Abstract
Purpose
To conduct a systematic review of preclinical and clinical peer-reviewed evidence linking alterations in oxidative stress biomarkers or outcome measures that were also prevalent in specific age-related lower urinary tract (LUT) disorders.
Methods
PubMed, Scopus, CINAHL, and Embase were searched for peer-reviewed studies published between January 2000 and March 2021. Animal and human studies that reported on the impact of oxidative stress in age-related LUT disorders through structural or functional changes in the LUT and changes in biomarkers were included. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol was followed.
Results
Of 882 articles identified, 21 studies (13 animal; 8 human) met inclusion criteria. Across LUT disorders, common structural changes were increased bladder and prostate weights, ischemic damage, nerve damage and detrusor muscle hypertrophy; common functional changes included decreased bladder contraction, increased bladder sensation and excitability, decreased perfusion, and increased inflammation. The disorders were associated with increased levels of biomarkers of oxidative stress that provided evidence of either molecular damage, protective mechanisms against oxidative stress, neural changes, or inflammation. In all cases, the effect on biomarkers and enzymes was greater in aged groups compared to younger groups.
Conclusions
Increased oxidative stress, often associated with mitochondrial dysfunction, plays a significant role in the pathogenesis of age-related LUT disorders and may explain their increasing prevalence. This systematic review identifies potential markers of disease progression and treatment opportunities; further research is warranted to evaluate these markers and the mechanisms by which these changes may lead to age-related LUT disorders.
Keywords: Oxidative stress, Lower urinary tract symptoms, Aging, Inflammation, Urologic diseases
• HIGHLIGHTS
- Age-related lower urinary tract disorders are associated with changes in biomarkers of oxidative stress and mitochondrial dysfunction that can be subcategorized as evidence of oxidative stress induced molecular damage, protective mechanisms against oxidative stress, inflammation, and neural changes.
INTRODUCTION
Over half of adults have experienced lower urinary tract (LUT) symptoms leading to many disorders, with prevalence expected to increase by at least 18% within the next decade [1,2]. Since LUT disorders cause burdensome symptoms, impacting quality of life [3], evaluation of the mechanisms of these disorders becomes important for management and treatment. While studies have examined some pathophysiological mechanisms, limited evidence exists for the role aging plays in these disorders [4].
Age-related LUT disorders are linked to ischemia, inflammation, and alterations in hormone levels (e.g., melatonin, vasopressin); oxidative stress and mitochondrial dysfunction connect these to aging [5-7]. Oxidative stress describes oxidant-antioxidant balance disturbances in cells due to increases in reactive oxygen species (ROS) or decreases in total antioxidant capacity (TAC). Excessive ROS causes oxidative damage to macromolecules through free radicals. With increasing age, mitochondria, key organelles in cellular respiration, may develop issues with metabolic capacity, which increases ROS, leading to cellular damage and apoptosis, mitochondrial DNA damage, inflammation, and fibrosis [4,8]. Identifying mechanisms and commonalities of LUT disorders can provide insight on pathogenesis and treatments. Inclusion of both clinical and preclinical (animal) studies were necessary to provide an in-depth analysis of specific cellular changes which strengthens translational potential.
Our goal was to conduct a systematic review of preclinical and clinical peer-reviewed evidence linking alterations in oxidative stress biomarkers or outcome measures that were also prevalent in specific age-related LUT disorders.
MATERIALS AND METHODS
This systematic review was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and checklist [9,10].
Literature Search
In March 2021, we designed and executed literature searches in PubMed, Scopus, CINAHL, and Embase for peer-reviewed studies indexed between 01/01/2000 and 03/01/2021. This date range was used in order to include relevant and recent studies. The search string included MeSH terms and keywords such as “lower urinary tract symptoms,” “ischemia,” and additional LUT disorders and “reactive oxygen species,” “mitochondrial dysfunction,” and other terms describing oxidative stress.
Study Selection
Two authors (LK and SG) independently screened articles for studies that met the following inclusion criteria in the review: (1) Study evaluated oxidative stress in LUT disorders; (2) Study was an original article published between January 2000 and March 2021; (3) Study evaluated any age-related LUT disorder (e.g., benign prostatic hyperplasia [BPH], overactive bladder [OAB], bladder outlet obstruction [BOO]); (4) Study was preclinical dealing with human/animal subjects or clinical dealing with human participants; (5) Study assessed results by appropriate statistical methods (e.g., analysis of variance, t-tests).
In title and abstract review, articles that did not meet the inclusion criteria were excluded (Fig. 1). Full-text articles of potential studies were screened and studies that focused on primary mitochondrial defects, had insufficient data (e.g., case studies), were narrative reviews, or fell under previous exclusion criteria were excluded.
Fig. 1.
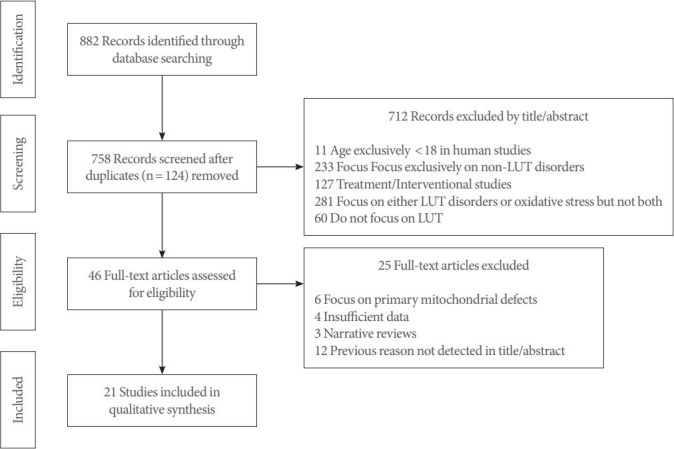
Study selection flow diagram showing the study selection process using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). All articles were screened using inclusion and exclusion criteria, by title/abstract review first and fulltext analysis next. All screened studies were published between January 2000 and March 2021. LUT, lower urinary tract.
BOO and changes in smooth muscle tone that can accompany BPH often results in LUTS. Since LUTS include storage disturbances (e.g., daytime urinary urgency, nocturia) and voiding disturbances (e.g., urinary hesitancy, weak stream), pathological processes such as BPH and symptoms complex such as OAB were all included.
Data Extraction
A data extraction sheet was created to record the study authors/name, publication year, sample characteristics (human/animal, sample size, age), study characteristics (design, duration, and outcomes measured), LUT disorder (s) evaluated, structural and functional changes, and changes in biomarkers. Missing information was retrieved by searching through cited protocols. Discrepancies in the review process and data extraction were resolved by consensus and with input from additional authors (JPW and LAB). Included studies were grouped based on the nature of the study (animal or human) and then organized in alphabetical order by the first author’s last name. Studies were labeled as 1–21 based on this ordering.
Assessment of Study Quality
Two authors (LK and SG) independently assessed the quality of the included studies using the Gradings of Recommendations, Assessment, Development, and Evaluations (GRADE) criteria and level of evidence was rated according to the criteria provided by the Oxford Centre for Evidence-Based Medicine (OCEBM) [11,12]. The GRADE criteria are suitable for animal studies in addition to human studies [13]. Studies with an assessment result of low quality were not excluded to avoid selection bias and allow for inclusion of more samples to strengthen findings.
RESULTS
The literature search yielded 882 articles. After removing duplicates, the titles and abstracts of 758 articles were reviewed, of which 46 studies were selected for full-text review. A total of 21 studies were selected for analysis (Fig. 1). These studies, published between 2003 and 2019, described oxidative stress in agerelated LUT disorders by examining the impact of ROS and mitochondrial dysfunction. Of the 21 studies, studies 1–13 were preclinical studies; study 8 also examined human prostate tissue [14-26]. Studies 14–21 were exclusively human studies (Table 1) [27-34].
Table 1.
