Abstract
Background
Preoperative anxiety is a common phenomenon in breast cancer, causing pain and tension, which is not conducive to the effective surgical treatment and postoperative recovery. It is believed that hypnosis can change the patient’s perception of pain, thereby improving the patient’s ability to control pain. However, the results of studies for this topic were controversy. In order to explore the effect of hypnosis in breast cancer surgery we included randomized controlled trials (RCTs) and conducted a meta analysis.
Methods
PubMed, Web of Science, Wiley online library, Elsevier, and Clinicaltrials.gov databases were searched by computer with the keywords “hypnosis/hypnotherapy” and “breast cancer” and “oncologic surgery/surgery/biopsy”. After screening, the meta-analysis was performed using RevMan 5.4 software, and the evidence was rated using GRADE profiler 3.6 software.
Results
A total of 1,242 patients were included in 8 studies, including 630 patients who received preoperative hypnosis and 612 patients who did not receive hypnosis. Meta-analysis showed that hypnosis before general anesthesia reduced the degree of preoperative anxiety (MD =−2.79, 95% CI: −3.93, −1.65, P<0.00001) and postoperative pain (MD =−1.25, 95% CI: −1.64, −0.86, P<0.00001) in patients undergoing breast cancer surgery, but had no effect on the operation time (MD =−6.30, 95% CI: −15.38, 2.78, P=0.17) and the incidence of postoperative nausea and vomiting (OR =0.68, 95% CI: 0.22, 2.07, P=0.49).
Discussion
The application of hypnosis before general anesthesia for breast cancer surgery can reduce the degree of anxiety of patients, also reducing postoperative pain.
Keywords: Breast cancer, general anesthesia, hypnosis, meta-analysis
Introduction
Breast cancer is a phenomenon in which uncontrolled proliferation of breast epithelial cells occurs under the action of a variety of carcinogenic factors. It is often characterized by symptoms such as breast lumps, nipple discharge, and axillary lymphadenopathy in the early stage, and distant metastasis with multiple organ lesions may occur due to cancer cells in the late stage, which directly threatens the patient’s life (1,2). Breast cancer has become one of the most common cancers with the highest mortality rate in women worldwide (3). Surgical treatment is still the treatment of choice for patients with breast cancer, but surgery usually elevates neuroendocrine, metabolic, and inflammation levels in patients, so that patients in the perioperative period produce physical and psychological stress responses, causing pain and tension, which is not conducive to the effective surgical treatment and postoperative recovery (4). Hypnosis uses direct or indirect cues to change the patient’s perception, sensation, emotion, thought, or behavior, so that the patient experiences reduced physiological pain and psychological pain during surgery (5). Hypnosis has been widely used in the medical field, and studies suggest that it can change patients’ perceptions of pain, thereby improving their ability to control pain (6), which could be measured by the Visual Analog Scale (VAS), Verbal Rating Scales (VRS) or other scales (7). It has also been suggested (8) that hypnosis may help reduce the incidence rate of chemotherapy-related side effects such as anticipatory nausea and vomiting by lowering the degree of treatment-related distress. In recent years, a number of controlled clinical studies have applied hypnosis before breast cancer surgery. However, due to the minimally invasive surgical technique of minor breast cancer surgery and the multimodal analgesic strategy (using several analgesic drugs together to containing the pain), the postoperative pain induced by the surgery was limited, which may weaken the effect of hypnosis (9). A study by Amraoui et al. (10) concluded that although patients received hypnosis had significantly lower degree of postoperative anxiety, but they had higher postoperative breast pain score. In this meta-analysis, 8 studies were included, all of which used hypnosis for about 15 minutes before general anesthesia for breast cancer surgery, and the outcome indicators after surgery were analyzed to provide an evidence-based foundation for the effectiveness of hypnosis as an adjuvant therapy.
We present the following article in accordance with the PRISMA reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-114/rc).
