Abstract
The metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97, a clinical isolate from Italy that was previously shown to produce an enzyme related to IMP-1, was isolated by means of a PCR methodology which targets amplification of gene cassette arrays inserted into class 1 integrons. Sequencing revealed that this determinant was an allelic variant (named blaIMP-2) of blaIMP found in Japanese isolates and that it was divergent from the latter by 12% of its nucleotide sequence, which evidently had been acquired independently. Similar to blaIMP, blaIMP-2 was also carried by an integron-borne gene cassette. However, the 59-base element of the blaIMP-2 cassette was unrelated to those of the blaIMP cassettes found in Japanese isolates, indicating a different phylogeny for the gene cassettes carrying the two allelic variants. Expression of the integron-borne blaIMP-2 gene in Escherichia coli resulted in a significant decrease in susceptibility to a broad array of β-lactams (ampicillin, carbenicillin, cephalothin, cefoxitin, ceftazidime, cefepime, and carbapenems). The IMP-2 enzyme was purified from an Escherichia coli strain carrying the cloned determinant, and kinetic parameters were determined with several β-lactam substrates. Compared to IMP-1, the kinetic parameters of IMP-2 were similar overall with some β-lactam substrates (cefoxitin, ceftazidime, cefepime, and imipenem) but remarkably different with others (ampicillin, carbenicillin, cephaloridine, and meropenem), revealing a functional significance of at least some of the mutations that differentiate the two IMP variants. Present findings suggest that the environmental reservoir of blaIMP alleles could be widespread and raise a question about the global risk of their transfer to clinically relevant species.
The integron-based recombination system is a powerful mechanism of discrete genetic rearrangement that operates in procaryotic genomes and that plays a major role in the spread of antibiotic resistance determinants (see references 13 and 30 for reviews). Although the most widespread secondary β-lactamases are not integron associated (see references 6 and 25 and references therein), various β-lactamase determinants are carried by integron-borne mobile cassettes (30). The repertoire of integron-associated β-lactamases has been shown to include also metallo-β-lactamases (2, 16, 20, 22), which is a most worrying development in the field of microbial drug resistance. In fact, these enzymes are able to hydrolyze virtually all β-lactam compounds (including carbapenems and expanded-spectrum cephalosporins) and are not susceptible to the mechanism-based serine-β-lactamase inhibitors (3, 5, 21, 22, 29, 32).
The blaIMP gene, which encodes the IMP-1 enzyme, was the first metallo-β-lactamase determinant to be identified as part of gene cassettes inserted into chromosomal or plasmid-borne integrons carried by nosocomial isolates of Serratia marcescens, Klebsiella pneumoniae, and Pseudomonas aeruginosa from Japan (2, 16, 20, 28; EMBL/GenBank database entry D29636). Spread of the blaIMP determinant has essentially remained limited to Japan, with single reports from Korea (K. Lee, Y. Chong, H. B. Shin, and D. Yong, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother. abstr. E85, p. 193, 1998) and Singapore (T. H. Koh, G. S. Babini, N. Woodford, L. H. Sng, L. M. Hall, and D. M. Livermore, Letter, Lancet 353:2162, 1999), until recently, when a multidrug-resistant Acinetobacter baumannii strain (strain AC-54/97) isolated from an Italian patient in Verona, Italy, was found to produce a metallo-β-lactamase and to carry blaIMP-related sequences (G. Cornaglia, M. L. Riccio, A. Mazzariol, P. Piccoli, L. Lauretti, R. Fontana, and G. M. Rossolini, Letter, Lancet 353:899–900, 1999). The unusual nature of this host (blaIMP has never been reported in Acinetobacter isolates in the Far East) and its different geographical origin raised a question about the nature and origin of this determinant.
In the work described here we cloned and characterized the blaIMP-related determinant acquired by A. baumannii AC-54/97. Since it was found to be an allelic variant (named blaIMP-2) of blaIMP found in Japanese isolates, we also purified the IMP-2 enzyme and analyzed its kinetic properties with various β-lactam substrates.
MATERIALS AND METHODS
Bacterial strains and genetic vectors.
A. baumannii AC-54/97 was isolated in 1997 from an Italian inpatient at the Intensive Care Unit of the Verona University Hospital. The properties of this strain and the clinical history of the patient have been described previously (Cornaglia et al., Letter, Lancet 353:899–900, 1999). Escherichia coli DH5α (GIBCO-BRL, Gaithersburg, Md.) was used as the host for recombinant plasmids. Bacterial strains were always grown aerobically at 37°C. Plasmid pBC-SK (Stratagene, La Jolla, Calif.) was used as a cloning vector.
