Abstract
Helicobacter pylori (H. pylori) infection is the most important risk factor for gastric cancer and plays an initiating role in the development of intestinal-type gastric cancer. Eradication of H. pylori significantly reduces the incidence and mortality of gastric cancer. International expert consensus recommends eradication treatment for all infected individuals unless competing considerations. However, large-scale H. pylori eradication treatments have led to increasing rates of resistance to multiple antibiotics, together with factors such as coccoid transformation, host CYP2C19 gene polymorphisms, and inappropriate treatment regimens, resulting in a gradual decline in H. pylori eradication rates. Currently, empirical and repeated eradication of H. pylori treatment is common in clinical practice, which will certainly lead to a further increase in antibiotic resistance, resulting in a great waste of medical resources and an increased psychological burden on patients and their relatives. Therefore, successful eradication of H. pylori on initial treatment should be given high priority, and the implementation of personalized treatment is essential.
Keywords: Helicobacter pylori, personalized treatment, eradication on initial treatment
Introduction
Helicobacter pylori (H. pylori) infection is the most important risk factor for gastric cancer with a prevalence of up to 50% in the world population [1]. The pathogenesis of most intestinal-type gastric cancers follows the Correa pattern of “normal gastric mucosa - non-atrophic gastritis - atrophic gastritis - intestinal metaplasia - dysplasia - gastric cancer”, and H. pylori plays an initiating role in this process [2]. The International Agency for Research on Cancer (IARC) and the U.S. Department of Health and Human Services both list H. pylori as a definite carcinogen [3,4]. Several large cohort studies have shown that the eradication of H. pylori significantly reduces the incidence and mortality of gastric cancer [5,6]. In addition to its role in gastric cancer prevention, H. pylori eradication can cure H. pylori-associated peptic ulcers as well as reduce their recurrence. The risk of NSAID-associated gastric complications and the chance for transmission are also decreased. These all minimized the cost for prevention, diagnosis, management, and outcome of H. pylori-associated disease [7]. Therefore, the Kyoto Global Consensus Report on H. pylori gastritis recommends eradication treatment when H. pylori infection is confirmed by testing, regardless of whether the infected patient has symptoms or complications unless competing considerations [7]. However, large-scale H. pylori eradication treatments have led to increasing resistance rates to multiple antibiotics, together with factors such as coccoid transformation, host CYP2C19 gene polymorphisms, and inappropriate treatment regimens, resulting in a gradual decline in H. pylori eradication rates. If this trend is not reversed, it will certainly further aggravate the burden of gastric cancer. Therefore, the idea of successful eradication of H. pylori on initial treatment and the adoption of personalized treatment guided by antibiotic phenotypic or genotypic resistance testing should be highly valued.
“Three highs and one low” with declining eradication rate
Despite several international or national expert consensuses on the importance of H. pylori eradication, in recent years, H. pylori have shown a “three highs and one low” situation in many regions, with high infection rates, high pathogenicity, high antibiotic resistance, and low eradication rates. Large-scale and inappropriate eradication treatments are occurring in many countries and regions, while H. pylori resistance (antibiotic-resistant mutations) is increasing, with rates of metronidazole resistance of around 80% and levofloxacin and clarithromycin resistance of >60% in some regions [8,9]. The resistance rate of antibiotics used to be low in resistance rates, such as amoxicillin and tetracycline, is gradually increased with more frequent use. The rate of dual and multiple antibiotic resistance is also rising, which requires great attention [10-12]. Together with the effects of H. pylori coccoid transformation and host CYP2C19 gene polymorphisms, the eradication rate of H. pylori has been declining (from nearly 90% to around 70%) [13-15]. “Antibiotic resistance” is currently the biggest problem in H. pylori infection treatment. Repeated eradication failures not only result in a huge waste of medical resources, but will also further burden antibiotic resistance. The speed of discovery and development of antibacterial drugs is far from keeping up with the growth of antibiotic resistance. In the long run, if action is not taken now to give high priority to the importance of successful eradication of H. pylori on initial treatment, we may face a situation where there are no effective antibiotics to treat H. pylori infection in the future. For patients, the adverse drug reactions (gastrointestinal reactions, liver damage, dysbiosis, etc.) derived from triple or quadruple eradication therapy cannot be ignored [16-18].
