Abstract
Stereotactic body radiation therapy (SBRT) is effective for the treatment of cancer. Neutrophil-to-lymphocyte ratio (NLR) is a common prognostic factor in predicting survival of patients with cancer. Previous studies have reported that NLR may be able to predict survival of patients with cancer treated with SBRT; however, the results are inconsistent. Therefore, the present study performed a meta-analysis to pool the data of prognostic prediction using NLR for patients with cancer who underwent SBRT. PubMed, Google Scholar, Embase and The Cochrane Library were used to search for articles published before October 2020. Pooled hazard radios (HRs) with 95% confidence intervals (CIs) were used to evaluate the association of NLR levels with patient outcome following SBRT. The primary endpoint was overall survival (OS). Subgroup analyses were used to detect sources of heterogeneity. Publication bias was assessed by Egger's test and Begg's test. A total of nine studies involving 1,010 participants were included in the present meta-analysis. Univariate and multivariate analyses revealed that elevated NLR predicted a worse outcome for OS (HR, 1.35; 95% CI, 1.22-1.49; P<0.001 and HR, 1.29; 95% CI, 1.16-1.44; P<0.001, respectively), regardless of pre- and post-treatment groups. Subgroup analysis demonstrated that the prospective group showed more significant heterogeneity (I2=57.7%; P=0.124) than the retrospective group (I2=0%) and overall (I2=47.5%). In conclusion, both pre- and post-SBRT elevated NLRs were revealed to be independently associated with poor survival in patients with cancer who received SBRT.
Keywords: NLR, SBRT, prognosis, cancer, meta-analysis
Introduction
At present, cancer is a major cause of death worldwide; in 2021, there were 19.3 million new cases of cancer and 10 million cancer-associated deaths. Approximately one in five men and one in six women will develop cancer during their lifetime (1). Stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), is an option for patients with cancer beyond chemotherapy or surgery (2). SBRT refers to the administration of high doses of radiation using several beams of various intensities aimed at different angles to precisely target the tumor. SBRT is a noninvasive technique that can deliver high precision and dose-escalated treatment throughout the body with excellent rates in local control. In addition, it has been widely used to treat various types of cancer, including gastrointestinal malignancies, prostate cancer and recurrent gastric cancer (3-6).
Although SBRT has been widely used to treat cancer for a number of years, the prognosis of the treatment is clinically heterogeneous, characterized by increased local recurrence and distant metastasis (7,8). Therefore, more effective and accurate indicators to assist clinicians with patient risk stratification and clinical therapy are required (9,10). In recent years, numerous studies have reported that tumor-associated inflammation and the tumor environment influence cancer development, progression and metastasis, which has led to much interest in the association between patient prognosis and inflammatory hematological markers (11,12). Among the inflammatory indexes, neutrophil-to-lymphocyte ratio (NLR) is an emerging biomarker of interest for several types of malignancy and is readily assessed from a serum complete blood count (CBC) with differential; notably, increased NLR has been reported to be associated with poor prognostic indicators, particularly poor overall survival (OS) in patients with advanced cancer (13).
Previous studies have shown that the number of participants included in individual studies is not large and the results are inconsistent (7,8). In addition, the association between inflammation-based biomarkers and oncological outcomes in patients with cancer who undergo SBRT is unclear. It is well known that patients receiving radiation therapy may experience a marked decline or a depletion of circulating lymphocytes (14,15), and a decreased lymphocyte count has been reported to be associated with a weaker anti-tumor immune response and a poor prognosis (16,17). It is therefore of great clinical importance to investigate the predictive roles of NLR before and after SBRT in patients with cancer.
The present study aimed to perform a meta-analysis to quantify the prognostic value of NLR on the outcome of tumors treated with SBRT. Furthermore, according to existing studies, the present study determined whether a statistical difference existed in the prognosis of cancer between pre-SBRT NLR and post-SBRT NLR.
Materials and methods
Registration number
The present study performed a systematic review and meta-analysis of the existing literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (18). The present study was registered in PROSPERO (registration no. CRD42020186132). All analyses were based on previously published studies; therefore, no ethics approval or patient consent were required.
Search strategy
A comprehensive retrieval of articles published between January 1, 1990 and October 5, 2020 was performed using the following databases: Embase (https://www.embase.com), PubMed (https://pubmed.ncbi.nlm.nih.gov), The Cochrane Library (https://www.cochranelibrary.com) and Google Scholar (https://scholar.google.com). Medical subject headings and abstract fields were searched combined with the related key words including ‘NLR’ (e.g., ‘neutrophil to lymphocyte ratio’ OR ‘NLR’ OR ‘neutrophil-to-lymphocyte ratio’) AND ‘SBRT’ (e.g., ‘stereotactic body radiotherapy’ OR ‘SBRT’ OR ‘stereotactic ablative radiotherapy’ OR ‘SABR’) AND ‘cancer’ (e.g., ‘cancer’ ‘carcinoma’ and ‘tumor’). No language restriction was applied.
Study selection
Original assessment was based on the title and abstract of each reference. Full articles of relevant references were then reviewed for qualification using the following criteria: i) Studies involving individuals with solid tumors who underwent SBRT; ii) the association between NLR and OS was discussed; iii) baseline levels of NLR were assessed before or after SBRT treatment; iv) studies providing the hazard ratio (HR) with 95% confidence interval (CI) for OS (19), or relevant information could be estimated by Engauge Digitizer (https://markummitchell.github.io/engauge-digitizer/) to obtain the aforementioned statistics; v) a Newcastle-Ottawa Scale (NOS) score >5 (20,21). Case reports, reviews, animal studies, conference proceedings, letters to editors, abstract only and duplicated studies were excluded.
