Abstract
Symptom severity in patients with human rhinovirus (HRV)-induced respiratory illness is associated with elevated levels of the inflammatory cytokines interleukin-6 (IL-6) and IL-8. AG7088 is a novel, irreversible inhibitor of the HRV 3C protease. In this study, AG7088 was tested for its antiviral activity and ability to inhibit the production of IL-6 and IL-8 in a human bronchial epithelial cell line, BEAS-2B. Infection of BEAS-2B cells with HRV 14 resulted in the production of both infectious virus and the cytokines IL-6 and IL-8. Treatment of HRV 14-infected cells with AG7088 resulted in a statistically significant (P, <0.05) dose-dependent reduction in the levels of infectious virus as well as IL-6 and IL-8 released into the cell supernatant compared to the results obtained for compound-free infected cells. AG7088 was also able to inhibit the replication of HRV 2 and 16 in BEAS-2B cells. In time-of-addition studies, AG7088 could be added as late as 14 to 26 h after HRV 14 infection of BEAS-2B cells and still result in a statistically significant (P, <0.05) reduction in the levels of infectious virus, IL-6, and IL-8 compared to the results obtained for compound-free infected cells. These findings have implications for the development of an antirhinovirus agent that may not only block virus replication but also diminish symptoms.
Human rhinoviruses (HRV), which include over 100 different virus serotypes, are responsible for a significant portion of common colds experienced each year (reviewed in references 7, 26, and 34). In patients with underlying respiratory disorders, HRV infections may lead to sinusitis, otitis media, and lower-respiratory-tract illnesses and also may lead to exacerbations of asthma, cystic fibrosis, and bronchitis (3). Many of the clinical symptoms observed in patients with HRV-induced respiratory illness, such as sore throat, nasal congestion, sneezing, and runny nose, are associated with elevated cytokine levels that can be detected in nasal washings (27, 31, 33). In addition, experimental HRV infections have been shown to produce an increase in the levels of one or more of the inflammatory cytokines and mediators, including interleukin-6 (IL-6), IL-8, IL-1β, kinins, tumor necrosis factor, histamine, and soluble intercellular adhesion molecule 1 (ICAM-1) (32, 38, 45; R. B. Turner, K. Weingand, C. H. Yeh, et al. Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother. abstr. H-48, 1996). Increased levels of both IL-6 (45) and IL-8 (39; Turner et al., 36th ICAAC) have been associated with symptom severity in HRV-infected patients.
Although a number of compounds have shown in vitro activity against HRV, no antiviral agent has been shown to be effective against disease caused by HRV infection in vivo (1, 2, 8, 15, 17, 28). To date, only one agent, pirodavir, has been shown to be effective when administered at the time of HRV challenge but not when given 24 h after HRV infection (19). Recent reports have described a new class of compounds directed toward a novel target, the HRV 3C protease (10–14, 20, 22–25, 29, 35–37, 40–42, 44). The HRV 3C protease is an enzyme responsible for the posttranslational cleavage of viral precursor polyproteins into their mature forms. AG7088 is a novel, irreversible inhibitor of HRV 3C protease which potently inhibits cytopathic effects induced in H1-HeLa cells by all HRV serotypes tested (48 of 48), with a mean 50% effective concentration (EC50) of 0.023 μM, a mean EC90 of 0.082 μM, and no cytotoxicity observed up to 1,000 μM (30). The EC50 refers to the concentration of compound that increased the percentage of live cells, as measured by formazan production, to 50% that of uninfected, non-compound-treated cells. In this study, we have extended these findings and have evaluated the ability of AG7088 to inhibit virus replication as well as cytokine production in a human bronchial epithelial cell line, BEAS-2B.
MATERIALS AND METHODS
Compound.
AG7088 was synthesized at Agouron Pharmaceuticals, Inc.
Cells and virus strains.
All HRV serotypes were purchased from the American Type Culture Collection (ATCC), Manassas, Va. HRV stocks were propagated in H1-HeLa cells (ATCC), and antiviral assays were performed with BEAS-2B cells (ATCC) incubated at 34°C. H1-HeLa cells were grown in minimal essential medium (Life Technologies, Gaithersburg, Md.) with 10% fetal bovine serum (HyClone, Logan, Utah), and BEAS-2B cells were grown in serum-free bronchial epithelial cell growth medium (Clonetics, San Diego, Calif.).
Antiviral assay.