Study characteristics for articles reporting on oxidative stress in age-related lower urinary tract disorders
| Study No. | Study | Number | Age | Study design | Study duration | Outcomes measured |
|---|---|---|---|---|---|---|
| Animal studies | ||||||
| 1 | Aikawa et al. (2003) [14] | 48 Rats | 3–12 Months | Experimental | Days | 1. ROS induced changes |
| 2. Bladder contractility | ||||||
| 3. MDA levels | ||||||
| 2 | Kirpatovsky et al. (2013) [15] | 25 Rats | 6–12 Months | Experimental | Days | 1. ROS induced changes |
| 2. Extra- and intracellular structure damage | ||||||
| 3. Effect of antioxidants | ||||||
| 4. Functional state of bladder detrusor | ||||||
| 3 | Lin et al. (2011) [16] | 16 Rabbits (100% male) | 15–20 Weeks | Experimental | 8 Weeks | 1. ROS induced changes |
| 2. MDA, TAC, and urinary 8-OHdG levels | ||||||
| 3. Bladder weight | ||||||
| 4 | Nomiya et al. (2012) [17] | 54 Rats (100% male) | 4 Months | Experimental | 8 Weeks | 1. ROS induced changes |
| 2. Cystometric changes | ||||||
| 3. 8-OHdG and N-(hexanoyl) lysine levels | ||||||
| 5 | Radu et al. (2011) [18] | 12 Rabbits (100% male) | 13–15 Weeks | Experimental | Days | 1. ROS induced changes |
| 2. Bladder contractility | ||||||
| 3. MDA levels | ||||||
| 6 | Sezginer et al. (2019) [19] | 30 Rats | 6–12 Months | Experimental | 6 Weeks | 1. ROS induced changes |
| 2. Bladder contractility and weight | ||||||
| 3. MDA, NF-kB, Nrf2, and hypoxia-inducible factors levels | ||||||
| 7 | Su et al. (2016) [20] | 10 Rats (100% male) | 2–12 Months | Experimental | 8 Weeks | 1. ROS induced changes |
| 2. Cystometric changes | ||||||
| 3. Proteomic analysis | ||||||
| 8 | Vital et al. (2016) [21] | Human and rat prostate tissuesa) | Men: older with BPH Rats: 2–18 months | Experimental | 1.5 Years | 1. ROS induced changes |
| 2. Prostate weight | ||||||
| 3. 8-OHdG levels | ||||||
| 9 | Witthaus et al. (2015) [22] | 24 Rats (100% male) | 2–12 Months | Experimental | 8 Weeks | 1. ROS induced changes |
| 2. Cystometric changes | ||||||
| 3. MDA, AOPP, and PI3K/Akt levels | ||||||
| 10 | Yang et al. (2017) [23] | 16 Rats (100% male) | 2–12 Months | Experimental | 8 Weeks | 1. ROS induced changes |
| 2. Cystometric changes | ||||||
| 3. Nrf2, heat shock protein 70, glucose-regulated protein 75, PI3K/Akt levels | ||||||
| 11 | Yuan et al. (2011) [24] | 24 Rats | 3–6 Months | Experimental | 6 Weeks | 1. ROS induced changes |
| 2. Bladder weight | ||||||
| 3. MDA levels | ||||||
| 4. Superoxide dismutase and nitric oxide activity | ||||||
| 12 | Zhang et al. (2014) [25] | 18 Rabbits (100% male) | 10–20 Weeks | Experimental | 8 Weeks | 1. ROS induced changes |
| 2. Cystometric changes | ||||||
| 3. MDA, AOPP, and P2X receptor level | ||||||
| 13 | Zhao et al. (2016) [26] | 32 Rats (100% male) | 2–12 Months | Experimental | 16 Weeks | 1. ROS induced changes |
| 2. Cytometric changes | ||||||
| 3. Muscarinic receptor levels | ||||||
| Human studies | ||||||
| 14 | Antunes-Lopes et al. (2019) [27] | 25 Males | > 60 Years | Case control | 13 Months | 1. ROS induced changes |
| 2. IPSS score | ||||||
| 3. Urinary nerve growth factor levels | ||||||
| 15 | Averbeck et al. (2018) [28] | 38 Patients (100% male) | ≥ 50 Years; mean: 66.4 | Prospective cohort | 2 Years | 1. ROS induced changes |
| 2. MDA levels | ||||||
| 3. Urodynamic parameters | ||||||
| 16 | Azadzoi et al. (2011) [29] | Human bladder tissue | N/A | Experimental | N/A | 1. ROS induced changes |
| 2. MDA, AOPP, 8-isoprostane, and nitrotyrosine levels | ||||||
| 3. Superoxide dismutase activity | ||||||
| 17 | Ener et al. (2015) [30] | 41 Females | Mean control: 42.0 years | Case control | 35 Months | 1. ROS induced changes |
| Mean disorder: 43.6 years | 2. TAC, IMA, immunoglobulin E, and C-reactive protein levels | |||||
| 18 | Guzel et al. (2012) [31] | 79 Patients (100% male; 25 BPH, 23 malignant prostate cancer, 16 LGPIN, 15 HGPIN) | 49–72 Years | Case control | N/A | 1. ROS induced changes |
| 2. Levels of Pb, Cd, and MDA, in whole blood and prostate tissue | ||||||
| 19 | Keske et al. (2019) [32] | 67 Patients (0% male; 38 detrusor overactivity, 29 healthy) | Mean: 42.7 years | Prospective cohort | 1.5 Years | 1. ROS induced changes |
| 2. Ischemia modified albumin and TAC levels | ||||||
| 20 | Merendino et al. (2003) [33] | 44 Patients (100% male; 1,022 BPH, 22 healthy,) | 55–79 Years | Prospective cohort | Days | 1. ROS induced changes |
| Mean control: 62.1 years | 2. MDA and prostate specific antigen levels | |||||
| Mean BPH: 65.8 years | 3. Prostate inflammation and modifications | |||||
| 21 | Ren et al. (2015) [34] | 60 Patients (100% male) | Control tissues: 37–46 years | Case control | N/A | 1. ROS induced changes |
| 2. Cell apoptosis | ||||||
| BPH tissues: 67–86 years | 3. Hypoxia-inducible factor, AR gene, vascular endothelial growth factor, IL-8 levels | |||||
ROS, reactive oxygen species; MDA, malondialdehyde; TAC, total antioxidant capacity; 8-OhdG, 8-hydroxy-2’-deoxyguanosine; NF-kB, nuclear factor kappa B; Nrf2, nuclear factor erythroid 2-related factor 2; AOPP, advanced oxidation protein products; PI3K/Akt, phosphoinositide 3-kinase/protein kinase B; IPSS, International Prostate System Score; IMA, ischemia modified albumin; LGPIN, low-grade prostatic intraepithelial neoplasia; HGPIN, high-grade prostatic intraepithelial neoplasia; BPH, benign prostatic hyperplasia; IL-8, interleukin-8; P2X, purinoceptor; N/A, not available.
Study 8 characterizes as both an animal and human study.
Study Design
The animal studies analyzed rats or rabbits with the sample size ranging from 12–54 (age range, 2–18 months). The human studies had population sizes of 25–79 patients with sex representation ranging from 100% male/0% female–0% male/100% female (age range, 37–79 years). All animal studies were experimental and had durations of days–1.5 years [21]. Human study designs were either experimental (study 8 and 16) [21,29], prospective cohort (studies 15, 19, and 20) [28,32,33], or case control studies (studies 14, 17–18, and 21) [27,30,31,34]. For prospective cohort studies, the duration ranged from days–2 years (Table 1) [28,32,33].
Outcomes Assessed
Structural LUT properties (e.g., weight, degeneration, cellular) were assessed in studies 2–4, 6–9, 11–13, and 16 [15-17,19-22,24-26,29]. Functional LUT properties (e.g., contractility, tension, uroflow) were assessed in studies 1, 2, 4–7, 9–10, 12–15, and 17–21 [14,15,17-20,22,23,25-28,30-34]. All studies examined oxidative stress biomarkers and some explored inflammatory biomarkers (Table 1).
Quality Assessment
Based on the GRADE criteria, which factors in an evaluation of publication bias, a quality of evidence rating of low (studies 13–17) [26-30], moderate (studies 1, 2, 4, 5, 7–9, 11, 12, 18, and 19) [14,15,17,18,20-22,24,25,31,32], or high (studies 3, 6, 10, 20, and 21) [16,19,23,33,34] was given (Table 2). With the OCEBM criteria applied to human clinical studies, 3 studies (15, 19, and 20) [28,32,33] were level 2b and 4 studies (14, 17, 18, and 21) [27,30,31,34] were level 3b.
