Abstract
Background
Transparent and robust real-world evidence sources are increasingly important for global health, including cardiovascular (CV) diseases. We aimed to identify global real-world data (RWD) sources for heart failure (HF), acute coronary syndrome (ACS), and atrial fibrillation (AF).
Methods
We conducted a systematic review of publications with RWD pertaining to HF, ACS, and AF (2010–2018), generating a list of unique data sources. Metadata were extracted based on the source type (e.g., electronic health records, genomics, and clinical data), study design, population size, clinical characteristics, follow-up duration, outcomes, and assessment of data availability for future studies and linkage.
Results
Overall, 11,889 publications were retrieved for HF, 10,729 for ACS, and 6,262 for AF. From these, 322 (HF), 287 (ACS), and 220 (AF) data sources were selected for detailed review. The majority of data sources had near complete data on demographic variables (HF: 94%, ACS: 99%, and AF: 100%) and considerable data on comorbidities (HF: 77%, ACS: 93%, and AF: 97%). The least reported data categories were drug codes (HF, ACS, and AF: 10%) and caregiver involvement (HF: 6%, ACS: 1%, and AF: 1%). Only a minority of data sources provided information on access to data for other researchers (11%) or whether data could be linked to other data sources to maximize clinical impact (20%). The list and metadata for the RWD sources are publicly available at www.escardio.org/bigdata.
Conclusions
This review has created a comprehensive resource of CV data sources, providing new avenues to improve future real-world research and to achieve better patient outcomes.
Keywords: Real-world data, Real-world evidence, Data sources, Cardiovascular
Background
Cardiovascular (CV) disease is the leading cause of death worldwide [1], accounting for >17 million deaths in 2015 alone [2]. According to the World Health Organization (WHO), the annual number of deaths due to CV diseases globally is projected to increase to 20.5 million by 2020 and 24.5 million by 2030 [3]. Moreover, in both high-income and middle-income countries, the main cause of death has shifted over time from communicable to non-communicable diseases, with a high burden on national health systems [4].
Real-world data (RWD) have played a key role in CV disease-related decision-making, especially in recent years, due to a widening range of new therapies and increasing demands for justification of their effectiveness. Translating RWD into real-world evidence (RWE) can provide information throughout a product's life cycle [5]. RWE can help design pivotal phase 3 trials by reducing the required sample size, supporting recruitment, and thereby saving time [6] and informing the appropriate selection criteria [7, 8]. RWE can provide outcomes of care in real-world settings, thus improving the external validity of clinical trial findings, and offer insights into coverage and payment decisions to support health authority decision-making [9, 10]. However, limitations of RWD should also be acknowledged, which broadly include bias and confounding, incomplete data, different legal frameworks leading to restricted data sharing, and lack of universally accepted methodological standards [9, 10, 11]. In addition, the evidence landscape is constantly evolving with respect to the conduct and reporting of RWE studies. The recent retraction from major medical journals of apparently fraudulent RWD on COVID-19 [12] highlights the urgent need for more transparency and access to global data sources.
This review aimed to identify global RWD sources pertaining to heart failure (HF), acute coronary syndrome (ACS), and atrial fibrillation (AF) in order to facilitate new evidence research and improve patient outcomes. Our objective was to help global researchers move toward the FAIR principles for RWD − Findable, Accessible, Interoperable, and Reusable [13].
Methods
The European Union Innovative Medicines Initiative (IMI) public-private consortium launched the BigData@Heart project with the goal of developing a big data-driven translational research platform from RWE focussing on HF, ACS, and AF. Through this translational research platform, BigData@Heart aims to deliver clinically relevant disease phenotypes and support drug development and personalized medicine [14, 15, 16]. One of the undertakings of this initiative is to identify and characterize available RWD sources that would serve as a starting point to identify existing datasets that could help address research questions at scale.
A systematic literature search was conducted in MEDLINE and EMBASE using the OvidSP platform for the period January 2010–March 2018 to identify publications using RWD sources for HF, ACS, and AF. The review was not prospectively registered. We did not include publications before 2010 because older RWD sources may not be relevant to current practice. Disease-specific search strategies (using Medical Subject Headings terms) were combined with study design terms to identify research publications that either generated primary RWD or used existing RWD sources. Identified data sources from these publications were categorized according to predefined geographical locations: Europe; USA; Latin America/Canada (LaCan); and Asia-Pacific, Middle East, and Africa (APMA).
Inclusion and Exclusion Criteria
We included English-language publications using different data sources as defined by the authors, such as structured data sources (administrative data and registries), medical records or charts, insurance claims, health surveys, and observational studies for HF, ACS, and AF. Publications that did not generate primary RWD or did not study existing RWD sources, as well as guidelines, editorials, letters, and reviews, were excluded. Additionally, we excluded clinical trials or interventional studies, in vitro/preclinical studies, and data sources with <50 patients.