Methods
Inclusion of studies
We defined the inclusion of related studies according to the PICOS criteria (Participants, Interventions, Control, Outcomes, Study). (I) Study type: we only included randomized controlled trials (RCTs); (II) participants: all subjects were female breast cancer patients, aged over 18 years, and patients underwent diagnostic breast biopsy or early lumpectomy; (III) intervention methods: there was a comparison of 2 groups of intervention methods in the study, namely hypnosis or no hypnosis before general anesthesia, and we did not include combined intervention methods (such as hypnosis + music therapy, hypnosis + mindfulness therapy, hypnosis + preoperative education, hypnosis + group psychotherapy); (IV) control: the control method was not limited when the study was included. If some studies used blinding, the experimental group and the control group were hypnotized by different therapists (the control group did not implement real hypnosis, but a dialogue similar to hypnosis, so that the patients could not distinguish whether they were hypnotized), while some studies did not have any blind control at all, meaning that patients knew whether they belonged to the hypnosis group. We then assessed patients for quality of intervention delivery after inclusion in the study. We preferred the inclusion of a hypnotic intervention guided by a professional psychotherapist rather than hypnosis guided by an anesthesiologist. We did not limit the hypnotist’s method of hypnosis, which can involve different tools and verbal cues, but the purpose of hypnosis was to guide the patient into a comfortable, safe, and relaxed state. In the experimental group, preoperative education and pain intervention remained consistent with the control group except for hypnosis before general anesthesia; (V) outcomes: we used the degree of postoperative pain, anxiety, operation time, and nausea and vomiting as the primary outcome indicators, while the degree of postoperative fatigue, anesthesiologist stay, hospital stay, anesthetic dosage, analgesic dosage, and satisfaction were the secondary outcome indicators. In order to contain the heterogeneity between studies, we only extracted the pain data measured by VAS, which was from 0 to 10. Also, all of the pain degree data was collected on the day after surgery.
Literature search strategy
From Dec 2021 to Jan 2022, PubMed, Web of Science, Wiley online library, Elsevier databases, CNKI (the China national knowledge infrastructure), Wanfang Data were searched by computer, and to include the latest clinical studies, we also searched the literature on this topic in Clinicaltrials.gov. The input keywords were: “hypnosis/hypnotherapy” and “breast cancer” and “oncologic surgery/surgery/biopsy”. We did not limit the time of literature publication. The starting time of the above databases was the initial time of database establishment.
Literature selection and data extraction
During Dec 2021 to Jan 2022, two of our co-workers independently completed the inclusion screening of the literature. In this process, if any of the inconsistency happened, a 3rd person was introduced to help reaching an agreement. Excel 2020 (published by Microsoft Corp) was used to extract the data. The extracted data included: (I) basic information: the author names, and region of the study, publication date; (II) basic characteristics of the participants: age, gender, disease type, surgical category, education level, preoperative pain level, occupation, and anxiety level; (III) literature intervention methods: specific implementation method of hypnosis, implementation time length, and number of cases grouped; (IV) outcome data: data for the outcome indicators. If there was no data provided in the article, we tried to contact, the original author of the article for it. We extracted mean value (plus standard deviation) for the continuous variables. For the graphical representation of data, unless there was specified data number appeared on the graphical, the data could not be counted.
Risk of bias, heterogeneity and sensitivity analysis
We used the tool Cochrane RoB 2.0 for the risk of bias assessment, which covered 5 aspects: (I) randomization process, (II) deviations from intended interventions, (III) missing outcome data, (IV) measurement of the outcome, (V) selection of the reported result. If there was heterogeneity in the statistical process, we tried to use subgroup analysis for investigation. If the source of heterogeneity could not be identified and confirmed, we only generally describe the situation. Sensitivity analysis was performed by comparing the results of the fixed effects model with the random effects model.
Statistical methods
I2 test analysis and the Q test were used to assess heterogeneity between different studies. I2<50% or P≥0.1 indicated no statistically significant heterogeneity. Mean difference (MD) was used for continuous variables (Postoperative pain, Postoperative anxiety, operation time etc.), odd ratio (OR) was used for binary variables (incidence of postoperative nausea and vomiting), and 95% CI was used as the confidence interval. For each outcome indicator, the results of studies reporting the indicator were pooled for statistics. The fixed effects model was applied if no statistical heterogeneity among studies, or else the random effects model was applied. RevMan 5.4 software provided by the Cochrane Collaboration was used as the analysis tool in this study to present the analysis results in the form of forest plots. Publication bias was depicted in funnel plots and was assessed by Egger’s test quantitatively. GRADE profiler 3.6 software was used for evaluation of the quality of evidence. All of the above were considered two-sided statistically significant when P<0.05.
