Abstract
Iron is the most abundant metal in the human body. No independent life forms on earth can survive without iron. However, excess iron is closely associated with carcinogenesis by increasing oxidative stress via its catalytic activity to generate hydroxyl radicals. Therefore, it is speculated that iron might play a dual role in cells, by both stimulating cell growth and causing cell death. Dietary iron is absorbed by the intestinal enterocytes in the form of ferrous ion which forms cLIP. Excess iron stored in the form of Ferritin serves as a reservoir under iron depletion conditions. Ferroptosis, is an iron-dependent non-mutational form of cell death process and is suppressed by iron-binding compounds such as deferoxamine. Blocking transferrin-mediated iron import or recycling of iron-containing storage proteins (i.e., ferritin) also attenuates ferroptosis, consistent with the iron-dependent nature of this process. Unsurprisingly, ferroptosis also plays a role in the development of cancer and maybe a beneficial strategy for anticancer treatment. Different lines of evidence suggest that ferroptosis plays a crucial role in the suppression of tumorigenesis. In this review, we have discussed the pros and cons of iron accumulation, utilization and, its role in cell proliferation, ferroptosis and pathophysiology of cancer.
Keywords: Iron, cancer, ferroptosis, reactive oxygen species, cytoplasmic labile iron pool (cLIP)
Introduction
Iron is one of the most requisite metal ions that regulates cellular metabolisms. The iron present in the body gets absorbed in the intestinal enterocytes and is utilised for various cellular processes such as nucleic acid synthesis, oxygen transportation, cellular respiration, enzyme activity, heme synthesis, detoxification, immune function and metabolism [1]. For instance, Ribonucleotide reductase is an iron containing enzyme that catalyses the synthesis [2]. The enzymes that require iron for their activities are listed in Table 1.
Table 1.
Types of proteins that require iron for their activity
| Iron containing proteins | Iron Proteins of the Mitochondrial Respiratory Chain and proteins involved in Oxygen-Dependent Reactions | Other Redox Enzymes Containing Iron |
|---|---|---|
| Haemoglobin | Proteins of Respiratory Chain | Ribonucleotide reductase |
| Cytochrome c | ||
| Myogloblin | Cytochrome oxidase | Xanthine oxidase |
| erythrocruorin | Ubiquinol: cytochrome-c reductase | Xanthine dehydrogenase |
| catalase | NADH: ubiquinone reductase | Aldehyde oxidase |
| cytochromes class B | Succinate: ubiquinone reductase | - |
| cytochromes class A | ETF dehydrogenase | - |
| Dihydro-orotate dehydrogenase | ||
| cytochromes class C | Oxygen-Dependent Reactions | - |
| Tryptophan oxygenase | ||
| cytochromes class D | Lipoxygenase | - |
| - | Cysteamine oxygenase | - |
| - | Phenylalanine hydroxylase | - |
| - | Tyrosine hydroxylase | - |
| - | Tryptophan hydroxylase | - |
| Fatty acid desaturase | ||
| Prolyl Oxygenase | ||
| Lysyl Oxygenase | ||
| Cysteine Oxygenase | ||
| Hydroxyanthranilate oxygenase | ||
| Indoleamine oxygenase |
Iron containing proteins can be classified as three main groups: i). Iron sulphur clusters, ii). Heme containing proteins, iii). Iron containing enzymes. Iron sulphur clusters are most predominantly found in the components of the mitochondrial electron transport chain. The most predominant heme containing proteins are haemoglobin and myoglobin which are majorly involved in oxygen transportation to various organs and cells via the blood stream. Hence these proteins are abundantly found in RBC [3].
Iron containing enzymes uses iron as co-factor to regulate its enzymatic activities for instance, mononuclear non-heme iron dependent (NHI) catalyses an array of chemical transformations which include hydroxylation, chlorination and epimerisation of organic substrates [4]. Iron also acts as a cofactor regulating the enzyme activity and acts as a major regulator of cell cycle by inhibiting the formation or activation of both cyclin and cyclin dependent kinases (CDKs). Lipoxygenase (LOX) requires ferrous ion as cofactor to regulate peroxidation of poly unsaturated fatty acids (PUFA) [5]. DNA polymerase α, δ & ε, DNA helicases, DNA primate regulation subunit Pri2 (PRIM-2) also requires iron as co factor [6].
Mechanism of iron absorption and its regulation
Cellular absorption of dietary iron
Dietary iron exists as organic heme iron in the form of ferrous ion and inorganic non heme iron in the form of ferric ion; Both are absorbed in the intestinal duodenal cells by divergent mechanisms.
Absorption of heme iron
Heme iron degraded in stomach and duodenum is transported to the cytoplasm of the enterocyte by heme carrier protein 1 (HCP1) and heme responsive gene (HCP1) importers [7].
Absorption of non-heme iron
Iron importers have high affinity towards ferrous ion and hence the inorganic non-heme iron is reduced to ferrous iron before it enters into enterocyte mediated by a brush border membrane ferrireductase enzyme called DcytB (duodenal cytochrome B). The reduced ferrous ion is then transported into the cell via an importer called Divalent metal ion transporter-1 (DMT-1) [8]. Once the ferrous ion enters the enterocyte these ions take hold of, other ferrous ions present in the cytosol to form a low molecular weight pool of cellular iron called the cytoplasmic labile iron pool (cLIP) [9]. This cLIP plays an indispensable role by mediating the biochemical events which require iron [10]. Iron that entered cLIP binds to the cytotoxic iron chaperons termed PCBP-1 and PCBP-2 (poly-(rc)-binding protein). These chaperons further modulate the delivery of the ferrous iron to their destinations [9]. Reduced ferrous ion can generate soluble radicals, lipid alkoxy, highly reactive oxygen species (ROS) or hydroxide ions via Fenton pathway which may lead to cellular damage. Hence the ferrous present in cLIP would be rapidly exported to various cellular organelles to meet the to meet the physiological requirements of the cell [9,11] (Figure 1).