Confluent BEAS-2B cells were infected at a multiplicity of infection (MOI) of 50 for HRV 14 or mock infected with medium only. After 2 h of incubation, cells were washed to remove virus inoculum and resuspended in medium containing appropriate concentrations of compound or medium only. The compound was added to the cells at the time of virus infection. After 3 days of infection, the cell supernatants were removed, clarified by centrifugation (2 min at 16,000 × g and 20°C), and either stored at −70°C for subsequent use or analyzed immediately for IL-6 and IL-8 by an enzyme-linked immunosorbent assay (ELISA) and for infectious virus by a virus yield assay. To determine the amount of intracellular virus present, the cells were washed two times, frozen and thawed two times, sonicated, clarified by centrifugation (2 min at 16,000 × g and 20°C), and either stored at −70°C for subsequent use or analyzed immediately for infectious virus. In certain experiments, uninfected BEAS-2B cells were treated with 50 μg of Escherichia coli lipopolysaccharide (LPS) (Sigma, St. Louis, Mo.) per ml for 2 h, washed two times, and incubated with compound for 3 days. Cell supernatants were analyzed for the presence of IL-8.
Cell cytotoxicity assay.
The cell cytotoxicity of AG7088 was measured by a dye reduction method (43). Briefly, BEAS-2B cells were resuspended at 5 × 104 cells per ml in medium containing appropriate concentrations of compound or medium only. Three days later, 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT) (Sigma)–phenazine methosulfate (Sigma) was added to the test plates, and the amount of formazan produced was quantified spectrophotometrically at 450 and 650 nm. Data were expressed as the percentage of formazan produced in compound-treated cells compared to that produced in compound-free cells. The 50% cytotoxic concentration was calculated as the concentration of compound that decreased the percentage of formazan produced in compound-treated cells to 50% that produced in compound-free cells.
Time-of-addition assay.
Confluent monolayers of BEAS-2B cells were infected with HRV at an MOI of 30 or mock infected with medium only. After 2 hours of virus adsorption, the monolayers were washed two times with medium and replenished with fresh medium. AG7088 was added at a concentration (10 μM) that was at least 10-fold above that needed to completely inhibit HRV 14 replication in BEAS-2B cells, either before virus infection or at various time points thereafter. Following 3 days of infection, cell supernatants were removed, clarified by centrifugation (2 minutes at 16,000 × g and 20°C), and either stored at −70°C for subsequent use or analyzed immediately for IL-6 and IL-8 content and for infectious virus.
Virus yield assay.
Infectious virus titers were determined by a virus plaque assay. Briefly, 0.2 ml of serial 10-fold dilutions of virus was allowed to adsorb to monolayers of H1-HeLa cells. After 1 h of incubation, the cell monolayers were washed twice with phosphate-buffered saline and overlaid with medium containing 0.5% SeaPlaque agarose (FMC Bioproducts, Rockland, Maine). After 3 days of incubation, the cell monolayers were fixed with EAF (65% ethanol, 22% acetic acid, 4% formaldehyde) and stained with 1% crystal violet, and virus plaques were enumerated. Data were expressed as PFU per milliliter.
ELISA.
Levels of both IL-6 and IL-8 were determined using a Quantikine ELISA kit (R&D Systems, Minneapolis, Minn.) according to the manufacturer's instructions. Data were expressed in picograms per milliliter and were derived by extrapolation from a standard curve that was generated in parallel with each experiment. The concentrations of each cytokine obtained in compound-treated infected cells were corrected by subtracting the concentrations of each cytokine obtained in compound-free uninfected cells.
Statistical analysis.
Statistical significance was determined with a one-way analysis of variance (SAS version 6.12; SAS Institute Inc., Cary, N.C.).
RESULTS
HRV 14 infection of BEAS-2B cells.
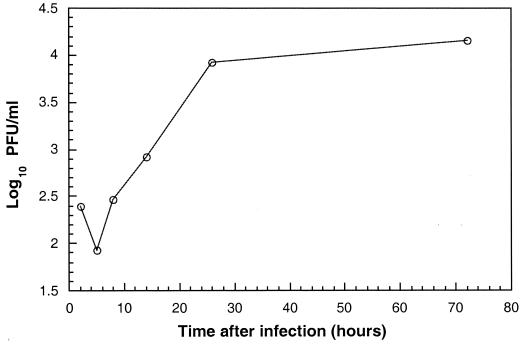
BEAS-2B cells were initially infected with a high MOI of HRV 14 to evaluate the time course of virus production during a single cycle of virus replication. Following an eclipse phase of approximately 4 h, levels of infectious virus released into the cell supernatant increased until reaching a plateau at 24 h after infection (Fig. 1). These levels of infectious virus were maintained throughout the 72-h time period studied. Comparable levels of infectious virus in cellular lysates were also detected after 72 h of infection (data not shown). Microscopic evaluation of infected BEAS-2B cells revealed a lack of virus-induced cytopathology; cells remained viable during the entire 72-h time period studied.