Repeated treatment failures will undoubtedly cause physical and psychological harm to patients and their families. In clinical practice, we often encounter patients with H. pylori infection seeking medical treatment due to “fear of gastric cancer”. The interaction of repeated treatment failure, adverse drug reactions, and high mental stress enabled patients to have difficulty in living and working normally. Therefore, the importance of successful eradication of H. pylori on initial treatment should be highly valued both in the long term and at present.
The key elements for improving the successful initial eradication rate of H. pylori
The main determinant of initial eradication success is pretreatment antibiotic resistance [19]. First, overall standardized management of antibiotics use is critical and patients should be educated for adverse consequences of antibiotic abuse. Second, gastroenterologists must know the patient’s antibiotic medication history and local antibiotic resistance (reliable knowledge of antibiotic resistance is still lacking in many regions) before prescribing the initial eradication treatment for H. pylori, to avoid administering potentially resistant antibiotics. The ideal solution to improve the initial eradication rate of H. pylori is to personalize the treatment based on antibiotic resistance (phenotypic and genotypic resistance) and coccoid transformation before treatment [20,21]. Meanwhile, a proton pump inhibitor can inhibit gastric acid secretion and increase gastric pH, thus enhancing the effect of antibiotics and playing an important role in eradication treatment. Therefore, choosing the appropriate PPI according to the patient’s CYP2C19 genotype can further improve the eradication rate [13].
Currently, an increasing number of gastroenterologists are advocating tailored treatment of H. pylori based on antibiotic resistance, coccoid transformation (for patients who have failed eradication), and individual PPI metabolic genotype obtained before treatment. Therefore, large-scale, multicentre epidemiological studies on H. pylori infection, antibiotic-resistant phenotypes and genotypes are necessary to meet the needs of personalized clinical care.
In the past, personalized treatment of H. pylori required solving the challenge of isolating and culturing the strain. The three-gas incubator provides a good microaerobic environment, which greatly improves the success rate of H. pylori culture. Antibiotic susceptibility testing can be performed by Kirby-Bauer or E-test method, which evaluates antibiotic susceptibility by the diameter of the inhibition ring.
Today, H. pylori antibiotic resistance gene mutation testing achieves higher sensitivity than culture-based antibiotic susceptibility testing, solving the challenge of culture failure for antibiotic susceptibility testing and providing insight into resistance at the molecular biological level. Molecular H. pylori antibiotic susceptibility testing is endorsed by the latest expert consensus and clinical practice guidelines, particularly for clarithromycin resistance, where the resistance genotype is highly concordant with the resistance phenotype and has a higher sensitivity and specificity [22-25]. A comparison of culture-based antibiotic susceptibility testing and antibiotic resistance gene mutation testing is described in Table 1. Nowadays, the commercial H. pylori 23S rRNA mutation test kit has been approved in some countries for rapid and sensitive detection of H. pylori infection and clarithromycin genotype resistance, providing rapid and accurate guidance for H. pylori infection treatment, and effectively increasing the initial eradication rate of H. pylori. This may change clinical practice in the management of H. pylori infection and individual treatment choices, as well as facilitate the surveillance of trends in resistance [19]. In China, the China Centre for Helicobacter pylori Molecular Medicine (CCHpMM), advocated by the late gastroenterologist Professor Xiao Shudong, not only conducts H. pylori culture and antibiotic susceptibility testing, but also antibiotic resistance gene research and testing. Based on the research foundation of CCHpMM, a kit for the detection of H. pylori 23S rRNA gene mutations (RT-PCR technology) was approved in China, bringing personalized eradication therapy derived from rapid resistance gene testing available.
Table 1.
