Abstract
Background and Objectives
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the CNS. CD8+ T cells are prominently found at inflammatory sites. Recent advances in understanding checkpoint molecules, including programmed cell death 1 (PD-1), expressed on CD8+ T cells, highlight the immune regulatory roles of this T-cell subset; however, the role of CD8+ T cells in MS is unclear. Thus, we aimed to reveal the characteristics of PD-1–expressed (PD-1+) CD8+ T cells in MS.
Methods
We performed a cohort, case-control study for phenotyping analysis of PD-1+CD8+ T cells in disease remission and flare states using CSF and peripheral blood samples of 45 patients with MS or clinically isolated syndrome and 12 healthy subjects. We further analyzed the transcriptome of sorted PD-1+CD8+ T cells obtained from interferon (IFN)-β–treated patients and validated their regulatory machinery using in vitro cell culture assays with lentiviral gene transfer.
Results
In the disease remission state, PD-1+CD8+ T cells were decreased in the peripheral blood of patients with MS and resolved in patients treated with IFN-β treatment who showed immune regulatory cytokine interleukin (IL)-10 expression. In the disease flare state, we found that PD-1+CD8+ T cells were enriched in the CSF, which predicted a good response to subsequent IV steroid therapy. Transcriptome analysis of sorted PD-1+CD8+ T cells revealed the transcription factor c-Maf as a potential major regulator of the gene module, including multiple coinhibitory molecules. Furthermore, c-Maf expressed in CD8+ T cells induced PD-1 expression and production of IL-10 as well as suppressed alloactivated CD4+ T-cell survival.
Discussion
This study uncovered a favorable role of PD-1+CD8+ T cells against MS and demonstrated that c-Maf–driven IL-10 is an immune regulatory machinery.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the CNS. An imbalance between autoimmunity and immune tolerance, particularly in T cells, is considered to play a key role in the pathogenesis of MS. CD8+ T cells are predominantly found in MS brain demyelinating lesions compared with CD4+ T cells, and their numbers correlate with axonal damage.1 Although researchers have focused on CD4+ T cells, which play pathogenic and regulatory roles in MS, recent studies have highlighted the regulatory role of CD8+ T cells in MS.2 Although the impairment of CD8+ T-cell tolerance is thought to play a crucial role in disease outcomes, the underlying mechanisms, such as the regulation of coinhibitory molecules, are unclear. According to recent studies on other autoimmune diseases or cancer immunology, the T-cell exhaustion signature, characterized by high levels of coinhibitory molecule expression, is found in T-cell tolerance and correlates with a favorable prognosis in autoimmune diseases.3,4
Programmed cell death 1 (PD-1) is expressed during T-cell activation as a coinhibitory molecule and decelerates excessive differentiation, proliferation, and cytokine production.5 Sequence variants in the programmed cell death protein 1 (PDCD1) locus are associated with disease progression in patients with MS.6 Cancer immunotherapy using anti–PD-1 blockade causes CNS demyelinating diseases.7 In experimental autoimmune encephalomyelitis (EAE) mice, a representative murine model of MS, PD-1, and its ligand PD-1 ligand 1 (PD-L1) are expressed on CNS-infiltrating cells, endothelial cells, and microglia.8,9 Furthermore, PD-1– and PD-L1–deficient mice are more susceptible to EAE induction than wild-type control mice.10 However, previous studies analyzing the role of the PD-1/PD-L1 axis in patients with MS have reported controversial results. Moreover, the PD-1/PD-L1 axis may not be associated with regulatory functions in CD4+ T cells.11 In a rodent model of PD-1, an interferon (IFN)-stimulated response element is present in the promoter region of PDCD1, and stimulation of type I IFN increases the expression of PD-1.12,13 IFN-β is a type I IFN that is known to facilitate an antiviral immune response. It is also involved in immune modulation in autoimmune conditions and is used as a classical disease-modifying therapy in MS.14 Recently, type I IFN has been identified as a key modulator of chronic neuroinflammation in neurodegenerative contexts, such as Alzheimer disease15 However, the impact of type I IFN on immune modulators in humans, particularly on tissue-oriented CD8+ T cells, has not been well described. In this study, we analyzed IFN-β–driven PD-1–expressing (PD-1+) CD8+ T cells in patients with MS and their immune regulatory functions.
