Abstract
Small cell lung cancer (SCLC) is a aggressive form of primary lung neoplasm that often presents in elderly smokers. While stage I SCLC can be managed with surgery, extensive-stage disease is managed with chemotherapy using etoposide and cisplatin among other agents, and often complemented by radiation therapy to the chest and cranium. Recent advances in pharmacological research have yielded novel antibody and peptide-conjugated adjunctive chemotherapy, of which bombesin and bombesin receptors have played an important role due to their overexpression in SCLC and other lung cancers. Chemotherapy agents conjugated to bombesin or bombesin-like peptides often demonstrate higher therapeutic efficacy, greater treatment specificity, as well as improved cytotoxicity towards SCLC cells that demonstrate drug resistance. Further modifications to the bombesin-drug conjugate, such as liposomal preparation, have further enhanced bio-availability and half-life of the compound. Additionally, bombesin-radioisotope conjugates can be used for early detection of SCLC using positron emission tomography, as well as subsequent targeted adjuvant radiotherapy to help minimize radiation-induced fibrosis of healthy tissue. Ultimately, further studies are imperative to capitalize on the various applications of bombesin conjugates in both the diagnosis and management of SCLC.
Keywords: Bombesin, cancer, SCLC, chemotherapy, conjugate, targetted therapy
Introduction
Lung cancer is the second most common primary malignancy and accounts for over 10% of new incident cancer cases worldwide [1]. The most used classification for subtypes of lung cancer divides it into non-small-cell lung cancer (NSCLC) and small cell lung cancer (SCLC). While NSCLC, which includes adenocarcinoma, squamous cell carcinoma and large cell carcinoma, makes up over 80% of total lung cancer cases, there are several unique characteristics that make SCLC a particular challenge to treat [2]. While surgery could be attempted for early-stage SCLC, most cases are typically diagnosed as an extensive-stage disease, often due to insidious signs and symptoms that are often masked by existing comorbidities of the patient. Fortunately, the chemo-sensitive nature of SCLC has spurred researchers and clinicians alike to study both more effective therapeutic agents as well as more targeted drug delivery methods to enhance chemotherapy efficacy and curb undesirable side effects [3]. As a result, bombesin (Bn) receptor targeting has been explored and developed as a novel delivery system for SCLC chemotherapy and has showed promise in recent years [4]. In this review, we introduce the various physical and biochemical properties of Bn, discuss more traditional chemotherapy modalities for SCLC, and finally recap the recent advances in Bn receptor targeted drug delivery methods.
Bombesin receptor structure and function
Bn is a hydrophilic tetra-decapeptide that has high permeability in peripheral cells yet unable to cross the blood-brain barrier in significant quantities [5]. Previous studies suggest that Bn and Bn receptors are involved with a wide array of complex physiological pathways and neuroendocrine signaling. The receptors for Bn are G-protein coupled receptors (GPCR), of which 3 subtypes have been studied: BB1, BB2, and BB3 [6]. These three subtypes of Bn-receptors share >50% of a common amino acid sequence [7]. The GPCRs use a classic seven-transmembrane-segment motif and involves several downstream signal transduction pathways, including phospholipase C (PLC), the release of inositol triphosphate (IP3) and the triggering of the subsequent calcium cascade and activation of protein kinase C (PKC) as a key step in the phosphorylation of other key signaling molecules.
The BB1 and BB2 subtypes are better-studied and can received signals from molecules other than Bn [8]. For example, the BB1 subtype also functions as a neuromedin B receptor and the BB2 subtype works to receive signals from gastrin-releasing peptide and other similar signaling molecules. In fact, the BB1 and BB2 receptor subtypes’ affinity for various signaling molecules have been studied extensively [9]. Researchers have concluded that the BB1 receptor subtype receptor shows a strong preference for binding litorin, neuromedin B and ranatensin, while showing a relative lower affinity for bombesin and GRP. This is in contrast to the preferences for the BB2 receptor subtype, which shows a strong affinity for litorin, ranatensin, bombesin and GRP, with a relative lower affinity for neuromedin B. Studies also examined exchanging certain amino acid positions in the BB1 receptor to increase its affinity towards bombesin and GRP. It turns out that amino acid position 3 was the single most important position for deciding receptor affinity towards GRP, but that a particular arrangement of the amino acid sequence Cys-Ala-Cys starting in position 6 through 14 was also crucial for the beta-sheet confirmation seen in BB2 receptors [10], which may also contribute towards its unique ligand-binding selectivity.
BB1 and BB2 have both been found to be overexpressed in primary cancers of the lung, pancreas, and colon. In particular, BB2 receptors have been found to be expressed in over 85% of SCLCs and 75% of NSCLCs [11], making it an excellent target for chemotherapy delivery. On the other hand, the physiological role of BB3 has yet to be fully appreciated, but its presence is also detected in neuroendocrine, lung and pancreatic cancers [12]. A demonstration of ligand-binding to a typical BB2 receptor can be seen in Figure 1.
Figure 1.
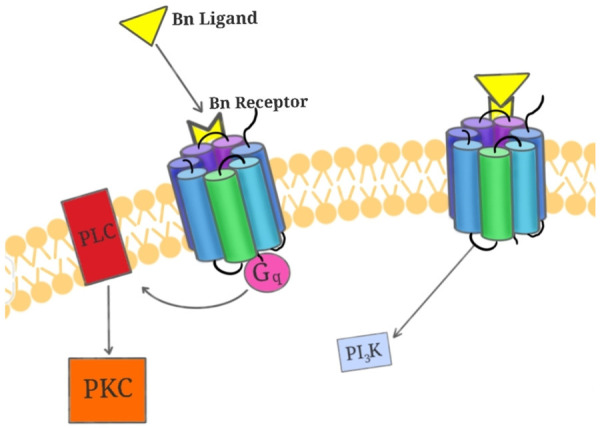
Bn-ligand binds to G-Protein coupled receptor to activate downstream PKC and PI3K pathways. Bn-ligand (in yellow) binding causes activation of the G-protein coupled receptor (multicolored seven-transmembrane structure) and the amplification of downstream PKC (in orange) pathway via PLC (in red) and the calcium-regulated PI3K pathways (in blue).