Results
Literature screening results
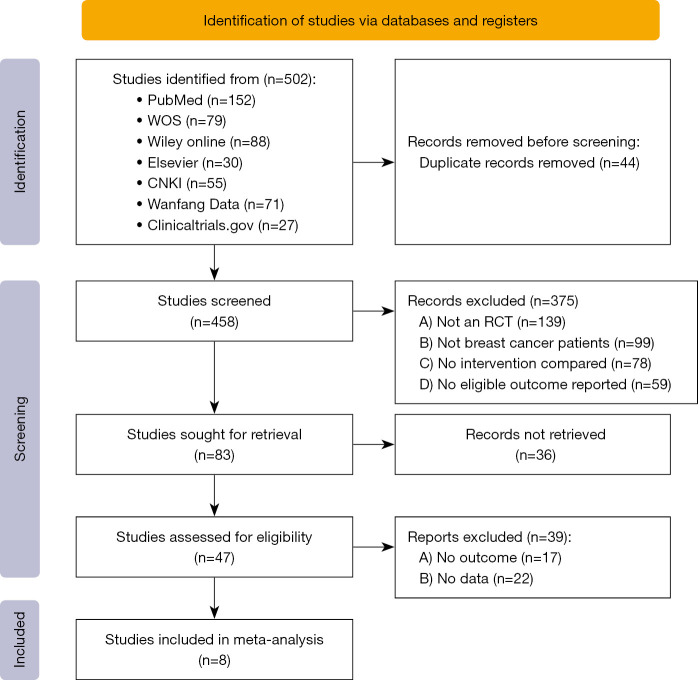
This study initially retrieved 502 articles and finally included 8 studies (10-17). The selection flow chart is shown in Figure 1. In the study by Elkins et al. (18), the effect of preoperative hypnosis on postoperative hot flashes was investigated and the study was excluded as lacking of identified outcome indicators. In the study by Lew et al. (19), the authors only had an experimental group, but no control group, and was therefore excluded. The intervention method in study by Sánchez-Jáuregui et al. (20) was hypnosis + music therapy and was excluded. The study by Grégoire et al. (21) focused on overall psychological intervention before surgery, rather than preoperative hypnosis, and was therefore excluded. The type of study by Potié et al. (22) was a systematic review rather than a clinical control study, and was also excluded. We did not list all the excluded articles, but only listed 5 representative articles.
Figure 1.
The study selection flow chart. WOS, Web of Science; CNKI, China national knowledge infrastructure; RCT, randomized controlled trials.
Basic characteristics of the studies
A total of 1,242 patients were included in this study, including 630 patients who received preoperative hypnosis and 612 patients who did not receive hypnosis. All patients underwent breast cancer surgery. The surgical types included breast lumpectomy and breast tissue biopsy. The youngest subject was 18 years old and the oldest was 92 years old. The shortest preoperative hypnosis time was 2 minutes and the longest was 20 minutes, as shown in Table 1.
Table 1. Basic characteristics, intervention measures, and outcome indicators of the included studies.
| Author | Year of publication | Procedure type | Mean age (years) | Population (E/C) | Experimental group | Hypnosis time (min) | Control group | Outcome indicators | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| Amraoui et al. (10) | 2018 | Tumorectomy or quadrantectomy | 57 (33–79) | 77/73 | Short session before anesthesia induction | 6 (2–15) | Standard care | (a)(b)(c)(d)(e)(f)(g)(h)(i)(j) | 3 |
| Berlière et al. (11) | 2018 | Lumpectomy or mastectomy | 58 | 150/150 | Session before anesthesia induction | N/A | Standard care | (e)(f)(g) | 2 |
| Berliere et al. (12) | 2021 | Oncologic surgery | 53 | 31/32 | Session before anesthesia induction | 20 | Standard care | (a) | 2 |
| Lang et al. (13) | 2000 | Breast biopsy | 57 (18–92) | 82/79 | Self-hypnotic relaxation exercise | N/A | Standard care | (a)(b)(e)(g) | 3 |
| Butler et al. (14) | 2009 | Breast biopsy | 53.1 (30–80) | 63/61 | Self-relaxation hypnotic exercise | N/A | Standard care | (a)(b)(e) | 2 |
| Montgomery et al. (15) | 2010 | Breast cancer surgery | 48.5 | 100/100 | Short session before anesthesia induction | 15 | Standard care | (a)(b)(c) | 2 |
| Schnur et al. (16) | 2008 | Excisional breast biopsy | 45.0 | 49/41 | 15-minute pre-surgery hypnosis session | 15 | Standard care | (a)(e) | 3 |
| Lang et al. (17) | 2006 | Breast biopsy | 50 (18–82) | 78/76 | Self-hypnotic relaxation exercise | N/A | Standard care | (a)(e) | 3 |
Outcome indicators: (a) VAS postsurgical pain; (b) postsurgical nausea/vomiting; (c) fatigue; (d) comfort/well-being; (e) anxiety; (f) PACU length of stay; (g) operative time; (h) use and dose of antiemetics; (i) analgesic consumption; (j) satisfaction. E represents experimental group, C represents control group. VAS, visual Analog scale; PACU, post anesthesia care unit; N/A, not applicable.
