Abstract
Inflammatory Breast Cancer (IBC) is a rare and aggressive type of breast cancer with a poor prognosis. Its management is challenging because of a lack of targeted therapies, increased metastatic potential, and high recurrence rates. Interest in using platinum agents such as carboplatin emerged from data suggesting frequent DNA repair defects in breast cancer. Because studies show that medicinal mushroom Ganoderma lucidum (GLE) sensitizes cancer cells to radiation and other drugs; herein, we aimed to investigate the therapeutic potential of GLE, alone or in combination with carboplatin in breast cancer models. Our studies were focused on the regulation of the DNA Damage Response (DDR) and on cancer cell stemness. Carboplatin and GLE were tested in vitro using the IBC cell line, SUM-149, breast cancer non-IBC cells, MDA-MB-231, and in vivo using IBC xenograft models. Our results show that the GLE/carboplatin combination decreased cell viability, induced cell death by two different mechanisms, and delayed the response to DNA damage. Furthermore, the combination suppressed mammosphere formation and the expression of cancer stemness proteins. In xenograft models, the combination showed significant tumor growth inhibitory effects without systemic toxicity. This study emphasizes the potential of this dual therapy for IBC patients.
Keywords: Inflammatory breast cancer, Ganoderma lucidum, cancer stem cells, DNA damage response, Reishi, ATM, ATR, Chk1, Chk2, tumor
Introduction
Inflammatory Breast Cancer (IBC) is the most rare and lethal form of advanced breast cancer. IBC accounts for 1-5% of breast cancers, where younger women are more likely to have metastatic disease at diagnosis and tend to display shorter survival [1]. The prognosis for IBC is bleak, with a median overall survival rate between 2.27 to 3.40 years in patients diagnosed with IBC stage III or IV, respectively [2]. Management of IBC is challenging because of a lack of specific therapies, aggressive tumor behavior, and a high recurrence rate. IBC is generally treated using a multimodal approach comprising preoperative systemic chemotherapy, HER-2 targeted therapy, modified radical mastectomy, and radiation [3]. Neoadjuvant therapy preferred regimens for IBC patients include anthracycline plus taxane and pertuzumab-containing program for HER-2+ tumors. The inclusion of platinum agents combined with paclitaxel or docetaxel has shown improved pathological complete response (pCR) rates [4]. However, long-term outcomes remain unknown.
Platinum-based agents are widely known for their cytotoxic effects, specifically by inducing DNA damage. The mechanism of action of these agents [e.g., cisplatin and carboplatin (cisplatin derivative)] begins by binding DNA forming mono- or di-adducts with intrastrand and interstrand cross-links. These disrupt the structure of DNA leading to inhibition of replication and transcription, induction of apoptosis, and extensive DNA damage [5]. Carboplatin is one of the leading platinum-based anti-cancer drugs used because of its efficacy and low toxicity. However, tumors can become resistant to these agents by the activation of DNA Damage Response (DDR) program [5,6]. Furthermore, recent investigations have identified the contribution of cancer stem cells to platinum-based drug chemoresistance in cancer [6-8]. Thus, studies are underway to identify low toxicity agents combined with carboplatin to improve cancer patients’ clinical response.
Two central kinases mediate DDR: Rad3-related protein (ATR) and Ataxia-Telangiectasia Mutated (ATM). ATM and ATR phosphorylate the serine-threonine kinases and cell cycle regulators Chk2 and Chk1, respectively. The activation of ATM responds to DNA double-strand break (DSBs) lesions. Upon activation, ATM is recruited to the DSB lesion subsequently phosphorylating local substrates such as the MRN complex (i.e., MRE11-RAD50-NBS1), P53BP1, and the variant histone H2AX at Ser139 to generate the DNA damage-associated γ-H2AX. Then, ATM phosphorylates Chk2 at the Thr68 residue, which acts on multiple substrates involved in cell death, cell cycle, and transcription regulation, including the tumor suppressors, BRCA1, and p53. Instead, the ATR-Chk1 pathway responds to single-strand breaks (SSBs). However, both signaling pathways can be activated simultaneously in cells exposed to ionizing radiation and cytotoxic chemotherapeutics [9]. Since DDR is an important chemoresistance mechanism, inhibition of ATM/Chk2 and ATR/Chk1 using therapies combined with platinum-based agents is a recently explored option to treat cancer [10,11].
Ganoderma lucidum is a medicinal mushroom with cytotoxic, antiproliferative, and antitumor effects on IBC and non-IBC [12,13]. Studies have shown that the triterpenoid ganoderic acid DM induces cell cycle arrest, DNA damage, and apoptosis in breast cancer cells. At the same time, total triterpenes and polysaccharides protected leucocytes from radiation-induced DNA damage and cell death [14-16]. Our previous study demonstrated that G. lucidum extract (GLE) sensitizes IBC cells to the tyrosine kinase inhibitor erlotinib, by inactivating AKT and ERK signaling pathways [17].
Here, we explored the mechanisms that underlie IBC cell responses to the combination of GLE and carboplatin in DDR signaling and tumorigenesis. This study presents evidence that GLE sensitizes IBC cells to carboplatin via the inhibition of DDR associated proteins. Notably, the combination of both drugs reduces IBC tumor growth and cancer cell stemness.
Materials and methods
Cell lines and reagents
SUM-149 was obtained from Asterand (Detroit, MI, USA). MDA-MB-231 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were authenticated and screened for Mycoplasma. Mycoplasma-free cells were used within 25 passages and cultured at 37°C in 5% CO2 using culture medium recommended by the suppliers. Carboplatin (Millipore Sigma, Burlington, MA, USA) 27 mM working stocks were dissolved in sterile water.