Data extraction
All candidate literature was evaluated and extracted by two independent authors. The two authors assessed all full articles for eligibility and extracted data using a preset spreadsheet. Any disagreement was resolved by a third researcher (LH) or through discussion. The primary endpoint was OS. Information summarized included: First author, publication year, research country, age, ethnicity, sample size, follow-up duration, primary location of the tumor, stage of cancer, method of treatment and NLR cut-off value. Outcome indicators, and HRs from multivariate and univariate analyses were preferred.
Data analysis
The present study evaluated the prognostic role of NLR by pooling the HRs and corresponding 95% CIs for survival analysis. I², calculated as follows: I² (%)=100 x (Q - df)/Q, where Q is Cochran's heterogeneity statistic and df is the degrees of freedom, and P-values were used to identify and quantify the degree of heterogeneity (22). When I² was ≥50% or P≤0.05 (significant heterogeneity) (23), random-effects model was used to combine HRs, otherwise a fixed-effects model was adopted. Subgroup analysis was used to detect sources of heterogeneity. Publication bias was assessed by Egger's test and Begg's test (24,25). Two-sided P<0.05 was considered statistically significant. Forest plot, Egger's test and Begg's test were conducted using STATA statistical software (version 12.0; StatCorp LLC). The flow diagram was generated using GraphPad Prism (version 8.0; GraphPad Software, Inc.). In addition, quality assessment was performed using RevMan (version 5.3; Cochrane Collaboration).
Results
Search and selection of studies
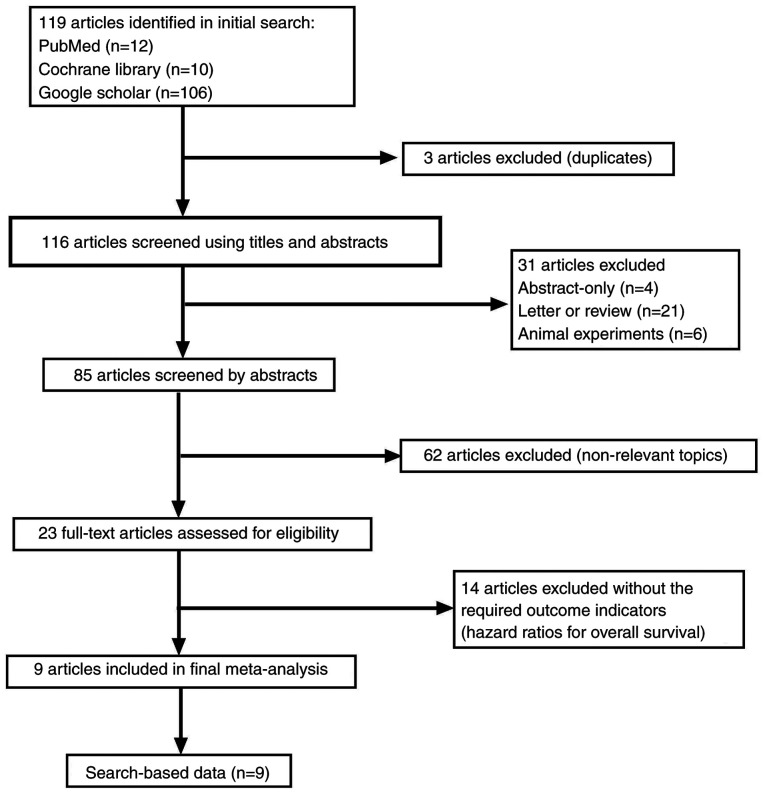
A total of 119 relevant articles were screened following the initial search. The process of the selection is shown in Fig. 1. Three of the studies were duplicated articles and 62 were revealed to be not relevant after scanning the abstract. A total of 31 studies were removed for other reasons (four studies were abstracts only; 21 studies were letters or reviews; and six studies were animal experiments). Subsequently, 23 full-text articles were assessed for eligibility; however, 14 articles failed to meet the inclusion criteria. Finally, nine studies (26-34) involving 1,010 participants were included for further assessment.
Figure 1.
Flow diagram of the literature search and study selection.
Study characteristics
Two studies were from China (26,30), six studies were from USA (27-29,32-34), and one study was performed in Canada (31). Four of these cohorts enrolled <100 participants (26,29,30,33) and five studies recruited >100 patients (27,28,31,32,34). Four studies investigated non-small cell lung cancer (NSCLC) (27,28,31,33), two studies investigated hepatocellular carcinoma (HCC) (26,30), and the remaining studies investigated pancreatic adenocarcinoma (34), brain metastases (32) and malignant adrenal lesions (29). Furthermore, two studies did not limit the stage of cancer (involved all disease stages) (26,28), four studies included only early-stage disease (I/I-II/I-III/II-IIIb) (27,30,31,33) and three studies included only late-stage disease (III-IV/IIIb-IV) (29,32,34). Two studies were prospective design (28,31) and seven studies were retrospective (26,27,29,30,32-34). Notably, six studies conducted both multivariate analysis and univariate analysis (26,28,29,31,32,34). In these six studies, some variables were used as covariates for Cox regression multivariate analysis; therefore, their covariates are listed separately. The characteristics of the included studies are shown in Table I.
