Abstract
Background: The environmental factors play a major role as risk factors of multiple sclerosis (MS). This study aimed at gathering environmental risk factors of MS in the Middle East and North Africa (MENA).
Methods: We used MEDLINE and EMBASE databases by a systematic review method. Out of a total of 123 studies, 16 studies met the eligibility criteria.
Results: Totally, 47 risk factors were assessed as follows: six studies found sunlight exposure as a protective factor with the odds ratio (OR) ranging from 0.06 to 0.57. Six studies evaluated smoking as a risk factor with the OR ranging from 1.69 in all patients to 6.48 in female patients. Four studies supported measles infection as a risk factor with the OR ranging from 1.60 to 3.77, and in 3 studies, stressful events had a significant association with the OR of 1.80, 1.90, and 32.57.
Conclusion: Among 47 assessed risk factors, sunlight exposure, cigarette smoking, measles infection, Epstein-Barr virus (EBV) infection, and stressful events had a significant association with MS.
Key Words: Multiple Sclerosis, Risk Factors, Environment, Middle East, North Africa
Introduction
Multiple sclerosis (MS), as a leading cause of a long-term disabling neurological disease in young adults, 1 is a chronic inflammatory disease of the central nervous system (CNS) with unclear cause and is believed to occur due to a combination of environmental and genetic causes.2,3
Based on a number of recent studies, the incidence of MS is rising worldwide.1,4 This disease is more prevalent in Northern America (140 cases per 100000 population) and less common in Sub-Saharan Africa (2.1 cases per 100000 population). 4 Moreover, MS has an overall prevalence of 51.52/100000 and the female/male ratio of 2.03 in the Middle East and North Africa (MENA) region. 5 It has been acknowledged that both genetic and environmental factors play a major role as risk factors of the disease, although the etiology of MS has not been fully understood. 6 The well-known mentioned risk factors are low sunlight exposure, Epstein-Barr virus (EBV), and smoking.2,7 These risk factors were mostly studied in Caucasian population rather than in the MENA population.5,8 Since MS has significant social and economic effects on the health system in many countries, detection of the possible risk factors is necessary to conceivably prevent the disease. 1 According to the rising trend of MS prevalence and incidence in the MENA region, 5 it is very important to detect the possible risk factors and their association with the prevalence of the disease.
To the best of authors’ knowledge, there is no comprehensive study on MS risk factors in the MENA region; therefore, the present study aimed at gathering the environmental risk factors mentioned in studies conducted in the MENA region performing a systematic review study.
Materials and Methods
Search strategy: To determine a clear and focused question addressing the environmental risk factors of MS in the MENA region, a systematic literature search of appropriate databases and interfaces such as MEDLINE and EMBASE was performed using the keywords of multiple sclerosis, environmental, risk factor, MENA sub regions (Middle EAST and North Africa), and each country name in the MENA region covering the period from January 1, 1950 to April 25, 2019.
The present study used database-appropriate syntax to optimize searching and evaluating the initial results. Moreover, this study conducted a manual hand-searching of the reference lists of the selected primary articles and related reviews to determine additional studies. 9
Screening and eligibility criteria: This study used computer databases to find data and statistics on MS environmental risk factors by merely addressing human population studies written in English. The observational studies including case-control and cohort studies were reviewed and analyzed to specify MS risk factors in the selected articles.
The titles and abstracts of all the studies identified by the databases were reviewed. Potentially eligible studies were read in full text and recovered to check if they could satisfy the following inclusion criteria in this systematic review: 10 being a population-based study and involving human samples of patients with MS living in the MENA region, 4 covering data on the environmental risk factors of MS in the target countries, 11 and documenting MS according to the global diagnostic criteria such as the McDonald, 12 Poser, 13 or Schumacher criteria that were used at the study period. 14
Two experts reviewed the eligible articles independently. If there was any disagreement between the two reviewers, a third expert reviewer would make the final decision in this respect. Review articles, editorials, and letters as well as articles about genetic risk factors were excluded.
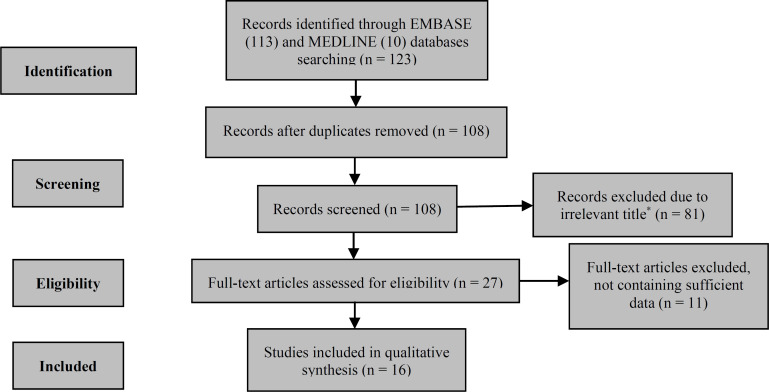
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart was established to demonstrate the method of study selection (Figure 1). The classification of the MENA countries in this study was based on the Global Burden of Disease (GBD) classification including 21 countries. 4
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram
*Studies with a title that was not about assessment of environmental risk factors
Data extraction and quality assessment: Quality assessment of each eligible article is summarized in the table 1. Repeated publications on the identical data were excluded. The following information was included in a form template by an expert reviewer: first author, year of study, study method, study region, risk factors mentioned in the study, and the sample size of the study.
Table 1.
Quality assessment
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdollahpour et al. 31 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 9/10 |
| Abbasi et al. 30 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | Yes | 8/9 |
| Abdollahpour et al. 34 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 |
| Shivappa et al. 21 | Yes | Yes | No | yes | Yes | Yes | Yes | No | NA | Yes | 8/9 |
| Eftekharian et al. 27 | Yes | Yes | Yes | No | Yes | NA | Yes | Yes | NA | Yes | 7/8 |
| Rejali et al. 26 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | 9/9 |
| Alonso et al. 32 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | 9/9 |
| Al-Shammri et al. 25 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | NA | 7/9 |
| Al-Afasy et al. 37 | Yes | Yes | Yes | No | Yes | No | Yes | Yes | NA | Yes | 7/9 |
| Al Wutayd et al. 24 | Yes | Yes | Yes | Yes | Yes | NA | No | Yes | Yes | Yes | 8/9 |
| Halawani et al. 23 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9/10 |
| Mansouri et al. 22 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 |
| Abdollahpour et al. 35 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 |
| Maghzi et al. 38 | Yes | Yes | Yes | Yes | Yes | NA | Yes | Yes | NA | Yes | 8/8 |
| Roudbari et al. 33 | No | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 7/10 |
| Mouhieddine et al. 36 | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | No | Yes | 8/9 |
Q1: Was the sample representative of the target population?
Q2: Were study participants recruited in an appropriate way?
Q3: Was the sample size adequate?
Q4: Were the study subjects and setting described in detail?
Q5: Is the data analysis conducted with sufficient coverage of the identified sample?
Q6: Were objective standard criteria used for measurement of the condition?
Q7: Was the condition measured reliably?
Q8: Was there an appropriate statistical analysis?
Q9: Are all important confounding factors/subgroups/differences identified and accounted for?
Q10: Were subpopulations identified using objective criteria?
NMO-IgG: Neuromyelitis optica immunoglobulin G; NA: Not available
The extracted information from each individual study was summarized in the respective tables. 15 The study first extracted the measures of effect [e.g., odds ratio (OR), relative risk (RR), confidence interval (CI), and hazard ratio (HR)] and the number of cases and controls from each eligible study and then summarized them in tables 2-5.
Table 2.
