Abstract
Background: Peripheral nerve injuries may result in pain, disability, and decreased quality of life (QoL). Pain is an incompletely understood experience and is associated with emotional and behavioral qualities. We hypothesized that pain following peripheral nerve surgery could be predicted by changes in emotions or QoL postoperatively. Methods: Using prospectively collected data, a retrospective study design was used to evaluate the relationships among pain, QoL, and psychosocial factors in patients who underwent peripheral nerve surgery. Patients completed questionnaires rating pain; impact of pain on QoL, sadness, depression, frustration, anger, and hopefulness before surgery; and each postoperative follow-up visit. Multilevel modeling was used to assess the concurrent and lagged relationships between pain and psychosocial factors. Results: Increased pain was concurrently associated with decreased hopefulness (P = .001) and increased the impact on QoL, sadness, depression, and anger (P < .001). In lagged analyses, the impact on QoL and anger prospectively predicted pain (P < .001 and P = .02, respectively). Pain predicted subsequent scores of QoL, sadness, depression, anger, and hopefulness (P < .01). Having an upper limb nerve injury and self-report of “no comment for childhood trauma” were predictors of postsurgical pain. Conclusion: Psychosocial measures and pain are reciprocally related among patients who underwent surgery for peripheral nerve injuries or compression. Our study provides evidence of the important relationships among psychosocial factors, pain, and outcome and identifies treatment targets following nerve surgery.
Keywords: quality of life, nerve injury, nerve, diagnosis, pain, psychosocial factors
Introduction
Upper extremity peripheral nerve injuries may cause not only a devastating loss of sensory and motor function, but can also lead to chronic, debilitating neuropathic pain and lifelong decreased quality of life (QoL).1-6 Surgical options for the treatment of peripheral nerve injuries have evolved significantly over the past 2 decades.7,8 Surgical options for the treatment of pain resulting from nerve injury include nerve decompression, neuroma excision, 9 nerve allografting, 10 and, more recently, targeted muscle regeneration and regenerative peripheral nerve interfaces.11,12
Chronic neuropathic pain is estimated to affect an estimated 7% to 10% of the general population, resulting in substantial physical dysfunction, suffering, and reduced QoL.13,14 Neuropathic pain occurs as a result of damage and dysfunction of the pain neuraxis (including the peripheral and central nervous systems). The exact mechanisms related to the development of chronic neuropathic pain have not been identified. However, neuropathic pain following a peripheral nerve injury results in maladaptive changes to the brain and spinal cord that maintain and amplify pain through a process known as central sensitization. 15 It is hypothesized that these changes to the central nervous system result in the persistence of pain. Psychosocial variables such as the pain attentional state, emotional factors, and the context and meaning of pain can alter the perception and experience of pain. 16 Studies have shown that individuals with upper extremity injuries experience emotional and physiological stress due to pain.17-19 In addition, negative emotions before surgery have been associated with adverse pain outcomes. 20
High levels of negative emotions, such as depression, anxiety, and catastrophizing before surgery, are associated with adverse pain outcomes. 20 Furthermore, a patient’s negative beliefs and decreased perceived QoL may increase the risk of persistent postsurgical pain and disability following major orthopedic trauma. 21 Individuals are also at greater risk of chronic pain following a history of childhood abuse or maltreatment, due to long-lasting changes to the developing brain.22,23 Thus, it may be helpful to measure health-related QoL, child abuse history, and current emotions when evaluating patients for nerve surgery and postoperative improvement. 24
Because pain is associated with emotions (such as sadness, anger, depression, and hopefulness) and QoL, some studies have recommended that these factors should be assessed preoperatively and postoperatively in patients undergoing peripheral nerve surgery. 24 We sought to identify whether emotional or QoL measures could reciprocally relate to a decrease in pain following surgery in patients with peripheral nerve injuries or compression. We hypothesized that emotional factors and QoL would be predictive of postoperative pain, and that pain would be predictive of emotional factors and QoL. The results of this study may provide insights that patient-reported measures could assist in informing patients, families, and surgeons after surgery.
