Abstract
Modern agriculture is becoming increasingly pollinator-dependent. However, the global stock of domesticated honeybees is growing at a slower rate than its demand, while wild bees are declining worldwide. This uneven scenario of high pollinator demand and low pollinator availability can translate into increasing pollination limitation, reducing the yield of pollinator-dependent crops. However, overall assessments of crop pollination limitation and the factors determining its magnitude are missing. Based on 52 published studies including 30 crops, we conducted a meta-analysis comparing crop yield in pollen-supplemented versus open-pollinated flowers. We assessed the overall magnitude of pollination limitation and whether this magnitude was influenced by (i) the presence/absence of managed honeybees, (ii) crop compatibility system (i.e. self-compatible/self-incompatible) and (iii) the interaction between these two factors. Overall, pollen supplementation increased yield by approximately 34%, indicating sizable pollination limitation. Deployment of managed honeybees and self-compatibility were associated with lower pollination limitation. Particularly, active honeybee management decreased pollination limitation among self-compatible but apparently not among self-incompatible crops. These findings indicate that current pollination regimes are, in general, inadequate to maximize crop yield, even when including managed honeybees, and stress the need of transforming the pollination management paradigm of agricultural landscapes.
Keywords: pollination limitation, pollinator-dependent crops, managed honeybees, crop compatibility system
1. Introduction
The agricultural area devoted to pollinator-dependent crops is growing worldwide [1,2], while the stock of managed honeybees (Apis mellifera L.), the main pollinator in most agroecosystems, is growing at a slower rate than its demand [3]. In parallel, wild bees and other non-bee pollinators are declining because of different and pervasive human disturbances [4–7]. This uneven scenario of high pollinator demand and low availability might represent a ‘pollination service bottleneck’, limiting the quantity and/or the quality of the fruits and seeds produced by many crops. However, the extent to which pollinator-dependent crops are pollination-limited at a global scale remains unknown.
Pollination limitation occurs when inadequate pollen transfer, either in quantitative or qualitative terms, limits sexual plant reproduction [8–10]. As a result, fewer seeds and/or fruits are produced than those that can be potentially produced given either available ovules or resources [11]. Inadequate quantity and/or quality of pollen transfer in pollinator-dependent crops can arise because of pollinator shortages and/or the presence of inefficient pollinators [9]. Different types of human disturbances, particularly the replacement of natural habitats by croplands and widespread use of pesticides, have caused declines in the abundance and/or diversity of pollinators at local, regional and global scales [5,7,12–14]. As a consequence, agriculture increasingly depends on managed bees.
The managed honeybee represents the most economically important pollinator for a wide variety of agricultural crops [15–17], being also the most abundant crop pollinator worldwide [18,19]. Honeybees have been historically the main pollinators for many crops due to their highly developed social behaviour, their generalist diet and large populations [20,21]. Farmers around the globe expend large amounts of money every year for their pollination services [22,23]. Surprisingly, and despite its relevance, the impact of managed bees, in general, and of honeybees in particular on alleviating pollination limitation has not been assessed.
The magnitude of pollination limitation is also expected to vary with a plant's compatibility system, which determines whether ovules can be successfully fertilized by self-pollen, outcross pollen or pollen from either source [24,25]. Self-incompatibility is a mechanism that prevents self-fertilization in hermaphroditic flowering plants based on the discrimination of self- versus outcross pollen in the pistil during pollen germination on the stigma or pollen-tube growth in the style or ovary [26]. Animal-pollinated crops exhibiting self-incompatibility (SI crops, hereafter) require the transfer of outcross pollen for successful ovule fertilization and seed formation and thus are expected to be highly dependent on pollinators. Therefore, the cultivation of SI crops commonly requires the planting of at least two varieties within a field and the presence of efficient pollinators for pollen transfer between them [27]. From an agricultural perspective, an efficient pollinator for a SI crop should be one that shows a low constancy to a single variety, limits geitonogamous pollination by visiting few flowers per plant and promotes cross-fertilization by flying relatively long distances between consecutive visits. Instead, crops exhibiting self-compatibility (SC crops, hereafter) do not possess a genetic-based mechanism to prevent selfing, showing variable dependence on pollinators based on their relative capacity to self-pollinate autonomously and achieving successful fertilization after self-pollination. Therefore, SC crops can be cultivated in monovarietal plantations usually without experiencing a significant yield reduction due to the transfer of self-pollen, a feature that also increases management options for efficient pollination [28]. Because of the high pollination demands and limited pollinator alternatives to maximize yield in SI crops together with the challenges of managing different varieties within the same plantation, selection for SC crop varieties in traditionally SI crops has been a priority in crop breeding programmes [29–31].
In this study, we present a global synthesis of the extent to which pollinator-dependent crops experience pollination limitation. Because the area devoted to pollinator-dependent crops is increasing while wild pollinators are declining and managed honeybees are growing but at a slower pace than their demand [2,3], we expected to find widespread pollination limitation in crops. After proving that pollination limitation in crops is the rule rather than the exception, we evaluated whether the use of domesticated honeybees, the most important and ubiquitous managed pollinator, mitigates pollination limitation. Given that the honeybee is a super-generalist pollinator and is being actively managed to pollinate most pollinator-dependent crops [32], we predicted lower levels of pollination limitation in crops with managed honeybees than in those crops without them. Then, we assessed if the magnitude of pollination limitation differed between SI and SC crops. Since SI crops require that pollinators transport outcross pollen for successful ovule fertilization, whereas SC crops can set seeds or fruits after receiving either outcross or self-pollen, we predicted higher levels of pollination limitation in SI than SC crops. Finally, we compared the extent to which managed honeybees may alleviate pollination limitation in SI versus SC crops (i.e. an interaction between presence/absence of managed honeybees and crop compatibility system). Because honeybees tend to favour the transfer of self-pollen by moving short distances between consecutive visits and visiting many flowers within the same plant (e.g. [33,34]), we expected active honeybee management to reduce pollination limitation more strongly in SC than SI crops. To address all these hypotheses, we conducted a meta-analysis using data from published literature covering phylogenetically diverse pollinator-dependent crops from studies distributed worldwide.