Figure 1.
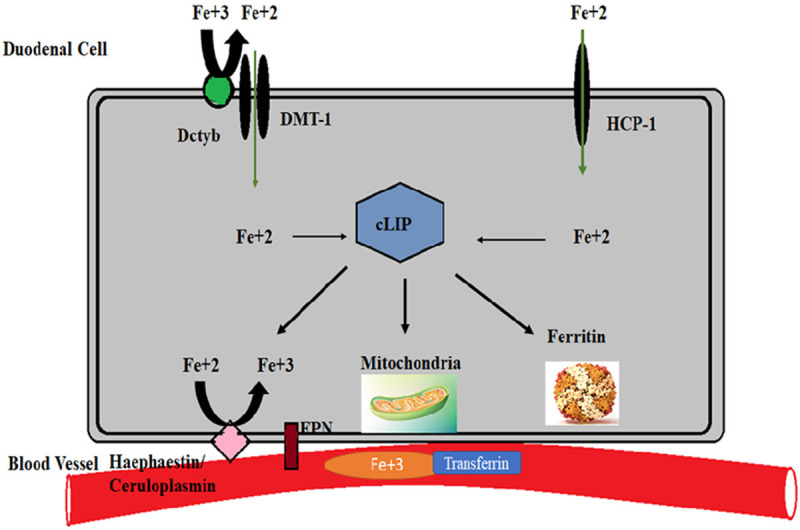
Iron import, regulation and export. Organic and inorganic iron enters into the intestinal duodenal cell through their respective ion channels in ferrous form. In the cytoplasm the ferrous ions gather together to form an iron pool called the cytoplasmic labile pool (cLIP). Depending upon the requirement cLIP will be utilized by different organelles and the extra ferrous iron is stored in ferritin. Ferrous iron cannot be transported and hence it should be reduced to ferric iron which can be transported into the circulation and is reached to other cells through a ferric ion exporter.
Importance of consuming iron through diet
S studies revealed that, intake of carotenoids lycopene, lutein and zeaxanthin improved iron absorption from a meal. Spinach (Spinacia oleracea L) a member of the family Amaranthaceae is a dark leafy vegetable rich in vitamins, minerals, phytochemicals and bioactive ingredients. 100 g serving of spinach provides high levels of magnesium, potassium and iron that meet 20%, 16% and 15% respectively of their recommended dietary allowance (RDA) respectively [12]. Epidemiological studies demonstrated that spinach consumption can combat breast, colon and esophageal cancers. In a case control study, females with higher intake of raw spinach (>52 servings/year) had a 45% decreased risk of breast cancer suggesting that the carotenoid lutein specifically associated with decreased risk of breast cancer suggesting that spinach has anti-cancer properties and inhibit cancer cell proliferation by increasing iron absorption [13].
Regulation of iron metabolism via cLIP in cancer cells
The amount of cLIP is sensed by iron regulatory RNA binding proteins 1 and 2 (IRP-aconitase-1 & IRP2-iron responsive element binding protein-2). These IRPs bind to the iron responsive element in the UTRs of TfR and DMT-1 which are the substantial proteins responsible for cLIP formation [14]. When cells have abundant amount of iron, in due course the cells activate TfR and DMT-1. During iron inadequacy, IRP binds to the IRE in the 3’UTR [15]. Binding stabilises the mRNA and license the translation of TfR and DMT1 which escort the accumulation of ferrous ions in cLIP [16]. When cells have profuse amount of iron then the IRP binds to IRE at the 5’UTR. Binding halts the translation thereby destabilising the mRNA [15] thereby preventing iron overload leading to non-expression of TfR and DMT1 genes [16].
Fate of iron absorbed by the enterocytes
Iron is the most required ion required to regulate cellular metabolism. Dietary iron absorbed in the intestinal epithelial cells will undergo the following fates. (1). Mitoferrin is an iron transporter protein that imports iron from the cytoplasm of the cells [17]. (2). Ferrous ion present in cLIP enters blood circulation so that it can be transported to various cell organelles. The absorbed iron is further transported to different cell organelles through an iron exporter called ferroportin (FPN).
The ferrous ion prior to its export by FPN is oxidised by the enzymes Hephaestin or ceruloplasmin. FPN is the cellular iron exporter encoded by SLS40A-1 [18], located on the basolateral membrane of intestinal enterocytes [19]. The oxidised ferric ion that is exported binds to transferrin, a protein of β-globulin gene family that transports circulating ferric ions to other cell organelles. In blood, transferrin protein exists in two forms Holo-Tf (free from ferric ion binding) and Apo-Tf (Tf associated with ferric ion). Transferrin receptor designated as TfR is a cell surface receptor which is present on almost all cells recognises holo Tf, recruit the Tf bound iron and engulfs the iron through receptor mediated endocytosis [20].
Ferric ions that are endocytosed by Tfr are reduced by endosomal six-transmembrane epithelial antigen of prostate-3 (STEAP-3) to ferrous iron and enters into cLIP of respective cells through DMT-1 protein [5,21]. If circulating iron levels exceed the levels of transferrin present in the blood circulation, then there is a chance of accumulation of non Tf bound iron which may lead to various health disorders [21].