Table I.
Main characteristics of all of the studies included in the meta-analysis.
| First author | Year | Study region | Ethnicity | Number of participants (M/F) | Median follow-up, months(range) | Disease type | Stage | Treatment | Median age, years (range) | NLR cut-off | Outcome | HR | Covariates | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alagappan | 2018 | USA | Caucasian | 208 (109/99) | 7.5 (4.6-12.0) | Pancreatic adenocarcinoma | Advanced | Combined | 75.2 (65.9-86.1) | 5 | OS/LR | R (M/U) | Albumin; RBC; prior chemotherapy (yes) | (34) |
| Cannon | 2015 | USA | Caucasian | 59 (31/28) | 17 | NSCLC | Early | SBRT | 70 (48-89) | 2.98 | OS | R (U) | - | (33) |
| Chowdhary | 2018 | USA | Caucasian | 188 (91/97) | 13.2 | Brain metastases | Advanced | Combined | NR | 6 | OS | R (M/U) | Active systemic disease; extracranial metastases; graded prognostic assessment; targeted therapy post-SRS; immunotherapy post-SRS | (32) |
| Giuliani | 2016 | Canada | Caucasian | 122 (60/62) | 26.9 (1.3-99.3) | NSCLC | Early | SBRT | 76 (48-90) | 3 | OS | R (M/U) | Female sex; tumor stage T2; hemoglobin | (31) |
| Lai | 2020 | China | Asian | 72 (61/11) | 67.2 (7.7-127.4) | HCC | Early | SBRT | 57 (30-84) | 1.88 | OS | R (M) | - | (30) |
| Mills | 2019 | USA | Caucasian | 27 (12-15) | 8 (1-66) | Malignant adrenal lesions | Advanced | Combined | 63 (51-78) | 4.1 | OS | R (M/U) | Pretreatment ALC >1x106/ml | (29) |
| Sebastian | 2019 | USA | Caucasian | 156 (89-67) | 13.4 | NSCLC | All stages | SBRT | 72 (51-92) | 3.6 | OS | R (M/U) | Age; sex; T stage; histology; ECOG performance status; Charlson's Comorbidity Index; smoking; BED Gy10 | (28) |
| Shaverdian | 2016 | USA | Caucasian | 118 | 28.9 | NSCLC | Early | SBRT | 76 | 2.18 | DMFS/DSS/OS | R (U) | - | (27) |
| Zhuang | 2019 | China | Asian | 60 (49/11) | 36.9 (4.1-73.5) | HCC | ALL stages | Combined | 61.0±12.8 | 2.7 | PFS/OS | R (M/U) | Presence of hepatitis; tumor size (≥1.5cm); pre-treatment pre-treatment AFP (≥20.0 ng/ml); pre-treatment RBC (≥4.5x1012/l); post-treatment PLR (≥263.0) | (26) |
M, male; F, female; USA, United States of America; NSCLC, non-small cell lung cancer; HCC, hepatocellular carcinoma; SBRT, stereotactic body radiation therapy; NR, not reported; OS, overall survival; LR, local recurrence; PFS, progression-free survival; DMFS, distant metastasis-free survival; DSS, disease-specific survival; NLR, neutrophil-to-lymphocyte ratio; HR, hazard ratio; R, obtained by reporting in text; M, multivariate analysis; U, univariate analysis; RBC, red blood cell; SRS, stereotactic radiosurgery; ALC, absolute lymphocyte count; ECOG, Eastern Cooperative Group; BED, biologically effective dose; AFP, α-fetoprotein; PLR, platelet-to-lymphocyte ratio.
Quality assessment
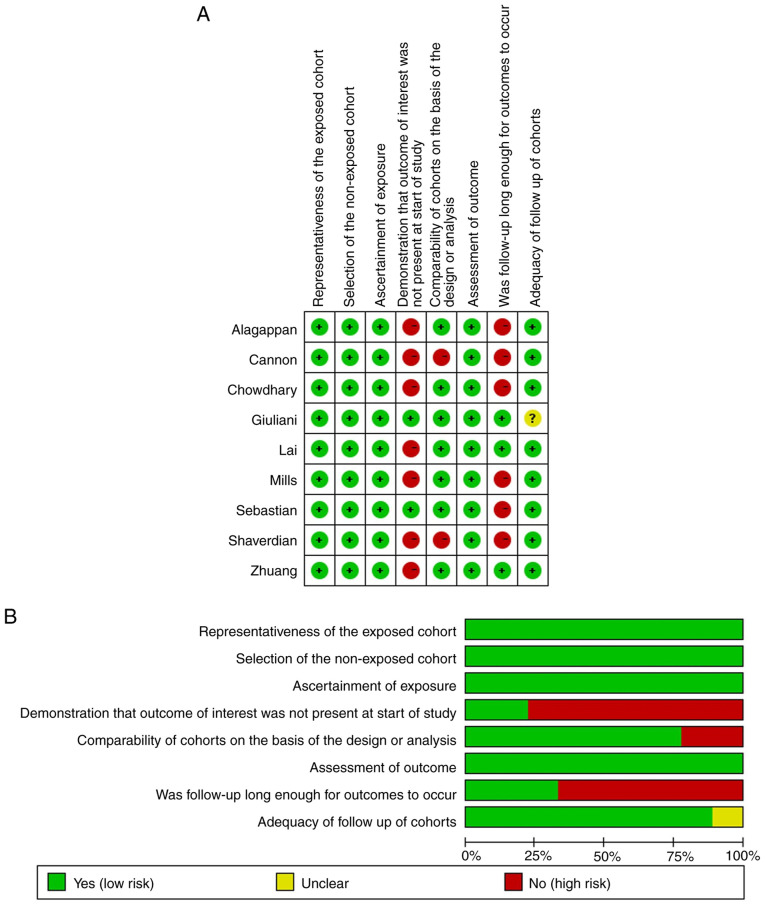
The NOS was used to assess the quality of each of the included studies by two independent authors. The NOS consists of three parts: Selection, comparability and outcome assessment. For the selection of cohort item, representative exposed groups were selected for inclusion, and the non-exposed and exposed groups were from the same population in all studies. Therefore, they were considered as being at low risk of bias. Only two of the studies were prospective and seven were retrospective, thus they were regarded as high risk of bias. As for intergroup comparability, seven studies applied multivariate analysis, whereas the remaining were considered as high risk of bias because they only used univariate analysis. With regard to outcome, all studies had record linkage. Nevertheless, the follow-ups in only three studies were long enough for outcomes to occur (median >2 years). Eight studies had complete follow-ups, only one was vague in details, which was labelled as unclear risk of bias. When items conform to NOS, the circle in the figure is green; non-conforming items are red; and unclear items are yellow (Fig. 2). Studies with ≥5 green circles were assigned as mid-quality studies and those with ≥6 green circles were assigned as high-quality studies. All of the studies assessed in the present study were mid-quality or high-quality.