Screening, Selection, and Extraction of Data Sources
The search strategies are presented in the additional files, available online at www.karger.com/doi/10.1159/000520674 (HF: Additional File 1 [online suppl. Table 1], ACS: Additional File 2 [online suppl. Table 2], and AF: Additional File 3 [online suppl. Table 3]). All publications identified from the literature searches were first screened based on the title and abstract by a single reviewer, and duplicates were removed. The inclusion and exclusion criteria were applied at this stage to generate a list of full-text reviews. Of the included publications, 10% were randomly selected and checked for discrepancies, which were reconciled through group discussions. For the included publications, names of identified data sources, type (single-centre or multicentre), and geographical location were extracted using a predefined screening tool. Publications with the same data source were grouped by name, and data were extracted into a single record to avoid double counting of data sources in subsequent analyses. Thereafter, a list of unique data sources available from the literature search was prepared for each indication.
From this list, selected data sources were further mapped and extracted in detail based on different criteria for each disease indication:
Data sources with larger samples sizes were prioritized with a view on big data and potentially more robust analyses. For the data sources identified based on the above criteria, information presented in the included publications was extracted, including additional information on data source details (description, coverage, and follow-up) and availability of clinically relevant key variables related to HF, ACS, and AF (diagnosis and staging, demographics, management [including procedures], test results and treatments, burden of disease [including costs], deaths and resource use, quality of life, and adverse events). In addition, publicly available information related to the data sources, such as the data source holder/owner, access and linkage possibility, supporting documentation, and its governance aspects, were extracted and recorded.
Results
Heart Failure
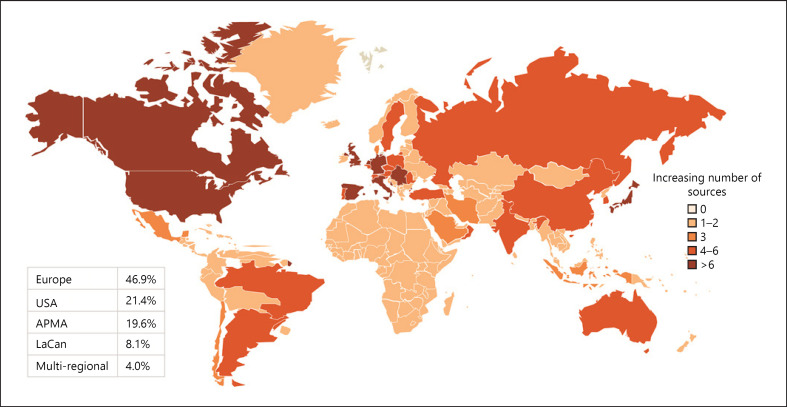
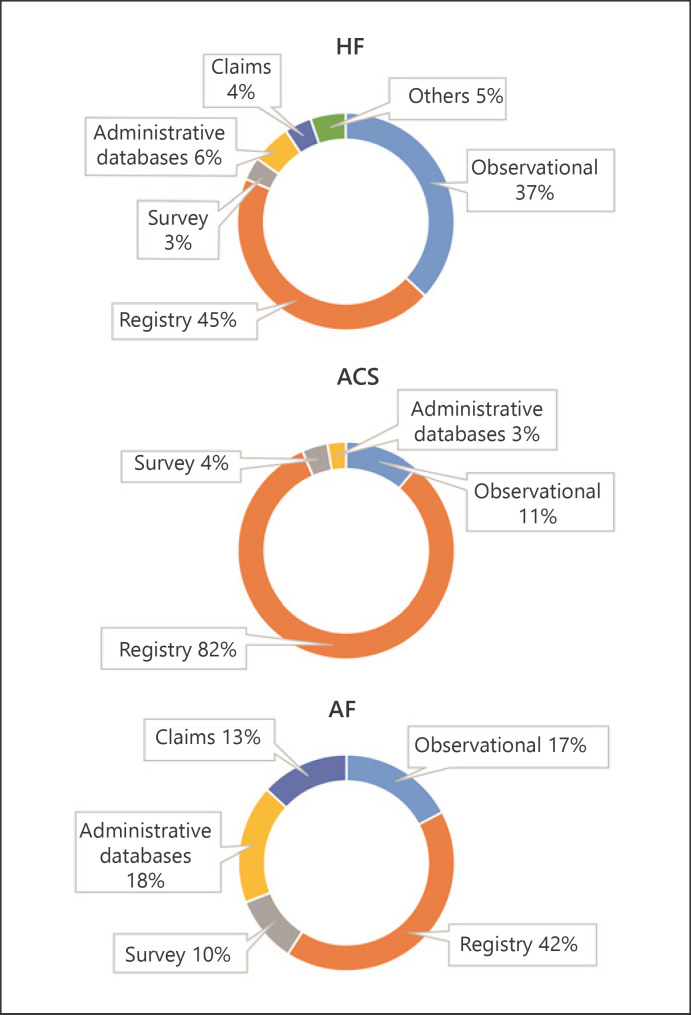
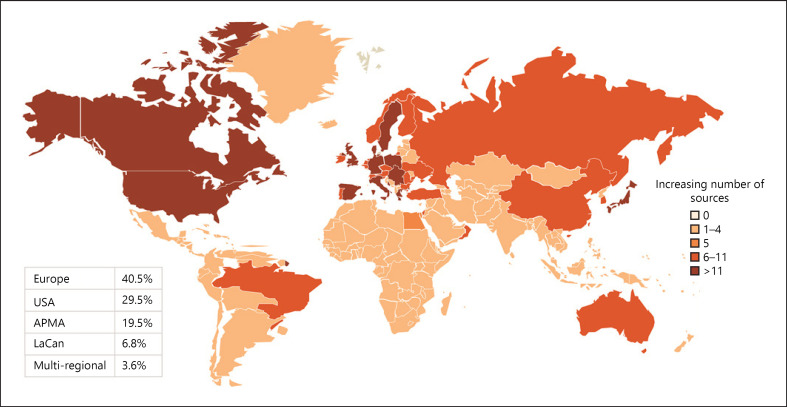