Antibiotics.
Antibiotics were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise specified. Nitrocefin was from Unipath (Milan, Italy), imipenem was from Merck Research Laboratories (Rahway, N.J.), meropenem was from Zeneca Pharmaceuticals (Cheshire, United Kingdom), ceftazidime was from Glaxo-Wellcome (Verona, Italy), and cefepime and aztreonam were from Bristol-Myers Squibb (Wallingford, Conn.). All antibiotic solutions were prepared immediately before use.
Recombinant DNA methodology.
Basic recombinant DNA procedures were performed as described by Sambrook et al. (33). Genomic DNA was extracted from A. baumannii as described previously (17). PCR for amplification of gene cassette arrays inserted into class 1 integrons (ICA-PCR) was performed with primers INT/5CS and INT/3CS designed on the 5′ conserved segment (5′-CS) and the 3′ conserved segment (3′-CS) of class 1 integrons, respectively (Fig. 1). Reactions were performed in a 100-μl volume with the Expand High-Fidelity PCR System (Boehringer Mannheim, Mannheim, Germany; 2.5 U of the enzyme per reaction) in the reaction buffer provided by the manufacturer, which contained 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 50 pmol of each primer, and 10 ng of bacterial genomic DNA as the template. Reaction parameters were as follows: annealing at 51°C for 60 s, extension at 70°C for 180 s (with an increment of 3 s for each cycle), and denaturation at 93°C for 40 s, which were repeated for 15 cycles, and then annealing at 55°C for 60 s, extension at 70°C for 180 s (with an increment of 3 s for each cycle), and denaturation at 93°C for 40 s, which were repeated for 20 cycles. Reactions were performed in 0.2-ml tubes with a Gene Amp PCR system 2400 (Perkin-Elmer, Rahway, N.J.).
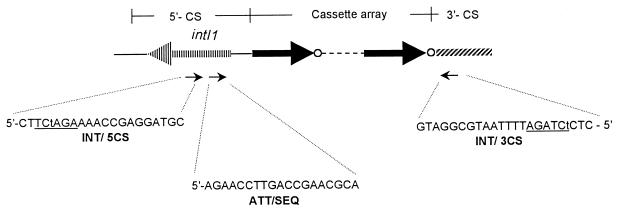
FIG. 1.
Design of primers INT/5CS and INT/3CS used in the ICA-PCR methodology to target amplification of the cassette arrays inserted into class 1 integrons and of primer ATT/SEQ used for direct sequencing of the attI site and downstream region of the amplification products. To facilitate cloning of the amplicons, an XbaI site (underlined) was engineered near the 5′ end of the amplification primers by introducing a point mutation in each of them (indicated by lowercase letters). Gene cassettes are represented by black arrows (coding sequences) followed by empty circles (59-base elements).
DNA sequencing and computer analysis of sequence data.
DNA sequences were determined either with crude PCR products or with plasmid templates by the dideoxy-chain termination method with an automatic DNA sequencer (model 4000; LI-COR Inc., Lincoln, Nebr.), the Thermosequenase DNA sequencing kit (Amersham Pharmacia Biotech, Milan, Italy), and IRD 800-labeled custom sequencing primers (MWG-Biotech, Munich, Germany). Similarity searches against sequence databases were performed with an updated version of the BLAST program (1). Computer analysis of sequence data was performed with the Wisconsin package (version 8.1; Genetics Computer Group Inc., Madison, Wis.) at the Italian EMBL Node of Bari. The cleavage site of the IMP-2 signal peptide was predicted with the SIGCLEAVE program of the EGCG extension of the Wisconsin package. Codon usage tables were compared as described by Grantham et al. (12) by using the CORRESPOND program of the Wisconsin package.
In vitro susceptibility testing.
MICs were determined by a broth macrodilution method (26) with cation-supplemented Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) and a bacterial inoculum of 5 × 105 CFU per tube. Results were recorded after incubation for 18 h at 37°C. MIC determinations were performed in triplicate. The results of the susceptibility tests were interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (27).
Protein analysis techniques.