Dietary habit and food consumption
| Risk factor | Study | Country of study | Study period | Study setting | Case number | Control number | OR | AOR # | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Fish | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) | 80 | 160 | - | 0.20 | 0.05-0.77 | < 0.050 |
| Dairy products | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.42 | - | 1.04-1.96 | 0.030 |
| Milk | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | - | 0.40 | 0.13-1.21 | - |
| Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | _ | 0.59 | - | 0.014 | |
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.32 | - | 0.92-1.91 | 0.140 | |
| Yogurt | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.35 | - | 0.001 |
| Cheese | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.70 | - | 0.120 |
| Conserved food | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.04 | 0.02-0.10 | < 0.001 |
| Fast food | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | - | 2.05 | 1.03-4.08 | 0.042 |
| Fruit | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | - | 0.25 | 0.16-0.38 | < 0.001 |
| Coffee | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | - | 0.46 | 0.31-0.68 | < 0.001 |
| Vegetable | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.57 | - | 0.42-0.79 | 0.001 |
| Date | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.94 | - | 0.68-1.29 | 0.690 |
| Red meat | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.97 | - | 0.60-1.57 | 0.900 |
| DII diet score* | Shivappa et al. 21 | Iran | 2011-2012 | Hospital-based## (single center) |
68 | 140 | - | 1.66 | 1.19-2.31 | 0.003 |
| Dairy consumption | ||||||||||
| Daily | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.49 | 0.33-0.74 | 0.001 |
| 2-3 times/week | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 1.50 | - | 1.06-2.49 | - |
| 4-7 times/week | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 1.10 | - | 0.72-1.60 | - |
| Seafood consumption | - | - | ||||||||
| 2-3 times/week | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.09 | - | 0.04-0.23 | - |
| 1-2 times/week | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.25 | - | 0.04-0.34 | - |
| 1-2 times/month | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.31 | - | 0.04-0.52 | - |
| 2-3 times/year | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.42 | - | 0.07-0.54 | - |
| Supplements | ||||||||||
| Vitamin D | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | 0.30 | - | 0.09-1.15 | - |
| Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.62 | 0.36-1.06 | 0.082 | |
| Calcium | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.44 | 0.27-0.71 | 0.001 |
| Infancy diet | - | |||||||||
| Breast feeding | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 2.90 | 1.49-5.65 | 0.002 |
| Dried milk | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.78 | 0.47-1.28 | 0.321 |
| Cow milk | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.82 | 0.34-1.93 | 0.642 |
| Goat milk | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.58 | 0.51-4.95 | 0.430 |
| Water and sugar | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.15 | 0.43-3.05 | 0.780 |
Significant results are bolded;
DII: Dietary inflammatory index; OR: Odds ratio; AOR: Adjusted odds ratio; CI: Confidence interval
The higher score shows more pro-inflammatory diet;
Adjusted mostly for age and sex;
Cases and controls were taken from the same hospital
Table 5.
Exposure and event
| Risk factors | Study | Country of study | Study period | Study setting | Case number | Control number | OR | AOR # | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sunlight exposure | |||||||||||
| Sunlight exposure | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | - | 0.06 | 0.00-0.65 | < 0.0500 | |
| Abdollahpour et al. 35 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 0.22 | 0.12-0.40 | < 0.0010 | ||
| Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.09 | 0.02-0.38 | < 0.0010 | ||
| Mansouri et al. 22 | Iran | 2008-2013 | Population-based | 1217 | 787 | - | 0.23 | 0.15-0.31 | < 0.0010 | ||
| Alonso et al. 32 * | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.62 | 0.53-0.73 | < 0.0001 | ||
| Alonso et al. 32 ** | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.34 | 0.19-0.60 | 0.0002 | ||
| Al Wutayd et al. 24 *** | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | - | 0.57 | 0.38-0.85 | < 0.0010 | ||
| Al Wutayd et al. 24 ¥ | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | - | 0.48 | 0.30-0.76 | < 0.0010 | ||
| Sunscreen | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 2.44 | 1.26-4.72 | 0.0080 | |
| Low sun exposure¥¥ | Al-Shammri et al. 25 | Kuwait | - | Hospital-based### (single center) |
195 | 146 | - | 5.30 | 2.70-10.50 | < 0.0010 | |
| Fully shrouded outdoor dressing | Al-Shammri et al. 25 | Kuwait | - | Hospital-based### (single center) |
195 | 146 | - | 2.20 | 1.00-5.00 | 0.0650 | |
| Sun exposure in childhood | |||||||||||
| 2 hours per day | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.51 | - | 0.22-1.17 | - | |
| 2-6 hours per day | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.44 | - | 0.20-0.98 | - | |
| 6-8 hours per day | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.66 | - | 0.30-1.46 | - | |
| Toxin exposure | - | ||||||||||
| Exposure to solvents€ | Al-Afasy et al. 37 | Kuwait | 2010-2012 | Population-based | 101 | 202 | 2.10 | - | 1.10-3.80 | 0.0200 | |
| Toxic fumes from oil wells | Al-Afasy et al. 37 | Kuwait | 2010-2012 | Population-based | 101 | 202 | 12.50 | - | 1.60-95.70 | 0.0150 | |
| Toxin | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.90 | 0.34-2.41 | 0.8350 | |
| Heavy metal | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.85 | 0.23-3.23 | 0.8150 | |
| Mercury lead | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.77 | 0.14-4.26 | 0.7660 | |
| Microwave exposure€€ | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 3.55 | 2.24-5.63 | < 0.0010 | |
| Animal exposure | |||||||||||
| Animal ownership (cat) | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.67 | 0.35-1.31 | - | |
| Dog | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.57 | 0.30-1.06 | - | |
| Bird | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 1.11 | 0.73-1.68 | - | |
| Sheep | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.70 | 0.32-1.54 | - | |
| Goat | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.97 | 0.36-2.59 | - | |
| Cow | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.64 | 0.30-1.40 | - | |
| Pet exposure | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.48 | 0.88-2.47 | 0.1370 | |
| Amount of pets | |||||||||||
| 1 | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 1.27 | 0.83-1.94 | - | |
| 2 | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.55 | 0.27-1.14 | - | |
| 3+ | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.56 | 0.23-1.36 | - | |
| Stressful events€€€ | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 32.57 | 17.21-61.64 | < 0.0010 | |
| Eftekharian et al. 27 Ֆ | Iran | 2013 | Population-based | 250 | 250 | 1.90 | - | 1.30-2.70 | - | ||
| Al-Afasy et al. 37 Ֆ Ֆ | Kuwait | 2010-2012 | Population-based | 101 | 202 | - | 1.80 | 1.10-3.50 | 0.0220 | ||
| Childhood hospital admission Ֆ Ֆ Ֆ | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.74 | 0.08-6.52 | 0.7840 | |
Significant results are bolded;
OR: Odds ratio; AOR: Adjusted odds ratio; CI: Confidence interval
Sunlight exposure more than 1 hours per day;
Sunlight exposure more than 2.5 hours per day;
High level of sunlight exposure during primary school;
High level of sunlight exposure during university;
Less than 15 minutes per day;
Paints and pesticides;
Using microwave less than 3 times a week;
Such as death of a parent or sibling or child, divorce of parents, getting married, divorced, unplanned pregnancy/abortion, major personal injury or illness, being ousted from work, retirement, major change in living conditions, major change in financial state,… 1 month prior to diagnosis;
Developing a sudden shock upon hearing bad news;
Being in Kuwait during Iraqi invasion in 1990;
Hospital admission for at least 24 hours during childhood;
Adjusted mostly for age and sex;
Cases and controls were recruited from the same hospital;
Controls were recruited from Kuwaiti community living in Kuwait
Results
As it is shown in figure 1, we reached to 28 articles after the removal of identical and irrelevant studies. Out of the 28 remaining articles, 12 studies were excluded due to incompatibility with the eligibility criteria. One of the mentioned 12 articles was removed because it was about Iranian immigrants in Canada; 16 hence, it was not considered as a study addressing the MENA region. Two studies were removed because they were only about MS demographic risk factors (month of birth).17,18 Nine of the mentioned studies did not contain sufficient data such as OR, adjusted OR (AOR), and RR to be included in the study.19,20
The remaining 16 eligible articles were all written in English and all of them were case-control studies, ranged in sample size from 68 cases with 140 controls to 1217 cases with 787 controls.21,22
Among these 16 articles, five articles were hospital-based studies21,23-26 and the rest were population-based studies. Among these five studies, all recruited the cases and controls from the same hospital, with the exception of one study which recruited controls from Kuwaiti community living in Kuwait. 25 Totally, the eligible articles assessed 47 environmental factors such as socioeconomic status (SES), dietary lifestyle, infancy diet, smoking status, alcohol use, drug abuse, comorbidity, viral infections, past surgical history, vaccination, fetal problems, head trauma, body size, sunlight exposure, toxin exposure, animal exposure, and stressful events. We summarized the results of the mentioned 16 articles in tables 2-5.