Of the 11,889 publications retrieved from the HF literature search, 1,326 unique data sources were identified, of which 322 RWD sources were selected for detailed mapping (Additional File 4: online suppl. Fig. 1). Overall, 74% of these data sources were disease-specific, with registries being the most common type of data source (45%). Geographic distribution is shown in Figure 1; 47% of the published HF data sources were from Europe, followed by the USA (21%), APMA (20%), LaCan (8%), and multiregional (4%). Germany had the highest number of data sources in Europe (n = 15); Japan, in APMA (n = 10); and Canada, in LaCan (n = 12). The top 5 HF data sources based on the highest number of publications are presented in Figure 2.
Fig. 1.
Geographical distribution of HF data sources. Global HF data source distribution, with darker shades of colour representing more data sources in each of the presented regions. HF, heart failure; LaCan, Latin America/Canada; APMA, Asia-Pacific, Middle East, and Africa; USA, United States of America.
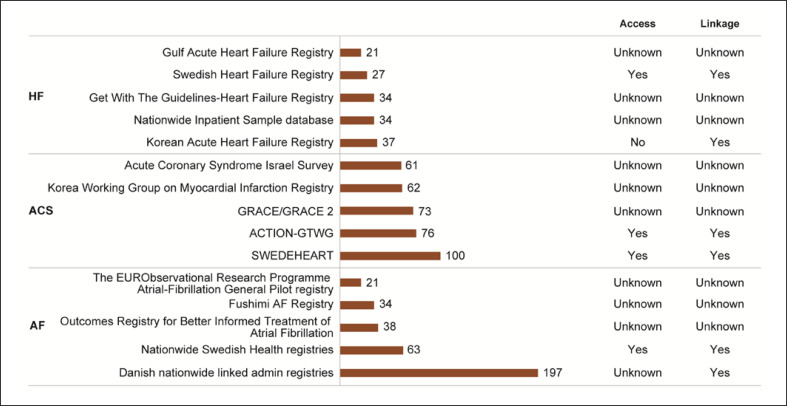
Fig. 2.
Top 5 data sources as per count of publications. Data sources with the highest number of publications during the search period of this review. On the right, the possibility to access or link these data sources is presented. ACTION-GTWG, Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With The Guidelines; GRACE/GRACE 2, Global Registry of Acute Coronary Events/Global Registry of Acute Coronary Events 2; HF, heart failure; ACS, acute coronary syndrome; AF, atrial fibrillation; Gulf CARE, Gulf aCute heArt failuRe rEgistry; SWEDEHEART, Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies; EORP, EUR Observational Research Programme.
Completeness of variables varied across the mapped data sources and ranged from 0 to 78%. The most commonly recorded variables were age and gender (94%), hospital admissions (81%), comorbidities (77%), mortality (75%), and LVEF (73%; increased by selection criteria). The least recorded data variables were drug codes (10%), dates of procedures and prescriptions (7%), and caregiver involvement (6%). In terms of comorbidities, the proportion of HF data sources reporting ACS and AF as a comorbidity was 16% and 28%, respectively. Information on access to these data sources through purchasing, licencing, or collaboration with the dataset owners was reported for 6% of the sources, whereas it was unknown for the remaining sources. Linking of these data with other data sources was reported in 18% of the sources, whereas the possibility of linkage was unknown for the remainder.