Diagnosis and prognosis for SCLC
SCLC most commonly presents in elderly males who have extensive history of smoking. Thus, the United States Preventative Services Task Force (USPSTF) recommends screening for lung cancer using low-dose chest CT annually for adults between age 50 and 80 and have had more than a 20-pack-year smoking history who are currently smokers, or recently quit within the past 15 years [13]. However, these screening guidelines are seldomly adhered to either due to limited access to primary care or patient unwillingness to undergo screening if they are asymptomatic. In fact, the insidious onset of the clinical signs and symptoms of lung cancer are a known culprit for delayed diagnosis and treatment of SCLC and lung cancers in general [14]. The most common presenting signs and symptoms of SCLC are cough, dyspnea, hemoptysis, and chest pain [15], all of which are non-specific and often overlap with symptoms of other comorbid conditions such as chronic obstructive pulmonary disease. Notably, SCLC can also present with certain paraneoplastic syndromes that help distinguish it from NSCLCs [16]. Syndrome of Inappropriate Antidiuretic Hormone and subsequent hyponatremia is the most common, affecting 15% of SCLC patients [17]. There have also been reported cases of ectopic secretion of adrenocorticotropin hormone or growth-hormone releasing hormone [18] and new onset Lambert-Eaton Syndrome [19] associated with SCLC.
Radiographic evaluation is the recommended next step in initial evaluation for all patients with suspected SCLC. A standard chest radiograph has poor sensitivity for detection of SCLC, unless the neoplasm has already developed to extensive stages. Because of this, chest CT is preferred and the detection of a lesion greater than 15 mm, with irregular borders, cavitation and/or septations confers a greater suspicion for malignancy [20]. Extra thoracic imaging should also be performed in the process of determining the TNM stage if there is strong evidence for malignancy [21]. Additionally, SCLC often exhibits a faster doubling-time and greater replicative aggression compared to other subtypes of lung cancer [22]. Thus, a rapidly enlarging lesion over the course of weeks to months can increases the suspicion of SCLC compared to NSCLC [23]. To confirm the diagnosis of SCLC, a lung tissue biopsy with subsequent microscopic examination is needed [24]. Furthermore, lymph node and distant metastasis biopsy should also be performed if there is evidence of advanced-stage disease [21]. Typical histopathological features of SCLC that help distinguish it from NSCLC are smaller cell sizes with a high nuclear-to-cytoplasmic ratio and a “salt and pepper”-like chromatin pattern [25].
The prognosis of SCLC is largely dependent on the staging of disease, similar to that of other neoplasms. Among patients with limited disease, the median survival ranges from 15-20 months, while those with extensive disease and distant metastasis have median survival of 8-13 months [26]. The five-year survival rate is around 10% for the former group and 1% for the latter group [27]. Given the insidious onset, challenging diagnosis, high aggressiveness, and poor prognosis of SCLC, there remains a pressing need in the search for more effective chemotherapy and more sophisticated drug delivery methods.
Traditional management for SCLC
The traditional management of SCLC is two-fold, largely dependent on the staging of the neoplasm. Additionally, a list of commonly used chemotherapy agents and a graphical representation of their mechanisms of action will be provided in Figure 2.
Figure 2.
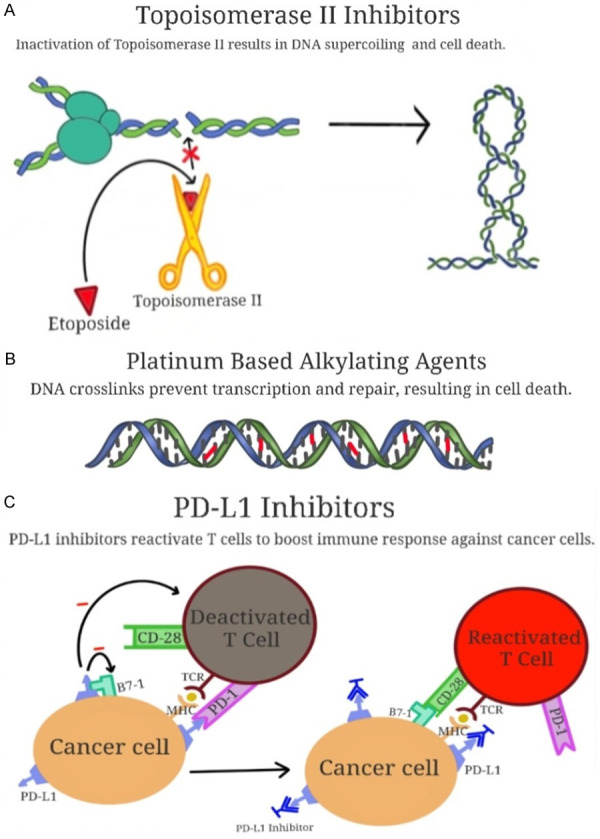
The mechanisms of action of topoisomerase II inhibitors, platinum-based alkylating agents, and PD-L1 inhibitors. Panel (A) depicts topoisomerase II inhibitors exacerbating DNA supercoiling, this leading to cell death. Panel (B) shows platinum-based alkylating agents forming DNA crosslinks (in red) to prevent transcription, thus resulting in cell death. Panel (C) depicts PD-L1 inhibitors blocking cancer cells’ ability to evade T-cell mediated immune surveillance, resulting in cell death.