Dietary habit and food consumption: Table 2 indicates the possible association between different dietary habits and MS development. The significant results are as follows: Al Wutayd et al. found that consumption of dairy products (OR = 1.42, 95% CI: 1.04-1.96) and fast foods (AOR = 2.05, 95% CI: 1.03-4.08) more than five times per week increased the risk of MS development; meanwhile, drinking coffee daily (AOR = 0.46, 95% CI: 0.31-0.68) and eating more than five servings of fruit weekly (AOR = 0.25, 95% CI: 0.16-0.38) lowered the risk of MS development. 24 Fish consumption for more than once weekly (AOR = 0.20, 95% CI: 0.05-0.77) 23 and seafood consumption 2-3 times per week (OR = 0.09, 95% CI: 0.04-0.23) 27 decreased the risk of MS development in Halawani et al.23 and Eftekharian et al.27 studies, respectively. Shivappa et al. found that pro inflammatory diets [the diets with higher score of dietary inflammatory index (DII)] were considered as pro-inflammatory diets;21 such as western-type diet, which is high in refined grains, simple carbohydrates, red meats, and high-fat dairy products. 28 These diets can potentially increase the inflammatory markers of the body such as C-reactive protein (CRP), interleukin-6 (IL-6), and homocysteine, 29 and were strongly associated with MS development (AOR = 1.66, 95% CI: 1.19-2.31). 21 In Abbasi et al. study, dairy (AOR = 0.49, 95% CI: 0.33-0.74) and calcium supplements (AOR = 0.44, 95% CI: 0.27-0.71) were revealed as protective factors. In addition, interestingly, this study found that breastfeeding the infants (AOR = 2.90, 95% CI: 1.49-5.65) increased the MS development in infants in the future. 30
Tobacco and waterpipe smoking, alcohol use, and drug abuse: Table 3 provides the summary of the association of tobacco and waterpipe smoking, alcohol use, and drug abuse with MS. Six of the studies found smoking as a risk factor of the disease with the OR ranging from 1.69 in both male and female patients to 6.48 in female patients.22,23,27,31-33 Abdollahpour et al. study not only considered smoking as a risk factor but also concluded that being a tobacco smoker (AOR = 1.69, 95% CI: 1.24-2.31), waterpipe smoker (AOR = 1.71, 95% CI: 1.36-2.31), and a passive smoker (AOR = 1.85, 95% CI: 1.48-2.32) had a significant clear dose-response association with MS both for duration of exposure and the amount of smoking.31 In Roudbari et al. study, smoking increased the risk of developing secondary progressive MS (SPMS) in patients with MS (OR = 2.43, 95% CI: 1.28-4.60). 33
Table 3.
Tobacco and waterpipe smoking, alcohol consumption, and drug abuse
| Risk factor | Study | Country of study | Study period | Study setting | Case number | Control number | OR | AOR # | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Cigarette smoking | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | - | 4.16 | 1.44-11.97 | < 0.050 |
| Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.62 | 0.36-1.05 | 0.075 | |
| Eftekharian et al. 27 * | Iran | 2013 | Population-based | 250 | 250 | 2.86 | - | 1.30-6.33 | - | |
| Eftekharian et al. 27 ** | Iran | 2013 | Population-based | 250 | 250 | 2.08 | - | 0.81-5.31 | - | |
| Mansouri et al. 22 | Iran | 2008-2013 | Population-based | 1217 | 787 | _ | 1.93 | 1.31-2.73 | < 0.001 | |
| Al-Afasy et al. 37 | Kuwait | 2010-2012 | Population-based | 101 | 202 | 1.70 | _ | 0.90-3.40 | 0.097 | |
| Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 1.72 | 0.90-3.30 | - | |
| Al-Shammri et al. 25 | Kuwait | - | Hospital-based### (single center) |
195 | 146 | - | 1.90 | 0.80-4.50 | 0.128 | |
| Roudbari et al. 33 *** | Iran | - | Population-based | 144 | 256 | 2.43 | - | 1.28-4.60 | 0.007 | |
| Female cigarette smoking | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 6.48 | 1.46-28.78 | 0.002 |
| Tobacco | Abdollahpour et al. 31 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.69 | 1.24-2.31 | < 0.001 |
| Passive smoking¥ | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | 1.28 | - | 0.75-2.20 | - |
| Abdollahpour et al. 35 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.48 | 1.14-1.93 | 0.004 | |
| Abdollahpour et al. 31 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.85 | 1.48-2.32 | < 0.001 | |
| Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.13 | 0.71-1.78 | 0.614 | |
| Passive smoking during childhood | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 2.25 | - | 1.57-3.20 | - |
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.00 | - | 0.72-1.39 | > 0.999 | |
| Ex-smoker* | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.32 | - | 0.84-1.56 | 0.430 |
| Current smoker | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.16 | - | 0.77-1.76 | 0.470 |
| Waterpipe smoking | Abdollahpour et al. 35 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.69 | 1.22-2.34 | 0.001 |
| Abdollahpour et al.31 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.77 | 1.36-2.31 | < 0.001 | |
| Alcohol | Abdollahpour et al. 35 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.61 | 1.11-2.33 | < 0.012 |
| Drug abuse | ||||||||||
| Life time drug abuse | Abdollahpour et al. 35 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 2.93 | 1.83-4.70 | < 0.001 |
| Drug abuse (opioids) | Abdollahpour et al. 35 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.00 | 0.52-1.92 | 0.998 |
| Drug abuse (cannabis) | Abdollahpour et al. 35 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.23 | 0.61-2.46 | 0.560 |
| Use of OTC antibiotics | ||||||||||
| Weekly | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 1.98 | - | 0.44-3.96 | |
| 1-2 times per month | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 1.55 | - | 0.62-3.84 | |
| Antibacterial soap usage | ||||||||||
| 1-2 per week | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.18 | - | 0.08-0.40 | - |
| 1-2 per month | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 0.43 | - | 0.22-0.82 | - |
| History of OCP usage | Rejali et al. 26 | Iran | 2014 | Hospital-based## (single center) |
200 | 200 | - | 0.49 | 0.31-0.77 | 0.002 |
| Duration of OCP usage | Rejali et al. 26 | Iran | 2014 | Hospital-based## (single center) |
200 | 200 | - | 0.88 | 0.80-0.96 | 0.008 |
| OCP starting age | Rejali et al. 26 | Iran | 2014 | Hospital-based## (single center) |
200 | 200 | - | 0.99 | 0.92-1.05 | 0.800 |
Significant results are bolded;
OTC: Over the counter; OR: Odds ratio; AOR: Adjusted odds ratio; CI: Confidence interval; OCP: Oral contraceptive pill
Taking 5 cigarette per day;
Taking 3-5 cigarette per day;
The study evaluated smoking as a risk factor for developing secondary progressive multiple sclerosis (SPMS);
Ever being exposed to passive smoking;
Adjusted mostly for age and sex;
Cases and controls were recruited from the same hospital;
Controls were recruited from Kuwaiti community living in Kuwait
However, only Alonso et al. found smoking as a risk factor for female patients (OR = 6.48, 95% CI: 1.46-28.78) and not for male patients (OR = 0.72, 95% CI: 0.31-1.68). 32 In contrast, three studies did not support smoking as a risk factor of MS.25,30,34 According to Abdollahpour et al. study, there was a significant association between MS and alcohol consumption (AOR = 1.61, 95% CI: 1.11-2.31) and lifetime drug abuse (AOR = 2.93, 95% CI: 1.83-4.70). 35
Medical history: Table 4 summarizes the possible association between medical histories and MS. According to Al-Shammri et al. study, the presence of any chronic medical illness (AOR = 2.40, 95% CI: 1.30-4.70) might increase the chance of MS development. 25 Mansouri et al. study supported allergy history (AOR = 1.92, 95% CI: 1.55-2.47) as a risk factor of the disease; 22 however, Halawani et al. study did not support the presence of any significant association between allergy history and MS development. 23 Abbasi et al. study revealed that the presence of diabetes mellitus (DM) (AOR = 0.11, 95% CI: 0.01-0.99) might decrease the risk of MS. 30 Al Wutayd et al. study supported thyroid disorder (OR = 0.55, 95% CI: 0.30-0.99) as a protective factor of the disease. 24 However, the other two studies revealed that there was not any significant association between MS and thyroid diseases such as hypothyroidism, hyperthyroidism, and thyroiditis.30,34 Migraine headache was evaluated in three studies, only one of which reported it as a risk factor (OR = 3.50, 95% CI: 1.61-7.59). 30 The other two studies did not find any significant association between MS and migraine headache.24,34 Eftekharian et al. study revealed that being depressed (OR = 3.30, 95% CI: 2.30-4.80), having obsessive-compulsive disorder (OCD) (OR = 1.93, 95% CI: 1.34-2.80), often (OR = 4.83, 95% CI: 3.03-7.71) and sometimes (OR = 1.74, 95% CI: 1.08-2.80) experiencing negative thoughts, and having anxiety disorder (OR = 3.82, 95% CI: 2.60-5.50) were all significantly associated with MS. 27 In four studies, measles infection was significantly associated with MS (OR range: 1.60-3.77 and OR mean = 2.70).23,24,27,30 In Eftekharian et al. study, childhood history of measles and rubella was revealed as a risk factor of MS (OR = 1.70, 95% CI: 1.20-2.40). In addition, there was a significant association between a history of chickenpox infection and MS (OR = 1.43, 95% CI: 1.02-2.00). 27
Table 4.