Table 2.
Assessment of study quality using the GRADE quality assessment criteria
| Study | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Quality rating |
|---|---|---|---|---|---|---|
| 1 | Not serious | Not serious | Serious | Not serious | Undetected | Moderate |
| 2 | Not serious | Not serious | Serious | Not serious | Undetected | Moderate |
| 3 | Not serious | Not serious | Not serious | Not serious | Undetected | High |
| 4 | Not serious | Serious | Not serious | Not serious | Undetected | Moderate |
| 5 | Not serious | Not serious | Serious | Not serious | Undetected | Moderate |
| 6 | Not serious | Not serious | Not serious | Not serious | Undetected | High |
| 7 | Not serious | Not serious | Serious | Not serious | Undetected | Moderate |
| 8 | Not serious | Not serious | Serious | Not serious | Undetected | Moderate |
| 9 | Not serious | Serious | Not serious | Not serious | Undetected | Moderate |
| 10 | Not serious | Not serious | Not serious | Not serious | Undetected | High |
| 11 | Not serious | Not serious | Serious | Not serious | Undetected | Moderate |
| 12 | Not serious | Not serious | Serious | Not serious | Undetected | Moderate |
| 13 | Not serious | Serious | Serious | Not serious | Undetected | Low |
| 14 | Not serious | Serious | Serious | Serious | Undetected | Low |
| 15 | Serious | Not serious | Serious | Serious | Undetected | Low |
| 16 | Not serious | Serious | Serious | Not serious | Undetected | Low |
| 17 | Not serious | Serious | Serious | Not serious | Undetected | Low |
| 18 | Not serious | Serious | Not serious | Not serious | Undetected | Moderate |
| 19 | Not serious | Serious | Not serious | Not serious | Undetected | Moderate |
| 20 | Not serious | Not serious | Not serious | Not serious | Undetected | High |
| 21 | Not serious | Not serious | Not serious | Not serious | Undetected | High |
LUT Changes
Evaluated LUT disorders included BPH (studies 1, 8, 15, 18, 20, and 21) [14,21,28,31,33,34], BOO/partial bladder outlet obstruction (PBOO) (studies 3, 5–6, 11, and 15) [16,18,19,24,28], ischemia-reperfusion (I/R) injury (studies 4–5, 7, 9–10, 12–14, and 16–17) [17,18,20,22,23,25-27,29,30], acute urinary retention (AUR) (study 2) [15], prostate neoplasia and cancer (study 18) [31], and detrusor overactivity (DO) (study 19) [32] (Table 3). In studies evaluating BPH, structural changes included increased prostate weight and stromal thickening; functional changes included decreased bladder contractions and increased bladder sensitivity, prostate inflammation, pro-neoplastic genes, and uroflow changes. In studies evaluating BOO or PBOO, structural changes included increased bladder weight, metabolic dysfunction, collagen deposits, and detrusor muscle hypertrophy; the functional change was decreased contractility. In studies evaluating I/R, structural changes included arterial fibrosis/thickening, nerve damage, mitochondrial damage, and collagen deposition; functional changes included increased voiding frequency, micturition frequency, and International Prostate System Score and decreased voided volume, micturition interval, bladder contractility and compliance, perfusion, and intravesical pressure fluctuations. Study 2 showed that oxidative stress in AUR caused structural changes such as deteriorating bladder blood supply, inflammation, intracellular structural damage, and epithelial cell degeneration; the functional change was increased detrusor excitability [15]. Study 19 examined patients with DO and showed that ischemia and oxidative damage led to this overactivity (Table 3) [32].
Table 3.
Evaluation of lower urinary tract and biomarker changes associated with oxidative stress
| Study No. | Study | Lower urinary tract disorder (s) evaluated | Lower urinary tract structural and functional changes | Molecular changes |
|---|---|---|---|---|
| Animal studies | ||||
| 1 | Aikawa et al. (2003) [14] | BPH | Functional: | MDA↑as H2O2↑and age↑ |
| - H2O2 caused dose dependent↓in maximal contraction of bladder strips. | MDA levels (pmol/mg protein) in 12-month-old vs. 3-month-old rats | |||
| - Older rat bladders were more sensitive to H2O2 damage. | Control: 400 vs. 420 | |||
| 0.25% H2O2: 1,000 vs. 800 | ||||
| 0.50% H2O2: 1,200 vs. 1,000 | ||||
| 1.00% H2O2: 1,600 vs. 1,400 | ||||
| 2 | Kirpatovsky et al. (2013) [15] | AUR | Structural: | ROS↑ |
| - Extra- and intracellular structure damage; antioxidants can↓severity. | ROS levels (arbitrary units via dichlorofluorescein fluorescence) in control vs. AUR | |||
| - AUR is associated with deterioration of the bladder blood supply. | ||||
| Functional: | Mucosa: 0.5 vs. 17.8 | |||
| - Detrusor excitability. | Detrusor: 4.9 vs. 34.7 | |||
| 3 | Lin et al. (2011) [16] | PBOO | Structural: | MDA↑, TAC↓, and 8-OHdG↑ |
| - PBOO mediates metabolic dysfunction and oxidative damage in bladder smooth muscle. | Levels in control, 4 weeks, 8 weeks | |||
| MDA (μM): 25-30, 45, 65 | ||||
| - Bladder weight↑due to PBOO (control: 2 g, 2 weeks: 7 g, 4 weeks: 9 g, 8 weeks: 12 g). | TAC (μmol/L): 2,500, 1,700, 1,500 | |||
| 8-OHdG (ng/creatinine): 150, 350, 375 | ||||
| 4 | Nomiya et al. (2012) [17] | I/R | Structural: | 8-OHdG↑, N-(hexanoyl) lysine↑(immunohistochemical staining; qualitative observation) |
| - Thickening and fibrosis in iliac artery and bladder arterioles. | ||||
| Functional: | ||||
| - 24- Hour void frequency↑(11.5 vs. 17.5). | ||||
| - VV↓(1.39 mL vs. 0.85 mL). | ||||
| - Micturition interval↓(7.5 min vs. 4.68 min). | ||||
| 5 | Radu et al. (2011) [18] | I/R; PBOO | Functional: | MDA↑due to ischemia-reperfusion |
| - Mitochondrial oxidative damage, associated with↓contractility. | MDA levels (μm/mg) in control vs. ischemia/reperfusion | |||
| Muscle mitochondria: 6 vs. 12 | ||||
| Muscle homogenate: 7 vs. 14 | ||||
| Mucosa mitochondria: 4 vs. 23 | ||||
| Mucosa homogenate: 17 vs. 30 | ||||
| 6 | Sezginer et al. (2019) [19] | PBOO | Structural: | MDA↑, NF-kB↑, Nrf2↑, HIF-1a↑, HIF-2B/Arnt2↑ |
| - Bladder weight↑in rats with severe obstruction (2–2.5×greater). | Levels in control vs. PBOO | |||
| MDA (nM/g protein): 15 vs. 31 | ||||
| Functional: | NF-kB mRNA expression (RFUs): 100 vs. 200 | |||
| - Contractile responses↓in bladder strips in severe obstruction (~82% smaller). | Nrf2 mRNA expression (RFUs): 100 vs. 180 | |||
| HIF-1a (HIF-1a/GAPDH): 0.6 vs. 1.1 | ||||
| HIF-2B/Arnt2 (Arnt2/GAPDH): 0.5 vs. 0.75 | ||||
| 7 | Su et al. (2016) [20] | I/R | Structural: | Ischemic bladder proteins↓(172 upregulated proteins and 527 downregulated proteins) |
| - Swollen mitochondria, mitochondrial membrane↓, mitochondrial granules↓, collagen deposition↑. | ||||
| Functional: | ||||
| - Spontaneous contractions. | ||||
| - Bladder blood perfusion in mL/min/100 g↓(9.8 vs. 3.8). | ||||
| - Bladder compliance in mL/cm H2O↓(0.132 vs. 0.093). | ||||
| 8 | Vital et al. (2016) [21] | BPH | Structural: | 8-OHdG↑ |
| - BPH↑prostate weight (< 12 months: 80 mg vs. 100 mg; > 12 months: 100 mg vs. 150 mg). | 8-OHdG levels (ng/5 μg DNA) in control vs. BPH | |||