First, for patients with stage I SCLC with no lymph node involvement and distant metastasis, surgery is recommended, which includes resection of the tumor, lung lobectomy and additional mediastinal lymph node dissection [28]. Post-surgery chemotherapy would consist of four rounds of cisplatin-based treatment. For certain patients that were identified to have lymph node involvement at the time of surgery, an additional course of post-surgical radiation therapy would be recommended [29].
For patients with Stage II or III SCLC, chemotherapy becomes the mainstay of treatment with etoposide and cisplatin (EP) as the most studied and preferred agents of choice [29]. The efficacy of EP has been compared to other alternative regimens, such as cyclophosphamide, doxorubicin and vincristine (CAV) or cyclophosphamide, epirubicin and vincristine (CEV). EP therapy was demonstrated to have a higher response rate (77% vs. 51% of CAV), longer overall survival (14.5 months vs. 9.7 months of CEV), and lower rates of reported myelotoxicity and cross-reaction with adjuvant radiotherapy [30]. Newer antibody-based adjuvant regiments are also being studied. Notably, bevacizumab, an antiangiogenic monoclonal antibody has been investigated for its concurrent used with chemoradiotherapy but showed high rates of tracheoesophageal fistulae, forcing the trial to end prematurely [31]. Other programmed cell death ligand 1 (PD-L1) inhibitors such as atezolizumab and durvalumab, are currently under investigation for their combined use with traditional EP therapy in this patient group, although their efficacy has already been demonstrated in those with extensive disease [32]. These Anti-PD-L1 antibodies are designed to reduce the ability of cancer cells to evade immune surveillance and thus evade apoptosis [30].
For patients with extensive-stage SCLC, current recommendations call for a combination of platinum-etoposide with immune modulating antibodies such as the aforementioned atezolizumab and durvalimumab [33]. Studies evaluating platinum-etoposide therapy vs. CAV or CEV regimens found that the former was at least as effective as the latter options, while resulting in less toxicity. There have also been studies comparing the efficacy of cisplatin vs. carboplatin but found no significant difference in response rates or overall survival. Other adjunctive therapies for this patient group include trilaciclib, which is a cyclin-dependent kinase inhibitor that is used to reduce effects of myelosuppression [34]. Additionally, both thoracic and prophylactic cranial radiation therapy is recommended for patients with a good response to chemotherapy. Continued imaging surveillance should be carried out in these patients using CT or MRI initially at 3-to-4-month intervals for the first two years, then at 6-month intervals thereafter [35]. Newer adjunctive therapies such as poly(ADP-ribose) polymerase inhibitors are also being explored for their combination with traditional chemotherapy in this patient group to help increase response rates and overall survival [32].
Use of Bombesin in targeted chemotherapy
SCLC has been studied to secrete numerous types of regulatory molecules, including Bn, GRP, and GRP-like peptides [36]. Many SCLC lines thus also express receptors for Bn and GRP. When additional concentrations of Bn and GRP were added to SCLC lines in vitro, researchers found that tumor cells were stimulated up to 150-fold at a Bn supplement concentration of just 50 nM [37]. What was particularly interesting about this finding was that while GRP, being a physiologically active analog of Bn, also demonstrated the ability to stimulate growth SCLC, des-Leu(13)-Met(14)-Bn, which is a physiologically inactive analog of Bn failed to demonstrate this stimulatory effect [38]. The combination of these observations suggest that the Bn receptors expressed by SCLC cells may be also receiving simultaneous autocrine signaling that help them discern the specific identity of the incoming signaling molecule, which is a topic worthy of exploring in future studies [39].
Due to the overexpression of Bn receptors in over 85% of SCLC, researchers have investigated targeted therapy towards the Bn receptor for improving delivery of chemotherapeutic agents, enhancing their efficacy and response rates, all the while mitigating unwanted side effects. Various animal models have been established to study this topic [40,41].
For example, paclitaxel was crosslinked with Bn using polyethylene glycol (PEG) in a scorpion-like formation [4], with two “claws” made of peptides and paclitaxel as a “tail”. This conjugate demonstrated greater cytotoxicity in GRP/BB2 receptor-positive cancer cells when compared to unmodified paclitaxel [42]. A demonstration of this unique Bn-PEG-paclitaxel conjugate can be seen in Figure 3. Doxorubicin is another agent that was crosslinked to a Bn ligand by an N-terminal glutamic acid [43,44]. This combination was tested in mice and inhibited the growth of various human cancer cell lines without downregulating the Bn receptors, which allows the potential for repeat regimens.
Figure 3.