Medical history
| Risk factor | Study |
Country of
study |
Study
period |
Study setting |
Case
number |
Control
number |
OR | AOR# | 95% CI | P | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| History of comorbidity* | Al-Shammri et al. 25 | Kuwait | - | Hospital-based### (single center) |
195 | 146 | - | 2.40 | 1.30-4.70 | < 0.0010 | ||||||||||||||
| Autoimmune disease history | ||||||||||||||||||||||||
| 1 autoimmune disease history | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 0.97 | 0.71-1.32 | 0.8550 | ||||||||||||||
| More than 1 autoimmune disease history | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 0.88 | 0.38-2.03 | 0.7630 | ||||||||||||||
| Allergy history | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | 1.06 | - | 0.73-1.89 | - | ||||||||||||||
| Mansouri et al. 22 | Iran | 2008-2013 | Population-based | 1217 | 787 | - | 1.92 | 1.55-2.47 | < 0.0010 | |||||||||||||||
| Type 1 DM | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 0.39 | 0.08-1.86 | 0.2360 | ||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | - | 0.67 | 0.34-1.31 | 0.2350 | |||||||||||||||
| DM | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.11 | 0.01-0.99 | 0.0490 | ||||||||||||||
| CVDs | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.54 | 0.08-3.69 | 0.5300 | ||||||||||||||
| SLE | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.19 | - | 0.02-1.69 | 0.2200 | ||||||||||||||
| RA | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 0.73 | 0.39-1.39 | 0.3400 | ||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.94 | - | 0.48-1.86 | 0.8600 | |||||||||||||||
| Thyroid disorder | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.55 | - | 0.30-0.99 | 0.0400 | ||||||||||||||
| Thyroiditis | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.95 | 0.44-2.05 | 0.8860 | ||||||||||||||
| Hypothyroidism | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.08 | 0.75-1.56 | 0.6580 | ||||||||||||||
| Hyperthyroidism | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 0.72 | 0.37-1.39 | 0.3250 | ||||||||||||||
| Crohn’s disease | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 6.64 | 0.59-74.12 | 0.1240 | ||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.00 | - | 0.06-16.06 | > 0.9999 | |||||||||||||||
| UC | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 6.64 | 0.59-74.12 | 0.1240 | ||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.41 | - | 0.68-2.93 | 0.3600 | |||||||||||||||
| Psoriasis | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 2.15 | 0.61-7.52 | 0.2300 | ||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.77 | - | 0.51-6.10 | 0.3610 | |||||||||||||||
| Kidney disease | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.02 | 0.61-1.71 | 0.9300 | ||||||||||||||
| Migraine | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.24 | 0.90-1.71 | 0.1940 | ||||||||||||||
| Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 3.50 | 1.61-7.59 | 0.0020 | |||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.91 | - | 0.56-1.49 | 0.7100 | |||||||||||||||
| HTN | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.89 | 0.31-2.53 | 0.8280 | ||||||||||||||
| Al-Afasy et al. 37 | Kuwait | 2010-2012 | Population-based | 101 | 202 | 0.10 | - | 0.04-0.04 | 0.0010 | |||||||||||||||
| Psychiatric disorder | ||||||||||||||||||||||||
| Stress and anxiety disorder | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 3.82 | - | 2.60-5.50 | - | ||||||||||||||
| Depression | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 3.30 | - | 2.30-4.80 | - | ||||||||||||||
| OCD | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 1.93 | - | 1.34-2.80 | - | ||||||||||||||
| Negative thoughts | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 4.83 | - | 3.03-7.71 | - | ||||||||||||||
| Eftekharian et al. 27 ** | Iran | 2013 | Population-based | 250 | 250 | 1.74 | - | 1.08-2.80 | - | |||||||||||||||
| Viral infection history | ||||||||||||||||||||||||
| Measles infection | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | - | 3.75 | 1.45-9.70 | < 0.0500 | ||||||||||||||
| Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.60 | 1.05-2.45 | 0.0290 | |||||||||||||||
| Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 1.12 | 0.66-1.90 | - | |||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | - | 3.77 | 2.05-6.96 | < 0.0010 | |||||||||||||||
| Chickenpox | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | 1.25 | - | 0.73-2.15 | - | ||||||||||||||
| Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.28 | 0.88-1.87 | 0.1900 | |||||||||||||||
| Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 1.43 | - | 1.02-2.00 | - | |||||||||||||||
| Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 1.15 | 0.78-1.69 | - | |||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.90 | - | 0.65-1.24 | 0.5700 | |||||||||||||||
| Rubella | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.47 | 0.85-2.55 | 0.1660 | ||||||||||||||
| Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 1.61 | 0.81-3.28 | - | |||||||||||||||
| Measles and rubella | Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 1.70 | - | 1.20-2.40 | - | ||||||||||||||
| Hepatitis | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.65 | 0.10-4.18 | - | ||||||||||||||
| Mumps | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.85 | 1.22-2.78 | 0.0030 | ||||||||||||||
| Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 1.52 | 0.93-2.49 | - | |||||||||||||||
| EBV | ||||||||||||||||||||||||
| Anti-VCA titer | Mouhieddine et al. 36 | Lebanon | 2012-2014 | Population-based | 249 | 230 | 1.02 | - | 1.01-1.03 | 0.0020 | ||||||||||||||
| Anti-EBNA-1 titer | Mouhieddine et al. 36 | Lebanon | 2012-2014 | Population-based | 249 | 230 | 1.14 | - | 1.10-1.19 | < 0.0001 | ||||||||||||||
| Past surgical history | ||||||||||||||||||||||||
| History of tonsillectomy | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | 0.86 | - | 0.40-1.85 | - | ||||||||||||||
| Al-Afasy et al. 37 | Kuwait | 2010-2012 | Population-based | 101 | 202 | 0.30 | - | 0.10-0.90 | 0.0290 | |||||||||||||||
| Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 0.65 | 0.32-1.34 | - | |||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 1.25 | - | 0.81-1.94 | 0.3700 | |||||||||||||||
| Appendectomy | Alonso et al. 32 | Iran | 2007-2010 | Population-based | 394 | 394 | - | 2.32 | 0.89-6.05 | - | ||||||||||||||
| Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.72 | - | 0.43-1.19 | 0.2500 | |||||||||||||||
| General anesthesia | Al-Afasy et al. 37 | Kuwait | 2010-2012 | Population-based | 101 | 202 | 0.60 | - | 0.30-1.00 | 0.0410 | ||||||||||||||
| Vaccination | ||||||||||||||||||||||||
| Childhood vaccination complete€ | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.53 | 0.28-1.00 | 0.0500 | ||||||||||||||
| Adulthood vaccination€€ | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 4.57 | 1.14-18.41 | 0.0320 | ||||||||||||||
| Varicella vaccine | Mansouri et al. 22 | Iran | 2008-2013 | Population-based | 1217 | 787 | - | 1.34 | 1.02-1.83 | 0.0400 | ||||||||||||||
| Head trauma | ||||||||||||||||||||||||
| Head trauma in adulthood | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 1.49 | 0.59-3.74 | 0.3950 | ||||||||||||||
| History of head trauma | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.27 | 0.94-1.70 | 0.1160 | ||||||||||||||
| Al-Afasy et al. 37 | Kuwait | 2010-2012 | Population-based | 101 | 202 | - | 2.60 | 1.20-5.50 | 0.0140 | |||||||||||||||
| Cumulative head trauma | Abdollahpour et al. 34 | Iran | 2013-2015 | Population-based | 547 | 1057 | - | 1.02 | 0.90-1.16 | 0.7700 | ||||||||||||||
| Childhood head trauma | Abbasi et al. 30 €€€ | Iran | 2013-2014 | Population-based | 660 | 421 | - | 8.21 | 1.56-43.06 | 0.0130 | ||||||||||||||
| Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 1.86 | - | 1.20-2.87 | - | |||||||||||||||
| Eftekharian et al. 27 | Iran | 2013 | Population-based | 250 | 250 | 2.11 | - | 1.38-4.41 | - | |||||||||||||||
| Body size | ||||||||||||||||||||||||
| Large body size | Halawani et al. 23 | Saudi Arabia | 2017-2018 | Hospital-based## (single center) |
80 | 160 | - | 8.97 | 1.03-77.98 | < 0.0500 | ||||||||||||||
| BMI > 30 kg/m2 | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.92 | - | 0.62-1.37 | 0.6700 | ||||||||||||||
| BMI (25-29.99 kg/m2) | Al Wutayd et al. 24 | Saudi Arabia | - | Hospital-based## (multi center) |
307 | 307 | 0.75 | - | 0.52-1.09 | 0.1300 | ||||||||||||||
| Obstetrics and gynecology | ||||||||||||||||||||||||
| Born in singleton pregnancy | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.94 | 0.08-11.68 | 0.9590 | ||||||||||||||
| Number of pregnancy | Rejali et al. 26 | Iran | 2014 | Hospital-based## (single center) |
200 | 200 | - | 0.58 | 0.46-0.74 | < 0.0010 | ||||||||||||||
| Born by vaginal delivery | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | _ | 0.80 | 0.41-1.58 | 0.5200 | ||||||||||||||
| Born by cesarean section | Maghzi et al. 38 | Iran | - | Population-based | 449 | 900 | 2.51 | _ | 1.43-4.41 | 0.0010 | ||||||||||||||