Internalisation of iron by other cells takes place only when the iron is exported by FPN. FPN mediated iron transport is tightly regulated by Hepcidin (Hcp) encoded by HAMP gene predominantly expressed is hepatocytes. Hcp inhibits cellular export of iron by binding the FPN [22]. The excess ferrous ion is stored in ferritin, an iron storage molecule that can store about 4500 ferrous ions and maintains balance when cell undergoes iron depletion [23]. Ferritin is a 24 sub-unit protein having heavy and light chain ferritin types [22]. Under iron depletion conditions, stored iron in ferritin is exported through FPN by the same mechanism.
Role of iron in the metabolism of oxygen
Genes that regulate the expression of iron metabolism play a crucial role in regulating the expression of genes required for oxygen metabolism. For instance, the genes HIF1 and HIF2 which are expressed under oxidative stress usually regulate hypoxia under low iron content [24]. Prolyl hydroxylases are the class of enzymes that inactivate HIF genes by hydroxylation of proline residues in HIF, resulting in recognition of HIF by ubiquitin ligases leading to HIF ubiquitination and degradation by proteasomes [25-28]. For catalysing hydroxylation reactions, the enzyme requires iron as a co-factor and hence iron scarcity results in activation of HIF genes apart from hypoxic conditions [29]. This implicates that when hypoxic conditions prevail in the cell prolyl hydroxylase is inhibited, because in hypoxia HIF genes are to be expressed to regulate angiogenesis [30], erythropoiesis, energy metabolism and cell motility [31]. In cancer cells this enzyme is suppressed resulting in continuous expression of HIF gene which mediates angiogenesis and other cellular functions [32].
Role of iron in the metabolism and pathophysiology of cancer cells
Iron metabolism and cancer
Malignant cellular phenotype is often found in association with dysregulated iron homeostasis [33], resulting in decreased RBC count [34] and anaemia [35]. Iron overload in cell promotes initiation and progression of tumours by generation of ROS [36]. Low concentration of ROS can contribute to cellular functions such as cell survival, differentiation or its death by targeting kinases and phosphatases [37] and can also induce HIF-α during hypoxic conditions. Similarly, low concentrations of ROS can also recruit other platelets at the site of injury [38]. Circulating tumour cells are encased in thrombus, protecting them from NK cells, the platelets present in them play a crucial role by providing shear forces [39]. In circulating tumour cells platelets play a crucial role by providing shear forces. Excess ROS concentration in the cell leads to oxidative stress eventually damaging the DNA [37], which when coupled with other mutations that disrupt the regulation of cell cycle checkpoints might lead to carcinogenesis.
Role of iron in cancer cell proliferation
Tumour cells have a greater metabolic demand for iron than normal cells [20]. Surplus amount of iron is required for tumour survival, proliferation and to regulate metastasis [40]. Iron in the tumour cells plays a key role in the remodelling of extra cellular matrix thereby increasing the motility of cancer cells promoting metastasis. Tumour cells avidly binds iron and enhances the expression of TfR that not only enhances iron uptake but also incites tumour cell survival [41]. Inhibition of FPN in various cancer cells enhanced availability of iron [9,42] proving that cancer cells require an abundant amount of iron for their survival [10]. Iron can also generate ROS via Fenton pathway. Tumour cells have higher basal ROS than normal cells [42] which confer growth to the tumour cells by facilitating mitogenic signalling via activation of several stress kinase pathways [43].
Regulation of iron metabolism in cancer
Expression of genes regulating iron metabolism in tumours, is a strong and independent predictor of prognosis. Ferritin down regulation is mediated by oncogene myc in B cells, adenovirus E1A and Ras genes [36]. Concomitant up regulation of iron import and down regulation of iron export and its storage results in higher iron availability and consequent faster growth in several types of tumour cells [10]. Interestingly, cancer stem cells have high levels of ferritin which indicates that these cells store a lot of iron than the tumour cells. Iron brings forth stemness in cancer stem cells and is responsible for higher malignant potential that makes these cells therapy resistance, priming the tumour cells for tumour recurrence [11].
Cancer cells are known to adapt a few alternate pathways to maintain cellular iron balance. In NSCLS (non-small cell lung carcinoma cells) EGFR, growth factor that regulates growth, survival, proliferation and differentiation in mammalian cells has been demonstrated to affect iron metabolism by directly binding and redistributing TfR-1 [41,44]. Inactivation of EGFR resulted in reduced TfR1 levels on the cellular surface resulting in decreased import of iron and cell cycle arrest [45]. FPN over expression induces autophagy and activates p53 along with its target p21 leading to cell cycle arrest and stress induced damage in prostate cancer [46]. FPN suppression can be correlated with increased amounts of hepcidin in tumour cells and is shown to be dramatically repressed in various cancer. Hepcidin contributes to cancer cell proliferation and progression by binding to FPN present on the membrane of iron exporting cells, which induces endocytosis and proteolysis of FPN thereby, inhibiting FPN expression and protecting the cancer cells by accumulating more iron reserves [47]. Histone deacetylase-1 (HDAC1) is associated with hepcidin suppression by binding to SMAD4 which of STEAP-3 family. This enzyme has also been reported to induce EMT (epithelial mesenchymal transition) in cancer cells by activating STAT-3-fox M1 axis, leading to upregulation in TfR1 expression and a surge in intracellular iron content [48].
