Abstract
The novobiocin biosynthetic gene cluster from Streptomyces spheroides NCIB 11891 was cloned by using homologous deoxynucleoside diphosphate (dNDP)-glucose 4,6-dehydratase gene fragments as probes. Double-stranded sequencing of 25.6 kb revealed the presence of 23 putative open reading frames (ORFs), including the gene for novobiocin resistance, gyrBr, and at least 11 further ORFs to which a possible role in novobiocin biosynthesis could be assigned. An insertional inactivation experiment with a dNDP-glucose 4,6-dehydratase fragment resulted in abolishment of novobiocin production, since biosynthesis of the deoxysugar moiety of novobiocin was blocked. Heterologous expression of a key enzyme of novobiocin biosynthesis, i.e., novobiocic acid synthetase, in Streptomyces lividans TK24 further confirmed the involvement of the analyzed genes in the biosynthesis of the antibiotic.
Novobiocin is produced by Streptomyces spheroides and Streptomyces niveus and belongs to the aminocoumarin antibiotics. Bacterial DNA gyrase represents the target of these coumarins (41), and novobiocin inhibits this enzyme by interaction with the N-terminal 24-kDa subdomain of the gyrB subunit (27). In addition to its antibacterial action, novobiocin shows synergistic effects with antitumor drugs such as etoposide or teniposide (37, 49).
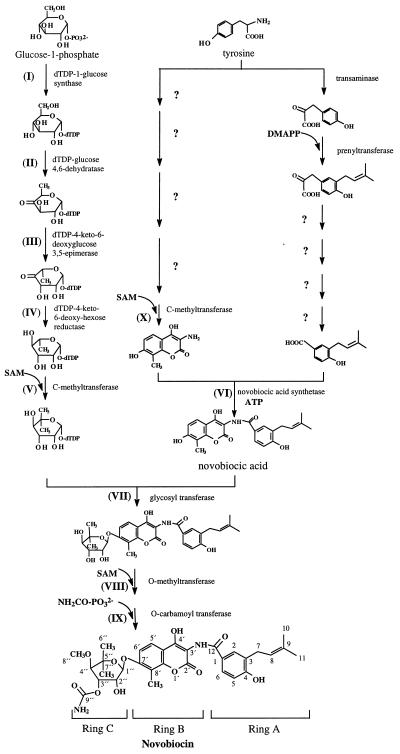
Little is known about the biosynthesis of novobiocin. Structurally, it is composed of three moieties, a noviose sugar (ring C), a substituted coumarin (ring B), and a prenylated 4-hydroxybenzoic acid (ring A), and these rings are linked by glycosidic and amide bonds (Fig. 1). Radioactive feeding experiments in the 1960s and 1970s showed that noviose is directly derived from d-glucose, whereas tyrosine serves as a precursor of ring A and ring B (3, 6, 31). This was recently confirmed by a feeding experiment with [1-13C]glucose (33) which also showed that the dimethylallyl moiety of novobiocin was formed through the nonmevalonate pathway.
FIG. 1.
Hypothetical biosynthetic pathway of novobiocin. Roman numerals refer to the putative assignments of genes identified in the biosynthetic reactions (see Table 1). SAM, S-adenosylmethionine.
Molecular biological studies have been restricted to the investigation of novobiocin resistance genes (43, 52), especially gyrBr (61, 62), and the production of novobiocin-deficient mutants (19). Discovery of the genetic basis of the biosynthesis of aminocoumarin antibiotics could provide a useful tool for drug development. “Combinatorial biosynthesis,” the interchange of genes involved in antibiotic biosynthesis between different microorganisms or the creation of hybrid genes and, consequently, proteins with new enzymatic properties, allows the production of modified or even novel antibiotics (23). In the past, much effort has been undertaken in the manipulation of the biosynthesis of polyketide antibiotics (25, 42, 56), and recently, progress has also been made in the construction of hybrid peptide synthetase genes (55, 59). The discovery of gene clusters for other types of secondary metabolites can offer additional possibilities for combinatorial biosynthesis.
Here we report on the identification of the novobiocin biosynthetic gene cluster from S. spheroides NCIB 11891. The gene cluster was analyzed by molecular cloning, DNA sequencing, an insertional inactivation experiment, and heterologous expression of a key enzyme of novobiocin biosynthesis, i.e., novobiocic acid synthetase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Plasmids pBluescript SK(−) [pBSK(−)], pBluescriptC SK(−) [pBCSK(−)], and SuperCosI were purchased from Stratagene (Heidelberg, Germany), Litmus 28 was purchased from New England Biolabs (Hertfordshire, United Kingdom), and pGEM-11Zf(+) was purchased from Promega (Madison, Wis.). pBSKT (36) was a gift from J. A. Salas (Oviedo, Spain). Shuttle vectors pEM4 (48) and pUWL201 were kindly provided by A. Bechthold and were originally obtained from J. A. Salas (Oviedo, Spain) and W. Piepersberg (Wuppertal, Germany), respectively.
S. spheroides NCIB 11891 and Streptomyces lividans TK24 were obtained from E. Cundliffe (Leicester, United Kingdom) and D. A. Hopwood (Norwich, United Kingdom), respectively, and were cultured at 28°C and 175 rpm for 2 to 4 days in baffled shake flasks. For preparation of genomic DNA and plasmid isolation, the Streptomyces strains were grown at 28°C in YMG medium containing 1.0% malt extract, 0.4% yeast extract, 0.4% glucose, and 1.0 mM CaCl2 (pH 7.3). For preparation of protoplasts of S. lividans TK24, CRM medium containing 10.3% sucrose, 2.0% tryptic soy broth, 1.0% MgCl2 · 6H2O, 1.0% yeast extract, and 0.75% glycine (pH 7.0) was used; for S. spheroides, TSB medium (20) containing 0.4% glycine and 10.0% sucrose was used (concentrations are given as wt/vol). Regeneration of S. lividans TK24 and of S. spheroides protoplasts was carried out on R2YE medium (20). For expression experiments and analysis of metabolites, cells were grown in CDM medium (31) for 3 to 4 days and 8 days, respectively.
Escherichia coli XL1 Blue MRF′ (Stratagene, Heidelberg, Germany) was used for the preparation of recombinant plasmids and for the construction of the cosmid library. For transformation of S. spheroides NCIB 11891, plasmid DNA was isolated after amplification in E. coli ET 12567.
Thiostrepton (20 μg/ml for S. lividans TK24 and 10 μg/ml for S. spheroides NCIB 11891) and carbenicillin (50 μg/ml for E. coli) were used for selection of recombinant strains.
DNA isolation, manipulation, and cloning.
Standard procedures were performed as described by Sambrook et al. (54) and Hopwood et al. (20). Isolation of DNA fragments from agarose gels was carried out with the Qiagen QIAEX II Gel Extraction Kit (Qiagen, Hilden, Germany). Genomic DNA was isolated from Streptomyces strains by lysozyme treatment and phenol-chloroform extraction as described elsewhere (1).
The S. spheroides NCIB 11891 cosmid library was constructed by partial Sau3A digestion of chromosomal S. spheroides DNA and ligation into the BamHI sites of SuperCosI. Packaging was performed with Gigapack II (Stratagene).