| Age of menarche | Rejali et al. 26 | Iran | 2014 | Hospital-based## | 200 | 200 | - | 0.78 | 0.67-0.89 | 0.0010 | ||||||||||||||
| Fetal problems | - | |||||||||||||||||||||||
| Post maturity | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.60 | 0.11-3.34 | 0.5610 | ||||||||||||||
| Prematurity | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 4.99 | 1.34-18.68 | 0.0170 | ||||||||||||||
| Low birth weight | Abbasi et al. 30 | Iran | 2013-2014 | Population-based | 660 | 421 | - | 0.63 | 0.17-2.38 | 0.4900 | ||||||||||||||
Significant results are bolded;
OR: Odds ratio; AOR: Adjusted odds ratio; CI: Confidence interval; DM: Diabetes mellitus; CVD: Collagen vascular disease; SLE: Systemic lupus erythematous; RA: Rheumatoid arthritis; UC: Ulcerative colitis; HTN: Hypertension; OCD: Obsessive compulsive disorder; EBV: Epstein-Barr virus; VCA: Viral capsid antigen; EBNA-1: Epstein-Barr nuclear antigen 1; BMI: Body mass index
Presence of any chronic medical illnesses;
Adjusted mostly for age and sex;
Cases and controls were recruited from the same hospital;
Controls were recruited from Kuwaiti community living in Kuwait;
Negative thoughts often;
Negative thoughts sometimes;
Complete vaccination according to the Iran Ministry of Health’s immunization program;
Vaccination after 18 years old and this vaccination must be 1 month prior to diagnosis;
Head trauma during childhood that needs at least 24-hour admission in emergency department in hospital;
History of head trauma during childhood for once;
History of head trauma during childhood for few times;
Based on a figure rating scale
Abbasi et al. study supported a significant association between mumps infection and MS (OR = 1.85, 95% CI: 1.22-2.78). 30 Meanwhile, Alonso et al. study indicated that there was not any significant association between measles infection and MS. 32 Mouhieddine et al. study evaluated the relation of anti-EBV antibody titers with environmental risk factors. This study showed a significant association between higher titer of anti-viral capsid antigen (anti-VCA) titer (OR = 1.02, 95% CI: 1.01-1.03) and anti-Epstein-Barr nuclear antigen (anti-EBNA)-1 titer (OR = 1.14, 95% CI: 1.10-1.19) with MS; furthermore, according to this study, smoking along with positive anti-VCA (OR = 1.39, 95% CI: 1.07-1.81) and anti-EBNA-1 (OR = 1.40, 95% CI: 1.07-1.83) had significant association with MS.36 The past surgical history section in table 4 examines the association between tonsillectomy and MS;23,24,32,37 however, only Al-Afasy et al. study reported that there was a significant association in this regard (OR = 0.30, 95% CI: 0.10-0.90). 37 In addition, two studies addressed appendectomy and did not indicate any significant association in this respect.24,32 According to Al-Afasy et al. study, general anesthesia (OR = 0.60, 95% CI: 0.30-1.00) may decrease the risk of MS.
Abbasi et al. study revealed that complete vaccination during childhood that was performed by immunization program launched by Iranian Ministry of Health (AOR = 0.53, 95% CI: 0.28-1.00) decreased the risk of MS. However, vaccination after 18 years old (AOR = 4.57, 95% CI: 1.14-18.41) increased the chance of MS development. 30 Three studies considered the history of head trauma as a risk factor.27,30,37 The AOR was 2.6 in Al-Afasy et al. study;37 however, it must be mentioned that Abbasi et al. 30 and Eftekharian et al. 27 studies only supported the history of head trauma in childhood as a risk factor with the AOR of 8.21 and OR of 1.86, respectively. Abdollahpour et al. study reported that head trauma was not significantly associated with MS development. 34 In Halawani et al. study from western region of Saudi Arabia, a large body size was significantly associated with MS (AOR = 8.97, 95% CI: 1.03-77.98), 23 while another study from Saudi Arabia did not report its significant association with MS. 24 According to Rejali et al. study, a higher number of pregnancies (AOR = 0.58, 95% CI: 0.46-0.74) and a higher menarche age (AOR = 0.78, 95% CI: 0.67-0.89) decreased the risk of MS significantly. 26 In addition, Maghzi et al. study reported that being born by cesarean section was significantly associated with MS in female population (OR = 2.69, 95% CI: 1.30-5.58), while no significant association was observed in male population (OR = 2.25, 95% CI: 0.90-5.63). 38 Abbasi et al. study revealed that premature birth (AOR = 4.99, 95% CI: 1.34-18.68) might increase the risk of developing MS.
Exposure and event: The possible association of exposure and events with MS is summarized in table 5. In six studies, sunlight exposure was considered as a protective factor with the OR ranging from 0.06 to 0.57 with the mean of 0.32.22-24,30,32,35 Furthermore, Al-Shammri et al.25 and Abbasi et al.30 studies revealed that the use of sunscreen (AOR = 2.44, 95% CI: 1.26-4.72) and less exposure to sunlight (AOR = 5.30, 95% CI: 2.70-10.50) increased the risk of MS (AOR = 5.30, 95% CI: 2.70-10.50), respectively. In contrast to the mentioned findings, Eftekharian et al. study revealed that there was no significant association between MS and sunlight exposure during childhood, with the exception of 2-6 hours of sunlight exposure per day (OR = 0.44, 95% CI: 0.20-0.98). 27 In Al-Afasy et al. study, the exposure to paints and pesticide (OR = 2.10, 95% CI: 1.10-3.80) and toxic fumes from oil wells (OR = 12.50, 95% CI: 1.60-95.70) was significantly associated with the risk of developing MS. 37 Moreover, Abbasi et al. study revealed that microwave exposure (AOR = 3.55, 95% CI: 2.24-5.63) was significantly associated with MS. There are two studies that addressed the association between animal exposure and MS and revealed no significant association in this regard.30,32 In three studies, stressful events were found to have a key role in development of MS with the OR of 1.90, 32.57, and 1.80, respectively.27,30,37
Discussion
The present study has summarized the major environmental risk factors associated with MS in the MENA region following a systematic review. Evaluated risk factors were mostly about MS disease onset except for one (cigarette smoking in Roudbari et al.33 study) which was about relapse-remitting MS (RRMS) progression to SPMS. According to the results, majority of the evaluated risk factors were milk consumption, smoking status, migraine, measles infection, chickenpox infection, tonsillectomy, sunlight exposure, and stressful events. Therefore, the current study focused on these risk factors in particular.
The present study holds neutral perception towards the association between MS and dairy products and milk consumption as various results were extracted from three studies that evaluated the mentioned two factors.23,24,30
According to an umbrella review of systematic reviews and meta-analyses addressing the association of environmental risk factors with MS, smoking had a strong association with the onset of MS. 2 However, our results in this study revealed that only six of the studies reported the significant association of smoking with MS, while three of the studies did not support a significant association in this regard. The studies with a larger sample size tended to support smoking as a risk factor;22,31 however, surprisingly one of the studies with a relatively large sample size did not find a significant association between smoking and MS. The mentioned study pointed that the mentioned finding could be attributed to the higher number of female patients with less tendency to smoking as compared with male patients. 30 It appears that smoking in susceptible individuals carrying certain genes increases the risk of MS development.39,40 Although the exact etiology of smoking effect on MS is indefinite, it is pointed that a sustained exposure to cigarette smoking can increase pro-inflammatory mediators that can lead to autoimmune diseases. 41 Nicotine may increase blood-brain barrier (BBB) permeability; hence, it may increase the infiltration of immune cells into CNS. 42 Overall, in line with findings of previous studies, our results mostly confirm smoking as a risk factor for MS.2,43,44 The possible reason for the mentioned controversial findings may be the genetic diversity of the study populations. For example, the presence of human leukocyte antigen-DRB1*15:01 (HLA-DRB*15:01) and the absence of HLA-A*02:01 in Swedish population make smokers 10 times more likely to develop MS. 45
Among three studies addressing the migraine-MS association, only one study revealed a robust association. 30 However, the researchers of the mentioned study noted that migraine could be a symptom of MS caused by plaques in the midbrain area. 46 Contrary to our results, a meta-analysis study indicated that patients with MS were twice more likely to have migraine without aura headache, though the causal relationship between migraine and MS was unclear. 47 In several studies, migraine often occurs prior to MS; hence, migraine may be considered as a possible risk factor of MS.48-50 According to a recent study, migraine also increases rates of MS relapse. 51 All of these studies suggest the possible association between MS and migraine.
