Abstract
Background: Cladribine tablets are the foremost oral immune-reconstitution therapy for high disease activity relapsing multiple sclerosis (HDA-RMS). We aimed to assess the cost-effectiveness of cladribine tablets compared to natalizumab in patients with HDA-RMS in Iran.
Methods: A 5-year cohort-based Markov model was developed with 11 expanded disability status score (EDSS) health states, including patients with HDA-RMS as on and off-treatment. All costs were identified from the literature and expert opinion and were measured in Iranian Rial rates, changed to the 2020 USD rate and were discounted by 7.2%. Quality adjusted life years (QALY), discounted by 3.5%, and life years gained (LYG) were adopted to measure efficacy. The final results were presented as incremental cost-effectiveness ratio that was compared to a national willingness to pay (WTP) threshold of 1 to 3 gross domestic product (GDP) per capita. Deterministic and probabilistic sensitivity analyses (D/PSA) were employed to evaluate uncertainty.
Results: Cladribine tablets dominated natalizumab and yielded 6,607 USD cost-saving and 0.003 additional QALYs per patient. LYG was comparable. The main cost component was drug acquisition cost in both arms. DSA indicated the sensitivity of the results to the cost discount rates and also the patients’ body weight; while they were less sensitive to the main clinical variables. PSA indicated that cladribine tablets were cost-effective in Iran, with a probability of 57.5% and 58.6% at lower and higher limits of threshold, respectively.
Conclusion: Cladribine tablets yielded higher QALYs and lower costs compared to natalizumab, in patients with HDA-RMS in Iran.
Key Words: Cladribine Tablets, Natalizumab, Relapsing Multiple Sclerosis, Cost-Utility Analysis, Pharmacoeconomics Study, Iran
Introduction
Multiple sclerosis (MS) is considered as a chronic autoimmune disorder, which is known by progressive neurodegeneration. The prevalence of MS is rising worldwide, along with the Middle East and North Africa (MENA) region.1 As socioeconomic factors play a considerable role in the epidemiology of MS, a higher prevalence can be seen in developing low- and middle-income countries.2 In Iran, the prevalence of MS was estimated to be 29.3 per 100000.3
MS is categorized into four clinical phenotypes: relapsing-remitting MS (RRMS), progressive-relapsing MS (PRMS), primary-progressive MS (PPMS), and secondary-progressive MS (SPMS). RRMS, the main subgroup, with 85% of the cases, is characterized by deterioration of neurological symptoms, for 45 to 90 days, and then reduction of symptoms.4-6 The relapsing MS (RMS) type with a minimum of one relapse in the former year and magnetic resonance imaging (MRI) results showing disease activity, albeit on disease modifying therapy (DMT), and also the RMS type with two or more relapses in the former year, on DMT or not, are known as high disease activity RMS (HDA-RMS).7,8 HDA-RMS accounts for about 8.5% of patients with RRMS.9 As both health and economic burdens increase with disability and the number of relapses,10 these burdens are substantial in patients with HDA-RMS.
In the last decade, novel DMTs have been introduced to the evolving field of MS therapeutics. In 2019, the Middle East and North Africa Committee for Treatment and Research in Multiple Sclerosis (MENACTRIMS) suggested fingolimod, natalizumab, siponimod, ocrelizumab or cladribine for patients with HDA-RMS, after relative risk stratification.1 At the time of this study, only the first two were available in Iran.
Cladribine tablets were approved by the Iran Food and Drug Administration (IFDA) and the European Medicines Agency (EMA) for relapsing forms of MS.7,11,12 Cladribine has been evaluated in two phase-III randomized clinical trials, CLARITY and CLARITY Extension; and indicated reduction in the 3- and 6-month confirmed disability progression (3M-CDP and 6M-CDP) and annualized relapse rate (ARR) and were characterized with an increase in the share of patients with relapse-free RMS, particularly in the HDA-RMS subgroup, compared to the placebo.13,14
The high cost of MS treatment, considered as the main component of cost in MS management,15,16 makes it vital to acknowledge the most cost-effective pharmaceutical strategy. Although the economic evaluation of cladribine tablets was assessed in few settings including England and the Netherlands,17,18 no study has been conducted in Iran or any of the developing Middle-Eastern countries. The objective of the present study was to analyse the cost-utility of cladribine tablets in patients with HDA-RMS, when compared to natalizumab, from the societal perspective in the Iranian setting. The results will hopefully support policymakers and healthcare providers in evidence-based decision-making regarding the essential list inclusion, adoption and compensation of cladribine tablets in Iran.