Apart from STEAP3, cancer cells also activate various proteins that belong to STEAP family depending on their requirement. For instance, STEAP1 & STEAP2 are usually expressed in various cancers while STEAP4 is activated under hypoxic conditions and is responsible for mitochondrial iron imbalance and enhanced ROS production [49]. It is shown to enhance the incidence of colitis associated colon cancer in mouse models [50]. Pharmacological inhibition of DMT1 halts colon tumour growth by suppressing JAK-STAT3 signalling pathway [51]. Studies revealed that increased mitochondrial iron accumulation lead to accumulation of mitochondrial NEET proteins NAF1 (CISD2) and mito NEET (CISD1) which play a critical role in cancer cell proliferation and metastasis. NEET proteins belong to a novel iron sulphur cluster family that regulates iron and redox homoeostasis [52]. Tumour cells alter iron metabolism by up-regulating the expression of genes responsible for elevating intra cellular iron content (TfR-1, DMT-1 and Hepcidin) while down regulating the gene expression of the proteins that are involved in exporting the iron content (FPN, hephaestin and ceruloplasmin) from the cell [31]. These studies suggest that regulation of iron metabolism related gene expression is consequential for a cancer cell to survive the harsh conditions.
Role of iron in the pathophysiology of cancer
Research has demonstrated the abnormality of iron homeostasis in several cancers including breast, ovarian, renal and lung cancers [9]. Cancer cells undergo malignancy by enhancing DNA synthesis and proliferation there by leading to increase in cell number. Although ferroptosis is an iron dependent cell death and cancer cells as well as cancer stem cells has bulk amount of iron these cells are protected from ferroptosis [53], because the iron present in these cells is utilized in various ways to promote cancer cell proliferation. One of the examples is, iron the prerequisite metal for the enzyme ribonucleotide reductase, an enzyme involved in DNA synthesis. Iron is very essential in proliferation of cell cycle particularly in late G1 and S-phase [54,55]. Iron enhances cyclin D1 protein expression, one of the cell cycle check-point that catalyses G1/S progression [55]. The mechanism through which iron enhances Cyclin D1 is not known yet. Studies proved that increased Cyclin D1 that assembles with cdk4 or cdk6 to promote cells from G1 to S phase plays a major role in tumorigenesis [56,57]. In their studies they showed that iron depletion via iron chelators inhibited tumor growth by targeting cyclin D1 through ubiquitin-independent proteasomal degradation [58]. Prominin2 a membrane glycoprotein that is involved in the organization of plasma membrane microdomains inhibits ferroptosis by promoting the formation of ferritin-containing multivesicular bodies in response to GPX4 inhibition and exosome formation which transport iron out of the cell there by impeding ferroptosis. Cells that cannot induce prominin2 and export ferritin are therefore highly sensitive to ferroptosis [59].
Studies also proved that patients with iron storage diseases are more prone to cancer [60]. For instance, hemochromatosis, a prototypical disease of excessive iron that arises due to the mutations in various genes involved in cellular iron uptake are at increased risk in attaining cancer including liver, colon, rectal, prostate and even breast cancers [61]. Another iron overload disease homozygous beta thalassemia is associated with increases risk of hepatocellular carcinoma [62]. To understand the relationship between cancer risk and iron levels in the body investigators examined cohorts of individuals undergoing blood donation or blood transfusion. Results showed that patients who has undergone blood donation were associated with less cancer risk [63,64] and those who underwent blood transfusion were more prone to cancer [65]. This is because blood containing haemoglobin an iron containing protein is reduced when patient donates blood while is elevated in person who is taking blood from another person [60].
Furthermore, a mutlicenter, randomized controlled study in patients with advanced peripheral arterial diseases showed that iron depletion by phelebotomy lowered cancer risk and mortality by 37% at a 5 years follow-up suggestion that increased iron levels in body promotes cancer risk [64].
Ferroptosis
Iron as a promising target in cancer therapy
Ferroptosis is a newly emerging method that targets tumour cell growth. It can be defined as non-apoptotic, caspase independent cell death. The process is dependent on iron concentration. Since cancer stem cells have bulk iron stored in ferritin when compared to both cancer cells as well as normal cells, ferroptosis can target the death of cancer stem cells which are solely responsible for chemotherapeutic resistance and cancer relapse [66]. This is because cancer stem cells will be present in G0-phase of the cell cycle and will not respond to primary therapy [67]. Iron chelators target and reduce the intracellular iron content by purging the metal iron outside the cell. This might result in hypo phosphorylation of pRb, decreased expression of cyclin, p21 and C-myc, leading to cell cycle arrest at G1/S cell phase [68]. Iron chelators selectively induce apoptosis in cancer cells in vitro and reduced tumour size in vivo. These chelators inhibit the enzyme ribonucleotide reductase which catalyses the rate limiting step of DNA synthesis and also increases the cytotoxicity in tumour cells by increasing ROS levels thereby resulting in caspase independent cell death. Iron chelators can be classified into two types: Redox active iron chelators and Redox inactive iron chelators. While redox active iron chelators generate ROS by allowing iron to interact with oxygen and hydrogen peroxide, the redox inactive chelators prevent iron from being exposed to oxygen and hydrogen peroxide thereby preventing the production of ROS [69].
Another approach that targets the growth of cancer stem cells is ferritinophagy which refers to autophagic degradation of ferritin protein. Nuclear receptor co activator-4 (NCOA4) is an autophagic cargo receptor that binds to ferritin heavy chain in autophagosome and delivers it for degradation in the lysosome there by reducing ferritin turnover [70,71]. Usually if a cell undergoes iron scarcity, an iron regulated metastasis suppressor gene called N-myc downstream regulated genes-1&2 (NDRG-1&2) are upregulated leading to tumor suppression. Both NDRG1 and NDRG2 are shown to decrease metastasis in breast, prostate, pancreas and colon cancers [70]. Accumulating evidence has shown that CD133, a stem cell marker which is used to identify tumour initiating cells in a wide number of human cancers is capable of inhibiting intra cellular uptake of iron by interacting with TfR1 gene and implicating in its endocytosis [72].