Comparison of culture-based antibiotic susceptibility testing and antibiotic resistance gene mutation testing
| Culture-based antibiotic susceptibility testing | Antibiotic resistance gene mutation testing | ||
|---|---|---|---|
|
| |||
| DNA sequencing | Polymerase chain reaction (PCR) | ||
| Specimens | Gastric mucosal biopsy specimens | Gastric mucosal biopsy specimen/paraffin-embedded specimen/fecal specimens | Gastric mucosal biopsy specimen/paraffin-embedded specimen/fecal specimens |
| Testing time | About 14 days | About 3 days | 2-3 hours |
| Transfer conditions | Strict transfer requirements, requiring special preservation solution, low temperature | Simple transfer requirements, low temperature | Simple transfer requirements, low temperature |
| Testing operation and instrument requirements | Complex operation, requiring specialized operators and three-gas incubation conditions | Simple operation, requires sequencing instrument, not available in most hospitals | Relatively simple operation, only requires PCR instrument, available in most hospitals |
| Sensitivity and specificity | Low sensitivity, high specificity | High sensitivity, high specificity | High sensitivity, high specificity |
| Applications | Mostly applied for antibiotic susceptibility testing in patients with refractory infections and scientific research, not suitable for large-scale application | Rapid diagnosis of H. pylori infection and understanding of antibiotic susceptibility, detection of heterogeneous antibiotic resistance, and identification of reinfection and recurrence | |
The host CYP2C19 gene polymorphism test can understand the metabolic genotype (extensive, intermediate, and poor metabolizer) of PPI in the population. Studies reported that the extensive metabolizer accounts for 26.4% of the population in China [26] while more than 40% of the patients were extensive metabolizers in CCHpMM database. Therefore, the type and dose of PPI should be selected appropriately to improve the eradication rate of H. pylori.
Coccoid transformation is the self-protective mechanism of H. pylori strain under the effect of sublethal doses of antibiotics leading to a significant decrease in the susceptibility of the strain to antibiotics and the consequence of treatment failure. When coccoid transformation occurs, it is difficult to achieve successful treatment with continued antibiotics administration, so it is recommended to stop the medication for 3 to 6 months before eradication. The coccoid transformation detection is performed by immunohistochemical staining to observe the morphology and number of H. pylori in the gastric mucosa and has significant advantages in the diagnosis of H. pylori coccoid transformation.
If applicable, the best procedure for initial treatment patients should be the testing for clarithromycin resistance genes (23S rRNA gene mutations), which identifies H. pylori infection and clarithromycin resistance at the same time. If clarithromycin-sensitive, clarithromycin-containing triple or quadruple therapy can achieve satisfactory efficacy. If clarithromycin-resistant, furazolidone-containing bismuth quadruple therapy regimen may be applied following the Chinese experience [27,28]. If initial treatment is failed, an advanced personalized treatment process, including culture-based antibiotic susceptibility testing, resistance gene testing, CYP2C19 gene polymorphism testing, and coccoid transformation testing, and the treatment referring to the personalized treatment decision-making process in Taipei global consensus should be applied [29]. The application process of H. pylori personalized treatment is shown in Figure 1.
Figure 1.