2. Material and methods
(a) . Literature search
Following the protocols and good practices stated by Nakagawa et al. [35], we conducted a systematic literature search of published studies in SCOPUS (https://www.scopus.com/home.uri), the largest abstract and citation multidisciplinary database of peer-reviewed literature, covering publications from 1900 to July 2021. We searched for studies reporting at least one estimate of crop yield (e.g. seed- and/or fruit-set, individual seed or fruit weight, fruit or seed production per plant or area unit) after natural (open) pollination and pollen supplementation (i.e. open flowers manually supplemented with outcross pollen) treatments in pollinator-dependent crops [36]. A pollinator-dependent crop was defined as any crop that exhibits at least some yield reduction when pollinators are excluded [36]. Studies were gathered using the following combinations of keywords: (crop* OR cultivation* OR culture* OR farming*) AND (control pollinat* OR open pollinat* OR free pollinat* OR cross pollinat* OR hand pollinat* OR hand cross pollinat* OR supplement poll* OR poll* limit* OR pollen supplementation) AND (fruit* OR seed* OR reproductive success* OR reproductive output*). This search yielded 1528 articles that were subsequently screened for eligibility and inclusion in our meta-analysis. Another group of papers were retrieved by checking references from the bibliography of papers fulfilling the inclusion criteria (see electronic supplementary material, figure S1).
(b) . Study selection and data collected
For a study to be included in the meta-analysis, it had to report the mean value, some measure of dispersion that could be ultimately transformed to standard deviation, and sample size for any of the above-mentioned measures of crop yield (‘yield’ hereafter) under both natural and supplemental pollination treatments in a pollinator-dependent crop. We only selected studies in which pollen supplementation was performed by adding outcross pollen manually or mechanically, at least once during flower lifespan, to the stigmas of otherwise open-pollinated flowers. For those studies reporting this information in figures, we extracted the data using WebPlotDigitalizer, an open web-based tool that extracts statistics from plots (https://automeris.io/WebPlotDigitizer/).
For each selected study, we also gathered information regarding (i) the presence or absence of managed honeybees in the study crop and (ii) crop compatibility system (i.e. SC or SI) (see electronic supplementary material, table S1). Studies that did not provide information regarding the presence/absence of managed honeybees were only considered for the evaluation of the overall pollination limitation (i.e. first question). Information regarding crop compatibility system (SC or SI) was gathered from each selected study when reported and from Klein et al. [36] or other literature sources when not reported in the selected study (see electronic supplementary material, table S1). Moreover, the crop Actinia deliciosa (kiwi) was included in the SI group, as it is dioecious and thus completely dependent on pollen transfer between plants for fruit production.
(c) . Effect size's estimation
We used the natural logarithm of the response ratio (i.e. LnRR) as a measure of effect size across the studies [37]. LnRR was estimated as , where and are the mean crop yield observed after supplemental and open pollination, respectively (see also [9]). To calculate the LnRR and its variance, we used the means, standard deviations and sample sizes of crop yield for the open-pollinated and pollen-supplemented treatments from each study. Several publications provided the opportunity of calculating more than one effect size per study as they compared open versus supplemented pollination treatments in independent samplings within the study (e.g. different sites, varieties). By contrast, other studies reported non-independent measures of crop yield (e.g. seed-set, fruit-set, fruit weight, seed number in the same plant). In these cases, effect sizes obtained from each of these non-independent reported measures were used to estimate a single within-study mean weighted effect (i.e. average effect) [38]. Response ratios were calculated using the ROM function from the metafor package [39] in R software [40]. Even though we used the LnRR, a metric that linearizes and normalizes the relative difference between two treatments [37], in all the statistical analyses, we report the per cent change of crop yield following pollen supplementation (i.e. ) for a more straightforward interpretation of the results (e.g. [41]). Positive values imply that pollen supplementation increases crop yield, while negative values indicate the opposite. This later outcome, even uncommon, may reflect experimental error (e.g. pollen supplementation out of the time of optimal stigmatic receptivity) or a true biological phenomenon (e.g. massive pollen-tube attrition in overcrowded styles; [42]). Values approximately 0 imply that both open and supplemental pollination result in similar crop yield, providing no evidence of pollination limitation.
(d) . Statistical analysis
We analysed data using meta-analytical mixed-effects models, which assume that all individual effect sizes within a category (i.e. the moderator variables: presence/absence of managed honeybees or SI versus SC) share a common effect, but that there is also random variation among studies in addition to within-study sampling variation [43,44]. These models incorporated the hierarchical dependence due to cases where more than one independent effect size was obtained per study and per crop species, by including ‘study’ and ‘crop’ identities, respectively, as random factors (e.g. [45]). The heterogeneity among effect sizes was assessed with Q statistics, which are weighted sums of squares that, under the null hypothesis, follow a χ2 distribution [37]. Specifically, we examined the p-values of Qbetween (Qb) statistics that describe the variation in effect sizes attributed to differences among categories of each moderator variable as well as their interaction. Effect sizes were considered significantly different from zero if their 95% bias-corrected bootstrap CIs did not overlap with zero [43,44]. All analyses were performed with the metafor [39] package in R software [40]. Datasets used in the meta-analyses are shown in electronic supplementary material, table S1.