Ganoderma lucidum extract (GLE)
A commercially available extract consisting of G. lucidum fruiting body and cracked spores, known as ReishiMax GLp™, was purchased from Pharmanex® Inc. (Provo, UT, USA). The extract is available in capsules, where the contents (500 mg) were dissolved in 10% sterile Dimethyl Sulfoxide (DMSO, Sigma Aldrich) at a working stock of 50 mg/mL. This concentration was chosen after a thorough review of the literature, and it is the concentration we demonstrate to be effective for anti-IBC effects [12]. For in vivo studies, GLE was dissolved in the vehicle solution of 10% ethanol and 90% ultrapure water at a concentration of 28 mg/kg BW as described in [13].
Cell survival assay
Cells were inoculated onto 12-well tissue culture plates at a density of 5×104-1×105 using media containing 10% FBS. To examine the anticancer effect of Carboplatin and/or GLE, we performed viability assays using two different human breast cancer cell lines: SUM-149 (IBC) and MDA-MB-231 (non-IBC). After incubation, Carboplatin and/or GLE were added to the cells at different concentrations (10, 20, 30, 40, 50 µM: Carboplatin and 0.2, 0.3 mg/mL: GLE). 0.1% DMSO and sterile water were added to controls (vehicle). The cells were treated in triplicates and maintained at 37°C containing 5% CO2 for 72 h. After treatment, cells were fixed with cold methanol, and nuclei stained with 0.4% propidium iodide (PI). Fluorescence units were measured using a GloMax® Microplate Reader (Promega, Madison, WI, USA). Cell viability was calculated as the percent of surviving cells after treatment relative to control wells.
Western blot
After being treated with vehicle, carboplatin, GLE, or the combination for 72 h, cells were lysed in lysis buffer (10% SDS, 10% sodium deoxycholate, 1% Triton-X 100, 1% Igepal, and protease inhibitors) and total protein was quantified using the Precision Red protein assay kit (Cytoskeleton, Inc. Denver, CO, USA). Equal total protein amounts were subject to separation by SDS-PAGE gels and transferred onto a PVDF membrane. After blocking with 5% milk, the membrane was incubated with the indicated primary antibodies at 4°C overnight. The membrane was then incubated with secondary antibody for 1 h. The membrane was developed with Pierce™ ECL Western Blotting Substrate kit (Thermo Fisher, Waltham, MA, USA) and visualized using the BioSpectrum Imaging System (UVP LLC, Upland, CA, USA).
Mammosphere formation assay
SUM-149 cells were cultured in Ham’s F-12 medium containing 20 ng/mL EGF, 20 ng/mL FGF2, 1X B27, 4 ng/mL Heparin, 5 µg/mL insulin, 1 µg/mL hydrocortisone, 100 units/mL penicillin and 100 units/mL streptomycin. Cells were trypsinized and passed through a 35 µm cell strainer (Corning, NY, USA). After, cells were inoculated onto ultra-low attachment 6-well plates (Corning, NY, USA) at a density of 1×104 cells/2 mL. Mammospheres were cultured for 10 days. The experiments were performed at the 3rd generation. After incubation, the formed mammospheres were treated with vehicle, 20 µM Carboplatin, 0.2 mg/mL GLE, and the combination of Carboplatin and GLE for 72 h. Micrographs were taken using an Olympus inverted microscope. The Mammospheres’ circularity was calculated with the formula 4π (Area/Perimeter2) using Image J V.1.48 [18].
Immunofluorescence
The fixing, permeabilization, and blocking solutions were prepared in 1X PBS. 1×105 cells were seeded in coverslips and treated with vehicle, 20 µM carboplatin, 0.2 mg/mL GLE and the combination for 48 h or 72 h. After the incubation, cells were fixed with 4% paraformaldehyde for 15 min, washed with 1X PBS, and permeabilized with 0.5% Triton X-100 for 10 min at room temperature. Cells were washed with 1X PBS and incubated with 5% BSA for 1 h at room temperature. For labeling, fixed cells were incubated 2 h with a primary antibody against γ-H2AX (1:200, Cell Signaling) and washed. Coverslips were incubated with anti-Rabbit Alexa 488 (1:750, #44125, Cell Signaling) for 1 h at room temperature. After three washes with 1X PBS, cells were incubated for 1min at room temperature with 1 µg/mL of DAPI (Life Technologies) and washed. Cells were mounted on slides with antifade medium (Life Technologies). Micrographs were taken using an Olympus inverted microscope. Cells containing >5 γ-H2AX distinct foci were quantified.
Mouse model
All studies were approved by the UCC and NYU School of Medicine Institutional Animal Care and Use Committee (IACUC) and conducted in accordance with IACUC guidelines. Female 21 d old severe combined immunodeficient (SCID) mice (Charles River Laboratories International, Wilmington, MA) were housed under pathogen-free conditions and were fed with 2920X Teklad Global Rodent Diet (Harlan Laboratories, Indianapolis, IN) and sterile water ad libitum.
To test the effects of treatments in tumor formation and progression, we injected 2.0×104 sorted (CD44+/CD24-) SUM-149 cells or 1.5×106 unsorted cells at 1:1 media:matrigel into the mammary fat pad of female SCID mice. After tumor establishment, treatment allocation was randomly made: (1) vehicle (10% ethanol/sterile water), (2) 30 mg/kg BW carboplatin, (3) 28 mg/kg BW GLE and (4) the combination of carboplatin and GLE. Mice were orally gavaged every day with vehicle or GLE when tumors measured ~150 mm3. Carboplatin treatment started when the tumors reached ~250 mm3 and was intraperitoneally injected once a week for three consecutive weeks. Mouse weight and tumor volume were measured weekly. Tumor volume (mm3) was calculated as follows: [(width)2 × length]/2. At the end of the study, the mice were euthanized, and the tumors were excised and maintained in optimal conditions for further experiments.