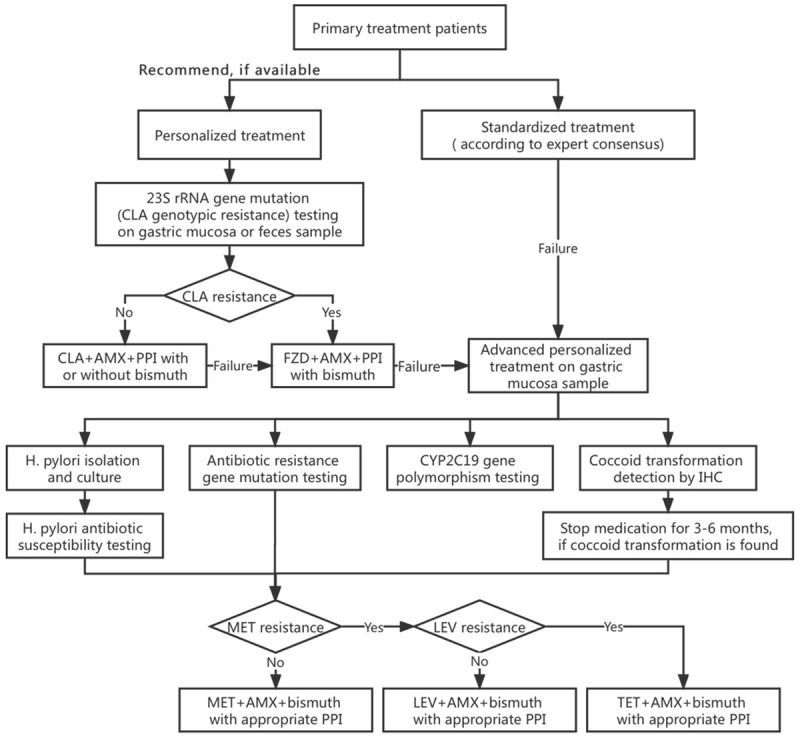
Application process for personalized treatment of H. pylori. CLA: clarithromycin, AMX: amoxicillin, FZD: furazolidone, MET: metronidazole, LEV: levofloxacin, TET: tetracycline, PPI: proton pump inhibitor, IHC: immunohistochemical stain.
Studies showed that empirical treatment from experienced clinicians also achieved a high eradication rate, which indicated the necessity of strengthening the ‘consensus’ education and quality training of physicians and promoted the organic integration of standardized empirical treatment and personalized treatment to effectively elevate the eradication rate of H. pylori [28,30]. CCHpMM has now developed a map of antibiotic-resistant phenotypes and genotypes of H. pylori in China, providing an important reference for the rational selection of H. pylori eradication treatment regimens in China [12].
Emphasis on the importance of successful eradication of H. pylori on initial treatment
Increasing rates of H. pylori antibiotic resistance (antibiotic-resistant gene mutations) due to large-scale eradication therapy, as well as coccoid transformation, host CYP2C19 gene polymorphisms, and inappropriate treatment regimens, have contributed to a significant decline in H. pylori eradication rates. Despite the increasing variety of combinations of eradication regimens and longer treatment courses or higher doses recommended by consensus, the overall trend in initial eradication rates has not been effectively controlled. The negative impact of repeated treatment failures on antibiotic resistance is far-reaching, and it is important to improve the initial treatment eradication rate. Therefore, personalized treatment of H. pylori is imperative, especially guided by antibiotic susceptibility testing in the first treatment. The awareness for successful eradication on initial treatment and personalized treatment strategies should be integrated into pre, during, and post-treatment of H. pylori infection. In addition, natural medicines (e.g. Traditional Chinese Medicine) are a treasure trove of new antibacterial drugs whose effects deserve further standardized research.
Acknowledgements
This work is funded by the Construction Project of Shanghai Molecular Medicine Engineering Technology Research Center (No. 19DZ2283100), Shanghai “Science and Technology Innovation Action Plan” Technical Standards Project (No. 19441911200), Postdoctoral Research Foundation of China (No. 2020M670068ZX), and National Natural Science Foundation of China (No. 82104604).
Disclosure of conflict of interest
None.
References
- 1.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon (FR): International Agency for Research on Cancer; 1994. Schistosomes, Liver Flukes and Helicobacter pylori; pp. 177–240. [Google Scholar]
- 4. https://ntp.niehs.nih.gov/go/roc15.
