auto-phosphorylation of the tyrosine residues 397 induced by integrin activation (see paragraph 4.1). The FERM domain has an auto-inhibitory function and integrin activation leads to FAK binding to talin and paxillin via FAT. This induces conformational changes that lead to displacement of the FERM domain, releasing the autoinhibition; at this point FAK can autophosphorylate itself at Y397. This autophosphorylation induces Src binding and phosphorylation of Y576 and Y577 in the catalytic domain (Fig 1A) [27–29].
The different phosphorylation sites of FAK modulate either its own catalytic activity or the affinity for binding proteins. Phosphorylation of Y397 creates a motif recognized by SH2-domain containing proteins (PLCγ, SOCS, GRB7, P120, and p85 of PI3K)[30–33]. Phosphorylation at Y397 induces Src binding and activation of downstream signaling pathway through both FAK and Src[34] and promotes the recruitment of PI3K and p130CAS[33–36]. Src phosphorylation of FAK increases affinity for SH3-domain mediated binding of p130CAS and for SH2- domain mediated binding for CRB2 adaptor proteins[37, 38]. Y925 can also activate myosin light chain kinase via ERK2[39, 40]. The best-known downstream targets of FAK are p130CAS and Paxillin. Recent experiments show that FAK plays a role in FAC dynamics and modulation[41] and promotes maturation of FAC with inhibition of α-actinin binding to actin filaments[42]. FAK localization at the Z-line suggests a role in sarcomere organization as well [43].
In isolated adult rat ventricular myocytes (ARVM) Nrg-1β is able to activate the Src/FAK signaling pathway. Nrg-1β treatment induces phosphorylation of FAK at Y861 and Y925 that is most prominent at the sites of the intercalated disk (Fig 1B). This is associated with formation of lamellipodia and ultimately cell-cell junctions[44]. This signal may mediate the cardioprotective role of Nrg-1β in stress conditions. In isolated hearts, ischemic injury leads to Nrg-1β cleavage and ErbB4 as well as FAK phosphorylation [11]. Evidence collected in other tissues shows similar findings. In the brain Nrg-1β induces FAK activation via ErbB2/ErbB3 heterodimer [45]. In different type of tumors (brain, breast, and ovary) positive for the ErbB2 receptor FAK is activated at baseline conditions [46–48] and promotes tumor cell motility [49–51], proliferation [52], formation of FAC [53, 54], resistance to ErbB2 specific chemotherapeutic agents [55].
3.3. Role of FAK in cardiac development
Cardiac morphogenesis is one of the first events that takes place during embryonic development and requires the complex coordination of recruitment, differentiation, and proliferation of cardiac and cardiac precursors cells. Like the Nrg/ErbB pathway[1–3], FAK signaling is involved in the embryonic development of the heart from its early stages. Systemic deletion of FAK in mice is lethal and shows cardiac defects in early embriogenesis as the heart fails to separate the mesocardial and the endocardial layers and lethality is associated with left ventricular non-compaction[56]. During heart development, a set of cells, the Neuronal Crest Cells (NCCs), migrate from the neuronal tube toward the developing heart to participate in the maturation of the cardiac outflow tract in to the aorta and pulmonary trunk. FAK expression is critical for the differentiation of the NCCs into smooth muscle cells (SMCs), which participate in the development of the aortic arch arteries. The failure of NCCs to develop in to SMCs results in the regression of the developing aortic branches rather than a premature halting of the process [57]. Embryonic myocyte chemotaxis is also impaired, suggesting the involvement of FAK in myocyte migration towards the cushion mesenchyme [58, 59]. Similar to what is observed in vivo, FAK regulates cardiogenesis and migration in cultured embryonic stem cells. Inhibition of FAK phosphorylation leads to decreased cell migration, which stimulates ES cells to differentiate in cardiac lineages, as assessed by expression of α-MHC [60]. Cardiac specific deletion of FAK with the use of nkx2.5 promotor-driven Cre-recombinase induces rapid cyanosis and mice die 10 to 120 min after birth. Analysis of the embryonic cardiac tissue shows that FAK is reduced as early as E13.5 and it is almost absent at E18.5. Histological analysis shows defect in ventricular septation and in few cases the presence of a double-outlet right ventricle, thickening of the semilunar valve leaflets but normal trabeculation[58].
Similar to what has been observed in nkx2.5-driven FAK cardiac-specific deletion, the use of MLC2a-Cre also leads to embryonic death at an early stage of development. At E13.5 all embryos appear normal, but at E14.5 mice show total body edema and nonspecific focal hemorrhages associated with cardiac failure. Histological analysis shows a thinning in the myocardium, septum, and trabeculae. At E16.5 there are ventricular septa defects and thin ventricular walls along with embryonic lethality. Analysis of the tissue with electron microscopy reveals a dilation of the rough endoplasmatic reticulum, mitochondria with irregular or disrupted cristae, and thin disorganized myofibrils. At E14.5 there are also reduced numbers of mitotic cells present in the heart of the FAK cardiac-restricted mice compared with genetic and age matched mice. The few mice with cardiac specific FAK deletion that survived into adulthood are fertile and they have a normal lifespan, but examination of the heart shows eccentric right ventricle hypertrophy [61].
3.4. Cardioprotective role of FAK in the adult heart
Several studies conducted so far demonstrate that in the adult heart FAK mediates mechanical and hypertrophic signaling, and exerts a critical role in cardiac survival, adaptation, and protections of myofilament structure under conditions of stress [62–65]. Cardiac specific deletion of FAK in mice at a perinatal stage does not alter baseline cardiac function and hemodynamics [66], and there are no differences seen in the posterior and intraventricular septal wall thickness or LV chamber size [67]. However, when treated with Angiotensin (Ang) II or subjected to trans-aortic constriction (TAC), these mice develop eccentric hypertrophy associated with re-expression of skeletal-actin, Atrial Natriuretic Factor (ANF), Brain Natriuretic Peptide (BNP), beta Myosin Heavy Chain (MHC), and collagen I and VI. These mice also display increased fibrosis, but no increase in cell death. In contrast to these findings, expression of a truncated form of FAK increases the basal level of apoptosis [68]. RNA analysis shows that TAC-induced ANF expression is abolished in FAK deficient mice concomitant with an increase in alpha but not in beta MHC [67]. FAK deletion leads to disorganized myofibrils with increased interspace filled with large aggregates of swollen mitochondrial [66, 68]. Long term exposure to TAC leads to an increase in wet lung weight, decreased cardiac output, and increased interstitial fibrosis. FAK cardiac deficiency blocks ERK1/2 activation induced by adrenergic stimulation [67], and phosphorylation of both p130cas and paxillin is reduced [66, 69]. In aging mice FAK deficiency leads to spontaneous decrease of heart weight/body weight and myocyte cross-sectional area, increase thickness of LV posterior wall and fibrosis [67].
