Abstract
Background: In an effort to reduce surgical complications, some institutions have implemented universal hemoglobin A1c (HbA1c) screening for all preoperative patients. However, the value of HbA1c screening for predicting clinically meaningful complications after elective carpal tunnel release (CTR) remains unclear. The purpose of this study was to investigate the clinically meaningful predicative value of HbA1c screening on postoperative complications following elective CTR. Methods: A retrospective cohort study of 790 patients who underwent CTR was performed. All patients had an HbA1c screening performed, regardless of whether they underwent the diagnosis for diabetes or not. Primary outcomes were overall complication rate, rates of major complications (readmission or reoperation), and rates of minor complications (surgical site infection and wound dehiscence). Patients were stratified into 3 groups based on HbA1c: HbA1c <7, HbA1c 7-8, and HbA1c >8. Results: The overall complication rate for all groups was 4.8%. Rates of major complications were 0.4% for readmission and 0.1% for reoperation. For minor complications, the odds ratio (OR) for the HbA1c 7-8 group was 0.6 (95% confidence interval [CI], 0.14-1.77), and for the HbA1c >8 group, the OR was 1.6 (95% CI, 0.66-3.60). All minor complications resolved with outpatient treatment. There were no statistically significant differences between the groups for any comparisons. Conclusions: Elective CTR has a low complication rate. Routine preoperative screening of HbA1c is of little value in predicting clinically meaningful complications.
Keywords: carpal tunnel syndrome, diabetes, risk factor, predictor, complications
Introduction
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy affecting approximately 3% to 6% of adults in the general population. 1 Diabetes is one of the major risk factors for CTS, and currently, 9.4% of the US population is diabetic.2,3 Diabetes has been reported as an established risk factor for elective surgery, including hand and upper extremity surgery.4,5 Current literature indicates that people with diabetes can improve following carpal tunnel release (CTR) compared with people without diabetes. 6 Large database studies have found that both diabetes and hemoglobin A1c (HbA1c) predict complication rates.5,7,8 However, there is a low complication rate following CTR, 9 and it is unclear whether these statistical findings represent clinically meaningful differences that would warrant procurement of the HbA1c level prior to surgical intervention.
Routine universal preoperative HbA1c testing has been advocated as a screening tool for all patients undergoing elective joint replacement surgery.10,11 The rationale is that routine testing of HbA1c screens patients for diabetes, identifies those who have poorly controlled diabetes, and avoids modifiable risk associated with elective surgery. The utility of screening HbA1c for CTR is less clear, given the low complication rate of CTR. 9 Thus, the objective of this study was to investigate whether routine screening of HbA1c is a useful tool in predicting clinically meaningful complications after CTR.
Materials and Methods
Our institutional preoperative protocol mandates universal HbA1c testing for all patients undergoing general anesthesia (GA) or sedation, regardless of the procedure type or known history of diabetes. A retrospective review of patients in our institution’s electronic medical record was conducted using our health analytics database. Search queries used the Current Procedural Terminology codes 64721 and 29848 for open CTR and endoscopic carpal tunnel release (ECTR), respectively. Inclusion criteria were patients older than 18 years with an HbA1c recorded within 90 days of surgery. Exclusion criteria included patients who underwent CTR as a result of trauma or infection and patients with no follow-up within 30 days of surgery. Patients who had CTR under local anesthesia were excluded as they are not required to have an HbA1c. The patients in this study received either monitored anesthesia care (MAC) or GA. The decision to perform CTR under local anesthesia versus under MAC or GA was directed by surgeon preference as well as part of an informed discussion with the patient on anesthetic options. Dates of surgery ranged from 2008 to 2018. A post hoc power calculation demonstrated the study was appropriately powered to detect a difference of an odds ratio (OR) of >3.0 with a proportionally unbalanced sample with α of 0.05 and β of 0.80.
The medical records of the patients were reviewed to obtain their demographic information, clinical presentation, medical history, and follow-up clinical visits for complications. Patient variables were recorded including presence and type of diabetes, chronic obstructive pulmonary disorder (COPD), cardiac disease, and active tobacco use. If concomitant procedures were performed during the same operation, these interventions were recorded as well. In addition, charts were reviewed for surgical site infection (SSI), wound dehiscence, readmission, reoperation, and hematoma. Patients were stratified into 3 cohorts based on their HbA1c: HbA1c <7, HbA1c 7-8, and HbA1c >8.