Mechanism of ferroptosis
Lysophosphotidtyl choline acyltransferase 3 (LPCAT-3) which belongs to the membrane bound O-acyltransferase family [73] incorporates Arachidonic acid and Adrenic acid into lipid bilayer [74]. These lipid molecules are then esterified by the enzyme Acetyl Co-A synthesis long chain family member-4 (ACSL-4), which further undergoes β-oxidation of poly unsaturated fatty acids (PUFA) biosynthesis [75-78]. The PUFAs are highly sensitive to peroxidation due to the presence of highly reactive hydrogen atoms that are generated by Fenton reaction. The enzyme lipoxygenase then catalyses the peroxidation of PUFA resulting in formation of lipid peroxides thereby triggering cell death. Lipoxygenase requires ferrous ion as co-factor which comes from cLIP [66].
Mechanism of ferroptosis inhibition in cancer cells
Cancer cells circumvent ferroptosis by elevating glutathione peroxidase-4 enzyme (GPX-4) levels. GPX-4 reduces lipid peroxides to lipid alcohols in presence of Glutathione (GSH) by serving as an electron donor, thereby limiting iron dependent formation of lipid alkoxy radicals from lipid peroxides generated by the ferroptosis machinery [79,80]. GSH is a cellular non protein tripeptide anti-oxidant consisting of glycine, glutamate and cysteine amino acids [81]. To prevent ferroptosis the cancer cells augment the production of GSH thereby elevating GPX4 enzyme activity. The GSH synthesis is dependent on cysteine/glutamate levels inside the cell which is mediated by sodium dependent antiporter called xCT system encoded by SLC7A11 gene. This antiporter imports one cystine (CySS) molecule in exchange for a glutamate molecule [9] (Figure 2). When GSH levels exhaust, the cancer cells activate ferroptosis suppressor protein-1 (FSP1), formerly called Apoptosis-inducing factor mitochondria associated-2 (AIFM2) to the plasma membrane by N-terminal myristylation. FSP1 reduces ubiquinone to ubiquinol, a lipid radical scavenger that reduces the accumulation of lipid free radicals within the membrane [82].
Figure 2.
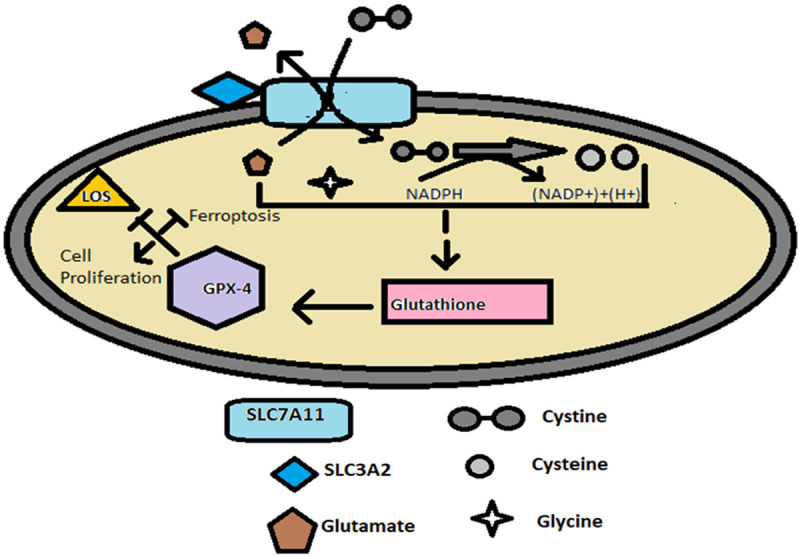
Mechanism of Ferroptosis Inhibition. SLC7A11 gene is a sodium-dependent antiporter responsible for Glutathione synthesis. The gene encodes a xCT system which imports one Cystine molecule by simultaneous export of Glutamate molecule. The transporter activity of SLC7A11 gene is stabilised by a chaperon encoded by SLC3A2 gene. Once cystine enter into the cytoplasm of the cell it is reduced to cysteine and contributes to the formation of Glutathione with Glycine and Glutamate. The Glutathione serves as a co-factor for the enzyme GPX-4 which inhibits the formation of lipid peroxides there by protecting the cells from ferroptosis and permitting cell proliferation.
Discussion and future prospects
Dysregulated ferroptosis is implicated in multiple physiological and pathological processes such as cancer cell death, tissue injury, and T-cell immunity. Also, ferroptosis plays an important role in sterile inflammatory conditions such as tissue acute injury, ischemic reperfusion injury, and neurotoxicity. Several epidemiological studies have suggested an association between endometriosis and ovarian cancer, demonstrating a high risk of ovarian cancer in women with a long-standing history (>10 years) of ovarian endometriosis. Toyokuni et al., have reported that ovarian endometriotic cysts are rich in catalytic iron, leading to increased oxidative DNA damage of the epithelia of those cysts which might culminate into clear cell adenocarcinomas of the ovary [83].
Similarly, hepatic iron is increased significantly in patients with chronic viral hepatitis due to persistent damage of hepatocytes leading to increased iron absorption and deposition eventually resulting in hepatocellular carcinoma [84]. In another interesting study, it has been demonstrated that local iron overload, endogenous or accumulated by other mechanisms, is important for asbestos-induced carcinogenesis since asbestos fibres contain iron that catalyses free radical generation responsible for lung cancer [83,85].