Materials and Methods
A retrospective review of prospectively collected data was used to identify patients who had undergone surgery for peripheral nerve injuries or compression between July 2015 and December 2017. Study participants were selected from patients who attended a center for nerve injury and paralysis. Data were collected on 383 participants, and 331 were included in the study. Patients were included if they had a peripheral nerve injury or compression, reported pain, underwent operative management, and returned for at least 2 postoperative follow-up visits. Exclusion criteria included non–English-speaking patients, and 52 participants were excluded due to multiple surgeries for pain relief. Demographic, nerve injury, and surgical data were obtained from patients’ medical records. This study was approved by our institutional review board.
Outcome Measures
At each pre- and postoperative visit, patients completed a Pain Questionnaire.1,3,25-27 This questionnaire contains 33 items, including visual analogue scales for subjective reporting of pain and the impact on QoL, sadness, depression, frustration, anger, and hopefulness; 20 descriptive pain adjectives; and a 21-item questionnaire to capture information about their pain experience, including a history of abuse as an adult and child.
Statistical Analyses
Multilevel modeling (MLM) was used to analyze the longitudinal relationships between pain and psychosocial factors (anger, hopefulness, depression, sadness, and QoL; Figure 1). Compared with conventional regression analyses, MLM is more complete and precise for analyzing longitudinal data. 28 The use of maximum likelihood estimation can also account for missing data and varying time intervals between outcome measurements and different numbers of observations in each individual. 29 A step-up approach was used in building the models. All models began with baseline visit pain ratings (random intercepts, model 0) and days since baseline visit (fixed slope, model 1; random slopes, model 2). If model 2 was found to be the best model, we explored the optimal effect of time by building and comparing different curvilinear models (linear, quadratic, and cubic models). Models were compared and selected based on the Akaike information criterion, Bayesian information criterion, and Wald tests for the change in deviance with α levels at 0.05. 30 Next, we examined the reciprocal relationships between pain and psychosocial factors. Specifically, 3 sets of analyses were conducted for each psychosocial variable to examine: (1) concurrent associations: psychosocial factors as predictors of same-visit pain; and (2) lagged associations on the next visit: psychosocial factors as predictors of pain on the next visit and pain as a predictor of psychosocial factors on the next visit. For these models, days since baseline visit (time) was nested within patients. Predictors and covariates including sex, symptom duration, surgical procedure, and childhood trauma were treated as fixed factors. Initial pain ratings at the baseline visit (intercepts), days since baseline visit (individual slopes), and covariance between intercepts and slopes were modeled as random effects. All statistical analyses were conducted using R (R Foundation for Statistical Computing, Vienna, Austria). 31
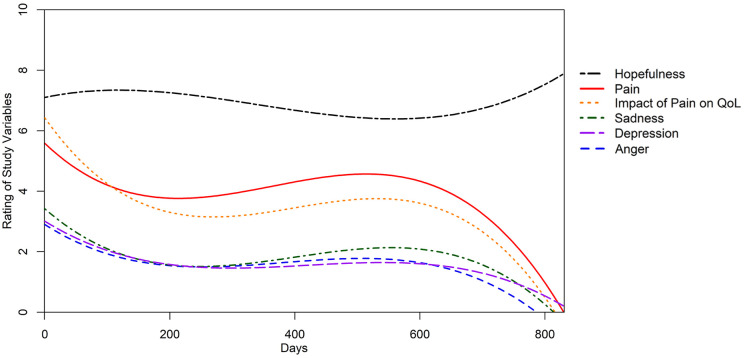
Figure 1.
The dynamic relationship of pain trajectory and all 5 psychosocial trajectories. With time, the intensity of the measured variables fluctuates in a sigmoidal fashion.
Note. QoL = quality of life.
