Abstract
Background:
The clinical effects of intranasal corticosteroids (INC) on nasal symptoms and the clinical course of coronavirus disease 2019 (COVID-19) in subjects with chronic rhinitis (CR) seem unclear.
Objective:
To evaluate the clinical effects of INCs on nasal symptoms in subjects with CR and with COVID-19.
Methods:
In subjects with CR and diagnosed with COVID-19 at four tertiary centers, quality of life and nasal symptoms were assessed by using the 22-item Sino-Nasal Outcome Test (SNOT-22) and the visual analog scale (VAS), respectively. In subjects with allergic rhinitis, nasal symptoms were also assessed on the total symptom score-6 (TSS-6) scale. The subjects were then allocated into two groups according to whether or not they used INCs while infected with the severe acute respiratory syndrome coronavirus 2 (group 1 and group 2, respectively). The subjects in group 2 were divided into two subgroups according to the use of antihistamines and/or leukotriene receptor antagonist or not (group 2a and group 2b, respectively). All the scores were compared before and during COVID-19 among the three groups.
Results:
A total of 71 subjects (21 in group 1, 24 in group 2a, and 26 in group 2b) were enrolled. The total scores of the SNOT-22 increased remarkably in all the groups during the infection when compared with the pre–COVID-19 scores (p < 0.001 in each group). However, the difference between the pre–COVID-19 and COVID-19 values revealed a lower decrease in the senses of smell and/or taste in group 1 than in group 2a and group 2b (p = 0.015, adjusted p = 0.045; and p = 0.001, adjusted p = 0.002, respectively). There were no significant differences in other COVID-19 findings, VAS, and TSS-6 scores among the groups (all p > 0.05).
Conclusion:
INCs in subjects with CR seemed protective against the decrease in smell and/or taste observed during COVID-19 and do not aggravate the clinical course of COVID-19.
Keywords: Allergic rhinitis, Chronic rhinitis, Pandemic, COVID-19, Intranasal corticosteroid, Quality of life, Rhinitis, SNOT-22, Steroid therapy, VAS score
A novel strain of severe acute respiratory syndrome related coronavirus (SARS-CoV-2) progressed to a pandemic referred to as coronavirus disease 2019 (COVID-19) almost a year ago.1 The most common symptoms are fever (83%), cough (82%), shortness of breath (31%), muscle ache (11%), confusion (9%), headache (8%), sore throat (5%), rhinorrhea (4%), chest pain (2%), diarrhea (2%), and nausea and/or vomiting(1%).2 Interestingly, nasal and ocular symptoms, including runny nose, sneezing, nasal pruritus, and ocular itch, were fewer in patients with COVID-19 infections compared with those with allergic rhinitis (AR), whereas symptoms of smell and/or taste dysfunction, dyspnea, and cough were reported more frequently according to a questionnaire filled out by the allergic rhinitis and impact on asthma (ARIA) members who saw patients with COVID-19.3
Chronic rhinitis (CR), defined as a symptomatic inflammation of the inner lining of the nose that causes at least two of the following symptoms of nasal obstruction, rhinorrhea, sneezing, or nasal and/or ocular itching for > 12 weeks per year, includes AR, infectious rhinitis, non-AR (NAR), and mixed rhinitis.4 During the pandemic, two recent consensus statements recommended the continued use of intranasal corticosteroids (INC).5,6 However, systemic corticosteroid therapy is not recommended for disorders of the sinuses and nasal cavities during the pandemic.6 In a European Academy of Allergy and Clinical Immunology position paper,5 the clinical use of INCs has been recommended to prevent sneezing and thereby spreading of SARS-CoV2. However, a real-life study that evaluates the clinical outcomes of the recommendations mentioned above is needed to reach definite conclusions. This observational study aimed to investigate the effects of INCs on sinonasal symptoms and clinical outcomes of COVID-19 in subjects with CR and infected with SARS-CoV-2.