More studies about ferroptosis, and iron-induced carcinogenesis in cancer and injury-associated diseases might create a new opportunity for diagnosis and therapeutic interventions for not cancer but for other iron overload-induced human pathological conditions like hemochromatosis induced hepatocellular carcinoma, chronic viral hepatitis B and C induced hepatocellular carcinoma, asbestos exposure induced malignant mesothelioma and lung carcinoma and ovarian endometriosis induced Ovarian carcinoma. Considering the recent report by Kato.J. et al., that iron reduction by phlebotomy decreased cancer risk in a supposedly normal population [86], the present review would be highly useful in designing efficient cancer prevention and therapeutic strategies.
Conclusion
Iron is one of the most essential nutrients that regulate cellular growth, development, differentiation as well as metabolism. Iron also plays an important role in regulating cell death during stress conditions by a process called ferroptosis. Cancer cells which are subjected to high stressful conditions do require iron to regulate cellular growth and metabolism, but these cells abscond ferroptosis by inhibiting lipid peroxidation through GPX-4. Cancer cells, in order to enhance the antioxidant defence mechanisms, upregulate SLC7A11 gene leading to elevated GSH levels. Over expression of SLC7A11 gene is mainly induced by two transcription factors. They are Activating Transcription Factor-4 (ATF-4) and Nuclear factor-2 related factor (Nrf-2). On the contrary, Tumour Protein 53 (TP53) and Activating Transcription Factor-3 (ATF-3) have been shown previously to suppress SLC7A11 gene in normal cells. These studies suggest that either limiting the availability of iron to the cancer cells or decreasing GSH accumulation might be helpful for cancer therapy.
Acknowledgements
SLP gratefully acknowledges DBT (BT/PR30629/BIC/101/1093/2018), New Delhi; UGC (Ref No: No.F.30-456/2018 (BSR)) and SERB (Ref No: PDF/2015/000867) for the financial support. PC gratefully acknowledges DBT (BT/PR30629/BIC/101/1093/2018), New Delhi for the Junior research fellowship.
Disclosure of conflict of interest
None.
References
- 1.Kerins MJ, Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal. 2018;29:1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan J, Ward DM. The essential nature of iron usage and regulation. Curr Biol. 2013;23:R642–R646. doi: 10.1016/j.cub.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta. 2012;1823:1468–1483. doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peck SC, van der Donk WA. Go it alone: four-electron oxidations by mononuclear non-heme iron enzymes. J Biol Inorg Chem. 2017;22:381–394. doi: 10.1007/s00775-016-1399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bebber CM, Müller F, Prieto Clemente L, Weber J, von Karstedt S. Ferroptosis in cancer cell biology. Cancers (Basel) 2020;12:164. doi: 10.3390/cancers12010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C. Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell. 2014;5:750–760. doi: 10.1007/s13238-014-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Fan Z, Yang Y, Gu C. Iron metabolism and its contribution to cancer. Int J Oncol. 2019;54:1143–1154. doi: 10.3892/ijo.2019.4720. [DOI] [PubMed] [Google Scholar]
- 8.Ascenzi P, Leboffe L, Polticelli F. Cyanide binding to human plasma heme-hemopexin: a comparative study. Biochem Biophys Res Commun. 2012;428:239–244. doi: 10.1016/j.bbrc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 9.El Hout M, Dos Santos L, Hamaï A, Mehrpour M. A promising new approach to cancer therapy: targeting iron metabolism in cancer stem cells. Semin Cancer Biol. 2018;53:125–138. doi: 10.1016/j.semcancer.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Recalcati S, Gammella E, Cairo G. Dysregulation of iron metabolism in cancer stem cells. Free Radic Biol Med. 2019;133:216–220. doi: 10.1016/j.freeradbiomed.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts JL, Moreau R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016;7:3337–3353. doi: 10.1039/c6fo00051g. [DOI] [PubMed] [Google Scholar]
- 13.Longnecker MP, Newcomb PA, Mittendorf R, Greenberg ER, Willett WC. Intake of carrots, spinach, and supplements containing vitamin A in relation to risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:887–892. [PubMed] [Google Scholar]
- 14.Jung M, Mertens C, Tomat E, Brüne B. Iron as a central player and promising target in cancer progression. Int J Mol Sci. 2019;20:273. doi: 10.3390/ijms20020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 16.McLaren GD, Nathanson MH, Jacobs A, Trevett D, Thomson W. Regulation of intestinal iron absorption and mucosal iron kinetics in hereditary hemochromatosis. J Lab Clin Med. 1991;117:390–401. [PubMed] [Google Scholar]
- 17.Lill R, Mühlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Torres C, Layrisse M. Iron absorption from veal muscle. Am J Clin Nutr. 1971;24:531–540. doi: 10.1093/ajcn/24.5.531. [DOI] [PubMed] [Google Scholar]
- 19.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Kovacevic Z, Richardson DR. Tuning cell cycle regulation with an iron key. Cell Cycle. 2007;6:1982–1994. doi: 10.4161/cc.6.16.4603. [DOI] [PubMed] [Google Scholar]
- 21.Frazer DM, Anderson GJ. The regulation of iron transport. Biofactors. 2014;40:206–214. doi: 10.1002/biof.1148. [DOI] [PubMed] [Google Scholar]