(e) . Phylogenetic bias
To ensure that our results were not phylogenetically biased, we compared the results of our first (overall) model with a phylogenetically explicit model that only considers one effect size estimate per crop species ([46]; see electronic supplementary material for details). Effect sizes calculated from the phylogenetically explicit model showed a similar response compared to the mixed-effects meta-analysis, and Bloomberg's K-test revealed that there was no evidence of a phylogenetic signal in the residuals of the mixed-effects model (see electronic supplementary material). Given that there was no indication of a phylogenetic bias (see electronic supplementary material), here we present results from the traditional hierarchical meta-analysis, which provided higher sample size (i.e. 107 versus 30 input values) and thus more robust estimations.
(f) . Publication bias
An intrinsic problem common to any quantitative systematic review is the potential presence of publication bias: studies reporting significant results may have higher chances of being published than those reporting non-significant results. To test for the presence of such bias in our dataset, we ran rank correlation tests between effect sizes and standard errors across the studies, where significant correlations imply a potential publication bias (i.e. studies with either large standard errors or small sample sizes are only published if they show large effects; [47]). We also performed the ‘trim and fill’ method, which provides an estimate of how the overall results would change if we were able to add all the potentially missing studies [38]. Finally, we calculated the Rosenberg's weighted fail-safe number, which estimates the number of non-significant, unpublished studies that would need to be incorporated into the meta-analysis to nullify the overall effect [48]. When the fail-safe number is greater than 5N + 10, with N being the number of studies, results are robust regardless of publication bias [48].
3. Results
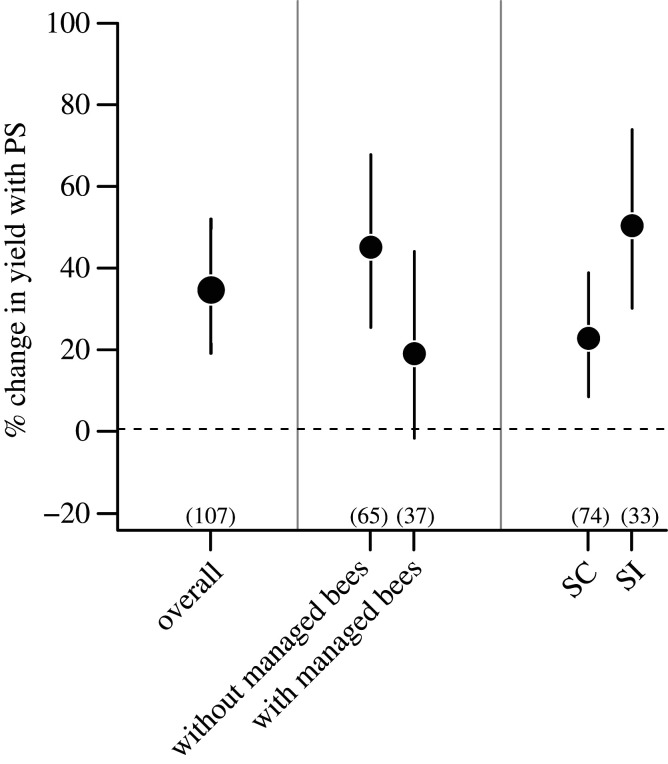
We retrieved data assessing crop pollination limitation from 52 studies conducted around the world (figure 1), encompassing 30 different pollinator-dependent crops, 18 SC and 13 SI (one of the crops studied, Prunus dulcis, has SC and SI varieties), which belong to 19 families (see electronic supplementary material, table S2 and figure S2). Our dataset included many economically important crops like apples, almonds, coffee, oil seed rape, sunflower and blueberries, among others. From these 52 studies, we estimated a total of 107 individual effect sizes. In terms of the magnitude of pollination limitation, we observed an average of 34.2% increase in yield when flowers were supplemented with outcross pollen (95% CI (18.53–51.43%), p < 0.001, figure 2). Model-adjusted mean effect sizes varied between an approximately 20% decrease to an approximately 80% increase in yield after pollen supplementation. Only one of the 107 individual estimates was negative (indicating lower yield after pollen supplementation), and nearly 95% of the 95% CIs associated with the 107 estimated positive effect sizes did not overlap with zero (electronic supplementary material, figure S3).
Figure 1.
World map showing the geographical location of the 52 studies (circles). (Online version in colour.)
Figure 2.
Average pollination limitation expressed as a percentage change (%) in yield after pollen supplementation (PS) for all crops, for crops with and without managed honeybees and for crops with different compatibility systems (SC: self-compatible; SI: self-incompatible). Dots indicate the estimated back-transformed mean LnRR values according to , while bars represent their respective 95% CIs. Numbers in parentheses indicate number of effect sizes. Pollen supplementation increases yield significantly if the corresponding 95% CI does not include zero.
Deployment of managed honeybees tends to circumvent pollination limitation. Without managed honeybees, pollen supplementation increased yield by an average of 44.5% (95% CI (24.8–67.19%), p < 0.001, figure 2), whereas crops with active honeybee management showed no evidence of pollination limitation as the 95% CIs around the mean yield increase in pollen-supplemented flowers overlapped with zero (18.4%, 95% CI (–2.26% to 43.47%), p = 0.084, figure 2). Therefore, the use of managed honeybees tended to reduce the extent of pollination limitation (Qb = 2.75, d.f. = 1, p = 0.09).
Both SI and SC crops showed signs of pollination limitation, although the effect was higher in SI crops. SC crops showed, on average, a 22.2% increase in yield when supplemented with pollen (95% CI (7.89–38.26%), p = 0.001, figure 2), whereas SI crops showed a much higher value, estimated in 49.7% (95% CI (29.56–73.32%), p < 0.001, figure 2). Therefore, yield was significantly more pollination-limited in SI than in SC crops (Qb = 5.91, d.f. = 1, p = 0.01).