FIG. 1.
HRV 14 production in BEAS-2B cells. BEAS-2B cells were infected with HRV 14 at an MOI of 30, and levels of infectious virus were determined at various times after infection (hours) as described in Materials and Methods. Data represent the mean of duplicate or triplicate determinations.
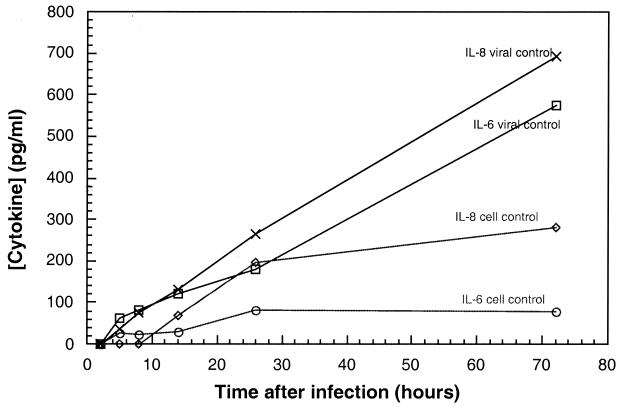
Levels of IL-6 and IL-8 released into the cell supernatant were assayed in parallel. In contrast to the levels of infectious virus, which increased until reaching a plateau at 24 h after infection, the levels of IL-6 and IL-8 continued to increase throughout the 72-h time period studied (Fig. 2). In some experiments, BEAS-2B cells were infected with HRV 2 and HRV 16. Levels of infectious virus as well as IL-6 and IL-8 comparable to those observed in HRV 14-infected cells were detected (data not shown).
FIG. 2.
Cytokine production in HRV 14-infected BEAS-2B cells. BEAS-2B cells were infected with HRV 14 at an MOI of 30 or mock infected with medium only, and levels of the cytokines IL-6 and IL-8 were determined at various times after virus infection (hours) as described in Materials and Methods. Data represent the mean of duplicate or triplicate determinations.
IL-6 and IL-8 were also produced in uninfected BEAS-2B cell supernatants. These levels were significantly lower (two- to fivefold) at 72 h than those detected in infected cell supernatants. In addition, in contrast to the levels produced in infected cells, which continued to increase throughout the 72-h time period studied, the levels in uninfected cells increased slowly until reaching a plateau at 24 h.
Antiviral activity of AG7088 against HRV infection of BEAS-2B cells.
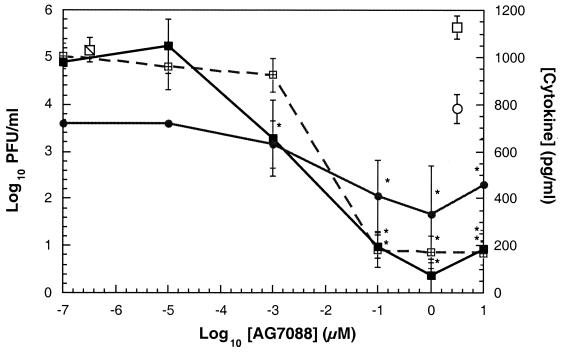
The antiviral activity and cytotoxicity of AG7088 were evaluated by use of BEAS-2B cells infected with HRV 14. The results indicated that AG7088 was able to produce a statistically significant (P, <0.05) dose-dependent reduction in the levels of infectious virus released into the cell supernatant compared to the results for compound-free infected cells (Fig. 3). Potent antiviral activity was observed, with concentrations of AG7088 of at least 0.1 μM being sufficient to produce a greater than 99.9% reduction in the levels of infectious virus released into the medium compared to the levels of infectious virus (5.15 ± 0.69 log10 PFU/ml) produced in compound-free infected cells. AG7088 was also able to produce a statistically significant reduction in the levels of intracellular virus compared to those in compound-free infected cells (data not shown). No cytotoxicity was observed with AG7088 up to the tested concentration of 320 μM in BEAS-2B cells by use of the XTT dye reduction method (data not shown).