METHODS
Study Design
In this multicenter study, patients with CR followed up at four adult allergy outpatient clinics in Istanbul (the adult immunology and allergy clinics at Istanbul Faculty of Medicine in Istanbul University, Okmeydanı Education and Research Hospital, Şişli Etfal Education and Research Hospital, and Yedikule Chest Diseases and Thoracic Surgery Education and Research Hospital) were contacted by telephone and asked to inform us during the COVID-19 period when they became infected with SARS-CoV-2. The study was approved by the Turkish Ministry of Health and the ethics committee of the coordinating center (approval date: July 27, 2020; approval 86358). Written informed consent was obtained from the participants of the study.
The patients with CR who became infected with SARS-CoV-2 were recruited into the study and allocated into two groups according to whether or not they used INCs during the COVID-19 period (group 1 and group 2, respectively). The subjects in group 1 were only using regular INCs and did not use any other medications such as antihistamines or leukotriene receptor antagonist (LTRA), or discontinued them at least 2 months before the pandemic. The subjects in group 2 were placed into two subgroups according to those who used antihistamines and/or LTRA and those who did not use antihistamines and/or LTRA (group 2a and group 2b, respectively). INCs (fluticasone propionate 50 μg or mometasone furoate 50 μg 2 sprays per nostril daily) and oral medications (levocetirizine 5 mg or desloratadine 5 mg and/or montelukast 10 mg daily) were being used at recommended doses.7 The subjects were permitted to continue with their current CR medications during the study. The subjects with CR were categorized as having AR when the clinically relevant sensitization to a common aeroallergen had been confirmed with skin-prick tests and/or specific serum immunoglobulin E measurements before the study8 and as NAR when the allergy diagnostic workup results had remained negative.
Patient Recruitment
Patients with CR, ages 18–65 years, without a history of recurrent sinusitis, mucosal obstruction, or nasal polyposis, and who were diagnosed with COVID-19 by using polymerase chain reaction by using nasopharyngeal swabs and/or SARS-CoV-2 antibody immunoglobulin M level in blood were included in the study. Patients with severe cardiovascular, renal, hepatic diseases, and malignancy were excluded.
The Evaluation of Quality of Life and Symptom Scores
The 22-item Sino-Nasal Outcome Test (SNOT-22) was assessed by using a total of 22 questions, including 12 questions about physical symptoms and 10 questions about quality of life (QoL). All questions are based on a scale from 0 to 5 points, in which 0 defines no problem and 5 defines maximum problems.9 The visual analog scale (VAS) scores that evaluate nasal symptoms ranged from 0 cm (no symptoms) to 10 cm (the highest level of symptoms). Evaluations < 5 cm were defined as mild rhinitis, and evaluations ≥ 5 cm were defined as moderate-severe rhinitis.10 The total symptom score-6 (TSS-6), which measures six symptoms (sneezing, rhinorrhea, nasal pruritus, nasal congestion, ocular symptoms, and itchy ears and/or palate) on a four-point scale (0 [no symptoms] to 3 [severe symptoms]) was used to evaluate the subjects with CR who were diagnosed to have AR.11
The QoL and physical symptoms concerning CR were assessed by using the SNOT-22 via messaging applications on the mobile phone and/or e-mail during the COVID-19 infection. Nasal symptoms were also assessed on the TSS-6 scale (in the subjects with AR) and the VAS (in all the subjects) by the same method. The SNOT-22, TSS-6, and VAS scores in the pre–COVID-19 period were collected from the subjects' routine control visits in their follow-up files. The pre–COVID-19 scores were the last recorded scores that occurred in the previous season for subjects with seasonal AR and at the time of the last visit for subjects with nonseasonal AR and NAR. Afterward, the pre–COVID-19 and COVID-19 CR QoL and symptom scores were compared, both within and among each to the three groups (group 1, group 2a, and group 2b).