Figure 2.
Risk of bias summary. (A) Judgements about each risk of bias item for each included study. (B) Judgements about each risk of bias item presented as percentages across all included studies. Green circle, items conform to Newcastle-Ottawa Scale; red circle, non-conforming items; yellow circle, unclear items.
Univariate analysis of NLR and OS
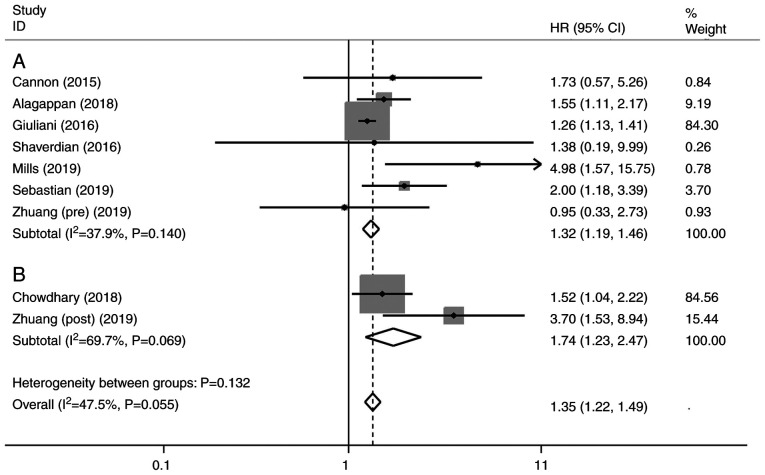
A total of eight studies were included in the univariate analysis of NLR and OS. Among them, one study evaluated both pre-treatment and post-treatment NLR (26), one study assessed only post-treatment NLR (32), and six studies included only pre-treatment NLR (27-29,31,33,34). The univariate analysis revealed that elevated NLR predicted a worse outcome for OS with a combined HR of 1.35 (95% CI, 1.22-1.49, P<0.001), without significant heterogeneity (I²=47.5%; P=0.055) (Fig. 3). The subgroup analysis by pre- or post-SBRT NLR showed that the pooled HRs were 1.32 (95% CI, 1.19-1.46; P<0.001; Fig. 3A) and 1.74 (95% CI, 1.23-2.47; P<0.005; Fig. 3B), respectively.
Figure 3.
Forest plot of univariate analysis of (A) pre-treatment and (B) post-treatment neutrophil-to-lymphocyte ratio. Results are presented as individual and pooled HRs, and 95% CIs. Grey square indicates weight of study. CI, confidence interval; HR, hazard ratio.
Multivariate analysis of NLR and OS
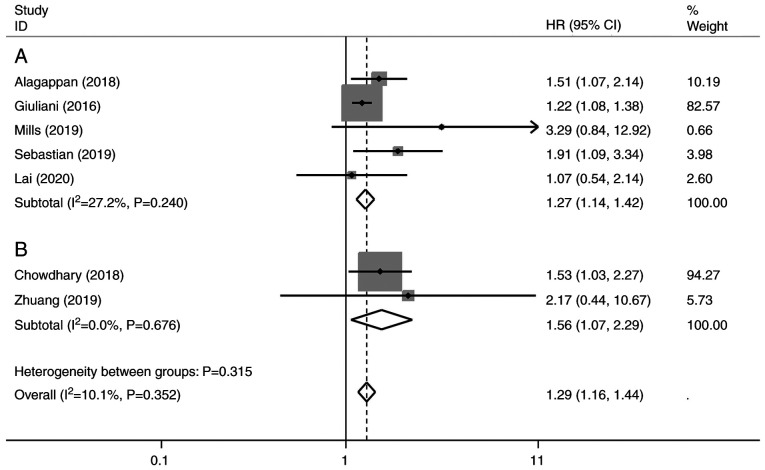
In the multivariate analysis, five studies assessed pre-treatment NLR (28-31,34) and two studies included post-treatment NLR (26,32). The results demonstrated that increased NLR was associated with a poorer OS (HR, 1.29; 95% CI, 1.16-1.44; P<0.001), without significant heterogeneity (I²=10.1%; P=0.352) (Fig. 4). Subgroup analysis by pre- or post-SBRT NLR revealed the pooled HR was 1.27 (95% CI, 1.14-1.42; P<0.001; Fig. 4A) and 1.56 (95% CI, 1.07-2.29; P<0.005; Fig. 4B).