Metallo-β-lactamase activity in crude cell extracts and in fractions during the purification procedure was assayed as described previously, with imipenem as the substrate (22). The protein concentration in the solution was assayed by the method of Bradford (4) with a commercial kit (Bio-Rad Protein Assay; Bio-Rad, Richmond, Calif.), with bovine serum albumin used as a standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (18), with final acrylamide concentrations of 15 and 5% (wt/vol) for the separating and the stacking gels, respectively. After electrophoresis the protein bands were stained with Coomassie brilliant blue R-250. Analytical isoelectric focusing was performed in precast 5% polyacrylamide gels containing ampholytes (pH range, 3.5 to 9.5; Ampholine PAGplate; Pharmacia) with a Multiphor II Apparatus (Pharmacia). Gels were focused at 0.1 W/cm2 for 2 h at 10°C. β-Lactamase activity was detected as purple bands after overlaying the gel with filter paper soaked with a 0.25 mM nitrocefin solution in 50 mM HEPES (pH 7.5) supplemented with 2 mM ZnCl2.
Purification of IMP-2 enzyme.
The IMP-2 enzyme was purified from E. coli DH5α(pBAUX-30) as follows. The strain was grown in 1 liter of brain heart infusion broth (Difco) containing chloramphenicol (60 μg/ml) for 16 h at 37°C. The cells were harvested by centrifugation, washed twice with 50 mM Tris-HCl (pH 8.7) (Tris buffer [TB]), resuspended in the same buffer, and disrupted by sonication (five times for 30 s each time at 60 W). Cell debris was removed by high-speed centrifugation (105,000 × g for 60 min at 4°C), and the clarified supernatant was loaded onto an S-Sepharose FF column (2.5 by 30 cm; Pharmacia) equilibrated with TB. After washing of the column with the same buffer, the bound proteins were eluted with a linear NaCl gradient (0 to 1 M) in TB (300 ml). The fractions showing metallo-β-lactamase activity were pooled, dialyzed against 50 mM HEPES (pH 7.5), concentrated 10-fold by ultrafiltration, and loaded onto a Superdex-75 column (1.6 by 75 cm; Pharmacia) that had been equilibrated and eluted with the same buffer. The β-lactamase-containing elution peak was concentrated at 0.5 mg/ml and was stored at −80°C until use.
Determination of kinetic parameters.
Kinetic parameters of the IMP-2 enzyme were determined by essentially the same methodology previously adopted for characterization of IMP-1 (21). Hydrolysis of β-lactams was monitored with a lambda 2 spectrophotometer (Perkin-Elmer) equipped with thermostatically controlled cells. The enzyme concentration in the reaction mixture was in the range 8 to 60 nM.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the EMBL/GenBank/DDBJ sequence databases and assigned the accession no. AJ243491.
RESULTS AND DISCUSSION
Cloning of the blaIMP-related determinant of A. baumannii AC-54/97.
A. baumannii AC-54/97 is a clinical isolate from Europe that was previously shown to produce a metallo-β-lactamase and to carry a chromosomal copy of a determinant related to blaIMP by means of Southern blot hybridization with a blaIMP-specific probe (Cornaglia et al., Letter, Lancet 353:899–900, 1999). AC-54/97 exhibits high-level resistance to carbapenems and is also resistant to several other β-lactam and aminoglycoside antibiotics (Cornaglia et al., Letter, Lancet 353:899–900, 1999) (Table 1).
TABLE 1.
MICs of various β-lactams and aminoglycosides for A. baumannii AC-54/97 and for E. coli DH5α(pBAUX-30), which carries the cloned cassette array of In42 in pBC-SKa
| Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| AC-54/97 | DH5α(pBAUX-30) | DH5α(pBC-SK) | |
| Ampicillin | >64 | >64 | 1 |
| Carbenicillin | >64 | >64 | 4 |
| Cephalothin | >64 | >64 | 4 |
| Cefoxitin | >64 | >64 | 2 |
| Ceftazidime | >64 | >64 | 0.12 |
| Cefepime | >64 | 8 | ≤0.06 |
| Imipenem | >64 | 2 | 0.12 |
| Meropenem | >64 | 1 | ≤0.06 |
| Aztreonam | >64 | 0.25 | 0.25 |
| Gentamicin | >64 | 2 | 0.5 |
| Tobramycin | 32 | 8 | 0.25 |
| Netilmicin | 32 | 2 | 0.25 |
| Amikacin | >64 | 1 | 1 |
The susceptibility of E. coli DH5α carrying the empty vector is also shown for comparison.