FIG. 3.
Activity of AG7088 against HRV 14 infection in BEAS-2B cells. BEAS-2B cells were infected with HRV 14 at an MOI of 50 and incubated with various concentrations of AG7088. Concentrations of the cytokines IL-6 (■) and IL-8 (●) and levels of infectious virus (log10 PFU/ml) (⊞) were determined as described in Materials and Methods and are expressed as the mean ± standard deviation of triplicate determinations. The levels of infectious virus ( ), IL-6 (□), and IL-8 (○) found in compound-free infected cells are shown. The IL-6 and IL-8 values were corrected for the cytokine levels found in compound-free uninfected cells as described in Materials and Methods. Statistical significance (P, <0.05; ∗) was determined by comparing each cytokine or infectious virus value to that obtained in compound-free infected cells.
), IL-6 (□), and IL-8 (○) found in compound-free infected cells are shown. The IL-6 and IL-8 values were corrected for the cytokine levels found in compound-free uninfected cells as described in Materials and Methods. Statistical significance (P, <0.05; ∗) was determined by comparing each cytokine or infectious virus value to that obtained in compound-free infected cells.
Concomitant with the decrease in the levels of infectious virus, AG7088 was also able to inhibit the HRV-induced production of IL-6 and IL-8 in a dose-dependent manner (Fig. 3). In these experiments, concentrations of at least 0.1 μM were sufficient to cause a statistically significant (P, <0.05) decrease in the levels of both cytokines compared to those in compound-free infected cells. This effect was specific for virus-infected cells, since AG7088 did not inhibit IL-8 production induced by LPS treatment or inhibit IL-8 production in uninfected BEAS-2B cells (data not shown). AG7088 also demonstrated comparable activity against the replication of HRV 2 and HRV 16 as well as against HRV 2- and HRV 16-induced production of IL-6 and IL-8 (data not shown).
Time-of-addition assay.
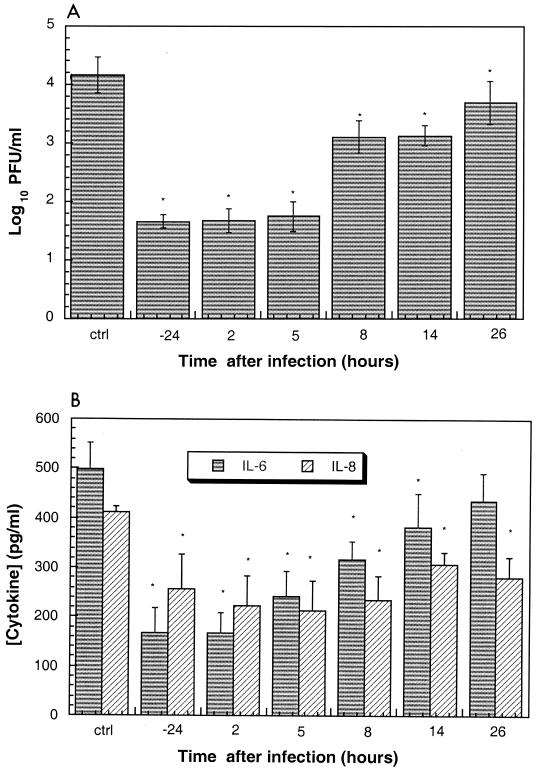
The ability of AG7088 to inhibit virus replication and HRV-induced cytokine production when added at various times during a single cycle of virus replication was also evaluated. Results indicated that AG7088 could be added as late as 26 h after virus infection and still achieve a statistically significant (P, <0.05) reduction in the levels of infectious virus compared to those in compound-free infected cells (Fig. 4A). Likewise, AG7088 could be added up to 14 and 26 h after virus infection and produce a statistically significant (P, <0.05) reduction in the amounts of IL-6 and IL-8, respectively, compared to those in compound-free infected cells (Fig. 4B).
FIG. 4.