Figure 4.
Forest plot of multivariate analysis of (A) pre-treatment and (B) post-treatment neutrophil-to-lymphocyte ratio. Results are presented as individual and pooled HRs, and 95% CI. Grey square indicates weight of study. CI, confidence interval; HR, hazard ratio.
Subgroup analysis to explore sources of heterogeneity
Subgroup analysis of univariate analysis was performed based on the extracted data (Table II). Subgroup analysis of retrospective or prospective data demonstrated that the pooled HRs were 1.47 (95% CI, 1.17-1.84) and 1.25 (95% CI, 1.10-1.40), respectively, and the prospective group showed more significant heterogeneity (I2=57.7%; P=0.124) than overall (I2=47.5%). The cut-off values applied in the studies were not consistent, ranging between 1.88 and 6. Five studies had a NLR cut-off value of ≤3, whereas four studies had a NLR cut-off value of >3. Heterogeneity was not detected between cut-off value ≤3 and cut-off value >3 groups (P=0.051), although the P-value was close to significance, and the pooled HRs were 1.22 (95% CI, 1.08-1.38) and 1.58 (95% CI, 1.26-1.98), respectively.
Table II.
Summary of the subgroup meta-analysis.
| Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|
| Analysis | N | References | Random-effects model HR (95% CI) | Fixed-effects model HR (95% CI) | I², % | P-value | P-value (between groups) |
| Subgroup 1: Study design | 0.210 | ||||||
| Retrospective | 7 | (26,27,29,30,32-34) | 1.47 (1.17-1.84) | 1.47 (1.17-1.84) | 0.00 | 0.817 | |
| Prospective | 2 | (28,31) | 1.40 (0.93-2.10) | 1.25 (1.10-1.40) | 57.70 | 0.124 | |
| Subgroup 2: Therapy | 0.158 | ||||||
| SBRT only | 5 | (27,28,30,31,33) | 1.25 (1.11-1.40) | 1.25 (1.11-1.40) | 0.00 | 0.577 | |
| Combined | 4 | (26,29,32,34) | 1.52 (1.18-1.95) | 1.52 (1.18-1.95) | 0.00 | 0.575 | |
| Subgroup 3: Stage | 0.147 | ||||||
| Early | 4 | (27,30,31,33) | 1.22 (1.08-1.38) | 1.22 (1.08-1.38) | 0.00 | 0.914 | |
| Advanced | 3 | (29,32,34) | 1.56 (1.21-2.02) | 1.56 (1.21-2.02) | 0.00 | 0.553 | |
| All stages | 2 | (26,28) | 1.56 (0.84-2.90) | 1.64 (1.00-2.69) | 23.90 | 0.252 | |
| Subgroup 4: Cut-off value | 0.051 | ||||||
| NLR ≤3 | 5 | (26,27,30,31,33) | 1.22 (1.08-1.38) | 1.22 (1.08-1.38) | 0.00 | 0.914 | |
| NLR >3 | 4 | (28,29,32,34) | 1.58 (1.26-1.98) | 1.58 (1.26-1.98) | 0.00 | 0.639 | |
| Subgroup 5: Tumor location | 0.230 | ||||||
| NSCLC | 4 | (27,28,31,33) | 1.25 (1.11-1.41) | 1.25 (1.11-1.41) | 0.00 | 0.440 | |
| HCC | 2 | (26,30) | 1.04 (0.58-1.84) | 1.04 (0.58-1.84) | 0.00 | 0.849 | |
| Others | 3 | (29,32,34) | 1.56 (1.21-2.02) | 1.56 (1.21-2.02) | 0.00 | 0.553 | |
| Subgroup 6: Ethnicity | 0.447 | ||||||
| Caucasian | 7 | (27-29,31-34) | 1.33 (1.17-1.51) | 1.30 (1.17-1.45) | 4.00 | 0.396 | |
| Asian | 2 | (26,29) | 1.04 (0.58-1.84) | 1.04 (0.58-1.84) | 0.00 | 0.849 | |
| Subgroup 7: Sample size | 0.941 | ||||||
| ≤100 | 4 | (26,29,30,33) | 1.31 (0.81-2.12) | 1.31 (0.81-2.12) | 0.00 | 0.448 | |
| >100 | 5 | (27,28,31,32,34) | 1.31 (1.16-1.48) | 1.29 (1.16-1.44) | 4.90 | 0.379 | |
CI, confidence interval; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; SBRT, stereotactic body radiation therapy; NSCLC, non-small cell lung cancer; HCC, hepatocellular carcinoma.
In addition, subgroup analyses were performed according to treatment methods (treatment by SBRT and combined), disease stage (early stage, advanced stage and all stages), tumor type (NSCLC, HCC and others), ethnicity (Caucasian and Asian) and sample size (≤100 and >100), but no significant differences were identified.