Since in members of the family Enterobacteriaceae and the genus Pseudomonas blaIMP was found to be carried on gene cassettes inserted into integrons (2, 16, 20, 28; EMBL/GenBank database entry D29636), a PCR methodology designed for amplification of integron cassette arrays (ICA-PCR; Fig. 1), modified after that previously developed by Lévesque et al. (23), was adopted to isolate the blaIMP-related determinant of AC-54/97. With the genomic DNA of AC-54/97 as the template, ICA-PCR yielded a 3-kb amplification product which was recognized by a blaIMP-specific probe that comprised the 0.5-kb HindIII fragment internal to the blaIMP gene (28) in a Southern blot hybridization (data not shown). Direct sequencing of this product with the primer ATT/SEQ, designed from the sequence upstream of the attI site of class 1 integrons (Fig. 1), yielded a sequence identical to that of the attI site of In1 from plasmid R46 (14) until the recombination core site of the first inserted cassette, from which a double sequence ladder began, preventing further reading (data not shown). This indicated that the 3-kb amplification product was actually contributed by a mixed population of two different amplicons of approximately the same size. Digestion of the amplification product with XbaI yielded a partial restriction pattern, suggesting that only one of the two amplicons contained an internal XbaI site that yielded two fragments of 1.75 and 1.25 kb. Southern blot hybridization with the blaIMP-specific probe showed that the blaIMP-related determinant was carried by the amplicon that did not contain the internal XbaI site (data not shown). Therefore, after digestion of the amplification product with XbaI, the 3-kb band was purified by agarose gel electrophoresis and was cloned into the pBC-SK vector to obtain recombinant plasmid pBAUX-30. Metallo-β-lactamase activity was detected in crude extracts of E. coli DH5α(pBAUX-30) (data not shown), confirming that the cloned gene encoded a functional product.
The ICA-PCR methodology developed for amplification of integron-borne cassette arrays, therefore, was successful in rapid isolation of the new metallo-β-lactamase determinant and could be used for rapid characterization of new class 1 integrons carried by antibiotic-resistant clinical isolates. If the amplification product obtained is unique, direct sequencing can be used for characterization of the entire cassette array. On the other hand, if two or more class 1 integrons are present in the strain studied, yielding multiple amplicons (as it was the case with A. baumannii AC-54/97), it may be necessary to separate them by size or by cloning to achieve their characterization. The introduction of a restriction site in the primers used for amplification in ICA-PCR facilitates cloning of the amplification products. Another difference of ICA-PCR compared to the approach previously developed by Lévesque et al. (23) is that the amplified region also contains the Pant promoter, whose configuration may be variable in different integrons (24, 34) and is relevant to the expression of the genes carried by the integrated cassettes (9, 24).
Sequence of the integron-borne metallo-β-lactamase determinant and flanking regions.
The nucleotide sequence of the cloned amplicon carried by pBAUX-30 was determined, and its fidelity was subsequently verified by direct sequencing of PCR products obtained from the genomic DNA of AC-54/97 with custom primers designed from the sequences of unique regions.
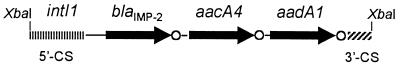
Analysis of the sequence data revealed the presence of three gene cassettes, which contained a blaIMP-related allele (named blaIMP-2), an aacA4 allele, and an aadA1 allele, respectively, inserted between the 5′-CS and the 3′-CS of a class 1 integron which was named In42 (Fig. 2).
FIG. 2.
Structure of the cloned amplicon carried by recombinant plasmid pBAUX-30, which contains the cassette array of In42 along with part of the 5′-CS and 3′-CS of the integron. Gene cassettes are indicated as described in the legend to Fig. 1.