Materials and Methods
Overview and model structure: A cohort-based multi-state Markov model of 1000 patients with HDA-RMS was developed using Microsoft Excel® 2008. A 5-year cost-utility analysis (CUA) was conducted to compare cladribine tablets and natalizumab in Iran. Quality adjusted life years (QALY), discounted by 3.5%19 and life years gained (LYG) were used to assess the treatment’s efficacy. Costs were captured locally and discounted by 7.2%.20 The final result indicator was the incremental cost effectiveness ratio (ICER, cost of intervention-cost of comparator)/(QALY of intervention-QALY of comparator). In addition, we calculated the incremental net health benefit as (ΔQALY-Δcost)/threshold for the cost-effectiveness of the intervention. On the basis of the World Health Organization (WHO) recommendations on thresholds for developing countries21 and Iran’s regulations, one to three gross domestic product (GDP) per capita (2,709-8,127 USD) was considered as the willingness to pay (WTP) threshold for one QALY.
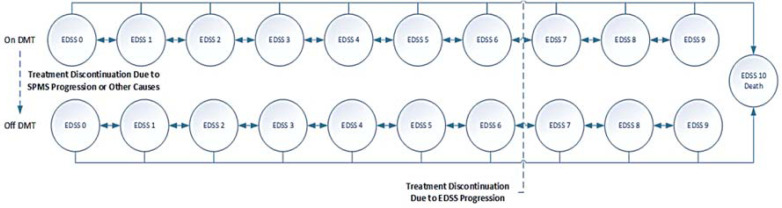
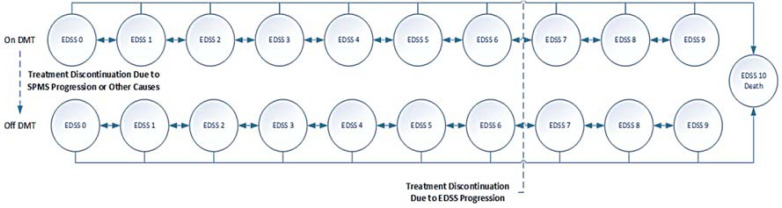
Due to the MS progressive disabling natural history, the “expanded disability status scale (EDSS) rating score”, which uses an ordinal rating system from 0 for death to 10 for normal health,22 was adopted for state modelling. The patients with RMS were on-treatment or off-treatment EDSS states and could stay on the same state, progress, regress, or die (absorbing state). Drug therapy continued until reaching EDSS < 7, treatment withdrawal, occurrence of adverse drug events, or lack of adequate response. Following treatment discontinuation, the patients remained on the best supportive care. Figure 1 illustrates the Markov model.
Figure 1.
Base case Markov model overview
DMT: Disease modifying therapy; EDSS: Expanded Disability Status Scale; SPMS: Secondary progressive multiple sclerosis
The patients could have experienced relapses in any cycle, grounded on data from the UK multiple technology appraisal assessment group and the Patzold et al. study.23,24 Grounded on the pooled analysis of relapses in Teriflunomide Multiple Sclerosis Oral (TEMSO) and Teriflunomide Oral in People with Relapsing Multiple Sclerosis (TOWER) studies, 34% of relapses required hospitalization.25 Mean duration for hospitalized and non-hospitalized relapses were assumed 34.41 and 38.64 days, respectively.13
Demographic inputs: Starting age (37 years), annual relapse amount (2.02), and baseline EDSS distribution data were extracted from the CLARITY trial.13 However, female to male ratio (2.88) and the average weight of the patients with MS (69.7 kg) were gathered from local studies.26,27
Clinical inputs: The efficacy and safety of the cladribine tablets were evaluated in CLARITY and CLARITY-Extension trials.13,14 Natalizumab was assessed in the "Natalizumab Safety and Efficacy in Relapsing–Remitting Multiple Sclerosis" (AFFIRM) study, a phase III placebo-controlled trial in patients with RRMS.28 Due to a lack of head-to-head trials between cladribine and natalizumab in patients with HDA-RMS, we adopted the network meta-analysis (NMA) by Siddiqui et al.30 and meta-regression by Berardi et al.29 for indirect treatment comparisons. In both studies, cladribine tablets were compared to alternative comparators (natalizumab, ocrelizumab, fingolimod, and alemtuzumab) in patients with active RMS. The results of the study by Siddiqui et al. indicated similar ARR.30 Due to a lack of subgroup specified data, comparison of 6M-CDP was assessed in the meta-regression analysis, which indicated an overlap in the hazard ratio (HR) confidence intervals of all comparators in 6M-CDP criteria, indicating that no DMT was superior to another. In the HDA-RMS subgroup, a non-significant difference was identified between cladribine tablets and natalizumab [HR = 1.08, 95% confidence interval (CI) (0.53-2.21)].29