Hypertrophy induced by Angiotensin II is blocked by the expression of FRNK, a naturally occurring dominant negative isoform of FAK. In these myocytes ANP and NF-κB expression is decreased, as well as Erk1/2 and Akt basal phosphorylation [70]. Treatment with calcium chelators effectively blocks AngII induced phosphorylation of FAK, ANF expression, and decreases expression of fatty acid oxidation-related genes. Activation of the receptor PPARδ also blocks FAK-dependent activation of Erk1/2 but not of c-Jun N-terminal Kinase (JNK) [71]. In neonatal cardiomyocytes stimulation with hypertrophic agonists induces activation of FAK at S910, which can interact with paxillin and it is involved in sarcomere assembly, cell migration, and heart failure. Further analysis shows that this activation depends on Erk1/2 as well as Src/Erk5 and Protein Kinase C (PKC)δ/Erk5 [72]. FAK overexpression, in absence of other stimuli, leads to concentric hypertrophy, associated with increased heart size, β-MHC expression, and left ventricular wall thickening, without changes in the left ventricle diameter or fractional shortening. In contrast FAK overexpression during pressure overload exerts a cardio-protective role via Akt, mTORC1, S6K, and rpS6 signaling [73]. Pressure overload alone can induce FAK activation [74] and it associates with Src, Grb2 [75], and ARHGAP21 [76, 77].
FAK also plays a critical role in linking events initiated by mechanical stress during hypertrophic responses in cardiomyocytes. Mechanical stretch activates and changes the localization of FAK, from the nucleus to the myofilament [78], as well as increasing the phosphorylation of Erk1/2, and paxillin [79, 80]. FAK accumulated in myocytes of failing hearts in spontaneously hypertensive rats [81, 82] and it is phosphorylated by integrin receptors [64, 83]. Inhibition of FAK blocks stretch-induced ANF expression [78]. In cultured Neonatal Rat Ventricular Myocytes (NRVM) FAK is associated with Shp2 and after stretch this complex is significantly reduced. Stretch reduces protein tyrosine phosphatase Shp2 phosphatese activity, and its inactivation leads to increased basal FAK phosphorylation, cell size, and expression of β-MHC [84]. Depletion of FAK with siRNA or inhibition of Src with the kinase inhibitor PP2 blocks stretch induced activation of Erk1/2, Akt S473, and S6K [84].
FAK is also involved in cardiomyocyte survival in the setting of metabolic stress including ischemic injury. In isolated NRVM chemical inhibition of glycolysis and myocardial respirationinduces phosphorylation of FAK, its association with PI3K, and Akt activity [85]. Overexpression of FAK is cardioprotective during ischemic injury by experimental myocardial infarction. FAK overexpressing mice have smaller infarct area, higher ejection fraction and fractional shortening after 8 weeks of remodeling. Further analysis shows reduced apoptosis and increased NF-κB translocation into the nucleus and transcription activity [86]. FAK cardiac restricted deletion in mice subjected to transient ligation of LAD coronary artery results in a higher infarct size and cell death, as well as in a decrease in heart function, and activation of NF-κB survival pathway [87]. Similar results were observed in mice overexpressing FRNK [85].
Stretch reduces basal phosphorylation of FAK at Y861, but it is increased with concomitant inhibition of the AngII receptor. Overexpression of FRNK or disruption of integrin β1D abolishes basal and stretch-mediated phosphorylation of FAK and ERK1/2 [88]. Tension-mediated focal adhesion maturation is a critical step for myocytes in adaptation to mechanical tension. Localization of vinculin at focal adhesion sites in myofibroblast depends on extracellular matrix stiffness and myosin II. Myosin II is also able to modulate recruitment of vinculin via FAK-dependent phosphorylation of paxillin [89].
4. ErbB/FAK/Integrin interaction
4.1. The role of Integrins in the heart
Integrins are transmembrane receptors able to sense alterations in the extracellular matrix and translate them to the cytoskeleton. They are formed by two different chains, α and β, non-covalently associated. Both subunits are present in different splicing variants (18 for α and 8 for β) leading to more than 24 possible heterodimers[90, 91]. Each splicing variant and heterodimer has a specific expression pattern, unique for tissue type and developmental stage[92–95]. Integrins can regulate the expression levels and the activation status of ion channels, as well as initiating specific ion currents directly or through the Src tyrosine kinase signaling[96, 97]. Hormone[98, 99] and growth factor receptors[100, 101] often interact with integrins. Integrins are essential for growth factor receptors and hormone mediated cell survival[102, 103], DNA synthesis[104, 105], and chemotherapy resistance[106].
Alterations in the ECM and integrin expression have been associated with various cardiac conditions. It has been observed that accumulation of ECM components in the myocardium and coronary arteries leads to cardiac failure[107, 108]. In pressure overload, integrin receptors subtypes change, suggesting a role in mechano-transduction[109–112]. Restricted deletion of β1 in myocytes leads to myocardial fibrosis and development of spontaneous dilated cardiomyopathy in 6 month old mice, as well as an exaggerate response to pressure overload without evidences of cell death[8]. A more severe phenotype has been observed in transgenic mice overexpressing a dominant negative isoform of β1. These transgenic mice die at perinatal stage and their hearts display extensive fibrotic replacement [113].
Upon activation, integrins associate at focal adhesion sites and bind actin filaments. The interaction with actin is mediated by proteins with structural (talin and vinculin)[114, 115], signaling (Fak, Src, and PIPKγ)[116–118], and adaptor (p130CAS and melusin) functions[119–123]. One of the best characterized pathways is the Src/FAK signaling, which also promotes actin anchoring (see paragraph 3.2) [24].