Over many years of conducting studies aimed at determining the association between measles infection and MS, the results of some studies have indicated a significant association, 52 while several studies have not supported a significant association in this regard.2,53 Two of the measles infection complications, namely acute disseminated encephalomyelitis (ADEM) and subacute sclerosing panencephalitis (SSPE), are about demyelination of CNS, which may suggest that measles infection with demyelinating lesions can develop MS. 54 Another study showed an increased level of anti-measles antibody in serum and cerebrospinal fluid (CSF) of patients diagnosed with MS over time. 55 However, according to a Swedish cohort study, no effect was observed on MS incidence after mass measles, mumps, and rubella (MMR) vaccination in Swedish population. The mentioned finding indicated the fact that the decreased incidence of measles infection did not change the incidence of MS; hence, this finding may discredit the association between MS and measles infection. 56 Interestingly, measles infection at older ages was more common among patients with MS as compared to healthy individuals. 57 The mentioned finding suggests that a mature immune system response against measles infection antigen can subsequently lead to MS development. 24 According to our result, all four studies, with the exception of one study, suggested a strong association between MS and measles infection.
Only one of the five studies addressing the history of chickenpox in the present systematic review study indicated a significant association between MS and chickenpox. 27 According to a cross-sectional study conducted in 2013 about varicella zoster virus (VZV) participation in MS relapses in Mexican population, a transient presence of VZV during the relapse phase of MS and a decrease of VZV during the remission phase proposed the possible role of VZV in the pathogenesis of MS. 58 A molecular mimicry between VZV antigens and neuronal cells is suggested. The mentioned molecular mimicry can cause autoimmune cross-reaction against neuronal cells of the CNS in this recent study;59 however, our study did not support a significant association in this respect, as only one of the five studies investigating the history of chickenpox supported the presence of a significant association. 27
According to a recent systematic review and meta-analysis study, the history of tonsillectomy under the age of 20 years can increase the risk of MS. 60 This finding may support the theory that removal of tonsils as one of the immune system organs can facilitate invasion of the upper respiratory tract infections that may trigger MS development. 60 Meanwhile, the results of the current study indicated that one study supported the history of tonsillectomy as a protective factor of MS, 37 and the other three studies did not find a significant association in this respect.23,24,32 Therefore, the reviewers of the present study hold a neutral perspective in this regard.
Based on the findings of recent studies, obesity is considered as one of the MS risk factors;43,61 however, the results of the current study revealed diverse results. For instance, two studies conducted in Saudi Arabia can be mentioned in this respect.23,24 Two variables of large body size 23 and a high amount of fast food consumption 24 that can lead to obesity supported obesity as a risk factor for MS; however, one of these studies did not find a significant association between high body mass index (BMI) and MS development. 24 Contrary to the mentioned finding, another study reviewing case-control studies from Sweden and America revealed that BMI ≥ 27 kg/m2 in individuals with susceptible genotypes (presence of HLA-DRB1*15 and absence of HLA-A*02), as compared with individuals not carrying the susceptible genotype, made them much more likely to develop MS. 62 It can be concluded that genotype might be a confounding factor resulting in different observations in various studies.
Almost all of the studies addressing sunlight exposure in the present study found it as a protective factor of MS. Sunlight exposure is considered as one of the major protective factors not only in our eligible studies conducted in the MENA region but also in most of the worldwide studies. 63 Many studies have already pointed that sunlight exposure can decrease MS development both independent from vitamin D 64 and by increasing the amount of cholecalciferol (vitamin D3) 65 which modulates the immune system.
Our results supported stressful events as a risk factor of MS since all three studies evaluating stressful events revealed a strong association between MS and stress.27,30,37 On the other hand, a cohort study conducted in 2014 in Danish population revealed that there was a little causal association between MS development and major stressful life events such as child death, divorce, and spouse death. 66 Furthermore, an American cohort study did not support a significant association between stressful events (e.g., history of physical and sexual abuse) and development of MS. 67 However, a nationwide cohort study focusing on bereaved parents in Denmark suggested that losing a child younger than 18 years old as a stressful event increased the risk of MS development in mournful parents. 68
In agreement with findings of the present study, a systematic review study conducted in 2011 indicated that stress could affect MS onset and its clinical course.69 However, our overall knowledge suggests that stress, instead of being MS etiological cause, is a triggering factor that leads to relapse attacks. 67
The following section presents the risk factors that are significantly associated with MS according to the results provided by multiple studies; however, they are not discussed in the current study due to either lack of data addressing them in the studies conducted in the MENA region or even non-consideration of them as risk factors of MS based on our results. These risk factors are EBV and vitamin D deficiency. EBV is considered as one of the prominent MS risk factors;43,70 however, unfortunately we only found one study about EBV that contained OR and 95% CI on MENA region with our search strategy. 36 This study indicates a significant association between higher anti-VCA, anti-EBNA-1 titers and MS development and also supports presence of smoking history along with positive mentioned EBV-related antibodies in individual’s serum as an amplifier for increased risk of MS. 36 However, there is still studies in MENA region that provide results showing significant association between higher anti-EBV antibody titers and MS without providing measures of effect such as OR and 95% CI.71,72 Based on a recent study investigating the findings from meta-analyses and Mendelian randomization studies, it can be concluded that a lower level of serum vitamin D is significantly associated with an increased risk of MS development. 43 However, our findings about vitamin D supplementation, which is considered as a potential protective factor for MS and can lead to a higher level of vitamin D, are not significantly associated with a lower risk of MS.23,30 Similarly, there is not strong evidence supporting vitamin D supplementation as a protective factor for MS based on the findings of previous studies.2,73
The present study had some limitations. Firstly, some of the covered studies had a small sample size.21,23,25,33 Secondly, the number of studies conducted in the MENA region, as compared with the European and American counterparts, was rather few, which made it more difficult to make precise conclusion.
One of the major possible reasons for different observed results in the MENA region, as compared to the findings of studies conducted in different parts of the world, is the ethnic and genetic diversity within different geographical regions. Since certain genotypes along with environmental risk factors make subjects prone to developing MS. Another explanation for the reported controversial findings might be the geographical differences. For instance, in a study about Iranian immigrants in British Colombia in Canada, the rate of standardized MS prevalence among Iranians living in Canada was three times higher than the rate of MS prevalence among Iranian population in Iran. 16 Other possible reasons can be differences in the methodological designs of the studies as well as the smaller sample size in some of the covered studies.
Conclusion
The review of 47 environmental risk factors in this study indicated that sunlight exposure, cigarette smoking, measles infection, EBV infection, and stressful events had a significant association with MS in the MENA region. Further studies are required to shed more light on the findings presented in this review study.
Acknowledgments
The present study was supported by Tehran University of Medical Sciences, Tehran, Iran (grant no. 98-01-188-41587).