Southern blot analysis was performed on Hybond-N nylon membranes (Amersham, Braunschweig, Germany) with digoxigenin-labeled probes by using the DIG high prime DNA labeling and detection starter kit II (Boehringer, Mannheim, Germany).
DNA sequencing and computer analysis.
For sequencing, fragments of approximately 200 to 3,000 bp in length were obtained from cosmid clones 9-6G and 10-9C and were subcloned into pBSK(−), pBCSK(−), or Litmus 28. Sequencing was performed by the dideoxynucleotide chain termination method with M13 primers or primers that consisted of fragments from within the sequenced region on a Molecular Dynamics Vistra 725 DNA sequencer or with a LI-COR automatic sequencer (MWG-Biotech, Ebersberg, Germany). Both strands of the region between novA and novW (a total of 25,616 bp) were sequenced.
The DNASIS software package (version 2.1, 1995; Hitachi Software Engineering, San Bruno, Calif.) was used for sequence analysis. Amino acid sequence homology searches were carried out in the GenBank database by using the BLAST program (release 2.0).
Construction of novobiocic acid synthetase expression plasmids.
To determine the genes involved in the novobiocic acid synthetase reaction, several fragments from cosmid 9-6G were cloned into the Streptomyces expression vectors pEM4 and pUWL201, both of which contain the ermE up promoter for foreign gene expression.
(i) pSL3(+).
A 9.7-kb EcoRI fragment containing the open reading frames (ORFs) from novH to novN was cloned into the EcoRI site of pEM4. The correct orientation of the insert in regard to the orientation of promoter was confirmed by restriction analysis.
(ii) pMS65.
A 1.95-kb EcoRI-BglII fragment, which was obtained from the 9.7-kb EcoRI fragment mentioned above and which contained the novH gene, was cloned into pUWL201 that had been digested with EcoRI and BamHI to give pSM65.
(iii) pMS71.
The 9.7-kb EcoRI fragment from cosmid 9-6G was cloned into the EcoRI site of pBSK(−), resulting in plasmid p9-6GE9-R; the SpeI site of pBSK(−) was located downstream of novN. After deletion of the fragment containing the genes from novK to novN by SnaBI and SpeI digestion of p9-6GE9-R, blunt ends were generated with T4 DNA polymerase (Amersham, Braunschweig, Germany), and the construct was religated. The insert of the resulting plasmid, comprising the genes from novH to novJ, was digested with EcoRI and XbaI and was cloned into the same sites of pUWL201 to give pMS71.
(iv) pMS75.
The 9.7-kb EcoRI fragment from cosmid 9-6G was cloned into the EcoRI site of pBSK(−); in this case, the orientation of the insert was such that the NotI site of pBSK(−) was located upstream of novH. A 5.5-kb NotI fragment from this construct, which contained the ORFs from novH to novK, was cloned into pBSK(−), providing a new XbaI site downstream of novK. The insert of the resulting plasmid was excised with EcoRI and XbaI and was cloned into the same sites of pUWL201 to give pMS75.
(v) pMS73.
After deletion of novM and novN from p9-6GE9-R by PflMI and SpeI digestion, blunt ends were created with T4 DNA polymerase and the construct was religated. The insert of the resulting plasmid, which comprised the genes from novH to novL, was excised with EcoRI and XbaI and was ligated into the same sites of pUWL201 to give pMS73.
Transformation of expression plasmids into S. lividans TK24.
Preparation of S. lividans TK24 protoplasts was carried out by the standard protocol (20) with cells grown for 48 h. Polyethylene glycol-induced protoplast transformation (20) was carried out with 10 μg of plasmid DNA per transformation. After protoplast regeneration on R2YE agar medium for 14 h at 28°C, transformants were selected by overlaying each R2YE plate with 200 μl of thiostrepton solution (5 mg/ml).
Preparation of cell-free extracts and novobiocic acid synthetase assay.
For expression of novobiocic acid synthetase, S. lividans TK24 transformants were cultured in CDM medium as mentioned above. All further operations were carried out at 4°C. Cells were harvested and washed twice with 50 mM Tris-HCl (pH 8.0). Cells (3 g [fresh weight]) and 3 ml of buffer A (50 mM Tris-HCl [pH 8.0], 5 mM dithiothreitol, 50 μM phenylmethylsulfonyl fluoride) were ground for 20 min in a mortar to which sea sand had been added. The mixture was centrifuged for 30 min at 27,000 × g, and the supernatant was passed through a Sephadex G-25 column (Nap-10; Pharmacia, Uppsala, Sweden), which had been equilibrated with buffer A. Protein contents were determined by the method of Bradford (5).
Ring A was obtained by hydrolysis of novobiocin (30). Ring B and novobiocic acid were kindly provided by Pharmacia & Upjohn, Inc. (Kalamazoo, Mich.). The enzyme assay (100 μl) contained 100 μg of protein and the reagents described elsewhere (30), plus 5 mM MnCl2. The reaction was stopped after incubation for 20 min at 30°C by addition of 5 μl of 1.5 M trichloroacetic acid. After extraction with 1 ml of ethyl acetate, the organic phase was evaporated, and the residue was dissolved in H2O-methanol (50:50; vol/vol) and analyzed by high-pressure liquid chromatography (HPLC) as described in the section Analysis of metabolites.
Construction of vector pAM1 for insertional gene inactivation.
A 2.1-kb EcoRI fragment from cosmid 9-6G, which comprises part of the novT gene, was cloned into the EcoRI site of vector pBSKT, a pBluescript SK(+) derivative containing the thiostrepton resistance gene (36), resulting in pAM1. Restriction analysis showed that the novT gene fragment had the same orientation as the thiostrepton and the ampicillin resistance genes of the vector.
Transformation of S. spheroides NCIB 11891.
Preparation of protoplasts was carried out as described elsewhere (22). Mycelia were grown for 48 h, harvested, and incubated with 10 mg of lysozyme in 5 ml of P buffer (20) for 60 to 90 min at 30°C.
For transformation, 10 to 50 μg of plasmid pAM1 was converted to the single-stranded form by incubation at 100°C for 10 min followed by rapid chilling on ice. The plasmid DNA was mixed with approximately 9.6 × 107 freshly prepared protoplasts (200 μl) under addition of 500 μl of T buffer (20) containing 50% (wt/vol) polyethylene glycol 1000 (Roth, Karlsruhe, Germany) and 30 μg of calf thymus DNA. Protoplasts were regenerated on R2YE agar medium overlaid with 3 ml of R3 agar (58), which was modified by inclusion of 0.8% (wt/vol) agar and 17.1% (wt/vol) sucrose and omission of disodium succinate and yeast extract. After 48 h at 25°C the plates were overlaid with 3 ml of soft nutrient agar (20) containing 0.33 mg of thiostrepton per ml for selection of integration mutants.
Analysis of metabolites.
Integration mutants of S. spheroides were cultured in CDM medium (see above) and were harvested by centrifugation. The medium (105 ml) was acidified with HCl to pH 2.0 and was extracted twice with 150 ml of ethyl acetate. The organic phase was washed twice with 100 ml of water and was dried with Na2SO4. The solvent was removed and the residue was dissolved in 1 ml of methanol.
Metabolites were analyzed by HPLC with a Multosphere RP18-5 column (250 by 4 mm; 5 μm; C+S Chromatographie Service, Düren, Germany) with a linear gradient from 60 to 100% methanol in 1% aqueous formic acid and detection at 305 nm. Authentic novobiocin (Fluka, Buchs, Germany) and novobiocic acid were used as standards.