4.2. Cross talk between integrins and ErbB receptors
Two different types of cross-talk between integrins and ErbB receptor tyrosine kinase (RTK) have been identified. The first is commonly called “collaborative”, where both integrins and RTK need to be activated by their respective ligand to form a cluster[124]. This interaction between RTK and integrins is mediated by FAK[44, 125]. In the second, called “direct”, integrins can directly phosphorylate RTK without the need of growth factors and FAK signaling[126, 127].
In cancer cells there is solid evidence for integrin/ErbB2 cross talk, whereas to date this has not been fully investigated in the heart. Cancer cells overexpressing both ErbB2 and integrin receptors α6β4 are highly aggressive and have a malignant phenotype[128]. In cell lines of breast carcinoma laminin induced phosphorylation of ErbB2 via integrin interaction[129]. Further analysis demonstrated that both integrins and ErbB2 co-localized[130, 131] and formed aggregates with tyrosine kinase proteins[44, 125]. These observations suggest a possible interaction between ErbB2 and integrin signaling. Expression of a constitutively active ErbB2 isoform in MFC-7 breast cancer cells leads to increase cell motility and it is associated with a higher expression of the integrin β1[132]. In human mammary and ovarian carcinoma cells the integrin receptor α6β4 co-immunoprecipitates with ErbB2. Further analysis demonstrated that upon binding to laminin α6β4 can also increase ErbB2 phosphorylation[128]. The co-activation of both receptors is required to induce PI3K activation and motility in NIH3T3 cells[124]. β4 integrin can also regulate ErbB2 dependent DNA synthesis[104] and ErbB2 translation[133], enhances ErbB2-dependendent expression of the growth factor VEGF, which in turn enhances tumour cell invasiveness[134, 135], and transactivates EGFR/ErbB2 signaling[133].
β1 integrin receptor is highly expressed in cardiomyocytes and is also the most abundant in the heart and may well interact with ErbB signaling according to literature in other cell types. Early on it was shown that in metastatic breast carcinoma cells cell adhesion is enhanced by activation of integrin β1[136]. In an epithelial tumor cell line overexpressing the ErbB2 receptor increases α5β1 expression and improves cell survival[137]. In earlier stages ErbB2 activation impairs spreading and adhesion on collagen surfaces by inactivating integrin β1 via PKB and PI3K/mTOR signaling[138, 139]. ErbB2 activation and overexpression can also induce scattering and apoptosis in human mammary epithelial cells cultured on collagen[140]. In contrast inhibition of laminin binding to integrin receptors (α6β4 or α3β1) sensitizes cancer cells toward ErbB2 specific cancer therapeutic agents Herceptin and Lapatinib[55].
In cardiac myocytes Nrg-1β induces specific phosphorylation of Src (Y215 and Y416) and FAK (Y867) and promotes the formation a protein complex between ErbB2 and Src, FAK, p130CAS, and paxillin[44]. These observations suggest the possibility of an ErbB/integrin cross-talk in cardiomyocytes. We hypothesize that the activation of FAK promotes the formation of an ErbB2/ErbB4/integrin complex, recruits and phosphorylates p130CAS, and modulates focal adhesion complex (FAC) and mechanical coupling (Fig 1). Further work will be necessary to fully explore this model in cardiac myocytes and understand the role that this plays in regulating cardiac structure and function.
Highlights.
Nrg/ErbB signaling is critical for cardiac protection under condition of stress.
Nrg/ErbB activates FAK signaling pathway in cardiac and cancer cells.
FAK is critical for cardiac differentiation and survival under condition of stress.
FAK mediated mechanical signal transduction initiated by integrins.
These observations suggest a possible ErbB/integrin cross-talk mediated by FAK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G, Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor, Nature, 378 (1995) 390–394. [DOI] [PubMed] [Google Scholar]
- [2].Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C, Requirement for neuregulin receptor erbB2 in neural and cardiac development, Nature, 378 (1995) 394–398. [DOI] [PubMed] [Google Scholar]
- [3].Meyer D, Birchmeier C, Multiple essential functions of neuregulin in development, Nature, 378 (1995) 386–390. [DOI] [PubMed] [Google Scholar]
- [4].Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J Jr., Chien KR, Lee KF, ErbB2 is essential in the prevention of dilated cardiomyopathy, Nat Med, 8 (2002) 459–465. [DOI] [PubMed] [Google Scholar]
- [5].Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, Lloyd KC, Hertig CM, Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle, Am J Physiol Heart Circ Physiol, 289 (2005) H1153–1160. [DOI] [PubMed] [Google Scholar]
- [6].Pentassuglia L, Sawyer DB, The role of Neuregulin-1beta/ErbB signaling in the heart, Exp Cell Res, 315 (2009) 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fassler R, Rohwedel J, Maltsev V, Bloch W, Lentini S, Guan K, Gullberg D, Hescheler J, Addicks K, Wobus AM, Differentiation and integrity of cardiac muscle cells are impaired in the absence of beta 1 integrin, J Cell Sci, 109 ( Pt 13) (1996) 2989–2999. [DOI] [PubMed] [Google Scholar]
- [8].Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS, Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure, Circ Res, 90 (2002) 458–464. [DOI] [PubMed] [Google Scholar]
- [9].Nakaoka Y, Nishida K, Narimatsu M, Kamiya A, Minami T, Sawa H, Okawa K, Fujio Y, Koyama T, Maeda M, Sone M, Yamasaki S, Arai Y, Koh GY, Kodama T, Hirota H, Otsu K, Hirano T, Mochizuki N, Gab family proteins are essential for postnatal maintenance of cardiac function via neuregulin-1/ErbB signaling, J Clin Invest, 117 (2007) 1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dunty JM, Gabarra-Niecko V, King ML, Ceccarelli DF, Eck MJ, Schaller MD, FERM domain interaction promotes FAK signaling, Mol Cell Biol, 24 (2004) 5353–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB, Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling, J Biol Chem, 279 (2004) 51141–51147. [DOI] [PubMed] [Google Scholar]
