Abstract
Background
Oral food challenge (OFC) is commonly used to diagnose food allergy. This test is time and resource intensive, and conclusions are not always unequivocal as this relies on the interpretation of symptoms. Therefore, an objective marker would improve the accuracy of the diagnostic workup of food allergy.
Objectives
The aim of this study was to investigate whether tryptase can be detected in saliva of children following OFC.
Method
Children from 3 to 18 years of age were eligible for inclusion if an OFC for peanut or tree nut had been recommended. Saliva samples were collected prior to the first dose and 5, 10, and 15 min following the last administered dose during OFC. Assay precision, spike-and-recovery, and assessment of lower limit of detection of the tryptase immunoassay were examined before analysis of tryptase in saliva was performed.
Results
A total of 30 children were included (median age 8 years, 63.3% male, 53.3% positive OFC outcome). Tryptase was detected in saliva samples. The mean of the change in baseline tryptase value to each saliva collecting time point was significantly different in patients with a positive OFC outcome compared to a negative outcome (p < 0.01).
Conclusions
This study showed that tryptase can be detected in saliva of children following OFC. Increased levels of tryptase compared to baseline were found if the OFC outcome was positive, suggesting that measuring tryptase in saliva may be useful in the diagnosis of food allergy. Further research is needed to evaluate the potential association between tryptase levels and symptoms.
Keywords: Allergy and immunology, Food hypersensitivity, Diagnosis, Saliva, Tryptase
Introduction
Food allergy has a substantial impact on the daily life of children and their families [1]. Therefore, it is important to accurately establish or exclude the diagnosis. Although sensitization to a specific food can be easily determined, it cannot adequately predict food allergy nor its severity [2, 3]. Today, the oral food challenge (OFC) is recommended to diagnose food allergies [4]. The OFC outcome is based on the interpretation of symptoms, which may be subject to variability [5]. Therefore, it would be valuable to have an objective parameter such as the release of specific allergic mediators [6].
Biomarkers may assist to diagnose food allergy [7, 8, 9, 10]. Tryptase can be detected in saliva of adults, although reported results were conflicting [7, 8]. To our knowledge, no data have been published on salivary tryptase levels in food allergic children. To explore the utility of salivary tryptase as a non-invasive objective tool to support interpretation of OFCs in children, we first aimed to investigate whether tryptase can be detected accurately in saliva of children following an OFC.
Materials and Methods
This prospective study took place between July 2018 and October 2018 at the paediatric allergy centre of a large teaching hospital. All children from 3 to 18 years of age were eligible for inclusion if referred for suspected peanut or tree nut allergy for which an OFC had been recommended by the attending physician. The Medical Ethics Committee (MEC) of Martini Hospital, Groningen, The Netherlands, approved the study protocol (MEC 2018-066). Written informed consent was obtained from parents and children aged 12 years and older.
Open or double-blind placebo controlled food challenges (DBPCFCs) were performed according to the European Academy of Allergy and Clinical Immunology (EAACI) guidelines [4]. In short, all food challenge days consist of a maximum of 7 steps with increasing dose (starting dose 3 mg, total amount 4443 mg) and 30-min waiting time between each dose. All OFCs were conducted by trained paediatric nurses. The occurrence of symptoms was registered based on the classification as mentioned in the EAACI guidelines [4]. Sensitization to the suspected food allergen was defined as serum specific IgE level ≥0.35 kU/L (Phadia 250, Uppsala, Sweden).
Saliva samples were planned to be collected prior to the first dose (baseline or T0) and at 5, 10, and 15 min (T1, T2, and T3, respectively) following the last administered dose, based on previous research [7, 8]. Saliva was collected by chewing on a synthetic swab (Salivette, Sartedt, Germany) for approximately 1 min. In case of DBPCFCs, saliva samples collected on the verum day were analysed. If allergic symptoms occurred and/or medication had to be administered, saliva was collected when deemed feasible. After collection, the saliva samples were labelled, stored at 4°C, and sent to the laboratory within 24 h. Hereafter, swabs were centrifuged according to the manufacturer's instructions, and saliva was stored at −20°C before analysis with ImmunoCAP Tryptase immunoassay on the UniCAP1000 (Phadia, Uppsala, Sweden). Details on the analysis of salivary tryptase can be found in online supplementary part A (see www.karger.com/doi/10.1159/000519374 for all online suppl. material).
All statistical analyses were performed using Analyse-it for Microsoft Excel Method Comparison Edition (v5.20; Analyse-it Software Ltd., Leeds, UK). Data were not normally distributed (Shapiro-Wilk's test, p < 0.05). Therefore, the Mann-Whitney U test was used to compare the salivary tryptase values in children with both positive and negative OFC outcome (p < 0.05 indicates statistical significance).