Health states utilities: Health state utilities (HSUs) were captured through the results of the study by Hawton and Green.31 This selection was due to the large number of patients who completed the EuroQol-5D (EQ-5D) questionnaire (7472 participants), and also due to the high recruitment and response rates (75% and 90%, respectively) of participants.31 A disutility of -0.071 was used for patients experiencing relapse events.32 The EDSS-based disutility of caregivers was captured from the study by Acaster et al.33 For disutility following the occurrence of treatment-related adverse events (TRAEs), relevant studies were identified, which are shown in table 1.
Table 1.
Duration and disutility of treatment-related adverse events (TRAEs)
| Period (days) | Disutility |
QALY
impact |
Reference
for duration |
Reference
for disutility |
||
|---|---|---|---|---|---|---|
| TRAE | ||||||
| Infusion-related reaction | 5 | -0.011 | -0.0002 | 39 | 34 | |
| PML | 93.1 | -0.200 | -0.0510 | * | 39 | |
| Infection (severe events) | 14 | -0.190 | -0.0073 | ** | 48 | |
| Autoimmune thyroid-related event | 365.25 | -0.110 | -0.1100 | 39 | 39 | |
| Influenza-like symptoms | 7 | -0.210 | -0.0040 | *** | 49 | |
| Malignancy | 365.25 | -0.116 | -0.1160 | 35 | 35 | |
| Immune thrombocytopenic purpura | 28 | -0.090 | -0.0069 | 39 | 39 | |
QALY: Quality adjusted life year; TRAE: Treatment-related adverse events; PML: Progressive multifocal leukoencephalopathy
*Based on steroid therapy duration for PML-related immune reconstitution syndrome, **Based on the assumption that most therapies for severe infections are taken for about two weeks, ***Based on the assumption that influenza symptoms persist for about seven days.
Costs: Due to the societal perspective, direct (medical and non-medical) and indirect costs were accounted. Drug acquisition (DAQ) costs were obtained from the IFDA official website.39 Iran’s official list of tariffs and national tariffs’ book were used to capture administration and monitoring (lab tests and physician visits) costs,40 with a ratio of 20-80% for public and private sectors. All costs were captured in Iranian Rial (IRR) rates and converted to USD, with the 2020 governmental conversion rate of 42,000 IRR/USD.
Natalizumab-related costs: The cost of treatment with natalizumab [a monoclonal antibody (mAb) infusion of 300 mg/15ml administered every 28 days] included DAQ, administration, hospitalization during administration, and monitoring costs (MRI, laboratory tests, and neurologist visit) based on the FDA label. For this arm, John Cunningham (JC) virus test costs were included in the model.
Cladribine tablets- related costs: Cladribine tablets (10 mg) are used based on a collective dose of 3.5 mg/kg over two years.41 Based on the mean weight of the Iranian patients with MS, an average of 5.38 tablets was assumed to be administered daily in the first week of the first month (0.875 mg/kg) and 5.38 tablets in the first week of the second month of therapy, in both years of the treatment period. Total costs included both DAQ and monitoring costs, based on the FDA label.
Relapse-related costs: These were based on the relapse-related costs in Iranian patients with RMS as reported in the study by Taheri et al.5
Adverse events: The management components of the aforementioned TRAEs in Iran and their costs were calculated based on experts’ opinion and the national book of tariffs.40
Direct non-medical costs: EDSS-based costs, including nursing, were calculated and inserted in the model calculations.