| - Stromal thickening. | 1.0 vs. 1.3 | |||
| 9 | Witthaus et al. (2015) [22] | I/R | Structural: | MDA↑, AOPP↑, PI3K/Akt↑ |
| - Swollen mitochondria with degraded granules, mitochondrial membrane↓in bladder tissue. | Levels in control, sham, ischemia | |||
| Functional: | MDA (pmol/mg): 17, 16, 27 | |||
| - Spontaneous contractile activity of the bladder. | AOPP (μM): 40, 50, 78 | |||
| - MF per 10 hours of sleep time↑(6.3 vs. 10.1). | PI3K/Akt (optical density): 1.0, 0.9, 1.7 | |||
| - Total urine production per 24 hours↑(13.2 mL vs. 19.9 mL). | ||||
| - Premicturition pressure↑(11.6 cm H2O vs. 15.7 cm H2O). | ||||
| - Bladder compliance↓(0.134 mL/cm H2O vs. 0.099 mL/cm H2O). | ||||
| 10 | Yang et al. (2017) [23] | I/R | Functional: | Nrf2 activity↓, heat shock protein 70↑, glucose-regulated protein 75↑, PI3K/Akt↑ |
| - Altered void patterns. | ||||
| - BBF↓in mL/m/100- g tissue (10.2 vs. 4.6). | Levels in control vs ischemia | |||
| - MF↑(11.7 vs. 17.6). | Nrf2 activity 25% control | |||
| - VV↓(1.30 mL vs. 0.86 mL). | Heat shock protein 70 (ng/mL): 0.5 vs. 1.9 | |||
| - BC↓(1.66 mL vs. 1.20 mL). | Glucose-related protein 75 (relative density): 0.4 vs. 1.0 | |||
| - Mitochondrial respiration rate↓in nmol/min/μg (0.68 vs. 0.38). | PI3K/Akt (relative density): 0.20 vs. 0.58 | |||
| 11 | Yuan et al. (2011) [24] | PBOO | Structural: | MDA↑, superoxide dismutase↓, nitric oxide synthase↓in PBOO |
| - PBOO is associated with↑bladder weight (control: 0.14 g, 3 weeks: 0.37 g, 6 weeks: 0.70 g). | Levels in control, 3 weeks PBOO, 6 weeks PBOO | |||
| - Collagen deposits and hypertrophy. | MDA (nmol/mg-prot): 0.24, 0.32, 0.52 | |||
| Superoxide dismutase activity (U/mg-prot): 17.67, 14.88, 11.72 | ||||
| Nitric oxide synthase activity(U/mg-prot): 1.373, 0.616, 0.558 | ||||
| 12 | Zhang et al. (2014) [25] | I/R | Structural: | MDA↑, AOPP↑, P2X↑ |
| - Intimal thickening and luminal narrowing of iliac arteries. | Levels in control vs. ischemia | |||
| Functional: | MDA (pmol/mg): 15 vs. 30 | |||
| - BBF↓in mL/min/100 g (7.5 vs. 3.2). | AOPP (μM): 38 vs. 70 | |||
| - BC↓(24.9 mL vs. 16.2 mL). | P2X levels (optical density): | |||
| - Spontaneous contractions/10 min↑(1.2 vs. 7.4). | P2X1: 0.3 vs. 0.5, P2X2: 0.5 vs. 0.7, P2X3: 0.4 vs. 0.7, P2X4: 0.5 vs. 0.7, P2X5: 0.3 vs. 0.5, P2X7: 0.2 vs. 0.3 | |||
| 13 | Zhao et al. (2016) [26] | I/R | Structural: | After 8 weeks, muscarinic M2 expression↑; after 16 weeks, muscarinic M1 and M2 expression↑, M3 expression↓ |
| - Swollen, degenerating axons with collagen invasion of nerve fibers. | Relative density in sham vs. ischemia | |||
| - Neural density↓. | 8 Weeks M1: 0.5 vs. 0.7 (Insignificant) | |||
| Functional: | 8 Weeks M2: 0.4 vs. 0.9 | |||
| - 8 Weeks: MF↑(13.3 vs. 18.2), VV↓(1.28 mL vs. 0.9 mL), BC↓(1.68 mL vs. 1.22 mL), BBF↓(10.8 mL vs. 5.3 mL). | 8 Weeks M3: 0.6 vs. 0.7 (Insignificant) | |||
| - 16 Weeks: MF↓(14.1 vs. 9.3), post- void residual volume↑(0.15 mL vs. 0.29 mL), BC↑(1.80 mL vs. 2.55 mL), micturition pressure↓(49.6 cm H2O vs. 41.5 cm H2O), BBF↓(8.9 mL vs. 3.4 mL). | 16 Weeks M1: 0.3 vs. 0.8 | |||
| 16 Weeks M2: 0.4 vs. 0.8 | ||||
| - Spontaneous detrusor activity and fluctuations in intravesical pressure. | 16 Weeks M3: 0.9 vs. 0.4 | |||
| Human studies | ||||
| 14 | Antunes-Lopes et al. (2019) [27] | I/R | Functional: | Nerve growth factor↑(normalized to urine creatinine): 2.9 vs. 3.7 |
| - Total IPSS↑(8 vs. 11). | ||||
| 15 | Averbeck et al. (2018) [28] | BOO due to BPH | Functional: | MDA↑ |
| - Bladder sensation↑34.2%, bladder compliance↓31.6%, cystometric capacity↓18.4%, detrusor overactivity 28.9%, urgency urinary incontinence↑13.2%, peak urinary flow↓92.1%, post- void residual volume↑57.9%, detrusor underactivity 44.7%. | MDA Levels (pmol/mg) in BOO and LUTS | |||
| Low grade BOO: 100 | ||||
| High grade BOO: 250 | ||||
| - 18.4% of patients presented with↓bladder sensation, which was associated with↓catalase enzymes in the bladder wall. | Mild LUTS: 111.93 | |||
| Severe LUTS: 290.93 | ||||
| 16 | Azadzoi et al. (2011) [29] | I/R | Structural: | MDA↑, AOPP↑, 8-isoprostane↑, nitrotyrosine↑, superoxide dismutase activity↓ |
| - Enlarged mitochondria with degraded or lost cristae and cytoplasmic lysosomes↑in cells exposed to oxidative stress. | Levels in normoxia vs. oxidative stress | |||
| MDA (pmol/mg): 0.4 vs. 0.7 | ||||
| AOPP (μM): 55 vs. 65 | ||||
| 8-isoprostane (pg/mL): 55 vs. 63 | ||||
| Nitrotyrosine (nM): 4.0 vs. 6.0 | ||||
| Superoxide dismutase activity (%): 29 vs. 25 | ||||
| 17 | Ener et al. (2015) [30] | I/R | Structural: | TAC↓, IMA↑, immunoglobulin E↑, C-reactive protein↑ |
| - Glomerulations↑ | Levels in control vs. ischemia | |||
| TAC (mM/L): 2.1 vs. 1.7 | ||||
| IMA (absorbance units): 0.51 vs. 0.56 | ||||
| Immunoglobulin E (IU/mL): 82.8 vs. 140 | ||||
| C-reactive protein (mg/L): 0.42 vs. 0.52 | ||||
| 18 | Guzel et al. (2012) [31] | BPH; malignant prostate cancer; LGPIN; HGPIN | Functional: | MDA↑as Cd↑and Pb↑in malignant prostate cancer, LGPIN, and HGPIN, and BPH |
| - Molecular damage and alteration of cell homeostasis. | Levels in BPH, malignant prostate cancer, LGPIN, and HGPIN | |||
| - Carcinogenic metals inhibit DNA repair proteins. | Tissue MDA (nmol/mg protein): 4.67, 5.43, 5.73, 5.96 | |||
| - ROS and reactive nitrogen species induce genes that promote proliferation and confer apoptosis resistance. | Plasma MDA (nmol/mg protein): 5.56, 6.64, 6.39, 6.66 | |||
| Tissue Cd (μg/g wet weight): 1.19, 1.29, 1.29, 1.29 | ||||
| Plasma Cd (μg/g wet weight): 1.10, 1.22, 1.22, 1.23 | ||||
| Tissue Pb (μg/g wet weight): 25, 34, 34, 36 | ||||
| Plasma Pb (μg/g wet weight): 25, 31, 31, 34 | ||||
| 19 | Keske et al. (2019) [32] | Detrusor overactivity | Functional: | IMA↑, TAC↓ |
| - Ischemia/oxidative damage leads to various lower urinary tract dysfunctions. | Levels in control vs. detrusor overactivity | |||
| IMA (absorbance units): 0.530 vs. 0.614 | ||||
| TAC (mM Trolox Eqv/L): 2.1 vs. 1.8 | ||||
| 20 | Merendino et al. (2003) [33] | BPH | Functional: | MDA↑and prostate specific antigen↑in BPH |
| - Circulating MDA level↑is a marker of lipid peroxidation and inflammation of prostate epithelium. | MDA levels (nmol/ml) in control vs. BPH | |||
| - MDA level↑may explain base modifications in BPH epithelium. | 0.97 vs. 2.12 | |||
| 21 | Ren et al. (2015) [34] | BPH | Functional: | ROS↑, HIF-1a↑, AR gene↑, vascular endothelial growth factor↑, IL-8↑in BPH prostatic tissues |
| - Vascular aging↑local ischemia and hypoxia. | Levels (RFUs) in control vs. BPH | |||
| - Hypoxic conditions lead to differential gene expression. | ROS: 40.947 vs. 82.727 | |||
| - ROS leads to greater apoptosis. | HIF-1a: 0.612 vs. 2.926 | |||
| AR: 1.236 vs. 2.918 | ||||
| Vascular endothelial growth factor: 1.094 vs. 3.035 | ||||
| IL-8: 1.201 vs. 1.970 | ||||