Statistical analysis
Statistical analyses for in vitro studies were done using GraphPad Prism® v.7.0 (San Diego, CA) via one-way ANOVA with Dunnett’s multiple comparisons estimator or two-way ANOVA with Bonferroni’s correction. Calculations of the IC50’s were done with dose-response curve fittings using the non-linear regression parameter: dose-response-inhibition (log [inhibitor] vs normalized response). Statistical significance was set at P≤0.05. Quantified data are expressed as mean ± SEM.
For in vivo experiments: Data were analyzed using a five week (W6-W10 of the study) statistical model with four comparison groups as follows: vehicle, Carbo, GLE and Carbo + GLE. Normality diagnostics were performed using the Shapiro-Wilk estimator. Frequencies, percentages, central tendency and dispersion measures were calculated to assess the raw distribution of the study variables. To account for the time horizon as a statistical unit, a General Linear Model Repeated Measures ANOVA design was used to calculate estimated marginal means. A Mauchly’s test of sphericity was performed to assess whether our models have or not the assumption of compound symmetry. If non-significant, we reported the univariate results with an Epsilon correction; if significant; we reported the multivariate results using the Pillai’s trace estimator. Either of the last explained factors were used to evaluate the time effect in our model.
A test of between-subjects effect was applied to perceive statistical differences between the groups per block. The estimated marginal means is reported. The significant level (α) was set to ≤0.05, excluding normality test criteria. IBM Statistical Package for Social Sciences (IBM-SPSS, Chicago, IL) v.26.0 for Windows and RStudio was used.
Results
GLE sensitizes breast cancer cells to carboplatin
To study the sensitizing effect of GLE on carboplatin-induced growth inhibition, we treated BC cells with the combination of GLE and carboplatin. Previously, our studies showed GLE’s anti-cancer effects on non-IBC (i.e., MDA-MB-231), and IBC (i.e., SUM-149) cells [12,13,17,18]. Thus, in the current study, we used the previously calculated half inhibitory concentrations (IC50s) of GLE (0.3 mg/mL; MDA-MB-231, and 0.2 mg/mL; SUM-149) at 72 h to test the efficacy of GLE in combination with carboplatin in BC cells [17]. When IC50s were calculated, we found that SUM-149 cells were more sensitive to carboplatin (IC50=20.07 µM) (Figure 1A, 1B) than MDA-MB-231 (IC50=25.37 µM) as well as to GLE (Figure 1C, 1D). Compared to cells exposed to carboplatin alone, the combination reduced carboplatin’s IC50 (SUM-149: IC50=15.50 µM and MDA-MB-231: IC50=11.78 µM). These results indicate that GLE enhanced the sensitivity of BC cells to carboplatin.
Figure 1.

GLE/carboplatin decreases breast cancer cell viability. (A, B) SUM-149 cells were treated with increasing carboplatin concentrations and 0.2 mg/mL GLE for 72 h (C, D) MDA-MB-231 cells were treated with increasing concentrations of carboplatin and 0.3 mg/mL GLE for 72 h. After treatment, cells were fixed with cold methanol, and nuclei stained with 0.4% propidium iodide. Cell viability was calculated as the percentage of surviving cells after treatment relative to control wells. IC50s were obtained from dose-response curve fittings using non-linear regression (n=3). *P=0.05, **P≤0.01; ****P≤0.0001.
GLE impairs the DNA damage repair machinery and induces apoptosis
To assess whether GLE sensitizes IBC cells to carboplatin via attenuating DNA repair, the immunofluorescence detection of γ-H2AX (phospho histone H2AX) foci formation was performed. The presence of γH2AX in nuclei foci represents the assembly of DNA repair proteins at the lesion sites. The ATM signaling cascade’s activation leads to recognition of chromatin by 53BP1, which accumulates proximal to DSBs [19]. As shown in Figure 2A, 2B, cells containing ≥5 γ-H2AX foci significantly increased when treated with carboplatin, GLE, and their combination after 48 h of treatment, while they decreased at 72 h. Interestingly, a low percentage of cells treated with GLE showed γ-H2AX foci compared with carboplatin or the mixture at 48 h, suggesting that GLE could induce DNA repair or cell death at earlier time points. However, when GLE is combined with carboplatin it induces a superior response. Importantly, GLE treatment combined with carboplatin showed a prolonged DNA damage repair response as evidenced by the highest percentage of cells containing γ-H2AX foci at 72 h (Figure 2B).
Figure 2.
GLE/carboplatin prolong the DNA damage repair response. A. Representative images show γ-H2AX foci in SUM-149 cells after 48 h and 72 h treatment with vehicle, carboplatin (20 µM), GLE (0.2 mg/mL), and their combination. B. Quantification of SUM-149 treated cells. Data represent the percent of SUM-149 cells containing >5 γ-H2AX distinct foci. Micrographs represent the average of 10 photos taken by treatment. Scale bars =20 µm. Columns represent means ± SEM. (n=3). Significance against vehicle (*), carboplatin (φ), and GLE (#) P≤0.05.
Next, we examined ATM and ATR protein levels, and their substrates Chk1 and Chk2 in both breast cancer models to assess whether the combination of treatment affects ATM and ATR activation. At a baseline level, we observed that ATR and ATM are endogenously activated in MDA-MB-231 and SUM-149 cells, respectively (Figure 3A, 3D). Based on the inhibitory role of GLE on DNA damage repair, we sought to determine whether the extract or its combination with carboplatin affects DNA repair factors involved in DDR. Our results show that both monotherapies and their combination affect DDR signaling by different mechanisms in both cell lines. As depicted in Figure 3A-C, in non-IBC cells, carboplatin increased the activation of ATM and Chk1, whereas GLE decreased the activation of Chk2. The combination increased the phosphorylation of Chk1 following our results obtained in cells treated with carboplatin. GLE reduced the phosphorylation of ATR and ATM compared with carboplatin. Interestingly, when GLE is added to carboplatin treated cells, the activation of Chk2 increased when compared with cells treated with GLE alone, suggesting that carboplatin is possibly inducing the activation of a feedback loop to circumvents the effects of GLE. As we observe in Figure 3A, 3B, the combination also decreased the expression of 53BP1.