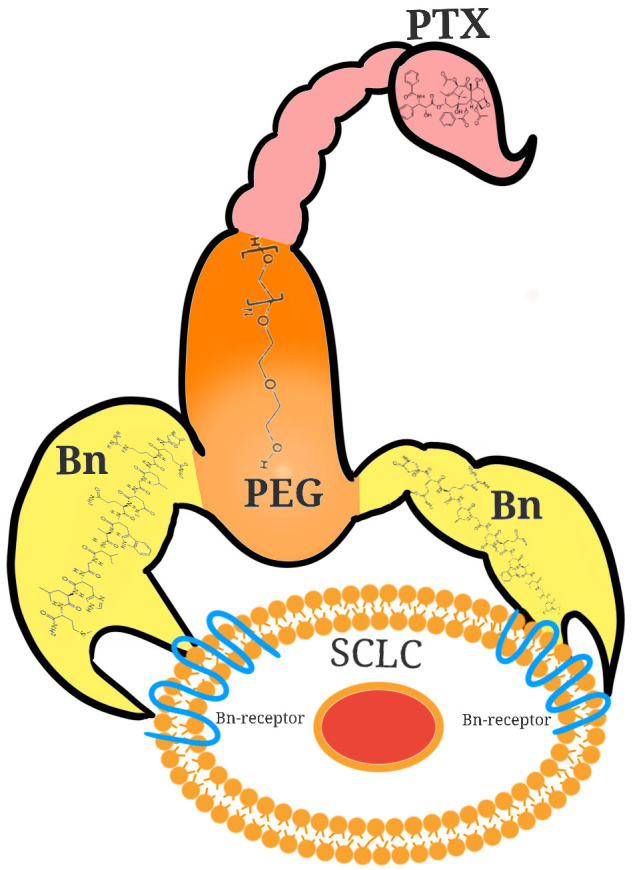
Bn-drug conjugate “scorpion” enhances targeted delivery of chemotherapy in SCLC. 2 Bn molecules (in yellow) are conjugated to paclitaxel (PTX, in pink) via polyethylene glycol (PEG, in yellow) in a “scorpion”-like configuration, which allows for better targeted chemotherapy delivery to SCLC cells expressing Bn-receptors.
The high affinity of Bn receptors, especially BB2, towards Bn-like ligands such as GRP allows for even more options in designing conjugate targeted chemotherapy for SCLC [45]. Kiaris et al. discovered that AN-215, a cytotoxic analog of bombesin could inhibit SCLC growth when conjugated to 2-pyrrolinodoxorubicin [46]. Cuttitta et al. found that by designing an antibody that targets the C-terminal region of Bn-like peptides such as GRP, the growth of Bn-receptor positive SCLC was inhibited both in-vitro and in-vivo [45]. This finding was further echoed by Zhou et al. in their investigation, which concluded that combined traditional EP chemotherapy and immunotherapy via monoclonal antibody against Bn-receptor improved cytotoxicity against SCLC when compared to traditional chemotherapy alone [47].
Using a murine model, Woll et al. demonstrated that substance P analogues, which are antagonists capable of blocking bombesin binding to its receptor, are also able to inhibit the growth of SCLC tumor cells [48]. This helps further demonstrate that Bn and Bn-analogues’ roles as important autocrine growth signals for SCLC cells. Yang et al. then demonstrated that 2A11, which is an anti-bombesin monoclonal antibody, demonstrated inhibitory effect on SCLC growth in a murine model [49]. Mahmoud et al. showed that three different analogues of Bn with peptide bond reductions were able to interrupt the growth of SCLC cells by interjecting in the autocrine growth pathway at varying concentrations [50]. Castellone et al. also recently showed that there may be extensive interaction between Bn signaling cascade and Sonic Hedgehog pathways, potentially suggesting a different angle of approach to designing pharmaceutical interventions that disrupt the activation of the former [51]. Draoui et al. found that downstream of Bn binding in SCLC cells, there was a stimulatory effect on c-fos and c-jun mRNA levels [52]. Based on this finding, novel therapies specifically targeting the modulation of nuclear gene expression can be designed and experimented upon. Kulhari [53], Wang [54], and Patil et al. [55] ran independent investigations on exploring the efficacy of conjugating Bn and Bn-like peptides to doxetaxel and doxorubicin respectively in SCLC, breast, and prostate cancers and found success with both improving cytotoxic efficacy of chemotherapy and reducing the overall toxic side effect profile. Notably, the well-known cardiotoxicity associated with doxorubicin use was markedly reduced by the use of Bn-drug conjugates [54], which can have significant implications for SCLC patients with comorbid cardiac dysfunction or comorbidities.
In addition to the direct conjugation of chemotherapeutic agents to Bn receptor ligands, researchers have also looked into modifying the Bn ligands themselves to further enhance the affinity of ligand-receptor interaction and consequently the efficacy of the chemotherapy [56]. Accardo et al. designed a liposome-based modification for a Bn peptide ligand that allowed for higher-load delivery of doxorubicin to cancer cells [57], a demonstration of which can be found in Figure 4. This liposomal-based modification also allowed for a longer half-life compared to standard chemotherapy delivery methods. Most interestingly, in an in-vivo animal model, the Bn-conjugated doxorubicin with liposomal modification was shown to be effective towards cancer cells that were resistant to standard doxorubicin therapy [54]. Akbar et al. also found that a liposomal preparation of a GRP receptor antagonist improved drug accumulation, especially in multi-drug resistant cancer cells [58]. Thus, it can be posited that Bn-drug conjugates can be studied as a method to overcome challenges that arise with initially chemo-refractory SCLC cases.
Figure 4.