Results
There were 331 patients (51% men; mean age, 50 years) included in this study (Table 1). Most of the nerve injuries occurred in the upper limb (n = 274, 83%). Patients underwent nerve decompression or transposition (n = 225, 68%), neuroma excision (n = 43, 13%), nerve grafting (n = 10, 3%), and nerve transfer (n = 53, 16%). There were 26 (8%) patients who reported childhood trauma, whereas 10 (3%) reported “no comment.” The longitudinal relationships of pain and psychosocial factors (anger, hopefulness, depression, sadness, and QoL) were distributed in a sigmoidal pattern, indicating the dynamic relationship of these factors over time (Figure 1).
Table 1.
Patient Demographics (N = 331).
| Variable | |
|---|---|
| Age, (mean ± SD) years | 50 ± 18 |
| Sex | 51% male, 49% female |
| Body mass index (median ± SD), kg/m2 | 27.5 ± 7.3 |
| Follow-Up, No. (%) | |
| First postoperative visit | 331 (100) |
| Second postoperative visit | 214 (64.7) |
| Affected limb, No. (%) | |
| Upper limb | 274 (82.8) |
| Lower limb | 57 (17.2) |
| Right side | 151 (45.6) |
| Left side | 142 (42.9) |
| Both sides | 38 (11.5) |
| Surgical procedure, % | |
| Decompression or transposition | 68.3 |
| Nerve transfer | 16 |
| Neuroma excision | 13 |
| Nerve grafting | 2.7 |
| Childhood trauma, % | |
| No | 89.3 |
| Yes | 7.9 |
| No comment | 2.8 |
Concurrent Associations: Psychosocial Factors as Predictors of Same-Visit Pain
The generalized linear mixed-effects models to assess the associations between same-visit pain and same-visit psychosocial variables (impact of pain on QoL, sadness, depression, anger, and hopefulness) are displayed in Table 2. Same-visit increased pain was associated with decreased hopefulness and increased impact on QoL, sadness, depression, and anger at the same visit. Upper limb nerve injury and selecting “no comment” when asked about a history of childhood trauma were significantly related to same-visit pain in all 5 psychosocial models. Sex and type of surgical procedure did not significantly predict same-visit pain.
Table 2.
Concurrent Associations: Psychosocial Factors as Predictors of Same-Visit Pain.
| Model |
Quality of life |
Sadness |
Depression |
Anger |
Hopefulness |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β | P value | β | P value | β | P value | β | P value | β | P value |
| Intercept | 2.81 | .001* | 5.10 | .001* | 5.12 | .001* | 5.43 | .001* | 6.57 | .001* |
| Time | −0.01 | .001* | −0.02 | .001* | −0.02 | .001* | −0.02 | .001* | −0.02 | .001* |
| Time2 | 0.001 | .001* | 0.001 | .001* | 0.001 | .001* | 0.001 | .001* | 0.001 | .001* |
| Time3 | 0.001 | .001* | 0.001 | .001* | 0.001 | .001* | 0.001 | .001* | 0.001 | .001* |
| Pain | 0.48 | .001* | 0.26 | .001* | 0.31 | .001* | 0.18 | .001* | −0.12 | .001* |
| Upper vs lower limb | −0.48 | .03* | −0.79 | .006* | −0.86 | .002* | −0.76 | .007* | −0.64 | .04* |
| Female vs male | −0.03 | .87 | 0.24 | .37 | 0.16 | .52 | 0.17 | .52 | 0.54 | .05* |
| Symptom duration | 0.003 | .06 | 0.004 | .09 | 0.004 | .06 | 0.004 | .08 | 0.004 | .10 |
| Neuroma excision | −0.27 | .35 | −0.29 | .45 | −0.20 | .58 | −0.25 | .50 | −0.27 | .51 |
| Nerve grafting | 0.94 | .11 | 0.95 | .25 | 1.12 | .16 | 1.13 | .17 | 1.03 | .24 |
| Nerve transfer | −0.33 | .21 | −0.42 | .24 | −0.39 | .25 | −0.64 | .07 | −0.34 | .38 |
| History of childhood trauma | 0.35 | .34 | 0.42 | .39 | 0.25 | .60 | 0.36 | .45 | 0.52 | .32 |
| No comment for childhood trauma | 1.42 | .008* | 2.16 | .003* | 2.37 | .001* | 2.35 | .001* | 2.28 | .002* |
Note. Cross-sectional model exploring the concurrent relationship between pain and all measured psychosocial factors. Pain is the dependent variable, and psychosocial factors are independent variables. Both variables were measured at the same visit.