Methods
Subjects and Specimens
We recruited 45 consecutive patients with MS or clinically isolated syndrome (CIS) who visited Kobe University Hospital from 2016 to 2020 as well as 12 age- and sex-matched healthy subjects (HSs). All patients with MS fulfilled the revised McDonald criteria.16 Patients who experienced a first episode of neurologic symptoms that lasts at least 24 hours and is caused by inflammation or demyelination were considered to have CIS. For patients with disease flare, peripheral blood and CSF samples were collected before treatment, and the patients were also assessed for their response to initial high-dose IV methylprednisolone (IVMP) therapy (1 g/d for 3 days for 1–3 times) based on the exacerbation of the Expanded Disability Status Scale (EDSS),17 a physical disability scale of MS, over the original EDSS score (responder, exacerbation of the EDSS = 0; nonresponder, exacerbation of the EDSS ≥0.5) at discharge. Length of hospitalization (days) was assessed for correlation with the proportion of PD-1+CD8+ T cells in the CSF.
Flow Cytometry
Peripheral blood mononuclear cells (PBMCs) were separated by density-gradient centrifugation using Ficoll-Paque PLUS density-gradient media (GE Healthcare, Uppsala, Sweden). PBMCs or CSF specimens were stained with anti-human CD4 PE/Cy7, CD8a-FITC, CD45RA-PerCP/Cy5.5, CD27-Brilliant Violet, TIGIT PE, PD-1 APC antibodies (BioLegend, San Diego, CA), and CD3− PerCP/Cy5.5 antibodies (BD Biosciences, Franklin Lakes, NJ) for flow cytometry. Data were obtained using fluorescence-activated cell sorting (FACS) Verse LSRFortessa X-20 or FACS Aria III (BD Biosciences). Dead cells were excluded using Fixable Viability Dye eFluor 506 (Thermo Fisher Scientific, Waltham, MA). Negative expression was defined as fluorescence minus one control (eFigure 1, links.lww.com/NXI/A710).
Culture Assays
For in vitro IFN-β stimulation, 8 × 104 PBMCs from HSs were cultured and stimulated with CD3/CD28 beads (Dynabeads Human T-Activator CD3/CD28; Thermo Fisher Scientific) in 96-well U-bottom plates for 4 days with or without IFN-β (R&D Systems, Minneapolis, MN). For lentiviral transfection, short and long forms of Maf (musculoaponeurotic fibrosarcoma) isoform complementary DNA were introduced into lentiviral vectors and transfected into primary human CD8+ T cells along with CD3/CD28 bead stimulation for 4 days and analyzed for either gene expression using quantitative real-time PCR (qRT-PCR) or protein expression using FACS. They were further cultured in replaced fresh medium for 3 days, and protein expression was analyzed in cultured supernatants (multiplex bead-based ELISA assay [LEGENDplex, BioLegend]). The details are discussed in the eMethods (links.lww.com/NXI/A710).
For the mixed lymphocyte reaction (MLR), 5 × 105 PBMCs from 2 different individuals were mixed in a ratio of 1:1 with culture supernatants from the lentivirus transfection experiments described above. PBMCs from one individual were labeled with Cell Trace® (Thermo Fisher Scientific) to distinguish cocultured PBMCs from another individual. The cells were cultured for 5 days with either 5 µg/mL of anti-human interleukin (IL)-10 receptor α (anti-IL-10R) antibody (MAB274; R & D systems) or mouse immunoglobulin G1 isotype control (MAB002; R & D systems) and analyzed by flow cytometry to determine the surface phenotype of cell viability (propidium iodide staining).
Quantitative RT-PCR and Microarray
Sorted PD-1+CD8+ T cells and PD-1–negative CD8+ T cells (PD-1–CD8+ T cells) from patients with MS treated with IFN-β using FACS Aria III were analyzed for global gene expression using Clariom S Arrays (Thermo Fisher Scientific). In addition, the gene expression of sorted cells was also determined by qRT-PCR using the Applied Biosystems 7900 HT Fast Real-Time PCR System (Thermo Fisher Scientific) and primers (eTable 1, links.lww.com/NXI/A710).