Figure 3.
GLE impairs the DDR and induces apoptosis. (A) MDA-MB-231 cells were treated for 72 h with vehicle, carboplatin (25 µM), GLE (0.3 mg/mL), or the combination. (B) Densitometric analysis of blots in (A) (53BP1, p-ATM, and p-ATR). (C) Densitometric analysis of blots in (A) (p-Chk1 and p-Chk2: phospho/total ratio). (D) SUM-149 cells were treated for 72 h with vehicle, carboplatin (20 µM), GLE (0.2 mg/mL), or the combination. (E) Densitometric analysis of blots in (D) (53BP1, p-ATM, and p-ATR). (F) Densitometric analysis of blots in (D) (p-Chk1 and p-Chk2: phospho/total ratio). (G) Immunoblot of Cleaved PARP in MDA-MB-231 cells using the same conditions as in (A). (H) Immunoblot of Cleaved PARP in SUM-149 cells using the same conditions as in (D). Columns represent means ± SEM. (n=3). Significance against vehicle (*), carboplatin (φ), and GLE (#) P≤0.05.
A different scenario was observed in IBC cells. Carboplatin increased the activation of Chk2, whereas GLE decreased p-ATM and p-ATR and did not affect the activation of their effectors compared to vehicle (Figure 3D-F). A study showed that Chk2 protein levels correlate with sensitivity to cisplatin in ovarian and small cell lung carcinoma cell lines [20]. Per Zhang et al., who reported that Chk2 is degraded in response to cisplatin, we found that similar to cisplatin, carboplatin induced downregulation of total Chk2 in SUM-149 cells but not in MDA-MB-231 (Figure 3A, 3D) [20]. Thus, is possible that DDR regulation in IBC cells by carboplatin occurs due to an increase in kinase activity, or a decrease in phosphatase activity. It is also possible that the phosphorylation observed is produced to compensate for the loss of total protein. In SUM-149 IBC cells, the combination decreased p-ATM and p-ATR levels similarly to that observed with GLE, and increased the activation of Chk2, as observed with carboplatin. In IBC cells, GLE inhibited the activation of ATM, ATR, and Chk2 compared to carboplatin, contrary to MDA-MB-231, where the activation of Chk2 was increased. The GLE/carboplatin combination abrogates carboplatin’s effects on the activation of Chk2 in IBC cells, differing from what we observed in MDA-MB-231 cells. These results evidence that GLE or the combination impair DSB cellular response by the modulation of DNA repair factors.
We examined the expression of cleaved PARP, which is a biomarker for early apoptotic cells. We found that cleavage induced by GLE and the GLE/carboplatin combination occurred in SUM-149 cells (Figure 3H). In MDA-MB-231 cells, treatments did not induce PARP cleavage (Figure 3G), confirming that GLE could be responsible for apoptosis-independent cell death in this cell line as we published previously [18]. Studies show that platinum-based salts cell death induction is concentration and cellular status dependent. Indeed, chronic low doses of cisplatin induce apoptosis, whereas high doses cause a defective apoptosis program or necrosis [21]. Our results indicate that GLE could induce cell death via two distinct mechanisms by either sensitizing breast cancer cells to carboplatin-induced apoptosis or by necrosis, depending on the cell line. The reduction of carboplatin-induced γ-H2AX foci accompanied by a sustained response to DNA damage, plus the inhibition of 53BP1 expression and the abrogation of DDR signaling cascade reflects the inability of IBC cells to repair DSB and undergo apoptosis, as our cell viability and PARP immunoblotting data suggest. Furthermore, GLE decreased ATR and ATM activation in MDA-MB-231 and SUM-149 cells, respectively. Altogether, these results reveal that GLE enhances the sensitivity of IBC cells to carboplatin through DDR signaling.
GLE/carboplatin combination reduce tumor growth and decrease the DDR
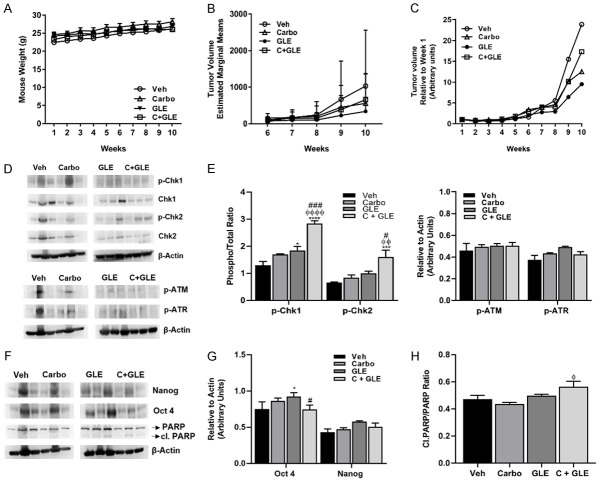
Because our data suggested that the GLE/carboplatin combination shows greater efficacy in reducing cell viability and inducing cell apoptosis than either treatment alone, we sought to determine the GLE/carboplatin efficacy in vivo. We previously showed that 28 mg/kg BW GLE reduces tumor volume in SUM-149 IBC xenograft models [13]. In the current study, we treated SCID mice with vehicle, 30 mg/kg BW carboplatin, or the GLE/carboplatin combination for nine weeks. Our data showed no differences in body weights indicating the treatments were not toxic to mice (Figure 4A). Importantly, the treatment combination significantly reduced tumor volume by 33% compared to vehicle (Figure 4B), while tumor weight decreased by ~40% (P=0.06) (Figure 4C).