Interestingly, honeybee management decreased pollination limitation significantly in SC but not in SI crops. In fact, SC crops with active honeybee management showed no evidence of pollination limitation as the 95% CIs around the mean yield increase in pollen-supplemented flowers overlapped with zero (8.3%, 95% CI (−11.08% to 31.91%), p = 0.42, figure 3). Instead, pollen supplementation translated into an average increase of 34.1% in yield among SC crops when no bee management was involved (95% CI (13.87–58.2%), p < 0.001, figure 3). On the contrary, SI crops with management of bees showed a 43.7% increase in yield when supplemented with pollen (95% CI (7.14–93.09%), p = 0.01, figure 3), which was similar to the estimated yield average increase of 54.5% observed without bee management (95% CI (30.34–83.30%), p < 0.001, figure 3). We actually detected an overall differential response to pollen supplementation between SC and SI crops depending on the use of managed honeybees (Qb = 8.12, d.f. = 3, p = 0.04). These results indicate that deployment of honeybees in fields cultivated with SI crops would not be enough to circumvent pollination limitation.
Figure 3.
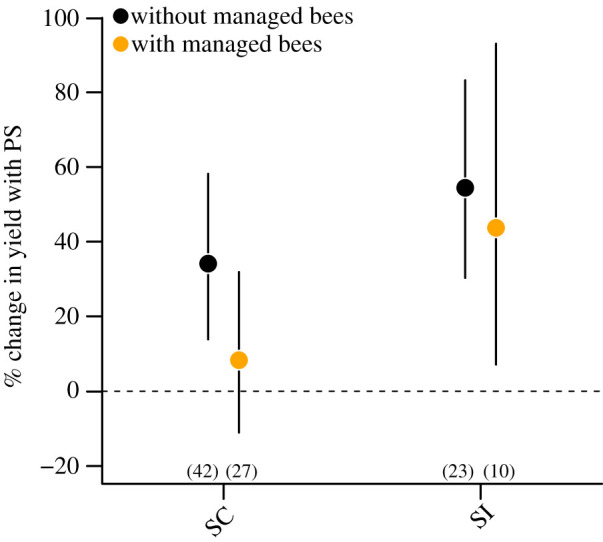
Average pollination limitation expressed as a percentage change (%) in yield after pollen supplementation (PS) for crops with and without managed honeybees in relation to compatibility systems (SC: self-compatible; SI: self-incompatible). Dots indicate the estimated back-transformed mean LnRR values according to , while bars represent their 95% CIs. Numbers in parentheses indicate number of effect sizes. Pollen supplementation increases yield significantly if the corresponding 95% CI does not include zero. (Online version in colour.)
(a) . Publication bias
The rank correlation test for funnel plot asymmetry suggests a potential publication bias (Kendall's tau = −0.42, p < 0.001) (i.e. studies showing small or nil effects might be missing from our database). However, the ‘trim and fill’ procedure showed that the overall effect size would only slightly decrease and would remain significantly different from zero (i.e. significant pollination limitation) after including the potential missing studies. Accordingly, the calculated weighted fail-safe number was 349 055, much larger than 5N + 10 = 550, indicating the robustness of our results, regardless of some evidence of publication bias.
4. Discussion
The findings of our meta-analysis based on 30 crops represent the first demonstration of a global scenario of pollination limitation among pollinator-dependent crops. Specifically, we found evidence of pollination limitation in about 94% of all estimated effects sizes, with pollen supplementation increasing crop yield by an average of approximately 35%. These results demonstrate that pollination limitation is widespread among pollinator-dependent crops and an important factor curtailing yield. Agreeing with our global results, pollination limitation has also been reported to be regionally widespread among several important crops across the United States [49]. We also found that the deployment of domesticated honeybees in crop fields and the cultivation of SC crops could ameliorate pollination limitation. However, while honeybee management seems to reduce pollination limitation in SC crops, it has a negligible effect in diminishing the large pollination deficits observed among SI crops. Therefore, our findings are indicative that the use of honeybees, the most widespread managed bee for crop pollination [16,19], will not be enough to decrease unrealized yields associated with pollination limitation in many crops, particularly in those that depend on cross-pollination to sustain high yields (e.g. almond, cacao, coffee and apples, among others). Even though the results of our quantitative review are of an associative nature, they agree with hypothetical expectations based on basic biological knowledge of how a pollinator's foraging behaviour can interact with a plant's compatibility system to determine the extent of quantitative and qualitative pollination limitation. Particularly, our results agree with the expectation that the use of a pollinator like the honeybee, which visits many flowers of the same plant in a row during the same foraging bout, cannot reduce pollination limitation to any large extent despite high stigmatic pollen receipt because of the preponderant transfer of self-pollen [10].
The pervasive pollination limitation revealed here implies that current regimens of biotic pollination are inadequate to maximize agricultural yield. This could be interpreted as evidence that pollination demands exceed the global pollination capacity [3]. In fact, it is expected that this pollination gap has been widening during the last decades as a consequence of a worldwide decline in pollinator abundance and diversity [7,16,50–52] driven, among other factors, by agriculture expansion and intensification [2,53]. Therefore, it is relevant to contrast the magnitude of pollination limitation in crops with estimates from wild plants [8,9]. Contrary to our expectations, the magnitude of pollen limitation we estimated here for crops, in terms of increase in reproductive output after pollen supplementation (i.e. approximately 34%), was somewhat lower than those estimated in wild plants (approx. 42% in Ashman et al., [8] and approx. 75% in Knight et al., [9]). Even though agricultural landscapes tend to be more depauperate of wild pollinators than those remaining landscapes dominated by natural and seminatural vegetation [4,54], the lower average magnitude of pollination limitation among crops could be reflecting the use of external pollinator subsidies such as the deployment of honeybee hives as well as the lower pollinator-dependence nature of most crops in comparison with wild plant communities. It has been estimated that only 10% of all crops would suffer a complete reproductive collapse in the absence of pollinators [9,55], which contrasts with the 30% estimated for wild plant species [56]. In any event, our results suggest that pollination is a key factor limiting yield of most animal-pollinated crops under different agricultural settings.
