Abstract
Radiation therapy (RT) continues to play an important role in the treatment of cancer. Adaptive RT (ART) is a novel method through which RT treatments are evolving. With the ART approach, computed tomography or magnetic resonance (MR) images are obtained as part of the treatment delivery process. This enables the adaptation of the irradiated volume to account for changes in organ and/or tumor position, movement, size, or shape that may occur over the course of treatment. The advantages and challenges of ART maybe somewhat abstract to oncologists and clinicians outside of the specialty of radiation oncology. ART is positioned to affect many different types of cancer. There is a wide spectrum of hypothesized benefits, from small toxicity improvements to meaningful gains in overall survival. The use and application of this novel technology should be understood by the oncologic community at large, such that it can be appropriately contextualized within the landscape of cancer therapies. Likewise, the need to test these advances is pressing. MR-guided ART (MRgART) is an emerging, extended modality of ART that expands upon and further advances the capabilities of ART. MRgART presents unique opportunities to iteratively improve adaptive image guidance. However, although the MRgART adaptive process advances ART to previously unattained levels, it can be more expensive, time-consuming, and complex. In this review, the authors present an overview for clinicians describing the process of ART and specifically MRgART.
Keywords: 0.35 Tesla MR Guidance, 1.5 Tesla MR Guidance, adaptive image guidance, adaptive radiation therapy, magnetic resonance (MR)-guided radiation, RT adaption, personalized radiation therapy, Unity, ViewRay
Introduction
Radiation therapy (RT) is a well established cornerstone of oncologic management. It is estimated that 40% to 60% of patients with cancer will benefit from RT at some point in their treatment course.1 Reflecting on this high utilization rate is both exciting and compelling. An estimated 1.8 million new cancer cases occurred in 2020 in the United States alone.2 This means that RT was potentially indicated as a modality in approximately 900,000 of those patients. The significant impact of this number places the RT modality as one of the most common single oncologic therapeutic options for patients with cancer. Thus meaningful improvements in RT will affect hundreds of thousands of patients with cancer annually. Studying the optimal use of RT is an exceedingly important part of understanding advancements in cancer therapy as a whole. Validated improvements in oncologic outcomes associated with technological improvements in RT will likewise provide evidence-based improvement for thousands of patients with cancer.
Like many cancer therapies, RT is rapidly evolving. The improvements in RT are the result of multiple technological advances. This reality should be considered in the broader context of improvements in cancer therapies.3 As computational advances occur along with imaging advances, each will have an important impact on the methods by which RT is given. There have been significant limitations to RT that these technological advances seek to overcome. Well designed, prospective, multi-institutional clinical trials will be needed to determine whether the proposed advances provide clinical benefit, and methods to improve RT will need to be continually introduced and evaluated. Radiation oncologists, medical oncologists, radiologists, clinical trialists, and patient advocates need to understand these advances to test them in a robust fashion. This review focuses on the concept of adaptive RT (ART) and, more specifically, magnetic resonance (MR)-guided ART (MRgART), as enabled by the integration of an MR imaging (MRI) scanner within the linear accelerators (linacs) that are used to deliver radiation. Our objective in this review is to illustrate how this novel category of RT differs from historic RT and how it is positioned to potentially improve outcomes associated with RT. Critical questions and potential applications associated with the use of these technologies are addressed.
Evolution of RT Technology Over the Past 30 Years
The past 30 years have seen dramatic technological changes in RT.4–6 Before 1990, RT used relatively simple techniques, in which image guidance was limited to using 2-dimensional (2D), plain film x-rays. In the most simplistic terms, a plain film x-ray was acquired during radiation planning, and the region of the body that contained malignancy was outlined and targeted for treatment. The field shape was typically drawn with straight lines, using bony anatomy that could be seen and referenced on x-rays as a guide. Lines were drawn to create a lead radiation portal, or port, that guided RT into the body. Because this planning process only used a 2D image, it was historically referred to as 2D RT. Although 2D-based RT seems primitive to many radiation oncologists today, it has dramatically influenced current RT practice. It was during the 2D era, from the 1950s through the 1980s, that RT formed its foundation as a cancer therapy.6 That foundation remains very influential in the modern era. Despite often being dismissed as technologically simplistic, the 2D era produced clinical outcome data that continue to influence current day RT. Indeed, many of our current standard-of-care RT doses, including normal organ dose tolerances, were derived largely during the 2D era. For example, despite technological advances over the past 30 years, postoperative RT doses for pancreatic cancer have remained largely unchanged.7,8 This is despite modern methods to control and deposit RT dose. A movement beyond the 2D-based normal tissue dose limits, total dose and fractionation schedules, and regional anatomic treatment volumes (as defined by surface anatomy and/or 2D imaging) is imperative to accelerate the capabilities of RT into the modern era.9
Three-Dimensional, Intensity-Modulated RT, Volumetric-Modulated Arc Therapy, and Particle Therapy (Protons/Carbon)
With the invention of computed tomography (CT) came the ability to define tumors and organs in 3 dimensions.6 Such technology and capabilities were quickly adopted by radiation oncologists. This introduced a volumetric dimension of data to the historic 2D perspective that allowed radiation oncologists to define entire organs in 3 dimensions. RT beams could be modeled to pass through organs with an understanding of how much radiation dose each beam contributed to the specific organs.6 Developing a 3D computational model of the patient using CT enabled relatively quick and accurate calculations of RT dose distributions throughout these organs. During this same time period, clinical outcomes began to emerge illustrating the relation between the volume of organs treated with RT and subsequent toxicity events.10 Such data formed a new basis for RT plan evaluation and review. Shortly after the introduction of CT-based, 3D-conformal RT came intensity-modulated RT (IMRT).9,11 The ability to modulate and sculpt the RT dose around normal organs was dramatically improved by IMRT. There have been demonstrations of reduced toxicity when using these novel techniques; however, pure randomized data are limited.12,13 IMRT has also expanded into more advanced methods, such as volumetric-modulated arc therapy (VMAT), which is an enhanced method of delivering IMRT using arcs rather than static gantry positions. During VMAT delivery, the RT treatment machine rotates around the patient, improving radiation delivery. It has been shown that VMAT is faster and provides better dose distributions than competing techniques.14
Shortly after the introduction of IMRT and VMAT came a more accelerated adoption of particle therapy.15 Particle therapy, essentially the use of accelerated heavy ions, can deliver very steep dose gradients because of the physical properties of its particles. This use of particle therapy also contributed to reductions in normal tissue doses and toxicity in specific indications.15 A schematic timeline of this information is provided in Figure 1.
FIGURE 1.
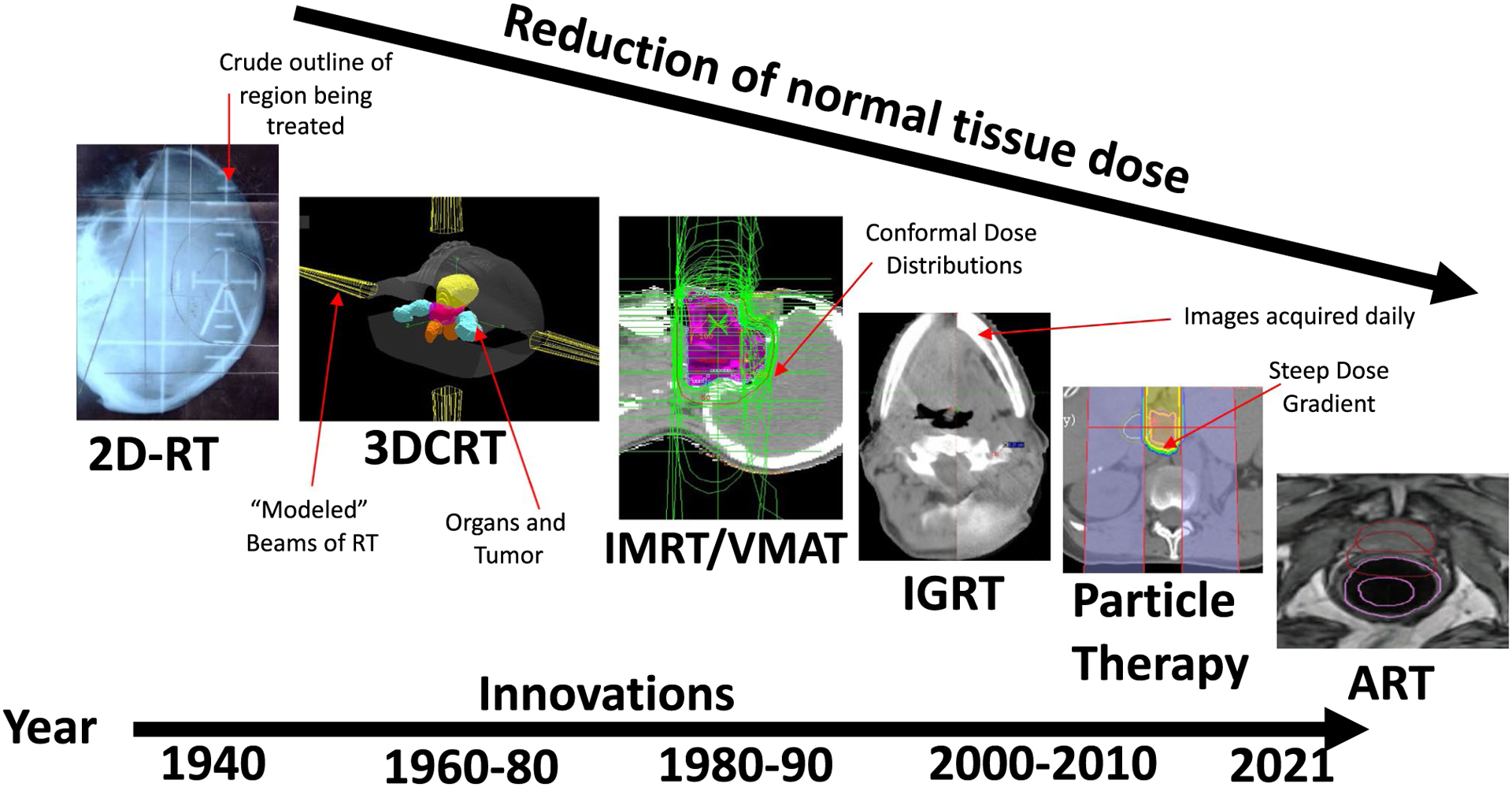
Historic Evolution of Radiation Technology Over the Past 30 Years: Image-Guidance Radiotherapy (IGRT), 2-Dimensional Radiotherapy (2D-RT), 3D-RT, Intensity-Modulated RT/Volumetric Modulated Arc Therapy (IMRT/VMAT), Particle Therapy, and Adaptive Therapy (ART).
The Use of Image Guidance in RT
Shortly after the introduction of 3D-based RT and, subsequently, IMRT came image-guided RT (IGRT). This relies first on the use of a CT simulation study, in which a patient model is derived from an immobilized patient, and external marks are placed on the patient for localization during daily treatment. This scan is also used for contouring and identifying normal organ positioning. When IGRT is implemented, patients are imaged immediately before each fraction of RT with on-board imaging systems that provide 3D (volumetric) images. The tumor and normal organ segmentations on these images are typically compared with those on the higher quality initial CT simulation images. The patient is then repositioned (adjusted slightly) to align these normal organs with their locations at the time of the CT simulation. In some circumstances, this is repeated at each fraction and thus enables slight adjustments for the location of the tumor along with normal organs. Figure 2 illustrates the concept of plain films versus daily image guidance using a CT scanner in the RT room. Figure 2B specifically demonstrates the use of daily CT to confirm the location of normal organs, such as the stomach. These organs are compared with their position from the original CT simulation image. Those structures are drawn on the CT simulation images, then their original position is superimposed onto each daily image to reflect the original position of these organs.
FIGURE 2.
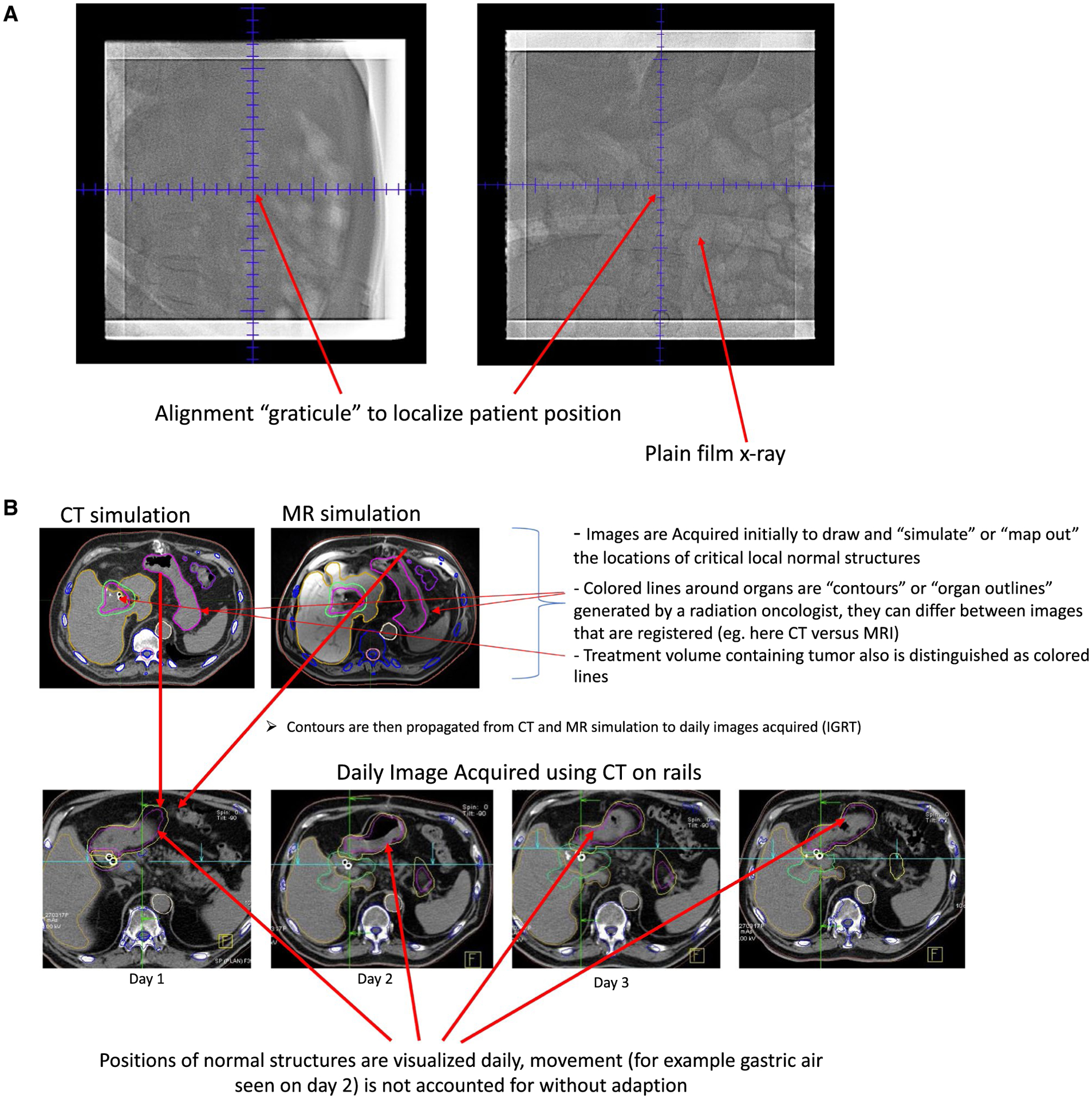
Illustrations of Radiation Treatment Given (A) Without Daily Computed Tomography (CT)-Based Image Guidance Using Plain Films and (B) Treatment Using CT on Rails.