Data Analysis
Flow cytometry data were analyzed using FlowJo software ver. 10 (BD Biosciences). Statistical analyses were performed using Prism software (GraphPad Software). In addition, the 2-sided t test, 2-sided Mann-Whitney U test, one-way analysis of variance (ANOVA) post hoc test (Tukey multiple comparison test), or repeated-measures ANOVA post hoc test (Tukey multiple comparison test) were used as appropriate.
For microarray experiments, individual raw data. CEL files were normalized and transformed to log space by considering log2 (intensity) using the Genomics Suite software (MOLSIS Inc, Tokyo, Japan). Differentially expressed (DE) genes were annotated as genes with false discovery rate–corrected ANOVA (<0.05) between PD-1+CD8+ and PD-1−CD8+ T cells. PD-1+CD8+ and PD-1−CD8+ cell data from HSs18 (GSE26495) were downloaded and subjected to robust multiarray average normalization. Gene set enrichment analysis of DE genes upregulated in PD-1+CD8+ T cells was performed using the Gene Ontology resource website (geneontology.org).19 c-Maf chromatin immunoprecipitation sequencing (ChIP-seq) data20 (GSE101389) were downloaded, and each peak was assigned to DE genes located within 185 kilobases, a reported median size of genomic interaction domains.21
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the ethics committee of Kobe University Hospital (No. 1381), and signed informed consent was obtained from all participants.
Data Availability
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Proportion of PD-1+CD8+ T Cells Increased in IFN-β–Treated Patients With MS and Exhibited an Immunoregulatory Gene Profile
To analyze the function of PD-1+CD8+ T cells in the disease remission state, we compared patients who were not treated (no treatment group) and patients treated with IFN-β. The proportion of PD-1+CD8+ T cells was significantly increased in the IFN-β treatment group compared with that in the no treatment group and recovered to the levels of HSs (Figure 1A), indicating that IFN-β, as a disease-modifying treatment for MS, induced PD-1 expression on CD8+ T cells, which is dysregulated in MS without treatment. We further analyzed the gene expression of PD-1+CD8+ T cells in patients with MS treated with IFN-β. We found that the expression of the immune regulatory cytokine IL-10 was increased in PD-1+CD8+ T cells compared with that in PD-1− CD8+ T cells, whereas the expression of other effector molecules of CD8+ T cells, such as IFN-γ, tumor necrosis factor-α, and granzyme B, did not differ between the groups (eFigure 2, links.lww.com/NXI/A710). Next, to show a direct link between IFN-β treatment and PD-1 expression, we stimulated CD8+ T cells from HSs with IFN-β in vitro and confirmed that the level of PD-1 expression was consistently higher in the IFN-β treatment group than in the no treatment group (Figure 1B).
Figure 1. Type 1 IFN-Induced PD-1 Expression on CD8+ T Cells From Patients With MS and the Immune Regulatory Cytokine IL-10.