Acute Coronary Syndromes
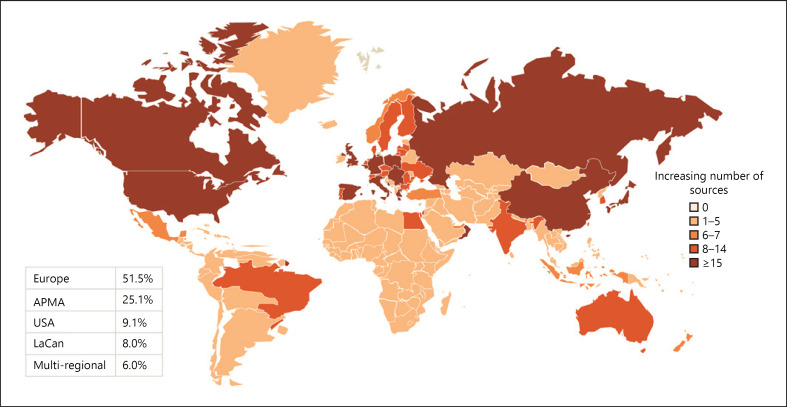
From the 10,729 publications retrieved through the literature search, 1,560 unique data sources were identified, of which 287 were further selected and mapped (Additional File 5: online suppl. Fig. 2). Over half of these data sources (52%) were from Europe; 25%, APMA; 9%, USA; and 8%, LaCan; 6% of the sources were multiregional (Fig. 3). The highest number of data sources was from Germany in Europe (n = 20), Japan in APMA (n = 21), and Canada in LaCan (n = 17). Over 80% of the mapped data sources were registries (Fig. 4). The Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies registry had the highest number of publications (n = 100) identified during the search period (Fig. 2).
Fig. 3.
Geographical distribution of ACS data sources. Global ACS data source distribution, with darker shades of colour representing more data sources in each of the presented regions. ACS, acute coronary syndrome; LaCan, Latin America/Canada; APMA, Asia-Pacific, Middle East, and Africa; USA, United States of America.
Fig. 4.
Distribution as per type of data sources. Data sources mapped in this review are categorized broadly into 6 different categories, comprising observational studies, registries, surveys, administrative databases, claims databases, and others. Observational studies include cohort studies, cross-sectional studies, prospective studies, retrospective studies, longitudinal studies, and population-based studies, as defined by the authors of the individual publications. HF, heart failure; ACS, acute coronary syndrome; AF, atrial fibrillation.
Completeness for recorded variables varied from 5% to 70%. The most commonly available clinical variables were age and gender (99%), mortality (95%), comorbidities (93%), inpatient diagnostic or therapeutic procedures (84%), and prescribed drugs (74%). The least recorded variables were date of ACS diagnosis (6%), dates of procedures and prescriptions (5%), procedure costs (3%), drug codes, and caregiver involvement and costs (1% each). The proportion of ACS data sources capturing the presence of HF and AF as a comorbidity was 27% and 8%, respectively. Information on access to these data sources was provided in 6% of the sources, and linkage of these data sources with other datasets was possible in 20%.
Atrial Fibrillation
From the 6,262 publications retrieved via the literature search, 701 unique data sources were identified, of which 220 data sources were further mapped (Additional File 6: online suppl. Fig. 3). Geographically, Europe had the highest number of data sources (40%), followed by the USA (30%), APMA (20%), and LaCan (7%); 4% of the sources were multiregional (Fig. 5). The highest number of data sources was from the United Kingdom in Europe (n = 13), Japan in APMA (n = 12), and Canada in LaCan (n = 14). Registries (42%) were the most common type of data sources, followed by administrative databases (18%), observational studies (17%), claims (13%), and surveys (10%) (Fig. 4). The top 5 data sources based on the highest number of publications are presented in Figure 2.
Fig. 5.
Geographical distribution of AF data sources. Global AF data source distribution, with darker shades of colour representing more data sources in each of the presented regions. AF, atrial fibrillation; LaCan, Latin America/Canada; APMA, Asia-Pacific, Middle East, and Africa; USA, United States of America.