Risk of bias assessment of the included studies
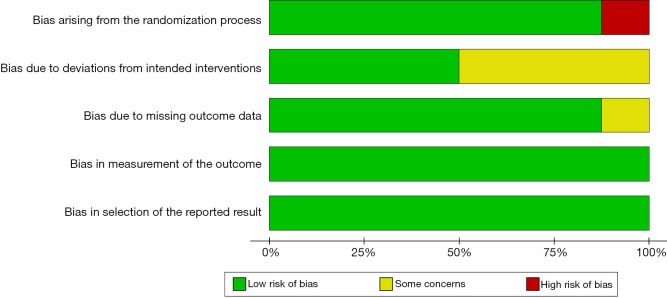
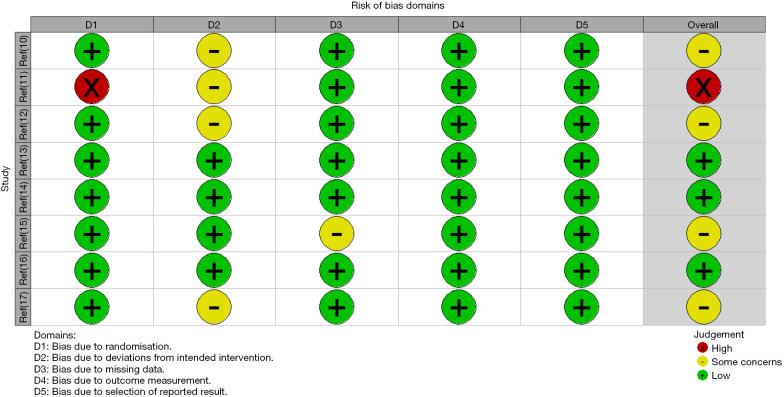
The risk of bias assessment of the included studies using RevMan 5.4 is shown in Figures 2,3. One study (11) only divided patients into the experimental group and control group according to the treatment method, without the random allocation method. There was a large selection bias. Allocation concealment was not described in 4 studies (10,11,12,17), which may introduce significant bias. All studies described the blinding method, and only one study (15) did not describe the dropout cases, which may lead to incomplete data. No selective reporting bias or other biases were found.
Figure 2.
Summary chart of the risk of bias assessment of the included studies.
Figure 3.
Risk of bias assessment chart of the included studies.
Meta-analysis results
Postoperative pain
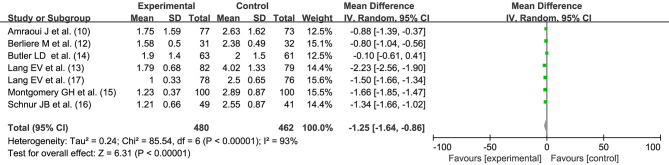
Seven studies (10,12-17) reported the effect of hypnosis before general anesthesia on postoperative pain after breast cancer surgery, with statistical heterogeneity between the studies (I2=93%, P<0.00001). Hypnosis before general anesthesia reduced the postoperative pain of patients undergoing breast cancer surgery (MD =−1.25, 95% CI: −1.64, −0.86, P<0.00001) performed by random effects model, as shown in Figure 4.
Figure 4.
Effect of hypnosis before general anesthesia on postoperative pain after breast cancer surgery. SD, standard deviation; IV, inverse variance; CI, confidence interval.
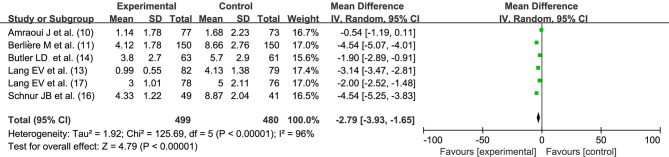
Preoperative anxiety
Six studies (10,11,13,14,16,17) reported the degree of preoperative anxiety, with the number of 499 and 480 for both the groups, respectively. There was statistical heterogeneity between the studies (I2=96%, P<0.00001), Hypnosis before general anesthesia reduced the degree of anxiety before breast cancer surgery (MD =−2.79, 95% CI: −3.93, −1.65, P<0.00001) performed by random effects model, as shown in Figure 5.