BPH, benign prostatic hyperplasia; MDA, malondialdehyde; AUR, acute urinary retention; ROS, reactive oxygen species; PBOO, partial bladder outlet obstruction; TAC, total antioxidant capacity; VV, voided volume; 8-OhdG, 8-hydroxy-2’-deoxyguanosine; I/R, ischemia/reperfusion; NF-kB, nuclear factor kappa B; RFUs, relative fluorescence units; BBF, bladder blood flow; BC, bladder capacity; Nrf2, nuclear factor erythroid 2-related factor 2; HIF-1a, hypoxia-inducible factor 1-alpha; HIF-2B/Arnt2, HIF-mediated aryl hydrocarbon receptor nuclear translocator 2; AOPP, advanced oxidation protein products; PI3K/Akt, phosphoinositide 3-kinase/protein kinase B; MF, micturition frequency; IPSS, International Prostate System Score; BOO, bladder outlet obstruction; LUTS, lower urinary tracts symptoms; LGPIN, low-grade prostatic intraepithelial neoplasia; HGPIN, high-grade prostatic intraepithelial neoplasia; IMA, ischemia modified albumin; IL-8, interleukin-8; P2X, purinoceptor; M1, M2, M3, muscarinic receptors.
Biomarkers
All studies noted molecular changes with increased oxidative stress. All studies showed that ROS increases in mitochondrial dysfunction. Additionally, malondialdehyde (MDA) in LUT disorders increased to 1.1-2.6 times levels in control (control was no hydrogen peroxide exposure to rats in study 1, no PBOO in rabbits in study 3, and no BOO in male patients in study 15) [14,16,28]. Higher MDA was noted with increasing age in studies 1, 3, and 11 [14,16,24]. Study 5 demonstrated that increased MDA is primarily within mitochondria in cells of rabbit bladder tissue (Table 3) [18].
In studies 3, investigating rabbits, and 8, analyzing human and rat prostate tissues, 8-hydroxy-2’-deoxyguanosine (8-OHdG) increased to 1.3–2.5 times levels in healthy samples without LUT dysfunctions, and increased with age [16,21]. N-(hexanoyl) lysine qualitatively increased in male rats in study 4 [17]. Nrf2, a transcription factor, increased to 1.8 times healthy rats in PBOO but decreased to 0.25 times healthy rats in I/R (studies 6 and 10) [19,23]. Ischemia modified albumin (IMA) increased to 1.1–1.2 times levels in patients without I/R or DO (studies 17 and 19) [30,32]. Hypoxia-inducible factors (HIFs) increased to 1.5–4.8 times levels in healthy counterparts (studies 6 and 21) [19,34].
TAC decreased by 0.1–0.4 times healthy controls in studies 3 (rabbits), 17 (female patients), and 19 (female patients) [16,20,32]. Superoxide dismutase (SOD) and nitric oxide synthase (NOS) activities decreased by 0.1–0.6 times levels in populations without LUT dysfunction and with increasing age (studies 11 and 16) [24,29]. Additionally, heavy metals (Cd and Pb) increased in prostate neoplasias by 1.1–1.4 times levels recorded in BPH (study 18) [31]. Three studies analyzed inflammatory biomarkers: Nuclear factor kappa B (NF-kB) doubled in PBOO when compared to healthy rats (study 6) [19], interleukin-8 (IL-8) increased in BPH to 1.6 times levels in healthy males (study 21) [34], IgE increased in I/R to 1.7 times levels in healthy females, and C-reactive protein (CRP) increased in I/R to 1.2 times levels in healthy females (study 17) [30].
Studies evaluating I/R noted specific biomarkers compared to controls without I/R. Study 7 showed that 172 proteins were upregulated and 527 proteins were downregulated [20]. Advanced oxidation protein products (AOPP) increased in the ischemia group to 1.2–1.8 times levels in healthy rat or human bladder tissues (studies 9 and 16) [22,29]. The PI3K/Akt expression increased to 1.7–2.9 times control (studies 9 and 10) [22,23]. Heat shock protein 70 (Hsp70) and GRP75 increased to 3.8- and 2.5-times control, respectively (study 10) [23]. Various isoforms of purinoceptors (P2X) increased to 1.4–1.75 times control (study 12) [25]. Study 13 showed muscarinic (M) receptor changes: M1 increased to 2.7 times control, M2 increased to 2.0 times control, and M3 decreased to 0.4 times control; differences increased with increasing age [26]. Nerve growth factor (NGF) increased to 1.3 times control (study 14) [27]. In study 16, 8-isoprostane and nitrotyrosine increased to 1.1- and 1.5-times control, respectively [29].
Overall trends in biomarkers were noted: ROS, MDA, 8-OHdG, IMA, HIFs, N-(hexanoyl) lysine, AOPP, PI3K/Akt, Hsp70, GRP75, P2X, M1, M2, NGF, 8-isoprostane, nitrotyrosine, heavy metals, and inflammatory biomarkers increased and M3, TAC, NOS, and SOD decreased in LUT dysfunction in both clinical and preclinical studies; Nrf 2 showed variability, increasing in PBOO but decreasing in I/R (Table 4).
Table 4.
Overview of noted changes in common biomarkers evaluated in oxidative stress
| Study No. | Biomarker |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROS | MDA | Other modified lipidsa) | Nitrotyrosine | 8-OHdG | TAC | Nrf2 | IMA | HIFs | AOPP | PI3K/Akt | Hsp 70 | GRP 75 | P2X | M receptorsb) | NGF | Enzymes (NOS, SOD) | Heavy Metals (Pb, Cd) | Inflammatory Markersc) | |
| 1 | ↑ | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 2 | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 3 | ↑ | ↑ | - | - | ↑ | ↓ | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 4 | ↑ | - | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 5 | ↑ | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 6 | ↑ | ↑ | - | - | - | - | ↑ | - | ↑ | - | - | - | - | - | - | - | - | - | ↑ |
| 7 | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 8 | ↑ | - | - | - | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 9 | ↑ | ↑ | - | - | - | - | - | - | - | ↑ | ↑ | - | - | - | - | - | - | - | - |
| 10 | ↑ | - | - | - | - | - | ↓ | - | - | - | ↑ | ↑ | ↑ | - | - | - | - | - | - |
| 11 | ↑ | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ↓ | - | - |
| 12 | ↑ | ↑ | - | - | - | - | - | - | - | ↑ | - | - | - | ↑ | - | - | - | - | - |
| 13 | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | ↑1,2;↓3 | - | - | - | - |
| 14 | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ↑ | - | - | - |
| 15 | ↑ | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 16 | ↑ | ↑ | ↑ | ↑ | - | - | - | - | - | ↑ | - | - | - | - | - | - | ↓ | - | - |
| 17 | ↑ | - | - | - | - | ↓ | - | ↑ | - | - | - | - | - | - | - | - | - | - | ↑ |
| 18 | ↑ | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ↑ | - |
| 19 | ↑ | - | - | - | - | ↓ | - | ↑ | - | - | - | - | - | - | - | - | - | - | - |
| 20 | ↑ | ↑ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 21 | ↑ | - | - | - | - | - | - | - | ↑ | - | - | - | - | - | - | - | - | - | ↑ |
ROS, reactive oxygen species; MDA, malondialdehyde; 8-OhdG, 8-hydroxy-2'-deoxyguanosine; TAC, total antioxidant capacity; Nrf2, nuclear factor erythroid 2-related factor 2; IMA, ischemia modified albumin; HIFs, hypoxia-inducible factors (HIF-1a, HIF-2b/Arnt2); AOPP, advanced oxidation protein products; PI3K/Akt, phosphoinositide 3-kinase/protein kinase B; Hsp70, heat shock protein 70; GRP75, glucose-regulated protein 75; P2X, purinoceptor; M, M1, M2, M3, muscarinic receptors; NGF, nerve growth factor; NOS, nitric oxide synthase; SOD, superoxide dismutase.