Significant relationship, P < .05.
Lagged Associations: Psychosocial Factors as Predictors of Pain on the Next Visit
Lagged associations were assessed by evaluating the psychosocial factors as predictors of pain reported on the next visit. The generalized linear mixed effects of the lagged models indicated that increased impact of pain on QoL (P < .001) and anger (P = .02) predicted higher levels of pain measured on the next visit (Table 3). Self-reported hopefulness, sadness, and depression were not significant predictors of next-visit pain. Nerve injury of the upper limb and selecting “no comment” when queried about history of childhood trauma (vs no trauma or admitted trauma) were significantly predictive of next-visit pain in every model.
Table 3.
Lagged Associations: Psychosocial Factors as Predictors of Pain.
| Model |
Quality of life |
Sadness |
Depression |
Anger |
Hopefulness |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β | P value | β | P value | β | P value | β | P value | β | P value |
| Intercept | 3.46 | .001* | 4.65 | .001* | 4.71 | .001* | 4.67 | .001* | 5.04 | .001* |
| Time | −0.005 | .29 | −0.01 | .11 | −0.01 | .08 | −0.01 | .09 | −0.01 | .05* |
| Time2 | 0.001 | .14 | 0.001 | .09 | 0.001 | .08 | 0.001 | .08 | 0.001 | .05 |
| Time3 | 0.001 | .10 | 0.001 | .08 | 0.001 | .08 | 0.001 | .08 | 0.001 | .06 |
| Pain | 0.18 | .001* | 0.05 | .15 | 0.06 | .19 | 0.06 | .02* | −0.03 | .39 |
| Upper vs lower limb | −0.69 | .04* | −0.78 | .034* | −0.78 | .03* | −0.77 | .04* | −0.74 | .05* |
| Female vs male | 0.02 | .95 | 0.09 | .80 | 0.09 | .80 | 0.06 | .87 | 0.098 | .77 |
| Symptom duration | 0.004 | .11 | 0.004 | .12 | 0.004 | .11 | 0.005 | .10 | 0.004 | .11 |
| Neuroma excision | 0.001 | .998 | 0.04 | .93 | 0.03 | .95 | 0.001 | .998 | 0.03 | .96 |
| Nerve grafting | 1.54 | .099 | 1.64 | .10 | 1.62 | .11 | 1.66 | .10 | 1.71 | .09 |
| Nerve transfer | −0.61 | .13 | −0.76 | .08 | −0.73 | .09 | −0.77 | .08 | −0.76 | .08 |
| History of childhood trauma | 0.36 | .53 | 0.50 | .42 | 0.43 | .50 | 0.38 | .54 | 0.54 | .38 |
| No comment for childhood trauma | 1.79 | .03* | 2.03 | .02* | 2.06 | .02* | 2.03 | .02* | 2.07 | .02* |
Note. Lagged model exploring the relationship between pain and all measured psychosocial factors. This lagged model contains the psychosocial factors (ie, time t) as the independent variables and pain measured at the next visit (ie, time t + 1) as the dependent variable.
Significant relationship, P < .05.
Lagged Associations: Pain as a Predictor of Psychosocial Factors on the Next Visit
Table 4 illustrates the generalized linear mixed-effects models which evaluated pain as a predictor of the impact of pain on QoL, sadness, depression, anger, and hopefulness assessed at the next visit. Pain was significantly predictive of all 5 next-visit psychosocial factors. Nerve injury of the upper limb (vs lower limb) was predictive of next-visit anger. Women (vs men) were more likely to have higher next-visit sadness (P = .01) and anger (P = .01). Self-report of a positive history of childhood trauma was predictive of higher next-visit sadness (P = .03) and depression (P = .002).
Table 4.