Figure 4.

GLE/carboplatin decreases tumor volume. 1.5×106 SUM-149 cells mixed with Matrigel (1:1) were injected into the mammary fat pad of female SCID mice. Once tumors measured ~150 mm3, mice were orally gavaged daily with vehicle or GLE. Once the tumors reached ~250 mm3, carboplatin was injected i.p. once a week for three consecutive weeks. (A) Weekly mouse weight. (B) Weekly tumor volume (mm3). (C) Final tumor weight. Estimated marginal means ± SEM. Vehicle and combination (n=8); carboplatin (n=9). P≤0.05. Significance against vehicle (*), carboplatin (φ), and GLE (#). P≤0.05. P=0.06 vs vehicle.
Tumor analysis revealed that the GLE/carboplatin treatment combination reduced ATR’s activation (Figure 5A-C) and induced the cleavage of PARP (Figure 5D, 5E). These results suggest that the combination of carboplatin and GLE treatment decreased tumor formation through the modulation of the DDR and induction of apoptosis.
Figure 5.
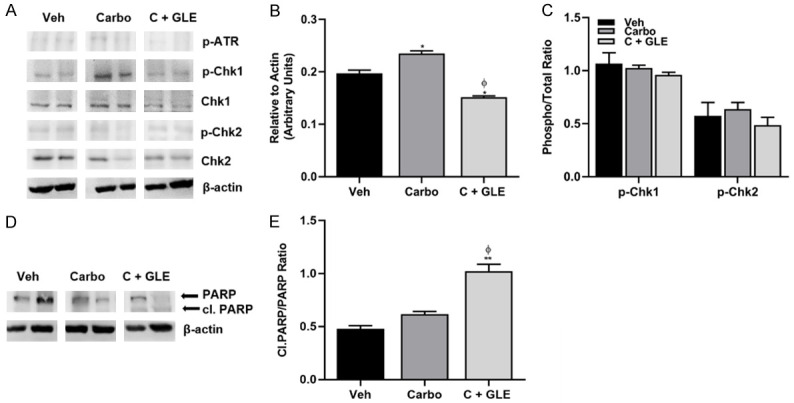
GLE/carboplatin affects the DDR in vivo. A. Immunoblot with the DDR associated primary antibodies listed on the right side of the panel. B. Densitometric analysis of p-ATR. C. Densitometric analysis of p-Chk1 and p-Chk2. Data are expressed as phospho/total protein ratio. D. Immunoblot of PARP and cleaved PARP protein. E. Densitometric analysis of Cleaved PARP. Data are expressed as Cleaved PARP/PARP. Each lane represents a different animal. β-actin was used as the loading control. Estimated marginal means ± SEM. P≤0.05. Significance against vehicle (*), and carboplatin (φ) P≤0.05.
GLE sensitizes IBC cells to carboplatin by inhibiting stemness
Since GLE sensitized IBC cells to carboplatin therapy in IBC in vitro and in vivo models, we wanted to explore the mechanism by which this occurs. Cancer stem cells or tumor-initiating cells represent the most tumorigenic and treatment-resistant cells in a heterogenous tumor environment [22]. Self-inflicted DNA DSB formation and ATM activation are necessary for sustaining cancer cell stemness [23]. Previously, our group published that GLE decreased both the CD44+/CD24- and ALDH1+ populations, reduced breast cancer stem cells’ capacity to form mammospheres, and decreased the expression of stemness associated transcription factors required to maintain self-renewal. GLE also impaired tumor growth of CD44+/CD24- breast cancer cells injected into mice [18]. Thus, we hypothesize that breast cancer stemness is affected by this therapy. We investigated whether the combination affects the capacity of IBC cells by evaluating mammosphere formation. As we expected, GLE and the combination disrupted mammospheres (Figure 6A, 6B) and significantly decreased the expression of stemness associated markers such as OCT4 and SOX2 (Figure 6C, 6D).
Figure 6.
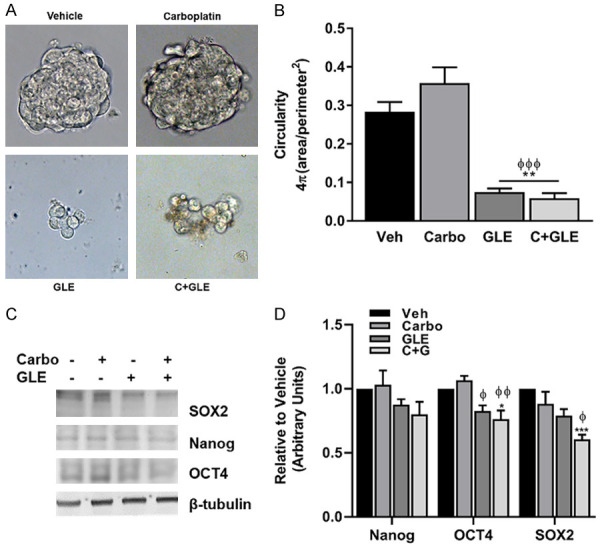
GLE/carboplatin inhibits IBC stemness. (A) SUM-149 IBC cells were seeded to form mammospheres in non-adherent culture conditions for three generations. Formed mammospheres were treated with vehicle, 20 µM carboplatin, 0.2 mg/mL GLE, and the combination of Carboplatin and GLE for 72 h. Micrographs were taken at a magnification of 40×. (B) Mammosphere circularity was calculated with the formula 4π (Area/Perimeter2) using Image J V.1.48. (C) Immunoblotting of stemness markers. (D) Densitometric analysis of blots in (C). β-tubulin was used as the loading control. Estimated marginal means ± SEM. (n=3). P≤0.05. Significance against vehicle (*), and carboplatin (φ) P≤0.05.