- 5.Pan K, Zhang L, Gerhard M, Ma J, Liu W, Ulm K, Wang J, Zhang L, Zhang Y, Bajbouj M, Zhang L, Li M, Vieth M, Liu R, Quante M, Wang L, Suchanek S, Zhou T, Guan W, Schmid R, Classen M, You W. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 2016;65:9–18. doi: 10.1136/gutjnl-2015-309197. [DOI] [PubMed] [Google Scholar]
- 6.Chiang T, Chang W, Chen SL, Yen AM, Fann JC, Chiu SY, Chen Y, Chuang S, Shieh C, Liu C, Chiu H, Chiang H, Shun C, Lin M, Wu M, Lin J, Chan C, Graham DY, Chen H, Lee Y. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut. 2021;70:243–250. doi: 10.1136/gutjnl-2020-322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P Faculty Members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460–466. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 9.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382. e1317. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW, Kim N, Kim JM, Nam RH, Chang H, Kim JY, Shin CM, Park YS, Lee DH, Jung HC. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013;18:206–214. doi: 10.1111/hel.12031. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, Jiang Y, Zhao Y. Promising in vitro and in vivo inhibition of multidrug-resistant Helicobacter pylori by linezolid and novel oxazolidinone analogues. J Glob Antimicrob Resist. 2016;7:106–109. doi: 10.1016/j.jgar.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhong Z, Zhang Z, Wang J, Hu Y, Mi Y, He B, Zhang Y, Zhang X, Xia X, Huang H, Lai Y, Lin M, Su C, Zhang Z, Wu Z, Lu L, Zhang B, Huang S, Zhong C, Zeng X, Peng Y, Chen G, Zhang H, Zhou G, Liu S, Yang C, Yan L, Chen A, Zhang G, Xu P, Wang S, Zheng P, Xu S, Gao H. A retrospective study of the antibiotic-resistant phenotypes and genotypes of Helicobacter pylori strains in China. Am J Cancer Res. 2021;11:5027–5037. [PMC free article] [PubMed] [Google Scholar]
- 13.Hong J, Shu X, Liu D, Zhu Y, Xie C, Xie Y, Zhang K, Wang A, Xiong H, Zeng H, Yu H, Ma J, Chen Y, Zhu X, Lu N. Antibiotic resistance and CYP2C19 polymorphisms affect the efficacy of concomitant therapies for Helicobacter pylori infection: an open-label, randomized, single-centre clinical trial. J Antimicrob Chemother. 2016;71:2280–2285. doi: 10.1093/jac/dkw118. [DOI] [PubMed] [Google Scholar]
- 14.Sisto F, Brenciaglia MI, Scaltrito MM, Dubini F. Helicobacter pylori: ureA, cagA and vacA expression during conversion to the coccoid form. Int J Antimicrob Agents. 2000;15:277–282. doi: 10.1016/s0924-8579(00)00188-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Lv Z, Wang Y, Wang H, Liu X, Xie Y, Zhou X. Standard triple therapy for Helicobacter pylori infection in China: a meta-analysis. World J Gastroenterol. 2014;20:14973–14985. doi: 10.3748/wjg.v20.i40.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell GD, Powell K, Burridge SM, Pallecaros A, Jones PH, Gant PW, Harrison G, Trowell JE. Experience with ‘triple’ anti-Helicobacter pylori eradication therapy: side effects and the importance of testing the pre-treatment bacterial isolate for metronidazole resistance. Aliment Pharmacol Ther. 1992;6:427–435. doi: 10.1111/j.1365-2036.1992.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 17.Ladirat SE, Schols HA, Nauta A, Schoterman MH, Keijser BJ, Montijn RC, Gruppen H, Schuren FH. High-throughput analysis of the impact of antibiotics on the human intestinal microbiota composition. J Microbiol Methods. 2013;92:387–397. doi: 10.1016/j.mimet.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Yap TW, Gan H, Lee Y, Leow AH, Azmi AN, Francois F, Perez-Perez GI, Loke M, Goh K, Vadivelu J. Helicobacter pylori eradication causes perturbation of the human gut microbiome in young adults. PLoS One. 2016;11:e0151893. doi: 10.1371/journal.pone.0151893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.worldgastroenterology.org/guidelines/helicobacter-pylori/helicobacter-pylori-english.