Results
In total, 30 patients were included with a median age of 8 years (IQR: 5.5–10.3) and 63.3% were male. Twenty-three (82.1%) patients were sensitized. A total of 30 OFCs were performed, of which 26 (86.7%) were open food challenges and 4 DBPCFCs. Fourteen (46.7%) out of 30 OFCs had a negative outcome and 16 (53.3%) positive. Most symptoms that occurred during the OFCs were categorized as ear, nose, and throat (e.g., itching of mouth, angioedema, or rhinitis) as well as cutaneous symptoms (e.g., erythema or urticaria). Detailed clinical information can be found in online supplementary part B.
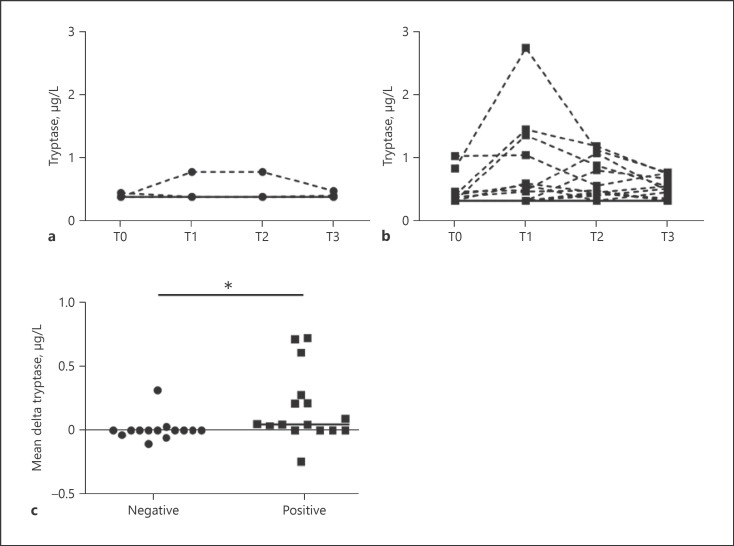
Salivary tryptase levels for all time points for both negative and positive OFC outcomes are shown in Figure 1a and b. Given the measurement uncertainty in absolute terms regarding the salivary tryptase levels, it was difficult to identify exact peak concentrations. Therefore, the mean of the change in baseline value to each saliva collecting time point was calculated (delta T1, T2, and T3). This was significantly higher in patients with a positive OFC outcome compared to those with a negative outcome (z = −2.678, p < 0.01; Fig. 1c).
Fig. 1.
Salivary tryptase levels. a Tryptase values from saliva samples collected prior to and following OFCs with negative outcome (n = 14). The continuous line represents the tryptase values of 12 out of 14 participants, all with values below 0.34 μg/L. b Tryptase values from saliva samples collected prior to and following OFCs with positive outcome (n = 16). The continuous line represents the tryptase values of 4 out of 16 participants, all with values below 0.34 μg/L. c Comparison of delta tryptase values calculated from saliva samples collected prior to and following OFCs with negative and positive outcome, respectively (n = 30). Delta tryptase values were calculated as the mean of the change in baseline value to each saliva collecting time point. T0: saliva sample collected at baseline; T1–T3: time points of collected saliva samples when OFC was ended (range 0–30 min); OFC: oral food challenge. *Significant difference.
Discussion
This study demonstrates that tryptase can be detected in saliva from children following OFC. The increase in salivary tryptase levels was significantly higher in OFCs with a positive outcome compared to those with a negative outcome.
While our study is the first to demonstrate that salivary tryptase levels can be detected after positive OFC in children, several limitations need to be considered. First, the kinetics of tryptase following OFC are largely unknown. Tryptase is mainly secreted by activated mast cells [6]. The molecular weight of the active form of tryptase makes transport from serum to other bodily fluids impossible [8]. Therefore, the amount of tryptase in saliva is fully dependent on release by local mast cells. The fixed time points we chose to collect saliva were consistent with previously published reports, but in practice turned out to be variable (range 0–30 min) due to several practical reasons (e.g., difficulties while collecting saliva if symptoms occurred). This makes it challenging to conclude whether or not we detected peak tryptase levels, as these may have either been on the rising or declining part of the kinetic curve. To gain more insight into the kinetics of tryptase, multiple saliva samples prior to, during, and following OFC should be collected.