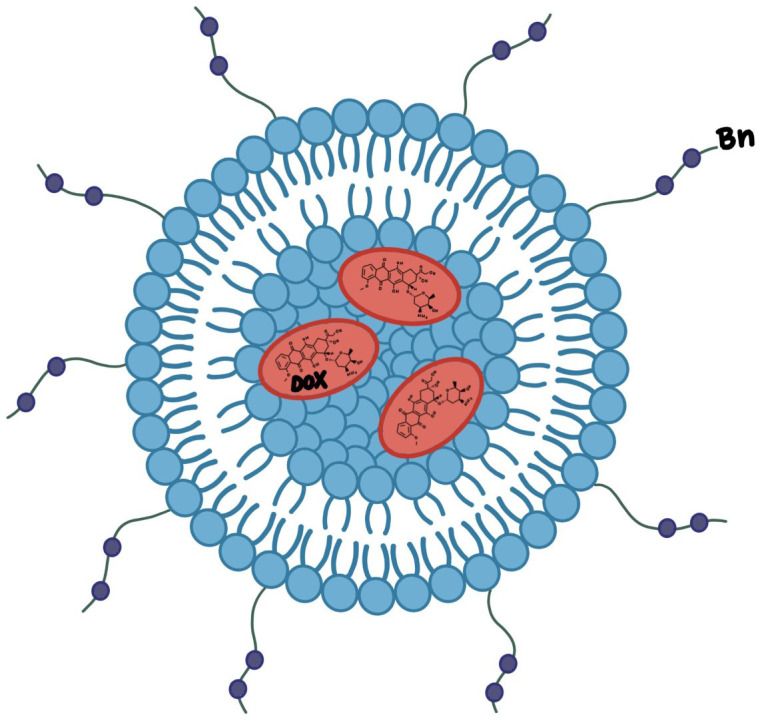
Liposomal preparation of bn-ligand for doxorubicin delivery enhances cytotoxic killing of SCLC. External conjugated Bn-ligand (in purple) allows for specific targeting of liposome (in blue and yellow) to SCLC, which releases concentrated Doxorubicin (DOX, in red) for enhanced therapeutic effect.
Until recently, most researchers explored the chemotherapy application potentials of the more well-known BB1 and BB2 receptor subtypes. However, recent investigations have also been launched to ascertain the role that the more “mysterious” BB3 subtype may play in SCLC [59]. Moreno et al. found remarkable levels of BB3 mRNA expression across 13 different human lung cancer cells lines [60]. In 54% of cell lines, BB3 mRNA expression even exceeded that of BB1 and BB2. They also showed that BB3 agonists stimulated the growth of BB3 receptor positive lung cancer cells lines, specifically through the transactivation of epidermal growth factor receptors. The overexpression of BB1, BB2 and BB3 receptors in SCLC allow for great potential in future conjugate chemotherapy development. The variety of Bn-like peptide ligands, such as GRP and neuromedin, also allow researchers plenty of options to choose in developing an appropriate “vehicle” for chemotherapy that enhances therapeutic accumulation, improves cytotoxic killing of cancer cells and minimizes toxicity to healthy neighboring cells. With further studies of Bn ligands and receptors, it may also become easier to overcome difficulties of treating SCLC lines that are traditionally refractory to chemotherapy.
In addition to conjugation to chemotherapy agents, Bn has also been explored for its application in in-vivo cancer imaging and targeted radiotherapy by way of coupling to various radioisotopes, such as 99-techteium, iodine and gadolinium [61]. Using positron emission tomography (PET), clinicians would be able to visualize Bn-radioisotope conjugates that localize to tumor cells at very early stages of disease- well before any radiographical evidence would present on chest CT screening. Given that one of the greatest obstacles for improving prognosis of SCLC is delayed detection, the improved sensitivity that Bn-radioisotope enhanced PET would provide could prove to be invaluable for early intervention and thus prolonged survival for many patients. Upon establishment of a diagnosis and a demonstrated response to chemotherapy, patients may also have the option to receive targeted radiotherapy in the same manner [62]. Targeted radiotherapy can have the potential to mitigate side effects such as aforementioned tracheoesophageal fistula or tissue fibrosis.
Future directions
The promising initial results of Bn-drug conjugates in SCLC as demonstrated in phase I thru III studies warrant further investigation on a larger-scale. More research efforts should be devoted towards studying the respective expression patterns of BB1, BB2 and BB3 in various SCLC lines and how to tailor individualized conjugate chemotherapy based on individual expression patterns [63]. Resources should be invested in exploring novel ways to modify Bn-ligands to achieve higher affinity and reduce unwanted effects, such as liposomal preparation or additional conjugation to hydrophobic or amphipathic molecules [64]. Further investigations should be launched to determine whether the three Bn receptor subtypes can be targeted simultaneously in a hybrid conjugate method. For example, would chemotherapeutic agents conjugated to both BB1 and BB3 antagonists mixed in a drug cocktail be more efficacious than the drug conjugated to BB1 antagonists alone? This “mix and match” strategy would potentially enhance treatment efficacy and response rates while overcoming rising rates of drug-resistant SCLC [65]. The ongoing Covid-19 has seen a significant increase in biotechnology investments [66], which will hopefully help bolster oncology research simultaneously using both animal and human models [40]. Lastly, it is also worthy to pursuit the application of Bn-radioisotope conjugation for the dual-purpose of early detection and better adjunctive treatment for SCLC [67].
Conclusion
Traditional chemotherapy remains a mainstay for most cases of SCLC. However, Bn-conjugated drug therapy has shown great promise in multiple studies and can help potentially enhance chemotherapy efficacy, increase treatment specificity, reduce unwanted side effects, and ultimately help prolong overall patient survival. Bn and Bn-ligands, such as GRP and Neuromedin, as well as BB1, BB2, and BB3 receptors can be further modified and cross-linked to achieve greater therapeutic effect. Bn-drug conjugates may prove to be particularly effective at overcoming rising drug resistance seen in certain SCLC lines. Furthermore, Bn-radioisotope conjugates may allow for greater sensitivity and early detection of SCLC, which can lead to timely intervention. Further investigation of Bn conjugates and their application in the diagnosis and management of SCLC is warranted.
Acknowledgements
We would greatly acknowledge the supports from Shenzhen Science and Technology Program (Grant No. KQTD20170810154011370), Xiangtan Institute of Industrial Technology Collaborative Innovation, and Xiangtan Science and Technology Bureau.
Disclosure of conflict of interest
None.