Coverage of variables differed across the mapped data sources, and their completeness ranged from 10% to 60%. The most widely reported data variables were age and gender (100%), comorbidities (97%), prescribed drugs (91%), stroke risk (81%), mortality (67%), and hospitalizations (66%), whereas the least reported variables were date of AF diagnosis (10%), drug codes (10%), quality of life (10%), and caregiver involvement (1%). HF and ACS as comorbidities were recorded for 92% and 49% of the AF data sources, respectively. Information on access to data sources was reported in 25% of the mapped sources, whereas for the remaining sources, the possibility of access was unknown. Linkage of these data sources with other data sources was possible in 28%.
Discussion
This review aimed to identify global RWD sources focussing on 3 common CV diseases and make them publicly available as a resource for researchers. Previous studies have identified RWD sources in disease areas such as chronic obstructive pulmonary disease [17] and Parkinson's disease [18] as well as generic RWD data sources [19], but to our knowledge, no study has reported RWD sources focussing on CV diseases across different geographies. We were able to map 322 RWD sources for HF, 287 for ACS, and 220 for AF. The mapping and provision of these sources in this review aims to enhance the generation of RWE across CV diseases. Importantly, we also define current limitations, such as lack of access to data, linkage with other sources, and insight on cross-comorbidity that should be improved in order to achieve maximum patient benefit from future RWE.
In December 2018, the US Food and Drug Administration (FDA) released a guidance document for the use of RWE to support regulatory decision-making for drugs and medical devices [20]. Similarly, in Europe, the European Medicines Agency (EMA), with its adaptive pathway initiative, highlighted RWE as an important source to further support evidence collected through randomized controlled trials (RCTs) [9]. In addition to the EMA and FDA, Health Technology Assessment International, in its global policy forum, presented the availability and use of RWE for health technology assessment [21], and the National Institute for Health and Care Excellence in the UK has documented the use of RWE in its decision-making [22]. In the context of the coronavirus pandemic, RWD has been used extensively to manage public health programmes, although the recent controversy and retraction of studies by leading journals have highlighted the need for robust evaluation before apparent RWD becomes RWE [12].
The growing importance of RWE can further be ascertained through many examples, including selected drug approvals during 1999–2014 by the FDA and EMA, which were largely based on uncontrolled studies for oncology and orphan indications [23]. For health technology assessments, certain outcomes such as costs and quality-adjusted life-years are often retrieved from non-RCT data [24]. With the growing need for RWE, we require varied, high-quality, and transparent sources of RWD to cater to different research objectives related to the epidemiology or burden of disease.
Across the 3 CV indications, we found that most data sources were currently from Europe and to North America, but a growing number are now presented from the Middle East, Asia, Russia, and South America. The collection of RWD requires relatively high upfront investment, which might be more feasible in high-income countries. Among the European HF data sources, and consistent with other published data, the Swedish Heart Failure Registry was the most frequent source for generating RWE [25]. The most published data sources for ACS and AF were the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies registry and the Danish nationwide-linked admin registries, respectively.
In the present review, for all the 3 CV conditions, demographics and comorbidities were the most commonly available variables, whereas costs and caregiver involvement were least reported. This could be because most of the identified data sources were registries. Moreover, cost to the healthcare system and caregiver involvement cannot be collected directly from patients, existing healthcare records, or medical charts. Data sources for HF also provided information on mortality, hospitalization, and LVEF. For ACS, information on mortality and prescribed drugs was captured in 95% and 74% of the data sources, respectively. For AF, other commonly reported variables were prescribed drugs, stroke risk, mortality, and hospitalization. Taken together, these data sources provide a wealth of information on patient characteristics and clinical burden; however, data pertaining to humanistic and economic burden are limited. These trends are similar to those observed in non-CV conditions [18, 25].
In the absence of universally accepted methodological standards for data models and infrastructure, the accessibility, linkage, and comparability of RWD sources are currently a challenge. This can prevent the establishment of larger datasets by linking RWD to generate more robust and representative RWE [9, 26]. In line with this, this review reports low accessibility and possibility of linkage based on information retrieved from the public domain. The alternative, i.e., personal communication with data holders, can be time-consuming and potentially unproductive. This challenge may be addressed through efforts in private-public collaborations and within the European framework; for example, dataset owners could be invited to the European Medical Information Framework catalogue [27], which allows users to explore population-based data sources. Linking of these data sources requires harmonization similar to that in other large IMIs such as the European Health Data & Evidence Network [28]. Translation to clinical practice and the development of new RWE will be aided by integration and linkage of molecular and genetic studies with RWD sources; this developing field has the potential to enable more rapid translation of mechanistic studies to improve patient care.