Lagged Associations: Pain as a Predictor of Psychosocial Factors.
| Model |
Quality of life |
Sadness |
Depression |
Anger |
Hopefulness |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β | P value | β | P value | β | P value | β | P value | β | P value |
| Intercept | 2.55 | .001* | 1.06 | .05 | 0.73 | .12 | 0.48 | .33 | 8.07 | .001* |
| Time | −0.003 | .54 | −0.003 | .61 | 0.001 | .68 | 0.001 | .82 | −0.004 | .001* |
| Time2 | 0.001 | .92 | 0.001 | .78 | −0.001 | .44 | −0.001 | .75 | NA | NA |
| Time3 | 0.001 | .95 | 0.001 | .88 | 0.001 | .35 | −0.001 | .76 | NA | NA |
| Pain | 0.33 | .001* | 0.08 | .03* | 0.08 | .009* | 0.09 | .01* | −0.10 | .03* |
| Upper vs lower limb | −0.52 | .13 | 0.49 | .12 | 0.54 | .08 | 0.65 | .04* | 0.32 | .37 |
| Female vs male | 0.58 | .06 | 0.74 | .009* | 0.51 | .06 | 0.77 | .008* | 0.04 | .91 |
| Symptom duration | 0.002 | .47 | −0.001 | .85 | −0.001 | .66 | −0.001 | .41 | 0.001 | .67 |
| Neuroma excision | 0.24 | .59 | −0.11 | .80 | 0.07 | .86 | −0.24 | .56 | −0.79 | .12 |
| Nerve grafting | 0.98 | .29 | 0.66 | .43 | 0.22 | .79 | 1.37 | .12 | 1.42 | .13 |
| Nerve transfer | −0.41 | .31 | −0.69 | .06 | −0.84 | .02* | −0.56 | .14 | −0.04 | .93 |
| History of childhood trauma | 0.23 | .70 | 1.19 | .03* | 1.6 | .002* | 0.62 | .25 | −0.25 | .71 |
| No comment for childhood trauma | 0.65 | .44 | −0.06 | .94 | −0.16 | .83 | −0.79 | .30 | −0.61 | .45 |
Note. Reverse lagged model to explore whether a bidirectional relationship exists between pain and psychosocial factors. This reverse lagged model contained pain (ie, time t) as the independent variable and psychosocial factors measured at the next visit (ie, time t + 1) as the dependent variable. NA = not applicable.
Significant relationship, P < .05.
Discussion
Our results reinforced the reciprocal nature of the relationships between pain and psychosocial factors. Increased pain was associated with decreased same-visit hopefulness, as well as increased impact of pain on QoL, sadness, depression, and anger. Changes in QoL and anger were predictive of future pain, and similarly a change in pain was predictive of changes in future QoL and anger and future sadness, depression, and hopefulness. We also found that having a nerve injury in the upper limb and self-report of “no comment” for experience of childhood trauma were predictors of postsurgical pain.
Each psychosocial factor (impact of pain on QoL, sadness, anger, depression, and hopefulness) was analyzed as the independent variable in the concurrent model and as both an independent and dependent variable in the lagged models. There were significant lagged relationships between pain and each subsequent psychosocial factor, suggesting that pain is the controlling factor, and an increase in pain leads to an increase in the negative effects of these psychosocial factors. The impact of pain on QoL and anger has a bidirectional relationship with pain, indicating that both QoL and anger have a closer relationship with pain than the other psychosocial factors. This warrants further investigation into the relationship between QoL, anger, and pain. Our findings suggest that the impact of pain on QoL and anger predicts subsequent pain. Clinically, helping patients to manage anger and positive appraisal of pain experiences may improve outcomes following nerve surgery.
A recent retrospective analysis of patients undergoing major orthopedic trauma demonstrates that optimism about surgical outcomes is linked to improved pain outcomes. 21 In our study, hopefulness was not predictive of pain in the future, but an increased level of hopefulness correlated with a decreased level of pain at the same visit. Many studies have demonstrated that preoperative depression and anxiety are predictors of persistent postsurgical pain.32-35 Our study identified pain as a predictive factor for self-reported depression and other negative mood symptoms. Timely treatment of postoperative pain may be warranted to prevent the development and progression of emotional problems for patients with peripheral nerve injuries.