For preparative isolation, the fractions from HPLC analysis were collected and the solvent was evaporated. To remove residual formic acid, the residue was dissolved in 50 ml of water and the products were extracted with ethyl acetate. The organic layer was washed with water until the pH was neutral. After evaporation of the ethyl acetate, the product was analyzed by mass spectrometry (MS) and 1H-nuclear magnetic resonance (1H-NMR) spectroscopy. Electron impact mass spectra were recorded on a TSQ70 spectrometer (Finnigan, Bremen, Germany) by using methanol as the solvent. A molecular weight of 395.1 was observed (novobiocic acid is C22H21NO6; molecular weight, 395.4).
1H-NMR spectrum was measured on an AMX 400 spectrometer (Bruker, Karlsruhe, Germany), and the isolated compound gave signals corresponding to novobiocic acid: δppm (CD3OD) 7.75 (d, 2.25 Hz, H-2), 7.71 (dd, 8.52 Hz, 2.25 Hz, H-6), 7.66 (d, J = 8.95 Hz, H-5′), 6.85 (d, 8.98 Hz, H-6′), 6.84 (d, 8.53 Hz, H-5), 5.35 (t, sep 7.63 Hz, 1.5 Hz, H-8), 3.34 (H-7, overlapped with signals of solvent), 2.26 (s, CH3-8′), 1.76 (s, H-11), 1.74 (s, H-10).
Nucleotide sequence accession number.
The nucleotide sequence reported on here is deposited in the GenBank database under accession no. AF170880.
RESULTS
Screening of a cosmid library for the novobiocin biosynthetic gene cluster.
Previous attempts to clone the novobiocin biosynthetic gene cluster by complementation experiments with novobiocin-deficient mutants of S. niveus were unsuccessful (9). Screening experiments with novobiocin resistance genes have been discussed as an alternative cloning strategy for this cluster (43, 52, 62), but no results have been reported so far. Therefore, we tried a different strategy. The sugar moiety of novobiocin (ring C) is a 6-deoxyhexose, and 6-deoxyhexoses are generally formed via a deoxynucleoside diphosphate (dNDP)-hexose 4,6-dehydratase reaction (Fig. 1, reaction II) (35, 46). Degenerate primers for the conserved N-terminal sequence of dNDP-glucose 4,6-dehydratase genes (10) were kindly provided by A. Bechthold, and PCR amplification of S. spheroides NCIB 11891 chromosomal DNA yielded two different fragments which could be identified as dNDP-glucose 4,6-dehydratases by sequence homology.
A cosmid library from S. spheroides was constructed in SuperCosI, and screening was carried out by using the two different dNDP-glucose 4,6-dehydratase gene fragments as probes. This led to the detection of two different groups of cosmids. Group 1 hybridized with a 2.1-kb EcoRI fragment; group 2 hybridized with a 6-kb EcoRI fragment.
The cosmids were analyzed by conventional restriction mapping, as well as by hybridization of partial digests of cosmids to the SuperCosI vector sequences flanking the cosmid inserts (50). This showed that each group consisted of four different overlapping cosmids and contained only a single gene that hybridized with the respective probe.
Random sequencing of fragments from both clusters revealed a sequence in cosmid group 1 with a very high degree of homology to gyrBr, which encodes a novobiocin-resistant gyrase B subunit and which has been described as the principal novobiocin resistance gene in S. spheroides (61). Hybridization experiments with this gene as the probe confirmed its presence in all four cosmids of group 1, but no homologous sequence was found in the cosmids of group 2 (data not shown). Since resistance genes are usually part of the biosynthetic gene clusters of the respective antibiotics, cosmid group 1 likely contained the biosynthetic genes for novobiocin and was further analyzed by sequencing.
Sequence analysis of novobiocin gene cluster.
A total span of 53.1 kb was sequenced by single-strand sequencing, and a core sequence of 25.6 kb was analyzed by double-strand sequencing. The core sequence has been deposited in the GenBank database under accession no. AF170880. The deduced gene organization of the identified 23 putative ORFs within the core region is shown in Fig. 2A. Remarkably, 22 of the ORFs are oriented in the same direction. Table 1 lists the ORFs identified and the homologies to database entries identified.
FIG. 2.
Organization of the novobiocin biosynthetic gene cluster. (A) Map of the core sequence, obtained by double-strand sequencing of S. spheroides genomic DNA (cosmid 9-6G). The roman numerals after the proposed gene functions refer to the reactions marked in Fig. 1. (B) Schematic representation of constructs used for heterologous expression of novobiocic acid synthetase. ABC, ATP-binding cassette.
TABLE 1.
Deduced functions of identified ORFs
| ORF | Size (no. of amino acids) | Homology toa: | Proposed functionb | % Identity of product | Origin | Reference(s) | Nucleotide sequence accession no. |
|---|---|---|---|---|---|---|---|
| novV | 297 | dTDP-1-glucose synthase | I | 62 | Mycobacterium tuberculosis | 39 | U55242 |
| novT | 336 | dTDP-glucose 4,6-dehydratase | II | 61 | Saccharopolyspora erythraea | 34 | L37354.1 |
| novW | 207 | dTDP-4-keto-6-deoxyglucose 3,5-epimerase | III | 50 | Streptomyces griseus | 47 | P29783 |
| novS | 288 | dTDP-4-keto-6-deoxyhexose reductase | IV | 53 | Streptomyces griseus | 47 | P29781 |
| novU | 420 | Methyltransferase | V | 36 | Saccharopolyspora erythraea | 17, 18 | X60379 |
| novH | 600 | Peptide synthetase | VI? | 46 | Amycolatopsis orientalis | 64 | AJ223998 |
| novL | 527 | Acyl-CoA-synthetase | VI? | 41 | Streptomyces coelicolor | 51 | AL049763.1 |
| novM | 379 | Glycosyl transferase | VII | 45 | Streptomyces argillaceus | 14 | AF077869.1 |
| novP | 262 | O-Methyltransferase | VIII | 54 | Micromonospora griseorubida | 24 | D16097.1 |
| novN | 677 | O-Carbamoyltransferase | IX | 45 | Rhizobium sp. | 16 | P55474 |
| novO | 230 | Methyltransferase | X | 27 | Streptomyces lincolnensis | 45 | S44970 |
| novA | 635 | ABC transporter | 42 | Amycolatopsis orientalis | 64 | AJ223999 | |
| novB | 284 | Aminodeoxychorismate lyase (?) | Unknown | 34 | Mycobacterium tuberculosis | 7 | Z83863 |
| novC | 352 | Oxidoreductase (?) | Unknown | 40 | Streptomyces coelicolor | 51 | AL078610.1 |
| novD | 143 | Hypothetical protein | Unknown | 57 | Mycobacterium tuberculosis | 7 | Q50742 |
| novE | 217 | lmbU | Unknown | 45 | Streptomyces lincolnensis | 45 | S44974 |
| novF | 362 | Oxidoreductase | Unknown | 44 | Streptomyces coelicolor | 51 | AL078610.1 |
| novG | 318 | Regulatory protein | Regulator (?) | 47 | Streptomyces griseus | 13 | P08076 |
| novI | 407 | Cytochrome P-450 | Unknown | 38 | Amycolatopsis orientalis | 64 | AJ223998 |
| 36 | Pseudomonas incognita | 53 | L23310 | ||||
| novJ | 262 | 3-Ketoacyl-[ACP]-reductase | Unknown | 46 | Vibrio harveyi | 57 | P55336 |
| novK | 244 | Reductase (?) | Unknown | 32 | Klebsiella terrigena | 4 | Q04520 |
| novQ | 271 | Unknown | |||||
| novR | 270 | Unknown |
CoA, coenzyme A; ABC, ATP-binding cassette.