Descriptive statistics were used to characterize the overall sample and by HbA1c groups. Comparisons across HbA1c groups were accomplished using analysis of variance (ANOVA) F tests and χ2 tests for continuous and categorical variables, respectively. Bivariate logistic regression models were estimated for each complication (any wound dehiscence and SSI), and variables demonstrating significant differences at the 0.20 level in any of the 3 models were included in final adjusted multivariable logistic regression models. Adjusted ORs are presented for HbA1c groups, with <7 as the reference group. Models were not estimated for readmission, reoperation, and hematoma, as the number of events was too few to generate reliable results. Due to the small number of events, sensitivity analyses were performed using HbA1c on a continuum. Note that the sample size of 790 patients results in minimum detectable ORs of 2.7 for complications at the expected rate of 5%, with β of 0.8 and α of 0.05.
This study was designed and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement on strengthening the reporting of observational studies and was approved by the institutional review board.
Results
After inclusion and exclusion criteria were applied, 790 patients were included in the study. The study included 586 patients with HbA1c <7, 104 patients with HbA1c 7-8, and 100 patients with HbA1c >8.
Demographics and comorbidities are demonstrated in Table 1. Groups were similar except that age was significantly lower in the HbA1c >8 group, and patients with HbA1c 7-8 and HbA1c >8 were significantly more likely to have a history of cardiac disease and COPD. Diabetes was more common in the HbA1c 7-8 and HbA1c >8 groups. Distributions of patients’ HbA1c are detailed in Figures 2 and 3.
Table 1.
Demographic and Clinical Characteristics Overall and by HbA1c Groups.
| Overall sample (N = 790) | HbA1c <7 (n = 586) | HbA1c 7-8 (n = 104) | HbA1c >8 (n = 100) | P value* | |
|---|---|---|---|---|---|
| Age, y | .005* | ||||
| n | 790 | 586 | 104 | 100 | |
| Mean | 58.05 | 58.65 | 58.77 | 53.83 | |
| Standard deviation | 13.98 | 14.34 | 13.23 | 11.86 | |
| Sex, No. (%) | .88 | ||||
| Female | 516 (65.3) | 382 (65.2) | 70 (67.3) | 64 (64.0) | |
| Male | 274 (34.7) | 204 (34.8) | 34 (32.7) | 36 (36.0) | |
| Diabetes mellitus diagnosis, No. (%) | <.001* | ||||
| Yes | 377 (47.7) | 180 (30.7) | 99 (95.2) | 98 (98.0) | |
| No | 413 (52.3) | 406 (69.3) | 5 (4.8) | 2 (2.0) | |
| History of cardiac disease, No. (%) | <.001* | ||||
| Yes | 225 (28.5) | 145 (24.7) | 42 (40.4) | 38 (38.0) | |
| No | 565 (71.5) | 441 (75.3) | 62 (59.6) | 62 (62.0) | |
| History of COPD, No. (%) | .03* | ||||
| Yes | 105 (13.3) | 67 (11.4) | 18 (17.3) | 20 (20.0) | |
| No | 685 (86.7) | 519 (88.6) | 86 (82.7) | 80 (80.0) | |
| Active smoking status, No. (%) | .95 | ||||
| Yes | 198 (25.1) | 147 (25.1) | 25 (24.4) | 26 (26.0) | |
| No | 592 (74.9) | 439 (74.9) | 79 (75.9) | 74 (74.0) | |
| Concomitant procedures performed, No. (%) | .08 | ||||
| Yes | 184 (23.3) | 127 (21.7) | 25 (24.0) | 32 (32.0) | |
| No | 606 (76.7) | 459 (78.3) | 79 (75.9) | 68 (68.0) | |
| Procedure performed, No. (%) | .72 | ||||
| Open CTR | 287 (36.4) | 210 (35.8) | 37 (35.6) | 40 (40.0) | |
| Endoscopic CTR | 502 (63.6) | 376 (64.2) | 67 (64.4) | 60 (60.0) |
Note. COPD = chronic obstructive pulmonary disorder; CTR = carpal tunnel release; HbA1c = hemoglobin A1c.
P value based on F test for age and χ2 tests for categorical variables.
Figure 2.
Incidence of complications across hemoglobin A1C.