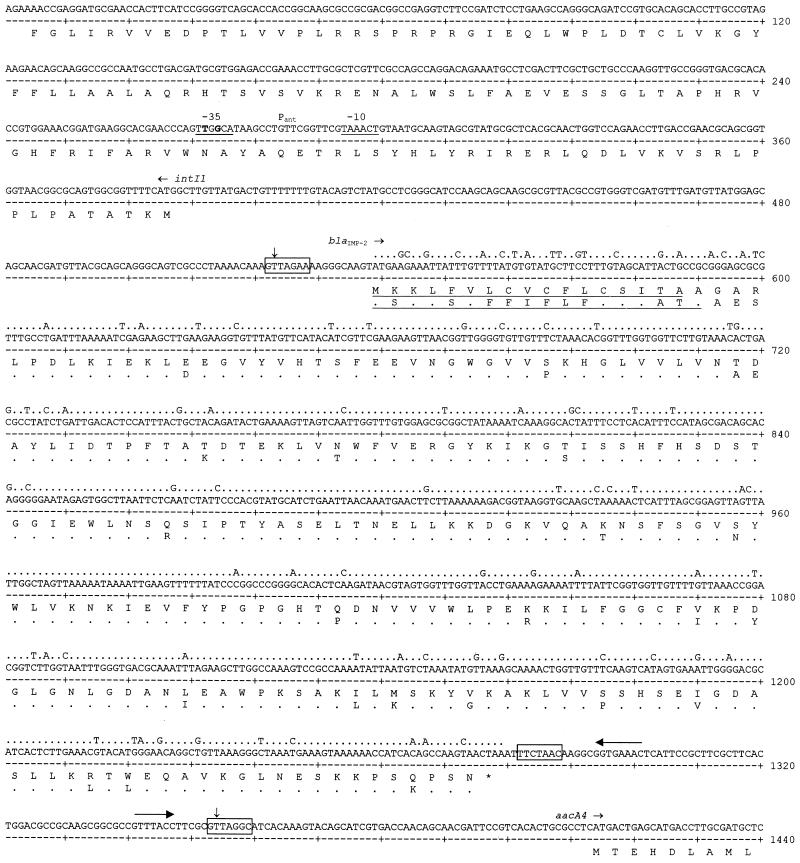
The partial 5′-CS of In42 contained in the cloned amplicon was identical to that of In1 (14) except for two differences located within the −35 hexamer of the Pant promoter (a T in place of a G in the second position and a G in place of an A in the fourth position; Fig. 3). The configuration of the Pant promoter found in this integron, with a TTGGCA −35 hexamer and a TAAACT −10 hexamer, is different from those present in other integrons (24, 34; results of a BLAST search on updated EMBL/GenBank sequence databases), with a −10 hexamer identical to that found in the strong and hybrid versions of Pant (24) and a −35 hexamer which is unique for the presence of a G in the fourth position. Since the Pant configuration of In42 is original, it will be interesting to investigate its functional behavior in comparison with that of Pant versions found in other integrons (9, 24).
FIG. 3.
Nucleotide sequence of the blaIMP-2 gene cassette of In42 and flanking regions. Initiation codons of the various ORFs are indicated, and protein translations are reported below the sequence. The nucleotide sequence of the blaIMP gene and the amino acid sequence of the IMP-1 protein (28) are also reported above that of blaIMP-2 and below that of IMP-2, respectively, with dots representing identical residues. The putative signal peptide of IMP-2, deduced with the SIGCLEAVE program, and the signal peptide of IMP-1 (21) are underlined. The blaIMP-2 cassette boundaries are indicated by vertical arrows. The conserved recombination core sites located at the cassette boundaries and the inverse core site are boxed. The internal 2L and 2R core sites of the 59-base element (35) are overlined with arrows. The −35 and −10 hexamers of Pant are underlined, and the two mutations in the −35 hexamer of the Pant promoter compared to that of In1 (14) are in boldface.
The partial 3′-CS of In42 contained in the cloned amplicon was identical to that of In1 (34).
The gene cassette containing blaIMP-2 is 831 bp long. The blaIMP-2 open reading frame (ORF) exhibits 88% nucleotide sequence identity to blaIMP found in Japanese isolates (Fig. 3). The deduced amino acid sequence of the IMP-2 enzyme is 85% identical to that of IMP-1, with several differences (10 of 36) being clustered within the signal peptide region (Fig. 3). Of the differences found in the mature protein, none falls in the highly conserved residues (His-86, His-88, Asp-90, His-149, Cys-168, and His-210, by use of the numbering for β-lactamase II [Bc-II] of Bacillus cereus 569/H [8]) that are known to be involved in the binding of zinc ions in the B. cereus and Bacteroides fragilis metallo-β-lactamases (7, 8, 10) and that are also conserved in IMP-1 (Fig. 4) and in the other enzymes of subclass B1 (3, 22, 29, 32). Only two of the differences between IMP-2 and IMP-1 (Gln-142 and Asp-164, by use of the numbering for IMP-2; Fig. 4) are found at positions that, in Bc-II, are known to be in or close to the active site (8). The G+C content of blaIMP-2 (39.0%) is similar to that of blaIMP (39.1%), and the codon usage of the two genes is not significantly different (D2 value = 1.26). The 59-base element of the blaIMP-2 cassette is 78 bp long and exhibits the features typical of these elements, with putative IntI1-binding domains at the left and right ends (Fig. 3). The 59-base element of the blaIMP-2 cassette is unrelated to those of the blaIMP cassettes found in Japanese isolates (Fig. 5).
FIG. 4.