COVID-19 Evaluation Methods
Demographic and clinical data were obtained from hospital records, through telephone interviews or e-mails. All the subjects were further classified as having severe or nonsevere COVID-19. The subjects with severe COVID-19 were distinguished by having one of the following criteria: (a) respiratory distress with respiratory frequency of ≥ 30 respirations/minute, (b) pulse oximeter oxygen saturation ≤ 93% at rest, and (c) oxygenation index (arteriel partial pressure of oxygen [Pao2]/fraction of inspired oxygen [Pio2]) ≤ 300 mm Hg.12 All clinical findings were compared between group 1 and group 2.
Statistical Analysis
Categorical variables were summarized as frequencies and percentages, and continuous variables were defined as median with interquartile ranges or mean ± standard deviation when appropriate. To compare the continuous variables for the data of the two groups, the two-tailed t-test and Mann-Whitney U test were used when appropriate. To compare the continuous variables for the data of more than two groups, Kruskal-Wallis and one-way analysis of variance tests were used as appropriate, and the Bonferroni correction was used. The frequencies of categorical variables were compared by using the χ2 and the Fisher exact tests as appropriate. All statistical analyses were performed by using Statistical Package for the Social Sciences version 24.0 (SPSS Inc., Chicago, IL), and graphs were generated by using Prism version 8.4.3 software (GraphPad Software Inc., San Diego, CA). P < 0.05 was considered to indicate a statistically significant result.
RESULTS
Demographic and Clinical Characteristics, and Medication Use
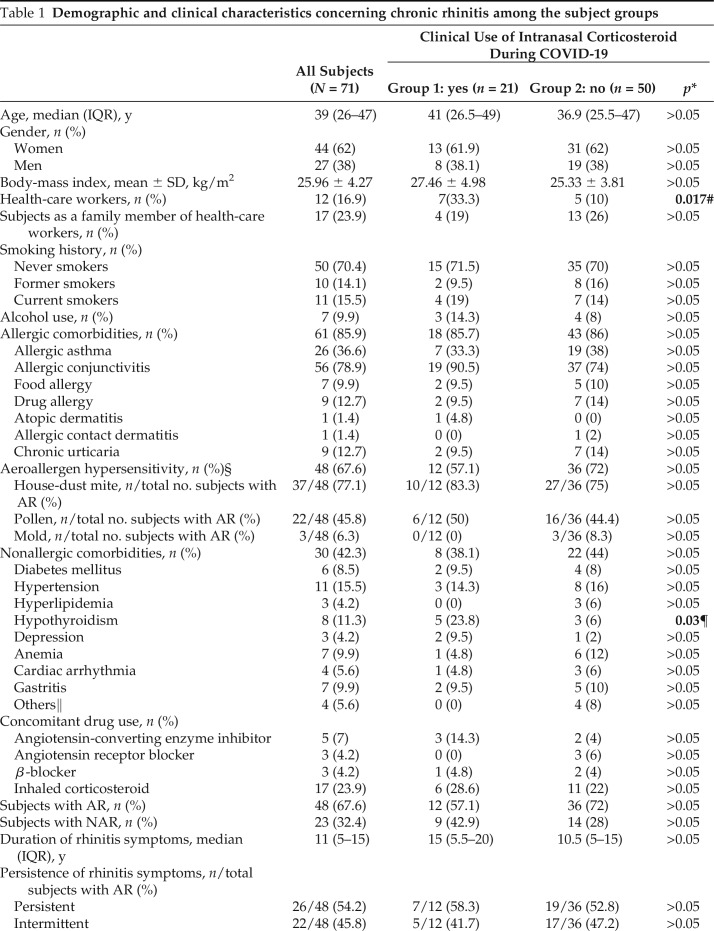
A total of 71 subjects were enrolled in the study. Of the 71 subjects, 48 (67.6%) were diagnosed to have AR and 23 (32.4%) to have NAR. Of 48 subjects, 12 (25%), 17 (35.4%), and 19 (39.6%) were in group 1, group 2a, and group 2b, respectively (p > 0.05). Forty-four subjects (62%) were women and the median (interquartile range) age was 39 years (26–47 years). The demographic and clinical characteristics are summarized in Table 1. While infected with SARS-CoV-2, only INCs were used by 21 subjects (group 1) and at least one oral drug (antihistamines and/or LTRA) was used by 24 subjects (group 2a). No oral or intranasal medication was used by 26 subjects (group 2b).