Publication bias
Begg's funnel plot and Egger's linear regression test were performed to evaluate publication bias. The publication biases were Pr>|z|=0.917 for Begg's test (Fig. 5A) and P>|t|=0.131 for Egger's test (Fig. 5B). The size of the circle indicates the weight of the article. No publication bias was found.
Figure 5.
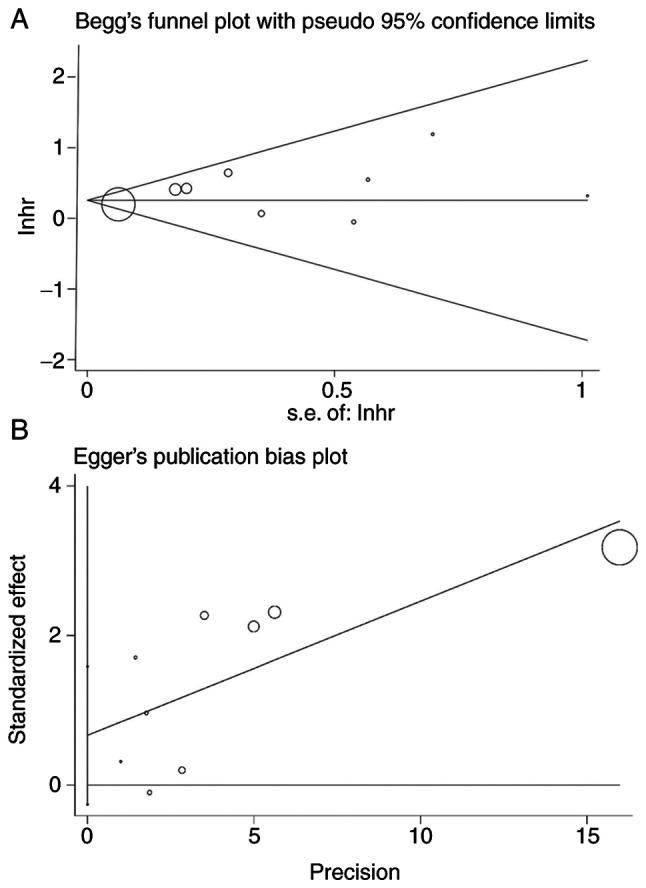
Publication bias. (A) Begg's funnel plot and (B) Egger's linear regression test. Circle size indicates weight of study. lnhr, logarithm of hazard ratio; s.e, standard error.
Discussion
The present meta-analysis demonstrated that elevated NLR was a significant predictor of poor survival outcomes in patients that underwent SBRT alone or in combination with chemotherapy or surgery. The results were consistent in both univariate and multivariate analyses, thus indicating that NLR may be an independent predictor for prognosis. Notably, the weights of Giuliani et al (31) and Chowdhary et al (32) were particularly large (>80%), because these studies yielded CIs of a smaller range and are thus considered more accurate. Subgroup analyses showed that both elevated pre- and post-treatment NLR could significantly reduce the survival of patients treated with SBRT. Meanwhile, post-treatment NLR predicted poorer survival than pre-treatment. Although there was no significance between pre- and post-treatment groups, the induction of a leukocyte-predominant inflammatory response after SBRT may predict a worse prognosis. Moreover, heterogeneity was not found between cut-off value ≤3 and cut-off value >3 groups (P=0.051), although the P-value was close to significance. This may be caused by an insufficient sample size.
The association of elevated NLR with a worse prognosis may be based on the immune/inflammatory response. Inflammation affects all stages of tumorigenesis; not only have researchers confirmed that inflammation and immunity govern the development of tumors (35), but they have also verified the therapeutic value when targeting the inflammasome for the prevention and treatment of cancer (36). The association between increased NLR and poor outcome is not yet understood; however, the potential mechanism may involve the association between NLR and inflammation. Notably, previous studies have revealed that neutrophils may be indicative of inflammation, which can induce production of chemokines and cytokines, and suppress the cytolytic activity of immune cells, such as activated T cells and natural killer cells (37,38). Cancer cells together with its host cells can produce inflammatory cytokines and chemokines that contribute to malignant progression (39). Neutrophils can produce an inflammatory response, which may stimulate the change of tumor microenvironment, thus resulting in the proliferation and metastasis of cancer cells. In addition, it has been reported that elevated NLR can lead to elevated tumor growth-promoting factors, such as TGF-β (40). Furthermore, inflammatory factors can increase the number of neutrophils and decrease the number of lymphocytes; in some reports, primary tumor infiltration was revealed to be positively linked with lymphopenia (41,42). Other studies have also reported that tumor-infiltrating lymphocytes (TIL) serve an essential role in guiding prognosis. Notably, CD3+ TILs have been reported to exert a positive effect on survival of patients with breast cancer and the importance of lymphocytes has been highlighted (43-45). Formerly regarded as a merely immunosuppressive treatment, pre- and clinical observations have indicated that radiotherapy can elicit an immune response against tumors (46,47). The response was first observed as infrequent abscopal effects emerged from the phenomenon of tumor remission outside the radiation field in satellite secondary tumors (48). Elevated lymphocytes and low NLR may be positive signs of abscopal effects.