Currently, most volumetric IGRT is performed with kilovoltage (kV) cone-beam CT (CBCT), megavoltage (MV) CT, and CT on rails. Other non-CT methods can include optical imaging, ultrasound imaging, orthogonal x-ray imaging (kV/kV or MV/kV), or fluoroscopic imaging (for guiding brachytherapy or intrafraction imaging). The concept and advantages of IGRT have been the subject of multiple separate review articles16,17; however, it is also important to understand current limitations. Although physicians can visualize anatomic organs and tumor volumes using image guidance, responding to anatomic variations, such as relative positional changes or changes in shape or size (such as peristalsis), using only patient positional shifts is suboptimal. This creates the principal limitation of IGRT, which is the absence of the ability to correct for the volume changes and deformations of the tumor and/or normal structures. This results in a discrepancy between the actual RT dose that is delivered to the tumor or normal structures and the treatment plan. Figure 3 highlights such uncertainty and the impetus for ART.
FIGURE 3.
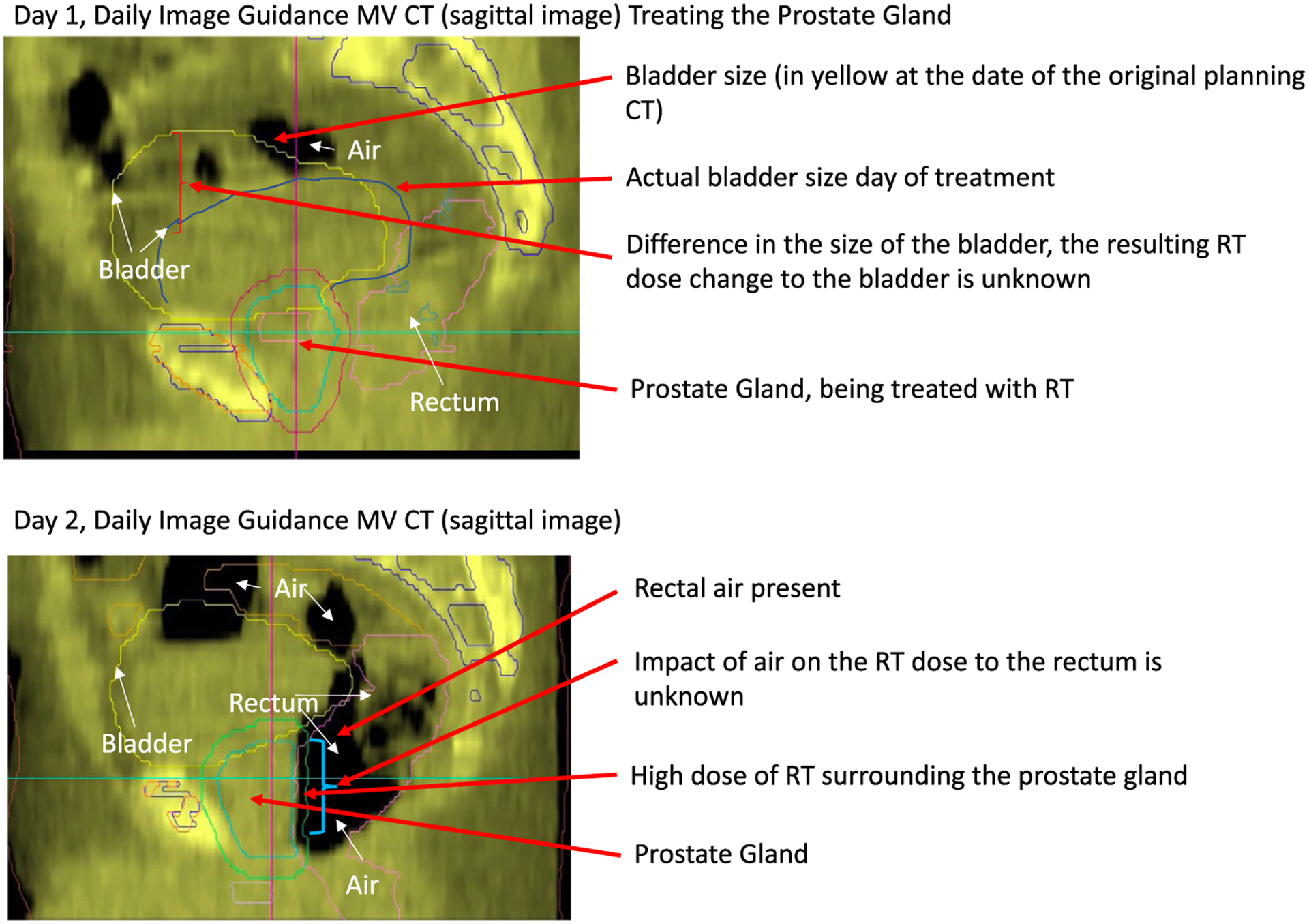
Limitations of Image Guidance Alone—Volume Change/Deformations Can Be Seen But Not Accounted for Dosimetrically: Treatment of the Prostate Gland Associated With Significant Rectal Movement and Gas Causing Distension. CT indicates computed tomography; RT, radiotherapy; MV, megavoltage.
There are also CT-based and MRI-based methods to account for movement of tumors, such as 4D-CT and 4D-MRI. These imaging methods allow for tumor movement to be visualized during the breathing cycle and subsequently accounted for with RT treatment volumes.18,19 However, it is difficult to account for normal organ movement during the time at which the beam is turned on. Movement of the radiation beam can allow for some tumor tracking; however, the ability to account for normal organ movement is limited to absent.
Adaptive Radiation Therapy
Background
ART represents the next advancing frontier of IGRT. In its most basic definition, ART enables the changing of RT dose delivery to account for changes in either the tumor or normal structures during the course of RT delivery. The concept of ART was first introduced in 199720 and, by 2010, ART was widespread within the radiation oncology literature.21,22 Its adoption has considerably accelerated recently with expanding technological innovations, including MR guidance.23,24 There are many different anatomic changes that would require the modification of an RT dose distribution, including motion of normal organs and variations in tumor or organ size, changes in patient external anatomy (such as weight loss or gain), and positioning variations associated with daily alignment. Moreover, ART can also signal the need to modify the total RT dose based on progressive biologic changes in the tumor seen on imaging acquired during the radiation course. As an example, some recent trials have adjusted treatment based on positron emission tomography (PET) response, enabling true biologic personalization of therapy.25 Overall, if changes are seen using the image guidance techniques detailed above, ART can correct the RT dose distribution to accommodate these changes. Under these circumstances, RT dose prescriptions can sometimes be increased based on tumor response or reduced to spare adjacent normal organs. This is a highly complex procedure and considerable technical challenges are associated with its implementation (Fig. 4).
FIGURE 4.
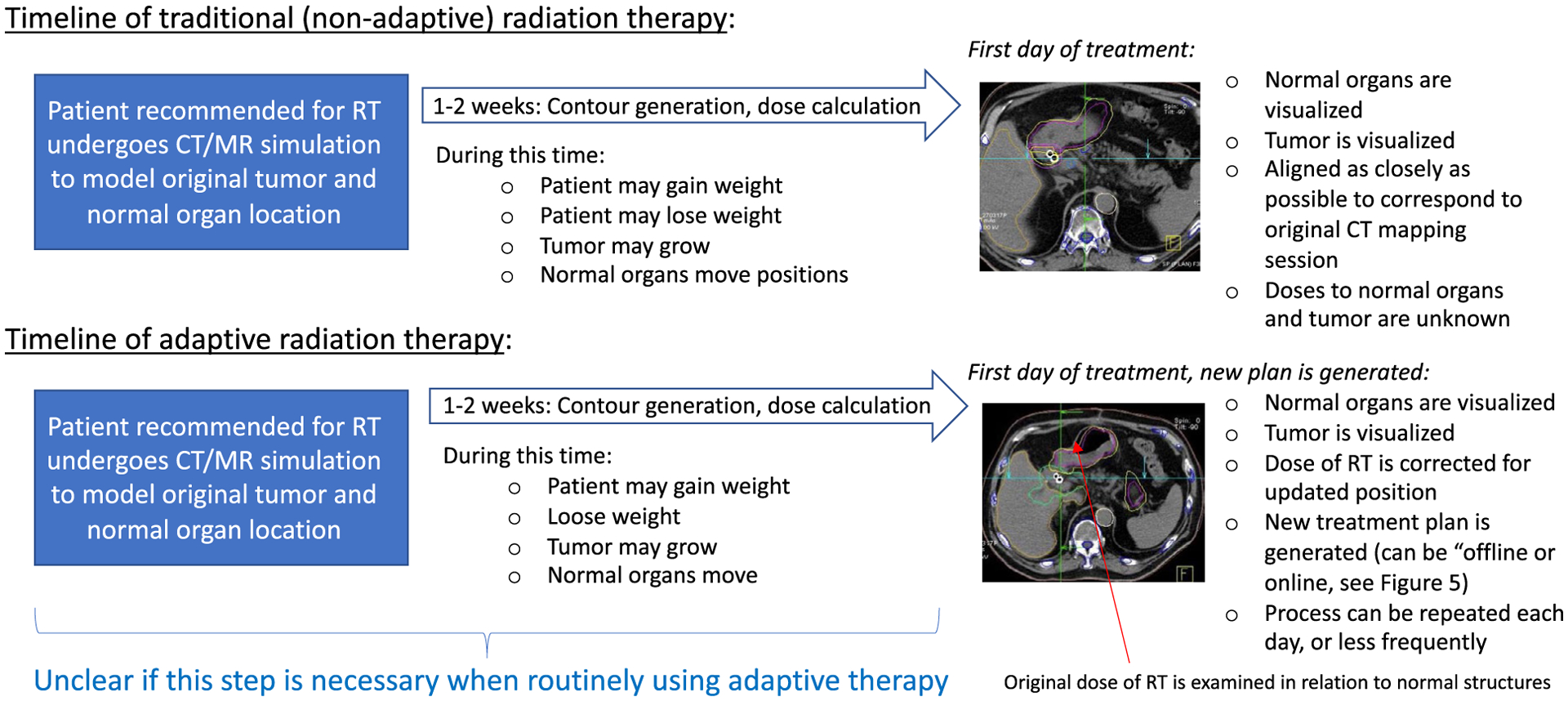
Traditional Radiotherapy (RT) Compared With Adaptive RT (ART). A comparative overview of the workflow associated with adaptive RT compared with traditional non-ART is presented. CT indicates computed tomography; MR, magnetic resonance.
There are several categories in which ART could be applied and may hold an important role. Each of these requires additional investigation and prospective evaluation before it is routinely recommended as a standard of care. It is important to recognize that, despite the existence of ART as a well described technique for at least a decade, the routine implementation and utilization of daily CT-based ART remains relatively limited.26 There are several reasons for this limited utilization. First is the tremendously labor-intensive and time-consuming nature of the adaptive process. Radiation oncologists often must be present for extended periods of time at each treatment to identify, modify, and approve new radiation plans. There is very limited reimbursement currently for the effort of involved personnel required to perform this adaptation. Second, the technical capability to perform adaptation is demanding. Imaging and adaptation technologies must be sophisticated enough such that they can be used to robustly detect and correct for the changes in tumor and normal organs. Such systems are not routinely available but are currently the subject of active research and development at high-volume academic centers and on a selective, case-by-case basis.27–31 Currently, this makes the widespread implementation of CT-based ART in a community practice limited. There are novel systems being introduced, such as the Varian Ethos system, that may affect this accessibility in the coming years.32 Another important consideration is the time associated with ART, which is a considerable limitation that should be considered. This can require a radiation oncologist to spend considerable time at the treatment machine re-contouring daily. Despite these limitations and relatively rare usage, the potential benefits of ART are significant. Moreover, MR guidance is introducing a highly feasible manner in which ART can be performed (Figs. 5 and 6).
FIGURE 5.

Detailed Overview of Workflow, Additional Steps, and Potential Dose Improvements Associated With Adaptive Radiotherapy (ART) Either Online or Offline. IGRT indicates image-guided radiotherapy; RT, radiotherapy.
FIGURE 6.
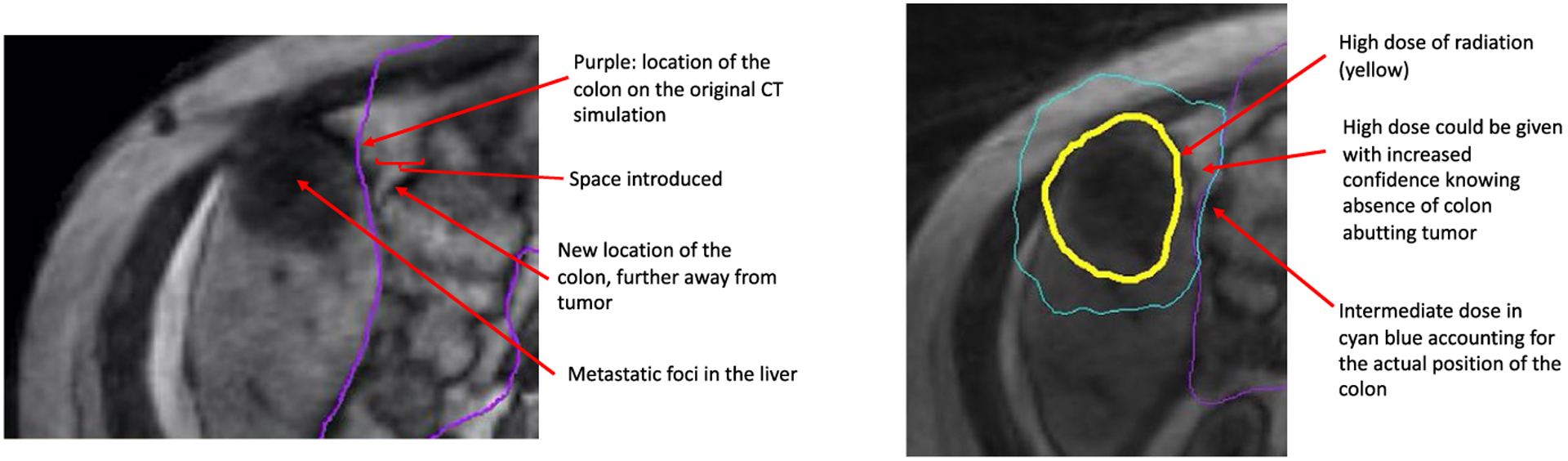
Detailed Example of Dose Distribution Differences Seen With Adaptive Therapy Using Magnetic Resonance Guidance. CT indicates computed tomography.