Multiple studies have demonstrated an increased incidence of chronic pain in women compared with men. 36 Our results did not show a gender difference in postoperative pain. However, women were found to have increased sadness and anger at future visits compared with men. The type of surgical procedure was not associated with pain in either the concurrent or lagged model, although undergoing a nerve transfer was associated with decreased next-visit sadness and depression. Participants who responded “no comment” to the question on childhood trauma had more pain compared with patients who reported no childhood trauma.
There are several limitations to this study. Due to the large national geographic referral base, the timing of postoperative assessment was not consistent for all patients. Another limitation of this study was that patients were not grouped based on the individual nerves injured. Instead, injuries were categorized as either upper or lower limb injuries, limiting the applicability of our findings to specific individual nerves and procedures. In addition, as an observational study exploring causality, the findings of this study may be subject to unseen confounders and should be verified in future studies.
This study contributes to our understanding of the relationships among pain, QoL, and psychosocial factors. The results exemplify the importance of capturing data on psychosocial factors as they relate to pain in patients with peripheral nerve injuries. Surgeons should assess and use psychosocial factors to better understand outcomes following nerve surgery.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all patients for being included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR002344.
ORCID iDs: Lara W. Crock  https://orcid.org/0000-0003-4913-9719
https://orcid.org/0000-0003-4913-9719
Benjamin A. Philip  https://orcid.org/0000-0001-5467-8384
https://orcid.org/0000-0001-5467-8384
Susan E. Mackinnon  https://orcid.org/0000-0002-5561-6027
https://orcid.org/0000-0002-5561-6027
References
- 1. Bailey R, Kaskutas V, Fox I, et al. Effect of upper extremity nerve damage on activity participation, pain, depression, and quality of life. J Hand Surg Am. 2009;34(9):1682-1688. [DOI] [PubMed] [Google Scholar]
- 2. Dolan RT, Butler JS, Murphy SM, et al. Health-related quality of life and functional outcomes following nerve transfers for traumatic upper brachial plexus injuries. J Hand Surg Eur Vol. 2012;37(7):642-651. [DOI] [PubMed] [Google Scholar]
- 3. Domeshek LF, Krauss EM, Snyder-Warwick AK, et al. Surgical treatment of neuromas improves patient-reported pain, depression, and quality of life. Plast Reconstr Surg. 2017;139(2):407-418. [DOI] [PubMed] [Google Scholar]
- 4. Jaquet JB, Luijsterburg AJ, Kalmijn S, et al. Median, ulnar, and combined median-ulnar nerve injuries: functional outcome and return to productivity. J Trauma. 2001;51(4):687-692. [DOI] [PubMed] [Google Scholar]
- 5. Ring D. Symptoms and disability after major peripheral nerve injury. Hand Clin. 2013;29(3):421-425. [DOI] [PubMed] [Google Scholar]
- 6. Taylor CA, Braza D, Rice JB, et al. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87(5):381-385. [DOI] [PubMed] [Google Scholar]
- 7. Mackinnon SE. Future perspectives in the management of nerve injuries. J Reconstr Microsurg. 2018;34(9):672-674. [DOI] [PubMed] [Google Scholar]
- 8. Mackinnon SE. Discussion: state-of-the-art techniques in treating peripheral nerve injury. Plast Reconstr Surg. 2018;141(3):711-712. [DOI] [PubMed] [Google Scholar]
- 9. Poppler LH, Mackinnon SE. The role of the peripheral nerve surgeon in the treatment of pain. Neurotherapeutics. 2019;16(1):9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong T, Wood I, Hunter DA, et al. Neuroma management: capping nerve injuries with an acellular nerve allograft can limit axon regeneration [published online ahead of print May 29, 2019]. Hand (N Y). doi: 10.1177/1558944719849115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019; 27(2): 238-246. [DOI] [PubMed] [Google Scholar]