- [12].Fang SJ, Wu XS, Han ZH, Zhang XX, Wang CM, Li XY, Lu LQ, Zhang JL, Neuregulin-1 preconditioning protects the heart against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism, Chin Med J (Engl), 123 (2010) 3597–3604. [PubMed] [Google Scholar]
- [13].Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB, Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt, J Mol Cell Cardiol, 35 (2003) 1473–1479. [DOI] [PubMed] [Google Scholar]
- [14].Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, Zuppinger C, Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes, J Mol Cell Cardiol, 41 (2006) 845–854. [DOI] [PubMed] [Google Scholar]
- [15].Bian Y, Sun M, Silver M, Ho KK, Marchionni MA, Caggiano AO, Stone JR, Amende I, Hampton TG, Morgan JP, Yan X, Neuregulin-1 attenuated doxorubicin-induced decrease in cardiac troponins, Am J Physiol Heart Circ Physiol, 297 (2009) H1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Suk Kim H, Hidaka K, Morisaki T, Expression of ErbB receptors in ES cell-derived cardiomyocytes, Biochem Biophys Res Commun, 309 (2003) 241–246. [DOI] [PubMed] [Google Scholar]
- [17].Kim HS, Cho JW, Hidaka K, Morisaki T, Activation of MEK-ERK by heregulin-beta1 promotes the development of cardiomyocytes derived from ES cells, Biochem Biophys Res Commun, 361 (2007) 732–738. [DOI] [PubMed] [Google Scholar]
- [18].Pentassuglia L, Graf M, Lane H, Kuramochi Y, Cote G, Timolati F, Sawyer DB, Zuppinger C, Suter TM, Inhibition of ErbB2 by receptor tyrosine kinase inhibitors causes myofibrillar structural damage without cell death in adult rat cardiomyocytes, Exp Cell Res, 315 (2009) 1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA, NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK, Am J Physiol, 277 (1999) H2026–2037. [DOI] [PubMed] [Google Scholar]
- [20].Giraud MN, Fluck M, Zuppinger C, Suter TM, Expressional reprogramming of survival pathways in rat cardiocytes by neuregulin-1beta, J Appl Physiol, 99 (2005) 313–322. [DOI] [PubMed] [Google Scholar]
- [21].Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA, Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes, J Biol Chem, 273 (1998) 10261–10269. [DOI] [PubMed] [Google Scholar]
- [22].Jacamo RO, Rozengurt E, A truncated FAK lacking the FERM domain displays high catalytic activity but retains responsiveness to adhesion-mediated signals, Biochem Biophys Res Commun, 334 (2005) 1299–1304. [DOI] [PubMed] [Google Scholar]
- [23].Parsons JT, Focal adhesion kinase: the first ten years, J Cell Sci, 116 (2003) 1409–1416. [DOI] [PubMed] [Google Scholar]
- [24].Schlaepfer DD, Hauck CR, Sieg DJ, Signaling through focal adhesion kinase, Prog Biophys Mol Biol, 71 (1999) 435–478. [DOI] [PubMed] [Google Scholar]
- [25].Hildebrand JD, Schaller MD, Parsons JT, Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions, J Cell Biol, 123 (1993) 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dixon RD, Chen Y, Ding F, Khare SD, Prutzman KC, Schaller MD, Campbell SL, Dokholyan NV, New insights into FAK signaling and localization based on detection of a FAT domain folding intermediate, Structure, 12 (2004) 2161–2171. [DOI] [PubMed] [Google Scholar]
- [27].Schlaepfer DD, Mitra SK, Multiple connections link FAK to cell motility and invasion, Curr Opin Genet Dev, 14 (2004) 92–101. [DOI] [PubMed] [Google Scholar]
- [28].Cox BD, Natarajan M, Stettner MR, Gladson CL, New concepts regarding focal adhesion kinase promotion of cell migration and proliferation, J Cell Biochem, 99 (2006) 35–52. [DOI] [PubMed] [Google Scholar]
- [29].Liu G, Guibao CD, Zheng J, Structural insight into the mechanisms of targeting and signaling of focal adhesion kinase, Mol Cell Biol, 22 (2002) 2751–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Han DC, Guan JL, Association of focal adhesion kinase with Grb7 and its role in cell migration, J Biol Chem, 274 (1999) 24425–24430. [DOI] [PubMed] [Google Scholar]
- [31].Endo M, Yamashita T, Inactivation of Ras by p120GAP via focal adhesion kinase dephosphorylation mediates RGMa-induced growth cone collapse, J Neurosci, 29 (2009) 6649–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang X, Chattopadhyay A, Ji QS, Owen JD, Ruest PJ, Carpenter G, Hanks SK, Focal adhesion kinase promotes phospholipase C-gamma1 activity, Proc Natl Acad Sci U S A, 96 (1999) 9021–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Reiske HR, Kao SC, Cary LA, Guan JL, Lai JF, Chen HC, Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration, J Biol Chem, 274 (1999) 12361–12366. [DOI] [PubMed] [Google Scholar]
- [34].Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT, Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src, Mol Cell Biol, 14 (1994) 1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ohba T, Ishino M, Aoto H, Sasaki T, Interaction of two proline-rich sequences of cell adhesion kinase beta with SH3 domains of p130Cas-related proteins and a GTPase-activating protein, Graf, Biochem J, 330 ( Pt 3) (1998) 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Polte TR, Hanks SK, Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas, Proc Natl Acad Sci U S A, 92 (1995) 10678–10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Calalb MB, Zhang X, Polte TR, Hanks SK, Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src, Biochem Biophys Res Commun, 228 (1996) 662–668. [DOI] [PubMed] [Google Scholar]
- [38].Schlaepfer DD, Broome MA, Hunter T, Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins, Mol Cell Biol, 17 (1997) 1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ishii I, Tomizawa A, Kawachi H, Suzuki T, Kotani A, Koshushi I, Itoh H, Morisaki N, Bujo H, Saito Y, Ohmori S, Kitada M, Histological and functional analysis of vascular smooth muscle cells in a novel culture system with honeycomb-like structure, Atherosclerosis, 158 (2001) 377–384. [DOI] [PubMed] [Google Scholar]