Notes:
How to cite this article: Maroufi H, Mortazavi SH, Sahraian MA, Eskandarieh S. Environmental risk factors of multiple sclerosis in the Middle East and North Africa region: A systematic review. Curr J Neurol 2021; 20(3): 166-84.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022–4. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–73. doi: 10.1016/S1474-4422(14)70267-4. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Liu Q, Zhu JM, Wang MR, Li J, Sun MG. Association between the IL7R T244I polymorphism and multiple sclerosis risk: A meta analysis. Neurol Sci. 2016;37(9):1467–74. doi: 10.1007/s10072-016-2608-8. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–80. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heydarpour P, Khoshkish S, Abtahi S, Moradi-Lakeh M, Sahraian MA. Multiple sclerosis epidemiology in middle east and north africa: A systematic review and meta-analysis. Neuroepidemiology. 2015;44(4):232–44. doi: 10.1159/000431042. [DOI] [PubMed] [Google Scholar]
- 6.Briggs FBS, Yu JC, Davis MF, Jiangyang J, Fu S, Parrotta E, et al. Multiple sclerosis risk factors contribute to onset heterogeneity. Mult Scler Relat Disord. 2019;28:11–6. doi: 10.1016/j.msard.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Tremlett H, Zhu F, Ascherio A, Munger KL. Sun exposure over the life course and associations with multiple sclerosis. Neurology. 2018;90(14):e1191–e1199. doi: 10.1212/WNL.0000000000005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eskandarieh S, Heydarpour P, Minagar A, Pourmand S, Sahraian MA. Multiple sclerosis epidemiology in East Asia, south East Asia and south Asia: A systematic review. Neuroepidemiology. 2016;46(3):209–21. doi: 10.1159/000444019. [DOI] [PubMed] [Google Scholar]
- 9.Volpato ESN, Betini M, Puga ME, Agarwal A, Cataneo AJM, Oliveira LD, et al. Strategies to optimize MEDLINE and EMBASE search strategies for anesthesiology systematic reviews An experimental study. Sao Paulo Med J. 2018;136(2):103–8. doi: 10.1590/1516-3180.2017.0277100917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hempel S, Graham GD, Fu N, Estrada E, Chen AY, Miake-Lye I, et al. A systematic review of modifiable risk factors in the progression of multiple sclerosis. Mult Scler. 2017;23(4):525–33. doi: 10.1177/1352458517690270. [DOI] [PubMed] [Google Scholar]
- 11.Eskandarieh S, Nedjat S, Abdollahpour I, Azimi AR, Moghadasi AN, Asgari N, et al. Environmental risk factors in neuromyelitis optica spectrum disorder: A case-control study. Acta Neurol Belg. 2018;118(2):277–87. doi: 10.1007/s13760-018-0900-5. [DOI] [PubMed] [Google Scholar]
- 12.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 13.Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher GA, Beebe G, Kibler RF, Kurland Lt, Kurtzke JF, Mcdowell F, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552–68. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- 15.Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst Rev. 2017;6(1):245. doi: 10.1186/s13643-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guimond C, Lee JD, Ramagopalan SV, Dyment DA, Hanwell H, Giovannoni G, et al. Multiple sclerosis in the Iranian immigrant population of BC, Canada: Prevalence and risk factors. Mult Scler. 2014;20(9):1182–8. doi: 10.1177/1352458513519179. [DOI] [PubMed] [Google Scholar]
- 17.Sidhom Y, Kacem I, Bayoudh L, Ben Djebara M, Hizem Y, Ben Abdelfettah S, et al. Season of birth and multiple sclerosis in Tunisia. Mult Scler Relat Disord. 2015;4(6):491–4. doi: 10.1016/j.msard.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Akhtar S, Alroughani R, Al-Shammari A, Al-Abkal J, Ayad Y. Month of birth and risk of multiple sclerosis in Kuwait: A population-based registry study. Mult Scler. 2015;21(2):147–54. doi: 10.1177/1352458514541578. [DOI] [PubMed] [Google Scholar]
- 19.Lauer K, Wahl A, Geilenkeuser M. A possible role of an interaction of the age at common childhood infections and selected dietary factors at young age, for the later risk of multiple sclerosis [Online] [cited 2016]. Available from: URL: https://www.oatext.com/a-possible-role-of-an-interaction-of-the-age-at-common-childhood-infections-and-selected-dietary-factors-at-young-age-for-the-later-risk-of-multiple-sclerosis.php.
- 20.Najafi S, Ghane M, Yousefzadeh-Chabok S, Amiri M. The high prevalence of the varicella zoster virus in patients with relapsing-remitting multiple sclerosis: A case-control study in the north of Iran. Jundishapur J Microbiol. 2016;9(3):e34158. doi: 10.5812/jjm.34158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivappa N, Hebert JR, Behrooz M, Rashidkhani B. dietary inflammatory index and risk of multiple sclerosis in a case-control study from Iran. Neuroepidemiology. 2016;47(1):26–31. doi: 10.1159/000445874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansouri B, Asadollahi S, Heidari K, Fakhri M, Assarzadegan F, Nazari M, et al. Risk factors for increased multiple sclerosis susceptibility in the Iranian population. J Clin Neurosci. 2014;21(12):2207–11. doi: 10.1016/j.jocn.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Halawani AT, Zeidan ZA, Kareem AM, Alharthi AA, Almalki HA. Sociodemographic, environmental and lifestyle risk factors for multiple sclerosis development in the Western region of Saudi Arabia. A matched case control study. Saudi Med J. 2018;39(8):808–14. doi: 10.15537/smj.2018.8.22864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Wutayd O, Mohamed AG, Saeedi J, Al Otaibi H, Al Jumah M. Environmental exposures and the risk of multiple sclerosis in Saudi Arabia. BMC Neurol. 2018;18(1):86. doi: 10.1186/s12883-018-1090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Shammri SN, Hanna MG, Chattopadhyay A, Akanji AO. Sociocultural and demographic risk factors for the development of multiple sclerosis in Kuwait: A Case - Control Study. PLoS One. 2015;10(7):e0132106. doi: 10.1371/journal.pone.0132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rejali M, Hosseini SM, Kazemi Tabaee MS, Etemadifar M. Assessing the risk factors for multiple sclerosis in women of reproductive age suffering the disease in Isfahan Province. Int J Prev Med. 2016;7:58. doi: 10.4103/2008-7802.178532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eftekharian MM, Ghannad MS, Taheri M, Roshanaei G, Mazdeh M, Musavi M, et al. Frequency of viral infections and environmental factors in multiple sclerosis. Hum Antibodies. 2016;24(1-2):17–23. doi: 10.3233/HAB-150289. [DOI] [PubMed] [Google Scholar]
- 28.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137(4):992–8. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 29.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139(12):2365–72. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbasi M, Nabavi SM, Fereshtehnejad SM, Jou NZ, Ansari I, Shayegannejad V, et al. Multiple sclerosis and environmental risk factors: A case-control study in Iran. Neurol Sci. 2017;38(11):1941–51. doi: 10.1007/s10072-017-3080-9. [DOI] [PubMed] [Google Scholar]
- 31.Abdollahpour I, Nedjat S, Sahraian MA, Mansournia MA, Otahal P, van der Mei I. Waterpipe smoking associated with multiple sclerosis: A population-based incident case-control study. Mult Scler. 2017;23(10):1328–35. doi: 10.1177/1352458516677867. [DOI] [PubMed] [Google Scholar]
- 32.Alonso A, Cook SD, Maghzi AH, Divani AA. A case-control study of risk factors for multiple sclerosis in Iran. Mult Scler. 2011;17(5):550–5. doi: 10.1177/1352458510397685. [DOI] [PubMed] [Google Scholar]
- 33.Roudbari SA, Ansar MM, Yousefzad A. Smoking as a risk factor for development of Secondary Progressive Multiple Sclerosis: A study in IRAN, Guilan. J Neurol Sci. 2013;330(1-2):52–5. doi: 10.1016/j.jns.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Abdollahpour I, Lizarraga AA, Nedjat S, Mansournia MA, Weinstock-Guttman B. Medical history and multiple sclerosis: A population-based incident case-control study. Neuroepidemiology. 2019;52(1-2):55–62. doi: 10.1159/000494257. [DOI] [PubMed] [Google Scholar]
- 35.Abdollahpour I, Nedjat S, Mansournia MA, Sahraian MA, van der Mei I. Lifestyle factors and multiple sclerosis: A population-based incident case-control study. Mult Scler Relat Disord. 2018;22:128–33. doi: 10.1016/j.msard.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Mouhieddine TH, Darwish H, Fawaz L, Yamout B, Tamim H, Khoury SJ. Risk factors for multiple sclerosis and associations with anti-EBV antibody titers. Clin Immunol. 2015;158(1):59–66. doi: 10.1016/j.clim.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Al-Afasy HH, Al-Obaidan MA, Al-Ansari YA, Al-Yatama SA, Al-Rukaibi MS, Makki NI, et al. Risk factors for multiple sclerosis in Kuwait: A population-based case-control study. Neuroepidemiology. 2013;40(1):30–5. doi: 10.1159/000341240. [DOI] [PubMed] [Google Scholar]