Indirect costs: The human capital method was used to calculate indirect costs in terms of productivity and wage loss based on work absence frequency and lowest government wage of a government worker per day (8.819 USD).36 It was assumed that a working patient (below 65 years old and with EDSS ≤ 6) will be on work leave for 1.9 and 14.4 days in EDSS < 3 and 3 ≤ EDSS < 5, respectively.42 To avoid double-counting, daily absence due to drug administration was not considered for natalizumab arm. A summary of cost components is presented in supplementary table 1.
Discontinuation and/or Treatment withdrawal: Patients were assumed to discontinue their treatment due to progression to EDSS > 7 and/or evidence of SPMS. Treatment withdrawal occurred with the lack of response or non-tolerability to treatment. All-cause discontinuation probabilities were derived from the CLARITY trial (4.85) and AFFAIR trial (6.4) for cladribine tablets and natalizumab, respectively.13,28
Mortality: Mortality was calculated using fixed standardized mortality ratios for patients with MS (1.680 versus general population)43 combined with Iran life-table statistics for the probability of death in the general population presented by age and gender.44
In order to assess the impact of individual and joint variable uncertainty on the results, deterministic and probabilistic sensitivity analysis (D/PSA) were conducted. DSA was conducted on the parameters by 95% confidence interval (CI) or 20%, if statistical measures of variance were not available. The variables included in DSA were: the DMT effect on 6M-CDP and ARR, discontinuation rates of costs and QALYs, patients’ baseline characteristics, mortality multiplier, and discontinuation rates. As a result of the DSA, a tornado diagram (figure 2) was developed to show the effects of variables on the net health benefit at the current threshold.
Figure 2.
Tornado diagram - Incremental net health effects
Note: Positive values equate to health gains for cladribine tablets versus comparator at the current threshold.
ARR: Annualized relapse rate; DP: Disability progression
To assess combined variable uncertainties, PSA was conducted. This was repeated for one thousand iterations based on tornado diagram Monte-Carlo simulation. As a result, a cost-effectiveness scatter plot (figure 2) was developed to identify the probability of cost-effectiveness at Iran’s WTP threshold.
Results
Base-case analysis: The cladribine tablets dominated natalizumab in patients with HDA-RMS in a 5-year time-horizon from the societal perspective in Iran. The total discounted cost per patient was 69,842 and 76449 USD for cladribine tablets and natalizumab, respectively. The DAQ costs were the main component of the total costs, accounting for 92.3 and 91.7% of the costs in the cladribine and natalizumab arms, respectively. Indirect costs were 2.43% and 2.25% of the total costs in the cladribine and natalizumab arms, respectively. The incremental discounted QALY was 0.003 per patient, favoring cladribine tablets (2.720 in cladribine arm versus 2.716 in natalizumab arm). In addition, the incremental net health benefit at the lower limit of threshold was 2.441. The results are shown in table 2.
Table 2.
Summary of base case analysis results
| Cladribine tablets | Natalizumab | |
|---|---|---|
| Total Discounted | ||
| Cost (USD) | 69.842 | 76.449 |
| LYG | 4.655 | 4.655 |
| QALY | 2.720 | 2.716 |
| Inc. (Cladribine Tablets vs. Natalizumab) | ||
| Inc. Cost (USD) | - | -6.606 |
| Inc. LYG | - | 0.000 |
| Inc. QALY | - | 0.003 |
| ICER vs Cladribine (QALY) | - | Cladribine tablets are dominant |
ICER: Incremental cost effectiveness ratio; Inc.: Incremental; LYG: Life years gained; QALY: Quality adjusted life years; USD: United States Dollar
DSA : Based on the current threshold, the net health benefit was mostly sensitive to the higher intervals of costs’ discount rate and patients’ baseline weight, both high and low intervals of the cladribine tablet effect on CDP and discontinuation rate of both arms. As shown in figure 3, all values were positive and in favor of cladribine.