- [40].Morla AO, Mogford JE, Control of smooth muscle cell proliferation and phenotype by integrin signaling through focal adhesion kinase, Biochem Biophys Res Commun, 272 (2000) 298–302. [DOI] [PubMed] [Google Scholar]
- [41].Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW, Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle, Am J Physiol, 277 (1999) C152–162. [DOI] [PubMed] [Google Scholar]
- [42].Cappello RE, Estrada-Gutierrez G, Irles C, Giono-Cerezo S, Bloch RJ, Nataro JP, Effects of the plasmid-encoded toxin of enteroaggregative Escherichia coli on focal adhesion complexes, FEMS Immunol Med Microbiol, 61 (2011) 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kovacic-Milivojevic B, Roediger F, Almeida EA, Damsky CH, Gardner DG, Ilic D, Focal adhesion kinase and p130Cas mediate both sarcomeric organization and activation of genes associated with cardiac myocyte hypertrophy, Mol Biol Cell, 12 (2001) 2290–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kuramochi Y, Guo X, Sawyer DB, Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes, J Mol Cell Cardiol, 41 (2006) 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vartanian T, Goodearl A, Lefebvre S, Park SK, Fischbach G, Neuregulin induces the rapid association of focal adhesion kinase with the erbB2-erbB3 receptor complex in schwann cells, Biochem Biophys Res Commun, 271 (2000) 414–417. [DOI] [PubMed] [Google Scholar]
- [46].Cance WG, Liu ET, Protein kinases in human breast cancer, Breast Cancer Res Treat, 35 (1995) 105–114. [DOI] [PubMed] [Google Scholar]
- [47].Ignatoski KM, Maehama T, Markwart SM, Dixon JE, Livant DL, Ethier SP, ERBB-2 overexpression confers PI 3’ kinase-dependent invasion capacity on human mammary epithelial cells, Br J Cancer, 82 (2000) 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vadlamudi RK, Sahin AA, Adam L, Wang RA, Kumar R, Heregulin and HER2 signaling selectively activates c-Src phosphorylation at tyrosine 215, FEBS Lett, 543 (2003) 76–80. [DOI] [PubMed] [Google Scholar]
- [49].Villa-Moruzzi E, Tyrosine phosphatases in the HER2-directed motility of ovarian cancer cells: Involvement of PTPN12, ERK5 and FAK, Anal Cell Pathol (Amst), 34 (2011) 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Roovers K, Wagner S, Storbeck CJ, O’Reilly P, Lo V, Northey JJ, Chmielecki J, Muller WJ, Siegel PM, Sabourin LA, The Ste20-like kinase SLK is required for ErbB2-driven breast cancer cell motility, Oncogene, 28 (2009) 2839–2848. [DOI] [PubMed] [Google Scholar]
- [51].Ponnusamy MP, Singh AP, Jain M, Chakraborty S, Moniaux N, Batra SK, MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells, Br J Cancer, 99 (2008) 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lahlou H, Sanguin-Gendreau V, Frame MC, Muller WJ, Focal adhesion kinase contributes to proliferative potential of ErbB2 mammary tumour cells but is dispensable for ErbB2 mammary tumour induction in vivo, Breast Cancer Res, 14 (2012) R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xu M, Bower KA, Chen G, Shi X, Dong Z, Ke Z, Luo J, Ethanol enhances the interaction of breast cancer cells over-expressing ErbB2 with fibronectin, Alcohol Clin Exp Res, 34 (2010) 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xu Y, Benlimame N, Su J, He Q, Alaoui-Jamali MA, Regulation of focal adhesion turnover by ErbB signalling in invasive breast cancer cells, Br J Cancer, 100 (2009) 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yang XH, Flores LM, Li Q, Zhou P, Xu F, Krop IE, Hemler ME, Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists, Cancer Res, 70 (2010) 2256–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S, Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK, Oncogene, 11 (1995) 1989–1995. [PubMed] [Google Scholar]
- [57].Vallejo-Illarramendi A, Zang K, Reichardt LF, Focal adhesion kinase is required for neural crest cell morphogenesis during mouse cardiovascular development, J Clin Invest, 119 (2009) 2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hakim ZS, DiMichele LA, Doherty JT, Homeister JW, Beggs HE, Reichardt LF, Schwartz RJ, Brackhan J, Smithies O, Mack CP, Taylor JM, Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment, Mol Cell Biol, 27 (2007) 5352–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zajac B, Hakim ZS, Cameron MV, Smithies O, Taylor JM, Quantification of myocyte chemotaxis: a role for FAK in regulating directional motility, Methods Mol Biol, 843 (2012) 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hakuno D, Takahashi T, Lammerding J, Lee RT, Focal adhesion kinase signaling regulates cardiogenesis of embryonic stem cells, J Biol Chem, 280 (2005) 39534–39544. [DOI] [PubMed] [Google Scholar]
- [61].Peng X, Wu X, Druso JE, Wei H, Park AY, Kraus MS, Alcaraz A, Chen J, Chien S, Cerione RA, Guan JL, Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice, Proc Natl Acad Sci U S A, 105 (2008) 6638–6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Taylor JM, Rovin JD, Parsons JT, A role for focal adhesion kinase in phenylephrine-induced hypertrophy of rat ventricular cardiomyocytes, J Biol Chem, 275 (2000) 19250–19257. [DOI] [PubMed] [Google Scholar]
- [63].Eble DM, Strait JB, Govindarajan G, Lou J, Byron KL, Samarel AM, Endothelin-induced cardiac myocyte hypertrophy: role for focal adhesion kinase, Am J Physiol Heart Circ Physiol, 278 (2000) H1695–1707. [DOI] [PubMed] [Google Scholar]
- [64].Laser M, Willey CD, Jiang W, Cooper G.t., Menick DR, Zile MR, Kuppuswamy D, Integrin activation and focal complex formation in cardiac hypertrophy, J Biol Chem, 275 (2000) 35624–35630. [DOI] [PubMed] [Google Scholar]
- [65].Pham CG, Harpf AE, Keller RS, Vu HT, Shai SY, Loftus JC, Ross RS, Striated muscle-specific beta(1D)-integrin and FAK are involved in cardiac myocyte hypertrophic response pathway, Am J Physiol Heart Circ Physiol, 279 (2000) H2916–2926. [DOI] [PubMed] [Google Scholar]