N-(hexanoyl) lysine & 8-isoprostane.
M1 and M2 increased; M3 decreased.
NF-kB increased in study 5, IL-8 increased in study 12, IgE and C-reactive protein increased in study 17.
DISCUSSION
Since the pathophysiological manifestation of LUT disorders requires elucidation [4], this review assessed 21 studies that examined oxidative stress-associated events to explain changes within the LUT. The human studies provided data on trends seen in the aging population experiencing LUT disorders while the animal studies helped confirm the clinical reports and provide additional mechanistic markers that helped elucidate reasons for the trends seen in the human studies.
LUT Structure and Function
Increased ROS explains many noted LUT structural and functional changes. Excess ROS damages cellular structures, macromolecules, mitochondrial DNA, and tissues through inflammation and fibrosis [8,35]. Chronic activation of ROS activates enzymes, damages DNA, and stimulates fibroblast proliferation, causing structural changes such as detrusor hypertrophy [36]. These ROS-driven mechanisms also explain the detrimental changes reported in the aged bladder [14].
A number of studies investigated the impact of ischemia, specifically by looking at structural and functional changes in the bladder. Initial changes showed bladder overactivity (e.g., increased contractility causing decreased bladder capacity) and later changes showed bladder underactivity (e.g., decreased contractility causing increased bladder capacity) [26]. Neural structural damage and M receptor expression may also explain the ischemic damage to the LUT [37]. I/R in humans is due in part to atherosclerosis, BOO, and infrequent voiding habits, which makes it difficult to characterize mechanisms. Nevertheless, there is ample support that chronic ischemia increases oxidative stress, especially with aging, in LUT disorders [5,37,38]. Previous studies support the claim that oxidative stress causes urinary dysfunction through BOO and I/R through an imbalance of oxidants/antioxidants [39].
Molecular Changes
Increased ROS with aging lead to changes in biomarkers of oxidative stress that can be subcategorized as evidence of oxidative stress induced molecular damage (ROS, MDA, 8-OHdG, IMA, N-(hexanoyl) lysine, 8-isoprostane, nitrotyrosine, AOPP, GRP75, NOS, Pb, and Cd), protective mechanisms against oxidative stress (HIFs, PI3K/Akt, Hsp70, TAC, and SOD), inflammation (NF-kB, IL-8, IgE, and CRP), and neural changes (P2X, M1, M2, M3, and NGF). Of these markers, I/R specifically caused changes in N-(hexanoyl) lysine, 8-isoprostane, nitrotyrosine, AOPP, GRP75, PI3K/Akt, Hsp70, P2X, M1, M2, M3, and NGF.
Oxidative stress induced molecular damage was marked by increased levels of ROS. In addition, MDA was found to be increased in a number of studies (Table 4). ROS-driven peroxidation of polyunsaturated fatty acids forms MDA, which then forms adducts and cross-links with macromolecules [40]. Another common biomarker of oxidative stress is 8-OHdG, which increased in PBOO and BPH [16,21]. 8-OHdG marks DNA damage; however, the exact role of DNA damage in the pathogenesis of LUT disorders requires further exploration [41]. Previous studies have shown that MDA levels reflect increased oxygen radical activity while 8-OHdG is a pivotal marker for measuring the effect of endogenous oxidative DNA damage [42,43]. IMA was also found to be increased, marking molecular damage [30,32]. The decrease in cobalt binding to IMA can lead to alteration in redox balance and disease [44]. Studies have demonstrated the importance of using IMA to monitor oxidative stress levels [45]. In I/R, N-(hexanoyl) lysine and 8-isoprostane, which can be modified by lipid peroxidation, increased [17,29]. Nitrotyrosine, made by tyrosine nitration in increased oxidative stress, also increased [29]. I/R increased proteins modified by oxidation, noted by the higher AOPP levels [22,25,29]. AOPP is commonly used to assess oxidative stress in a number of organs and diseases [45]. Upregulation of GRP75 was associated with impaired mitochondrial respiration in I/R [23]. NOS, an enzyme responsible for generating nitric oxide, decreased in studies analyzing oxidative stress in PBOO and I/R, potentially due to increased free radical damage to the enzyme [24,29]. Pb and Cd, increasing ROS and mediating effects of oxidative stress, increased in study 18, a clinical study [31,46]. Studies have supported these observations as heavy metal-induced oxidative stress in cells can be partially responsible for cellular toxicity [47].
Other studies showed changes in molecules that indicated protective mechanisms against oxidative stress. HIFs increased over time in PBOO and BPH, respectively [19,34]. HIF-1a and HIF-2b may protect against oxidative stress induced apoptosis [48]. TAC, the capacity to scavenge ROS, decreased due to increased use and depletion of antioxidant molecules in protecting against oxidative stress [16,30,32]. These biomarkers also signal cell stress and consequent bladder dysfunction. SOD activities decreased in models for PBOO and I/R due to increased ROS [24,29], which resulted in a decreased ability to scavenge ROS [49]. Additionally, LUT dysfunctions such as I/R increase stress on mitochondria to drive processes needed for cell survival such as the PI3K/Akt pathway, which inhibits expression of antioxidant proteins to facilitate apoptosis [22,23]. Hsp70 increased, indicating stress of the cell during protein folding [23].
Inflammatory biomarkers (NF-kB, IL-8, IgE, and CRP) increased in PBOO, BPH, and I/R, suggesting a role of inflammation in oxidative stress related LUT disorders [19,30,34]. NF-kB is a transcription factor that upregulates genes involved in immune functions. IL-8 is a cytokine involved in proinflammatory functions [50]. IgE and CRP increases were seen for painful bladder syndrome and interstitial cystitis, signaling potential for allergy-mediated inflammation [30]. Previous studies have shown that oxidative stress mediates the NF-kB pathway, upregulates IL-8 synthesis by human dendritic cells, increases production of IgE, and shows increases in CRP [51-54]. With age-related LUT disorders, oxidative stress can also increase expression of genes in inflammatory pathways to consistently high levels and chronic inflammation, in turn, induces oxidative stress and decreases TAC [55]. Additionally, Nrf2 was more expressed in preclinical study 6 analyzing PBOO compared to healthy rats, signaling antioxidant protein expression in inflammatory states [56]. However, Nrf2 was lower in rats with I/R in preclinical study 10, hypothesized to lead to increased damage from oxidative stress in I/R instead [23].
Neural changes were seen in studies investigating I/R. Purinoceptor (P2X isoforms), receptors mediating hypersensitivity and pain in the bladder, also increased, contributing to bladder noncompliance and spontaneous contractions [25]. P2X receptors interact with M receptors to decrease bladder contractions. M1 receptors contribute to acetylcholine release from cholinergic nerves, while M2 and M3 receptors mediate bladder contraction. With acute ischemia, M2 receptors increase and correlate with an overactive state. In chronic ischemic states, M1 receptors increase and M3 receptors decrease, which is associated with a transition to an underactive bladder [26]. NGF also increases, further suggesting neural involvement in oxidative stress [27].