References
- 1.Van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 2.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 3.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–672. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pooja D, Gunukula A, Gupta N, Adams DJ, Kulhari H. Bombesin receptors as potential targets for anticancer drug delivery and imaging. Int J Biochem. 2019;114:105567. doi: 10.1016/j.biocel.2019.105567. [DOI] [PubMed] [Google Scholar]
- 5.Wharton J, Polak JM, Bloom S, Ghatei M, Solcia E, Brown M, Pearse A. Bombesin-like immunoreactivity in the lung. Nature. 1978;273:769–770. doi: 10.1038/273769a0. [DOI] [PubMed] [Google Scholar]
- 6.Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, two analogous active peptides from the skin of the European amphibians bombina and alytes. Experientia. 1971;27:166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- 7.Fathi Z, Way JW, Corjay MH, Viallet J, Sausville EA, Battey JF. Bombesin receptor structure and expression in human lung carcinoma cell lines. J Cell Biochem Suppl. 1996;63:237–246. doi: 10.1002/jcb.240630519. [DOI] [PubMed] [Google Scholar]
- 8.Ohki-Hamazaki H, Iwabuchi M, Maekawa F. Development and function of bombesin-like peptides and their receptors. Int J Dev Biol. 2003;49:293–300. doi: 10.1387/ijdb.041954ho. [DOI] [PubMed] [Google Scholar]
- 9.Lin JT, Coy DH, Mantey SA, Jensen RT. Comparison of the peptide structural requirements for high affinity interaction with bombesin receptors. Euro J Pharm. 1995;294:55–69. doi: 10.1016/0014-2999(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 10.Battey J, Wada E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 1991;14:524–528. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez N, Moody TW, Igarashi H, Ito T, Jensen RT. Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diabetes Obes. 2008;15:58–64. doi: 10.1097/MED.0b013e3282f3709b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghatei M, Jung R, Stevenson JC, Hillyard CJ, Adrian TE, Lee YC, Christofides ND, Sarson DL, Mashiter K, MacIntyre I, Bloom SR. Bombesin: action on gut hormones and calcium in man. J Clin Endocrinol Metab. 1982;54:980–985. doi: 10.1210/jcem-54-5-980. [DOI] [PubMed] [Google Scholar]
- 13.Colson YL, Shepard J, Lennes IT. New USPSTF guidelines for lung cancer screening: better but not enough. JAMA Surg. 2021;9:513–514. doi: 10.1001/jamasurg.2021.0242. [DOI] [PubMed] [Google Scholar]
- 14.Beckles MA, Spiro SG, Colice GL, Rudd RM. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest. 2003;123(Suppl):97S–104S. doi: 10.1378/chest.123.1_suppl.97s. [DOI] [PubMed] [Google Scholar]
- 15.Crane M, Scott N, O’Hara BJ, Aranda S, Lafontaine M, Stacey I, Varlow M, Currow D. Knowledge of the signs and symptoms and risk factors of lung cancer in Australia: mixed methods study. BMC Public Health. 2016;16:508. doi: 10.1186/s12889-016-3051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darnell RB, DeAngelis LM. Regression of small-cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet. 1993;341:21–22. doi: 10.1016/0140-6736(93)92485-c. [DOI] [PubMed] [Google Scholar]
- 17.Bordi P, Tiseo M, Buti S, Regolisti G, Ardizzoni A. Efficacy and safety of long-term tolvaptan treatment in a patient with SCLC and SIADH. Tumori J. 2015;101:e51–e53. doi: 10.5301/tj.5000249. [DOI] [PubMed] [Google Scholar]
- 18.Terzolo M, Reimondo G, Ali A, Bovio S, Daffara F, Paccotti P, Angeli A. Ectopic ACTH syndrome: molecular bases and clinical heterogeneity. Ann Oncol. 2001;12(Suppl 2):S83–S87. doi: 10.1093/annonc/12.suppl_2.s83. [DOI] [PubMed] [Google Scholar]
- 19.Sabater L, Titulaer M, Saiz A, Verschuuren J, Güre A, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology. 2008;70:924–928. doi: 10.1212/01.wnl.0000281663.81079.24. [DOI] [PubMed] [Google Scholar]
- 20.Lee D, Rho JY, Kang S, Yoo KJ, Choi HJ. CT findings of small cell lung carcinoma: can recognizable features be found? Medicine (Baltimore) 2016;95:e5426. doi: 10.1097/MD.0000000000005426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sone S, Nakayama T, Honda T, Tsushima K, Li F, Haniuda M, Takahashi Y, Hanaoka T, Takayama F, Koizumi T, Kubo K, Yamanda T, Kondo R, Fushimi H, Suzuki T. CT findings of early-stage small cell lung cancer in a low-dose CT screening programme. Lung Cancer. 2007;56:207–215. doi: 10.1016/j.lungcan.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Harris K, Khachaturova I, Azab B, Maniatis T, Murukutla S, Chalhoub M, Hatoum H, Kilkenny T, Elsayegh D, Maroun R. Small cell lung cancer doubling time and its effect on clinical presentation: a concise review. Clin Med Insights Oncol. 2012;6:199–203. doi: 10.4137/CMO.S9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waqar SN, Morgensztern D. Treatment advances in small cell lung cancer (SCLC) Pharmacol Ther. 2017;180:16–23. doi: 10.1016/j.pharmthera.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Gore EM, Hu C, Sun AY, Grimm DF, Ramalingam SS, Dunlap NE, Higgins KA, Werner-Wasik M, Allen AM, Iyengar P, Videtic GMM, Hales RK, McGarry RC, Urbanic JJ, Pu AT, Johnstone CA, Stieber VW, Paulus R, Bradley JD. Randomized phase II study comparing prophylactic cranial irradiation alone to prophylactic cranial irradiation and consolidative extracranial irradiation for extensive-disease small cell lung cancer (ED SCLC): NRG Oncology RTOG 0937. J Thorac Oncol. 