- [66].Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, Chen J, Chien K, Gu H, Guan JL, Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice, J Clin Invest, 116 (2006) 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].DiMichele LA, Doherty JT, Rojas M, Beggs HE, Reichardt LF, Mack CP, Taylor JM, Myocyte-restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy, Circ Res, 99 (2006) 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rafiq K, Kolpakov MA, Abdelfettah M, Streblow DN, Hassid A, Dell’Italia LJ, Sabri A, Role of protein-tyrosine phosphatase SHP2 in focal adhesion kinase down-regulation during neutrophil cathepsin G-induced cardiomyocytes anoikis, J Biol Chem, 281 (2006) 19781–19792. [DOI] [PubMed] [Google Scholar]
- [69].Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM, GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes, Circ Res, 90 (2002) 1282–1289. [DOI] [PubMed] [Google Scholar]
- [70].Qin J, Liu ZX, FAK-related nonkinase attenuates hypertrophy induced by angiotensin-II in cultured neonatal rat cardiac myocytes, Acta Pharmacol Sin, 27 (2006) 1159–1164. [DOI] [PubMed] [Google Scholar]
- [71].Lee KS, Park JH, Lee S, Lim HJ, Park HY, PPARdelta activation inhibits angiotensin II induced cardiomyocyte hypertrophy by suppressing intracellular Ca2+ signaling pathway, J Cell Biochem, 106 (2009) 823–834. [DOI] [PubMed] [Google Scholar]
- [72].Chu M, Iyengar R, Koshman YE, Kim T, Russell B, Martin JL, Heroux AL, Robia SL, Samarel AM, Serine-910 phosphorylation of focal adhesion kinase is critical for sarcomere reorganization in cardiomyocyte hypertrophy, Cardiovasc Res, 92 (2011) 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Clemente CF, Xavier-Neto J, Dalla Costa AP, Consonni SR, Antunes JE, Rocco SA, Pereira MB, Judice CC, Strauss B, Joazeiro PP, Matos-Souza JR, Franchini KG, Focal adhesion kinase governs cardiac concentric hypertrophic growth by activating the AKT and mTOR pathways, J Mol Cell Cardiol, 52 (2012) 493–501. [DOI] [PubMed] [Google Scholar]
- [74].Franchini KG, Torsoni AS, Soares PH, Saad MJ, Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart, Circ Res, 87 (2000) 558–565. [DOI] [PubMed] [Google Scholar]
- [75].Domingos PP, Fonseca PM, Nadruz W Jr., Franchini KG, Load-induced focal adhesion kinase activation in the myocardium: role of stretch and contractile activity, Am J Physiol Heart Circ Physiol, 282 (2002) H556–564. [DOI] [PubMed] [Google Scholar]
- [76].Borges L, Bigarella CL, Baratti MO, Crosara-Alberto DP, Joazeiro PP, Franchini KG, Costa FF, Saad ST, ARHGAP21 associates with FAK and PKCzeta and is redistributed after cardiac pressure overload, Biochem Biophys Res Commun, 374 (2008) 641–646. [DOI] [PubMed] [Google Scholar]
- [77].Fonseca PM, Inoue RY, Kobarg CB, Crosara-Alberto DP, Kobarg J, Franchini KG, Targeting to C-terminal myosin heavy chain may explain mechanotransduction involving focal adhesion kinase in cardiac myocytes, Circ Res, 96 (2005) 73–81. [DOI] [PubMed] [Google Scholar]
- [78].Torsoni AS, Constancio SS, Nadruz W Jr., Hanks SK, Franchini KG, Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes, Circ Res, 93 (2003) 140–147. [DOI] [PubMed] [Google Scholar]
- [79].Senyo SE, Koshman YE, Russell B, Stimulus interval, rate and direction differentially regulate phosphorylation for mechanotransduction in neonatal cardiac myocytes, FEBS Lett, 581 (2007) 4241–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sbroggio M, Bertero A, Velasco S, Fusella F, De Blasio E, Bahou WF, Silengo L, Turco E, Brancaccio M, Tarone G, ERK1/2 activation in heart is controlled by melusin, focal adhesion kinase and the scaffold protein IQGAP1, J Cell Sci, 124 (2011) 3515–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yi XP, Zhou J, Huber L, Qu J, Wang X, Gerdes AM, Li F, Nuclear compartmentalization of FAK and FRNK in cardiac myocytes, Am J Physiol Heart Circ Physiol, 290 (2006) H2509–2515. [DOI] [PubMed] [Google Scholar]
- [82].Yi XP, Wang X, Gerdes AM, Li F, Subcellular redistribution of focal adhesion kinase and its related nonkinase in hypertrophic myocardium, Hypertension, 41 (2003) 1317–1323. [DOI] [PubMed] [Google Scholar]
- [83].Umar S, Hessel M, Steendijk P, Bax W, Schutte C, Schalij M, van der Wall E, Atsma D, van der Laarse A, Activation of signaling molecules and matrix metalloproteinases in right ventricular myocardium of rats with pulmonary hypertension, Pathol Res Pract, 203 (2007) 863–872. [DOI] [PubMed] [Google Scholar]
- [84].Marin TM, Clemente CF, Santos AM, Picardi PK, Pascoal VD, Lopes-Cendes I, Saad MJ, Franchini KG, Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/Src and mTOR pathways, Circ Res, 103 (2008) 813–824. [DOI] [PubMed] [Google Scholar]
- [85].Wei H, Vander Heide RS, Heat stress activates AKT via focal adhesion kinase-mediated pathway in neonatal rat ventricular myocytes, Am J Physiol Heart Circ Physiol, 295 (2008) H561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cheng Z, DiMichele LA, Hakim ZS, Rojas M, Mack CP, Taylor JM, Targeted focal adhesion kinase activation in cardiomyocytes protects the heart from ischemia/reperfusion injury, Arterioscler Thromb Vasc Biol, 32 (2012) 924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hakim ZS, DiMichele LA, Rojas M, Meredith D, Mack CP, Taylor JM, FAK regulates cardiomyocyte survival following ischemia/reperfusion, J Mol Cell Cardiol, 46 (2009) 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, Dostal DE, Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase, J Mol Cell Cardiol, 43 (2007) 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM, Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation, J Cell Biol, 188 (2010) 877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Arnaout MA, Mahalingam B, Xiong JP, Integrin structure, allostery, and bidirectional signaling, Annu Rev Cell Dev Biol, 21 (2005) 381–410. [DOI] [PubMed] [Google Scholar]