Aging
Chronic changes in oxidative stress significantly impairs urinary bladder function in both adult and aged animals. Studies show aging is associated with higher levels of MDA, 8-OHdG, M1/M2 receptors and a number of structural and functional LUT deficits. Further, they also reported lower levels of TAC, the M3 receptor, SOD and NOS activities in rats and rabbits [14,16,24,26]. Oxidative stress may be a contributing factor underlying LUT dysfunctions, which increases in prevalence with age [4,57]. Disease progression changes with advanced age, along with dynamic changes in multiple factors. This was supported by studies showing alterations in NGF and M receptors, which may play a role in transitioning the bladder from overactive to an underactive state. The effects of aging on the bladder are complex and associated with multiple risk factors. Thus, the use of animal models (including use of genetically modified mice) permits detailed investigation of specific factors in the context of aging [4].
Considerations and Limitations
Due to the increasing prevalence of LUT disorders in the older adult, we examined common manifestations and biomarkers to explain the influence of oxidative stress induced changes in outcome measures. Identifying clusters of biomarkers that can be linked with LUT dysfunction in the older adult can aid prognosis and treatment. However, these studies require further exploration. Pharmaceutical agents and natural antioxidants have been used to modify oxidative stress in LUT disorders, although underlying mechanisms are unclear [39,58]. Most of the identified biomarkers require exploration before being considered as treatment targets. Future studies should consider inflammation in the pathogenesis of oxidative stress, as inflammation is associated with a number of age-related diseases [55].
The findings of our review have several limitations. By using stringent criteria, many studies may have been excluded. Also, the impact of primary mitochondrial dysfunction on the LUT is represented while not being completely understood. Additionally, despite conducting comprehensive searches, articles that were published in journals not indexed in databases used in our analysis could have been missed.
Only 2 studies included in this review provided analysis on females and additional demographic data was lacking; therefore, findings may be insufficient for generalized judgements. Additionally, the systematic review’s protocol was not registered on Prospero, which is a drawback we acknowledge.
Despite these limitations, our review provides a systematic analysis which associated a number of outcomes associated with age-associated increases in oxidative stress in LUT disorders. We tested several search constructions and selected the ones that allowed for the broadest selection of articles. Additionally, we searched 4 databases and had 2 reviewers independently screen studies to reduce selection bias. We used 2 quality assessment methods, GRADE and OCEBM criteria. Finally, we incorporated findings from both animal studies, which allowed for a controlled setting, and human studies, in order to provide translational relevance. Nevertheless, further studies are warranted, such as exploration of biomarker clusters and progression in the context of demographics, LUT disorders, prognosis, and treatments.
The increasing prevalence of LUT disorders with aging shows an unmet need for better understanding of mechanisms of pathogenesis, in order to provide safe and effective treatments for these conditions. This systematic review focuses on factors associated with oxidative stress in age-related LUT disorders. In addition to mechanical, vascular, neural, and functional changes of the LUT, oxidative stress is associated with increased ROS, MDA, 8-OHdG, IMA, HIFs, N-(hexanoyl) lysine, AOPP, PI3K/Akt, Hsp70, GRP75, P2X, M1, M2, NGF, 8-isoprostane, nitrotyrosine, heavy metals, and inflammatory biomarkers, and decreased TAC, M3, NOS, and SOD. Our findings may serve as an initial list of potential targets that may be useful for the investigation of a number of age-associated LUT disorders.
Footnotes
Fund/Grant Support
This research did not receive any funding support in the form of grants from any public, commercial, or not-for-profit funding agencies.
Conflict of Interest
LK has no conflict of interests to declare and certifies that any potential conflict of interests for all authors are the following: Outside this systematic review, JPW is a consultant for Ferring and the Institute for Bladder and Prostate Research. All other authors have nothing to disclose.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: LK, JPW, LAB
·Data curation: LK, SG, JPW, LAB
·Formal analysis: LK, SG, JPW, LAB
·Methodology: LK, SG, JPW, LAB
·Project administration: LK, JPW, LAB
·Visualization: LK, SG, LAB
·Writing-original draft: LK, SG, JPW, LAB
·Writing-review & editing: LK, SG, JPW, LAB
REFERENCES
- 1.Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and sweden: results from the epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104:352–60. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 2.Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur Urol. 2006;50:1306–5. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Zhang AY, Xu X. Prevalence, burden, and treatment of lower urinary tract symptoms in men aged 50 and older: a systematic review of the literature. SAGE Open Nurs. 2018;4:2377960818811773. doi: 10.1177/2377960818811773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birder LA. Is there a role for oxidative stress and mitochondrial dysfunction in age-associated bladder disorders? Ci Ji Yi Xue Za Zhi. 2020;32:223–6. doi: 10.4103/tcmj.tcmj_250_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vahabi B, Wagg AS, Rosier PFWM, Rademakers KLJ, Denys MA, Pontari M, et al. Can we define and characterize the aging lower urinary tract?-ICI-RS 2015. Neurourol Urodyn. 2017;36:854–8. doi: 10.1002/nau.23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fathollahi A, Daneshgari F, Hanna-Mitchell AT. Melatonin and its role in lower urinary tract function: an article review. Curr Urol. 2015;8:113–8. doi: 10.1159/000365701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birder LA, Wolf-Johnston AS, Jackson EK, Wein AJ, Dmochowski R. Aging increases the expression of vasopressin receptors in both the kidney and urinary bladder. Neurourol Urodyn. 2019;38:393–7. doi: 10.1002/nau.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudryavtseva AV, Krasnov GS, Dmitriev AA, Alekseev BY, Kardymon OL, Sadritdinova AF, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7:44879–905. doi: 10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and metaanalysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Higgins JPT. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open. 2018;8:e019703. doi: 10.1136/bmjopen-2017-019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.University of Oxford . Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009) [Internet] Oxford: University of Oxford; 2022. [cited 2021 Apr 4]. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidencebased-medicine-levels-of-evidence-march-2009. [Google Scholar]
- 13.Wei D, Tang K, Wang Q, Estill J, Yao L, Wang X, et al. The use of GRADE approach in systematic reviews of animal studies. J Evid Based Med. 2016;9:98–104. doi: 10.1111/jebm.12198. [DOI] [PubMed] [Google Scholar]
- 14.Aikawa K, Leggett R, Levin RM. Effect of age on hydrogen peroxide mediated contraction damage in the male rat bladder. J Urol. 2003;170:2082–5. doi: 10.1097/01.ju.0000081461.73156.48. [DOI] [PubMed] [Google Scholar]
- 15.Kirpatovsky VI, Plotnikov EY, Mudraya IS, Golovanov SA, Drozhzheva VV, Khromov RA, et al. Role of oxidative stress and mitochondria in onset of urinary bladder dysfunction under acute urine retention. Biochemistry (Mosc) 2013;78:542–8. doi: 10.1134/S0006297913050131. [DOI] [PubMed] [Google Scholar]
- 16.Lin WY, Chen CS, Wu SB, Lin YP, Levin RM, Wei YH. Oxidative stress biomarkers in urine and plasma of rabbits with partial bladder outlet obstruction. BJU Int. 2011;107:1839–43. doi: 10.1111/j.1464-410X.2010.09597.x. [DOI] [PubMed] [Google Scholar]
- 17.Nomiya M, Yamaguchi O, Andersson KE, Sagawa K, Aikawa K, Shishido K, et al. The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol Urodyn. 2012;31:195–200. doi: 10.1002/nau.21073. [DOI] [PubMed] [Google Scholar]