- 22.Qiao B, Sugianto P, Fung E, del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T, Nemeth E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15:918–924. doi: 10.1016/j.cmet.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourseau-Guilmain E, Griveau A, Benoit JP, Garcion E. The importance of the stem cell marker prominin-1/CD133 in the uptake of transferrin and in iron metabolism in human colon cancer Caco-2 cells. PLoS One. 2011;6:e25515. doi: 10.1371/journal.pone.0025515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallah J, Rini BI. HIF inhibitors: status of current clinical development. Curr Oncol Rep. 2019;21:6. doi: 10.1007/s11912-019-0752-z. [DOI] [PubMed] [Google Scholar]
- 25.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 27.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 28.Scheuermann TH, Li Q, Ma HW, Key J, Zhang L, Chen R, Garcia JA, Naidoo J, Longgood J, Frantz DE. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. 2013;9:271–276. doi: 10.1038/nchembio.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver DJ. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 30.Coffman LG, Parsonage D, D’Agostino R, Torti FM, Torti SV. Regulatory effects of ferritin on angiogenesis. Proc Natl Acad Sci U S A. 2009;106:570–575. doi: 10.1073/pnas.0812010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasricha SR, Lim PJ, Duarte TL, Casu C, Oosterhuis D, Mleczko-Sanecka K, Suciu M, Da Silva AR, Al-Hourani K, Arezes J. Hepcidin is regulated by promoter-associated histone acetylation and HDAC3. Nat Commun. 2017;8:1–15. doi: 10.1038/s41467-017-00500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. A review. Curr Mol Med. 2018;18:343–351. doi: 10.2174/1566524018666181109121849. [DOI] [PubMed] [Google Scholar]
- 33.Kwok JC, Richardson DR. The iron metabolism of neoplastic cells: alterations that facilitate proliferation? Crit Rev Oncol Hematol. 2002;42:65–78. doi: 10.1016/s1040-8428(01)00213-x. [DOI] [PubMed] [Google Scholar]
- 34.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–393. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludwig H, Evstatiev R, Kornek G, Aapro M, Bauernhofer T, Buxhofer-Ausch V, Fridrik M, Geissler D, Geissler K, Gisslinger H. Iron metabolism and iron supplementation in cancer patients. Wien Klin Wochenschr. 2015;127:907–919. doi: 10.1007/s00508-015-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakhlon O, Gruenbaum Y, Cabantchik ZI. Ferritin expression modulates cell cycle dynamics and cell responsiveness to H-ras-induced growth via expansion of the labile iron pool. Biochem J. 2002;363:431. doi: 10.1042/0264-6021:3630431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bystrom LM, Guzman ML, Rivella S. Iron and reactive oxygen species: friends or foes of cancer cells? Antioxid Redox Signal. 2014;20:1917–1924. doi: 10.1089/ars.2012.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krötz F, Sohn HY, Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler Thromb Vasc Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 39.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11:1–15. doi: 10.1186/s13045-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauckman K, Haller E, Taran N, Rockfield S, Ruiz-Rivera A, Nanjundan M. Iron alters cell survival in a mitochondria-dependent pathway in ovarian cancer cells. Biochem J. 2015;466:401–413. doi: 10.1042/BJ20140878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jian J, Yang Q, Huang X. Src regulates Tyr20 phosphorylation of transferrin receptor-1 and potentiates breast cancer cell survival. J Biol Chem. 2011;286:35708–35715. doi: 10.1074/jbc.M111.271585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang B, Zhang J, Song F, Tian M, Shi B, Jiang H, Xu W, Wang H, Zhou M, Pan X. EGFR regulates iron homeostasis to promote cancer growth through redistribution of transferrin receptor 1. Cancer Lett. 2016;381:331–340. doi: 10.1016/j.canlet.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Yin X, Wu Q, Monga J, Xie E, Wang H, Wang S, Zhang H, Wang ZY, Zhou T, Shi Y. HDAC1 governs iron homeostasis independent of histone deacetylation in iron-overload murine models. Antioxid Redox Signal. 2018;28:1224–1237. doi: 10.1089/ars.2017.7161. [DOI] [PubMed] [Google Scholar]
- 46.Deng Z, Manz DH, Torti SV, Torti FM. Effects of ferroportin-mediated iron depletion in cells representative of different histological subtypes of prostate cancer. Antioxid Redox Signal. 2019;30:1043–1061. doi: 10.1089/ars.2017.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu XN, Su D, Wang L, Yu FL. Roles of the hepcidin-ferroportin axis and iron in cancer. Eur J Cancer Prev. 2014;23:122–133. doi: 10.1097/CEJ.0b013e3283627f14. [DOI] [PubMed] [Google Scholar]
- 48.Han M, Xu R, Wang S, Yang N, Ni S, Zhang Q, Xu Y, Zhang X, Zhang C, Wei Y. Six-transmembrane epithelial antigen of prostate 3 predicts poor prognosis and promotes glioblastoma growth and invasion. Neoplasia. 2018;20:543–554. doi: 10.1016/j.neo.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder CM, Chandel NS. Mitochondrial regulation of cell survival and death during low-oxygen conditions. Antioxid Redox Signal. 2009;11:2673–2683. doi: 10.1089/ars.2009.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C, Zhang F. Iron homeostasis and tumorigenesis: molecular mechanisms and therapeutic opportunities. Protein Cell. 2015;6:88–100. doi: 10.1007/s13238-014-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, Ismail T, Berx G, McKie AT, Hotchin N, Anderson GJ. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut. 2006;55:1449–1460. doi: 10.1136/gut.2006.094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipper CH, Karmi O, Sohn YS, Darash-Yahana M, Lammert H, Song L, Liu A, Mittler R, Nechushtai R, Onuchic JN. Structure of the human monomeric NEET protein MiNT and its role in regulating iron and reactive oxygen species in cancer cells. Proc Natl Acad Sci U S A. 2018;115:272–277. doi: 10.1073/pnas.1715842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spangler B, Morgan CW, Fontaine SD, Vander Wal MN, Chang CJ, Wells JA, Renslo AR. A reactivity-based probe of the intracellular labile ferrous iron pool. Nat Chem Biol. 2016;12:680–685. doi: 10.1038/nchembio.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- 55.Green D, Antholine W, Wong S, Richardson D, Chitambar C. Ribonucleotide reductase as a preferential target for the inhibition of leukemic cell growth by 311, a novel iron chelator of the pyridoxal isonicotinoyl hydrazone class. Clin Cancer Res. 2001;7:3574–3579. [PubMed] [Google Scholar]