Table 1.
Demographic and clinical characteristics concerning chronic rhinitis among the subject groups
COVID-19 = Coronavirus disease 2019; IQR = Interquartile range; SD = standard deviation; AR = allergic rhinitis; NAR = non–allergic rhinitis; OR = odds ratio; CI = confidence interval.
*The p values that compared the subjects in groups 1 and 2 were from the χ2 test, Fisher exact test, Mann-Whitney U test, or two-tailed t-test.
#OR 4.5 (95% CI, 1.23–16.42).
§Evaluated by skin-prick tests and/or specific immunoglobulin E measurement.
¶OR 4.89 (95% CI, 1.05–22.83).
‖Familial Mediterranean Fever, hyperthyroidism, mitral regurgitation, migraine;
Significant p values are in bold.
Comparison of SNOT-22 and Symptom Scores among Groups
SNOT-22 Results.
In all the subjects, the most common five symptoms before COVID-19 infection were “runny nose” (n = 57 [80.3%]), “nasal blockage” (n = 57 [80.3%]), “nose blowing” (n = 53 [74.6%]), “sneezing” (n = 52 [73.2%]), and “postnasal discharge” (n = 36 [50.7%]). Conversely, during the COVID-19 infection, the most common symptoms were “fatigue” (n = 36 [50.7%]), “decreased senses of smell and/or taste” (n = 36 [50.7%]), “cough” (n = 28 [39.4%]), “nasal blockage” (n = 25 [35.2%]), and “dizziness” (n = 21 [29.6%]). During the COVID-19 infection, the total scores of SNOT-22 increased remarkably in all the groups when compared with the pre–COVID-19 scores (p < 0.001 in each group). Also, the scores of the total physical symptoms that related to CR increased significantly in group 2a and group 2b (both p < 0.001), whereas they did not change in group 1 (p > 0.05) when compared with the pre–COVID-19 scores.
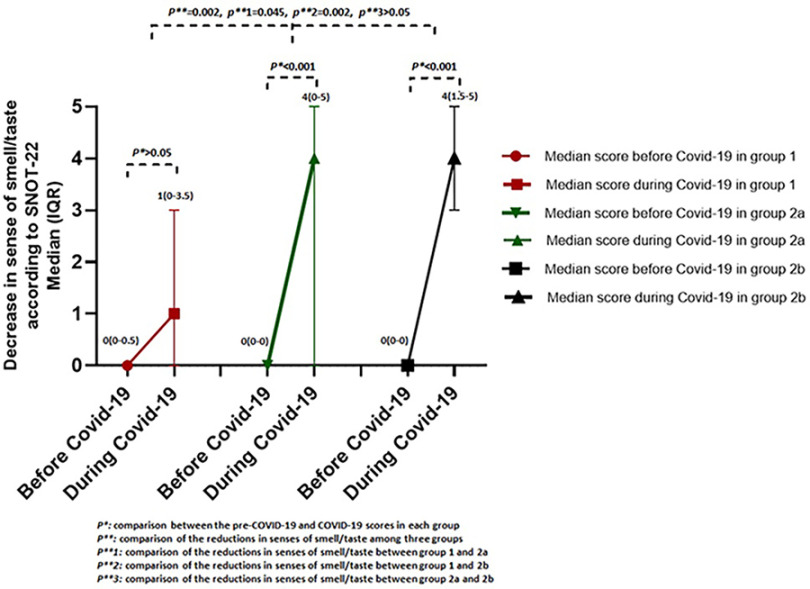
In addition, the impairment of QoL increased significantly in all the groups during the COVID-19 infection when compared with pre–COVID-19 values (p < 0.001 in each group). Among the physical symptoms of SNOT-22, no significant increase was detected in the scores of reduction in the sense of smell and/or taste during COVID-19 in group 1 (p > 0.05). However, there was a significant increase in groups 2a and 2b (both p < 0.001) (Fig. 1). When the differences in the scores between the pre–COVID-19 and COVID-19 periods were compared, the reduction in the sense of smell and/or taste scores was significantly less for group 1 than for groups 2a and 2b (p = 0.002). The comparisons of all the scores that relate to SNOT-22 are shown in Table 2.