Second, the aforementioned limitations hampered further analysis regarding the possible association between symptoms and salivary tryptase. However, the aim of this study was to evaluate whether tryptase could be detected in saliva of children following OFC. Further research should evaluate the association between salivary tryptase levels and symptoms (both severity and type) and use a controlled study design using DBPCFCs. This is of special interest because it may be helpful to draw more firm conclusions about the OFC outcome since the broad spectrum of possible symptoms during OFC can be challenging to interpret. It might be interesting to determine whether salivary tryptase levels can differentiate between objective and subjective symptoms and/or local and systemic allergic reactions. Furthermore, additional analyses in a larger population of healthy subjects may be of interest to better characterize sensitivity and specificity of salivary tryptase.
Third, a sandwich immunoassay intended for blood-derived matrices in the range of 1–200 μg/L was used to measure salivary tryptase levels, as previously described in 2 studies [7, 8]. Within our study, a significant number of samples were below the detection limit, which is similar to the result previously reported by Vila et al. [7] Therefore, a partial method validation was performed to validate the measurement in saliva in the lower range of the assay (for details see online suppl. part A). Expanding the calibration curve to lower (salivary) tryptase levels in future studies will strengthen the results.
In conclusion, the results of our study demonstrate that tryptase can be detected in saliva samples of children following OFC. Further research is needed to define optimal sampling times and evaluate the potential association between tryptase levels and symptoms.
Statement of Ethics
The study was conducted in accordance with the World Medical Association Declaration of Helsinki. The study protocol was reviewed and approved by the Medical Ethics Committee (MEC) of Martini Hospital, Groningen, The Netherlands (Approval No. MEC 2018-066). Written informed consent was obtained from parents and children aged 12 years and older.
Conflict of Interest Statement
The authors report no proprietary or commercial interest in any product mentioned, concept discussed, or personal relationships with other people or organizations that could influence their work and conclusions in this article.
Funding Sources
The Paediatric Department of Martini Hospital, Groningen, is supported by an unrestricted research grant from Nutricia, The Netherlands. The funding sources had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or the decision to submit the article for publication.
Author Contributions
W.W.W., V.M.B., E.M.L., L.L., J.H.G., C.E.M.H., G.N.M., and A.W.A.K. contributed to conceptualization; W.W.W. and E.M.L. contributed to data acquisition; V.M.B. contributed to formal analysis; W.W.W. and V.M.B. contributed to writing − original draft preparation; E.M.L., L.L., J.H.G., A.B.S., G.H.K., and A.W.A.K. contributed to writing − review and editing; A.W.A.K. and J.H.G. contributed to supervision.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Supplementary data
References
- 1.Polloni L, Muraro A. Anxiety and food allergy a review of the last two decades. Clin Exp Allergy. 2020;50:420–41. doi: 10.1111/cea.13548. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141:41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Anvari S, Miller J, Yeh CY, Davis CM. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244–60. doi: 10.1007/s12016-018-8710-3. [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA, Van Wijk RG, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, et al. Standardizing double-blind placebo-controlled oral food challenges American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260–74. doi: 10.1016/j.jaci.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Van Erp FC, Knulst AC, Meijer Y, Gabriele C, Van Der Ent CK. Standardized food challenges are subject to variability in interpretation of clinical symptoms. Clin Transl Allergy. 2014;4:43–6. doi: 10.1186/s13601-014-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitte J. Human mast cell tryptase in biology and medicine. Mol Immunol. 2015;63:18–24. doi: 10.1016/j.molimm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Vila L, Sanz ML, Sánchez-López G, García-Avilés C, Diéguez I. Variations of serum eosinophil cationic protein and tryptase, measured in serum and saliva, during the course of immediate allergic reactions to foods. Allergy. 2001;56:568–72. doi: 10.1034/j.1398-9995.2001.056006568.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruëff F, Friedl T, Arnold A, Kramer M, Przybilla B. Release of mast cell tryptase into saliva a tool to diagnose food allergy by a mucosal challenge test? Int Arch Allergy Immunol. 2011;155:282–8. doi: 10.1159/000320492. [DOI] [PubMed] [Google Scholar]
- 9.Wongkaewpothong P, Pacharn P, Sripramong C, Boonchoo S, Piboonpocanun S, Visitsunthorn N, et al. The utility of serum tryptase in the diagnosis of food-induced anaphylaxis. Allergy Asthma Immunol Res. 2014;6:304–9. doi: 10.4168/aair.2014.6.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dua S, Dowey J, Foley L, Islam S, King Y, Ewan P, et al. Diagnostic value of tryptase in food allergic reactions a prospective study of 160 adult peanut challenges. J Allergy Clin Immunol Pract. 2018;6:1692.e1–8.e1. doi: 10.1016/j.jaip.2018.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.