MR-Guided Adaptive RT
A central limitation of ART is the ability to routinely perform it with the state-of-the-art and most widely disseminated, 3D, on-table imaging modality, CBCT. The images that CBCT can generate on a daily basis often do not provide sufficient soft tissue contrast to accurately identify the required precise boundaries between normal organs and tumors. These images also suffer from organ motion-induced artifacts that further degrade image quality. CT on rails, a system that is essentially a diagnostic CT collocated with the RT delivery device, can offer a higher contrast image of the tumor and normal organs than CBCT provides. However, these systems are uncommon, and their use is relatively cumbersome and time-consuming. On-board MRI provides the superior soft tissue contrast of MRI, allowing ART to be performed with a much greater degree of confidence. An example of such imaging differences is presented in Figure 7 for a tumor that moves because of respiration. This is an example of a patient undergoing treatment with RT for a pancreatic tumor using CBCT guidance compared with a similar patient undergoing treatment using MRI guidance. The clarity of these daily MR images offers a more realistic and feasible method to perform ART using available images.
FIGURE 7.
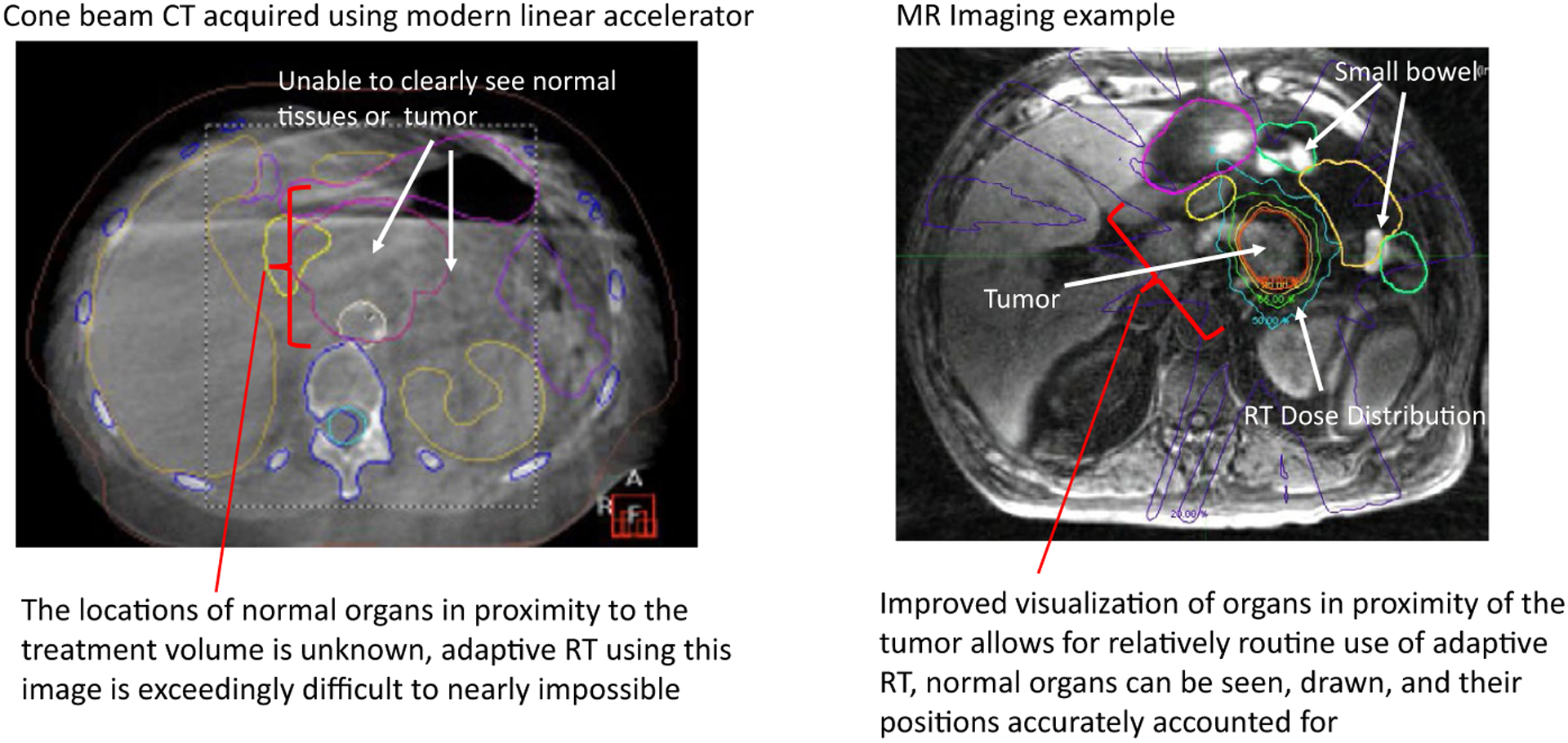
Example of Cone-Beam Computed Tomography (CT), Which Is Typical for Daily Image Guidance on Linear Accelerators, Compared With Daily Magnetic Resonance Imaging.
Some of the most common current uses for MR-guided RT include prostate tumors, oligometastatic disease, pancreatic tumors, central lung tumors, brain tumors, and rectal tumors.33 It is important to consider that the precise utilization of this technology is rapidly evolving and, as the technological capabilities develop, the utilization may change.
Randomized Trials Evaluating Adaptive RT
Despite the timeline and availability of ART, the currently published randomized clinical trials evaluating ART compared with non-ART are very limited. Only a small handful of trials have been performed attempting to prospectively quantify the benefits of this technology. Fortunately, this area is growing, and additional data should be available in the coming years. There are previous retrospective studies demonstrating that ART can improve target coverage and also reduce the doses of RT delivered to organs at risk in a variety of tumor sites, including head and neck, lung, breast, abdomen, and pelvis.34–36 Multiple historic trials have evaluated the feasibility of performing ART, and multiple reviews have been published on the topic.26 A few examples for consideration include the following: An early prospective trial conducted by Vargas et al demonstrated that ART could be performed in patients with prostate cancer and that higher doses of RT could be delivered to the prostate without higher rates of toxicity if normal organs are identified and accounted for in the process of RT delivery.35 In other tumor sites, such as lung, ART has also been attempted; however, clear clinical benefits of such adaptation are limited.37 There have been a few recent examples of biologic imaging during a course of treatment that have shown promise using fluorodeoxyglucose-PET (ClinicalTrials.gov identifier NCT01333033).38 There are also ongoing trials for which long-term outcomes are pending, such as NRG Oncology/Radiation Therapy Oncology Group trial 1106 (NRG/RTOG 1106) and the ARTFORCE Trial (ClinicalTrials.gov identifier NCT01504815).39–41 Specifically, RTOG 1106 adapted the radiation dose during treatment based on PET. The results of the study have been presented in abstract form and showed no significant differences in grade 3 or worse toxicity of the lung, esophagus, or heart. In addition, there was no difference in overall survival (OS), progression-free survival, or lung cancer-specific survival between treatment arms (adaptive vs nonadaptive). ART did improve in-field local-regional tumor control by 11% and in-field primary tumor control by 17% during the trial. The full publication of this experience is anticipated. Considerable additional effort is needed in the form of prospective trials to demonstrate the value of performing ART. This is primarily because the effort associated with the process is exceedingly time-consuming with the current technology. Before sufficient support of that time and effort can be provided or more robust technologies become available, there should be commensurate compensation for the considerable additional time and effort associated with this work.42–51 Table 1 summarizes select prospective trials that have recently examined different adaptive strategies.35,37,38,41
TABLE 1.
Recent Prospective Trials Examining Different Adaptive Strategies
| REFERENCE | NO. OF PATIENTS | DESIGN/RANDOMIZATION/QUESTION ADDRESSED | CONCLUSIONS |
|---|---|---|---|
| Vargas 200535 | 331 |
|
|
| Spoelstra 200937 | 24 |
|
|
| Goodman 202138 | 225 |
|
|
| Kong 202141 | 138 |
|
|
Abbreviations; 4D, 4-dimensional; ART, adaptive radiotherapy; CT, computed tomography; FDG-PET, fluorodeoxyglucose-positron emission tomography; GEJ, gastroesophageal junction; Gy, grays; pCR, partial complete remission; PET, positron emission tomography; RT, radiotherapy.
Hypothesized Advantages of MR-Guided ART
The image quality and soft tissue contrast limitations highlighted above with non-MRI–based imaging have led many groups to consider incorporating MRI into the process of treatment planning and RT delivery. There are important novel aspects to consider about this process. The first is understanding how MR guidance differs from MR registration. For over a decade, MRI has been used as an additive to CT-based RT planning to help define soft tissues; this is described as MR simulation.52 However, the concept and process of adaptive MR guidance differs from this historic process of registering an MRI to a CT, which is outlined in Figure 8. Clinical implementation of MR simulation has been expanding over the past 8 years.
FIGURE 8.
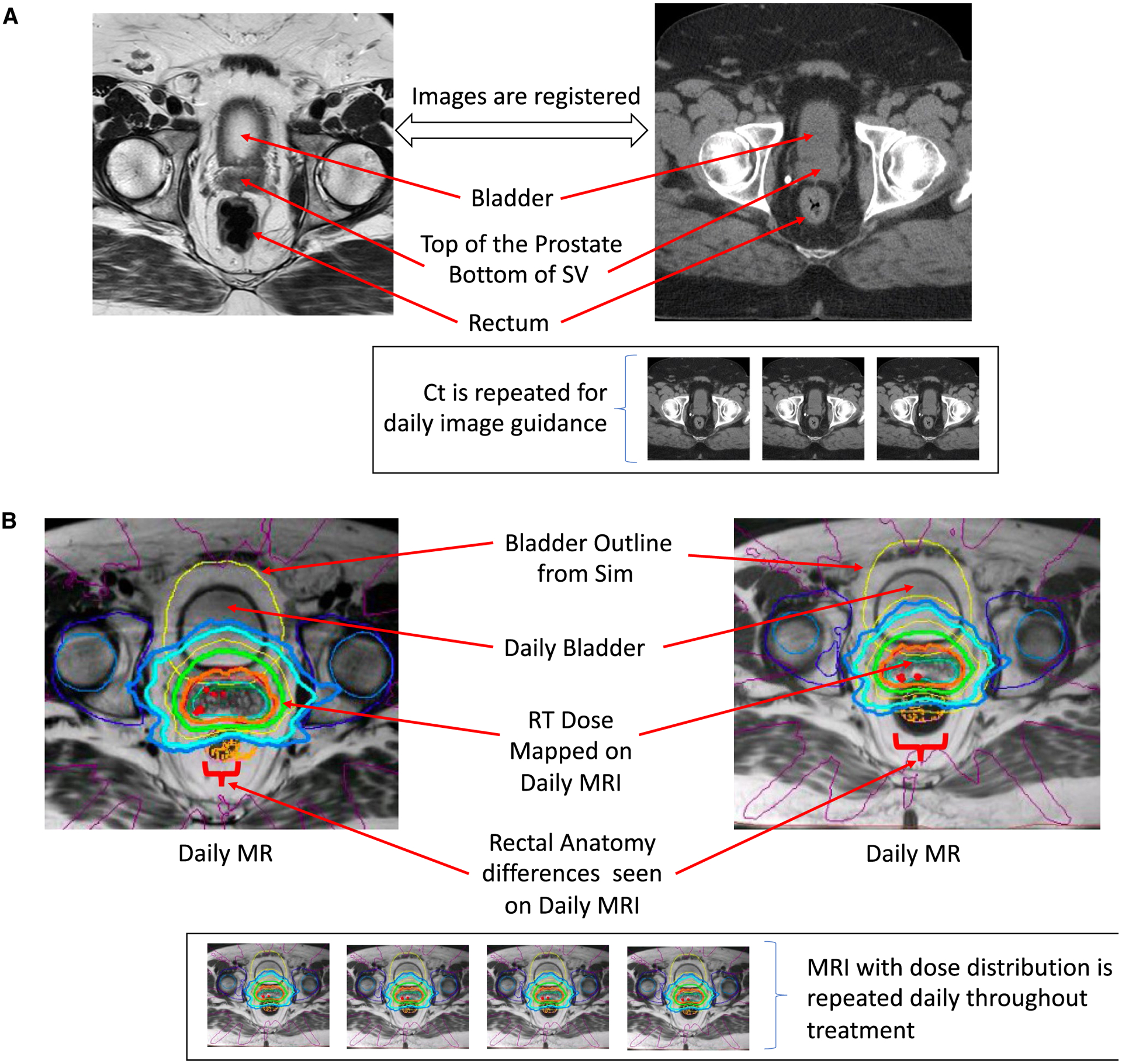
(A) Computed Tomography (Ct) to Magnetic Resonance (MR) Registration Is Compared With Daily MR Acquisition for the Purpose of MR Guidance. (B) MR-Guided Radiotherapy (RT) Acquires an MR Image Each Day. This enables clear visualization of targets as they change daily. MRI indicates magnetic resonance imaging; Sim, simulation; SV, seminal vesicle.
There are several advantages to using MRI as part of the daily image guidance to align and treat a patient using RT. It is well known from diagnostic radiology that MR offers superior soft tissue contrast. This improved soft tissue visualization is particularly helpful during the process of RT delivery in distinguishing tumors from the adjacent normal organs. It is critical to understand that soft tissues move during the radiation treatment because of physiologic anatomic changes, such as bowel peristalsis, respiration, or changes in tumor size.53–55 Therefore, improved visualization of these moving soft tissues enables radiation oncologists to precisely understand changes in dose distribution to these structures over the course of treatment. This improved soft tissue visualization also allows for the routine use of adaptation based on changes in daily anatomy. In addition to daily visualization of the soft tissues, MR guidance offers the ability to actively monitor and visualize these organs in real time while the beam is turned on. This allows radiation oncologists to see any unexpected movements while the treatment is being delivered that could result in higher or lower than anticipated doses delivered to these organs. Both of the commercially available MR-guided RT systems include adaptive capabilities to account for changes in normal organs and tumors that are seen on a daily basis. One important consideration is that MR systems often can account for tumor movement during treatment with gating. Gating refers to turning a beam on or off, depending on the position of a tumor. Normal organs, however, can move differently than a primary tumor—second to second during treatment—and this is not accounted for with gating or even with daily adaptation. The impact of this movement is not fully understood but is often visualized (as seen in Fig. 9).
FIGURE 9.