Sequence comparison between the IMP-1 (28) and IMP-2 proteins and additional comparison with Bc-II of B. cereus 569/H (15). Identical residues are indicated by an asterisk; conserved amino acid substitutions are indicated by a colon. Identity and similarity symbols reported below the Bc-II sequence refer to the comparison among the three sequences, while those reported below the IMP-2 sequence refer to the comparison between IMP-1 and IMP-2. The highly conserved residues known to be involved in the binding of zinc ions in the B. cereus and B. fragilis metallo-β-lactamases (7, 8, 10) are boxed. Secondary structure elements of Bc-II (8) are also indicated above the sequence.
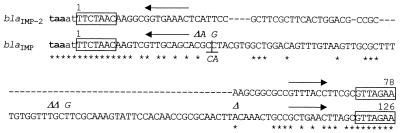
FIG. 5.
Comparison of the structure of the 59-base elements of the blaIMP-2 and blaIMP gene cassettes. The trinucleotides in boldface at the beginning of each sequence correspond to the stop codons of the blaIMP-2 and blaIMP ORFs. The 59-base elements are in uppercase letters and are those present in the circular forms of the cassettes. The inverse core sites and the recombination core sites are boxed, and the internal 2L and 2R core sites of the 59-base elements (35) are overlined with arrows. Identical residues are indicated by asterisks. The blaIMP 59-base element reported in the figure is that of the blaIMP cassette inserted in In31 (20). The minor differences observed between this sequence and those of previously sequenced blaIMP cassettes (2, 28) are indicated above (for comparison with data from reference 28) or below (for comparison with data from reference 2) the sequence and are italicized. ▵, a deletion from that position; ⊥, an insertion at that position. It should be noted that sequence data from reference 28 are available for comparison only until nucleotide 101 (in the numbering of the blaIMP 59-base element).
The aacA4 gene cassette inserted in In42 (Fig. 2) contains an aacA4 allele that encodes an aminoglycoside acetyltransferase identical to that encoded by plasmid pIP1855 of Pseudomonas fluorescens BM2687 (19).
The aadA1 cassette inserted in In42 (Fig. 2) contains an aadA1 allele that encodes a protein which is nearly identical (except for the substitution Ala 240→Ser) to the aminoglycoside adenylyltransferase encoded by In1 of plasmid R46 (14).
Characterization of the metallo-β-lactamase determinant carried by A. baumannii AC-54/97 therefore revealed that it is an allelic variant of the blaIMP gene that is circulating in Japan (2, 20, 28; EMBL/GenBank database entry D29636) and, evidently, had been acquired independently. A similar finding demonstrates that different allelic variants of this resistance determinant exist in nature and can be acquired by clinically relevant species. It also suggests that the environmental reservoir of blaIMP alleles could be more widespread than was originally believed. In fact, finding of different blaIMP alleles in isolates from different epidemiological settings raises a question concerning their geographical distribution and their degree of genetic variability, which could be relevant when screening for similar resistance determinants by PCR-based assays. Recently, two A. baumannii clinical isolates that produce a metallo-β-lactamase of alkaline pI have been reported in Portugal (11). It would be interesting to investigate the nature of the acquired metallo-β-lactamase determinants carried by those isolates to ascertain their potential relationships with blaIMP or blaIMP-2.
Similar to blaIMP (2, 16, 20, 28; EMBL/GenBank database entry D29636) blaIMP-2 was also found to be carried on an integron-borne gene cassette. Interestingly, however, the sequence similarity between the cassettes containing the two blaIMP allelic variants was essentially limited to the coding sequences, while their 59-base elements were unrelated to each other, revealing different phylogenies of the two cassettes. A similar situation, which has also been observed with gene cassettes containing different alleles of class A and class D β-lactamases (31), is consistent with the hypothesis that gene cassettes could be assembled from individual pools of genes and 59-base elements or that 59-base elements can be shuffled during cassette evolution (31). Identification of the environmental source(s) of blaIMP alleles not only would be relevant from the epidemiological standpoint but also could provide an interesting model for study of the evolution of similar cassette-borne resistance determinants. The strong similarity of base composition and of codon usage pattern that exists between blaIMP-2 and blaIMP suggests that their original hosts could belong to closely related, although not identical, taxa, considering the consistent divergence between the signal peptides of IMP-1 and IMP-2.
In vitro susceptibility of E. coli carrying the cloned cassette array of In42.
The susceptibility of E. coli DH5α(pBAUX-30), which carries the cloned cassette array of In42, to various β-lactams and aminoglycosides was determined and compared to that of A. baumannii AC-54/97 and to that of the E. coli host carrying an empty vector.