There are some limitations in the present study. Firstly, the number and sample size of the included eligible studies were small. In addition, two (27,33) of the HRs and 95% CIs were extracted from Kaplan-Meier survival curves due to the unavailability of original data using Engauge Digitizer, which could lead to imprecise risk estimates. Secondly, among the included studies, only three studies were followed up for >2 years. Insufficient follow-up may overestimate the survival and prognosis of patients with cancer in the cohort to some extent. Thirdly, the NLR cut-off value for the present study was inconsistent; each study varied from another. The optimal NLR cut-off value for various tumors needs to be investigated in further large-scale prospective cohort studies. In addition, it is well known that SBRT processing has an impact on NLR; however, with the exception of Sebastian et al (28), the original studies did not provide the specific measurement time of NLR. Sebastian et al (28) mentioned that all patients had an available CBC with differential within 6 months of completion of treatment. Therefore, we cannot know whether the post-NLR value given in these studies was obtained after the first SBRT or measured after all SBRT was completed; this affects the accuracy of the results to a certain extent. Finally, the discrepancies between pre- and post-SBRT NLR require further research; although the present results revealed there was no statistical significance, this may be caused by insufficient sample size.
Notably, more well-designed, large-scale studies with a longer follow-up are required in the future. Furthermore, further research is needed to clarify the mechanism underlying the systemic inflammatory response to SBRT based on the change of pre- and post-SBRT NLR.
In conclusion, both pre- and post-SBRT elevated NLR may be considered an independent predictor of poor survival in patients with cancer who received SBRT; the higher level of NLR predicts a worse outcome. Therefore, NLR may be considered a promising index for appropriately individualizing SBRT and assessing prognosis.
Acknowledgements
Not applicable.
Funding Statement
Funding: This work was supported by grants from the China Postdoctoral Science Foundation (grant no. 2020M682578), the Science and Technology Innovation Program of Hunan Province (grant no. 2020RC2061), the Hunan Cancer Hospital Climb Plan (grant no. YF2020006), the Hunan Cadres Health Care Department (grant no. NCC2017A17), the Hunan Provincial Health Commission (grant no. B2019098) and the Chinese National Cancer Center (grant no. NCC2017A17, No NCC2017L01).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YY, DT and HL designed the research and extracted data. CL, ZH and PY performed the statistical analysis, and the data visualization and interpretation. YY and DT drafted the first manuscript. HL, PY and ZH made critical revisions to the manuscript for key intellectual content and reviewed the data analysis. YY and DT confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32:2847–2854. doi: 10.1200/JCO.2014.55.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tandberg DJ, Tong BC, Ackerson BG, Kelsey CR. Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: A comprehensive review. Cancer. 2018;124:667–678. doi: 10.1002/cncr.31196. [DOI] [PubMed] [Google Scholar]
- 4.Abdelfatah MM, Gochanour EM. Fiducial placement for recurrent gastric cancer. Arab J Gastroenterol. 2019;20:56–58. doi: 10.1016/j.ajg.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Schaub SK, Hartvigson PE, Lock MI, Hoyer M, Brunner TB, Cardenes HR, Dawson LA, Kim EY, Mayr NA, Lo SS, Apisarnthanarax S. Stereotactic body radiation therapy for hepatocellular carcinoma: Current trends and controversies. Technol Cancer Res Treat. 2018;17(1533033818790217) doi: 10.1177/1533033818790217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 7.Mathew AS, Atenafu EG, Owen D, Maurino C, Brade A, Brierley J, Dinniwell R, Kim J, Cho C, Ringash J, et al. Long term outcomes of stereotactic body radiation therapy for hepatocellular carcinoma without macrovascular invasion. Eur J Cancer. 2020;134:41–51. doi: 10.1016/j.ejca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schonewolf CA, Heskel M, Doucette A, Singhal S, Frick MA, Xanthopoulos EP, Corradetti MN, Friedberg JS, Pechet TT, Christodouleas JP, et al. Five-year long-term outcomes of stereotactic body radiation therapy for operable versus medically inoperable stage I non-small-cell lung cancer: Analysis by operability, fractionation regimen, tumor size, and tumor location. Clin Lung Cancer. 2019;20:e63–e71. doi: 10.1016/j.cllc.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Costa-Pinheiro P, Montezuma D, Henrique R, Jeronimo C. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics. 2015;7:1003–1015. doi: 10.2217/epi.15.56. [DOI] [PubMed] [Google Scholar]
- 10.Cai D, Xu Y, Ding R, Qiu K, Zhang R, Wang H, Huang L, Xie X, Yan H, Deng Y, et al. Extensive serum biomarker analysis in patients with non-small-cell lung carcinoma. Cytokine. 2020;126(154868) doi: 10.1016/j.cyto.2019.154868. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, Wang Q, Yang W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13. doi: 10.1016/j.ctrv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Raben M, Walach N, Galili U, Schlesinger M. The effect of radiation therapy on lymphocyte subpopulations in cancer patients. Cancer. 1976;37:1417–1421. doi: 10.1002/1097-0142(197603)37:3<1417::aid-cncr2820370324>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol. 2018;3:512–519. doi: 10.1016/j.adro.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. doi: 10.1016/j.critrevonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Maehata Y, Onishi H, Kuriyama K, Aoki S, Araya M, Saito R, Tominaga L, Oguri M, Araki T. Immune responses following stereotactic body radiotherapy for stage I primary lung cancer. Biomed Res Int. 2013;2013(731346) doi: 10.1155/2013/731346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(e1000097) doi: 10.1371/journal.pmed.1000097. PRISMA Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Deng C, Ma X, Liu X. rs10865331 in 2p15 increases susceptibility to ankylosing spondylitis: A HuGE review and meta-analysis. Clin Exp Rheumatol. 2020;38:993–1000. [PubMed] [Google Scholar]