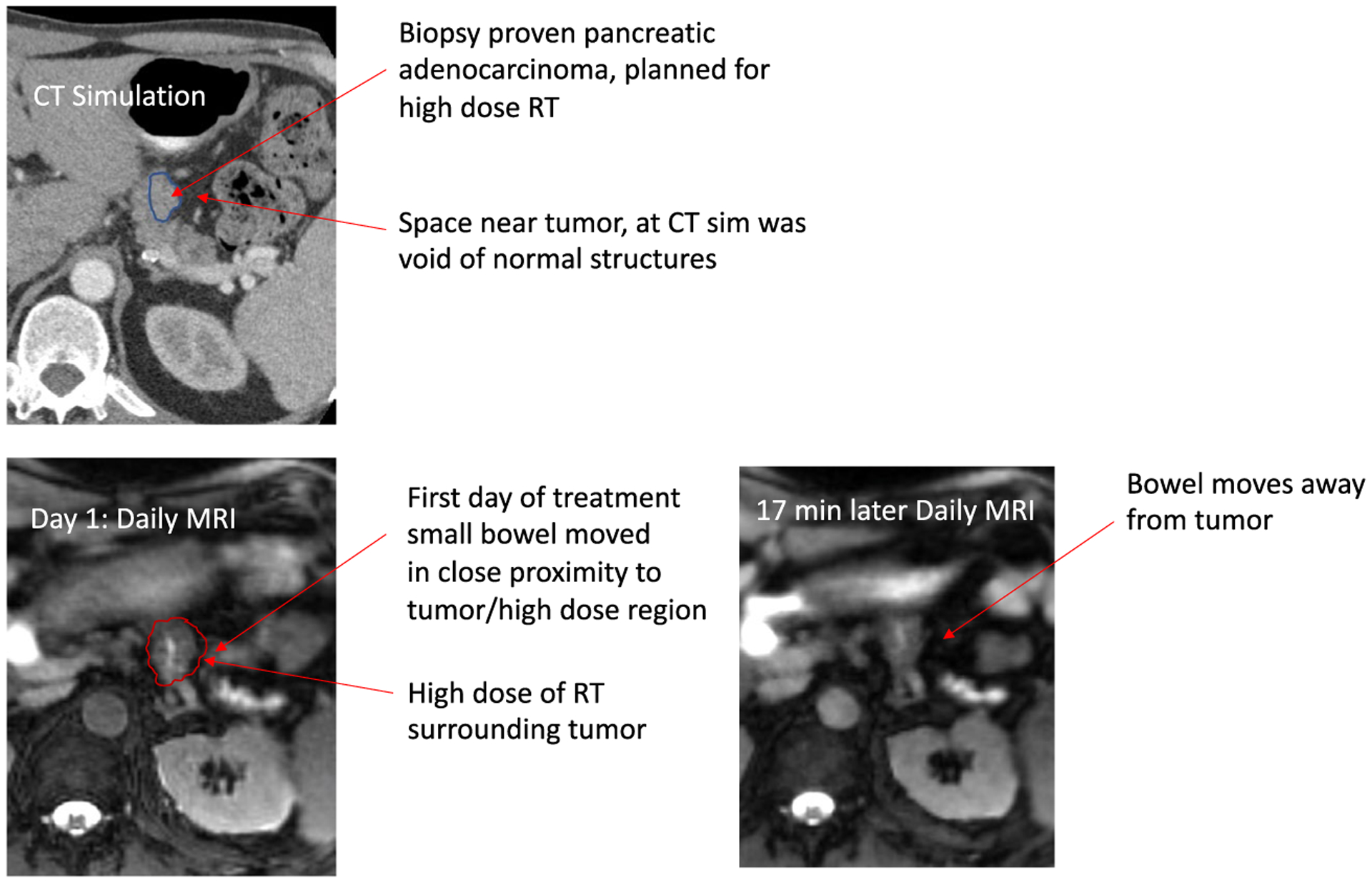
Illustration of Bowel Movement Intrafraction on Close Proximity to High-Dose Radiotherapy (RT) Into an Otherwise Voided Space. CT indicates computed tomography; MRI, magnetic resonance imaging; sim, simulation.
Overview of Currently Available MR-Guidance Technology
Currently, 2 commercially available technologies combine an MRI device with a radiation delivery machine. Each of these technologies has important distinguishing features but overall they represent a common goal, which is the integration of improved soft tissue imaging capabilities (using MR) with a radiation machine to deliver RT. These devices are manufactured by 2 different companies: ViewRay Technologies Inc and Elekta AB. There are also 2 devices that are in development, one is by an Australian-based development group56 and the second is the Aurora-RT system (MagnetTx Oncology Solutions).57 The commercially available devices are gaining rapid and widespread adoption, with >26 Elekta systems clinically operational (>90 sold) and >41 ViewRay systems installed and in use globally (personal email communication from both companies). Key differences between devices are presented in Table 1. The ViewRay MRI-cobalt device has been cleared by the US Food and Drug Administration (FDA) since May 2012, and the ViewRay MRIdian linac has been FDA cleared since February 2017, with most of the installed cobalt systems subsequently being upgraded to the linac version. The Elekta Unity system received FDA clearance in December 2018. To date, ViewRay has produced 2 different systems consisting of their first device, a split 0.35-Tesla MR scanner with a ring gantry and 3 multileaf, collimator-equipped cobalt-60 heads. The cobalt-based device is no longer in production, although a few are still in clinical operation worldwide. The subsequent MRIdian linac is capable of 6-MV photon production combined, again, with a 0.35-T MR scanner.58 The Elekta Unity system is a 1.5-T MR scanner produced by Philips that is combined with a 7-MV linac produced by Elekta.59,60 Details regarding each of these systems are summarized in Table 2.56,58,61–63
TABLE 2.
Currently Approved Devices and Features
| COMMERCIAL NAME (REFERENCE) | MANUFACTURE | MRI FIELD STRENGTH | BORE DIAMETER | BEAM STRENGTH | NO. OF DEVICES OPERATIONAL GLOBALLY |
|---|---|---|---|---|---|
| Commercially available | |||||
| ViewRay (Mutic & Dempsey 201458) | ViewRay Technologies Inc (Oakwood, Ohio) | 0.35 Tesla | 70 cm | 41 | |
| • Co-60 | Co-60 source | ||||
| • Linac | 6 MV | ||||
| Elekta Unity (Raaymakers 2009,61 201762) | Elekta AB (Stockholm, Sweden) | 1.5 Tesla | 70 cm | 7 MV | 26 |
| In development | |||||
| Australian MRI linac system (Keall 201456) | Australian MRI-linac program | 1 Tesla | 82 cm | 6 MV | NA |
| Aurora-RT System (Fallone 201463) | MagnetTx (Edmonton, Alberta, Canada) | 0.5 Tesla | 60 cm | 6 MV | NA |
Abbreviations: Co-60, cobalt-60; linac, linear accelerator; MRI, magnetic resonance imaging; MV, megavolts; NA, not applicable; RT, radiotherapy.
Hypothesized Improvements in the Therapeutic Index Using MR Guidance
There are several categories of hypothesized improvements that are most commonly discussed in association with adaptive MR guidance. These consist of better visualization, routine access to and use of anatomic adaptation, motion management, and incorporation of biologic image guidance. Each of these topics is discussed in greater detail in the sections below.
Better visualization and routine access to ART
There are multiple aspects of RT that could be improved by the routine use of ART and, specifically, MR guidance. MR guidance presents an excellent opportunity to introduce ART into clinical practice for radiation oncology. The ability to see and adapt to changing normal anatomy is theoretically helpful for a variety of reasons. One of the greatest limitations to RT accomplishing a durable cure for patients is the proximity of the tumor to normal dose-limiting structures. This proximity results in the inability of RT to accomplish durable, long-term control. For example, control of solid tumors, such as pancreatic adenocarcinoma (PAC), with RT have historically been very limited secondary to the proximity of the small bowel, stomach, and colon. These structures have dramatically limited the doses of RT that could be used. An excellent contrary example of this circumstance is seen in early stage nonsmall cell lung cancer. Patients treated with ablative (very high) doses of RT for early stage nonsmall cell lung cancer routinely accomplish minimally invasive cure rates equivalent to those accomplished with surgical resection.64 A similar example is seen in patients with prostate cancer. When these patients are treated with high doses of RT, they routinely accomplish cure rates identical to those of surgical resection.65 Understandably, radiation oncologists are reluctant to deliver high doses to pancreatic tumors because of the significant risk of bowel toxicity. Theoretically, adaptive MR guidance can help to overcome this limitation.
The narrow therapeutic index (detriments to benefit with increased toxicity) for many tumor types, such as pancreatic tumors, head and neck cancer, primary liver cancer, and rectal cancer, presents limitations for the success of current RT treatment modalities. Tumors require specific doses of RT to be fully eradicated; however, those doses to the tumor often cannot be achieved secondary to excessive toxicity to the adjacent dose-limiting organs. The potential to routinely apply an adaptive dose of radiation accounting for normal structures is very appealing.
Beam-on motion monitoring
Because MRI does not use ionizing radiation, MR guidance can monitor tumors as the beam is delivering RT using fast cine imaging. Repeated imaging has historically been avoided using CT-based image guidance because of the increased radiation risks, and there is no real-time analog to CBCT. Such monitoring can enable visualization when a critical normal organ moves in close proximity to a tumor. With effective motion management technology, this motion cannot only be detected but potentially can be reacted to with gating or plan adaptation. An example of this circumstance can be seen in Figure 9.
Routine access to and potential incorporation of quantitative and biologic imaging
This last category is one that will require time and commitment, however, but without question presents an immense opportunity for an improvement in outcomes.66 Continually in cancer therapy, capturing the response to treatment and adjusting such treatment accordingly results in considerable improvements in outcomes. When the biologic response of a tumor can be accounted for in a treatment strategy, patients may have considerable improvements in their treatment outcomes. Currently, this is seen most commonly for circumstances in which PET imaging is assessed before and after treatment.67 Patients who have a response with regard to posttreatment imaging have a tendency toward better outcomes than those without a response. This is consistently true in an extensive number of tumors, including lymphomas, head and neck cancer, and primary brain tumors. This is also true with MRI, and there is extensive evidence that changes in biologic data imaged with a variety of MRI sequences, such as perfusion and diffusion, significantly correlate with outcomes.66 With the use of MR guidance, these data will become routinely available at the time of RT delivery. With additional confirmation, signals associated with response may become a new standard that can be collected and accounted for during, rather than after, the treatment delivery.66 If this biologic-response approach to radiation dose selection can be validated, it could theoretically eliminate the current standard of dosing with historical radiation doses. Instead, this would lead to imaging response-directed doses. This presents a novel radiation dosing strategy. In other words, rather than giving an arbitrary and historic radiation dose, such a dose could be correlated with validated biologic imaging markers.66 However, it is important to note that MR sequences specifically for response evaluation need to be further developed and validated on existing MR-guided RT systems.
Limitations of MR Guidance
Although many of the advantages of MR guidance are theoretically compelling, it should be recognized that there are also numerous limitations to this technology. Many of these limitations apply to ART in general and are magnified by the presence of an MRI-equipped linac with adaptive capabilities. Most importantly, it remains unknown with any level of certainty whether these improved visualization capabilities translate into meaningful clinical outcome improvements. If they do improve clinical outcomes, the precise magnitude of that improvement has yet to be fully quantified because of the absence of technology-driven, randomized clinical trials. Although radiation oncologists may believe or hypothesize that outcomes will be improved with the introduction of MR guidance, this remains an area of active inquiry. Measuring such an improvement over CT-based techniques remains relatively absent in the current landscape of clinical trials. An example of a trial testing this in prostate cancer is the MIRAGE trial (ClinicalTrials.gov identifier NCT04384770). In this phase 3 study, patients are being randomized between CT-based and MRI-based treatment for prostate cancer, with a primary end point of acute genitourinary toxicity. More trials like the MIRAGE study with clinically meaningful end points are needed to demonstrate the potential benefits of MR guidance. Another vendor-supported, multi-institutional clinical trial for pancreatic cancer using MRgART and real-time treatment monitoring is actively recruiting that seeks to evaluate gastrointestinal toxicity, survival end points, and patient-reported quality of life (ClinicalTrials.gov identifier NCT03621644).
Unique Physics and Dosimetric Considerations
One of the features of MR guidance that should be commonly considered is the impact of the magnetic field on the RT dose distribution. RT dose is deposited in tissue when the x-rays interact with matter and emit energetic charged particles (typically electrons), which bombard the tissues and cause corresponding damage. The paths of those charged particles are affected by the presence of the magnetic field. Although the impact in tissues is relatively small and easily calculable, there can be profound changes in the dose distributions at and near air-tissue interfaces. This effect is known as the electron-return effect and is a unique consideration associated with the use of MR guidance (Fig. 10). Although these effects can be largely accounted for in the treatment-planning systems,68,69 they increase in magnitude with increasing magnetic field strength.68,70 It should also be noted that MR-guided RT systems currently lack some of the more advanced IMRT implementations, such as more couch positions, VMAT-based or arc-based treatment delivery, and noncoplanar beams.
FIGURE 10.
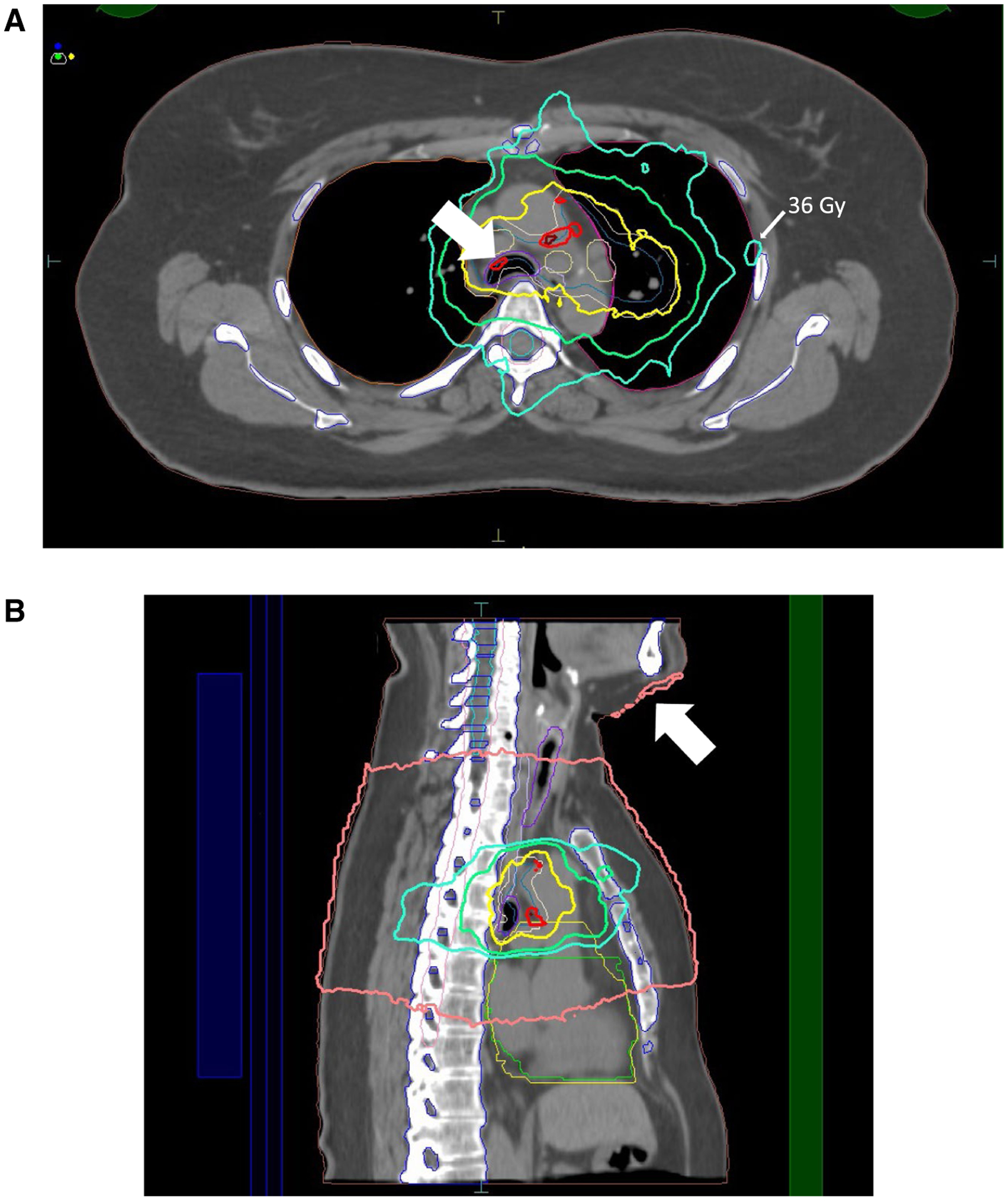
(A) The Electron-Return Effect. Dose was deposited at the lung/tissue interface (3600-centigray [cGy] line) and at the trachea/tissue interface (6600-cGy line). (B) The Electron Air-Stream Effect (White Arrow; 400-cGy Isodose Line). Dose was deposited to protruding anatomic structures from electrons swept away from the treatment field by the magnetic field.