Figure 3.
Incidence of complications of hemoglobin A1c 6-9.
Thirty-eight patients experienced a complication across all groups for an overall complication rate of 4.8%. Wound dehiscence occurred in 15 patients (1.9%). Surgical site infection occurred in 28 patients (3.5%). Three patients (0.4%) were readmitted, and 1 patient underwent reoperation (0.1%). No patients experienced hematoma complication. The major complication rate was 0.5%, and the minor complication rate was 4.3% across all groups. For the 34 patients who had minor complications in this study, all resolved with outpatient treatment, including oral antibiotics and local wound care. None required a secondary operation. Detailed rates of complications stratified by HbA1c cohort are seen in Table 2.
Table 2.
Complications Overall and by HbA1c Groups.
| Any complications, No. (%) | Wound dehiscence, No. (%) | Surgical site infection, No. (%) | Readmission, No. (%) | Reoperation, No. (%) | Hematoma, No. (%) | |
|---|---|---|---|---|---|---|
| Overall sample (N = 790) | 38 (4.8) | 15 (1.9) | 28 (3.5) | 3 (0.4) | 1 (0.1) | 0 |
| HbA1c <7 (n = 586) | 27 (4.6) | 9 (1.5) | 20 (3.4) | 3 (0.5) | 1 (0.2) | 0 |
| HbA1c 7-8 (n = 104) | 3 (2.9) | 1 (0.9) | 2 (1.9) | 0 | 0 | 0 |
| HbA1c >8 (n = 100) | 8 (8.0) | 5 (5.0) | 6 (6.0) | 0 | 0 | 0 |
| P value * | .24 | .06 | .26 |
Note. HbA1c = hemoglobin A1c.
P value based on χ2 test statistic.
Simple bivariate logistic regression models found that none of the following significantly predicted complications: sex, cardiac disease, and COPD. Other factors were weakly predicative and included age, smoking, diabetes, concomitant procedures, and open versus ECTR. These factors were included in the multivariate model.
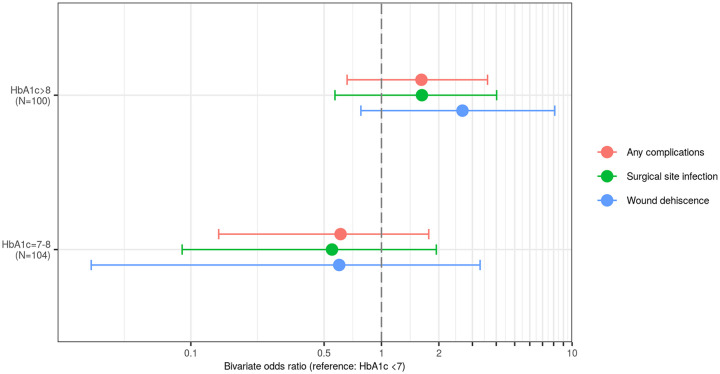
Table 3 provides estimates of adjusted ORs derived from multivariable logistic regression models with 3 HbA1c groups (HbA1c <7, HbA1c 7-8, and HbA1c >8) as the predictor of interest and adjusting for variables demonstrating significance in bivariate models at the 0.20 level. None of the ORs were statistically significantly different from 1.0. Figure 1 demonstrates the OR for complications of the HbA1c 7-8 and HbA1c > 8 groups compared with the HbA1c <7 group.
Table 3.
Logistic Regression Model Results for Complications Regressed on HbA1c Groups: Adjusted a Odds Ratios.
| Any complications |
Wound dehiscence |
Surgical site infection |
Readmission, No. (%) | Reoperation, No. (%) | Hematoma, No. (%) | |
|---|---|---|---|---|---|---|
| OR (95% CI) P value |
OR (95% CI) P value |
OR (95% CI) P value |
||||
| HbA1c <7 (N = 586) | REF | REF | REF | 3 (0.51) | 1 (0.17) | 0 |
| HbA1c 7-8 (n = 104) | 0.61 (0.14-1.77) .43 |
0.60 (0.03-3.29) .63 |
0.55 (0.09-1.94) .43 |
0 | 0 | 0 |
| HbA1c >8 (n = 100) | 1.62 (0.66-3.60) .16 |
2.66 (0.78-8.11) .09 |
1.63 (0.57-4.02) .32 |
0 | 0 | 0 |
Note. OR = odds ratios; CI = confidence interval; HbA1c = hemoglobin A1c.