Time-of-addition assay showing the effects on infectious virus replication and cytokine production. BEAS-2B cells were infected with HRV 14 at an MOI of 30, and AG7088 (10 μM) was added at various times before and after virus infection as indicated (hours). Following 3 days of infection, levels of infectious virus (A) or IL-6 and IL-8 (B) were determined as described in Materials and Methods. Data represent the mean ± standard deviation of triplicate determinations. The levels of IL-6 and IL-8 were corrected for the levels found in compound-free uninfected cells as described in Materials and Methods. Levels of infectious virus (A) or IL-6 and IL-8 (B) found in compound-free infected cells are designated ctrl. Statistical significance (P, <0.05; ∗) was determined by comparing each value to that obtained in compound-free infected cells.
DISCUSSION
The symptoms associated with both natural and experimental rhinovirus infections, such as sneezing, nasal congestion, sore throat, cough, headache, and malaise, begin shortly after infection and follow the course of virus replication (9). These symptoms are associated with a number of inflammatory mediators, such as histamine, bradykinin, prostaglandins, IL-1β, IL-6, IL-8, tumor necrosis factor, kinins, and soluble ICAM-1 (27, 31, 33). IL-6 and IL-8 have been shown to be associated with symptom severity in both natural and experimental rhinovirus infections (39, 45; Turner et al., 36th ICAAC). In this study, we evaluated the ability of an HRV 3C protease inhibitor, AG7088, to inhibit HRV replication and HRV-induced cytokine production in a human bronchial epithelial cell line, BEAS-2B. In vivo, nasal epithelial cells represent the target host cell for HRV replication (5). Although BEAS-2B cells are bronchial epithelial cells that have been transformed with an adenovirus type 12-simian virus 40 hybrid, they share many properties in common with normal respiratory epithelial cells and thus represent a biologically relevant system for studying HRV infections in vitro (38).
In these experiments, infection of BEAS-2B cells with HRV resulted in the production of significant levels of both infectious virus and the inflammatory cytokines IL-6 and IL-8, while cell viability was maintained throughout the time period studied. These findings are consistent with previous studies that have shown that productive infection of BEAS-2B cells or A549 cells, a cell line of epithelial origin, with HRV results in the production of IL-6 and/or IL-8 in the absence of cell cytopathic effects (6, 21, 38). The continued viability of BEAS-2B cells is in contrast to the complete destruction of H1-HeLa cells observed during an HRV infection in vitro (30). It is not clear which of these two pathways is followed during an HRV infection in patients. In one study, although a few small foci of cell destruction in the nasal epithelium were observed, the majority of the cell layer still appeared intact (4).
AG7088 not only demonstrated potent antiviral activity in inhibiting HRV 14 replication in BEAS-2B cells but was also efficacious against the replication of other HRV serotypes tested. These results are consistent with data generated in H1-HeLa cells, in which AG7088 demonstrated antiviral activity against all HRV serotypes tested, but are in contrast to data obtained for capsid binding inhibitors, which demonstrated extensive variability in antiviral activity against HRV serotypes (1, 28, 30).
AG7088 was also able to concomitantly reduce the levels of both inflammatory cytokines, IL-6 and IL-8. The inhibition was specific for virus infection, since the compound had no effect on the cytokines induced in LPS-treated BEAS-2B cells or produced by uninfected cells. This result is consistent with studies demonstrating a reduction of IL-8 levels after inhibition of HRV infection in BEAS-2B cells by an antibody to ICAM-1 (38). The relevance of the ability of AG7088 to inhibit virus replication and cytokine production in vitro can be ascertained from recent human clinical trials with zamanivir, a sialic acid analogue with activity against influenza virus infection (18). In these studies, treatment with zamanivir delivered intravenously prior to viral challenge caused a statistically significant reduction in both upper-respiratory-tract symptoms and cytokine levels (16; D. P. Calfee, A. W. Peng, L. M. R. Cass, M. Lobo, and F. G. Hayden, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H58, 1998; R. S. Fritz, F. G. Hayden, D. P. Clafee, L. M. R. Cass, A. W. Peng, W. G. Alvord, and S. E. Straus, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H57, 1998).
The efficacy of AG7088 when its addition was delayed until several hours after virus infection was demonstrated in a time-of-addition assay. The results showing that AG7088 was still active even when it was added late in the infection cycle are consistent with the requirement for 3C protease activity throughout the virus life cycle (30). The evaluation of AG7088 as an antiviral compound in human clinical trials has recently begun. The finding that AG7088 is able to inhibit HRV-induced cytokine production when added throughout the virus life cycle in vitro indicates that the compound not only may be effective when administered prophylactically but also may be effective therapeutically when administered after symptoms have begun.