Figure 5.
Effect of hypnosis before general anesthesia on postoperative anxiety after breast cancer surgery. SD, standard deviation; IV, inverse variance; CI, confidence interval.
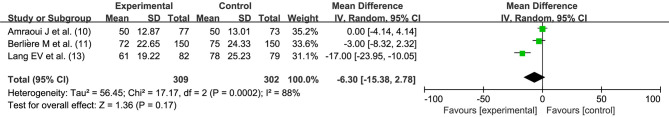
Operation time (minutes)
Three studies (10,11,13) performed comparisons of operation time between the 2 intervention methods. There was statistical heterogeneity between the studies (I2=88%, P=0.0002). Hypnosis before general anesthesia had no effect on the operation time (MD =−6.30, 95% CI: −15.38, 2.78, P=0.17) performed by random effects model, as shown in Figure 6.
Figure 6.
Effect of hypnosis before general anesthesia on the operation time of breast cancer surgery. SD, standard deviation; IV, inverse variance; CI, confidence interval.
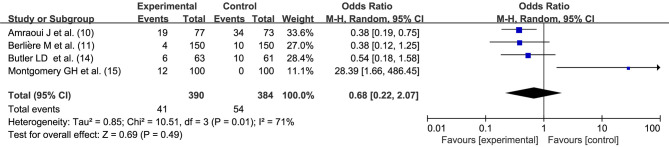
Incidence of postoperative nausea and vomiting
Four studies (10,11,14,15) reported postoperative nausea and vomiting adverse reactions. There was statistical heterogeneity between the studies (I2=71%, P=0.01). Hypnosis before general anesthesia had no effect on the incidence rate of postoperative nausea and vomiting (OR =0.68, 95% CI: 0.22, 2.07, P=0.49) performed by random effects model, as shown in Figure 7.
Figure 7.
Effect of hypnosis before general anesthesia on the incidence of postoperative nausea and vomiting after breast cancer surgery. SD, standard deviation; M-H, Mantel Haenszel; CI, confidence interval.
Heterogeneity investigation and sensitivity analysis
Significant heterogeneity among the studies ocurred in the case of meta analysing all the 4 indicators. The source of heterogeneity may be from the different types of surgery for patients. However, some studies included multiple surgery types, while some studies did not describe surgery type, we could not perform a subgroup analysis by the type of surgery. Patient age stratification may also be a source of heterogeneity. In addition, different evaluation criteria for outcome indicators were also sources of heterogeneity. The random effects model results were consistent with the fixed effects model results, indicating that the results were stable.
Analysis of publication bias
In the analysis of postoperative pain outcome, the funnel plot showed a evenly distribution for both sides, suggesting that there was little publication bias, as shown in Figure 8.
Figure 8.
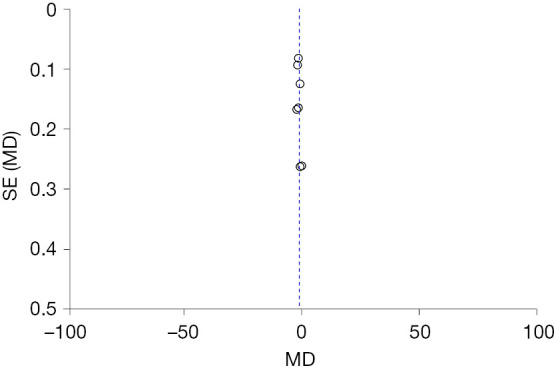
Funnel plot for the analysis of hypnosis before general anesthesia for postoperative pain after breast cancer surgery. MD, mean difference, SE, standard error.
The P value of Egger’s test was 0.280, which showed that there was little publication bias, as shown in Figure 9.
Figure 9.
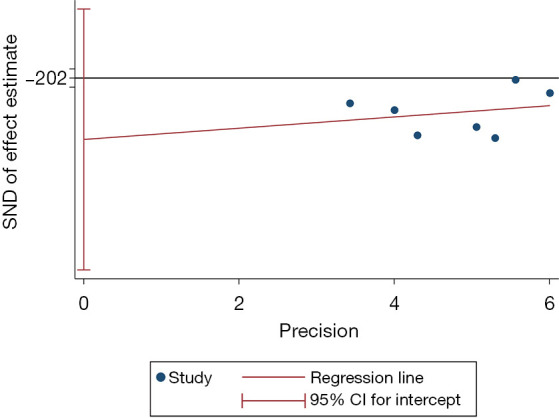
Egger’ test result for the analysis of hypnosis before general anesthesia for postoperative pain after breast cancer surgery. SND, standard normal deviate; CI, confidence interval.