- [91].Takada Y, Ye X, Simon S, The integrins, Genome Biol, 8 (2007) 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cavani A, Zambruno G, Marconi A, Manca V, Marchetti M, Giannetti A, Distinctive integrin expression in the newly forming epidermis during wound healing in humans, J Invest Dermatol, 101 (1993) 600–604. [DOI] [PubMed] [Google Scholar]
- [93].Rahilly MA, Fleming S, Differential expression of integrin alpha chains by renal epithelial cells, J Pathol, 167 (1992) 327–334. [DOI] [PubMed] [Google Scholar]
- [94].Virtanen I, Laitinen A, Tani T, Paakko P, Laitinen LA, Burgeson RE, Lehto VP, Differential expression of laminins and their integrin receptors in developing and adult human lung, Am J Respir Cell Mol Biol, 15 (1996) 184–196. [DOI] [PubMed] [Google Scholar]
- [95].Wu JE, Santoro SA, Differential expression of integrin alpha subunits supports distinct roles during lung branching morphogenesis, Dev Dyn, 206 (1996) 169–181. [DOI] [PubMed] [Google Scholar]
- [96].Arcangeli A, Becchetti A, Complex functional interaction between integrin receptors and ion channels, Trends Cell Biol, 16 (2006) 631–639. [DOI] [PubMed] [Google Scholar]
- [97].Kawasaki J, Davis GE, Davis MJ, Regulation of Ca2+-dependent K+ current by alphavbeta3 integrin engagement in vascular endothelium, J Biol Chem, 279 (2004) 12959–12966. [DOI] [PubMed] [Google Scholar]
- [98].Basak S, Dhar R, Das C, Steroids modulate the expression of alpha4 integrin in mouse blastocysts and uterus during implantation, Biol Reprod, 66 (2002) 1784–1789. [DOI] [PubMed] [Google Scholar]
- [99].Cody V, Davis PJ, Davis FB, Molecular modeling of the thyroid hormone interactions with alpha v beta 3 integrin, Steroids, 72 (2007) 165–170. [DOI] [PubMed] [Google Scholar]
- [100].Vellon L, Menendez JA, Lupu R, AlphaVbeta3 integrin regulates heregulin (HRG)-induced cell proliferation and survival in breast cancer, Oncogene, 24 (2005) 3759–3773. [DOI] [PubMed] [Google Scholar]
- [101].Yao K, Tan J, Ye P, Wang K, Xu W, ShenTu X, Tang X, Integrin beta1-mediated signaling is involved in transforming growth factor-beta2-promoted migration in human lens epithelial cells, Mol Vis, 13 (2007) 1769–1776. [PubMed] [Google Scholar]
- [102].Haenssen KK, Caldwell SA, Shahriari KS, Jackson SR, Whelan KA, Klein-Szanto AJ, Reginato MJ, ErbB2 requires integrin alpha5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells, J Cell Sci, 123 (2010) 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Agouni A, Sourbier C, Danilin S, Rothhut S, Lindner V, Jacqmin D, Helwig JJ, Lang H, Massfelder T, Parathyroid hormone-related protein induces cell survival in human renal cell carcinoma through the PI3K Akt pathway: evidence for a critical role for integrin-linked kinase and nuclear factor kappa B, Carcinogenesis, 28 (2007) 1893–1901. [DOI] [PubMed] [Google Scholar]
- [104].Kawano S, Mizutani K, Miyata M, Ikeda W, Takai Y, Interaction of integrin alpha(6)beta(4) with ErbB3 and implication in heregulin-induced ErbB3/ErbB2-mediated DNA synthesis, Genes Cells, 15 (2010) 995–1001. [DOI] [PubMed] [Google Scholar]
- [105].Scarlett A, Parsons MP, Hanson PL, Sidhu KK, Milligan TP, Burrin JM, Thyroid hormone stimulation of extracellular signal-regulated kinase and cell proliferation in human osteoblast-like cells is initiated at integrin alphaVbeta3, J Endocrinol, 196 (2008) 509–517. [DOI] [PubMed] [Google Scholar]
- [106].Huang C, Park CC, Hilsenbeck SG, Ward R, Rimawi MF, Wang YC, Shou J, Bissell MJ, Osborne CK, Schiff R, beta1 integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib, Breast Cancer Res, 13 (2011) R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Berk BC, Fujiwara K, Lehoux S, ECM remodeling in hypertensive heart disease, J Clin Invest, 117 (2007) 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bonapace S, Rossi A, Cicoira M, Golia G, Zanolla L, Franceschini L, Conte L, Marino P, Zardini P, Vassanelli C, Aortic stiffness correlates with an increased extracellular matrix turnover in patients with dilated cardiomyopathy, Am Heart J, 152 (2006) 93 e91–96. [DOI] [PubMed] [Google Scholar]
- [109].Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F, Silengo L, Tarone G, Lembo G, Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload, Nat Med, 9 (2003) 68–75. [DOI] [PubMed] [Google Scholar]
- [110].Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S, Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands, Proc Natl Acad Sci U S A, 98 (2001) 1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].van der Wees CG, Bax WH, van der Valk EJ, van der Laarse A, Integrin stimulation induces calcium signalling in rat cardiomyocytes by a NO-dependent mechanism, Pflugers Arch, 451 (2006) 588–595. [DOI] [PubMed] [Google Scholar]
- [112].Suryakumar G, Kasiganesan H, Balasubramanian S, Kuppuswamy D, Lack of beta3 integrin signaling contributes to calpain-mediated myocardial cell loss in pressure-overloaded myocardium, J Cardiovasc Pharmacol, 55 (2010) 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Keller RS, Shai SY, Babbitt CJ, Pham CG, Solaro RJ, Valencik ML, Loftus JC, Ross RS, Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance, Am J Pathol, 158 (2001) 1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Critchley DR, Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion, Biochem Soc Trans, 32 (2004) 831–836. [DOI] [PubMed] [Google Scholar]
- [115].Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C, Vinculin controls focal adhesion formation by direct interactions with talin and actin, J Cell Biol, 179 (2007) 1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Sieg DJ, Hauck CR, Schlaepfer DD, Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration, J Cell Sci, 112 ( Pt 16) (1999) 2677–2691. [DOI] [PubMed] [Google Scholar]
- [117].Bertagnolli ME, Hudson LA, Stetsenko GY, Selective association of the tyrosine kinases Src, Fyn, and Lyn with integrin-rich actin cytoskeletons of activated, nonaggregated platelets, Biochem Biophys Res Commun, 260 (1999) 790–798. [DOI] [PubMed] [Google Scholar]