To test our results in an in vivo model, we isolated the CD44+/CD24- stem cell population from SUM-149 IBC cells, then subsequently injected them into female SCID mice. Mice were then treated with vehicle, GLE, carboplatin, and their combination using the same concentrations as detailed above and in our previous studies [13]. As expected, treatments did not affect mouse weights (Figure 7A). Although we did not obtain statistically significant results in tumor volume due to high variability of the data (Figure 7B), our results suggest GLE or the combination reduces tumor progression in comparison with vehicle when treatments’ effects are analyzed relative to week one of the experiment (Figure 7C). Future studies with a larger sample size are needed to avoid Type II error. At the molecular level, GLE/carboplatin increased the activation of Chk1 and Chk2 (Figure 7D, 7E) and decreased the expression of the stemness marker OCT4 (Figure 7F, 7G). The combination also induced PARP cleavage (Figure 7H).
Figure 7.
GLE/carboplatin affects DDR and stemness in vivo. 2.0×104 sorted (CD44+/CD24-) SUM-149 cells in 1:1 media:Matrigel were injected into the mammary fat pad of female SCID mice. Once tumors measured ~150 mm3, mice were orally gavaged daily with vehicle (n=7) or GLE [28 mg/kg BW (n=8)]. Carboplatin (30 mg/kg BW [carboplatin alone: n=8; combination (n=9)] treatment started when the tumors reached ~250 mm3 and were injected i.p. once a week for three consecutive weeks. (A) Weekly mouse weight. (B) Tumor volume (mm3). (C) Tumor volume relative to week one. (D) Immunoblotting of DDR associated proteins. (E) Densitometric analysis of blots in (D). (F) Immunoblotting of stemness associated proteins and PARP. (G) Densitometric analysis of blots in (F). (H) Densitometric analysis of Cleaved PARP. Data are expressed as Cleaved PARP/PARP. Each lane represents a different animal. β-actin was used as the loading control. Estimated marginal means ± SEM. P≤0.05. Significance against vehicle (*), carboplatin (φ), and GLE (#) P≤0.05.
Discussion
Chemotherapy is still the most prevailing treatment option used to treat cancer patients. Although, chemotherapy is efficient to treat multiple cancers, it shows high risk of side effects and therapy resistance development. A novel strategy designed to optimize chemotherapy programs is the combination of traditional chemotherapeutics along with natural products.
Platinum-based agents such as cisplatin and carboplatin are still part of the regimen used to treat breast cancer. However, only a subset of patients has a definite response from these drugs because of the development of chemoresistance. Defects in DNA repair machinery, abnormal activation of DDR proteins, and CSC activity in breast cancer are strongly correlated with resistance to genotoxic chemotherapies [9,24-27]. Our present study demonstrated that Ganoderma lucidum extract (GLE) sensitized breast cancer cells to carboplatin. Furthermore, GLE treatment resulted in cell death through the inactivation of ATR/Chk1 and ATM/Chk2 DDR signaling pathways and contributed to the reduction of inflammatory breast cancer (IBC) CSC self-renewal. More importantly, the combination of GLE and carboplatin exhibited a reinforced antitumor action in breast cancer cell lines and in IBC xenograft models.
Natural products have been effective in sensitizing cancer cells to therapy drugs. Over the past decades, several pre-clinical studies have focused on characterizing the molecular pathways of GLE anti-tumor effects. IBC is the most lethal type of advanced breast cancer, that presents unique and different characteristics from non-IBC. A key challenge for this disease is the active search for IBC-specific treatments because of the heterogeneity of tumors. Accordingly, our group has actively evaluated the role of GLE in IBC. Previously, we published that GLE decreases IBC cell proliferation, induces apoptosis, and reduces cell migration, metastasis, and tumor growth [12,13,18]. GLE also synergizes with the EGFR TKI, erlotinib, to sensitize IBC cells to treatment via Akt/ERK signaling regulation [17].
During recent years, a small handful of studies have been focused on studying the effects of this medicinal mushroom or its bioactive compounds in combination with conventional therapy. Investigators have geared their efforts on describing the effects of bioactive compounds within G. lucidum in its ability to potentiate radiation sensitivity of cancer cells and on identifying how GLE minimizes the side effects of toxic drugs [28-30]. The innovation of our current study relies in the need of evidence based studies that identify natural antitumor products that increase conventional drug efficacy. Thus, herein we hypothesized that GLE could sensitize IBC to platinum-based treatments through DDR modulation.
Activation of the DDR is the mechanism that eukaryotic cells use to maintain genome stability and respond to DNA damage due to endogenous or exogenous factors that either promote DNA repair, trigger cell cycle arrest or cell death pathways. DDR signaling initiates with phosphorylation of three members of the phosphoinositide 3-kinases (PI3K)-like protein kinases family, ATM, ATR, and DNA-PKcs as sensors of DNA lesions. ATM and ATR then activate DNA-damage mediators and downstream kinases; BRCA1, γ-H2AX, 53BP1, Chk1, and Chk2. Signals from transducers spread the damage signal to effectors p-53 and cell division cycle 25 (CDC25) [26]. Defects in DNA repair machinery and abnormal activation of DDR proteins in breast cancer are strongly correlated with resistance to genotoxic chemotherapies [9,26-28].