GRADE evidence quality analysis
GRADE was used to evaluate the quality of the evidence, and the 4 outcome indicators were all graded as moderate, as shown in Table 2.
Table 2. Grading of evidence for 4 outcome indicators.
| Outcomes | Number of studies | Number of participants | Relative effect (95%CI) | Quality of the evidence |
|---|---|---|---|---|
| Post surgical pain | 7 | 942 | MD =−1.25 (−1.64, −0.86) | Moderate |
| Post surgical anxiety | 6 | 979 | MD =−2.79 (−3.93, −1.65) | Moderate |
| Operating time | 3 | 611 | MD =−6.30 (−15.38, 2.78) | Moderate |
| Post nausea rate | 4 | 774 | OR =0.68 (0.22, 2.07) | Moderate |
MD, mean difference; CI, confidence interval.
Discussion
The diagnosis of breast cancer places great pressure on patients, and surgery causes changes to a patient’s appearance, which will impact gender identity in female patients and may cause anxiety and depression (23). In addition, fear of surgery, fear of pain during surgery, inability to wake up from anesthesia, and postoperative discomfort increase the patient’s anxiety. Clinical study (24) has shown that preoperative mental health (positive affect, optimism) can predict postoperative acute pain and is a protective factor for postoperative chronic pain, and this protection can even continue until 4 months after surgery. The effect of hypnotherapy on anxiety reduction has been demonstrated by several studies and applied in different surgeries. The study by Hirzlı et al. (25) applied hypnotherapy for 10 minutes before ultrasound-guided needle biopsy in patients with prostate cancer to relax patients and reduce tension, and the results showed that both tension and pain were reduced in patients. A study by Hemmerling et al. (26) showed that patients who underwent hypnosis before surgery had a better sense of pain control than those who did not, which reduced the amount of analgesia required. The use of hypnosis in breast cancer surgery was first seen in a controlled clinical study published by Spiegel and Bloom et al. (27), which showed for the first time that hypnosis can reduce pain in patients undergoing breast cancer surgery. Since then, the effectiveness of hypnotic oncological therapy has expanded from initial pain control to improvement in a variety of outcome indicators.
In this meta-analysis, 8 controlled clinical studies were included. The intervention measure was hypnosis before general anesthesia for surgery. The pooled effect size showed that the implementation of hypnotic surgery was beneficial for reducing the degree of postoperative pain and anxiety. However, in this analysis, it did not improve the operation time and postoperative nausea and vomiting side effects. Jackson et al. (28) showed that anxiety prior to surgery was strongly associated with multiple outcome indicators of surgery, such as pain intensity, pain medication use, and functional impairment. From a neurocognitive and neuroscientific point of view, hypnosis is thought to be mediated by the right cerebral hemisphere, while from a neuroanatomical point of view, it has been possible to find local gray matter related to hypnotic cues in some regions of the frontal, temporal, and occipital cortices. Although the neurobiological basis of hypnosis has not been elucidated, the results suggest that the attention skills involved in hypnosis may be related to central dopaminergic activity, and hypnotic cues cause changes in the brain’s perception and interpretation of input elements, accompanied by changes in neural connection (29). After hypnosis, the patient’s perception of pain changes and their threshold is increased, so the degree of pain decreases (30).
In addition to reducing pain perception, several studies have shown that hypnosis can be applied in the treatment of hot flashes in breast cancer patients, improving symptoms and the quality of life of patients (31). The study by Lang et al. (17) compared the medical costs of hypnosis with 2 other interventions (standard care versus structured empathic attention) and concluded that hypnosis provides more powerful anxiety relief without excessive costs and is therefore more attractive for outpatient pain management. The study (32) has explored the applicable population for hypnosis, concluding that elderly patients are also able to be hypnotized and that the role of hypnotic analgesia does not appear to diminish with age during invasive medical procedures.