ACKNOWLEDGMENTS
We thank Min Zhang for helping with the statistical analysis and Jules Beardsley for help in preparation of the manuscript.
REFERENCES
- 1.Andries K, Dewindt B, Snoeks J, Willebrords R, Van Eemeren K, Stokbroekx R, Janssen P A J. In vitro activity of pirodavir ( R77975), a substituted phenoxy-pyridazinamine with broad-spectrum antipicornaviral activity. Antimicrob Agents Chemother. 1992;36:100–107. doi: 10.1128/aac.36.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arruda E, Crump C E, Marlin S D, Merluzzi V J, Hayden F G. In vitro studies of the antirhinovirus activity of soluble intercellular adhesion molecule-1. Antimicrob Agents Chemother. 1992;36:1186–1191. doi: 10.1128/aac.36.6.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruda E, Boyle T R, Winther B, Pevear D C, Gwaltney J M, Jr, Hayden F G. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 4.Arruda E, Hayden F G. Clinical studies of antiviral agents for picornaviral infections. In: Jeffries D J, De Clerq E, editors. Antiviral chemotherapy. Chichester, N.Y: John Wiley & Sons Ltd.; 1995. pp. 321–355. [Google Scholar]
- 5.Bardin P G, Johnston S L, Sanderson G, Robinson B S, Pickett M A, Fraenkel D J, Holgate S T. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am J Respir Cell Biol. 1994;10:207–213. doi: 10.1165/ajrcmb.10.2.8110476. [DOI] [PubMed] [Google Scholar]
- 6.Biagioli C M, Kaul P, Singh I, Turner R B. The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Rad Biol Med. 1999;26:454–462. doi: 10.1016/s0891-5849(98)00233-0. [DOI] [PubMed] [Google Scholar]
- 7.Couch R B. Rhinoviruses. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 607–629. [Google Scholar]
- 8.Diana G D, Rudewicz P, Pevear D C, Nitz T J, Aldous S C, Aldous D J, Robinson D T, Draper T, Dutko F J, Aldi C, Gendron G, Oglesby R C, Volkots D L, Reuman M, Bailey T R, Czerniak R, Block T, Roland R, Oppermann J. Picornavirus inhibitors: trifluoromethyl substitution provides a global protective effect against hepatic metabolism. J Med Chem. 1995;38:1355–1371. doi: 10.1021/jm00008a014. [DOI] [PubMed] [Google Scholar]
- 9.Douglas R G, Cate T R, Gerone P J, Couch R B. Quantitative rhinovirus shedding patterns in volunteers. Am Rev Respir Dis. 1966;94:159–167. doi: 10.1164/arrd.1966.94.2.159. [DOI] [PubMed] [Google Scholar]
- 10.Dragovich P S, Prins T J, Zhou R, Fuhrman S A, Patick A K, Matthews D A, Ford C E, Meador III J W, Ferre R A, Worland S T. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 3. Structure-activity studies of ketomethylene-containing peptidomimetics. J Med Chem. 1999;42:1203–1212. doi: 10.1021/jm980537b. [DOI] [PubMed] [Google Scholar]
- 11.Dragovich P S, Prins T J, Zhou R, Webber S E, Marakovits J T, Fuhrman S A, Patick A K, Matthews D A, Lee C A, Ford C E, Burke B J, Rejto P A, Hendrickson T F, Tuntland T, Brown E L, Meador III J W, Ferre R A, Harr J E V, Kosa M B, Worland S T. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as l-glutamine replacements. J Med Chem. 1999;42:1213–1224. doi: 10.1021/jm9805384. [DOI] [PubMed] [Google Scholar]
- 12.Dragovich P S, Webber S E, Babine R E, Fuhrman S A, Patick A K, Matthews D A, Lee C A, Reich S H, Prins T J, Marakovits J T, Littlefield E S, Zhou R, Tikhe J, Ford C E, Wallace M, Meador III J W, Ferre R A, Brown E L, Binford S L, Harr J E V, DeLisle D M, Worland S T. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 1. Michael acceptor structure-activity studies. J Med Chem. 1998;41:2806–2818. doi: 10.1021/jm980068d. [DOI] [PubMed] [Google Scholar]
- 13.Dragovich P S, Webber S E, Babine R E, Fuhrman S A, Patick A K, Matthews D A, Reich S H, Marakovits J T, Prins T J, Zhou R, Tikhe J, Littlefield E S, Bleckman T M, Wallace M, Little T, Ford C E, Meador III J W, Ferre R A, Brown E L, Binford S L, DeLisle D M, Worland S T. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 2. Peptide structure-activity studies. J Med Chem. 1998;41:2819–2834. doi: 10.1021/jm9800696. [DOI] [PubMed] [Google Scholar]