This review has certain limitations, including incomplete information on the RWD sources because of the limited information available in the public domain. Many databases may contain more data than are currently reported in the tool, and inversely, some variables may be recorded for only a subset of patients (e.g., LVEF). This review reflects the current state-of-the-art; however, RWD sources are continually being generated and revised. Key recent publications from the identified data sources are presented in Table 1 and demonstrate the broad impact that RWE can have on clinical practice. The consortium will update this review periodically (see www.escardio.org/bigdata for future updates), and the European Medical Information Framework catalogue is open for investigators to add or update information on their data sources. The risk of bias in the data sources was not assessed, and the selection of predominantly disease-specific registries may have introduced a bias with respect to the type of variables available; for example, we reported a large number of data sources with LVEF due to the selection criteria for detailed mapping. For some research questions, however, other data sources could be more suitable. Furthermore, this review was limited to English-language publications and may consequently underrepresent data sources from other regions. Finally, RWD are observational in nature and cannot replace RCTs to determine the unbiased efficacy of therapy. Treatment choices in clinical practice are dependent on a large array of prescription biases and confounding factors that limit the value of observational data [29]. However, RWE can complement clinical trial data, and allows an understanding of the epidemiology and interaction of diseases.
Table 1.
Recent key RWD publications in HF, ACS, and AF
| Data source | Participants | Findings | Reference |
|---|---|---|---|
| Gulf CARE | 5,005 patients hospitalized with acute HF | ACS was the most common precipitating factor for new-onset HF (39.2%) and non-compliance with medications the most common precipitating factor for decompensated chronic HF (27.8%) | [30] |
|
| |||
| Swedish Heart Failure Registry | 21,496 patients with HF | Iron deficiency testing only performed in 27% of patients; 49% of those tested had iron deficiency which was associated with recurrent hospitalization | [31] |
|
| |||
| GTWG-Heart Failure Registry | 1,551 patients hospitalized for HFrEF and discharged on sacubitril/valsartan and 7,857 discharged on ACEi/ARB | Prescription of sacubitril/valsartan was associated with reduced post-discharge mortality and all-cause hospitalization compared with ACEi/ARB | [32] |
|
| |||
| Korean Registry of Acute Myocardial Infarction for Regional Cardio-Cerebrovascular Centres | 11,700 patients with acute MI | ST-elevation and non-ST-elevation MI occurred in 43% and 57%, with case fatality within 12 months of 10% | [33] |
|
| |||
| EORP-Atrial Fibrillation III Registry | 8,306 patients with AF | Median age of the registry cohort was 69 years, with patients enrolled across 31 participating countries with future follow-up to assess adherence to guidelines and adverse events | [34] |
ACEi, angiotensin converting enzyme inhibitor; ACS, acute coronary syndrome; AF, atrial fibrillation; ARB, angiotensin receptor blocker; HF, heart failure; MI, myocardial infarction; RWD, real-world data; Gulf CARE, Gulf aCute heArt failuRe rEgistry; GTWG, Get With The Guidelines; EORP, EUR Observational Research Programme.
Conclusions
In summary, this review identified and mapped worldwide RWD sources pertaining to HF, ACS, and AF, thus providing researchers with a knowledge base to conduct feasibility assessments of these data sources for RWE studies. The list of and metadata for the data sources are publicly available at www.escardio.org/bigdata. Epidemiological research can be conducted using the wealth of individual data sources available. However, further details and access to the RWD sources, enhanced collaboration and harmonization between data holders (academia and industry), as well as integration of datasets would allow for the generation of more complex and impactful evidence. This could support CV disease drug development, market access, and use of interventions in clinical practice, eventually leading to improved CV outcomes and patient well-being.
Statement of Ethics
An ethics statement was not required for this study type; no human or animal subjects or materials were used.
Conflict of Interest Statement
Prof. Kotecha reports, outside of this study, grants from the National Institute for Health Research (NIHR CDF-2015-08-074 RATE-AF; NIHR HTA-130280 DaRe2THINK; NIHR EME-132974 D2T-NV), the British Heart Foundation (PG/17/55/33087 and AA/18/2/34218), EU/EFPIA IMI (BigData@Heart 116074), the European Society of Cardiology supported by educational grants from Boehringer Ingelheim/BMS-Pfizer Alliance/Bayer/Daiichi Sankyo/Boston Scientific, the NIHR/the University of Oxford Biomedical Research Centre, and British Heart Foundation/the University of Birmingham Accelerator Award (STEEER-AF NCT04396418), Amomed Pharma, and IRCCS San Raffaele/Menarini (beta-blockers in Heart Failure Collaborative Group NCT0083244), in addition to personal fees from Bayer (Advisory Board), AtriCure (Speaker fees), Protherics Medicines Development (Advisory Board), and Myokardia (Advisory Board). Prof. Kotecha is one of the associate editors to Cardiology. Dr. Sartini, Dr. Agrawal, Dr. Natani, Prof. Denaxas, Prof. Asselbergs, and Prof. Dobson have nothing to disclose. Dr. Gill reports funding through the BigData@Heart IMI, grant No. 116074. Dr. Suzart-Woischnik reports personal fees from Bayer AG, during the conduct of the study, and other from Bayer AG, outside the submitted work. Dr. Wirta reports other from Novartis, during the conduct of the study. Dr. Studer reports personal fees from Novartis Pharmaceuticals, during the conduct of the study, and other from Novartis Pharmaceuticals, outside the submitted work.