In this study, GRADE evidence quality analysis showed that the evidence grading of the 4 outcome indicators was moderate. The funnel plot showed that both sides were evenly distributed, indicating no significant publication bias, which was proved by the Egger’s test results. Sensitivity analysis showed that the results were stable. However, there were studies that did not use the random allocation method, which may cause randomization bias. In addition, in this meta-analysis, there was significant heterogeneity among studies, and we found no improvement in operation time and postoperative nausea and vomiting after hypnosis. More controlled clinical studies using the same evaluation method with the same case characteristics are needed for deeper investigations in the future due to the risk of bias.
Conclusions
In this meta-analysis, 8 studies were included. Meta-analysis showed that the application of hypnosis before general anesthesia for breast cancer surgery could reduce the degree of anxiety of patients, thereby reducing postoperative pain.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-114/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-114/coif). The authors have no conflicts of interest to declare.
(English Language Editor: C. Betlazar-Maseh)
References
- 1.Fahad Ullah M. Breast Cancer: Current Perspectives on the Disease Status. Adv Exp Med Biol 2019;1152:51-64. 10.1007/978-3-030-20301-6_4 [DOI] [PubMed] [Google Scholar]
- 2.Liang Y, Zhang H, Song X, et al. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol 2020;60:14-27. 10.1016/j.semcancer.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 3.Anastasiadi Z, Lianos GD, Ignatiadou E, et al. Breast cancer in young women: an overview. Updates Surg 2017;69:313-7. 10.1007/s13304-017-0424-1 [DOI] [PubMed] [Google Scholar]
- 4.Tosello G, Torloni MR, Mota BS, et al. Breast surgery for metastatic breast cancer. Cochrane Database Syst Rev 2018;3:CD011276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montgomery GH, Schnur JB, Erblich J, et al. Presurgery psychological factors predict pain, nausea, and fatigue one week after breast cancer surgery. J Pain Symptom Manage 2010;39:1043-52. 10.1016/j.jpainsymman.2009.11.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash MR, Perez N, Tasso A, et al. Clinical research on the utility of hypnosis in the prevention, diagnosis, and treatment of medical and psychiatric disorders. Int J Clin Exp Hypn 2009;57:443-50. 10.1080/00207140903099153 [DOI] [PubMed] [Google Scholar]
- 7.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073-93. 10.1016/j.jpainsymman.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 8.Joudi M, Fathi M, Izanloo A, et al. An Evaluation of the Effect of Hypnosis on Postoperative Analgesia following Laparoscopic Cholecystectomy. Int J Clin Exp Hypn 2016;64:365-72. 10.1080/00207144.2016.1171113 [DOI] [PubMed] [Google Scholar]
- 9.Montgomery GH, Bovbjerg DH, Schnur JB, et al. A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. J Natl Cancer Inst. 2007;99:1304-12. 10.1093/jnci/djm106 [DOI] [PubMed] [Google Scholar]
- 10.Amraoui J, Pouliquen C, Fraisse J, et al. Effects of a Hypnosis Session Before General Anesthesia on Postoperative Outcomes in Patients Who Underwent Minor Breast Cancer Surgery: The HYPNOSEIN Randomized Clinical Trial. JAMA Netw Open 2018;1:e181164. 10.1001/jamanetworkopen.2018.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berlière M, Roelants F, Watremez C, et al. The advantages of hypnosis intervention on breast cancer surgery and adjuvant therapy. Breast 2018;37:114-8. 10.1016/j.breast.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 12.Berliere M, Piette N, Bernard M, et al. Hypnosis Sedation Reduces the Duration of Different Side Effects of Cancer Treatments in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Cancers (Basel) 2021;13:4147. 10.3390/cancers13164147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang EV, Benotsch EG, Fick LJ, et al. Adjunctive non-pharmacological analgesia for invasive medical procedures: a randomised trial. Lancet 2000;355:1486-90. 10.1016/S0140-6736(00)02162-0 [DOI] [PubMed] [Google Scholar]
- 14.Butler LD, Koopman C, Neri E, et al. Effects of supportive-expressive group therapy on pain in women with metastatic breast cancer. Health Psychol 2009;28:579-87. 10.1037/a0016124 [DOI] [PubMed] [Google Scholar]