To minimize pollination inefficiencies and maximize yields, farmers pay for the service provided by managed bees, mostly honeybees, representing a key species for agriculture [15,16,18,19,22]. Our review shows that although the use of managed honeybees does not fully circumvent unrealized yields caused by pollination limitation, it significantly decreases its magnitude. In fact, deployment of managed honeybee hives reduced pollination limitation to almost one half (figure 2). On the one hand, this result validates the importance of managed honeybees and their contribution to global agriculture [3,57,58]. But, on the other hand, it suggests that single-species management (i.e. the honeybee) is not sufficient to compensate for the lack of wild pollinators (see also [19]). Diverse pollinator assemblages can increase pollination efficiency and reduce pollination limitation through several mechanisms, including increased spatial and temporal niche complementarity, likelihood to include one or more effective pollinator, and interference competition between pollinators promoting inter-plant movement and outcrossing [59–62]. Additionally, overstocking fields with managed bees may promote honeybee spill over into natural ecosystems, with negative consequences for wild plants and other pollinators [63,64]. Therefore, our findings call for an integrated pollinator management to sustain high yields. This integrated management should consider the use of domesticated pollinators, together with the fostering of diverse wild pollinator assemblages through enhancing habitat heterogeneity and floral and nesting resources at different spatial scales [65].
Our findings also demonstrate that a crop's compatibility system, as a proxy of mating system and outcrossing requirements, is a key trait determining the extent of pollination limitation and thus of realized yield. Specifically, we found that pollination limits the yield of SI crops twice as much as the yield of SC crops (figure 2). This result could be explained by the fact that, in SI crops, pollen needs to be transferred between plants, more commonly between different varieties, for successful pollination [27]. SC crops, on the other hand, do not have strict barriers to self-fertilization, which reduces the pollen transfer distance for effective pollination, for example, within a flower and between flowers within a plant [66]. Also, SC crops exhibit variable dependency levels on animal pollinators based on their differential capacity to self-pollinate autonomously, as it has been shown in new SC varieties of almond trees [30].
Most interestingly, we demonstrated that the deployment of managed honeybee hives in crop fields ameliorates pollination limitation depending on a crop's compatibility system. Specifically, the use of honeybees could circumvent pollination limitation and maximize yield among SC crops but not among SI crops. This latter result could be explained by the foraging behaviour of Apis mellifera. First, in agricultural contexts, honeybees tend to forage on single plant rows [34,67,68], while SI crops are typically sown planting a different variety in each row [20]. This fact also points to the relevance of population genetic aspects of crop pollination management, particularly the inter-compatibility of the different admixed varieties and how these varieties are spatially intermixed [69–72]. Second, honeybees show high floral constancy depending on flower rewards [59,73], morphology [74,75] and colour ([76] and references therein), and crop varieties may differ strongly in these traits (see for example [77]). An extreme example of honeybee constancy related to phenotypic differences can be found in kiwi, where male plants offer pollen as a reward to floral visitors while female plants do not, generating a strong preference for male flowers by honeybees [78]. This example represents only one out of many examples of honeybees foraging behaviour, showing that honeybees rarely move between varieties because of their high constancy, which decreases the transfer of outcross pollen [34,67,68].
Fortunately, research has shown that honeybee constancy can be disrupted when other pollinators are present, increasing their pollination efficiency [34,59]. Consequently, the presence of both wild and managed pollinators in crop fields could be important for increasing cross-pollination because of either their additive or interactive effects. On the other hand, the use of just one all-purpose species of managed pollinator like the honeybee will have a negligible effect in reducing pollination deficits, particularly in SI crops. Moreover, reliance on a single pollinator species for crop pollination will increase enormously the risk of sudden yield collapses [79].
5. Concluding remarks
Modern agriculture is becoming increasingly pollinator-dependent, and animal-pollinated crops account for the majority of the expansion in agricultural area [1,2]. However, the increasing demand of pollination services has not been attended with proper agricultural management practices that promote diversity in terms of pollen sources (i.e. varieties/cultivars) and pollinators. On the plant side, even though the effects of enhancing genetic diversity within plantations had been relatively unexplored, Kron & Husband [80] showed that increasing the diversity of pollen donors could decrease pollination limitation by enhancing the number of ovules fertilized with high-quality pollen (see also [10]). More specifically, well intermixed multi-varietal plantations, particularly of SI crops, may diminish pollination limitation by decreasing the chance of morphological and phenological flowering mismatches, thus increasing the effective availability of alternative sources of compatible pollen. On the pollinator side, here we show that single-species management (i.e. honeybees) does not completely solve yield losses associated with pollination limitation for many crops. This limitation could be circumvented by the application of well-developed protocols to diversify pollinator options (see [65]), such as the enhancement of wild pollinator assemblages through restauration of natural and seminatural habitats. Overall, our study suggests that crop pollination management for increasing yield should consider the joint diversification of both the plant and pollinator sides as positive synergistic effects could derive from such an integrative approach.
Acknowledgements
We thank Valeria Martin-Albarracin for her help during data extraction and two anonymous reviewers for their helpful comments on the initial manuscript.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rfj6q57ct [81].
Authors' contributions
A.S.: conceptualization, data curation, formal analysis, writing—original draft; R.A.: data curation, formal analysis, writing—review and editing; L.A.: data curation, writing—review and editing; G.G.: formal analysis, writing—review and editing; C.L.M.: conceptualization, data curation, writing—review and editing; A.T.: writing—review and editing; M.A.A.: conceptualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Scientific and Technical Research Council (CONICET) – Argentina; CONICET (PIP 2015-0371); FONCyT (PICT 2016-0764; PICT 2019-1897) and SURPASS2 ‘Safeguarding pollination services in a changing world’, project funded under the Newton Fund Latin America Biodiversity Programme: Biodiversity–Ecosystem Services for Sustainable Development, grants awarded by the NERC, Great Britain [NE/S011870/1] and CONICET, Argentina [RD 1984/19].