- [118].Chao WT, Daquinag AC, Ashcroft F, Kunz J, Type I PIPK-alpha regulates directed cell migration by modulating Rac1 plasma membrane targeting and activation, J Cell Biol, 190 (2010) 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, et al. , Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs, J Biol Chem, 270 (1995) 15398–15402. [DOI] [PubMed] [Google Scholar]
- [120].Ohmori T, Yatomi Y, Inoue K, Satoh K, Ozaki Y, Tyrosine dephosphorylation, but not phosphorylation, of p130Cas is dependent on integrin alpha IIb beta 3-mediated aggregation in platelets: implication of p130Cas involvement in pathways unrelated to cytoskeletal reorganization, Biochemistry, 39 (2000) 5797–5807. [DOI] [PubMed] [Google Scholar]
- [121].Sansing HA, Sarkeshik A, Yates JR, Patel V, Gutkind JS, Yamada KM, Berrier AL, Integrin alphabeta1, alphavbeta, alpha6beta effectors p130Cas, Src and talin regulate carcinoma invasion and chemoresistance, Biochem Biophys Res Commun, 406 (2011) 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Vuori K, Ruoslahti E, Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix, J Biol Chem, 270 (1995) 22259–22262. [DOI] [PubMed] [Google Scholar]
- [123].Critchley DR, Biochemical and structural properties of the integrin-associated cytoskeletal protein talin, Annu Rev Biophys, 38 (2009) 235–254. [DOI] [PubMed] [Google Scholar]
- [124].Gambaletta D, Marchetti A, Benedetti L, Mercurio AM, Sacchi A, Falcioni R, Cooperative signaling between alpha(6)beta(4) integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion, J Biol Chem, 275 (2000) 10604–10610. [DOI] [PubMed] [Google Scholar]
- [125].Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD, FAK integrates growth-factor and integrin signals to promote cell migration, Nat Cell Biol, 2 (2000) 249–256. [DOI] [PubMed] [Google Scholar]
- [126].Han J, Jenq W, Kefalides NA, Integrin alpha2beta1 recognizes laminin-2 and induces C-erb B2 tyrosine phosphorylation in metastatic human melanoma cells, Connect Tissue Res, 40 (1999) 283–293. [DOI] [PubMed] [Google Scholar]
- [127].Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, Godovac-Zimmermann J, Conti A, Schaefer E, Beguinot L, Tacchetti C, Gaggini P, Silengo L, Tarone G, Defilippi P, Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines, J Biol Chem, 277 (2002) 9405–9414. [DOI] [PubMed] [Google Scholar]
- [128].Falcioni R, Antonini A, Nistico P, Di Stefano S, Crescenzi M, Natali PG, Sacchi A, Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines, Exp Cell Res, 236 (1997) 76–85. [DOI] [PubMed] [Google Scholar]
- [129].Tagliabue E, Ardini E, Pellegrini R, Campiglio M, Bufalino R, Jeschke M, Groner B, Colnaghi MI, Menard S, Laminin activates the p185HER2 oncoprotein and mediates growth inhibition of breast carcinoma cells, Br J Cancer, 74 (1996) 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Campiglio M, Tagliabue E, Srinivas U, Pellegrini R, Martignone S, Menard S, Colnaghi MI, Lombardi L, Marchisio PC, Colocalization of the p185HER2 oncoprotein and integrin alpha 6 beta 4 in Calu-3 lung carcinoma cells, J Cell Biochem, 55 (1994) 409–418. [DOI] [PubMed] [Google Scholar]
- [131].Mocanu MM, Fazekas Z, Petras M, Nagy P, Sebestyen Z, Isola J, Timar J, Park JW, Vereb G, Szollosi J, Associations of ErbB2, beta1-integrin and lipid rafts on Herceptin (Trastuzumab) resistant and sensitive tumor cell lines, Cancer Lett, 227 (2005) 201–212. [DOI] [PubMed] [Google Scholar]
- [132].Lauritzen G, Stock CM, Lemaire J, Lund SF, Jensen MF, Damsgaard B, Petersen KS, Wiwel M, Ronnov-Jessen L, Schwab A, Pedersen SF, The Na+/H+ exchanger NHE1, but not the Na+, HCO3(−) cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2, Cancer Lett, 317 (2012) 172–183. [DOI] [PubMed] [Google Scholar]
- [133].Yoon SO, Shin S, Lipscomb EA, A novel mechanism for integrin-mediated ras activation in breast carcinoma cells: the alpha6beta4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling, Cancer Res, 66 (2006) 2732–2739. [DOI] [PubMed] [Google Scholar]
- [134].Fan J, Cai B, Zeng M, Hao Y, Giancotti FG, Fu BM, Integrin beta4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF, Ann Biomed Eng, 39 (2011) 2223–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG, Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis, Cell, 126 (2006) 489–502. [DOI] [PubMed] [Google Scholar]
- [136].Adelsman MA, McCarthy JB, Shimizu Y, Stimulation of beta1-integrin function by epidermal growth factor and heregulin-beta has distinct requirements for erbB2 but a similar dependence on phosphoinositide 3-OH kinase, Mol Biol Cell, 10 (1999) 2861–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Spangenberg C, Lausch EU, Trost TM, Prawitt D, May A, Keppler R, Fees SA, Reutzel D, Bell C, Schmitt S, Schiffer IB, Weber A, Brenner W, Hermes M, Sahin U, Tureci O, Koelbl H, Hengstler JG, Zabel BU, ERBB2-mediated transcriptional up-regulation of the alpha5beta1 integrin fibronectin receptor promotes tumor cell survival under adverse conditions, Cancer Res, 66 (2006) 3715–3725. [DOI] [PubMed] [Google Scholar]
- [138].Lindberg LE, Hedjazifar S, Baeckstrom D, c-erbB2-induced disruption of matrix adhesion and morphogenesis reveals a novel role for protein kinase B as a negative regulator of alpha(2)beta(1) integrin function, Mol Biol Cell, 13 (2002) 2894–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hedjazifar S, Jenndahl LE, Shimokawa H, Baeckstrom D, PKB mediates c-erbB2-induced epithelial beta1 integrin conformational inactivation through Rho-independent F-actin rearrangements, Exp Cell Res, 307 (2005) 259–275. [DOI] [PubMed] [Google Scholar]
- [140].Baeckstrom D, Lu PJ, Taylor-Papadimitriou J, Activation of the alpha2beta1 integrin prevents c-erbB2-induced scattering and apoptosis of human mammary epithelial cells in collagen, Oncogene, 19 (2000) 4592–4603. [DOI] [PubMed] [Google Scholar]