- 15.Montgomery GH, Hallquist MN, Schnur JB, et al. Mediators of a brief hypnosis intervention to control side effects in breast surgery patients: response expectancies and emotional distress. J Consult Clin Psychol 2010;78:80-8. 10.1037/a0017392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnur JB, Bovbjerg DH, David D, et al. Hypnosis decreases presurgical distress in excisional breast biopsy patients. Anesth Analg 2008;106:440-4, table of contents. 10.1213/ane.0b013e31815edb13 [DOI] [PubMed] [Google Scholar]
- 17.Lang EV, Berbaum KS, Faintuch S, et al. Adjunctive self-relaxation hypnotic for outpatient medical procedures: a prospective randomized trial with women undergoing large core breast biopsy. Pain 2006;126:155-64. 10.1016/j.pain.2006.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elkins G, Marcus J, Stearns V, et al. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. J Clin Oncol 2008;26:5022-6. 10.1200/JCO.2008.16.6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lew MW, Kravits K, Garberoglio C, et al. Use of preoperative hypnosis to reduce postoperative pain and anesthesia-related side effects. Int J Clin Exp Hypn 2011;59:406-23. 10.1080/00207144.2011.594737 [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Jáuregui T, Téllez A, Juárez-García D, et al. Clinical Hypnosis and Music In Breast Biopsy:A Randomized Clinical Trial. Am J Clin Hypn 2019;61:244-57. 10.1080/00029157.2018.1489776 [DOI] [PubMed] [Google Scholar]
- 21.Grégoire C, Faymonville ME, Vanhaudenhuyse A, et al. Effects of hypan intervention combining self-care and self-intervention on fatigue and associated symptoms in post-treatment cancer patients: A randomized-controlled trial. Psychooncology 2020;29:1165-73. 10.1002/pon.5395 [DOI] [PubMed] [Google Scholar]
- 22.Potié A, Roelants F, Pospiech A, et al. Hypnosis in the Perioperative Management of Breast Cancer Surgery: Clinical Benefits and Potential Implications. Anesthesiol Res Pract 2016;2016:2942416. 10.1155/2016/2942416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery GH, Bovbjerg DH. Presurgery distress and specific response expectancies predict postsurgical outcomes in surgery patients confronting breast cancer. Health Psychol 2004;23:381-7. 10.1037/0278-6133.23.4.381 [DOI] [PubMed] [Google Scholar]
- 24.Powell R, Scott NW, Manyande A, et al. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database Syst Rev 2016;(5):CD008646. 10.1002/14651858.CD008646.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hızlı F, Özcan O, Selvi İ, et al. The effects of hypnotherapy during transrectal ultrasound-guided prostate needle biopsy for pain and anxiety. Int Urol Nephrol 2015;47:1773-7. 10.1007/s11255-015-1111-0 [DOI] [PubMed] [Google Scholar]
- 26.Hemmerling TM, Charabati S, Zaouter C, et al. A randomized controlled trial demonstrating that a novel closed-loop propofol system performs better hypnosis control than manual administration. Can J Anaesth 2010;57:725-35. 10.1007/s12630-010-9335-z [DOI] [PubMed] [Google Scholar]
- 27.Spiegel D, Bloom JR. Group therapy and hypnosis reduce metastatic breast carcinoma pain. Psychosom Med 1983;45:333-9. 10.1097/00006842-198308000-00007 [DOI] [PubMed] [Google Scholar]
- 28.Jackson T, Tian P, Wang Y, et al. Toward Identifying Moderators of Associations Between Presurgery Emotional Distress and Postoperative Pain Outcomes: A Meta-Analysis of Longitudinal Studies. J Pain 2016;17:874-88. 10.1016/j.jpain.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 29.McGeown WJ, Mazzoni G, Vannucci M, et al. Structural and functional correlates of hypnotic depth and suggestibility. Psychiatry Res 2015;231:151-9. 10.1016/j.pscychresns.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 30.Rizzo RRN, Medeiros FC, Pires LG, et al. Hypnosis Enhances the Effects of Pain Education in Patients With Chronic Nonspecific Low Back Pain: A Randomized Controlled Trial. J Pain 2018;19:1103.e1-9. 10.1016/j.jpain.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 31.Elkins G, Marcus J, Palamara L, et al. Can hypnosis reduce hot flashes in breast cancer survivors? A literature review. Am J Clin Hypn 2004;47:29-42. 10.1080/00029157.2004.10401473 [DOI] [PubMed] [Google Scholar]
- 32.Lutgendorf SK, Lang EV, Berbaum KS, et al. Effects of age on responsiveness to adjunct hypnotic analgesia during invasive medical procedures. Psychosom Med 2007;69:191-9. 10.1097/PSY.0b013e31803133ea [DOI] [PubMed] [Google Scholar]