- 56.Le NT, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim Biophys Acta. 2002;1603:31–46. doi: 10.1016/s0304-419x(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 57.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 58.Nurtjahja-Tjendraputra E, Fu D, Phang JM, Richardson DR. Iron chelation regulates cyclin D1 expression via the proteasome: a link to iron deficiency-mediated growth suppression. Blood. 2007;109:4045–4054. doi: 10.1182/blood-2006-10-047753. [DOI] [PubMed] [Google Scholar]
- 59.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, Dixon SJ, Mercurio AM. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell. 2019;51:575–586. e4. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and cancer. Annu Rev Nutr. 2018;38:97–125. doi: 10.1146/annurev-nutr-082117-051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elliott R, Stjernholm R, Elliott MJ. Preliminary evaluation of platinum transferrin (MPTC-63) as a potential nontoxic treatment for breast cancer. Cancer Detect Prev. 1988;12:469–480. [PubMed] [Google Scholar]
- 62.Musallam KM, Cappellini MD, Wood JC, Taher AT. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev. 2012;26:S16–S19. doi: 10.1016/S0268-960X(12)70006-1. [DOI] [PubMed] [Google Scholar]
- 63.Merk K, Mattsson B, Mattsson A, Holm G, Gullbring B, Björkholm M. The incidence of cancer among blood donors. Int J Epidemiol. 1990;19:505–509. doi: 10.1093/ije/19.3.505. [DOI] [PubMed] [Google Scholar]
- 64.Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, Lavori PW. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002. doi: 10.1093/jnci/djn209. [DOI] [PubMed] [Google Scholar]
- 65.Hjalgrim H, Edgren G, Rostgaard K, Reilly M, Tran TN, Titlestad KE, Shanwell A, Jersild C, Adami J, Wikman A. Cancer incidence in blood transfusion recipients. J Natl Cancer Inst. 2007;99:1864–1874. doi: 10.1093/jnci/djm248. [DOI] [PubMed] [Google Scholar]
- 66.Nakayama K, Kataoka N. Regulation of gene expression under hypoxic conditions. Int J Mol Sci. 2019;20:3278. doi: 10.3390/ijms20133278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pandrangi SL, Chikati R, Chauhan PS, Kumar CS, Banarji A, Saxena S. Effects of ellipticine on ALDH1A1-expressing breast cancer stem cells-an in vitro and in silico study. Tumour Biol. 2014;35:723–737. doi: 10.1007/s13277-013-1099-y. [DOI] [PubMed] [Google Scholar]
- 68.Tang M, Chen Z, Wu D, Chen L. Ferritinophagy/ferroptosis: iron-related newcomers in human diseases. J Cell Physiol. 2018;233:9179–9190. doi: 10.1002/jcp.26954. [DOI] [PubMed] [Google Scholar]
- 69.Chaston TB, Watts RN, Yuan J, Richardson DR. Potent antitumor activity of novel iron chelators derived from di-2-pyridylketone isonicotinoyl hydrazone involves fenton-derived free radical generation. Clin Cancer Res. 2004;10:7365–7374. doi: 10.1158/1078-0432.CCR-04-0865. [DOI] [PubMed] [Google Scholar]
- 70.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 72.Ryu MS, Duck KA, Philpott CC. Ferritin iron regulators, PCBP1 and NCOA4, respond to cellular iron status in developing red cells. Blood Cells Mol Dis. 2018;69:75–81. doi: 10.1016/j.bcmd.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Yu L, Ding J, Chen Y. Iron metabolism in cancer. Int J Mol Sci. 2019;20:95. doi: 10.3390/ijms20010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Chen YQ, Bonacci TM, Bredt DS, Li S, Bensch WR, Moller DE, Kowala M, Konrad RJ, Cao G. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J Biol Chem. 2008;283:8258–8265. doi: 10.1074/jbc.M710422200. [DOI] [PubMed] [Google Scholar]
- 75.Pérez-Chacón G, Astudillo AM, Ruipérez V, Balboa MA, Balsinde J. Signaling role for lysophosphatidylcholine acyltransferase 3 in receptor-regulated arachidonic acid reacylation reactions in human monocytes. J Immunol. 2010;184:1071–1078. doi: 10.4049/jimmunol.0902257. [DOI] [PubMed] [Google Scholar]
- 76.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, Stockwell BR. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 78.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 81.Stoyanovsky D, Tyurina Y, Shrivastava I, Bahar I, Tyurin V, Protchenko O, Jadhav S, Bolevich S, Kozlov A, Vladimirov Y. Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic Biol Med. 2019;133:153–161. doi: 10.1016/j.freeradbiomed.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9–16. doi: 10.1111/j.1349-7006.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Georgopoulou U, Dimitriadis A, Foka P, Karamichali E, Mamalaki A. Hepcidin and the iron enigma in HCV infection. Virulence. 2014;5:465–476. doi: 10.4161/viru.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toyokuni S. Iron overload as a major targetable pathogenesis of asbestos-induced mesothelial carcinogenesis. Redox Rep. 2014;19:1–7. doi: 10.1179/1351000213Y.0000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]