Figure 1.
Comparison of scores for reduction in senses of smell and/or taste according to 22-item Sino-Nasal Outcome Test (SNOT-22) in three groups.
Table 2.
The results of SNOT-22 in groups 1, 2a, and 2b
SNOT-22 = 22-Item Sino-Nasal Outcome Test; COVID-019 = coronavirus disease 2019; SD = standard deviation; IQR = interquartile range; QoL = quality of life.
*From the paired sample t-test, Wilcoxon signed rank test; comparison of scores before and during COVID-19 in group 1.
#From the paired sample t-test, Wilcoxon signed rank test; comparison of scores before and during and COVID-19 in group 2a.
§From the paired sample t-test, Wilcoxon signed rank test; comparison of scores before and during COVID-19 in group 2b.
¶From the Kruskal Wallis test and one-way analysis of variance test; comparison of differences between the scores before and during COVID-19 among the three groups.
‖Values are mean ± SD or median (IQR). Significant p values are in bold.
Nasal Symptom Scores.
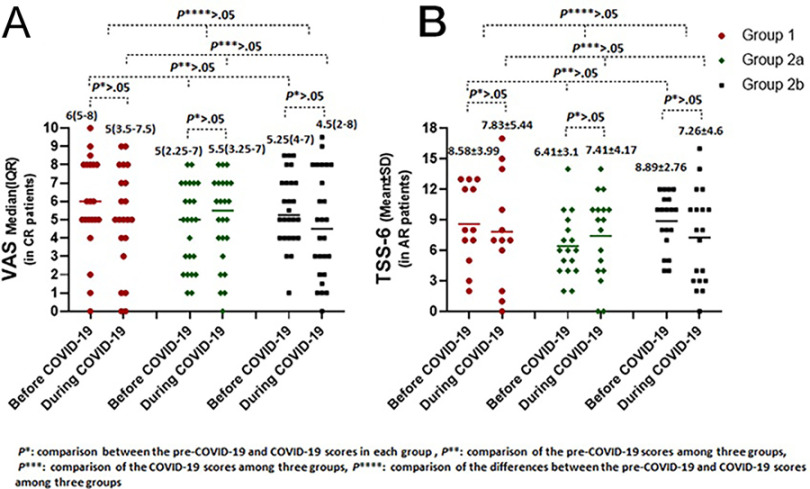
When compared, the TSS-6 (in the subjects with AR) and VAS (in all the subjects) before and during the COVID-19 infection, there was no significant difference among the three study groups. Furthermore, the TSS-6 and VAS scores obtained before and during the COVID-19 infection did not change within each of the three groups (Fig. 2).
Figure 2.
Comparison of (A) visual analog scale (VAS) scores and (B) total symptom score-6 (TSS-6) before and during coronavirus disease 2019 (COVID-19) in group 1, group 2a, and group 2b.
Clinical Outcomes and Treatments Concerning COVID-19
Only seven subjects (9.9%) were identified as having severe COVID-19 infection. The distribution of COVID-19 symptoms is shown in Table 3. The frequency of COVID-19–related symptoms was similar in group 1 and 2 (all p > 0.05) except the reduction in the sense of smell and/or taste (p = 0.02) (Table 3). Two of the subjects (2.8%) were asymptomatic and had no diagnostic sign on the computed tomography of the chest despite having a positive polymerase chain reaction result. A reduction in the sense of smell and/or taste was found in 9 subjects in group 1 (42.9%) and 36 subjects in group 2 (72%) (p = 0.02) (odds ratio 0.292 [95% confidence interval, 0.101–0.844]).