Logistic regression model adjusted for age, active smoking, concomitant procedures, and open versus endoscopic carpal tunnel release.
Figure 1.
Odds ratios for complications using hemoglobin A1c <7 as the reference cohort.
Discussion
Despite the known low complication rate of CTR, some institutions require that all patients undergo universal presurgical testing of HbA1c within 90 days preoperatively. For the hand surgeon, it is unclear whether this is a useful practice and it is unclear how to proceed when the HbA1c is found to be elevated. Our hospital protocol recommends delay of any surgery for 30 days to try to optimize glucose control. There is no guidance in the current literature of how to proceed regarding elective carpal tunnel surgery with a low risk of known complications.
Several studies have examined the relationship between diabetes and SSIs after CTR.5,7,8,12 Werner et al 5 found that for 450 000 Medicare patients who underwent open CTR, the SSI rate was 0.32%. However, the database study by Werner et al was unable to capture minor complications treated with outpatient treatment such as those reported here. In addition, the relationship between complications and HbA1c was unknown. In studying more than 3000 patients who underwent CTR surgery, Harness et al 9 reported an SSI rate of 0.36% and demonstrated no difference between diabetic and nondiabetic patients. Cunningham et al found that an HbA1c of >7.8 predicted wound complications and reported a rate of 4%. Our study found a similar rate of major complications (0.51%) as the large database studies by Werner et al and Harness et al and a similar rate of minor complications (4.3%) as that reported by Cunningham et al. However, we demonstrated no significant difference in complication rates among people with and without diabetes. The advantage of this study is the known association with preoperative HbA1c and additional information on minor complications managed with outpatient treatment.
In this study, we considered a major complication to be one that required reoperation or readmission. With this definition, the rate of major complications was too low for statistical analysis between groups. A study to detect a significant difference in major complications would require tens of thousands of patients between groups with HbA1c <7 and HbA1c 7-8 or to conclusively demonstrate their equivalence. Even with large numbers, the detected difference would likely be very small and have minimal impact on clinical decision making.
For minor complications, this study was adequately powered to detect an OR of >3 between groups. We considered minor complications managed with outpatient treatment with minimal intervention. For minor complications, we considered an OR >3.0 to be clinically meaningful, as an OR lower than 3.0 is tolerable for infrequent minor complications and unlikely to have an impact on preoperative clinical decision making. Here, we found that routine preoperative HbA1c testing did not predict the likelihood of minor complication with an OR >3.0.
The results of our study do not indicate a significant impact of preoperative HbA1c on complications. With this in mind, we advocate the procurement of preoperative HbA1c be withheld to the surgeon’s discretion. In addition, the clinical results for CTR in diabetic and nondiabetic patients are both consistent and excellent, leading us to advocate that surgical treatment should not be reserved or delayed without clear evidence of a clinically meaningful increased risk of complications.6,13 Cunningham et al 8 reported an OR of 4.2 for minor complications in people with diabetes with an HbA1c >7.8. However, due to the low incidence, this was a rate of 1% in the well-controlled versus 4% in the poorly controlled group. For a minor complication managed with outpatient antibiotics or local wound care, we do not see this as a clinically meaningful difference.
In this study, we chose cutoff points for the 3 cohorts of interest (<7, 7-8, and >8) based on recommendations from the American Diabetes Association Standards of Care. General recommendations are an HbA1c goal of <7% for most patients. 14
A limitation of this study is the selection bias toward patients who underwent surgery. Patients who may have had extremely high HbA1c values and did not have surgery were not captured in this study. In this study, the distribution of patients’ HbA1c was skewed toward lower values as shown in Figures 2 and 3. In addition, patients who underwent local anesthetic only were not included in this study. Further study on direct relationship between HbA1c and complication risk would be needed to further elucidate the effect of very high HbA1c, but would also require high numbers of patients given the low complication rate. Thus, the risk of very high HbA1c on complications after CTR remains unknown.
Although our study is one of the largest cohorts at a single institution to date, the sample size is also a limiting factor, given the overall low incidence of complication after CTR. As stated previously, a much larger study cohort would be needed to demonstrate a statistically significant difference in complication risk based on HbA1c or to demonstrate equivalence. Such a study is not easily feasible nor would it likely provide any more informative data than the current study and current literature. Thus, we do not plan or recommend future study examining the relationship between HbA1c and surgical complications following elective CTR.