References
- 1.Aizen MA, Garibaldi LA, Cunningham SA, Klein AM. 2008. Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr. Biol. 18, 1572-1575. ( 10.1016/j.cub.2008.08.066) [DOI] [PubMed] [Google Scholar]
- 2.Aizen MA, et al. 2019. Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob. Chang. Biol. 25, 3516-3527. ( 10.1111/gcb.14736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aizen MA, Harder LD. 2009. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19, 915-918. ( 10.1016/j.cub.2009.03.071) [DOI] [PubMed] [Google Scholar]
- 4.Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. 2009. A meta-analysis of bees' responses to anthropogenic disturbance. Ecology 90, 2068-2076. ( 10.1890/08-1245.1) [DOI] [PubMed] [Google Scholar]
- 5.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends. Ecol. Evol. 25, 345-353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 6.Soroye P, Newbold T, Kerr J. 2020. Among bumble bees across continents. Science 367, 685-688. ( 10.1126/science.aax8591) [DOI] [PubMed] [Google Scholar]
- 7.Zattara EE, Aizen MA. 2021. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4, 114-123. ( 10.1016/j.oneear.2020.12.005) [DOI] [Google Scholar]
- 8.Ashman T-L, et al. 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408-2421. ( 10.1890/03-8024) [DOI] [Google Scholar]
- 9.Knigh TM, et al. 2005. Pollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst. 36, 467-497. ( 10.1146/annurev.ecolsys.36.102403.115320) [DOI] [Google Scholar]
- 10.Aizen MA, Harder LD. 2007. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88, 271-281. ( 10.1890/06-1017) [DOI] [PubMed] [Google Scholar]
- 11.Harder LD, Aizen MA. 2010. Floral adaptation and diversification under pollen limitation. Phil. Trans. R. Soc. B 365, 529-543. ( 10.1098/rstb.2009.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brittain CA, Vighi M, Bommarco R, Settele J, Potts SG. 2010. Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl. Ecol. 11, 106-115. ( 10.1016/j.baae.2009.11.007) [DOI] [Google Scholar]
- 13.Gill RJ, Ramos-Rodriguez O, Raine NE. 2012. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105-108. ( 10.1038/nature11585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 1126, 1-10. ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 15.McGregor S. 1976. Insect pollination of cultivated crop plants (No. 496). Agricultural Research Service, US Department of Agriculture, Washington DC. [Google Scholar]
- 16.Watanabe ME. 1994. Pollination worries rise as honey bees decline. Science 265, 1170-1171. ( 10.1126/science.265.5176.1170) [DOI] [PubMed] [Google Scholar]
- 17.Calderone NW. 2012. Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992–2009. PLoS ONE 7, e37235. ( 10.1371/journal.pone.0037235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rader R, Howlett BG, Cunningham SA, Westcott DA, Newstrom-Lloyd LE, Walker MK, Teulon DAJ, Edwards W. 2009. Alternative pollinator taxa are equally efficient but not as effective as the honeybee in a mass flowering crop. J. App. Ecol. 46, 1080-1087. ( 10.1111/j.1365-2664.2009.01700.x) [DOI] [Google Scholar]
- 19.Garibaldi LA, et al. 2012. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608-1611. ( 10.1126/science.1230200) [DOI] [PubMed] [Google Scholar]
- 20.Free J. 1993. Insect pollination of crops, 2nd edn. London, UK: Academic Press. [Google Scholar]
- 21.Partap U. 2011. The pollination role of honeybees. In Honeybees of Asia (eds Hepburn HR, Radloff SE), pp. 227-255. Berlin, Germany: Springer. [Google Scholar]
- 22.Morse RA, Calderon NW. 2000. The value of honey bees as pollinators of US crops. Bee Cult. 128, 1-15. [Google Scholar]
- 23.Allsopp MH, de Lange WJ, Veldtman R. 2008. Valuing insect pollination services with cost of replacement. PLoS ONE 3, e3128. ( 10.1371/journal.pone.0003128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannell J, Labouche AM. 2013. The incidence and selection of multiple mating in plants. Phil. Trans. R. Soc. B 368, 20120051. ( 10.1098/rstb.2012.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett SCH, Harder LD. 2017. The ecology of mating and its evolutionary consequences in seed plants. Annu. Rev. Ecol. Evol. Syst. 48, 135-157. ( 10.1146/annurev-ecolsys-110316-023021) [DOI] [Google Scholar]
- 26.Franklin-Tong VE. 2008. Self-incompatibility in flowering plants. Evolution, diversity, and mechanisms.. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 27.Muñoz-Sanz JV, Zuriaga E, Cruz-García F, McClure B, Romero C. 2020. Self-(in)compatibility systems: target traits for crop-production, plant breeding, and biotechnology. Front. Plant. Sci. 11, 195. ( 10.3389/fpls.2020.00195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortega E, Dicenta F. 2006. Self-fertilization in homozygous and heterozygous self-compatible almonds. Sci. Hortic. 109, 288-292. ( 10.1016/j.scienta.2006.04.017) [DOI] [Google Scholar]
- 29.Gradziel TM, Kester DE. 1997. Breeding for self-fertility in California almond cultivars. Acta Hortic. 470, 109-117. ( 10.17660/ActaHortic.1998.470.15) [DOI] [Google Scholar]