Table 3.
Clinical, radiographic, and laboratory findings of COVID-19 among the subject groups
COVID- 19 = Coronavirus disease 2019; SD = standard deviation; IL = interleukin; CT = computed tomography; CRP = C-reactive protein; AST = aspartate aminotransferase; ALT = alanine aminotransferase; LDH = lactate dehydrogenase.
*The p values for the comparison of the data of groups 1 and 2 are taken from the χ2 test, Fisher exact test, Mann-Whitney U test, or two-tailed t-test.
#Odds ratio 0.292 (95% confidence interval, 0.101–0.844).
§Data are expressed as n/total no. subjects with available data (%). Significant p values are in bold.
Three subjects who were asymptomatic and one pregnant subject chose not to undergo COVID-19 treatment. Sixty-four subjects (91.5%) were treated with hydroxychloroquine for 5 days. The number of the subjects who received a specific antiviral drug and the duration of use were as follows: (1) oseltamivir (25 subjects, 5 days), (2) favipravir (6 subjects, 5 days); and (3) lopinavir-ritonavir (2 subjects, one subject 10 days, one subject 8 days). Two subjects in group 2 (2.8%) were treated with anti-interleukin 6 receptor antibody due to a cytokine storm. The frequencies of abnormal laboratory levels and radiographic findings concerning COVID-19 at admission were not different between both groups (all p > 0.05). All clinical outcomes related to COVID-19 are shown in Table 3.
DISCUSSION
This study contributes significant information to the literature that the use of INCs by patients with CR and infected with SARS-CoV-2 seems effective in preventing the impairment in the sense of taste and/or smell and does not present a harmful impact on the clinical outcomes or the severity of COVID-19. As our knowledge on COVID-19 has improved, many risk groups, such as patients with hypertension, diabetes, and other chronic diseases, have been shown to have more-severe COVID-19 infections.13 In addition, there have been concerns that other chronic diseases, especially those that affect the respiratory tract, e.g., CR, may increase the severity of COVID-19 infections. In the present study, the QoL assessment scores in the SNOT-22 revealed an impairment of QoL in each of the three groups during the COVID-19 infection. Also, the physical symptoms as assessed in the SNOT-22 revealed a worsening in the subjects in groups 2a and 2b. However, the VAS scores (in all the subjects) and TSS-6 score (in subjects with AR) did not change in any of the three groups during the COVID-19 infection. These findings suggest that SNOT-22 is useful to assess sinonasal symptoms of subjects with CR during COVID-19.