- 18.Radu F, Leggett RE, Schuler C, Levin RM. The effect of in vitro ischemia/reperfusion on contraction, free fatty acid content, phospholipid content, and malondialdehyde levels of the rabbit urinary bladder. Mol Cell Biochem. 2011;346:179–86. doi: 10.1007/s11010-010-0603-6. [DOI] [PubMed] [Google Scholar]
- 19.Sezginer EK, Yilmaz-Oral D, Lokman U, Nebioglu S, Aktan F, Gur S. Effects of varying degrees of partial bladder outlet obstruction on urinary bladder function of rats: a novel link to inflammation, oxidative stress and hypoxia. Low Urin Tract Symptoms. 2019;11:O193–201. doi: 10.1111/luts.12211. [DOI] [PubMed] [Google Scholar]
- 20.Su N, Choi HP, Wang F, Su H, Fei Z, Yang JH, et al. Quantitative proteomic analysis of differentially expressed proteins and downstream signaling pathways in chronic bladder ischemia. J Urol. 195:515–23. doi: 10.1016/j.juro.2015.09.079. [DOI] [PubMed] [Google Scholar]
- 21.Vital P, Castro P, Ittmann M. Oxidative stress promotes benign prostatic hyperplasia. Prostate. 2016;76:58–67. doi: 10.1002/pros.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witthaus MW, Nipa F, Yang JH, Li Y, Lerner LB, Azadzoi KM. Bladder oxidative stress in sleep apnea contributes to detrusor instability and nocturia. J Urol. 2015;193:1692–9. doi: 10.1016/j.juro.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 23.Yang JH, Siroky MB, Yalla SV, Azadzoi KM. Mitochondrial stress and activation of PI3K and akt survival pathway in bladder ischemia. Res Rep Urol. 2017;9:93–100. doi: 10.2147/RRU.S132082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan X, Wu S, Lin T, He D, Li X, Liu S, et al. Role of nitric oxide synthase in bladder pathologic remodeling and dysfunction resulting from partial outlet obstruction. Urology. 2011;77:1008.e1–8. doi: 10.1016/j.urology.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Siroky M, Yang JH, Zhao Z, Azadzoi K. Effects of ischemia and oxidative stress on bladder purinoceptors expression. Urology. 2014;84:1249.e1–7. doi: 10.1016/j.urology.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Azad R, Yang JH, Siroky MB, Azadzoi KM. Progressive changes in detrusor function and micturition patterns with chronic bladder ischemia. Investig Clin Urol. 2016;57:249–59. doi: 10.4111/icu.2016.57.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antunes-Lopes T, Vasconcelos A, Costa D, Charrua A, Neves J, Silva J, et al. The impact of chronic pelvic ischemia on LUTS and urinary levels of neuroinflammatory, inflammatory, and oxidative stress markers in elderly men: a case-control study. Urology. 2019;123:230–4. doi: 10.1016/j.urology.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Averbeck MA, de Lima NG, Motta GA, Beltrão L, Abboud NJ F, Rigotti CP, et al. Oxidative stress in the bladder of men with LUTS undergoing open prostatectomy: a pilot study. Int Braz J Urol. 2018;44:1182–93. doi: 10.1590/S1677-5538.IBJU.2018.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azadzoi KM, Yalla SV, Siroky MB. Human bladder smooth muscle cell damage in disturbed oxygen tension. Urology. 2011;78:967.e9–15. doi: 10.1016/j.urology.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Ener K, Keske M, Aldemir M, Özcan MF, Okulu E, Özayar A, et al. Evaluation of oxidative stress status and antioxidant capacity in patients with painful bladder syndrome/interstitial cystitis: preliminary results of a randomised study. Int Urol Nephrol. 2015;47:1297–302. doi: 10.1007/s11255-015-1021-1. [DOI] [PubMed] [Google Scholar]
- 31.Guzel S, Kiziler L, Aydemir B, Alici B, Ataus S, Aksu A, et al. Association of pb, cd, and se concentrations and oxidative damage-related markers in different grades of prostate carcinoma. Biol Trace Elem Res. 2012;145:23–32. doi: 10.1007/s12011-011-9162-2. [DOI] [PubMed] [Google Scholar]
- 32.Keske M, Gok B, Ener K, Ozcan MF, Ozayar A, Okulu E, et al. Relationship between oxidative stress and detrussor overactivity: a case control study. Urol J. 2019;16:371–4. doi: 10.22037/uj.v0i0.5090. [DOI] [PubMed] [Google Scholar]
- 33.Merendino RA, Salvo F, Saija A, Di Pasquale G, Tomaino A, Minciullo PL, et al. Malondialdehyde in benign prostate hypertrophy: a useful marker? Mediators Inflamm. 2003;12:127–8. doi: 10.1080/0962935031000097745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren H, Li X, Cheng G, Li N, Hou Z, Suo J, et al. The effects of ROS in prostatic stromal cells under hypoxic environment. Aging Male. 2015;18:84–8. doi: 10.3109/13685538.2015.1018159. [DOI] [PubMed] [Google Scholar]
- 35.Levin RM, Leggett RE, Schuler C, Rehfuss A, Hass M. Oxidative stress and lower urinary tract dysfunctions primarily found in women. Urol Sci. 2010;21:8–18. [Google Scholar]
- 36.Andersson KE. Oxidative stress and its possible relation to lower urinary tract functional pathology. BJU Int. 2018;121:527–33. doi: 10.1111/bju.14063. [DOI] [PubMed] [Google Scholar]
- 37.Speich JE, Tarcan T, Hashitani H, Vahabi B, McCloskey KD, Andersson KE, et al. Are oxidative stress and ischemia significant causes of bladder damage leading to lower urinary tract dysfunction? Report from the ICI-RS 2019. Neurourol Urodyn. 2020;39 Suppl 3:S16–22. doi: 10.1002/nau.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyata Y, Matsuo T, Mitsunari K, Asai A, Ohba K, Sakai H. A review of oxidative stress and urinary dysfunction caused by bladder outlet obstruction and treatments using antioxidants. Antioxidants (Basel) 2019;8:132. doi: 10.3390/antiox8050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 42.Cherian DA, Peter T, Narayanan A, Madhavan SS, Achammada S, Vynat GP. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J Pharm Bioallied Sci. 2019;11(Suppl 2):S297–300. doi: 10.4103/JPBS.JPBS_17_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 44.Coverdale JPC, Katundu KGH, Sobczak AIS, Arya S, Blindauer CA, Stewart AJ. Ischemia-modified albumin: crosstalk between fatty acid and cobalt binding. Prostaglandins Leukot Essent Fatty Acids. 2018;135:147–57. doi: 10.1016/j.plefa.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao L, Zhang F, Zhao Y, Zhang L, Xie Z, Huang K, et al. Using advanced oxidation protein products and ischaemia-modified albumin to monitor oxidative stress levels in patients with drug-induced liver injury. Scientific Reports. 2020;10:18128. doi: 10.1038/s41598-020-75141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–39. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 48.Li HS, Zhou YN, Li L, Li SF, Long D, Chen XL, et al. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019;25:101109. doi: 10.1016/j.redox.2019.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiffrin EL. Oxidative stress, nitric oxide synthase, and superoxide dismutase: A matter of imbalance underlies endothelial dysfunction in the human coronary circulation. Hypertension. 2008;51:31–2. doi: 10.1161/HYPERTENSIONAHA.107.103226. [DOI] [PubMed] [Google Scholar]
- 50.Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993;64(5 Suppl):456–60. [PubMed] [Google Scholar]
- 51.Lingappan K. NF-κB in oxidative stress. Curr Opin Toxicol. 2018;7:81–6. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verhasselt V, Goldman M, Willems F. Oxidative stress up-regulates IL-8 and TNF-alpha synthesis by human dendritic cells. Eur J Immunol. 1998;28:3886–90. doi: 10.1002/(SICI)1521-4141(199811)28:11<3886::AID-IMMU3886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 53.Emin O, Hasan A, Aysegul D, Rusen D. Total antioxidant status and oxidative stress and their relationship to total IgE levels and eosinophil counts in children with allergic rhinitis. J Investig Allergol Clin Immunol. 2012;22:188–92. [PubMed] [Google Scholar]
- 54.Cottone S, Mulè G, Nardi E, Vadalà A, Guarneri M, Briolotta C, et al. Relation of C-reactive protein to oxidative stress and to endothelial activation in essential hypertension. Am J Hypertens. 2006;19:313–8. doi: 10.1016/j.amjhyper.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 57.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–8. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 58.Birder LA, Wolf-Johnston A, Wein AJ, Grove-Sullivan M, Stoltz D, Watkins S, et al. A uro-protective agent with restorative actions on urethral and striated muscle morphology. World J Urol. 2021;39:2685–90. doi: 10.1007/s00345-020-03492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