- 20.Cosme A, Montes M, Ibarra B, Tamayo E, Alonso H, Mendarte U, Lizasoan J, Herreros-Villanueva M, Bujanda L. Antimicrobial susceptibility testing before first-line treatment for Helicobacter pylori infection in patients with dual or triple antibiotic resistance. World J Gastroenterol. 2017;23:3367–3373. doi: 10.3748/wjg.v23.i18.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SM, O’Morain C, McNamara D. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol. 2014;20:9912–9921. doi: 10.3748/wjg.v20.i29.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jehanne Q, Bénéjat L, Mégraud F, Bessède E, Lehours P. Evaluation of the Allplex™ H pylori and ClariR PCR Assay for Helicobacter pylori detection on gastric biopsies. Helicobacter. 2020;25:e12702. doi: 10.1111/hel.12702. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Lv T, He C, Wang H, Cram DS, Zhou L, Zhang J, Jiang W. Evaluation of multiplex ARMS-PCR for detection of Helicobacter pylori mutations conferring resistance to clarithromycin and levofloxacin. Gut Pathogens. 2020;12:35. doi: 10.1186/s13099-020-00373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrero Rolon R, Cunningham SA, Mandrekar JN, Polo ET, Patel R. Clinical evaluation of a real-time PCR assay for simultaneous detection of helicobacter pylori and genotypic markers of clarithromycin resistance directly from stool. J Clin Microbiol. 2021;59:e03040. doi: 10.1128/JCM.03040-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichon M, Pichard B, Barrioz T, Plouzeau C, Croquet V, Fotsing G, Chéron A, Vuillemin É, Wangermez M, Haineaux PA, Vasseur P, Thiebault Q, Lefèvre C, de Singly A, Cremniter J, Broutin L, Michaud A, Silvain C, Burucoa C. Diagnostic accuracy of a noninvasive test for detection of helicobacter pylori and resistance to clarithromycin in stool by the Amplidiag H. pylori+ClariR real-time PCR assay. J Clin Microbiol. 2020;58:e01787. doi: 10.1128/JCM.01787-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada S, Onda M, Kato S, Matsuda N, Matsuhisa T, Yamada N, Miki M, Matsukura N. Genetic differences in CYP2C19 single nucleotide polymorphisms among four Asian populations. J Gastroenterol. 2001;36:669–672. doi: 10.1007/s005350170029. [DOI] [PubMed] [Google Scholar]
- 27.Song C, Qian X, Zhu Y, Shu X, Song Y, Xiong Z, Ye J, Yu T, Ding L, Wang H, Lu N, Xie Y. Effectiveness and safety of furazolidone-containing quadruple regimens in patients with Helicobacter pylori infection in real-world practice. Helicobacter. 2019;24:e12591. doi: 10.1111/hel.12591. [DOI] [PubMed] [Google Scholar]
- 28.Xie Y, Zhang Z, Hong J, Liu W, Lu H, Du Y, Wang W, Xu J, Wang X, Huo L, Zhang G, Lan C, Li X, Li Y, Wang H, Zhang G, Zhu Y, Shu X, Chen Y, Wang J, Lu N. Furazolidone-containing triple and quadruple eradication therapy for initial treatment for Helicobacter pylori infection: a multicenter randomized controlled trial in China. Helicobacter. 2018;23:e12496. doi: 10.1111/hel.12496. [DOI] [PubMed] [Google Scholar]
- 29.Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, Yeoh KG, Hsu PI, Goh KL, Mahachai V, Gotoda T, Chang WL, Chen MJ, Chiang TH, Chen CC, Wu CY, Leow AH, Wu JY, Wu DC, Hong TC, Lu H, Yamaoka Y, Megraud F, Chan FKL, Sung JJ, Lin JT, Graham DY, Wu MS, El-Omar EM Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM) Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093–2112. doi: 10.1136/gutjnl-2020-322368. [DOI] [PubMed] [Google Scholar]
- 30.Yeo YH, Shiu S, Ho HJ, Zou B, Lin J, Wu M, Liou J, Wu C Taiwan Gastrointestinal Disease and Helicobacter Consortium. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta-analysis. Gut. 2018;67:20–27. doi: 10.1136/gutjnl-2016-311868. [DOI] [PubMed] [Google Scholar]