Practical Considerations of MR Guidance
The logistical considerations associated with the daily acquisition of MRIs during a treatment course are not to be minimized. They are extensive and require considerable infrastructure, expertise, and, most of all, a substantial time commitment over traditional RT. Images need to be reviewed and dose distributions need to be considered in light of the specific oncologic case. In addition, the selection of appropriate patients for MR guidance is an important first step. Not all patients are candidates for MR guidance. Patients must be able to undergo an MRI examination, meaning they cannot have some types of implanted metal, electronic devices, or significant claustrophobia, and they must meet body habitus and size requirements. In addition, therapists and radiation oncologists must become familiar with MR-based imaging that can subsequently be used in the process of treatment delivery. Furthermore, MR safety of patients and personnel requires careful consideration in the establishment of a new program.
Artificial Intelligence and MR Guidance
Many of the limitations associated with both ART and MR-guided RT are surrounding time-consuming manual tasks. These tasks require the attention of radiation therapists, radiation oncologists, and physicists. This is a prime area for development and partnership with artificial intelligence (AI), with a focus on methodologies to expand the feasibility of daily adaptation. This area is undergoing rapid investigation, and publications will be expanding in number over the coming years.71,72 Plan quality-assurance review, dosimetric tasks, and normal organ contouring are all areas that will likely be immediately improved through the partnership with AI. The amount this will expand and enable adaptation currently is difficult to quantify but is likely substantial.
Adaptive MR Guidance Results in Clinical Practice
Because 2 MR-guided RT devices have been approved for clinical use for at least 3 years, there are multiple clinical publications that have used MR guidance. Most of these are retrospective; however, understanding their proposed value, along with the potential advantages associated with specific use cases, is illustrative for both the ViewRay (0.35 Tesla) and Elekta Unity (1.5 Tesla) MR-guided systems.
General Clinical Use of MR-Guided RT and Daily Adaptation
The overall clinical utilization of MR-guided RT, along with tumor types and locations selected for daily adaptation, is an important topic of consideration. Notable is that uses of MR guidance and adaptation are rapidly evolving as the technology becomes more widely distributed and accessible. As radiation oncologists gain familiarity with the technology and its capabilities, their specific utilization will likely evolve. Data have been presented for each of the commercially available vendors of different MR-guided RT solutions. For both vendors, the most common site of treatment tends to be prostate tumors, followed by oligometastatic lymph nodes. There is often a heterogenous mix of tumors that follow on, including pancreatic tumors, head and neck tumors, rectal cancer, adrenal gland metastases, liver tumors, primary lung tumors, and kidney tumors.33,73 These tend to be equally distributed across both vendors. Malignancies for which the use of MR-guided RT and daily adaptation remains relatively uncommon include breast tumors, sarcoma, and gynecologic cancers. With regard to the use of daily adaptation (recontouring each local organ and tumor on a daily basis), the precise data for this are evolving but have been very recently published by users of the Elekta 1.5-Tesla MR linac.33 The most common tumors that use daily adaptive recontouring include pancreatic tumors, rectal tumors, and lymph nodes.33 This is likely secondary to the close proximity of these treatment sites to highly mobile and radiosensitive structures, such as the small bowel. Primary or secondary central nervous system (CNS) tumors, prostate tumors, and primary liver tumors seem less likely to use daily adaptation and recontouring. The precise reason for this is uncertain and likely multifactorial. In the prostate, the size and position of the rectum and bladder can often be managed with pretreatment instructions on timing of bowel movements, enema use as needed, and fluid consumption. With regard to primary brain tumors, lung tumors, and liver tumors, these are often surrounded by a parallel organ (ie, an organ that can function with a portion removed or damaged, such as the lung or liver) that is in a relatively stable position. This results in perhaps reduced need to adaptively recontour these sites on a daily basis because the organs in close proximity are not highly mobile or dramatically influenced by small changes in position. Overall, approximately 30% to 50% of treated tumors tend to be selected for daily adaptation. This selection for the process of daily adaptive recontouring has been previously published.74 It will be important to continue to follow emerging evidence on the best methods to use MR-guided RT and adaptive recontouring. Specific evidence by tumor site is summarized in the sections below.
Primary Central Nervous System
The brain offers an appealing opportunity for MR guidance.75 MRI is the standard imaging study to evaluate the normal brain parenchyma as well as primary brain tumors, thus having MRI daily during a course of treatment offers advantages over CT. Changes in the tumor, such as interval growth or shrinkage, during a course of RT are very likely to be better appreciated on MR compared with CT. In addition, the physiologic information provided by MR is possibly the most validated and widely used in CNS-based imaging studies.76,77 It is important to note that these studies have historically used diagnostic MRI image data sets, and such data would need to be collected with the field strengths and specific features of the MRIs used for MR-guided RT. Although primary CNS tumors are not often considered to be highly mobile, there are examples of CNS tumors, such as craniopharyngiomas or surgical resection cavities, that change in size quickly, either before or even during RT.78,79 There have been several small, mostly single-institution reports published on MR guidance for primary brain tumors.78,80 An early series of patients being treated for primary brain tumors with MR guidance was published by Jones et al. In that series, in total, 14 patients were treated using a ViewRay treatment device and were imaged using its 0.35-Tesla MRI. It was noted that at least 3 patients in the series demonstrated growth of their tumor during RT, as seen on their daily MRI, and this was associated with a differential outcome. Such data could enable adaptive therapy in patients with glioblastoma.81 A second recently published series by Stewart et al of 61 patients with serial MRI during treatment (including at planning, at fraction 10, at fraction 20, and 1 month after treatment) demonstrated that meaningful tumor dynamics were observed during chemo-RT for glioblastoma.82 Such information regarding tumor dynamics could be routinely collected and accounted for using MR guidance. Moreover, these data could enable an improved understanding of where to deposit higher doses of RT. Emerging data have also been presented for the promise of longitudinal, quantitative MRI of the brain during MR-guided RT, offering new areas for functional response-based adaptation.83
Head and Neck Tumors
Primary tumors of the head and neck present an interesting and promising opportunity for the clinical use and application of MR-guided RT. MRI is well established as an imaging modality for head and neck tumors.84,85 The use of MRI offers several advantages for these tumors. First, they often regress quickly during the course of RT. Some types of squamous cell carcinomas of the head and neck, particularly those that are human papillomavirus-positive, are quite sensitive to treatment with chemotherapy and external-beam RT. This makes them excellent candidates for adaptive therapy, either online or offline, secondary to rapid tumor shrinkage. As the tumors decrease in size during RT, the ability to decrease the volume of surrounding normal organs receiving high doses could mitigate toxicities. In addition to rapid tumor regression, patients undergoing head and neck cancer treatment often experience significant weight loss during therapy because of difficulty swallowing and eating secondary to the acute reactions associated with chemotherapy and RT in the oropharyngeal structures. These reasons make ART a highly appealing option for patients with head and neck tumors. As a patient loses weight, without ART, the dose of RT can change to either the tumor or normal organs at risk. This impact of weight loss needs to be accounted for; therefore, ART is commonly used for these patients. The MR soft tissue resolution seen in the head and neck makes this a highly appealing option for patients over CT-based image guidance.
Currently, there have been several published experiences using MR-guided RT for head and neck cancers.86–90 A few of these have been early retrospective series, but there have also been some feasibility studies as well as prospective institutional registries. There are several ongoing phase 2 trials prospectively examining the potential benefits of MR-guided adaptive therapy. One example is the MARTHA trial (ClinicalTrials.gov identifier NCT03972072), which intends to examine the ability of MR-guided RT to reduce xerostomia. A second example of an ongoing prospective trial is the MR-ADAPTOR trial (ClinicalTrials.gov identifier NCT03224000), a Bayesian phase 2 trial that will examine adaptive MR guidance compared with standard IMRT with a primary aim of assessing noninferiority of the MR-guided adaptive-therapy approach.91
Thoracic Tumors, Including Primary Lung Tumors, Secondary Metastatic Lesions, and Esophageal Malignancies
There are some challenges associated with MR-based imaging and subsequent treatment of thoracic tumors.92–94 As mentioned above, the electron-return effect is of great consideration in this setting. Thoracic tumors tend to be well visualized using standard CT-based imaging and tend to have a considerable amount of movement. The significant amount of air present in the thorax also leads to additional dosimetric difficulties.94 It is important to note that MRI is less well established in the staging and diagnostic evaluation of primary lung tumors, metastatic lesions to the thorax, and esophageal tumors. This lack of establishment introduces slight challenges to the routine use of MR-based adaptive treatments. Despite these challenges, there are several well conducted, early experiences of using MR guidance for primary thoracic tumors, esophageal tumors, and secondary metastatic lesions to the lung.95 There are also important hypothesized benefits of this technology in the thorax. Some of those benefits are specifically for central lung tumors, in which the visualization of soft tissues (such as vascular structures or airways) in close proximity to tumors could prove advantageous.96,97 In addition, for esophageal primary tumors, soft tissue visualization, with potential adaptation using MRI during the treatment course, may provide advantages over standard CT-based imaging. An example of a series demonstrating the safety and feasibility of this approach was recently published by Finazzi et al.95 In that series, >50 consecutive patients were treated with MR-guided ablative radiation for either centrally located primary tumors or metastatic lesions to the lung.95 The authors demonstrated that such a treatment could be conducted safely and feasibly. However, measurable benefits of this technique over CT-based techniques need to be more clearly demonstrated. Of note, it has been shown in small feasibility case series that, compared with nonadaptive therapy, ART using MR guidance may be helpful to spare normal tissues; however, additional work is needed to assess its clinical benefits.98 Similar hypothesis-generating series have been published for esophageal cancers, an area that also needs further work and evaluation.99
Upper Abdominal Tumors, Including Pancreatic and Primary and Second Liver Tumors
Perhaps one of the most promising and widely used areas for adaptive MR guidance has been the upper abdomen. There are several reasons for this. First, many abdominal tumors routinely treated with RT are very difficult to visualize with CT-based imaging. Examples include primary and secondary liver cancers as well as pancreatic primary tumors.100,101
The inability to see these lesions on CT-based imaging relates directly to the limitations of CT-based simulation imaging. These limitations in the ability to image these tumors are considerably improved with MRI.101 Upper abdominal tumors also have a considerable amount of movement during radiation treatments and are located in close proximity to exquisitely radiosensitive structures, such as the small bowel, stomach, and duodenum. This makes ART a highly appealing option for these tumors. As the normal organs move in close proximity to the tumors, ART can account for this movement and change the RT dose distribution accordingly (Fig. 9). For these important reasons, multiple series have been published examining the role of adaptive MR-guided RT for these tumors.
Pancreatic Adenocarcinoma
PAC has been the subject of multiple publications, both retrospective and prospective, examining the role of adaptive MR guidance. Multiple different retrospective experiences have been published that assessed feasibility, local control, and OS associated with MR-guided RT in PAC.102–104 Those series deliver insight into potential clinical outcomes when MR-guided RT is applied to patients with pancreatic tumors. A retrospective example of the potential benefits of adaptive MR guidance in PAC is presented by Rudra et al.102 In their series, the results from 44 patients who were treated for inoperable, nonmetastatic PAC are presented.102 This was a multi-institutional series using a variety of dose and fractionation schedules that focused specifically on the potential benefits of high-dose RT delivered with adaptive MR guidance for patients with PAC. Accordingly, the 2-year OS rate was improved with the use of high-dose MR-guided RT in the series (49% vs 30%; P = .03). It is important to note that the series also reported impressive rates of local control without any grade 3 toxicity seen in the high-dose cohort. Given the retrospective nature of the series by Rudra et al, there are limitations that must be considered. Such limitations include patient selection bias, the potential for selective reporting bias, and other unappreciated biases.102,105 Notwithstanding the limitations of a retrospective study, these results clearly offer promise for prospective investigation because other methods of classically delivered, low-dose, nonadaptive, concurrent chemotherapy and RT have largely failed patients with PAC.106 Hassanzadeh et al recently reported their institutional data using the ViewRay MR-linac examining patients who received with high-dose ablative radiation for locally advanced, inoperative PAC.103 Again, high rates of local control (>80%) with acceptable grade 3 toxicity were demonstrated. Median OS rates in the series by Hassanzadeh et al were modest at 15.7 months. This result is rather comparable to the results achieved with low-dose, conventionally fractionated, concurrent chemotherapy and RT series from multi-institutional prospective trials.106,107 Other recently published retrospective series have also demonstrated higher OS outcomes associated with high-dose RT.108 This illustrates that further work is needed to optimally understand which patients benefit from the use of adaptive MR guidance and high-dose RT.
These disparate results could be secondary to biologic selection or differences in the RT procedures. Chuong et al recently published a retrospective analysis of 35 patients who were treated using the ViewRay technology.104 They demonstrated excellent rates of local control and reported low rates of toxicity. Again, despite these seemingly strong outcomes, the median OS and progression-free survival were relatively similar to previously published series, including those using CT-based image guidance. It is important to note that the time point from which follow-up data are being measured is from the end of RT (vs the time of diagnosis). These results also contrast with other recently published ablative RT series. It is notable that some of these series have not used MR guidance. Although cross-study comparisons are fraught with impediments, rates of grade 3 toxicity are numerically slightly higher in these series.108 Clearly, the specialty must assertively move into prospective comparative studies that robustly evaluate these different techniques.
Ongoing Prospective Pancreatic Adaptive MR-Guidance Trials
Fortunately, there are multiple ongoing prospective trials to robustly collect and address outcomes associated with adaptive MR guidance in PAC. Future trials will need to focus on randomization between these different strategies, such as adaptive MR-guided RT compared with CT-based guidance or other novel local/regional therapies. These trials are vital given some of the limitations associated with retrospective series. The Stereotactic MRI-guided on-table Adaptive Radiation Therapy for Locally Advanced Pancreatic Cancer (SMART) trial is an example of a single-arm phase 2 trial examining the use of MR-guided RT for locally advanced PAC and is currently accruing (ClinicalTrials.gov identifier NCT03621644). In total, 133 patients are planned for enrollment with a primary end point of grade ≥3 gastrointestinal toxicity within 90 days of completing RT. Ideally, this trial will provide a robust signal for future studies that could potentially focus on randomizations between different treatment strategies, such as adaptive MR guidance and nonadaptive CT-based image guidance. Such data will very likely be necessary to justify these expensive and time-consuming procedures in the future.
In addition to the SMART trial, a few broader prospective trials and registries are capturing clinical and outcome data for patients with pancreatic cancer. An example of such a registry is a currently ongoing study at Dana Farber Cancer Institute. This is a phase 1/2 study involving patients with either adrenal, prostate, liver, lymph node, lung, pancreatic, or renal cancer (ClinicalTrials.gov identifier NCT04115254). The primary end point for the initial phase of the study is delivery success across multiple tumor types. Such data will be critical for understanding outcomes associated with this novel and highly complex procedure. Another example is the MOMENTUM study (ClinicalTrials.gov identifier NCT04075305), which is an ongoing, prospective registry trial that is currently collecting outcomes among patients treated for multiple solid tumors, including PAC, using 1.5-Tesla MR guidance. In this multi-institutional study, currently consisting of 8 centers with 1.5-Tesla linacs, patients are being prospectively enrolled and followed for a multitude of outcomes.