The presence of the resistance genes carried by In42 was associated with a decrease in the in vitro susceptibility to several β-lactams (ampicillin, carbenicillin, cephalothin, cefoxitin, ceftazidime, cefepime, imipenem, and meropenem) and aminoglycosides (gentamicin, tobramycin, and netilmicin), while the susceptibility to aztreonam and amikacin was apparently unaffected (Table 1).
The pattern of decreased aminoglycoside susceptibility exhibited by E. coli DH5α(pBAUX-30) was consistent with the pattern for the aacA4 allelic variant carried by In42, which encodes an AAC(6′)-II aminoglycoside acetyltransferase active on gentamicin but not on amikacin (19). The pattern of decreased β-lactam susceptibility exhibited by E. coli DH5α(pBAUX-30) indicated that IMP-2 has a broad substrate specificity and was overall consistent with the kinetic parameters determined with the purified enzyme (see below). The higher MICs of some antibiotics for A. baumannii AC-54/97 compared to those for E. coli DH5α(pBAUX-30) (Table 1) are likely due to the contribution of additional resistance mechanisms in the former strain. We are investigating the nature of the gene cassettes carried by the other integron detected by ICA-PCR in AC-54/97 (see above).
Purification and characterization of IMP-2 enzyme.
The IMP-2 enzyme was purified from E. coli DH5α(pBAUX-30) by means of an anion-exchange chromatography step, followed by a gel permeation chromatography step. By SDS-PAGE the purified protein appeared as a single 26-kDa band. The pI of the purified protein, determined by analytical isoelectric focusing, was 8.1 (data not shown). This value is in agreement with the theoretical pI calculated for mature IMP-2 (7.96), assuming the presence of a signal peptide of 17 amino acids (Fig. 3), and is similar to the alkaline pI value (9 ± 0.2) previously reported for IMP-1 (21).
The purified IMP-2 protein hydrolyzed many β-lactam substrates including penicillins, narrow- to expanded-spectrum cephalosporins including cephamycins, and carbapenems. No hydrolysis of aztreonam was detected (Table 2). Compared to IMP-1, the kinetic parameters of IMP-2 were similar to those of some β-lactam substrates but were remarkably different from those of others (Table 2). Hydrolysis of ampicillin and carbenicillin by IMP-2 showed differences in both Km values and turnover rates for the two substrates but resulted in similar kcat/Km ratios, whereas IMP-1 exhibits a much higher (240-fold) kcat/Km ratio with ampicillin than with carbenicillin. Moreover, IMP-2 exhibited Km and kcat values considerably lower than those of IMP-1 with cephaloridine and meropenem. With the former substrate these variations resulted in a nearly 10-fold lower kcat/Km ratio for IMP-2, while with meropenem the much higher affinity of IMP-2 actually resulted in a higher value for the kcat/Km ratio (Table 2).
TABLE 2.
Kinetic parameters of the purified IMP-2 enzyme
| Substrate | Km (μM)ab | kcat (s−1)a | kcat/Km (μM−1 s−1) |
|---|---|---|---|
| Ampicillin | 110 ± 10 (200)c | 23 ± 2 (950) | 0.21 (4.8) |
| Carbenicillin | 700 ± 65 (NAd) | 252 ± 20 (NA) | 0.36 (0.02) |
| Nitrocefin | 95 ± 7 (27) | 275 ± 22 (63) | 2.9 (2.3) |
| Cephaloridine | 3 ± 0.2 (22) | 0.8 ± 0.06 (53) | 0.27 (2.4) |
| Cefoxitin | 7 ± 0.5 (8) | 7 ± 0.7 (16) | 1.0 (2.0) |
| Ceftazidime | 111 ± 9 (44) | 21 ± 2 (8) | 0.19 (0.18) |
| Cefepime | 7 ± 0.6 (11) | 4 ± 0.2 (7) | 0.57 (0.66) |
| Imipenem | 24 ± 2 (39) | 22 ± 2 (46) | 0.92 (1.2) |
| Meropenem | 0.3 ± 0.03 (10) | 1 ± 0.08 (5) | 3.3 (0.5) |
| Aztreonam | NDe | NHf | ND |
Values are means ± standard deviations of three measurements.
Determined as Ki when Km was lower than 10 μM.
Data in parentheses report the corresponding values previously measured for the IMP-1 enzyme (21).
NA, not available.
ND, not determined.
NH, no hydrolysis detected.