In a report from Italy, 20 of 59 hospitalized patients (33.9%) reported at least one taste or olfactory sense alteration and 11 subjects (18.6%) reported alterations in both senses.14 In another report, from Germany, 18 of 45 hospitalized patients with COVID-19 (40%) were anosmic, 20 (44%) were hyposmic, and 7 (15%) were normosmic.15 In the latter study, the Sniffin' test (Sniffin' Sticks, Burghart GmbH, Wedel, Germany) showed that > 80% of the patients with COVID-19 were hyposmic or anosmic, whereas only 49% of these subjects self-reported having a smelling dysfunction.15 This finding showed that the patients who were hyposmic and anosmic seemed to be underestimated among patients infected with SARS-CoV-2. Similarly, in our study, a decrease in the sense of smell and/or taste was found in 45 of 71 subjects (63.4%) and would probably have been higher if the quantitative Sniffin' test had been used. Several immunologic mechanisms have been proposed to explain the hyposmia observed during COVID-19. The expression of angiotensin-converting enzyme 2 and transmembrane protease serine type 2 in olfactory epithelium seemed to play a role in the entry of SARS-CoV-2.16 In addition, the pro-inflammatory cytokine tumor necrosis factor α level was detected to be elevated in the olfactory epithelium in patients with COVID-19 compared with healthy subjects. Concordantly, the inflammation of the olfactory epithelium by tumor necrosis factor α may be responsible for acute olfactory loss.17
Before the pandemic, several studies conducted on postinfectious diseases and nasal polyposis demonstrated the effectiveness of oral corticosteroids for anosmia.18–20 Moreover, INCs have been shown to improve olfactory functions in patients with AR.21 Some treatment options other than steroid therapy, such as olfactory training, theophylline, and sodium citrate, are also recommended for postinfectious olfactory dysfunctions. However, the use of oral steroids is still not recommended until the full clinical presentation (respiratory distress or systemic cytokine storm) of COVID-19 can be identified in patients infected with SARS-CoV2 and with anosmia.22 Oral corticosteroids can aggravate immunosuppression in patients infected with SARS-CoV-2, and the use of oral corticosteroids in patients with Middle East respiratory syndrome caused a delay in RNA reduction in Middle East respiratory syndrome coronavirus.23 However, a case report showed the effectiveness of oral corticosteroid for post–COVID-19 anosmia.24
A study during the pandemic showed earlier improvement in the reduced sense of smell and/or taste in subjects who used INCs.25 In contrast, another study of patients who had recovered from COVID-19 but who continued to have symptoms of anosmia or hyposmia showed that olfactory training alone or when combined with mometasone furoate nasal spray significantly improved smell scores after 3 weeks (52% and 62%, respectively).26 These treatment groups did not differ in terms of the duration of anosmia, smell scores, or time to recovery.26 In concordance with the majority of these studies, results of the present study suggested that INCs may diminish the reduced sense of smell and/or taste during COVID-19. Furthermore, angiotensin-converting enzyme 2 messenger RNA expression has been shown to decrease in the subjects with asthma who were taking inhaled corticosteroids27 as well as in subjects with chronic obstructive pulmonary disease.28 These findings led some to suggest that the use of inhaled corticosteroids in asthma and chronic obstructive pulmonary disease may reduce the susceptibility to COVID-19 infections. Also, the above findings led us to explore the possibility that INCs may have a beneficial effect on the prevention and/or treatment of COVID-19 in patients with CR.
In the present study, one limitation was the lack of a quantitative method, e.g., the Sniffin' test, for measuring the senses of smell and taste. A second limitation was not using nasal provocation to distinguish AR from NAR. However, it was felt that the administration of the Sniffin' test and/or the nasal provocation to the patients infected with SARS-CoV-2 would add too much risk to those administering the tests. The third limitation was the relatively small number of subjects, but when considering the urgency of the pandemic, we felt that any knowledge that could help guide our clinical practice would be of value. Also, it was not possible to create a placebo control group in patients infected with SARS-CoV-2. If possible, the findings in the present study may be supported by placebo controlled studies.
CONCLUSION
INCs should be considered in patients with CR who are infected with SARS-CoV-2 to minimize the impairment in olfactory function observed during COVID-19 infections. We showed that, in the subjects with CR, the continued use of INCs was both safe and effective for the management of sinonasal symptoms.