2017;12:1561–1570. doi: 10.1016/j.jtho.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, Sheppard MN, Falk R, Travis WD. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26:1184–1197. doi: 10.1097/00000478-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Yang L, Ci B, Maclean M, Gerber DE, Xiao G, Xie Y. Development and validation of a nomogram prognostic model for SCLC patients. J Thorac Oncol. 2018;13:1338–1348. doi: 10.1016/j.jtho.2018.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf M, Holle R, Hans K, Drings P, Havemann K. Analysis of prognostic factors in 766 patients with small cell lung cancer (SCLC): the role of sex as a predictor for survival. Br J Cancer. 1991;63:986–992. doi: 10.1038/bjc.1991.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E ESMO Guidelines Working Group. Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi99–vi105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- 29.Saltos A, Shafique M, Chiappori A. Update on the biology, management, and treatment of small cell lung cancer (SCLC) Front Oncol. 2020;10:1074. doi: 10.3389/fonc.2020.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: beyond traditional treatment approaches. J Thorac Oncol. 2013;8:587–598. doi: 10.1097/JTO.0b013e318286cf88. [DOI] [PubMed] [Google Scholar]
- 31.Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14:549–561. doi: 10.1038/nrclinonc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oronsky B, Reid TR, Oronsky A, Carter CA. What’s new in SCLC? A review. Neoplasia. 2017;19:842–847. doi: 10.1016/j.neo.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas A, Vilimas R, Trindade C, Erwin-Cohen R, Roper N, Xi L, Krishnasamy V, Levy E, Mammen A, Nichols S, Chen Y, Velcheti V, Yin F, Szabo E, Pommier Y, Steinberg SM, Trepel JB, Raffeld M, Young HA, Khan J, Hewitt S, Lee JM. Durvalumab in combination with olaparib in patients with relapsed SCLC: results from a phase II study. J Thorac Oncol. 2019;14:1447–1457. doi: 10.1016/j.jtho.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel D, Kuchava V, Bondarenko I, Ivashchuk O, Spigel D, Dasgupta A, Reddy S, Melkadze T, Jaal J, Kudaba I, Hart L, Matitashvili A, Koynov KD, Yang Z, Wolfe SG, Malik R, Morris SR, Antal JM, Goldschmidt J. 1742PD-Trilaciclib (T) decreases myelosuppression in extensive-stage small cell lung cancer (ES-SCLC) patients receiving first-line chemotherapy plus atezolizumab. Ann Oncol. 2019;30(Suppl 5):v713. [Google Scholar]
- 35.Ahmed Z, Kujtan L, Kennedy KF, Davis JR, Subramanian J. Disparities in the management of patients with stage i small cell lung carcinoma (SCLC): a surveillance, epidemiology and end results (SEER) analysis. Clin Lung Cancer. 2017;18:e315–e325. doi: 10.1016/j.cllc.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Carney DN, Cuttitta F, Moody TW, Minna JD. Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin-releasing peptide. Cancer Res. 1987;47:821–825. [PubMed] [Google Scholar]
- 37.Layton JE, Scanlon DB, Soveny C, Morstyn G. Effects of bombesin antagonists on the growth of small cell lung cancer cells in vitro. Cancer Res. 1988;48:4783–4789. [PubMed] [Google Scholar]
- 38.Staley J, Coy D, Taylor JE, Kim S, Moody TW. [Des-Met14] bombesin analogues function as small cell lung cancer bombesin receptor antagonists. Peptides. 1991;12:145–149. doi: 10.1016/0196-9781(91)90181-n. [DOI] [PubMed] [Google Scholar]
- 39.Davis TP, Crowell S, Taylor J, Clark DL, Coy D, Staley J, Moody TW. Metabolic stability and tumor inhibition of bombesin/GRP receptor antagonists. Peptides. 1992;13:401–407. doi: 10.1016/0196-9781(92)90128-p. [DOI] [PubMed] [Google Scholar]
- 40.Gong S, Zhang Y, Tian A, Deng WM. Tumor models in various drosophila tissues. WIREs Mech Dis. 2021;13:e1525. doi: 10.1002/wsbm.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Rouggly L, You M, Lubet R. Chapter 7. Animal models of lung cancer: characterization and use for chemoprevention Res. In: Conn PM, editor. Progress in molecular biology and translational science. Academic Press; 2012. pp. 211–226. [DOI] [PubMed] [Google Scholar]
- 42.Safavy A, Raisch KP, Matusiak D, Bhatnagar S, Helson L. Single-drug multiligand conjugates: synthesis and preliminary cytotoxicity evaluation of a paclitaxel-dipeptide “scorpion” molecule. Bioconjug Chem. 2006;17:565–570. doi: 10.1021/bc050224c. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Sun L. Sweetening the deal: glycosylation and its clinical applications. J Biomed Sci. 2020;9:3–9. [Google Scholar]
- 45.Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 46.Kiaris H, Schally A, Nagy A, Sun B, Armatis P, Szepeshazi K. Targeted cytotoxic analogue of bombesin/gastrin-releasing peptide inhibits the growth of H-69 human small-cell lung carcinoma in nude mice. Brit J Cancer. 1999;81:966–971. doi: 10.1038/sj.bjc.6690794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Chen J, Mokotoff M, Zhong R, Shultz LD, Ball ED. Bombesin/gastrin-releasing peptide receptor: a potential target for antibody-mediated therapy of small cell lung cancer. Clin Cancer Res. 2003;9:4953–4960. [PubMed] [Google Scholar]