Primary and Secondary Liver Tumors
External-beam RT has been used for decades in the treatment of both primary and secondary liver tumors. In many different circumstances, RT offers an appealing and minimally invasive strategy for these tumors.109–112 However, there are historic limitations associated with currently existing, non–MR-guided techniques for the treatment of liver tumors that are important to recognize and understand. The liver presents another optimal clinical circumstance for the use of MR-guided RT. MRI is a well established imaging modality in the treatment of both primary and secondary liver tumors. Secondary to this ideal union, treatment of the liver is an area of rapidly expanding investigation and utilization specifically for MR guidance. Multiple review articles have been published explicitly on the use of MR-guided RT for primary and secondary liver tumors.113 There are a few advantages of MR guidance that warrant special consideration when it comes to treatment of the liver. Historically, to treat liver tumors, implanted fiducial markers were typically used to visualize the area of the liver to be targeted with external-beam RT. Because the soft tissue boundaries of liver tumors are difficult to visualize using CT, radiopaque markers had to be inserted in the vicinity of the tumor to localize these lesions. The placement of these radiopaque fiducials is an invasive procedure that ideally would be avoided given the potential for complications associated with such invasive procedures in the liver. Figure 11 presents an example of how clearly metastatic lesions can be seen in the liver using MRI compared with CT. This clarity of RT dose deposition can potentially be further enhanced with the use of contrast before, during, and after RT. Such contrast agents can clearly accentuate the location of the tumor as well as the distribution of RT dose. Such a distribution can be seen in Figure 11, and experiences with using contrast to specifically enhance the lesion visualization have been previously published by Wojcieszynski et al.114
FIGURE 11.
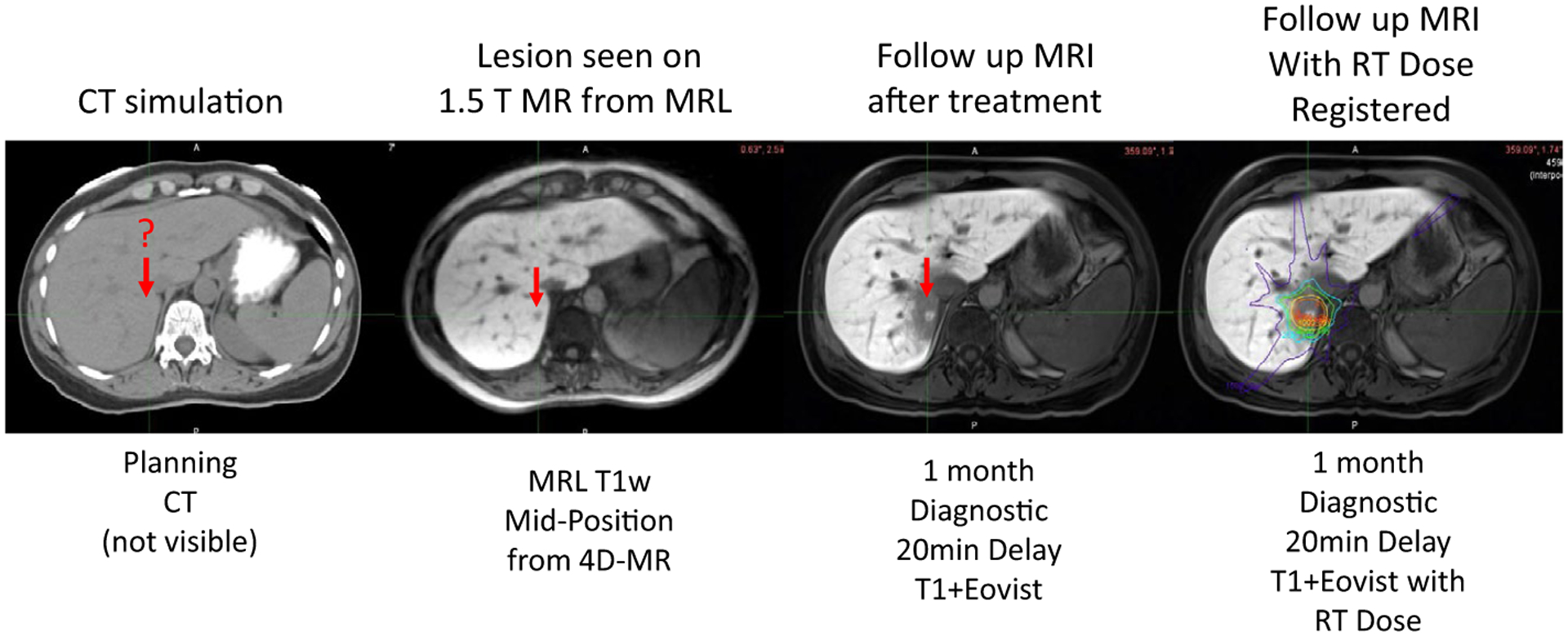
Example of Liver Imaging Improvement With Magnetic Resonance (MR) Compared With Computed Tomography (CT). 4D indicates 4-dimensional; Eovist, gadoxetate disodium; MRI, magnetic MR resonance imaging; MRL, magnetic resonance lymphangiography; RT, radiotherapy; T, Tesla; T1w, T1-weighted.
A detailed clinical experience of the implementation of a fiducial-free treatment technique was published recently by Gani et al.115 Several, large, retrospective, clinical experiences have been published presenting early outcomes of patients treated for liver tumors using adaptive MR guidance. Rosenberg et al conducted a multi-institutional experience of 26 patients who were treated using the ViewRay system. This was a somewhat heterogenous collection of tumors, including a total of 26 patients with either primary liver tumors or secondary metastatic lesions to the liver. Treatment was well tolerated, and freedom from local progression was high; however, the median follow-up was relatively short, and the cohort was rather heterogenous and small.116 Another example of a small series using the Elekta Unity device has also recently been published demonstrating feasibility of the treatment of both primary and secondary liver tumors.74 Additional prospective research is needed in primary and secondary liver cancers to optimally define the use of MR guidance in this setting.
Rectal Cancer
Rectal cancer is currently a rapidly evolving area of management regarding the use of RT. In one regard, ongoing clinical trials are examining the elimination of RT entirely for some stages of rectal cancer (ClinicalTrials.gov identifier NCT01515787). Conversely, novel strategies of applying higher RT doses to rectal tumors are emerging as a potential method to allow organ preservation, eliminating surgery in some patients.117 In addition, recent data have demonstrated that rectal tumors regress during treatment with RT, and this can be seen optimally on MRI.118 Historically, patients with rectal cancer have been treated with preoperative therapy followed by surgical resection (in some cases, leading to permanent colostomy); however, that paradigm is starting to significantly change. More frequently, patients are being considered for systemic chemotherapy and concurrent chemo-RT. This strategy is resulting in high rates of organ preservation for these patients (the OPRA Trial; ClinicalTrials.gov identifier NCT02008656).119 These patients may be optimally treated with careful consideration of MR-based imaging changes for several reasons. Rectal tumors are commonly staged with MRI, and MRI is being used increasingly for response assessment after treatment. This presents an appealing option for conformal and ART-boosting strategies associated with rectal tumors. Already, the feasibility of using adaptive MR guidance has been published using the ViewRay device. Bostel et al presented their series of approximately 22 patients who were treated using a ViewRay device and concluded that MR-guided RT is a feasible option for the treatment of rectal cancer, demonstrating several potential benefits.120 Interesting radiomic markers of potential response in rectal cancer have been reported using a 0.35-Tesla MR linac.121 Finally, a study showing the feasibility of these treatments using the Elekta Unity device has also recently been published.122
Prostate Cancer
Prostate cancer is one of the most common, established, and widely used indications for RT. RT is a solitary curative modality for many men afflicted with prostate cancer. There exists high-level, randomized evidence (Level I) that surgical resection and RT offer men with prostate cancer the same curative outcomes achieved by those who are suitable for active surveillance.65 Outcomes may be better with RT and hormone-blocking therapy in patients who have more advanced disease, but this remains to be tested in a randomized trial.123 The use of MRI is now very common for the diagnosis, staging, and management of prostate cancer.124,125 MRI helps with the visualization of particularly malignant regions of the prostate, and it also provides exquisite detail of the regional normal structures, such as the bladder and rectum.126 Therefore, the combination of an MRI device with a linac is appealing for potentially addressing prostate cancer.127 There are several hypothesized reasons for this potential advantage. Recently, using MR images to guide boosting of dominate prostate cancer nodules has emerged as a method to improve outcomes for men with prostate cancer.128 Rather than treating the whole prostate gland to a homogenous dose of RT, there are differentially boosted regions within the gland where the higher RT doses are focused, with the goal of eradicating cancer in those areas. In addition, MR reveals the precise boundaries of the bladder and the rectum, which are often difficult to visualize on CT. These structures constitute the most important sources for potential toxicity associated with RT for prostate cancer. The ability to visualize and account for the positions of these organs could be very helpful in potentially reducing toxicity. Of note, most men treated with RT for prostate cancer are doing very well after treatment. The incidence of grade ≥3 effects associated with this treatment are fortunately very rare when using CT-based treatment.129,130 Some of the promising areas for potential application of MR guidance include reducing acute urinary or bowel toxicity associated with RT, which can be rather common. There is interest in attempting to reduce the total number of fractions over which RT is given. Finally, the ability to identify and potentially spare the neural and vascular structures involved in erectile function is of significant interest, given the ability to clearly visualize these structures using MRI.131 There have been several early experiences published demonstrating the feasibility of treating patients with prostate cancer using MR guidance. These have included both Elekta Unity and ViewRay center experiences.132,133 An important conclusion from these early data is that the treatment is both feasible and well tolerated. Intriguingly, the rates of grade ≥2 urinary and bowel toxicities at the end of treatment were quantitatively lower in the setting of MRI-guided urethral-sparing stereotactic body radiotherapy (SBRT) on a phase 2 trial compared with the rates reported on a large multicenter trial of CT-guided SBRT.134,135 The ongoing MIRAGE trial is randomizing patients between CT-based and MRI-based prostate SBRT. Long-term follow-up and further studies will be needed to assess control and late toxicity outcomes from these treatment strategies.
Breast Cancer
There have been several recent publications focused on the use of MR guidance in the treatment of breast cancer.136–138 This area is both promising and interesting for future exploration. Several of these publications have demonstrated that both acute and late toxicity profiles are rather favorable when using MR-guided radiation to perform partial breast irradiation. Specifically, this has been examined using both a 0.35-Tesla and 1.5-Tesla MR linac with novel and interesting dose/fractionation schedules.136–138 Additional work will be needed in this space to demonstrate clear improvements over CT-based RT.
Muscle-Invasive Bladder Cancer
An interesting and evolving application of MR guidance is in the management of bladder cancer. Treatment with chemotherapy and RT represents a long-standing management strategy for cure in patients with muscle-invasive bladder cancer.139 However, bladder tumors can be difficult to visualize using CT. MR can provide exquisite visualization of bladder tumors, and its routine use in the diagnostic evaluation of bladder cancer is expanding.140 The use of MR is particularly appealing because strategies to improve localization using CT-based markers have largely failed. Attempts have been made to place radio-opaque markers in close proximity to tumors; however, the markers often migrate or fall out.141 Improved visualization of tumors in the bladder could result in the ability to perform partial bladder boosting, which may improve the ability to safely deliver higher doses of RT to regions of the bladder. The capability to include diffusion-weighted imaging, along with adaptive boosting, as the bladder increases in size during treatment is a highly appealing aspect of MR guidance for bladder cancer.142 In addition, these tumors are often in close proximity to critical normal structures, such as the small or large bowel. The movement of these normal structures during treatment can result in toxicities secondary to high doses of RT afflicting these organs. MR linacs have the ability to address both of these current challenges associated with RT use in muscle-invasive bladder cancer. Like many other treatment sites, the feasibility of these treatment strategies has been published; however, further investigation is needed before these can become standard of care.143
Conclusions
The use of RT for numerous solid tumors remains an essential component of organ-sparing and/or multimodality cancer treatment. Image guidance, in fact, has increased the indication for RT across a variety of circumstances. The role of ART, and specifically MRgART, is continuing to evolve. Here, we have presented an overview of many different clinical circumstances in which ART, and more specifically MRgART, could be clinically advantageous. Such advantages will include potentially reducing acute and late toxicity, improving local control, and ideally improving OS in some malignancies. The identification of biologic rather than strictly anatomic targets represents an exciting aspect of MR-based ART that could have wide-reaching implications, including a transition from the current use of historical radiation doses based on tumor histology and tumor stage, and may lead to imaging response-mediated doses. However, it is critical for oncologists to recognize the need for prospective randomized data to evaluate these types of novel treatment strategies. MR guidance is expensive, labor-intensive, and time-consuming. Therefore, to justify this effort on the part of radiation oncologists and their treatment teams, high-quality prospective data are needed.144
There are several limitations and challenges associated with the collection of high-quality, prospective data for novel RT devices. In fact, these challenges are common to the introduction of many innovations across the radiation oncology specialty, including IMRT and proton therapy. However, MR guidance and adaptive therapy present even further challenges because they require a considerable amount of time and effort for the radiation oncologist and treatment team on a daily basis. Clarity is needed regarding whether this time is justified or needed. Currently, device approvals are based largely on the safety of clinical use. Once these devices are approved, they can be used at the discretion of medical providers with relatively limited evidence indicating their efficacy or superiority. Novel reimbursement strategies may affect the ability to routinely use expensive technologies without commensurate supporting evidence.
The future is exciting as we contemplate the integration of AI with adaptive therapy and MR guidance.71 There is a perception in medicine that AI may replace physicians, technicians, and physicists for tasks that have been routinely performed by humans. However, rather than consider the ability for AI to replace humans, oncologists should focus on the ability of AI to enhance the capabilities of our current treatment strategies. Looking at AI as a new and compelling partner in the treatment of cancer is a more productive strategy than considering AI as a competitor. For the specialty of radiation oncology specifically, partnering with AI to expand and revolutionize what is capable with RT is an incredible opportunity. A future in which ART is performed on each and every patient treated becomes feasible. Highly futuristic concepts, such as 4D cine MR images being routinely analyzed for any movement, segmented in real time, and precise RT doses calculated and accounted for by AI-based software solutions, become more realistic. The implications of the coming AI revolution on the capabilities of RT are astonishing to imagine. Radiation oncologists will likely be presented with considerable amounts of additional data in the future, including biologic imaging data, daily adaptive data, as well as real-time intratreatment information. The meaning of these data must be collected, analyzed, and understood. Benefits to patients must be quantified and tested robustly. Whether this approach can ultimately improve clinical outcomes for patients with a variety of malignancies will require considerable prospective evaluation.
Acknowledgements:
This project represents independent research supported by the National Institute for Health Research Biomedical Research Center at The Royal Marsden National Health Service Foundation Trust and the Institute of Cancer Research, London.