Recently, a study of the mutational profile of IBC showed variants in BRCA1, BRCA2, and ATM genes that were associated with an overall worse survival [31]. These results are aligned with studies by Bertucci et al., where investigators found actionable genetic alterations of ATM in 9% of IBC cases, a higher percentage of IBC patients with alterations of DNA repair associated genes, and a higher homologous recombination deficiency (HRD) score in IBC than in non-IBC patients [32]. Accordingly, our results showed a high baseline activation of ATM in IBC cells. Furthermore, the constitutive activation of ATR in MDA-MB-231 cells has been associated with cell survival [23], and here we found an elevated activation of ATR in MDA-MB-231 non-IBC cells. Hence, our data confirmed that IBC and non-IBC cell survival relies on unique DDR specific pathways.
Breast cancer stem cells (BCSCs) are one of the mechanisms used by tumors to promote resistance to platinum-based agents. Previously, our group published that GLE decreased ALDH1 and CD44+/CD24- stem-like population, reduced BC’s capacity to form BCSC mammospheres, and decreased the expression of stemness associated transcription factors required to maintain self-renewal. GLE also impaired tumor growth of CD44+/CD24- triple negative breast cancer cells [18]. Here, we investigated whether the combination affects the stemness capacity of IBC cells. As we expected, GLE and the combination disrupted BCSC mammospheres, and decreased stemness-associated markers, OCT4 and SOX2 in IBC cells.
To our knowledge, this is the first study demonstrating that GLE potentiates the effects of carboplatin in breast cancer through the modulation of the DDR and cancer cell stemness. These findings strongly support that GLE, in combination with other platinum-based agents or DDR targeted inhibitors, may enhance therapeutic responsiveness in BC tumors with an abnormal repair program. Our findings confirm the anti-cancer therapeutic potential of G. lucidum and provide new knowledge regarding the molecular mechanism of action behind its effectiveness. Our results could lead to new insight for developing a novel therapeutic program for IBC patients.
Acknowledgements
We thank Ms. Mercedes Lacourt and Abhilash Gadi for their technical contributions. This research was funded by the National Institutes of Health SC3GM111171 (MMMM), #U54GM133807 (MMMM), #R25GM110513 (MMMM), Title-V-Cooperative P031S130068 (MMMM, IJSA), PR-INBRE #P20GM103475 (IJSA). RJS funding: NIH R01CA207893; R01CA178509; Breast Cancer Research Foundation BCRF-20-146.
Disclosure of conflict of interest
None.
References
- 1.Robertson FM, Bondy M, Yang W, Yamauchi H, Wiggins S, Kamrudin S, Krishnamurthy S, Le-Petross H, Bidaut L, Player AN, Barsky SH, Woodward WA, Buchholz T, Lucci A, Ueno NT, Cristofanilli M. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60:351–375. doi: 10.3322/caac.20082. [DOI] [PubMed] [Google Scholar]
- 2.Fouad TM, Barrera AM, Reuben JM, Lucci A, Woodward WA, Stauder MC, Lim B, DeSnyder SM, Arun B, Gildy B, Valero V, Hortobagyi GN, Ueno NT. Inflammatory breast cancer: a proposed conceptual shift in the UICC-AJCC TNM staging system. Lancet Oncol. 2017;18:e228–e232. doi: 10.1016/S1470-2045(17)30192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan Y, Tang L, Tong W, Zhou J. The role and indications of aggressive locoregional therapy in metastatic inflammatory breast cancer. Sci Rep. 2016;6:25874. doi: 10.1038/srep25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueno NT, Espinosa Fernandez JR, Cristofanilli M, Overmoyer B, Rea D, Berdichevski F, El-Shinawi M, Bellon J, Le-Petross HT, Lucci A, Babiera G, DeSnyder SM, Teshome M, Chang E, Lim B, Krishnamurthy S, Stauder MC, Parmar S, Mohamed MM, Alexander A, Valero V, Woodward WA. International consensus on the clinical management of inflammatory breast cancer from the Morgan Welch inflammatory breast cancer research program 10th anniversary conference. J Cancer. 2018;9:1437–1447. doi: 10.7150/jca.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sousa GFD, Wlodarczyk SR, Monteiro G. Carboplatin: molecular mechanisms of action associated with chemoresistance. Brazilian Journal of Pharmaceutical Sciences. 2014;50:693–701. [Google Scholar]
- 6.Zhou J, Kang Y, Chen L, Wang H, Liu J, Zeng S, Yu L. The drug-resistance mechanisms of five platinum-based antitumor agents. Front Pharmacol. 2020;11:343. doi: 10.3389/fphar.2020.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, Zhang K, Conner M, Landen CN. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012;18:869–881. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Liu X, Ren Y, Zhang J, Chen J, Zhou W, Guo W, Wang X, Chen H, Li M, Yuan X, Zhang X, Yang J, Wu C. Cisplatin-enriching cancer stem cells confer multidrug resistance in non-small cell lung cancer via enhancing TRIB1/HDAC activity. Cell Death Dis. 2017;8:e2746. doi: 10.1038/cddis.2016.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 10.Khalil HS, Tummala H, Chakarov S, Zhelev N, Lane DP. Targeting ATM pathway for therapeutic intervention in cancer. Biodiscovery. 2012;1:3. [Google Scholar]