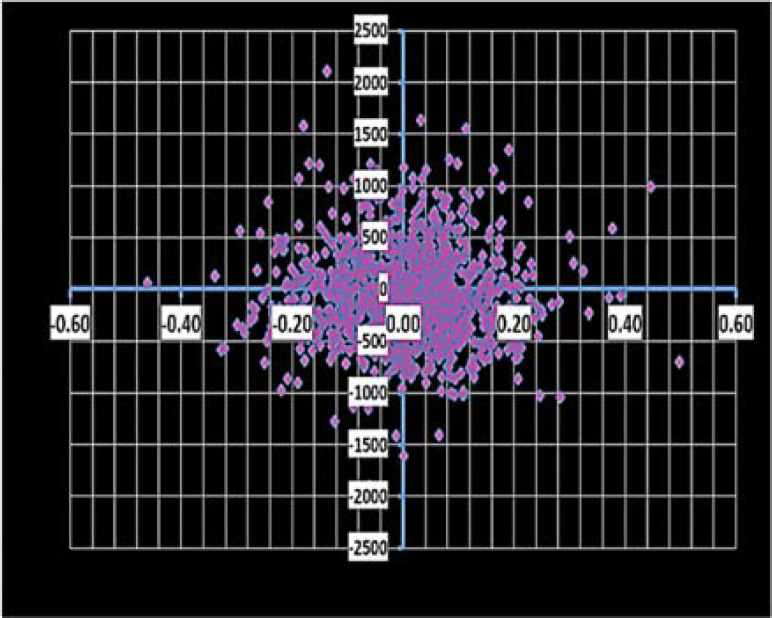
Figure 3.
Cost-effectiveness scatter plot in the lower willingness to pay threshold (1 GDP per Capita- 2709 USD)
GDP: Gross domestic product; QALY: Quality adjusted life years
PSA: PSA showed a mean discounted QALY of 2.71 [2.35-3.01] and 2.71 [2.40-2.99], for cladribine tablets and natalizumab, respectively. The analysis indicated that in a cost-effectiveness WTP threshold of one and three GDP per capita (2,709-8,127 USD), which are the lower and higher limits in Iran, cladribine tablets are cost-effective in 57.5% and 58.6% of iterations, respectively. The cost effectiveness scatter plot in the lower limit of threshold is shown in figure 2.
Discussion
We found that cladribine tablets dominated natalizumab, when used in patients with HDA-RMS. Cladribine yielded additional QALYs and was associated with cost savings as compared to natalizumab. The cost saved by cladribine tablets (6606 USD per patient per year) was mainly attributed to the exceptional posology of the cladribine tablets, with an extended duration of efficacy over 4 years for a two-year oral treatment and a low monitoring burden compared to natalizumab.
Our findings were in line with previously published economic analyses of cladribine tablets compared to natalizumab in HDA-RMS.17,18,45 Hettle et al. found that cladribine tablets dominated natalizumab in HDA-RMS with a 93% cost-effectiveness probability from the national health system perspective in England.17
The main cost components affecting the results in the latter study were similar to the current findings and were shown to be the DAQ and administration costs. However, in contrast to the present study, the final results were significantly influenced by indirect treatment comparison results on disability progression which was reported as a study limitation. In the study by Hettle et al.,17 a life-time horizon was considered, whereas in the current study a time horizon of 5 years was selected. This selection was due to the short-term efficacy assessment of cladribine tablets in the CLARITY and CLARITY-Extension studies and also economic uncertainties associated with costs and WTP threshold in Iran. In longer time horizons, however, cladribine tablets yield higher cost savings and QALYs compared to natalizumab; because of the extended efficacy of the intervention. When a 10-year time horizon was considered, the incremental costs and QALYs were 36,561 USD and 0.006, respectively.
The Markov model structure used in this study was similar to that employed by Hettle et al.17 and different from many MS pharmacoeconomics studies which use a 21-health state model. In this study, a simplified 11-health state model structure was used including 10 EDSS-based states for RMS (both RRMS and SPMS) and an absorbing state of death. This pooling approach was consistent with the study by Palace et al., which assumed SPMS as a later state of RRMS.46
Our study focused on the population of patients with HDA-RMS; although, other populations such as rapidly evolving severe (RES) MS and active RRMS can be assessed. Michels et al. found that cladribine tablets dominated natalizumab in RES-MS in the Netherlands with a cost-effectiveness probability of 94.1% in a life-time horizon.18 Djambazov et al. identified cladribine tablets as the most cost-effective option compared to fingolimod, alemtuzumab, and natalizumab; and dominant as compared to natalizumab and fingolimod in patients with active RRMS in Bulgaria.45
The key limitation of this analysis was the reliance on an NMA of clinical data in the absence of head-to-head clinical trials comparing the intervention and the comparator in HAD-RMS. This meta-analysis included studies that are different in design, time frame, and population characteristics. However, the sensitivity analysis results of this study and further comprehensive studies29 alleviated the limitation with their consistent results, which were aligned with the methodology used in the NICE pharmacoeconomics study.47
The second limitation was the time-horizon of 5 years for this chronic disorder; whereas a longer horizon is usually advised by the pharmacoeconomics’ guidelines,48,49 but it should be noted that the longer time horizons would have yielded more QALYs and less costs due to the unique approach with a short-course treatment of oral cladribine tablets. The third limitation was the assumption that no further DMT treatment was required after cessation of therapy with cladribine tablets and natalizumab.