DISCLOSURES:
William A. Hall is supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) (award KL2TR001438) and reports institutional grants and meeting support from Elekta AB outside the submitted work. Eric Paulson reports institutional research grants from Elekta AB and Siemens Healthineers; service as a committee member of the American Association for Physicists in Medicine; and in-kind software support from Elekta AB and Siemens Healthineers outside the submitted work. X. Allen Li reports institutional grants from Elekta AB, Manteia Medical Technologies, and Siemens Healthineers; and honoraria from Elekta AB for an education lecture outside the submitted work. Beth Erickson serves as vice chair of the Education Council for American Society for Radiation Oncology (ASTRO), chair of the Annual ASTRO Refresher Course, and co-chair of the American Brachytherapy Society GYN Brachytherapy School outside the submitted work. Christopher Schultz reports research grants from Elekta AB, Siemens Healthineers, Acuray, and Manteia Imaging Technologies; meeting expenses from Elekta AB; service on the Clinical Steering Committee Magnetic Resonance Linac Consortium for Elekta AB; and service as a member of the Magnetic Resonance-Integrated Radiation Therapy Circle of Siemens Healthineers outside the submitted work. Alison Tree is supported by Cancer Research UK (C33589/A28284 and C7224/A28724) and the National Institute for Health Research Cancer Research Network; she reports financial support for a clinical trial (MOMENTUM) and occasional honoraria for lectures from Elekta AB outside the submitted work. Musaddiq Awan reports a grant from NIH R21 (CA256144-01) funding for a phase 1 clinical trial of magnetic resonance-guided radiation; honoraria from Elekta AB; and free drug support from Genentech for NIH R21 (CA256144-01) funded trial of magnetic resonance-guided radiation (ClinicalTrials.gov identifier NCT04477759) outside the submitted work. Brigid A. McDonald is supported by an NIH F31 fellowship (F31DE029093) and reports speaking honoraria from Elekta AB outside the submitted work. Travis Salzillo reports support from The University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences TL1 Program (TL1 TR003169) outside the submitted work. Carri K. Glide-Hurst reports grants from the NIH (R01CA204189 and R01HL153720) and GE Healthcare; and the receipt of equipment from Modus Medical Devices and GE Healthcare outside the submitted work. Amar U. Kishan reports research funding from ViewRay Technologies Inc and the ASTRO-Prostate Cancer Foundation; consulting fees from ViewRay Technologies Inc, Varian Medical Systems Inc, and Intelligent Automation Inc; speaker’s fees from ViewRay Technologies Inc and Varian Medical Systems Inc; service as a committee member of the American Society of Clinical Oncology and ASTRO; and owns stock in ViewRay Technologies Inc outside the submitted work. Clifton D. Fuller reports institutional funding from the NIH, National Institute of Dental and Craniofacial Research (NIDCR) Academic Industrial Partnership grant (R01DE028290); an NIH/National Cancer Institute (NCI) Cancer Center Support Grant (CCSG) Pilot Research Program Award to the University of Texas MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672); the National Science Foundation (NSF)/NCI Smart Connected Health Program (R01CA257814); the NCI Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Program (R01CA214825); an NSF/NIH Joint Initiative on Quantitative Approaches to Biomedical Big Data Program (R01CA225190); an NIH National Institute of Biomedical Imaging and Bioengineering Research Education Programs for Residents and Clinical Fellows grant (R25EB025787); an NIDCR Establishing Outcome Measures for Clinical Studies of Oral and Craniofacial Diseases and Conditions award (R01DE025248); an NCI Parent Research Project grant (R01CA258827); the NIH NIDCR Exploratory/Developmental Research Grant Program (R21DE031082); a Small Business Innovation Research Grant Program subaward from Oncospace, Inc (R43CA254559); a Human BioMolecular Atlas Program Integration, Visualization, and Engagement Initiative (OT2OD026675) subaward; a Patient-Centered Outcomes Research Institute (PCS-1609-36195) subaward from Princess Margaret Hospital; an NSF Division of Civil, Mechanical, and Manufacturing Innovation grant (NSF 1933369), and an Elekta AB Institutional research grant; speaker’s fees from Elekta AB, the American Association for Physicists in Medicine, the University of Alabama-Birmingham, ASTRO, the Radiological Society of North America, and the European Society for Radiation Oncology; honoraria from the American Society for Clinical Oncology and the NIH; US Patent Application No. 16/631,662, based on International Patent Application No. PCT/US2018/042364; service as committee member for The American Association for Physicists in Medicine, ASTRO, the Radiological Society of North America, and the American Society of Clinical Oncology; and the receipt of in-kind software from Elekta AB outside the submitted work. Daniel Low had no disclosures.
References
- 1.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005; 104:1129–1137. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Alvarnas J From the editor-in-chief: oncology in the time of “Moore’s Law.” Am J Manag Care. 2019;25(5 spec no.): Sp140. [PubMed] [Google Scholar]
- 4.Abshire D, Lang MK. The evolution of radiation therapy in treating cancer. Semin Oncol Nurs. 2018;34:151–157. [DOI] [PubMed] [Google Scholar]
- 5.Gardner SJ, Kim J, Chetty IJ. Modern radiation therapy planning and delivery. Hematol Oncol Clin North Am. 2019; 33:947–962. [DOI] [PubMed] [Google Scholar]
- 6.Bucci MK, Bevan A, Roach M III. Advances in radiation therapy: conventional to 3D, to IMRT, to 4D, and beyond. CA Cancer J Clin. 2005;55:117–134. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. [DOI] [PubMed] [Google Scholar]
- 8.Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic adenocarcinoma, version 1.2019. J Natl Compr Canc Netw. 2019; 17:202–210. [DOI] [PubMed] [Google Scholar]
- 9.Dearnaley DP, Khoo VS, Norman AR, et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet. 1999;353:267–272. [DOI] [PubMed] [Google Scholar]
- 10.Emami B, Lyman J, Browe A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. [DOI] [PubMed] [Google Scholar]
- 11.Tubiana M, Eschwege F. Conformal radiotherapy and intensity-modulated radiotherapy— clinical data. Acta Oncol. 2000;39:555–567. [DOI] [PubMed] [Google Scholar]
- 12.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:27–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klopp AH, Yeung AR, Deshmukh S, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG Oncology-RTOG 1203. J Clin Oncol. 2018;36:2538–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu C, Chen DJ, Li A, et al. Clinical implementation of intensity-modulated arc therapy. Proceedings of the 22nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (cat. no. 00CH37143), Vol 4. IEEE Engineering in Medicine and Biology Society; 2000;3035–3057. [Google Scholar]
- 15.Durante M, Orecchia R, Loeffler JS. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol. 2017;14:483–495. [DOI] [PubMed] [Google Scholar]
- 16.Dawson LA, Sharpe LA. Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol. 2006;7: 848–858. [DOI] [PubMed] [Google Scholar]
- 17.Huntzinger C, Munro P, Johnson S, et al. Dynamic targeting image-guided radiotherapy. Med Dosim. 2006;31:113–125. [DOI] [PubMed] [Google Scholar]
- 18.Rietzel E, Pan T, Chen GT. Four-dimensional computed tomography: image formation and clinical protocol. Med Phys. 2005;32:874–889. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Camphausen K, Mueller B, et al. Advances in 4D medical imaging and 4D radiation therapy. Technol Cancer Res Treat. 2008;7:67–81. [DOI] [PubMed] [Google Scholar]
- 20.Yan D, Vicini F, Wong J, Martinez A. Adaptive radiation therapy. Phys Med Biol. 1997;42:123–132. [DOI] [PubMed] [Google Scholar]
- 21.Sonke JJ, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol. 2010;20:94–106. [DOI] [PubMed] [Google Scholar]
- 22.Yan D Adaptive radiotherapy: merging principle into clinical practice. Semin Radiat Oncol. 2010;20:79–83. [DOI] [PubMed] [Google Scholar]
- 23.Brock KK. Adaptive radiotherapy: moving into the future. Semin Radiat Oncol. 2019;29:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glide-Hurst CK, Lee P, Yock AD, et al. Adaptive radiation therapy (ART) strategies and technical considerations: a state of the ART review from NRG Oncology. Int J Radiat Oncol Biol Phys. 2021;109: 1054–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman KA, Hall N, Bekaii-Saab TS, et al. Survival outcomes from CALGB 80803 (Alliance): a randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer [abstract]. J Clin Oncol. 2018;36(15 suppl):4012. [Google Scholar]
- 26.Li XA, ed. Adaptive Radiation Therapy. CRC Press; 2011. [Google Scholar]
- 27.Li XA, Liu F, Tai A, et al. Development of an online adaptive solution to account for inter- and intra-fractional variations. Radiother Oncol. 2011;100:370–374. [DOI] [PubMed] [Google Scholar]
- 28.Ahunbay EE, Li XA. Gradient maintenance: a new algorithm for fast online replanning. Med Phys. 2015;42:2863–2876. [DOI] [PubMed] [Google Scholar]
- 29.Ates O, Ahunbay EE, Moreau M, Li XA. Technical note: a fast online adaptive replanning method for VMAT using flattening filter free beams. Med Phys. 2016;43:2756–2764. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Ahunbay E, Li XA. Technical note: acceleration of online adaptive replanning with automation and parallel operations. Med Phys. 2018;45:4370–4376. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Paulson E, Lim S, et al. A patient-specific autosegmentation strategy using multi-input deformable image registration for magnetic resonance imaging-guided online adaptive radiation therapy: a feasibility study. Adv Radiat Oncol. 2020;5:1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varian. Ethos therapy. Accessed August 24, 2021. varian.com/products/adaptive-therapy/ethos
- 33.de Mol van Otterloo SR, Christodouleas JP, Blezer ELA, et al. Patterns of care, tolerability and safety of the first cohort of patients treated on a novel high-field MR-linac within the MOMENTUM study: initial results from a prospective multi-institutional registry. Int J Radiat Oncol Biol Phys. 2021;111:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head- and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas C, Yan D, Kestin LL, et al. Phase II dose escalation study of image-guided adaptive radiotherapy for prostate cancer: use of dose-volume constraints to achieve rectal isotoxicity. Int J Radiat Oncol Biol Phys. 2005;63:141–149. [DOI] [PubMed] [Google Scholar]
- 36.Sonke JJ, Aznar M, Rasch C. Adaptive radiotherapy for anatomical changes. Semin Radiat Oncol. 2019;29:245–257. [DOI] [PubMed] [Google Scholar]
- 37.Spoelstra FOB, Pantarotto JR, van Sorensen de Koste JR, Slotman BJ, Senan S. Role of adaptive radiotherapy during concomitant chemoradiotherapy for lung cancer: analysis of data from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2009;75:1092–1097. [DOI] [PubMed] [Google Scholar]
- 38.Goodman KA, Ou FS, Hall NC, et al. Randomized phase II study of PET response-adapted combined modality therapy for esophageal cancer: mature results of the CALGB 80803 (Alliance) trial. J Clin Oncol. 2021;39:2803–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong FM, Machtay M, Bradley JD, et al. NRG Oncology ECOG-ACRIN RTOG 1106/ACRIN 6697 randomized phase II trial of individualized adaptive radiotherapy using during-treatment FDG-PET/CT and modern technology in locally advanced non-small cell lung cancer. Update, 2014. Accessed August 24, 2021. acr.org/-/media/ACR/NOINDEX/Research/ACRIN/Legacy-Trials/ACRIN-6697_RTOG1106.pdf
- 40.Kong FMS, Hu C, Matchtay M. Abstract OA01.04: Results of RTOG 1106/ACRIN-6697: A randomized phase II trial of individualized adaptive radiotherapy using mid-treatment FDG-PET/CT and modern technology in locally advanced non-small cell lung cancer (NSCLC). Paper presented at: International Association for the Study of Lung Cancer 2020 World Conference on Lung Cancer of the; January 28–31, 2021; Virtual Meeting. [Google Scholar]
- 41.Kong FMS, Hu C, Ten Haken R, et al. NRG-RTOG 1106/ACRIN 6697: a phase IIR trial of standard versus adaptive (mid-treatment PET-based) chemoradiotherapy for stage III NSCLC—results and comparison to NRG-RTOG 0617 (non-personalized RT dose escalation) [abstract]. J Clin Oncol. 2021;39(15 suppl): 8548. [Google Scholar]
- 42.Liu F, Tai A, Ahunbay E, Gore E, Johnstone C, Li XA. Management of independent motion between multiple targets in lung cancer radiation therapy. Pract Radiat Oncol. 2017;7:26–34. [DOI] [PubMed] [Google Scholar]
- 43.Ahunbay EE, Peng C, Godley A, Schultz C, Li XA. An online replanning method for head and neck adaptive radiotherapy. Med Phys. 2009;36:4776–4790. [DOI] [PubMed] [Google Scholar]
- 44.Ahunbay EE, Peng C, Holmes S, Godley A, Lawton C, Li XA. Online adaptive replanning method for prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:1561–1572. [DOI] [PubMed] [Google Scholar]
- 45.Bobic M, Lalonde A, Sharp GC, et al. Comparison of weekly and daily online adaptation for head and neck intensity-modulated proton therapy. Phys Med Biol. 2021;66:055023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Qiao Q, DeVries A, et al. Adaptive replanning to account for lumpectomy cavity change in sequential boost after whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2014;90:1208–1215. [DOI] [PubMed] [Google Scholar]
- 47.Hijab A, Tocco B, Hanson I, et al. MR-guided adaptive radiotherapy for bladder cancer. Front Oncol. 2021;11:637591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoegen P, Lang C, Akbaba S, et al. Cone-beam-CT guided adaptive radiotherapy for locally advanced non-small cell lung cancer enables quality assurance and superior sparing of healthy lung. Front Oncol. 2020;10:564857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu F, Erickson B, Peng C, Li XA. Characterization and management of interfractional anatomic changes for pancreatic cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:e423–e429. [DOI] [PubMed] [Google Scholar]
- 50.Magallon-Baro A, Milder MTW, Granton PV, Nuyttens JJ, Hoogeman MS. Comparison of daily online plan adaptation strategies on a cohort of pancreatic cancer patients treated with SBRT. Int J Radiat Oncol Biol Phys. 2021;111:208–219. [DOI] [PubMed] [Google Scholar]
- 51.Rogowski P, von Bestenbostel R, Walter F, et al. Feasibility and early clinical experience of online adaptive MR-guided radiotherapy of liver tumors. Cancers (Basel). 2021;13:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metcalfe P, Liney GP, Holloway L, et al. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013;12:429–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nonaka H, Onishi H, Watanabe M, Nam VH. Assessment of abdominal organ motion using cine magnetic resonance imaging in different gastric motilities: a comparison between fasting and postprandial states. J Radiat Res. 2019;60:837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbas H, Chang B, Chen ZJ. Motion management in gastrointestinal cancers. J Gastrointest Oncol. 2014;5:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33:3874–3900. [DOI] [PubMed] [Google Scholar]
- 56.Keall PJ, Barton B, Crozier S, et al. The Australian magnetic resonance imaging-linac program. Semin Radiat Oncol. 2014;24:203–206. [DOI] [PubMed] [Google Scholar]
- 57.Fallone BG, Murray B, Rathee S, et al. First MR images obtained during mega-voltage photon irradiation from a prototype integrated linac-MR system. Med Phys. 2009;36:2084–2088. [DOI] [PubMed] [Google Scholar]