- 38.Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18(4):468–71. doi: 10.1177/1352458511424904. [DOI] [PubMed] [Google Scholar]
- 39.Hedstrom AK, Olsson T, Alfredsson L. Smoking is a major preventable risk factor for multiple sclerosis. Mult Scler. 2016;22(8):1021–6. doi: 10.1177/1352458515609794. [DOI] [PubMed] [Google Scholar]
- 40.Briggs FB, Acuna B, Shen L, Ramsay P, Quach H, Bernstein A, et al. Smoking and risk of multiple sclerosis: Evidence of modification by NAT1 variants. Epidemiology. 2014;25(4):605–14. doi: 10.1097/EDE.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J Dent Res. 2012;91(2):142–9. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petecchia L, Sabatini F, Varesio L, Camoirano A, Usai C, Pezzolo A, et al. Bronchial airway epithelial cell damage following exposure to cigarette smoke includes disassembly of tight junction components mediated by the extracellular signal-regulated kinase 1/2 pathway. Chest. 2009;135(6):1502–12. doi: 10.1378/chest.08-1780. [DOI] [PubMed] [Google Scholar]
- 43.Belbasis L, Bellou V, Evangelou E, Tzoulaki I. Environmental factors and risk of multiple sclerosis: Findings from meta-analyses and Mendelian randomization studies. Mult Scler. 2020;26(4):397–404. doi: 10.1177/1352458519872664. [DOI] [PubMed] [Google Scholar]
- 44.Handel AE, Williamson AJ, Disanto G, Dobson R, Giovannoni G, Ramagopalan SV. Smoking and multiple sclerosis: An updated meta-analysis. PLoS One. 2011;6(1):e16149. doi: 10.1371/journal.pone.0016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hedstrom AK, Hossjer O, Hillert J, Stridh P, Kockum I, Olsson T, et al. The influence of human leukocyte antigen-DRB1*15:01 and its interaction with smoking in MS development is dependent on DQA1*01:01 status. Mult Scler. 2020;26(13):1638–46. doi: 10.1177/1352458519877685. [DOI] [PubMed] [Google Scholar]
- 46.Gee JR, Chang J, Dublin AB, Vijayan N. The association of brainstem lesions with migraine-like headache: An imaging study of multiple sclerosis. Headache. 2005;45(6):670–7. doi: 10.1111/j.1526-4610.2005.05136.x. [DOI] [PubMed] [Google Scholar]
- 47.Pakpoor J, Handel AE, Giovannoni G, Dobson R, Ramagopalan SV. Meta-analysis of the relationship between multiple sclerosis and migraine. PLoS One. 2012;7(9):e45295. doi: 10.1371/journal.pone.0045295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kister I, Munger KL, Herbert J, Ascherio A. Increased risk of multiple sclerosis among women with migraine in the Nurses' Health Study II. Mult Scler. 2012;18(1):90–7. doi: 10.1177/1352458511416487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez Sobrepera HJ, Cabrera Gomez JA, Tuero Iglesias A. Exogenous factors in the aetiology of multiple sclerosis in Cuba. A study of cases and controls. Rev Neurol. 2001;33(10):931–7. [PubMed] [Google Scholar]
- 50.Zorzon M, Zivadinov R, Nasuelli D, Dolfini P, Bosco A, Bratina A, et al. Risk factors of multiple sclerosis: A case-control study. Neurol Sci. 2003;24(4):242–7. doi: 10.1007/s10072-003-0147-6. [DOI] [PubMed] [Google Scholar]
- 51.Kowalec K, McKay KA, Patten SB, Fisk JD, Evans C, Tremlett H, et al. Comorbidity increases the risk of relapse in multiple sclerosis: A prospective study. Neurology. 2017;89(24):2455–61. doi: 10.1212/WNL.0000000000004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krone B, Pohl D, Rostasy K, Kahler E, Brunner E, Oeffner F, et al. Common infectious agents in multiple sclerosis: A case-control study in children. Mult Scler. 2008;14(1):136–9. doi: 10.1177/1352458507082069. [DOI] [PubMed] [Google Scholar]
- 53.Sundstrom P, Juto P, Wadell G, Hallmans G, Svenningsson A, Nystrom L, et al. An altered immune response to Epstein-Barr virus in multiple sclerosis: A prospective study. Neurology. 2004;62(12):2277–82. doi: 10.1212/01.wnl.0000130496.51156.d7. [DOI] [PubMed] [Google Scholar]
- 54.Sips GJ, Chesik D, Glazenburg L, Wilschut J, De Keyser J, Wilczak N. Involvement of morbilliviruses in the pathogenesis of demyelinating disease. Rev Med Virol. 2007;17(4):223–44. doi: 10.1002/rmv.526. [DOI] [PubMed] [Google Scholar]
- 55.Ahlgren C, Oden A, Bergstrom T, Lycke J. Serum and CSF measles antibody levels increase over time in patients with multiple sclerosis or clinically isolated syndrome. J Neuroimmunol. 2012;247(1-2):70–4. doi: 10.1016/j.jneuroim.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Ahlgren C, Oden A, Toren K, Andersen O. Multiple sclerosis incidence in the era of measles-mumps-rubella mass vaccinations. Acta Neurol Scand. 2009;119(5):313–20. doi: 10.1111/j.1600-0404.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- 57.Bachmann S, Kesselring J. Multiple sclerosis and infectious childhood diseases. Neuroepidemiology. 1998;17(3):154–60. doi: 10.1159/000026167. [DOI] [PubMed] [Google Scholar]
- 58.Sotelo J, Ordonez G, Pineda B, Flores J. The participation of varicella zoster virus in relapses of multiple sclerosis. Clin Neurol Neurosurg. 2014;119:44–8. doi: 10.1016/j.clineuro.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Kattimani Y, Veerappa AM. Complex interaction between mutant HNRNPA1 and gE of varicella zoster virus in pathogenesis of multiple sclerosis. Autoimmunity. 2018;51(4):147–51. doi: 10.1080/08916934.2018.1482883. [DOI] [PubMed] [Google Scholar]
- 60.Lunny C, Knopp-Sihota JA, Fraser SN. Surgery and risk for multiple sclerosis: A systematic review and meta-analysis of case-control studies. BMC Neurol. 2013;13:41. doi: 10.1186/1471-2377-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z, Zhang TT, Yu J, Liu YL, Qi SF, Zhao JJ, et al. Excess body weight during childhood and adolescence is associated with the risk of multiple sclerosis: A meta-analysis. Neuroepidemiology. 2016;47(2):103–8. doi: 10.1159/000450854. [DOI] [PubMed] [Google Scholar]
- 62.Hedstrom AK, Lima Bomfim I, Barcellos L, Gianfrancesco M, Schaefer C, Kockum I, et al. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology. 2014;82(10):865–72. doi: 10.1212/WNL.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKay KA, Jahanfar S, Duggan T, Tkachuk S, Tremlett H. Factors associated with onset, relapses or progression in multiple sclerosis: A systematic review. Neurotoxicology. 2017;61:189–212. doi: 10.1016/j.neuro.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 64.Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. 2011;76(6):540–8. doi: 10.1212/WNL.0b013e31820af93d. [DOI] [PubMed] [Google Scholar]
- 65.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9(6):599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 66.Nielsen NM, Bager P, Simonsen J, Hviid A, Stenager E, Bronnum-Hansen H, et al. Major stressful life events in adulthood and risk of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85(10):1103–8. doi: 10.1136/jnnp-2013-307181. [DOI] [PubMed] [Google Scholar]
- 67.Riise T, Mohr DC, Munger KL, Rich-Edwards JW, Kawachi I, Ascherio A. Stress and the risk of multiple sclerosis. Neurology. 2011;76(22):1866–71. doi: 10.1212/WNL.0b013e31821d74c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Johansen C, Bronnum-Hansen H, Stenager E, Koch-Henriksen N, Olsen J. The risk of multiple sclerosis in bereaved parents: A nationwide cohort study in Denmark. Neurology. 2004;62(5):726–9. doi: 10.1212/01.wnl.0000113766.21896.b1. [DOI] [PubMed] [Google Scholar]
- 69.Artemiadis AK, Anagnostouli MC, Alexopoulos EC. Stress as a risk factor for multiple sclerosis onset or relapse: A systematic review. Neuroepidemiology. 2011;36(2):109–20. doi: 10.1159/000323953. [DOI] [PubMed] [Google Scholar]
- 70.Jacobs BM, Giovannoni G, Cuzick J, Dobson R. Systematic review and meta-analysis of the association between Epstein-Barr virus, multiple sclerosis and other risk factors. Mult Scler. 2020;26(11):1281–97. doi: 10.1177/1352458520907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laribi B, Shekarabi M, Zarnani AH, Ghaffarpour M, Marzban M. Differential serostatus of Epstein-Barr virus in Iranian MS patients with various clinical patterns. Med J Islam Repub Iran. 2018;32:118. doi: 10.14196/mjiri.32.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karampoor S, Zahednasab H, Pirkouh AA, Monavari SH, Ramagopalan S, Keyvani H. Serostatus of Epstein-Barr virus in Iranian MS patients. Acta Neurol Belg. 2016;116(1):43–6. doi: 10.1007/s13760-015-0496-y. [DOI] [PubMed] [Google Scholar]
- 73.Sintzel MB, Rametta M, Reder AT. Vitamin D and multiple sclerosis: A comprehensive review. Neurol Ther. 2018;7(1):59–85. doi: 10.1007/s40120-017-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]