Conclusion
Based on literature-reported efficacy data and local costs and tariffs, cladribine tablets were dominant versus natalizumab in patients with HDA-RMS in Iran, with lower costs and higher QALYs gained. This study filled the gap of the unavailability of any study assessing the pharmacoeconomics of cladribine tablets in Iran or other developing Middle-Eastern countries. Our results may help policy makers and healthcare providers for efficient allocations of scarce resources and more informed decision-making processes.
Acknowledgments
The authors would like to appreciate Merck Serono Middle East FZ-Ltd for the allocation of cladribine tablets pharmacoeconomics dossier to be used in this study.
Notes:
How to cite this article: Ayati N, Fleifel L, Sharifi S, Sahraian MA, Nikfar S. Cladribine tablets are a cost-effective strategy in high-disease activity relapsing multiple sclerosis patients in Iran. Curr J Neurol 2021; 20(3): 146-53.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Yamout B, Sahraian M, Bohlega S, Al-Jumah M, Goueider R, Dahdaleh M, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult Scler Relat Disord. 2020;37:101459. doi: 10.1016/j.msard.2019.101459. [DOI] [PubMed] [Google Scholar]
- 2.Stenager E. A global perspective on the burden of multiple sclerosis. Lancet Neurol. 2019;18(3):227–8. doi: 10.1016/S1474-4422(18)30498-8. [DOI] [PubMed] [Google Scholar]
- 3.Azami M, YektaKooshali MH, Shohani M, Khorshidi A, Mahmudi L. Epidemiology of multiple sclerosis in Iran: A systematic review and meta-analysis. PLoS One. 2019;14(4):e0214738. doi: 10.1371/journal.pone.0214738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg MM. Multiple sclerosis review. P T. 2012;37(3):175–84. [PMC free article] [PubMed] [Google Scholar]
- 5.Taheri S, Sahraian MA, Yousefi N. Cost-effectiveness of alemtuzumab and natalizumab for relapsing-remitting multiple sclerosis treatment in Iran: decision analysis based on an indirect comparison. J Med Econ. 2019;22(1):71–84. doi: 10.1080/13696998.2018.1543189. [DOI] [PubMed] [Google Scholar]
- 6.Burkill S, Montgomery S, Hajiebrahimi M, Hillert J, Olsson T, Bahmanyar S. Mortality trends for multiple sclerosis patients in Sweden from 1968 to 2012. Neurology. 2017;89(6):555–62. doi: 10.1212/WNL.0000000000004216. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Mavenclad: Annex I: Summary of Product Characteristics [Online] [cited 2018]. Available from: https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf.
- 8.Hughes R, Brainin M, Gilhus NE. European Handbook of Neurological Management. Oxford, UK: Wiley-Blackwell; 2008. [Google Scholar]
- 9.Ohlmeier C, Gothe H, Haas J, Osowski U, Weinhold C, Blauwitz S, et al. Epidemiology, characteristics and treatment of patients with relapsing remitting multiple sclerosis and incidence of high disease activity: Real world evidence based on German claims data. PLoS One. 2020;15(5):e0231846. doi: 10.1371/journal.pone.0231846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naci H, Fleurence R, Birt J, Duhig A. Economic burden of multiple sclerosis: A systematic review of the literature. Pharmacoeconomics. 2010;28(5):363–79. doi: 10.2165/11532230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Wiendl H. Cladribine - an old newcomer for pulsed immune reconstitution in MS. Nat Rev Neurol. 2017;13(10):573–4. doi: 10.1038/nrneurol.2017.119. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. FDA-Approved Drugs: Mavenclad [Online] [cited 2019 Apr 26]. Available from: URL: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022561.