- 14.Dragovich P S, Zhou R, Skalitzky D J, Fuhrman S A, Patick A K, Ford C E, Meador III J W, Worland S T. Solid-phase synthesis of irreversible human rhinovirus 3C protease inhibitors. 1. Optimization of tripeptides incorporating N-terminal amides. Bioorg Med Chem. 1999;7:589–598. doi: 10.1016/s0968-0896(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 15.Fromtling R A, Castañer J. VP-63843 Pleconaril WIN-63843. Drugs Future. 1997;22:40–44. [Google Scholar]
- 16.Hayden F G, Fritz R S, Lobo M C, Alvord W G, Strober W, Straus S E. Local and systemic cytokine responses during experimental human influenza A virus infection: relation to symptom formation and host defense. J Clin Investig. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden F G, Hipskind G J, Woerner D H, Eisen G F, Janssen M, Janssen P A J, Andries K. Intranasal pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob Agents Chemother. 1995;39:290–294. doi: 10.1128/aac.39.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden F G, Osterhaus A D M E, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zamanivir in the treatment of influenzavirus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 19.Hayden F G, Andries K, Janssen P A J. Safety and efficacy of intranasal pirodavir ( R77975) in experimental rhinovirus infection. Antimicrob Agents Chemother. 1992;36:727–732. doi: 10.1128/aac.36.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinz B A, Tang J, Labus J M, Chadwell F W, Kaldor S W, Hammond M. Simple in vitro translation assay to analyze inhibitors of rhinovirus proteases. Antimicrob Agents Chemother. 1996;40:267–270. doi: 10.1128/aac.40.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston S L, Papi A, Bates P J, Mastronarde J G, Monick M M, Hunninghake G W. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol. 1998;160:6172–6181. [PubMed] [Google Scholar]
- 22.Kaldor S W, Hammond M, Dressman B A, Labus J M, Chadwell F W, Kline A D, Heinz B A. Glutamine-derived aldehydes for the inhibition of human rhinovirus 3C protease. Bioorg Med Chem Lett. 1995;5:2021–2026. [Google Scholar]
- 23.Kong J-S, Venkatraman S, Furness K, Nimkar S, Shepherd T A, Wang Q M, Aube J, Hanzlik R P J. Synthesis and evaluation of peptidyl Michael acceptors that inactivate human rhinovirus 3C protease and inhibit virus replication. J Med Chem. 1998;41:2579–2587. doi: 10.1021/jm980114+. [DOI] [PubMed] [Google Scholar]
- 24.Matthews D A, Dragovich P S, Webber S E, Fuhrman S A, Patick A K, Zalman L S, Hendrickson T, Prins T J, Marakovits J T, Zhou R, Tikhe J, Ford C E, Meador J W, Ferre R A, Brown E L, Binford S L, Brothers M A, DeLisle D M, Worland S T. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc Natl Acad Sci USA. 1999;96:11000–11007. doi: 10.1073/pnas.96.20.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews D A, Smith W W, Ferre R A, Condon B, Budahazi G, Sisson W, Villafranca J E, Janson C A, McElroy H E, Gribskov C L, Worland S. Structure of human rhinovirus 3C protease reveals a trypsin-like polypeptide fold, RNA-binding site, and means for cleaving precursor polyprotein. Cell. 1994;77:761–771. doi: 10.1016/0092-8674(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 26.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 549–605. [Google Scholar]
- 27.Noah T L, Henderson F W, Wortman I A, Devlin R B, Handy J, Koren H S, Becker S. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 28.Otto M J, Fox M P, Fancher M J, Kuhrt M F, Diana G D, McKinlay M A. In vitro activity of WIN 51711, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1985;27:883–886. doi: 10.1128/aac.27.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patick A, Potts K. Protease inhibitors as antiviral agents. Clin Microbiol Rev. 1998;11:614–627. doi: 10.1128/cmr.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patick A K, Binford S L, Brothers M A, Jackson R L, Ford C E, Diem M D, Maldonado F, Dragovich P S, Zhou R, Prins T J, Fuhrman S A, Meador J W, Zalman L S, Matthews D A, Worland S T. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother. 1999;43:2444–2450. doi: 10.1128/aac.43.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitkaranta A, Hayden F G. What's new with common colds? Pathogenesis and diagnosis. Infect Med. 1998;15:50–59. [Google Scholar]