The structural polymorphism that exists between IMP-1 and IMP-2 therefore appears to be relevant to their functional properties, as shown by the notable differences in their kinetic parameters with some β-lactam substrates. For this reason, the two IMP variants could be an interesting model for study of the structure-function relationships of these clinically important enzymes.
ACKNOWLEDGMENTS
This work was supported by the European research network on metallo-β-lactamases within the “Training and Mobility of Researchers” Program (contract FMRX-CT98-0232) and by grant 9906404271 from MURST (ex-40%).
We acknowledge the excellent technical support of Tiziana di Maggio and Michela Cappelli and the secretarial assistance of Francesco Lissi and Elena Sestini.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellais S, Léotard S, Poirel L, Naas T, Nordmann P. Molecular characterization of a carbapenem-hydrolyzing β-lactamase from Chryseobacterium (Flavobacterium) indologenes. FEMS Microbiol Lett. 1999;171:127–132. doi: 10.1111/j.1574-6968.1999.tb13422.x. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Bush K. Metallo-β-lactamases: a class apart. Clin Infect Dis. 1998;27(Suppl. 1):S48–S53. doi: 10.1086/514922. [DOI] [PubMed] [Google Scholar]
- 6.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfi A, Duée E, Galleni M, Frère J-M, Dideberg O. 1.85 Å resolution structure of the zinc(II) β-lactamase from B. cereus. Acta Crystallog Sect D. 1998;54:313–323. doi: 10.1107/s0907444997010627. [DOI] [PubMed] [Google Scholar]
- 8.Carfi A, Pares S, Duée E, Galleni M, Duez C, Frère J-M, Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collis C M, Hall R M. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–162. doi: 10.1128/aac.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Concha N, Rasmussen B A, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 11.Da Silva G D, Leitão R, Peixe L. Emergence of carbapenem-hydrolyzing enzymes in Acinetobacter baumannii clinical isolates. J Clin Microbiol. 1999;37:2109–2110. doi: 10.1128/jcm.37.6.2109-2110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grantham R, Gautier C, Gouy M, Jacobzone M, Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981;9:43–74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 14.Hall R M, Vockler C. The region of the IncN plasmid R46 coding for resistance to β-lactam antibiotics, streptomycin/spectinomycin and sulphonamides is closely related to antibiotic resistance segments found in IncW plasmids and in Tn21-like transposons. Nucleic Acids Res. 1987;15:7491–7501. doi: 10.1093/nar/15.18.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain M, Carlino A, Madonna M J, Lampen J O. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J Bacteriol. 1985;164:223–229. doi: 10.1128/jb.164.1.223-229.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyobe S, Yamada H, Minami S. Insertion of a carbapenemase gene cassette into an integron of a Pseudomonas aeruginosa plasmid. J Antimicrob Chemother. 1996;38:1114–1115. doi: 10.1093/jac/38.6.1114. [DOI] [PubMed] [Google Scholar]
- 17.Johnson J L. Similarity analysis of DNAs. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 655–682. [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lambert T, Ploy M-C, Courvalin P. A spontaneous mutation in the aac(6′)-Ib gene results in altered substrate specificity of aminoglycoside 6′-N-acetyltransferase of a Pseudomonas fluorescens strain. FEMS Microbiol Lett. 1994;115:297–304. doi: 10.1111/j.1574-6968.1994.tb06654.x. [DOI] [PubMed] [Google Scholar]
- 20.Laraki N, Galleni M, Thamm I, Riccio M L, Amicosante G, Frère J-M, Rossolini G M. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laraki N, Franceschini N, Rossolini G M, Santucci P, Meunier C, de Pauw E, Amicosante G, Frère J-M, Galleni M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauretti L, Riccio M L, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini G M. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999;43:1584–1590. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lévesque C, Piché L, Larose C, Roy P H. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–191. doi: 10.1128/aac.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lévesque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 25.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 1997. Approved standard. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Supplement M100-S9. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 28.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Recchia G D, Hall R M. Gene cassettes: a new class of mobile elements. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 31.Recchia G D, Hall R M. Origins of the mobile gene cassettes found in integrons. Trends Microbiol. 1997;5:389–394. doi: 10.1016/S0966-842X(97)01123-2. [DOI] [PubMed] [Google Scholar]
- 32.Rossolini G M, Franceschini N, Riccio M L, Mercuri P S, Perilli M, Galleni M, Frère J-M, Amicosante G. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. Biochem J. 1998;332:145–152. doi: 10.1042/bj3320145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Stokes H W, Hall R M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 35.Stokes H W, O'Gorman D B, Recchia G D, Parsekhian M, Hall R M. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol. 1997;26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]