- 13.Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg SP, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–26. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 14.Giovannoni G, Soelberg SP, Cook S, Rammohan K, Rieckmann P, Comi G, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24(12):1594–604. doi: 10.1177/1352458517727603. [DOI] [PubMed] [Google Scholar]
- 15.Khanizadeh H, Izham M, Akmal A. PND12 the costs analysis of multiple sclerosis at different stages in Iran. Value Health. 2012;15(4):A143. [Google Scholar]
- 16.Torabipour A, Asl ZA, Majdinasab N, Ghasemzadeh R, Tabesh H, Arab M. A study on the direct and indirect costs of multiple sclerosis based on expanded disability status scale score in Khuzestan, Iran. Int J Prev Med. 2014;5(9):1131–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Hettle R, Harty G, Wong SL. Cost-effectiveness of cladribine tablets, alemtuzumab, and natalizumab in the treatment of relapsing-remitting multiple sclerosis with high disease activity in England. J Med Econ. 2018;21(7):676–86. doi: 10.1080/13696998.2018.1461630. [DOI] [PubMed] [Google Scholar]
- 18.Michels RE, de FM, Mahajan K, Hengstman GJD, Schiffers KMH, Budhia S, et al. Cost effectiveness of cladribine tablets for the treatment of relapsing-remitting multiple sclerosis in the netherlands. Appl Health Econ Health Policy. 2019;17(6):857–73. doi: 10.1007/s40258-019-00500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robberstad B. Estimation of private and social time preferences for health in northern Tanzania. Soc Sci Med. 2005;61(7):1597–607. doi: 10.1016/j.socscimed.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Abdoli G, Heydari H. An estimation of social discount rate based on hazard rate for Iran and selected countries. Iranian Economic Research. 2009;13(38):1–29. [In Persian] [Google Scholar]
- 21.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–24. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. doi: 10.1186/1471-2377-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patzold T, Schwengelbeck M, Ossege LM, Malin JP, Sindern E. Changes of the MS functional composite and EDSS during and after treatment of relapses with methylprednisolone in patients with multiple sclerosis. Acta Neurol Scand. 2002;105(3):164–8. doi: 10.1034/j.1600-0404.2002.1o135.x. [DOI] [PubMed] [Google Scholar]
- 24.Lambe T, Duarte R, Mahon J, Nevitt S, Greenhalgh J, Boland A, et al. Cladribine tablets for the first-line treatment of relapsing-remitting multiple sclerosis: an evidence review group perspective of a nice single technology appraisal. Pharmacoeconomics. 2019;37(3):345–57. doi: 10.1007/s40273-018-0718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer M, Comi G, Freedman MS, Kappos L, Olsson TP, Wolinsky JS, et al. Multiple sclerosis relapses are associated with increased fatigue and reduced health-related quality of life - A post hoc analysis of the TEMSO and TOWER studies. Mult Scler Relat Disord. 2016;7:33–40. doi: 10.1016/j.msard.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Behaeein B, Yadolazadeh A, Same H, Etemadi S, Sadegi H, Salehian MH. Relation of anthropometric characteristics in women with different types of ms and comparison with health ones. Scholars Research Library. 2011;2(5):14–21. [Google Scholar]
- 27.Eskandarieh S, Heydarpour P, Elhami SR, Sahraian MA. Prevalence and incidence of multiple sclerosis in Tehran, Iran. Iran J Public Health. 2017;46(5):699–704. [PMC free article] [PubMed] [Google Scholar]
- 28.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 29.Berardi A, Siddiqui MK, Treharne C, Harty G, Wong SL. Estimating the comparative efficacy of cladribine tablets versus alternative disease modifying treatments in active relapsing-remitting multiple sclerosis: Adjusting for patient characteristics using meta-regression and matching-adjusted indirect treatment comparison approaches. Curr Med Res Opin. 2019;35(8):1371–8. doi: 10.1080/03007995.2019.1585779. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui MK, Khurana IS, Budhia S, Hettle R, Harty G, Wong SL. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2018;34(8):1361–71. doi: 10.1080/03007995.2017.1407303. [DOI] [PubMed] [Google Scholar]
- 31.Hawton A, Green C. Health utilities for multiple sclerosis. Value Health. 2016;19(4):460–8. doi: 10.1016/j.jval.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Orme M, Kerrigan J, Tyas D, Russell N, Nixon R. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10(1):54–60. doi: 10.1111/j.1524-4733.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 33.Acaster S, Perard R, Chauhan D, Lloyd AJ. A forgotten aspect of the NICE reference case: An observational study of the health related quality of life impact on caregivers of people with multiple sclerosis. BMC Health Serv Res. 2013;13:346. doi: 10.1186/1472-6963-13-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–30. doi: 10.1007/s10198-010-0224-8. [DOI] [PubMed] [Google Scholar]
- 35.Trogdon JG, Ekwueme DU, Chamiec-Case L, Guy GP. Breast cancer in young women: Health state utility impacts by race/ethnicity. Am J Prev Med. 2016;50(2):262–9. doi: 10.1016/j.amepre.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parliament Research Center of the Islamic Republic of Iran. Work Law [Online] [cited 2019 April 26]. Available from: https://rc.majlis.ir/fa/law/show/99612.