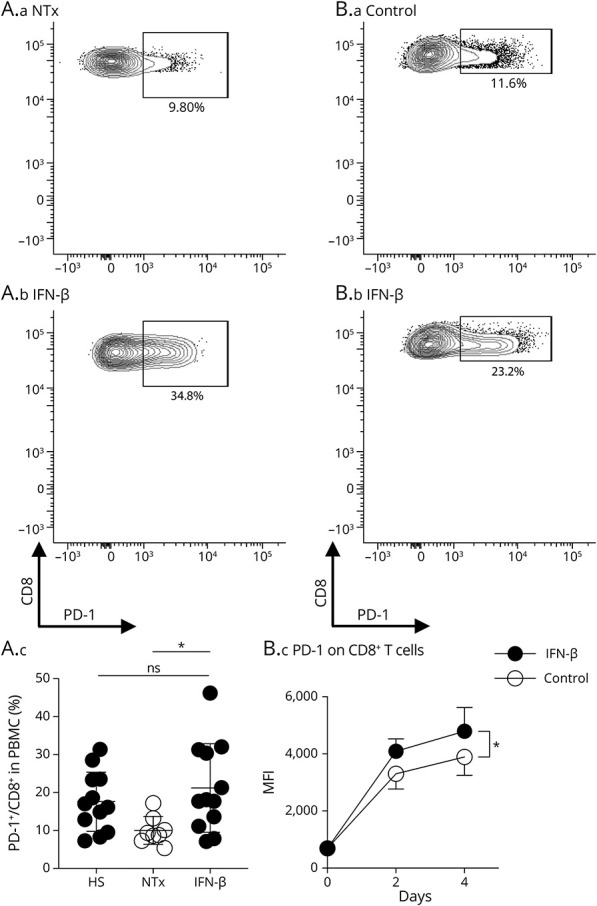
(A) Representative flow cytometry data and summary of the proportions of PD-1 expression on CD8+ T cells from patients with MS (A.a, NTx and A.b, IFN-β). The proportion of PD-1–expressing cells was evaluated on whole CD8+ T cells in PBMCs were obtained from HSs (n = 12), patients with MS in remission receiving NTx (n = 8) (A.c), and patients with MS treated with IFN-β (n = 12). Values are expressed as mean ± SD; *p < 0.05, 2-sided Mann-Whitney U test. (B) Representative flow cytometry data and summary of PD-1 expression on CD8+ T cells from HSs stimulated by anti-CD3 and anti-CD28 beads (B.a) or with IFN-β (B.b) for 4 days. MFI was calculated based on all CD8+T cells (n = 4) (B.c). Values represent as mean ± SD; *p < 0.05, one-way ANOVA followed by the Tukey multiple comparison test. ANOVA = analysis of variance; HS = healthy subject; IFN = interferon; IL = interleukin; MFI = mean fluorescence intensity; MS = multiple sclerosis; NTx = no treatment group; PBMC = peripheral blood mononuclear cell; PD-1 = programmed cell death 1.
Prominent PD-1+CD8+ T Cells in the CSF Correlated With Responsiveness to Corticosteroid Therapy in the Disease Flare State
In MS, the memory T-cell population contains autoreactive T cells that play a crucial role in defining the imbalance between autoimmunity and immune tolerance.22 We analyzed the peripheral blood and CSF distribution of CD8+ T-cell subpopulations in patients with MS and CIS. The clinical profiles of the patients and HSs are presented in table 1. There were no significant differences among the groups in terms of age, disease duration, or EDSS scores. In contrast, we found that PD-1 was mainly expressed in the memory cell population of CD8+ T cells and was less expressed in naive cells (CD27+CD45RA+) or terminally differentiated cells (CD27−CD45RA+) in line with the previous report23 (eFigure 3, links.lww.com/NXI/A710). We found a higher frequency of PD-1+CD8+ T cells in the patient group experiencing a disease flare, which was evident in the CSF specimens (Figure 2A). Of note, CD8+ T cells in the CSF were mainly those with a central memory phenotype (CD27+CD45RA−), and PD-1 expression was prominently observed in that subpopulation. Furthermore, the proportion of PD-1+CD8+ T cells in the CSF of IVMP treatment responders was higher than that in nonresponders; however, there was no difference in the proportion of cells in the peripheral blood between the groups, suggesting that the immune state of the CSF may predict the response to subsequent treatments (Figure 2B and C). We also analyzed PD-1 expression on CD8+ T cells in the CSF of noninflammatory neurologic diseases, such as idiopathic normal pressure hydrocephalus, which is approximately 50% (eFigure 4), suggesting that the high frequency of PD-1+CD8+ T cells in treatment responders may play active immune regulatory roles. Indeed, the proportion of PD-1+CD8+ T cells before treatment in patients experiencing a disease flare was correlated with short-term hospitalization (Figure 2D). In contrast, CSF parameters, such as cell number, total protein level, immunoglobulin G index, and myelin basic proteins, were not significantly different between responders and nonresponders (eTable 2, links.lww.com/NXI/A710).
Table 1.