See Fig. 1.
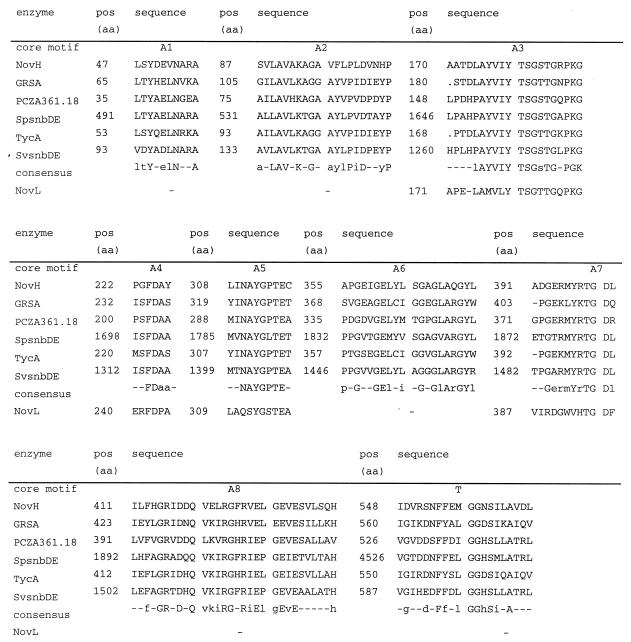
The deduced amino acid sequence of novH displays significant homology to several peptide synthetases (Fig. 3). Besides novH, novL was also found to be a member of the superfamily of adenylate-forming enzymes. Two conserved ATP binding motifs and the ATPase motif (TGD) could be detected in this ORF (Fig. 3). novM has sequence similarity to glycosyltransferases and contains the characteristic UDP-glycosyltransferase motif close to the C terminus of the enzyme (14). The protein encoded by novN is similar to the nodulation proteins of Rhizobium sp. (16) and Bradyrhizobium japonicum (38), which are responsible for the O-carbamoylation of nodulation factors, as well as to the protein encoded by cmcH of Streptomyces clavuligerus, which carries out the O-carbamoylation within cephamycin biosynthesis (8). novO, as well as novU, exhibited similarity to C-methyltransferases (17, 18, 45). novP is homologous to the O-methyltransferases involved in mycinamicin III and tylosin biosynthesis, i.e., mycF (24) and tylF (15), respectively. Both macrolides contain the O-methylated sugar mycinose. novP exhibits a region with similarity to a conserved methyltransferase motif [hh(D/E)hGxGxG, with h representing a hydrophobic residue] involved in S-adenosylmethionine binding (26). As in mycF and tylF, the glycine at position 7 in novP is replaced by a tryptophan.
FIG. 3.
Comparison of the conserved core motifs of the deduced amino acid (aa) sequence of NovH with other peptide synthetase modules and with NovL. Alignment was performed by using the DNASIS program. GRSA, gramicidin S-synthetase I from Bacillus cereus (32); PCZA361.18, peptide synthetase from Amycolatopsis orientalis (64); SpsnbDE, pristinamycin I synthetase 3 from Streptomyces pristinaespiralis (11); TycA, tyrocidine synthetase 1 from Bacillus brevis (44); SvsnbDE, virginiamycin S synthetase from Streptomyces virginiae (12). The consensus line contains capital letters for amino acids with 100% conservation and lowercase letters for conservative amino acid substitutions. Motifs A1 to A8 are core motifs of the adenylation domain, and motif T represents the 4′-phosphopantetheine cofactor attachment site (40).
Finally, immediately upstream of the novobiocin resistance gene gyrBr, five ORFs with homology to genes involved in 6-deoxyhexose biosynthesis were discovered (novS, novT, novU, novV, and novW).
In total, 11 of the 23 ORFs upstream of the gyrB gene could be assigned to a putative function in novobiocin biosynthesis (Table 1) on the basis of their similarity to known genes.
Gene insertional inactivation of the novobiocin biosynthetic gene cluster.
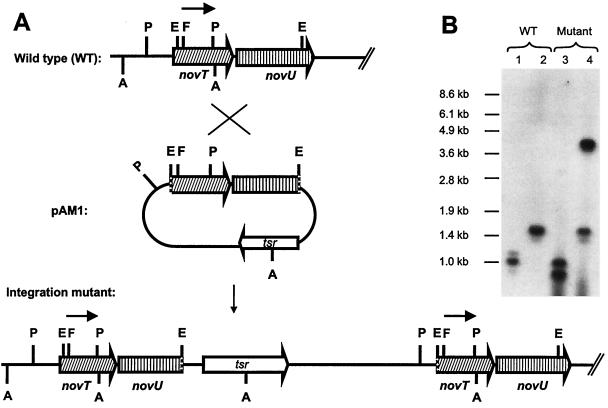
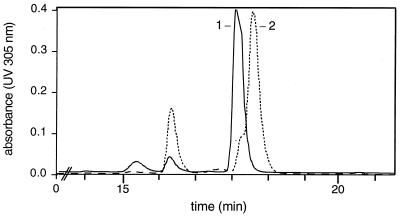
In order to confirm that the cloned sequence indeed represented the novobiocin biosynthetic gene cluster, an inactivation experiment was carried out with a 2.1-kb EcoRI fragment containing parts of the dTDP-glucose 4,6-dehydratase gene (novT) and of the putative C-methyltransferase gene (novU). This fragment was cloned into pBSKT, which carries a thiostrepton resistance gene, and the resulting plasmid was introduced into S. spheroides protoplasts. Resistant clones that arose from homologous recombination were isolated and analyzed by Southern hybridization with a 520-bp FspI-PstI probe derived from the dTDP-glucose 4,6-dehydratase gene; hybridization bands appeared as expected at 3.98 and 1.47 kb after restriction with AccI and at 1.07 and 0.91 kb after restriction with PvuII, proving integration of the 2.1-kb EcoRI fragment into chromosomal S. spheroides DNA by homologous recombination (Fig. 4). Thiostrepton-resistant transformants were tested for their secondary product formation by HPLC analysis. In contrast to the wild type, no novobiocin was detected, but another peak of similar UV absorption was observed (Fig. 5). This substance was isolated on a preparative scale. Both 1H-NMR and MS analyses (see Materials and Methods) allowed the unequivocal identification of this substance as novobiocic acid, i.e., the aglycone moiety of novobiocin (Fig. 1).
FIG. 4.