- 37.National Institute for Health and Care Excellence (NICE) Alemtuzumab for treating highly active relapsing remitting multiple sclerosis [Online] [cited 2014 May 28]. Available from: URL: https://www.nice.org.uk/guidance/ta312/chapter/3-the-manufacturers-submission.
- 38.Shingler S, Fordham B, Evans M, Schroeder M, Thompson G, Dewilde S, et al. Utilities for treatment-related adverse events in type 2 diabetes. J Med Econ. 2015;18(1):45–55. doi: 10.3111/13696998.2014.971158. [DOI] [PubMed] [Google Scholar]
- 39.Iran food and Drug Administration. National Formulary of Iran: Natalizumab [Online] [cited 2020 Jun 15]. Available from: URL: https://irc.fda.gov.ir/nfi/Detail/15324.
- 40.Iran Ministry of Health and Medical Education. Tariff of healthcare services in public and private sectors in Iran (2018-2019) Tehran, Iran: Iran Ministry of Health and Medical Education; 2018. [Google Scholar]
- 41.Food and Drug Administration. Mavenclad FDA Label, 2019 (revised) [Online] [cited 2019 Apr 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022561s000lbl.pdf.
- 42.Orlewska E, Mierzejewski P, Zaborski J, Kruszewska J, Wicha W, Fryze W, et al. A prospective study of the financial costs of multiple sclerosis at different stages of the disease. Eur J Neurol. 2005;12(1):31–9. doi: 10.1111/j.1468-1331.2004.00950.x. [DOI] [PubMed] [Google Scholar]
- 43.Jick SS, Li L, Falcone GJ, Vassilev ZP, Wallander MA. Mortality of patients with multiple sclerosis: a cohort study in UK primary care. J Neurol. 2014;261(8):1508–17. doi: 10.1007/s00415-014-7370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. Life tables by country- Iran (Islamic Republic of) [Online] [cited 2016]. Available from: https://apps.who.int/gho/data/?theme=main&vid=60760.
- 45.Djambazov S, Slavchev G, Dineva T, Panayotov P, Vekov T. Cost-effectiveness analysis of cladribine tablets for treatment of patients with relapsing-remitting multiple sclerosis in Bulgaria. Value Health. 2018;21(Suppl 1):S206. [Google Scholar]
- 46.Palace J, Bregenzer T, Tremlett H, Oger J, Zhu F, Boggild M, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4(1):e004073. doi: 10.1136/bmjopen-2013-004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institute for Health and Care Excellence. Cladribine for treating relapsing-remitting multiple sclerosis. Technology appraisal guidance [TA616] [Online] [cited 2019 Dec 19]. Available from: URL: https://www.nice.org.uk/guidance/ta616.
- 48.Glennie JL, Torrance GW, Baladi JF, Berka C, Hubbard E, Menon D, et al. The revised Canadian Guidelines for the Economic Evaluation of Pharmaceuticals. Pharmacoeconomics. 1999;15(5):459–68. doi: 10.2165/00019053-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 49.National Institute for Health and Care Excellence (NICE) Guide to the Methods of Technology Guide to the Methods of Technology Apprappraisal: Process and Methods [Online] [cited 2013 Apr 4]. Available from: URL: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. [PubMed]