- 30.Sáez A, Aizen MA, Medici S, Viel M, Villalobos E, Negri P. 2020. Bees increase crop yield in an alleged pollinator-independent almond variety. Scient. Rep. 10, 1-7. ( 10.1038/s41598-019-56847-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novoselov M, Starshinova OA, Drobysheva LV. 2021. The possibility of using self-compatible forms of meadow clover (Trifolium pratense L.) in breeding to increase seed productivity. IOP Conf. Ser.: Earth. Env. Sci. 663, 012016. ( 10.1088/1755-1315/663/1/012016) [DOI] [Google Scholar]
- 32.Aizen MA, et al. 2020. Invasive bees and their impact on agriculture. Adv. Ecol. Res. 63, 49-92. ( 10.1016/bs.aecr.2020.08.001) [DOI] [Google Scholar]
- 33.Aizen MA, Feisinger P. 1994. Habitat fragmentation, native insect pollinators, and feral honey bees in Argentine ‘Chaco Serrano’. Ecol. Appl. 4, 378-392. ( 10.2307/1941941) [DOI] [Google Scholar]
- 34.Brittain C, Williams N, Kremen C, Klein AM. 2013. Synergistic effects of non-Apis bees and honey bees for pollination services. Proc. R. Soc. B 280, 20122767. ( 10.1098/rspb.2012.2767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa S, Noble DW, Senior AM, Lagisz M. 2017. Meta-evaluation of meta-analysis: ten appraisal questions for biologists. BMC Biol. 15, 1-14. ( 10.1186/s12915-017-0357-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303-313. ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedges L, Olkin I. 2014. Statistical methods for meta-analysis. London, UK: Academic Press. [Google Scholar]
- 38.Jennions MD, Lortie CJ, Rosenberg MS, Rothstein HR. 2013. Publication and related biases. In Handbook of meta-analysis in ecology and evolution (eds Koricheva J, Gurevitch J, Mengersen K), pp. 207-236. Princeton, NJ: Princeton University Press. [Google Scholar]
- 39.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Soft. 36, 1-48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 40.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 41.Ainsworth E, Rogers A, Nelson R, Long SP. 2004. Testing the ‘source–sink’ hypothesis of down-regulation of photosynthesis in elevated in the field with single gene substitutions in Glycine max. Agric. For. Meteo. 122, 85-94. ( 10.1016/j.agrformet.2003.09.002) [DOI] [Google Scholar]
- 42.Harder LD, Aizen MA, Richards SA. 2016. The population ecology of male gametophytes: the link between pollination and seed production. Ecol. Lett. 19, 497-509. ( 10.1111/ele.12596) [DOI] [PubMed] [Google Scholar]
- 43.Borenstein M, Hedges L, Higgins J, Rothstein H. 2009. Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 44.Koricheva J, Gurevitch J. 2014. Uses and misuses of meta-analysis in plant ecology. J. Ecol. 102, 828-844. ( 10.1111/1365-2745.12224) [DOI] [Google Scholar]
- 45.Rossetti M, Tscharntke T, Aguilar R, Batary P. 2017. Responses of insect herbivores and herbivory to habitat fragmentation: a hierarchical meta-analysis. Ecol. Lett. 20, 264-272. ( 10.1111/ele.12723) [DOI] [PubMed] [Google Scholar]
- 46.Lajeunesse MJ. 2009. Meta-analysis and the comparative phylogenetic method. Am. Nat. 174, 369-381. ( 10.1086/603628) [DOI] [PubMed] [Google Scholar]
- 47.Begg CB. 1994. Publication bias. In The handbook of research synthesis, (eds Cooper H, Hedges LV), New York, NY: Rusell Sage Foundation. [Google Scholar]
- 48.Rosenberg MS. 2005. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59, 464-468. ( 10.1111/j.0014-3820.2005.tb01004.x) [DOI] [PubMed] [Google Scholar]
- 49.Reilly JR, et al. 2020. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B 287, 20200922. ( 10.1098/rspb.2020.0922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen-Wardell G, et al. 1998. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv. Biol. 12, 8-17. ( 10.1046/j.1523-1739.1998.97154.x) [DOI] [Google Scholar]
- 51.Gallai N, Salles J, Settele J, Vaissière BE. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810-821. ( 10.1016/j.ecolecon.2008.06.014) [DOI] [Google Scholar]
- 52.Bauer DM, Wing IS. 2010. Economic consequences of pollinator declines: a synthesis. Agric. Econ. Res. Rev. 39, 368-383. ( 10.1017/S1068280500007371) [DOI] [Google Scholar]
- 53.Hemberger J, Crossley MS, Gratton C. 2021. Historical decrease in agricultural landscape diversity is associated with shifts in bumble bee species occurrence. Ecol. Lett. 24, 1800-1813. ( 10.1111/ele.13786) [DOI] [PubMed] [Google Scholar]
- 54.Garibaldi LA, et al. 2011. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 14, 1062-1072. ( 10.1111/j.1461-0248.2011.01669.x) [DOI] [PubMed] [Google Scholar]
- 55.Aizen MA, Garibaldi LA, Cunningham SA, Klein AM. 2009. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 103, 1579-1588. ( 10.1093/aob/mcp076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogers HS, Donoso I, Traveset A, Fricke EC. 2021. Cascading impacts of seed disperser loss on plant communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 52, 641-666. ( 10.1146/annurev-ecolsys-012221-111742) [DOI] [Google Scholar]
- 57.Southwick EE, Southwick L Jr. 1992. Estimating the economic value of honey bees (Hymenoptera: Apidae) as agricultural pollinators in the United States. J. Econ. Entomol. 85, 621-633. ( 10.1093/jee/85.3.621) [DOI] [Google Scholar]
- 58.Carreck N, Williams I. 1998. The economic value of bees in the UK. Bee World 79, 115-123. ( 10.1080/0005772X.1998.11099393) [DOI] [Google Scholar]