Insertional gene inactivation of deoxysugar biosynthesis. (A) Schematic presentation of the integration event. The 520-bp FspI-PstI-DNA fragment used as the probe is indicated as a black arrow. Relevant restriction sites are identified as follows: A, AccI; E, EcoRI; F, FspI; P, PvuII. tsr, Thiostrepton resistance. Hatched arrows indicate genes. (B) Southern analysis of S. spheroides NCIB 11891 wild type (WT) and a disrupted mutant. Lanes 1 and 2, genomic DNA of the wild type restricted with PvuII in lane 1 (expected band at 1.07 kb) and with AccI in lane 2 (expected band at 1.47 kb); lanes 3 and 4, genomic DNA of an integration mutant restricted with PvuII in lane 3 (expected bands at 1.07 and 0.91 kb) and AccI in lane 4 (expected bands at 1.47 and 3.98 kb).
FIG. 5.
HPLC chromatogram of secondary metabolites of S. spheroides NCIB 11891 (wild type, dotted line) and of an integration mutant (solid line). Peak 1, novobiocic acid; peak 2, novobiocin. See text for further explanations.
Heterologous expression of novobiocic acid synthetase in S. lividans TK24.
A key reaction in the biosynthesis of novobiocin is the formation of the amide bond between ring A and ring B in an ATP-dependent reaction catalyzed by novobiocic acid synthetase (Fig. 1). An assay method for this enzyme, as well as the chemical synthesis of ring A and ring B from novobiocin, has been described previously (30). In our sequence analysis, NovH was shown to have homology to peptide synthetases and therefore was a likely candidate for the enzyme catalyzing the novobiocic acid synthetase reaction.
A 9.7-kb EcoRI fragment containing the genes from novH to novN was cloned into pEM4, a Streptomyces-E. coli shuttle vector containing the ermE up promoter for foreign gene expression, resulting in plasmid pSL3(+) (Fig. 2B). This construct was transformed into S. lividans TK24. Novobiocic acid synthetase activity could clearly be detected in cell extracts from that transformant but not in extracts from S. lividans transformed with an empty vector (Table 2).
TABLE 2.
Heterologous expression of novobiocic acid synthetase in S. lividans TK24
| Organism/plasmid | ORF includeda
|
Novobiocic acid synthetase activity (pkat/mg of protein)b | ||||||
|---|---|---|---|---|---|---|---|---|
| novH | novI | novJ | novK | novL | novM | novN | ||
| S. spheroidesc | + | + | + | + | + | + | + | 53.97 |
| S. lividans TK24/pSL3(+): | ||||||||
| Full assay | + | + | + | + | + | + | + | 9.07 |
| ring A | <0.014 | |||||||
| ring B | <0.014 | |||||||
| ATP | <0.014 | |||||||
| Heat-denaturated enzyme | <0.014 | |||||||
| S. lividans TK24/pMS73 | + | + | + | + | + | 0 | 0 | 229.63 |
| S. lividans TK24/pMS75 | + | + | + | + | 0 | 0 | 0 | <0.014 |
| S. lividans TK24/pMS71 | + | + | + | 0 | 0 | 0 | 0 | <0.014 |
| S. lividans TK24/pMS65 | + | 0 | 0 | 0 | 0 | 0 | 0 | <0.014 |
| S. lividans TK24/pUWL201d | 0 | 0 | 0 | 0 | 0 | 0 | 0 | <0.014 |
See Fig. 2 for the exact length of the plasmid insert. +, ORF is included; 0, ORF is not included.
Data are mean values from experiments with two independent transformants, with each assay performed in duplicate.
S. spheroides is the producer of novobiocin and contains the novobiocin biosynthetic genes in the genomic DNA.
Empty vector.
In order to determine exactly which ORFs are required for novobiocic acid synthetase activity, additional constructs were prepared by successive shortening of the 9.7-kb insert of pSL3(+) (Fig. 2). These constructs were expressed in S. lividans under control of the ermE up promoter, and novobiocic acid synthetase activity was examined. For each construct, two independent transformants were analyzed, and each plasmid was reisolated from S. lividans and was confirmed to be intact by restriction analysis. Of the constructs examined, only pSL3(+) and pMS73 conferred novobiocic acid synthetase activity, proving that novL is essential for the reaction (Table 2).
DISCUSSION
The present study describes, for the first time, the cloning of a biosynthetic gene cluster of an aminocoumarin antibiotic. This class of antibiotics comprises novobiocin and two closely related compounds, coumermycin A1 and clorobiocin (41); the characteristic 3-amino-4,7-dihydroxycoumarin moiety is also found in a number of other antibiotics, such as protorubradirin (2).
In the novobiocin producer S. spheroides, we detected two different dNDP-glucose 4,6-dehydratase genes. Screening of a cosmid library with these two genes as probes revealed two distinct cosmid groups. The novobiocin resistance gene gyrBr was found in only one of the two cosmid groups, and the gyrB gene-containing group was shown to contain the novobiocin biosynthetic gene cluster. The other dNDP-glucose 4,6-dehydratase gene may therefore be involved in the biosynthesis of a metabolite different from novobiocin.
Sequence analysis of the novobiocin biosynthetic gene cluster revealed several genes for which possible roles in novobiocin biosynthesis could be putatively assigned by their homology to known genes in the database. Especially, an analogy to the biosynthesis of other deoxysugars and the results of earlier feeding experiments with novobiocin (3, 33) permit one to make a detailed hypothesis about the biosynthesis of the noviose moiety and the genes involved (Fig. 1). Glucose-1-phosphate is expected to be activated by nucleotidylation to give dTDP-glucose (Fig. 1, reaction I) and to be further converted to dTDP-4-keto-6-deoxyglucose under catalysis of an dNDP-glucose 4,6-dehydratase (reaction II). Subsequent steps include 3,5-epimerization and 4-ketoreduction (reactions III and IV). C-methylation at C-5" of the sugar (reaction V) may be the last of these five steps, but it could also occur at an earlier stage.
As shown in Table 1 and Fig. 2, four ORFs, grouped closely together immediately upstream of the gyrBr gene, could be fitted perfectly to the proposed noviose biosynthetic pathway: NovV, NovT, NovW, and NovS may catalyze reactions I to IV, respectively, of the reaction sequence shown in Fig. 1.
Both novU and novO show similarity to methyltransferases and represent candidates for the C-methylation reactions at C-5" of noviose (Fig. 1, reaction V) and at C-8′ of ring B (Fig. 1, reaction X). NovU resembles EryBIII, which has been functionally shown to catalyze the C-methylation in the biosynthesis of dTDP-l-mycarose, the deoxysugar moiety of erythromycin, in Saccharopolyspora erythraea (17). This, as well as its association with the four other deoxyhexose biosynthetic genes, suggests that NovU rather than NovO catalyzes the C-5" methylation in noviose biosynthesis. The 4.8-kb fragment that comprises novS, novT, novU, novV, and novW therefore most likely contains the entire biosynthetic information for the biosynthesis of the deoxysugar noviose except 4-O-methylation, which takes place at a later stage.
C-4" O-methylation and C-3" O-carbamoylation (Fig. 1, reactions VIII and IX, respectively) are regarded as the last steps in novobiocin biosynthesis (31). Sequence data show two ORFs, novP and novN, which can putatively be assigned as O-methyltransferase and O-carbamoyltransferase, respectively.