- 32.Proud D, Gwaltney J M, Jr, Hendley J O, Dinarello C A, Gillis S, Schleimer R P. Increased levels of interleukin-1 are detected in nasal secretions of volunteers during experimental rhinovirus colds. J Infect Dis. 1994;169:1007–1013. doi: 10.1093/infdis/169.5.1007. [DOI] [PubMed] [Google Scholar]
- 33.Roseler S, Holtappels G, Wagenmann M, Bachert C. Elevated levels of interleukins IL-1b, IL-6 and IL-8 in naturally acquired viral rhinitis. Eur Arch Otorhinolaryngol. 1995;252(Suppl. 1):S61–S63. doi: 10.1007/BF02484437. [DOI] [PubMed] [Google Scholar]
- 34.Rueckert R R. Picornaviridae and their replication. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 507–548. [Google Scholar]
- 35.Shepherd T A, Cox G A, McKinney E, Tang J, Wakulchik M, Zimmerman R E, Villarreal E C. Small peptidic aldehyde inhibitors of human rhinovirus 3C protease. Bioorg Med Chem Lett. 1996;6:2893–2896. [Google Scholar]
- 36.Skern T, Sommergruber W, Blaas D, Gruendler P, Fraundorfer F, Pieler C, Fogy I, Kuechler E. Human rhinovirus 2: complete nucleotide sequence and proteolytic processing signals in the capsid protein region. Nucleic Acids Res. 1985;13:2111–2126. doi: 10.1093/nar/13.6.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanway G, Hughes P J, Mountford R C, Minor P D, Almond J W. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984;12:7859–7875. doi: 10.1093/nar/12.20.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subauste M C, Jacoby D B, Richards S M, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus; induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Investig. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner R B, Weingand K W, Yeh C-H, Leedy D W. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin Infect Dis. 1998;26:840–846. doi: 10.1086/513922. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q M, Johnson R B, Hungheim L N, Cohen J D, Villarreal E C. Dual inhibition of human rhinovirus 2A and 3C proteases by homophthalimides. Antimicrob Agents Chemother. 1998;42:916–920. doi: 10.1128/aac.42.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webber S, Tikhe J, Worland S T, Fuhrman S A, Hendrickson T F, Matthews D A, Love R A, Patick A K, Meador J W, Ferre R A, Brown E L, DeLisle D M, Ford C E, Binford S L. Design, synthesis, and evaluation of nonpeptidic inhibitors of human rhinovirus 3C protease. J Med Chem. 1996;39:5072–5082. doi: 10.1021/jm960603e. [DOI] [PubMed] [Google Scholar]
- 42.Webber S E, Okano K, Little T L, Reich S, Xin Y, Worland S T, Fuhrman S A, Matthews D A, Hendrickson T F, Love R A, Patick A K, Meador III J W, Ferre R A, Brown E L, Ford C E, Binford S L. Tripeptide aldehyde inhibitors of human rhinovirus 3C protease: design, synthesis, biological evaluation, and cocrystal structure solution of P1 glutamine isosteric replacements. J Med Chem. 1998;41:2786–2805. doi: 10.1021/jm980071x. [DOI] [PubMed] [Google Scholar]
- 43.Weislow O S, Kiser R, Fine D L, Bader J, Shoemaker R H, Boyd M R. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J Natl Cancer Inst. 1989;81:577–586. doi: 10.1093/jnci/81.8.577. [DOI] [PubMed] [Google Scholar]
- 44.Werner G, Rosenwirth B, Bauer E, Seifert J M, Werner F J, Besemer J. Molecular cloning and sequence determination of the genomic regions encoding protease and genome-linked protein of three picornaviruses. J Virol. 1986;57:1084–1093. doi: 10.1128/jvi.57.3.1084-1093.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Z, Tang W, Ray A, Wu Y, Einarsson O, Landry M L, Gwaltney J J, Elias J A. Rhinovirus stimulation of interleukin-6 in vivo and in vitro: evidence for nuclear factor kappa B dependent transcriptional activation. J Clin Investig. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]