- 58.Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. [DOI] [PubMed] [Google Scholar]
- 59.Lagendijk JJW, Raaymakers BW, Raaijmakers AJE, et al. MRI/linac integration. Radiother Oncol. 2008;86:25–29. [DOI] [PubMed] [Google Scholar]
- 60.Lagendijk JJW, Raaymakers BW, van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. 2014;24:207–209. [DOI] [PubMed] [Google Scholar]
- 61.Raaymakers BW, Lagendijk JJW, Overweg J, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54:N229–N237. [DOI] [PubMed] [Google Scholar]
- 62.Raaymakers BW, Jurgenliemk-Schultz IM, Bol GH, et al. First patients treated with a 1.5 T MRI-linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017;62:L41–L50. [DOI] [PubMed] [Google Scholar]
- 63.Fallone BG. The rotating biplanar linac-magnetic resonance imaging system. Semin Radiat Oncol. 2014;24:200–202. [DOI] [PubMed] [Google Scholar]
- 64.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375: 1415–1424. [DOI] [PubMed] [Google Scholar]
- 66.van Houdt PJ, Yang Y, van der Heide UA. Quantitative magnetic resonance imaging for biological image-guided adaptive radiotherapy. Front Oncol. 2020;10:615643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. [DOI] [PubMed] [Google Scholar]
- 68.Prior P, Chen X, Botros M, et al. MRI-based IMRT planning for MR-linac: comparison between CT- and MRI-based plans for pancreatic and prostate cancers. Phys Med Biol. 2016;61:3819–3842. [DOI] [PubMed] [Google Scholar]
- 69.Chen X, Prior P, Chen GP, Schultz CJ, Li XA. Technical note: dose effects of 1.5 T transverse magnetic field on tissue interfaces in MRI-guided radiotherapy. Med Phys 2016;43:4797. [DOI] [PubMed] [Google Scholar]
- 70.Raaijmakers AJE, Raaymakers BW, Lagendijk JJW. Magnetic-field-induced dose effects in MR-guided radiotherapy systems: dependence on the magnetic field strength. Phys Med Biol. 2008;53:909–923. [DOI] [PubMed] [Google Scholar]
- 71.Huynh E, et al. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol. 2020;17:771–781. [DOI] [PubMed] [Google Scholar]
- 72.Van Dieren EB, Zwart LGM, Bhawanie A, et al. Adaptive radiotherapy can be applied routinely, using an artificial intelligence solution, to treat prostate cancer patients. Int J Radiat Oncol Biol Phys. 2020;108(suppl):E274–E275. [Google Scholar]
- 73.ViewRay. Scientific Presentations. Accessed August 24, 2021. viewray.com/clinical-spotlight/scientific-presentations/
- 74.Hall WA, Straza MW, Chen X, et al. Initial clinical experience of stereotactic body radiation therapy (SBRT) for liver metastases, primary liver malignancy, and pancreatic cancer with 4D-MRI based online adaptation and real-time MRI monitoring using a 1.5 Tesla MR-linac. PLoS One. 2020;15:e0236570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maziero D, Straza MW, Ford JC, et al. MR-guided radiotherapy for brain and spine tumors. Front Oncol. 2021;11:626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ellingson BM, Malkin MG, Rand SD, et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31:538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017;81:397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winkfield KM, Linsenmeier C, Yock TI, et al. Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:716–721. [DOI] [PubMed] [Google Scholar]
- 79.Atalar B, Choi CYH, Harsh BR 4th, et al. Cavity volume dynamics after resection of brain metastases and timing of post-resection cavity stereotactic radiosurgery. Neurosurgery. 2013;72:180–185; discussion 185. [DOI] [PubMed] [Google Scholar]
- 80.Mehta S, Gajjar SR, Padgett KR, et al. Daily tracking of glioblastoma resection cavity, cerebral edema, and tumor volume with MRI-guided radiation therapy. Cureus. 2018;10:e2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones KK, Dooley S, Maziero D, et al. MRI-guided radiotherapy identifies early pseudoprogression of glioblastoma. Front Oncol. Published online March 8, 2021. doi: 10.3389/fonc.2021.626100 [DOI] [Google Scholar]
- 82.Stewart J, Sahgal A, Lee Y, et al. Quantitating interfraction target dynamics during concurrent chemoradiation for glioblastoma: a prospective serial imaging study. Int J Radiat Oncol Biol Phys. 2021;109:736–746. [DOI] [PubMed] [Google Scholar]
- 83.Nejad-Davarani SP, Zakariaei N, Chen Y, et al. Rapid multicontrast brain imaging on a 0.35T MR-linac. Med Phys 2020;47:4064–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jensen K, Al-Farra G, Dejanovic D, et al. Imaging for target delineation in head and neck cancer radiotherapy. Semin Nucl Med. 2021;51:59–67. [DOI] [PubMed] [Google Scholar]
- 85.Szyszko TA, Cook GJR. PET/CT and PET/MRI in head and neck malignancy. Clin Radiol. 2018;73:60–69. [DOI] [PubMed] [Google Scholar]
- 86.Raghavan G, Kishan AU, Cao M, Chen AM. Anatomic and dosimetric changes in patients with head and neck cancer treated with an integrated MRI-tri-(60) Co teletherapy device. Br J Radiol. 2016;89:20160624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen AM, Cao M, Hsu S, et al. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Adv Radiat Oncol. 2017;2:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen A, Hsu S, Lamb J, et al. MRI-guided radiotherapy for head and neck cancer: initial clinical experience. Clin Transl Oncol. 2018;20:160–168. [DOI] [PubMed] [Google Scholar]
- 89.Mohamed ASR, Bahig H, Aristophanous M, et al. Prospective in silico study of the feasibility and dosimetric advantages of MRI-guided dose adaptation for human papillomavirus positive oropharyngeal cancer patients compared with standard IMRT. Clin Transl Radiat Oncol. 2018;11:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McDonald BA, Vedam S, Yang J, et al. Initial feasibility and clinical implementation of daily MR-guided adaptive head and neck cancer radiation therapy on a 1.5T MR-linac system: prospective R-IDEAL 2a/2b systematic clinical evaluation of technical innovation. Int J Radiat Oncol Biol Phys. 2021;109:1606–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bahig H, Yuan Y, Mohamed ASR, et al. Magnetic resonance-based response assessment and dose adaptation in human papilloma virus positive tumors of the oropharynx treated with radiotherapy (MR-ADAPTOR): an R-IDEAL stage 2a-2b/Bayesian phase II trial. Clin Transl Radiat Oncol. 2018;13:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Menten MJ, Wetscherek A, Fast MF. MRI-guided lung SBRT: present and future developments. Phys Med. 2017;44: 139–149. [DOI] [PubMed] [Google Scholar]
- 93.Li Z, Yin Y, Zhu J. MR guided esophageal cancer radiotherapy: quantifying the impact of the magnetic field. Int J Radiat Oncol Biol Phys. 2019;105:E190. [Google Scholar]
- 94.Kurz C, Buizza G, Landry G, et al. Medical physics challenges in clinical MR-guided radiotherapy. Radiat Oncol. 2020;15:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Finazzi T, Haasbeek CJA, Spoelstra FOB, et al. Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors. Int J Radiat Oncol Biol Phys. 2020;107:270–278. [DOI] [PubMed] [Google Scholar]
- 96.Finazzi T, Palacios MA, Spoelstra FOB, et al. Role of on-table plan adaptation in MR-guided ablative radiation therapy for central lung tumors. Int J Radiat Oncol Biol Phys. 2019;104:933–941. [DOI] [PubMed] [Google Scholar]
- 97.Henke LE, Kashani R, Hilliard J, et al. In silico trial of MR-guided midtreatment adaptive planning for hypofractionated stereotactic radiation therapy in centrally located thoracic tumors. Int J Radiat Oncol Biol Phys. 2018;102:987–995. [DOI] [PubMed] [Google Scholar]
- 98.Padgett KR, Simpson GN, Llorente R, Samuels MA, Dogan N. Feasibility of adaptive MR-guided stereotactic body radiotherapy (SBRT) of lung tumors. Cureus. 2018;10:e2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nachbar M, Monnich D, Kalwa P, Zips D, Thorwarth D, Gani C. Comparison of treatment plans for a high-field MRI-linac and a conventional linac for esophageal cancer. Strahlenther Onkol. 2019;195:327–334. [DOI] [PubMed] [Google Scholar]
- 100.Arvold ND, Neimierko A, Mamon HJ, Fernandez-del Castillo C, Hong TS. Pancreatic cancer tumor size on CT scan versus pathologic specimen: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys. 2011;80:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hall WA, Mikell JL, Mittal P, et al. Tumor size on abdominal MRI versus pathologic specimen in resected pancreatic adenocarcinoma: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys. 2013;86:102–107. [DOI] [PubMed] [Google Scholar]
- 102.Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8:2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hassanzadeh C, Rudra S, Bommireddy A, et al. Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation. Adv Radiat Oncol. 2020;6:100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2021;11:134–147. [DOI] [PubMed] [Google Scholar]
- 105.Nohr EA, Liew Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand. 2018;97:407–416. [DOI] [PubMed] [Google Scholar]
- 106.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. [DOI] [PubMed] [Google Scholar]
- 107.Hassanzadeh C, Rudra S, Bommireddy A, et al. Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation. Adv Radiat Oncol. 2021;6:100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reyngold M, O’Reilly EM, Varghese AM, et al. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 2021;7:735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol. 2016;34:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117:4060–4069. [DOI] [PubMed] [Google Scholar]
- 111.Jackson WC, Tao Y, Mendiratta-Lala M, et al. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiat Oncol Biol Phys. 2018;100:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wahl DR, Stenmark MH, Tao Y, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Witt JS, Rosenberg SA, Bassetti MF. MRI-guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol. 2020;21:e74–e82. [DOI] [PubMed] [Google Scholar]
- 114.Wojcieszynski AP, Rosenberg SA, Brower JV, et al. Gadoxetate for direct tumor therapy and tracking with real-time MRI-guided stereotactic body radiation therapy of the liver. Radiother Oncol. 2016;118:416–418. [DOI] [PubMed] [Google Scholar]
- 115.Gani C, Boeke S, McNair H, et al. Marker-less online MR-guided stereotactic body radiotherapy of liver metastases at a 1.5 T MR-linac—feasibility, work-flow data and patient acceptance. Clin Transl Radiat Oncol. 2021;26:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosenberg SA, Henke LE, Shaverdian N, et al. A multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Adv Radiat Oncol. 2019;4:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Appelt AL, Ploen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:919–927. [DOI] [PubMed] [Google Scholar]
- 118.Bostel T, Dreher C, Wollschlager D, et al. Exploring MR regression patterns in rectal cancer during neoadjuvant radiochemotherapy with daily T2- and diffusion-weighted MRI. Radiat Oncol. 2020;15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the Organ Preservation of Rectal Adenocarcinoma (OPRA) trial [abstract]. J Clin Oncol. 2020;38(15 suppl):4008. [Google Scholar]
- 120.Chiloiro G, Boldrini L, Meldolesi E, et al. MR-guided radiotherapy in rectal cancer: first clinical experience of an innovative technology. Clin Transl Radiat Oncol. 2019;18:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cusumano D, Boldrini L, Yadav P, et al. Delta radiomics for rectal cancer response prediction using low field magnetic resonance guided radiotherapy: an external validation. Phys Med. 2021;84:186–191. [DOI] [PubMed] [Google Scholar]
- 122.Intven MPW, de Mol van Otterloo SR, Mook S, et al. Online adaptive MR-guided radiotherapy for rectal cancer; feasibility of the workflow on a 1.5T MR-linac: clinical implementation and initial experience. Radiother Oncol 2021;154:172–178. [DOI] [PubMed] [Google Scholar]
- 123.Murthy V, Maitre P, Kanan S, et al. Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): outcomes from phase III randomized controlled trial. J Clin Oncol. 2021;39:1234–1242. [DOI] [PubMed] [Google Scholar]
- 124.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sidaway P MRI improves diagnosis. Nat Rev Clin Oncol. 2018;15:345. [DOI] [PubMed] [Google Scholar]
- 126.Thompson J, Lawrentschuk N, Frydenberg M, Thompson L, Striker P, USANZ. The role of magnetic resonance imaging in the diagnosis and management of prostate cancer. BJU Int. 2013;112(suppl 2):6–20. [DOI] [PubMed] [Google Scholar]
- 127.Tocco BR, Kishan AU, Ma TM, Kerkmeijer LGW, Tree AC. MR-guided radiotherapy for prostate cancer. Front Oncol. 2020;10:616291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: results from the FLAME randomized phase III trial. J Clin Oncol. 2021;39:787–796. [DOI] [PubMed] [Google Scholar]
- 129.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016; 17:1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 2018;4:e180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cassidy RJ, Yang X, Liu T, Thomas M, Nour SG, Jani AB. Neurovascular bundle-sparing radiotherapy for prostate cancer using MRI-CT registration: a dosimetric feasibility study. Med Dosim. 2016;41:339–343. [DOI] [PubMed] [Google Scholar]
- 132.Dunlop A, Mitchell A, Tree A, et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Transl Radiat Oncol. 2020;23:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tetar SU, Bruynzeel AME, Oei SS, et al. Magnetic resonance-guided stereotactic radiotherapy for localized prostate cancer: final results on patient-reported outcomes of a prospective phase 2 study. Eur Urol Oncol. 2021;4:628–634. [DOI] [PubMed] [Google Scholar]
- 134.Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20:1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bruynzeel AME, Tetar SU, Oei SS, et al. A prospective single-arm phase 2 study of stereotactic magnetic resonance guided adaptive radiation therapy for prostate cancer: early toxicity results. Int J Radiat Oncol Biol Phys. 2019;105:1086–1094. [DOI] [PubMed] [Google Scholar]
- 136.Acharya S, Fischer-Valuck BW, Mazur TR, et al. Magnetic resonance image guided radiation therapy for external beam accelerated partial-breast irradiation: evaluation of delivered dose and intrafractional cavity motion. Int J Radiat Oncol Biol Phys. 2016;96:785–792. [DOI] [PubMed] [Google Scholar]
- 137.Groot Koerkamp ML, Vasmel JE, Russell NS, et al. Optimizing MR-guided radiotherapy for breast cancer patients. Front Oncol. 2020;10:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee HI, Kim K, Kim JH, Chang JH, Shin KH. The acute and late toxicities of MRI-guided external beam partial breast irradiation delivered using a once-per-day regimen. Front Oncol. 2021;11:649301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McBain CA, Logue JP. Radiation therapy for muscle-invasive bladder cancer: treatment planning and delivery in the 21st century. Semin Radiat Oncol. 2005;15:42–48. [DOI] [PubMed] [Google Scholar]
- 140.Panebianco V, Narumi Y, Altun E, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur Urol. 2018;74:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chan MF, Cohen GN, Deasy JO. Qualitative evaluation of fiducial markers for radiotherapy imaging. Technol Cancer Res Treat. 2015;14:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Al Johi RS, Seifeldein GS, Moeen AM, et al. Diffusion weighted magnetic resonance imaging in bladder cancer, is it time to replace biopsy? Cent European J Urol. 2018;71:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hunt A, Hanson I, Dunlop A, et al. Feasibility of magnetic resonance guided radiotherapy for the treatment of bladder cancer. Clin Transl Radiat Oncol. 2020;25:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verkooijen HM, Kerkjeijer LGW, Fuller CD, et al. R-IDEAL: a framework for systematic clinical evaluation of technical innovations in radiation oncology. Front Oncol. 2017;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]