Little information is available on the biosynthetic steps involved in the biosynthesis of ring A and ring B (Fig. 1). At present, an assignment of the genes detected in our study to reactions in the biosynthesis of rings A and B would be purely speculative. Formation of ring A includes a prenylation reaction, and the responsible dimethylallyl diphosphate:4-hydroxyphenylpyruvate dimethylallyl transferase has been identified in cell extracts of S. spheroides (60). Unfortunately, the presently known prenyltransferases that attach a prenyl group to an aromatic ring share very little homology. So far, none of the ORFs detected in our study can be suggested to be likely candidates for this enzyme.
Downstream of the gyrBr gene, several ORFs with homology to primary metabolic enzymes were detected, e.g., ORFs with homology to glyceraldehyde 3-phosphate dehydrogenase and to peptide and sugar transporters (data not shown). This suggests that the gyrBr gene may represent the right border of the cluster in Fig. 2. In contrast, the position of the border on the left end remains ambiguous, since, except for a subtilisin-like protease and two ATP-binding cassette transporters, no meaningful homologies were found in the region to the left of the core sequence depicted in Fig. 2 (data not shown).
Functional proof that the sequence that was identified contains the novobiocin gene cluster was provided by an insertional gene inactivation experiment. The resulting integration mutants contain a tandem duplication of novT and novU, including one intact and one truncated copy of each gene (Fig. 4). The integration led to a complete abolishment of novobiocin production, and the mutants accumulated the aglycone of novobiocin, i.e., novobiocic acid, instead. Since no promoter is apparently located between novT and novU (intergenic region, 54 bp), integration most likely disrupted the transcription unit that comprises the genes involved in deoxysugar formation.
Conflicting hypotheses have been made about the sequence of the linkage of the three rings, rings A, B, and C (Fig. 1), in the biosynthesis of novobiocin (19, 29). The fact that novobiocic acid (ring A and ring B) accumulated after disruption of deoxysugar (ring C) biosynthesis suggests that formation of the amide bond between ring A and ring B takes place prior to glycosylation. The gene product of novM is a likely candidate for the glycosyltransferase that connects ring C to novobiocic acid (Fig. 1, reaction VII).
Further proof for the function of the sequence was given by the heterologous expression of novobiocic acid synthetase. This enzyme catalyzes the linkage of ring A and ring B by an ATP-dependent amide bond formation (Fig. 1, reaction VI). Novobiocic acid synthetase activity could be demonstrated upon heterologous expression of a 9.7-kb fragment which included novH and six other ORFs of the cluster (Table 2). novH showed significant homologies to several peptide synthetases involved in the biosynthesis of biologically active peptides and contained eight conserved motifs expected in the adenylation domain of a single peptide synthetase module, as well as the 4′-phosphopantetheine attachment motif (Fig. 3). The general scheme of nonribosomal peptide bond formation (for reviews, see references 28 and 40) includes activation of each amino acid (or amino acid derivative) as an aminoacyl adenylate, followed by covalent thioester linkage with an enzyme-attached 4′-phosphopantetheine cofactor and transfer of the activated carboxyl group to the amino group of the next acyl intermediate; for each amino acid (or derivative) included in the peptide, these functions are assembled on a distinct peptide synthetase module. Amide bond formation between ring A and ring B of novobiocin by a similar mechanism may therefore require the existence of a second peptide synthetase module, and in fact, NovH alone was found to be unable to catalyze novobiocic acid formation (Table 2). Successive shortening of the 9.7-kb fragment (Table 2) showed that the inclusion of novL was essential for the enzymatic activity. novL is a further member of the superfamily of adenylate-forming enzymes (63). The deduced amino acid sequence exhibited ATP binding and ATPase motifs (Fig. 3) and showed homology to acyl-coenzyme A synthetases (51, 21), which are similar to peptide synthetases in their adenylation domain. Further enzymatic and genetic investigations are now in progress to elucidate the exact roles of novH and novL for the novobiocic acid synthetase reaction.
ACKNOWLEDGMENTS
We thank D. A. Hopwood and E. Cundliffe for providing Streptomyces strains, A. Bechthold, J. A. Salas, and W. Piepersberg for donation of shuttle vectors pEM4, pBSKT, and pUWL201, and Pharmacia & Upjohn for supply of novobiocic acid and ring B. A. Bechthold, W. Wohlleben, and coworkers provided important technical advice for this project. We also thank Susanne Hennig and Emmanuel Wemakor for excellent technical assistance.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (to L. Heide and S.-M. Li).
REFERENCES
- 1.Altenbuchner J, Cullum J. DNA amplification and an unstable arginine gene in Streptomyces lividans 66. Mol Gen Genet. 1984;195:134–138. doi: 10.1007/BF00332735. [DOI] [PubMed] [Google Scholar]
- 2.Bannister B, Zapotocky B A. Protorubradirin, an antibiotic containing a C-nitroso-sugar fragment, is the true secondary metabolite produced by Streptomyces achromogenes var. rubradiris. Rubradirin, described earlier, is its photo-oxidation product. J Antibiot Tokyo. 1992;45:1313–1324. doi: 10.7164/antibiotics.45.1313. [DOI] [PubMed] [Google Scholar]
- 3.Birch A J, Holloway P W, Rickards R W. The biosynthesis of noviose, a branched-chain monosaccharide. Biochim Biophys Acta. 1962;57:143–145. doi: 10.1016/0006-3002(62)91090-9. [DOI] [PubMed] [Google Scholar]
- 4.Blomqvist K, Nikkola M, Lehtovaara P, Suihko M-L, Airaksinen U, Straby K B, Knowles J K C, Penttilä M E. Characterization of the genes of the 2,3-butanediol operons from Klebsiella terrigena and Enterobacter aerogenes. J Bacteriol. 1993;175:1392–1404. doi: 10.1128/jb.175.5.1392-1404.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Bunton C A, Kenner G W, Robinson M J T, Webster B R. Experiments related to the biosynthesis of novobiocin and other related coumarins. Tetrahedron. 1963;19:1001–1010. [Google Scholar]
- 7.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver S, Osborne J, Quail M A, Rajandream M A, Rogers J, Rutter S, Seeger K, Skelton S, Squares S, Sqares R, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 8.Coque J J R, Pérez-Llarena F J, Enguita F J, Fuente J L, Martin J F, Liras P. Characterization of the cmcH genes of Nocardia lactamdurans and Streptomyces clavuligerus encoding a functional 3′-hydroxymethylcephem O-carbamoyltransferase for cephamycin biosynthesis. Gene. 1995;162:21–27. doi: 10.1016/0378-1119(95)00308-s. [DOI] [PubMed] [Google Scholar]