- 11.Nam AR, Jin MH, Park JE, Bang JH, Oh DY, Bang YJ. Therapeutic targeting of the DNA damage response using an ATR inhibitor in biliary tract cancer. Cancer Res Treat. 2018;51:1167–1179. doi: 10.4143/crt.2018.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Montemayor MM, Acevedo RR, Otero-Franqui E, Cubano LA, Dharmawardhane SF. Ganoderma lucidum (Reishi) inhibits cancer cell growth and expression of key molecules in inflammatory breast cancer. Nutr Cancer. 2011;63:1085–1094. doi: 10.1080/01635581.2011.601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suarez-Arroyo IJ, Rosario-Acevedo R, Aguilar-Perez A, Clemente PL, Cubano LA, Serrano J, Schneider RJ, Martinez-Montemayor MM. Anti-tumor effects of Ganoderma lucidum (reishi) in inflammatory breast cancer in in vivo and in vitro models. PLoS One. 2013;8:e57431. doi: 10.1371/journal.pone.0057431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu GS, Lu JJ, Guo JJ, Li YB, Tan W, Dang YY, Zhong ZF, Xu ZT, Chen XP, Wang YT. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia. 2012;83:408–414. doi: 10.1016/j.fitote.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Smina TP, De S, Devasagayam TP, Adhikari S, Janardhanan KK. Ganoderma lucidum total triterpenes prevent radiation-induced DNA damage and apoptosis in splenic lymphocytes in vitro. Mutat Res. 2011;726:188–194. doi: 10.1016/j.mrgentox.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 16.González A, Atienza V, Montoro A, Soriano JM. Use of Ganoderma lucidum (ganodermataceae, basidiomycota) as radioprotector. Nutrients. 2020;12:1143. doi: 10.3390/nu12041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suárez-Arroyo IJ, Rios-Fuller TJ, Feliz-Mosquea YR, Lacourt-Ventura M, Leal-Alviarez DJ, Maldonado-Martinez G, Cubano LA, Martínez-Montemayor MM. Ganoderma lucidum combined with the EGFR tyrosine kinase inhibitor, erlotinib synergize to reduce inflammatory breast cancer progression. J Cancer. 2016;7:500–511. doi: 10.7150/jca.13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rios-Fuller TJ, Ortiz-Soto G, Lacourt-Ventura M, Maldonado-Martinez G, Cubano LA, Schneider RJ, Martinez-Montemayor MM. Ganoderma lucidum extract (GLE) impairs breast cancer stem cells by targeting the STAT3 pathway. Oncotarget. 2018;9:35907–35921. doi: 10.18632/oncotarget.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirman Z, de Lange T. 53BP1: a DSB escort. Genes Dev. 2020;34:7–23. doi: 10.1101/gad.333237.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P, Wang J, Gao W, Yuan BZ, Rogers J, Reed E. CHK2 kinase expression is down-regulated due to promoter methylation in non-small cell lung cancer. Mol Cancer. 2004;3:14. doi: 10.1186/1476-4598-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol. 2001;59:657–663. doi: 10.1124/mol.59.4.657. [DOI] [PubMed] [Google Scholar]
- 22.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Li F, Huang Q, Zhang Z, Zhou L, Deng Y, Zhou M, Fleenor DE, Wang H, Kastan MB, Li CY. Self-inflicted DNA double-strand breaks sustain tumorigenicity and stemness of cancer cells. Cell Res. 2017;27:764–783. doi: 10.1038/cr.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majidinia M, Yousefi B. DNA repair and damage pathways in breast cancer development and therapy. DNA Repair (Amst) 2017;54:22–29. doi: 10.1016/j.dnarep.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Aktas BY, Guner G, Guven DC, Arslan C, Dizdar O. Exploiting DNA repair defects in breast cancer: from chemotherapy to immunotherapy. Expert Rev Anticancer Ther. 2019;19:589–601. doi: 10.1080/14737140.2019.1631162. [DOI] [PubMed] [Google Scholar]
- 26.Neizer-Ashun F, Bhattacharya R. Reality CHEK: understanding the biology and clinical potential of CHK1. Cancer Lett. 2020;497:202–211. doi: 10.1016/j.canlet.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Ji P, Zhang Y, Wang SJ, Ge HL, Zhao GP, Xu YC, Wang Y. CD44hiCD24lo mammosphere-forming cells from primary breast cancer display resistance to multiple chemotherapeutic drugs. Oncol Rep. 2016;35:3293–3302. doi: 10.3892/or.2016.4739. [DOI] [PubMed] [Google Scholar]
- 28.Hassan HM, Al-Wahaibi LH, Elmorsy MA, Mahran YF. Suppression of cisplatin-induced hepatic injury in rats through alarmin high-mobility group Box-1 pathway by Ganoderma lucidum: theoretical and experimental study. Drug Des Devel Ther. 2020;14:2335–2353. doi: 10.2147/DDDT.S249093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Qian L, Du N, Liu Y, Zhao X, Zhang X. Ganoderma lucidum polysaccharide enhances radiosensitivity of hepatocellular carcinoma cell line HepG2 through Akt signaling pathway. Exp Ther Med. 2017;14:5903–5907. doi: 10.3892/etm.2017.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao CS, Feng N, Zhou S, Zheng XX, Wang P, Zhang JS, Huang Q. Ganoderic acid T improves the radiosensitivity of HeLa cells via converting apoptosis to necroptosis. Toxicol Res (Camb) 2021;10:531–541. doi: 10.1093/toxres/tfab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faldoni FLC, Villacis RAR, Canto LM, Fonseca-Alves CE, Cury SS, Larsen SJ, Aagaard MM, Souza CP, Scapulatempo-Neto C, Osório CABT, Baumbach J, Marchi FA, Rogatto SR. Inflammatory breast cancer: clinical implications of genomic alterations and mutational profiling. Cancers (Basel) 2020;12:2816. doi: 10.3390/cancers12102816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertucci F, Rypens C, Finetti P, Guille A, Adélaïde J, Monneur A, Carbuccia N, Garnier S, Dirix P, Gonçalves A, Vermeulen P, Debeb BG, Wang X, Dirix L, Ueno NT, Viens P, Cristofanilli M, Chaffanet M, Birnbaum D, Van Laere S. NOTCH and DNA repair pathways are more frequently targeted by genomic alterations in inflammatory than in non-inflammatory breast cancers. Mol Oncol. 2020;14:504–519. doi: 10.1002/1878-0261.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]