Funding Sources
This work was supported by the BigData@Heart project, which has received funding from the IMI 2 Joint Undertaking (Grant Agreement No. 116074). This joint undertaking receives support from the European Union's Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA).
Author Contributions
R.S. conceptualized the study and critically reviewed the manuscript. D.K. analyzed the data and critically reviewed the manuscript. H.N. analyzed the data and drafted the manuscript. C.S., K.S.-W., R.A., S.K.G., S.B.W., F.W.A., R.D., and S.D. critically reviewed the manuscript. All authors have made substantial contribution in critically reviewing and writing the manuscript. All authors have read and approved the submission of the paper.
Data Availability Statement
The list of metadata of publicly available data sources can be found at www.escardio.org/bigdata.
Supplementary Material
Supplementary data
Supplementary data
References
- 1.World Health Organization Cardiovascular diseases (CVDs) 2020. Available from: http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. The Lancet. 2016;388((10053)):1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation . The future of CVD. WHO; 2020. Available from: https://www.who.int/cardiovascular_diseases/en/cvd_atlas_25_future.pdf?ua=1. [Google Scholar]
- 4.World Health Organisation Global status report on noncommunicable diseases 2010: description of the global burden of NCDs, their risk factors and determinants. 2020. Available from: https://www.who.int/nmh/publications/ncd_report2010/en/
- 5.U.S. Food & Drug Administration Real world evidence. 2020. Available from: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence.
- 6.Martina R, Jenkins D, Bujkiewicz S, Dequen P, Abrams K. The inclusion of real world evidence in clinical development planning. Trials. 2018;19((1)):468. doi: 10.1186/s13063-018-2769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khosla S, White R, Medina J, Ouwens M, Emmas C, Koder T, et al. Real world evidence (RWE): a disruptive innovation or the quiet evolution of medical evidence generation? F1000Research. 2018;7:111. doi: 10.12688/f1000research.13585.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miksad RA, Abernethy AP. Harnessing the power of real‐world evidence (RWE): a checklist to ensure regulatory‐grade data quality. Clin Pharmacol Ther. 2018;103((2)):202–5. doi: 10.1002/cpt.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampson G, Towse A, Dreitlein WB, Henshall C, Pearson SD. Real-world evidence for coverage decisions: opportunities and challenges. J Comp Eff Res. 2018;7((12)):1133–43. doi: 10.2217/cer-2018-0066. [DOI] [PubMed] [Google Scholar]
- 10.Garrison LP, Jr, Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real‐world data for coverage and payment decisions: the ISPOR real‐world data task force report. Value Health. 2007;10((5)):326–35. doi: 10.1111/j.1524-4733.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen AT, Goto S, Schreiber K, Torp-Pedersen C. Why do we need observational studies of everyday patients in the real-life setting? Eur Heart J Suppl. 2015;17((Suppl l_D)):D2–8. [Google Scholar]
- 12.Rabin EG, The New York times . USA: The New York Times; 2020. Two huge Covid-19 studies are retracted after scientists sound alarms. Available from: https://www.nytimes.com/2020/06/04/health/coronavirus-hydroxychloroquine.html. [Google Scholar]
- 13.John W, Ian H, Rafael J, Eric L, Kees VB, Gaspare M, et al. Implementation and relevance of FAIR data principles in biopharmaceutical research and development. Drug Discov Today. 2019 doi: 10.1016/j.drudis.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Grobbee D. Innovative medicines innitiative. BigData@Heart. 2020. Available from: https://www.imi.europa.eu/projects-results/project-factsheets/bigdataheart.