- 9.Cushing K E. Genetic and biochemical studies of the regulation of novobiocin biosynthesis in Streptomyces niveus. Ph.D. thesis. Liverpool, United Kingdom: University of Liverpool; 1989. [Google Scholar]
- 10.Decker H, Gaisser S, Pelzer S, Schneider P, Westrich L, Wohlleben W, Bechthold A. A general approach for cloning and characterizing dNDP-glucose dehydratase genes from actinomycetes. FEMS Microbiol Lett. 1996;141:195–201. doi: 10.1111/j.1574-6968.1996.tb08384.x. [DOI] [PubMed] [Google Scholar]
- 11.De Crécy-Lagard V, Blanc V, Gil P, Naudin L, Lorenzon S, Famechon A, Bamas-Jaques N, Crouzet J, Thibaut D. Pristinamicin I biosynthesis in Streptomyces pristinaespiralis: molecular characterization of the first two structural peptide synthetase genes. J Bacteriol. 1997;179:705–713. doi: 10.1128/jb.179.3.705-713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De-Cécy-Lagard V, Saurin W, Thibaut D, Gil P, Naudin L, Crouzet J, Blanc V. Streptogramin B biosynthesis in Streptomyces pristinaespiralis and Streptomyces virginiae: molecular characterization of the last structural peptide synthetase gene. Antimicrob Agents Chemother. 1997;41:1904–1909. doi: 10.1128/aac.41.9.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Distler J, Ebert A, Mansouri K, Pissowotzki K, Stockmann M, Piepersberg W. Gene cluster for streptomycin biosynthesis in Streptomyces griseus: nucleotide sequence of three genes and analysis of transcriptional activity. Nucleic Acids Res. 1987;15:8041–8056. doi: 10.1093/nar/15.19.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández E, Weißbach U, Reillo C S, Braña A F, Méndez C, Rohr J, Salas J A. Identification of two genes from Streptomyces argillaceus encoding glycosyltransferases involved in transfer of a disaccharide during biosynthesis of the antitumor drug mithramycin. J Bacteriol. 1998;180:4929–4937. doi: 10.1128/jb.180.18.4929-4937.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouces R, Mellado E, Díez B, Barredo J L. The tylosin biosynthetic cluster from Streptomyces fradiae: genetic organization of the left region. Microbiology. 1999;145:855–868. doi: 10.1099/13500872-145-4-855. [DOI] [PubMed] [Google Scholar]
- 16.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 17.Gaisser S, Böhm G A, Doumith M, Raynal M-C, Dhillon N, Cortés J, Leadlay P F. Analysis of eryBI, eryBIII and eryBVII from the erythromycin biosynthetic gene cluster in Saccharopolyspora erythraea. Mol Gen Genet. 1998;258:77–88. doi: 10.1007/s004380050709. [DOI] [PubMed] [Google Scholar]
- 18.Haydock S F, Dowson J A, Dhillon N, Roberts G A, Cortes J, Leadlay P F. Cloning and sequence analysis of genes involved in erythromycin biosynthesis in Saccharopolyspora erythraea: sequence similarities between EryG and a family of S-adenosylmethionine-dependent methyltransferases. Mol Gen Genet. 1991;230:120–128. doi: 10.1007/BF00290659. [DOI] [PubMed] [Google Scholar]
- 19.Hoggarth J H, Cushing K E, Ritchie D A. Genetic and functional analysis of novobiocin-producing mutants of Streptomyces niveus. J Appl Bacteriol. 1995;79:625–630. doi: 10.1111/j.1365-2672.1995.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 20.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces—a laboratory manual. Norwich, England: The John Innes Foundation; 1985. [Google Scholar]
- 21.Hu W J, Kawaoka A, Tsai C J, Lung J, Osakabe K, Ebinuma H, Chiang V L. Compartmentalized expression of two structurally and functionally distinct 4-coumarate:CoA ligase genes in aspen (Populus tremuloides) Proc Natl Acad Sci USA. 1998;95:5407–5412. doi: 10.1073/pnas.95.9.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain H A, Ritchie D A. High frequency transformation of Streptomyces niveus protoplasts by plasmid DNA. J Appl Bacteriol. 1991;71:422–427. doi: 10.1111/j.1365-2672.1991.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 23.Hutchinson C R. Combinatorial biosynthesis for new drug discovery. Curr Opin Microbiol. 1998;1:319–329. doi: 10.1016/s1369-5274(98)80036-2. [DOI] [PubMed] [Google Scholar]
- 24.Inouye M, Suzuki H, Takada Y, Muto N, Horinouchi S, Beppu T. A gene encoding mycinamicin III O-methyltransferase from Micromonospora griseorubida. Gene. 1994;141:121–124. doi: 10.1016/0378-1119(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson J R, Hutchinson C R, Cane D E, Khosla C. Precursor-directed biosynthesis of erythromycin analogs by an engineered polyketide synthase. Science. 1997;277:367–369. doi: 10.1126/science.277.5324.367. [DOI] [PubMed] [Google Scholar]
- 26.Kagan R M, Clarke S. Widespread occurrence of three sequence motifs in diverse S-adenosylmethionine-dependent methyltransferases suggests a common structure for these enzymes. Arch Biochem Biophys. 1994;310:417–427. doi: 10.1006/abbi.1994.1187. [DOI] [PubMed] [Google Scholar]
- 27.Kampranis S C, Gormley N A, Tranter R, Orphanides G, Maxwell A. Probing the binding of coumarins and cyclothialidines to DNA gyrase. Biochemistry. 1999;38:1967–1976. doi: 10.1021/bi982320p. [DOI] [PubMed] [Google Scholar]
- 28.Kleinkauf H, von Döhren H. A nonribosomal system of peptide biosynthesis. Eur J Biochem. 1996;236:335–351. doi: 10.1111/j.1432-1033.1996.00335.x. [DOI] [PubMed] [Google Scholar]
- 29.Kominek L A. Biosynthesis of novobiocin by Streptomyces niveus. Antimicrob Agents Chemother. 1972;1:123–134. doi: 10.1128/aac.1.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kominek L A, Meyer H F. Novobiocic acid synthetase. Methods Enzymol. 1975;43:502–508. doi: 10.1016/0076-6879(75)43111-1. [DOI] [PubMed] [Google Scholar]
- 31.Kominek L A, Sebek O K. Biosynthesis of novobiocin and related coumarin antibiotics. Dev Ind Microbiol. 1974;15:60–69. [Google Scholar]
- 32.Krätzschmar J, Krause M, Marahiel M A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J Bacteriol. 1989;171:5422–5429. doi: 10.1128/jb.171.10.5422-5429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S-M, Hennig S, Heide L. Biosynthesis of the dimethylallyl moiety of novobiocin via a non-mevalonate pathway. Tetrahedron Lett. 1998;39:2717–2720. [Google Scholar]
- 34.Linton K J, Jarvis B W, Hutchinson R C. Cloning of the genes encoding thymidine diphosphoglucose 4,6-dehydratase and thymidine diphospho-4-keto-6-deoxyglucose 3,5-epimerase from the erythromycin-producing Saccharopolyspora erythraea. Gene. 1995;153:33–40. doi: 10.1016/0378-1119(94)00809-7. [DOI] [PubMed] [Google Scholar]
- 35.Liu H-W, Thorson J S. Pathways and mechanisms in the biogenesis of novel deoxysugars by bacteria. Annu Rev Microbiol. 1994;48:223–256. doi: 10.1146/annurev.mi.48.100194.001255. [DOI] [PubMed] [Google Scholar]