Footnotes
The authors have no conflict of interest to declare pertaining to this article
No external funding sources reported
REFERENCES
- 1. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020; 5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hagemann J, Onorato GL, Jutel M, et al. Differentiation of COVID-19 signs and symptoms from allergic rhinitis and common cold: an ARIA-EAACI-GA2 LEN consensus. Allergy. 2021; 76:2354–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hellings PW, Klimek L, Cingi C, et al. Non-allergic rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2017; 72:1657–1665. [DOI] [PubMed] [Google Scholar]
- 5. Bousquet J, Akdis CA, Jutel M, et al. Intranasal corticosteroids in allergic rhinitis in COVID-19 infected patients: an ARIA-EAACI statement. Allergy. 2020; 75:2440–2444. [DOI] [PubMed] [Google Scholar]
- 6. Herman P, Vincent C, Parietti Winkler C, et al. Consensus statement. Corticosteroid therapy in ENT in the context of the COVID-19 pandemic. Eur Ann Otorhinolaryngol Head Neck Dis. 2020; 137:315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015; 152(suppl):S1–S43. [DOI] [PubMed] [Google Scholar]
- 8. Greiner AN, Hellings PW, Rotiroti G, et al. Allergic rhinitis. Lancet. 2011; 378:2112–2122. [DOI] [PubMed] [Google Scholar]
- 9. Cakir Cetin A, Kumus O, Keskinoglu P, et al. Turkish validation of the Sino-Nasal Outcome Test-22. Clin Otolaryngol. 2019; 44:557–564. [DOI] [PubMed] [Google Scholar]
- 10. Bousquet PJ, Combescure C, Neukirch F, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007; 62:367–372. [DOI] [PubMed] [Google Scholar]
- 11. Demoly P, Bousquet PJ, Mesbah K, et al. Visual analogue scale in patients treated for allergic rhinitis: an observational prospective study in primary care: asthma and rhinitis. Clin Exp Allergy. 2013; 43:881–888. [DOI] [PubMed] [Google Scholar]
- 12. Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020; 75:1730–1741. [DOI] [PubMed] [Google Scholar]
- 13. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 in patients in Wuhan. J Allergy Clin Immunol. 2020; 146:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020; 71:889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hornuss D, Lange B, Schröter N, et al. Anosmia in COVID-19 patients. Clin Microbiol Infect. 2020; 26:1426–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020; 6:eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torabi A, Mohammadbagheri E, Akbari Dilmaghani N, et al. Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. ACS Chem Neurosci. 2020; 11:1909–1913. [DOI] [PubMed] [Google Scholar]
- 18. Stevens MH. Steroid-dependent anosmia. Laryngoscope. 2001; 111:200–203. [DOI] [PubMed] [Google Scholar]
- 19. Blomqvist EH, Lundblad L, Bergstedt H, et al. Placebo-controlled, randomized, double- blind study evaluating the efficacy of fluticasone propionate nasal spray for the treatment of patients with hyposmia/anosmia. Acta Otolaryngol. 2003; 123:862–868. [DOI] [PubMed] [Google Scholar]
- 20. Heilmann S, Huettenbrink KB, Hummel T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am J Rhinol. 2004; 18:29–33. [PubMed] [Google Scholar]
- 21. Stuck BA, Blum A, Hagner AE, et al. Mometasone furoate nasal spray improves olfactory performance in seasonal allergic rhinitis. Allergy. 2003; 58:1195. [DOI] [PubMed] [Google Scholar]
- 22. Addison AB, Wong B, Ahmed T, et al. Clinical Olfactory Working Group consensus statement on the treatment of postinfectious olfactory dysfunction. J Allergy Clin Immunol. 2021; 147:1704–1719. [DOI] [PubMed] [Google Scholar]
- 23. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018; 197:757–767. [DOI] [PubMed] [Google Scholar]
- 24. Touisserkani SK, Ayatollahi A. Oral corticosteroid relieves post-COVID-19 anosmia in a 35-year-old patient. Case Rep Otolaryngol. 2020; 2020:5892047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. İşlek A, Balcı MK. Evaluation of effects of chronic nasal steroid use on rhinological symptoms of COVID-19 with SNOT-22 questionnaire. Pharmacol Rep. 2021; 73:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abdelalim AA, Mohamady AA, Elsayed RA, et al. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am J Otolaryngol. 2021; 42:102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peters MC, Sajuthi S, Deford P, et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020; 202:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finney LJ, Glanville N, Farne H, et al. Inhaled corticosteroids downregulate the SARS- CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol. 2021; 147:510–519.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]