- 15.Innovative Medicines Innitiative BigData@Heart. 2020. Available from: https://www.bigdata-heart.eu/
- 16.Anker S, Asselbergs FW, Brobert G, Vardas P, Grobbee DE, Cronin M. Big data in cardiovascular disease. Eur Heart J. 2017;24((38)):1863–5. doi: 10.1093/eurheartj/ehx283. [DOI] [PubMed] [Google Scholar]
- 17.Gruenberger J-B, Castelo-Branco A, Keininger D, Gerardo M, Yadav V, Goyal P. An inventory of real-world data sources to address the current management of patients with chronic obstructive pulmonary disease (COPD) Am Thorac Soc. 2015:A5812-A. [Google Scholar]
- 18.Tanguy A, Jönsson L, Ishihara L. Inventory of real world data sources in Parkinson's disease. BMC Neurol. 2017;17((1)):213. doi: 10.1186/s12883-017-0985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Lahoz R, Jawla S, Przybysz R, Kahler KH, Burdukova L, et al. Identification and mapping of worldwide sources of generic real-world data. Pharmacoepidemiol Drug Safe. 2019 doi: 10.1002/pds.4782. [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration Use of real-world evidence to support regulatory decision-making for medical devices. Guidance for industry and Food and Drug Administration staff. 2017.
- 21.Oortwijn W. Real-world evidence in the context of health technology assessment processes − from theory to action. 2020. Available from: https://htai.org/wp-content/uploads/2018/11/Policy_Brief_GPF_2019_051118_final_line-numbers.pdf.
- 22.Bell H, Wailoo A, Hernandez M, Grieve R, Faria R, Gibson L, et al. The use of real world data for the estimation of treatment effects in NICE decision making. Nice Dsu Tech Sup. 2016 [Google Scholar]
- 23.Hatswell AJ, Baio G, Berlin JA, Irs A, Freemantle N. Regulatory approval of pharmaceuticals without a randomised controlled study: analysis of EMA and FDA approvals 1999–2014. BMJ open. 2016;6((6)):e011666. doi: 10.1136/bmjopen-2016-011666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George E. How real-world data compensate for scarce evidence in HTA. Z Evid Fortbild Qual Gesundhwes. 2016;112:S23–6. doi: 10.1016/j.zefq.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Du X, Khamitova A, Kyhlstedt M, Sun S, Sengoelge M. Utilisation of real-world data from heart failure registries in OECD countries-A systematic review. Int J Cardiol Heart Vasc. 2018 doi: 10.1016/j.ijcha.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alemayehu D, Ali R, Alvir JMJ, Cappelleri JC, Cziraky MJ, Jones B, et al. Examination of data, analytical issues and proposed methods for conducting comparative effectiveness research using” real-world data. J Manag Care Spec Pharm. 2011;17((9 Suppl A)):1–37. [Google Scholar]
- 27.European Meetings Industry Fair EMIF catalogue. 2020. Available from: http://www.emif.eu/emif-catalogue/
- 28.Ehden I. Innovative medicines initiative. 2020. Available from: https://www.imi.europa.eu/projects-results/project-factsheets/ehden.
- 29.Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GY, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351:h4451. doi: 10.1136/bmj.h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salam AM, Sulaiman K, Alsheikh-Ali AA, Singh R, AlHabib KF, Al-Zakwani I, et al. Precipitating Factors for Hospitalization with Heart Failure: Prevalence and Clinical Impact Observations from the Gulf CARE (Gulf aCute heArt failuRe rEgistry) Med Princ Pract. 2020;29((3)):270–278. doi: 10.1159/000503334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becher PM, Schrage B, Benson L, Fudim M, Corovic Cabrera C, Dahlström U, et al. Phenotyping heart failure patients for iron deficiency and use of intravenous iron therapy: data from the Swedish Heart Failure Registry. Eur J Heart Fail. 2021;23((11)):1844–1854. doi: 10.1002/ejhf.2338. [DOI] [PubMed] [Google Scholar]
- 32.Greene SJ, Choi S, Lippmann SJ, Mentz RJ, Greiner MA, Hardy NC, et al. Clinical Effectiveness of Sacubitril/Valsartan Among Patients Hospitalized for Heart Failure With Reduced Ejection Fraction. J Am Heart Assoc. 2021;10((16)):e021459. doi: 10.1161/JAHA.121.021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim RB, Hwang JY, Park HW, Her AY, Lee JH, Kim MH, et al. Contemporary Status of Acute Myocardial Infarction in Korean Patients: Korean Registry of Acute Myocardial Infarction for Regional Cardiocerebrovascular Centers. J Clin Med. 2021;10((3)):498. doi: 10.3390/jcm10030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potpara TS, Lip GYH, Dagres N, Crijns HJMG, Boriani G, Kirchhof P, et al. Cohort profile: the ESC EURObservational Research Programme Atrial Fibrillation III (AF III) Registry. Eur Heart J Qual Care Clin Outcomes. 2021;7((3)):229–237. doi: 10.1093/ehjqcco/qcaa050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
The list of metadata of publicly available data sources can be found at www.escardio.org/bigdata.