- 21.Rezapour M, Rezapour HA, Chegeni M, Khanjani N. Exposure to cadmium and head and neck cancers: A meta-analysis of observational studies. Rev Environ Health. 2021;36:577–584. doi: 10.1515/reveh-2020-0109. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 26.Zhuang Y, Yuan BY, Hu Y, Chen GW, Zhang L, Zhao XM, Chen YX, Zeng ZC. Pre/post-treatment dynamic of inflammatory markers has prognostic value in patients with small hepatocellular carcinoma managed by stereotactic body radiation therapy. Cancer Manag Res. 2019;11:10929–10937. doi: 10.2147/CMAR.S231901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaverdian N, Veruttipong D, Wang J, Schaue D, Kupelian P, Lee P. Pretreatment immune parameters predict for overall survival and toxicity in early-stage non-small-cell lung cancer patients treated with stereotactic body radiation therapy. Clin Lung Cancer. 2016;17:39–46. doi: 10.1016/j.cllc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Sebastian N, Wu T, Bazan J, Driscoll E, Willers H, Yegya-Raman N, Bond L, Dwivedi A, Mo X, Tan Y, et al. Pre-treatment neutrophil-lymphocyte ratio is associated with overall mortality in localized non-small cell lung cancer treated with stereotactic body radiotherapy. Radiother Oncol. 2019;134:151–157. doi: 10.1016/j.radonc.2019.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills MN, Reddy AV, Richardson L, Richardson KM, Kersh CR. The prognostic role of pretreatment neutrophil to lymphocyte ratio (NLR) in malignant adrenal lesions treated with stereotactic body radiation therapy (SBRT) Am J Clin Oncol. 2019;42:945–950. doi: 10.1097/COC.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 30.Lai L, Su T, Liang Z, Lu Y, Hou E, Lian Z, Gao H, Zhu X. Development and assessment of novel predictive nomograms based on APRI for hepatitis B Virus-associated small solitary hepatocellular carcinoma with stereotactic body radiotherapy. J Cancer. 2020;11:6642–6652. doi: 10.7150/jca.47291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giuliani M, Sampson LR, Wong O, Gay J, Le LW, Cho BC, Brade A, Sun A, Bezjak A, Hope AJ. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes on outcomes in lung stereotactic body radiotherapy. Curr Oncol. 2016;23:e362–e368. doi: 10.3747/co.23.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhary M, Switchenko JM, Press RH, Jhaveri J, Buchwald ZS, Blumenfeld PA, Marwaha G, Diaz A, Wang D, Abrams RA, et al. Post-treatment neutrophil-to-lymphocyte ratio predicts for overall survival in brain metastases treated with stereotactic radiosurgery. J Neurooncol. 2018;139:689–697. doi: 10.1007/s11060-018-2914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon NA, Meyer J, Iyengar P, Ahn C, Westover KD, Choy H, Timmerman R. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10:280–285. doi: 10.1097/JTO.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 34.Alagappan M, Pollom EL, von Eyben R, Kozak MM, Aggarwal S, Poultsides GA, Koong AC, Chang DT. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol. 2018;41:242–247. doi: 10.1097/COC.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 35.Singh R, Mishra MK, Aggarwal H. Inflammation, immunity, and cancer. Mediators Inflamm. 2017;2017(6027305) doi: 10.1155/2017/6027305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karki R, Man SM, Kanneganti TD. Inflammasomes and cancer. Cancer Immunol Res. 2017;5:94–99. doi: 10.1158/2326-6066.CIR-16-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol. 1985;134:230–234. [PubMed] [Google Scholar]
- 38.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–2413. [PubMed] [Google Scholar]
- 39.Balkwill F, Mantovani A. Inflammation and cancer: Back to virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 40.Yuksel OH, Verit A, Sahin A, Urkmez A, Uruc F. White blood cell counts and neutrophil to lymphocyte ratio in the diagnosis of testicular cancer: A simple secondary serum tumor marker. Int Braz J Urol. 2016;42:53–59. doi: 10.1590/S1677-5538.IBJU.2014.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura Y, Watanabe R, Katagiri M, Saida Y, Katada N, Watanabe M, Okamoto Y, Asai K, Enomoto T, Kiribayashi T, Kusachi S. Neutrophil/lymphocyte ratio has a prognostic value for patients with terminal cancer. World J Surg Oncol. 2016;14(148) doi: 10.1186/s12957-016-0904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi Y, Horio H, Hato T, Harada M, Matsutani N, Morita S, Kawamura M. Prognostic significance of preoperative neutrophil-lymphocyte ratios in patients with stage I non-small cell lung cancer after complete resection. Ann Surg Oncol 22 Suppl. 2015;3:S1324–S1331. doi: 10.1245/s10434-015-4735-5. [DOI] [PubMed] [Google Scholar]
- 43.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 44.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4(59) doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boustani J, Grapin M, Laurent PA, Apetoh L, Mirjolet C. The 6th R of radiobiology: Reactivation of anti-tumor immune response. Cancers (Basel) 2019;11(860) doi: 10.3390/cancers11060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, Mourtada F, Sims-Mourtada J. The impact of radiation on the tumor microenvironment: Effect of dose and fractionation schedules. Cancer Growth Metastasis. 2018;11(1179064418761639) doi: 10.1177/1179064418761639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.