- 48.Woll PJ, Rozengurt E. Bombesin and bombesin antagonists: studies in Swiss 3T3 cells and human small cell lung cancer. Brit J Cancer. 1988;57:579–586. doi: 10.1038/bjc.1988.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang HK, Scott FM, Trepel JB, Battey JF, Johnson BE, Kelley MJ. Correlation of expression of bombesin-like peptides and receptors with growth inhibition by an anti-bombesin antibody in small-cell lung cancer cell lines. Lung Cancer. 1998;21:165–175. doi: 10.1016/s0169-5002(98)00054-3. [DOI] [PubMed] [Google Scholar]
- 50.Mahmoud S, Palaszynski E, Fiskum G, Coy DH, Moody TW. Small cell lung cancer bombesin receptors are antagonized by reduced peptide bond analogues. Life Sci. 1989;44:367–373. doi: 10.1016/0024-3205(89)90231-2. [DOI] [PubMed] [Google Scholar]
- 51.Castellone MD, Laukkanen MO, Teramoto H, Bellelli R, Alì G, Fontanini G, Santoro M, Gutkind JS. Cross talk between the bombesin neuropeptide receptor and sonic hedgehog pathways in small cell lung carcinoma. Oncogene. 2015;34:1679–1687. doi: 10.1038/onc.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Draoui M, Chung P, Park M, Birrer M, Jakowlew S, Moody TW. Bombesin stimulates c-fos and c-jun mRNAs in small cell lung cancer cells. Peptides. 1995;16:289–292. doi: 10.1016/0196-9781(94)00173-1. [DOI] [PubMed] [Google Scholar]
- 53.Kulhari H, Pooja D, Singh MK, Kuncha M, Adams DJ, Sistla R. Bombesin-conjugated nanoparticles improve the cytotoxic efficacy of docetaxel against gastrin-releasing but androgen-independent prostate cancer. Nanomedicine (Londa) 2015;10:2847–2859. doi: 10.2217/nnm.15.107. [DOI] [PubMed] [Google Scholar]
- 54.Wang C, Sun X, Wang K, Wang Y, Yang F, Wang H. Breast cancer targeted chemotherapy based on doxorubicin-loaded bombesin peptide modified nanocarriers. Drug Deliv. 2016;23:2697–2702. doi: 10.3109/10717544.2015.1049721. [DOI] [PubMed] [Google Scholar]
- 55.Patil V, Gada K, Panwar R, Majewski S, Tekabe Y, Varvarigou A, Khaw BA. In vitro demonstration of enhanced prostate cancer toxicity: pretargeting with bombesin bispecific complexes and targeting with polymer-drug-conjugates. J Drug Target. 2013;21:1012–1021. doi: 10.3109/1061186X.2013.818675. [DOI] [PubMed] [Google Scholar]
- 56.Accardo A, Salsano G, Morisco A, Aurilio M, Parisi A, Maione F, Cicala C, Tesauro D, Aloj L, De Rosa G, Morelli G. Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: a potential theranostic agent. Int J Nanomedicine. 2012;7:2007–17. doi: 10.2147/IJN.S29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Accardo A, Mansi R, Salzano G, Morisco A, Aurilio M, Parisi A, Maione F, Cicala C, Ziaco B, Tesauro D, Aloj L, De Rosa G, Morelli G. Bombesin peptide antagonist for target-selective delivery of liposomal doxorubicin on cancer cells. J Drug Target. 2013;21:240–249. doi: 10.3109/1061186X.2012.741138. [DOI] [PubMed] [Google Scholar]
- 58.Akbar MJ, Ferreira PCL, Giorgetti M, Stokes L, Morris CJ. Bombesin receptor-targeted liposomes for enhanced delivery to lung cancer cells. Beilstein J Nanotechnol. 2019;10:2553–2562. doi: 10.3762/bjnano.10.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreno P, Mantey SA, Lee SH, Ramos-Álvarez I, Moody TW, Jensen RT. A possible new target in lung-cancer cells: the orphan receptor, bombesin receptor subtype-3. Peptides. 2018;101:213–226. doi: 10.1016/j.peptides.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, Spigel DR, Moreno V, Chau I, Hann CL, Eder JP, Steele NL, Pieters A, Fairchild J, Antonia SJ. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14:237–244. doi: 10.1016/j.jtho.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banerjee SR, Pomper MG. Clinical applications of Gallium-68. Appl Radiat Isot. 2013;76:2–13. doi: 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand 125I-[D-TYR6, β-ALA11, PHE13, NLE14] bombesin (6-14) Clinical Cancer Res. 2002;8:1139–1146. [PubMed] [Google Scholar]
- 63.Cortazar P, Gazdar AF, Woods E, Russell E, Steinberg SM, Williams J, Ihde DC, Johnson BE. Survival of patients with limited-stage small cell lung cancer treated with individualized chemotherapy selected by in vitro drug sensitivity testing. Clinical Cancer Res. 1997;3:741–7. [PubMed] [Google Scholar]
- 64.Santos AO, da Silva LCG, Bimbo LM, de Lima MCP, Simões S, Moreira JN. Design of peptide-targeted liposomes containing nucleic acids. Biochim Biophys Acta. 2010;1798:433–441. doi: 10.1016/j.bbamem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–4471. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Nguyen A, Chun M, Lin Z, Ross J, Sun L. Ninety days in: a comprehensive review of the ongoing COVID-19 outbreak. Health Science J. 2020;14:1–13. [Google Scholar]
- 67.Rezazadeh F, Sadeghzadeh N. Tumor targeting with (99m)Tc radiolabeled peptides: clinical application and recent development. Chem Biol Drug Desn. 2019;93:205–221. doi: 10.1111/cbdd.13413. [DOI] [PubMed] [Google Scholar]