- 36.Lombó F, Siems K, Braña A F, Méndez C, Bindseil K, Salas J A. Cloning and insertional inactivation of Streptomyces argillaceus genes involved in the earliest steps of biosynthesis of the sugar moieties of the antitumor polyketide mithramycin. J Bacteriol. 1997;179:3354–3357. doi: 10.1128/jb.179.10.3354-3357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorico A, Rappe G, Sartorelli A C. Novobiocin-induced accumulation of etoposide (VP-16) in WEHI-3B D+ leukemia cells. Int J Cancer. 1992;52:903–909. doi: 10.1002/ijc.2910520613. [DOI] [PubMed] [Google Scholar]
- 38.Luka S, Sanjuan J, Carlson R W, Stacey G. nolMNO genes of Bradyrhizobium japonicum are co-transcribed with nodYABCSUIJ, and nolO is involved in the synthesis of the lipo-oligosaccharide nodulation signals. J Biol Chem. 1993;268:27053–27059. [PubMed] [Google Scholar]
- 39.Ma Y, Mills J A, Belisle J T, Vissa V, Howell M, Bowlin K, Scherman M S, McNeil M. Determination of the pathway for rhamnose biosynthesis in mycobacteria: cloning, sequencing and expression of the Mycobacterium tuberculosis gene encoding alpha-d-glucose-1-phosphate thymidylyltransferase. Microbiology. 1997;143:937–945. doi: 10.1099/00221287-143-3-937. [DOI] [PubMed] [Google Scholar]
- 40.Marahiel M A, Stachelhaus T, Mootz H D. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem Rev. 1997;97:2651–2673. doi: 10.1021/cr960029e. [DOI] [PubMed] [Google Scholar]
- 41.Maxwell A. DNA gyrase as a drug target. Trends Microbiol. 1997;5:102–109. doi: 10.1016/S0966-842X(96)10085-8. [DOI] [PubMed] [Google Scholar]
- 42.McDaniel R, Thamchaipenet A, Gustafsson C, Fu H, Betlach M, Betlach M, Ashley G. Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel “unnatural” natural products. Proc Natl Acad Sci USA. 1999;96:1846–1851. doi: 10.1073/pnas.96.5.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell J I, Logan P G, Cushing K E, Ritchie D A. Novobiocin-resistance sequences from the novobiocin-producing strain Streptomyces niveus. Mol Microbiol. 1990;4:845–849. doi: 10.1111/j.1365-2958.1990.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 44.Mootz H D, Marahiel M A. The tyrocidine biosynthesis operon of Bacillus brevis: complete nucleotide sequence and biochemical characterization of functional internal adenylation domains. J Bacteriol. 1997;179:6843–6850. doi: 10.1128/jb.179.21.6843-6850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peschke U, Schmidt H, Zhang H-Z, Piepersberg W. Molecular characterization of the lincomycin-production gene cluster of Streptomyces lincolnensis 78-11. Mol Microbiol. 1995;16:1137–1156. doi: 10.1111/j.1365-2958.1995.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 46.Piepersberg W. Pathway engineering in secondary metabolite-producing actinomycetes. Crit Rev Biotechnol. 1994;14:251–285. doi: 10.3109/07388554409079835. [DOI] [PubMed] [Google Scholar]
- 47.Pissowotzki K, Mansouri K, Piepersberg W. Genetics of streptomycin production in Streptomyces griseus: molecular structure and putative function of genes strELMB2N. Mol Gen Genet. 1991;231:113–123. doi: 10.1007/BF00293829. [DOI] [PubMed] [Google Scholar]
- 48.Quirós L M, Aguirrezabalaga I, Olano C, Méndez C, Salas J A. Two glycosyltransferases and a glycosidase are involved in oleandomycin modification during its biosynthesis by Streptomyces antibioticus. Mol Microbiol. 1998;28:1177–1185. doi: 10.1046/j.1365-2958.1998.00880.x. [DOI] [PubMed] [Google Scholar]
- 49.Rappa G, Lorico A, Sartorelli A C. Potentiation by novobiocin of the cytotoxic activity of etoposide (VP-16) and teniposide (VM-26) Int J Cancer. 1992;51:780–787. doi: 10.1002/ijc.2910510519. [DOI] [PubMed] [Google Scholar]
- 50.Redenbach M, Ikeda K, Yamasaki M, Kinashi H. Cloning and physical mapping of the EcoRI fragments of the giant linear plasmid SCP1. J Bacteriol. 1998;180:2796–2799. doi: 10.1128/jb.180.10.2796-2799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 52.Ritchie D A, Cushing K E, Logan P G, Mitchell J I. Expression of genes coding for novobiocin biosynthesis and novobiocin resistance in Streptomyces niveus. In: Zelinka J, Balan J, editors. Proceedings of the 6th International Symposium on Metabolism and Enzymology of Nucleic Acids Including Gene Manipulation. New York, N.Y: Plenum Press; 1988. pp. 155–159. [Google Scholar]
- 53.Ropp J D, Gunsalus I C, Sligar S G. Cloning and expression of a member of a new cytochrome P-450 family: cytochrome P-450lin (CYP111) from Pseudomonas incognita. J Bacteriol. 1993;175:6028–6037. doi: 10.1128/jb.175.18.6028-6037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 55.Schneider A, Stachelhaus T, Marahiel M A. Targeted alteration of the substrate specificity of peptide synthetases by rational module swapping. Mol Gen Genet. 1998;257:308–318. doi: 10.1007/s004380050652. [DOI] [PubMed] [Google Scholar]
- 56.Shen Y, Yoon P, Yu T W, Floss H G, Hopwood D, Moore B S. Ectopic expression of the minimal whiE polyketide synthase generates a library of aromatic polyketides of diverse sizes and shapes. Proc Natl Acad Sci USA. 1999;96:3622–3627. doi: 10.1073/pnas.96.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen Z, Byers D M. Isolation of Vibrio harveyi acyl carrier protein and the fabG, acpP, and fabF genes involved in fatty acid biosynthesis. J Bacteriol. 1996;178:571–573. doi: 10.1128/jb.178.2.571-573.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirahama T, Furumai T, Okanishi M. A modified regeneration method for streptomycete protoplasts. Agric Biol Chem. 1981;45:1271–1273. [Google Scholar]
- 59.Stachelhaus T, Schneider A, Marahiel M A. Rational design of peptide antibiotics by targeted replacement of bacterial and fungal domains. Science. 1995;269:69–72. doi: 10.1126/science.7604280. [DOI] [PubMed] [Google Scholar]
- 60.Steffensky M, Li S-M, Vogler B, Heide L. Novobiocin biosynthesis in Streptomyces spheroides: identification of a dimethylallyl diphosphate:4-hydroxyphenylpyruvate dimethylallyl transferase. FEMS Microbiol Lett. 1998;161:69–74. [Google Scholar]
- 61.Thiara A S, Cundliffe E. Cloning and characterization of a DNA gyrase B gene from Streptomyces sphaeroides that confers resistance to novobiocin. EMBO J. 1988;7:2255–2259. doi: 10.1002/j.1460-2075.1988.tb03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thiara A S, Cundliffe E. Expression and analysis of two gyrB genes from the novobiocin producer, Streptomyces sphaeroides. Mol Microbiol. 1993;8:495–506. doi: 10.1111/j.1365-2958.1993.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 63.Turgay K, Krause M, Marahiel M A. Four homologous domains in the primary structure of GrsB are related to domains in a superfamily of adenylate-forming enzymes. Mol Microbiol. 1992;6:529–546. doi: 10.1111/j.1365-2958.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 64.Van Wageningen A M, Kirkpatrick P N, Williams D H, Harris B R, Kershaw J K, Lennard N J, Jones M, Jones S J, Solenberg P J. Sequencing and analysis of genes involved in the biosynthesis of a vancomycin group antibiotic. Chem Biol. 1998;5:155–162. doi: 10.1016/s1074-5521(98)90060-6. [DOI] [PubMed] [Google Scholar]