- 12. Woo SL, Kung TA, Brown DL, et al. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open. 2016;4(12):e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654-662. [DOI] [PubMed] [Google Scholar]
- 14. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2016;18(1):20-30. [DOI] [PubMed] [Google Scholar]
- 16. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gustafsson M, Ahlstrom G. Emotional distress and coping in the early stage of recovery following acute traumatic hand injury: a questionnaire survey. Int J Nurs Stud. 2006;43(5):557-565. [DOI] [PubMed] [Google Scholar]
- 18. Gustafsson M, Persson LO, Amilon A. A qualitative study of stress factors in the early stage of acute traumatic hand injury. J Adv Nurs. 2000;32(6):1333-1340. [DOI] [PubMed] [Google Scholar]
- 19. Jaquet JB, Kalmijn S, Kuypers PD, et al. Early psychological stress after forearm nerve injuries: a predictor for long-term functional outcome and return to productivity. Ann Plast Surg. 2002;49(1):82-90. [DOI] [PubMed] [Google Scholar]
- 20. Jackson T, Tian P, Wang Y, et al. Toward identifying moderators of associations between presurgery emotional distress and postoperative pain outcomes: a meta-analysis of longitudinal studies. J Pain. 2016;17(8):874-888. [DOI] [PubMed] [Google Scholar]
- 21. Busse JW, Heels-Ansdell D, Makosso-Kallyth S, et al. Patient coping and expectations predict recovery after major orthopaedic trauma. Br J Anaesth. 2019;122(1):51-59. [DOI] [PubMed] [Google Scholar]
- 22. Tesarz J, Eich W, Treede RD, et al. Altered pressure pain thresholds and increased wind-up in adult patients with chronic back pain with a history of childhood maltreatment: a quantitative sensory testing study. Pain. 2016;157(8):1799-1809. [DOI] [PubMed] [Google Scholar]
- 23. Sachs-Ericsson N, Kendall-Tackett K, Hernandez A. Childhood abuse, chronic pain, and depression in the National Comorbidity Survey. Child Abuse Negl. 2007;31(5):531-547. [DOI] [PubMed] [Google Scholar]
- 24. Padua L, Schenone A, Aprile I, et al. Quality of life and disability assessment in neuropathy: a multicentre study. J Peripher Nerv Syst. 2005;10(1):3-10. [DOI] [PubMed] [Google Scholar]
- 25. Chen DL, Novak CB, Mackinnon SE, et al. Pain responses in patients with upper-extremity disorders. J Hand Surg Am. 1998;23(1):70-75. [DOI] [PubMed] [Google Scholar]
- 26. Novak CB, Mackinnon SE. Evaluation of cold sensitivity, pain, and quality of life after upper extremity nerve injury. Hand (N Y). 2016;11(2):173-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wojtkiewicz DM, Saunders J, Domeshek L, et al. Social impact of peripheral nerve injuries. Hand (N Y). 2015;10(2):161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lohse K, Bland MD, Lang CE. Quantifying change during outpatient stroke rehabilitation: a retrospective regression analysis. Arch Phys Med Rehabil. 2016;97(9):1423-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. Stata Press;2008. [Google Scholar]
- 30. Long JD. Longitudinal Data Analysis for the Behavioral Sciences Using R. Thousand Oaks, CA: Sage; 2012. [Google Scholar]
- 31. Pinheiro J, Bates D, DebRoy S, et al. nlme: linear and nonlinear mixed effects models. R package version. 2013;3(1):111. [Google Scholar]
- 32. Horn-Hofmann C, Scheel J, Dimova V, et al. Prediction of persistent post-operative pain: pain-specific psychological variables compared with acute post-operative pain and general psychological variables. Eur J Pain. 2018;22(1):191-202. [DOI] [PubMed] [Google Scholar]
- 33. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618-1625. [DOI] [PubMed] [Google Scholar]
- 34. Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93(4):1123-1133. [DOI] [PubMed] [Google Scholar]
- 35. Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105(suppl 1):i69-i85. [DOI] [PubMed] [Google Scholar]
- 36. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]