- 59.Greenleaf SS, Kremen C. 2006. Wild bees enhance honey bees' pollination of hybrid sunflower. Proc. Natl Acad. Sci. USA 103, 13 890-13 895. ( 10.1073/pnas.0600929103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albrecht M, Schmid B, Hautier Y, Müller CB. 2012. Diverse pollinator communities enhance plant reproductive success. Proc. R. Soc. B 279, 4845-4852. ( 10.1098/rspb.2012.1621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winfree R, Reilly JR, Bartomeus I, Cariveau DP, Williams NM, Gibbs J. 2018. Species turnover promotes the importance of bee diversity for crop pollination at regional scales. Science 359, 791-793. ( 10.1126/science.aao2117) [DOI] [PubMed] [Google Scholar]
- 62.Senapathi D, et al. 2021. Wild insect diversity increases inter-annual stability in global crop pollinator communities. Proc. R. Soc. B 288, 20210212. ( 10.1098/rspb.2021.0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González-Varo JP, Vila M. 2017. Spillover of managed honeybees from mass-flowering crops into natural habitats. Biol. Conser. 212, 376-382. ( 10.1016/j.biocon.2017.06.018) [DOI] [Google Scholar]
- 64.Magrach A, González-Varo J, Boiffier M, Vila M, Bartomeus I. 2017. Honeybee spillover reshuffles pollinator diets and affects plant reproductive success. Nat. Ecol. Evol. 1, 1299-1307. ( 10.1038/s41559-017-0249-9) [DOI] [PubMed] [Google Scholar]
- 65.Garibaldi LA, et al. 2014. From research to action: enhancing crop yield through wild pollinators. Front. Ecol. Environ. 12, 439-447. ( 10.1890/130330) [DOI] [Google Scholar]
- 66.Dicenta F, Ortega E, Canovas JA, Egea J. 2001. Self-pollination versus cross-pollination of six self-compatible almond cultivars: pollen tube growth and fruit set. Cah. Options Mediterr. 56, 369-372. [Google Scholar]
- 67.Free JB. 1966. The pollinating efficiency of honey-bee visits to apple flowers. J. Hortic. Sci. 41, 91-94. ( 10.1080/00221589.1966.11514158) [DOI] [Google Scholar]
- 68.Quinet M, Jacquemart AL. 2017. Cultivar placement affects pollination efficiency and fruit production in European pear (Pyrus communis) orchards. Eur. J. Agron. 91, 84-92. ( 10.1016/j.eja.2017.09.015) [DOI] [Google Scholar]
- 69.Kester DE, Gradziel TM, Micke WC. 1994. Identifying pollen incompatibility groups in California almond cultivars. J. Am. Soc. Hortic. Sci. 119, 106-109. ( 10.21273/JASHS.119.1.106) [DOI] [Google Scholar]
- 70.Kron P, Brian C, Peter G, Kevan G. 2001. Across- and along-row pollen dispersal in high-density apple orchards: insights from allozyme markers. J. Hortic. Sci. Biotech. 76, 286-294. ( 10.1080/14620316.2001.11511365) [DOI] [Google Scholar]
- 71.Garratt MPD, et al. 2016. Apple pollination: demand depends on variety and supply depends on pollinator identity. PLoS ONE 11, e0153889. ( 10.1371/journal.pone.0153889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sáez A, di Virgilio A, Tiribelli F, Geslin B. 2018. Simulation models to predict pollination success in apple orchards: a useful tool to test management practices. Apidologie 49, 551-561. ( 10.1007/s13592-018-0582-2) [DOI] [Google Scholar]
- 73.Free JB. 1963. The flower constancy of honeybees. J. Anim. Ecol. 32, 119-1311. ( 10.2307/2521) [DOI] [Google Scholar]
- 74.Martin CS, Farina WM. 2016. Honeybee floral constancy and pollination efficiency in sunflower (Helianthus annuus) crops for hybrid seed production. Apidologie 47, 161-170. ( 10.1007/s13592-015-0384-8) [DOI] [Google Scholar]
- 75.Estravis-Barcala MC, Palottini F, Farina WM. 2019. Honey bee and native solitary bee foraging behavior in a crop with dimorphic parental lines. PLoS ONE 14, e0223865. ( 10.1371/journal.pone.0223865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill PS, Wells PH, Wells H. 1997. Spontaneous flower constancy and learning in honey bees as a function of colour. Anim. Behav. 54, 615-627. ( 10.1006/anbe.1996.0467) [DOI] [PubMed] [Google Scholar]
- 77.Sepahvand E, Khadivi-Khub A, Momenpour A, Fallahi E. 2015. Evaluation of an almond collection using morphological variables to choose superior trees. Fruits 70, 53-59. ( 10.1051/fruits/2014044) [DOI] [Google Scholar]
- 78.Goodwin R, Steven D. 1993. Behaviour of honey bees visiting kiwifruit flowers. N. Z. J. Crop Hortic. 21, 17-24. ( 10.1080/01140671.1993.9513741) [DOI] [Google Scholar]
- 79.Winfree R. 2008. Pollinator-dependent crops: an increasingly risky business. Curr. Biol. 18, 968-969. ( 10.1016/j.cub.2008.09.010) [DOI] [PubMed] [Google Scholar]
- 80.Kron P, Husband BC. 2006. The effects of pollen diversity on plant reproduction: insights from apple. Sex. Plant Reprod. 19, 125-131. ( 10.1007/s00497-006-0028-2) [DOI] [Google Scholar]
- 81.Sáez A, Aguilar R, Ashworth L, Gleiser G, Morales CL, Traveset A, Aizen MA. 2022. Database from: Managed honeybees decrease pollination limitation in self-compatible but not in self-incompatible crops. Dryad Digital Repository. ( 10.5061/dryad.rfj6q57ct) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sáez A, Aguilar R, Ashworth L, Gleiser G, Morales CL, Traveset A, Aizen MA. 2022. Database from: Managed honeybees decrease pollination limitation in self-compatible but not in self-incompatible crops. Dryad Digital Repository. ( 10.5061/dryad.rfj6q57ct) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rfj6q57ct [81].