Based on the data here, routine HbA1c screening prior to CTR should not be performed as an institutional preoperative protocol as the results are unlikely to provide clinically meaningful information. Obtaining preoperative HbA1c before CTR should, therefore, remain at the surgeon’s discretion. In the event that HbA1c is performed and found to be elevated, the surgeon should consider the well-documented benefit of CTR weighted against the low risk of a minor complication.
Acknowledgments
The authors thanks Noah J. Orfield (Virginia Tech-Carilion) and Alexandra L. Hanlon (Virginia Tech).
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: Informed consent was not required for our study due to the retrospective nature of the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The statistical analysis within the study is associated with funding from the National Center for Advancing Translational Sciences of the National Institutes of Health. Research reported in this publication/presentation/work was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR003015. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: Patrick S. Collins  https://orcid.org/0000-0001-8818-4761
https://orcid.org/0000-0001-8818-4761
Albert Y. Truong  https://orcid.org/0000-0002-9551-4519
https://orcid.org/0000-0002-9551-4519
Alicia J. Lozano  https://orcid.org/0000-0001-8122-1312
https://orcid.org/0000-0001-8122-1312
References
- 1. LeBlanc KE, Cestia W. Carpal tunnel syndrome. Am Fam Physician. 2011;83(8):952-958. http://www.ncbi.nlm.nih.gov/pubmed/21524035. Accessed September 13, 2019. [PubMed] [Google Scholar]
- 2. Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379(9833):2252-2261. doi: 10.1016/S0140-6736(12)60480-2. [DOI] [PubMed] [Google Scholar]
- 4. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2011;93(17):1627-1633. doi: 10.2106/JBJS.J.00039. [DOI] [PubMed] [Google Scholar]
- 5. Werner BC, Teran VA, Deal DN. Patient-related risk factors for infection following open carpal tunnel release: an analysis of over 450,000 Medicare patients. J Hand Surg Am. 2018;43(3):214-219. doi: 10.1016/j.jhsa.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 6. Zimmerman M, Eeg-Olofsson K, Svensson AM, et al. Open carpal tunnel release and diabetes: a retrospective study using PROMs and national quality registries. BMJ Open. 2019;9(9):e030179. doi: 10.1136/bmjopen-2019-030179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Werner BC, Teran VA, Cancienne J, et al. The association of perioperative glycemic control with postoperative surgical site infection following open carpal tunnel release in patients with diabetes. Hand. 2019;14(3):324-328. doi: 10.1177/1558944717743594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cunningham DJ, Baumgartner RE, Federer AE, et al. Elevated preoperative hemoglobin A1c associated with increased wound complications in diabetic patients undergoing primary, open carpal tunnel release. Plast Reconstr Surg. 2019;144:632e-638e. doi: 10.1097/PRS.0000000000006023. [DOI] [PubMed] [Google Scholar]
- 9. Harness NG, Inacio MC, Pfeil FF, et al. Rate of infection after carpal tunnel release surgery and effect of antibiotic prophylaxis. J Hand Surg Am. 2010;35(2):189-196. doi: 10.1016/j.jhsa.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 10. Shohat N, Goswami K, Tarabichi M, et al. All patients should be screened for diabetes before total joint arthroplasty. J Arthroplasty. 2018;33(7):2057-2061. doi: 10.1016/j.arth.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 11. Capozzi JD, Lepkowsky ER, Callari MM, et al. The prevalence of diabetes mellitus and routine hemoglobin A1c screening in elective total joint arthroplasty patients. J Arthroplasty. 2017;32(1):304-308. doi: 10.1016/j.arth.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 12. Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;80:112-117. doi: 10.1097/TA.0000000000001724. [DOI] [PubMed] [Google Scholar]
- 13. Zyluk A, Puchalski P. A comparison of outcomes of carpal tunnel release in diabetic and non-diabetic patients. J Hand Surg Eur Vol. 2013;38(5):485-488. doi: 10.1177/1753193412469781. [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association. 6. Glycemic targets. Diabetes Care. 2017;40:S48-S56. doi: 10.2337/dc17-S009. [DOI] [PubMed] [Google Scholar]