Clinical Profilesa
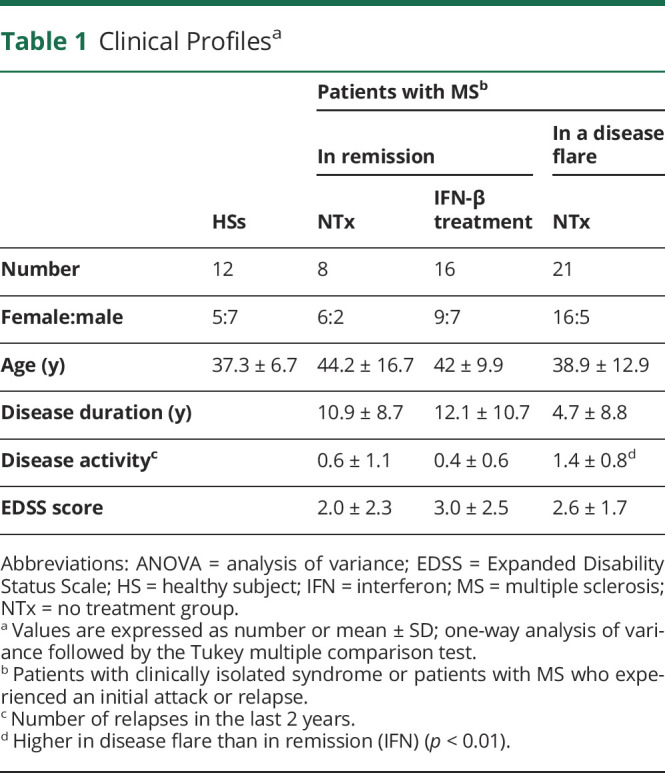
Figure 2. Prominent PD-1+CD8+ T Cells in the CSF From Patients With Disease Flare Correlates With Responsiveness to Corticosteroid Therapy.
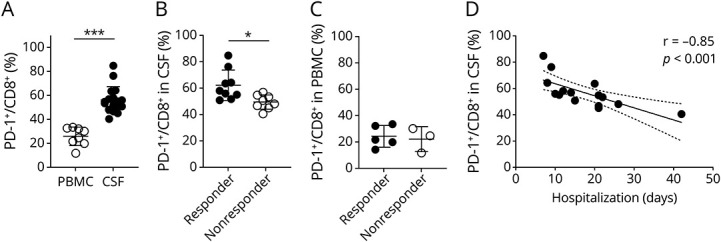
(A) The proportion of PD-1 expression on CD8+ T cells in PBMCs (n = 8) and CSF (n = 17) from patients with relapsed MS or CIS. Values are expressed as mean ± SD; ***p < 0.001, 2-sided unpaired t test. (B and C) Summary plot of (B) CSF data of responders and nonresponders (n = 9 and n = 8, respectively) and (C) PBMC data of responders (n = 5) and nonresponders (n = 3). Values are expressed as mean ± SD; *p < 0.05, 2-sided unpaired t test. (D) Scatter plot and Spearman correlation coefficient (r) of the proportion of PD-1+CD8+ T cells in the CSF and hospitalization period (days) were plotted (n = 15; hospitalized patients in disease flare); simple linear regression with 95% CI is shown. CIS = clinically isolated syndrome; MS = multiple sclerosis; PBMC = peripheral blood mononuclear cell; PD-1 = programmed cell death 1.
IFN-β–Driven PD-1+CD8+ T Cells Harbored Immune Regulatory Phenotype
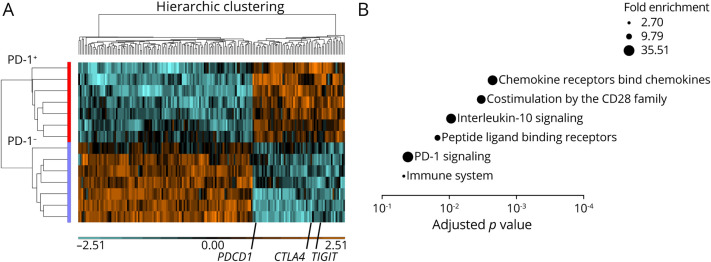
Because our data suggested that the proportion of PD-1+CD8+ T cells correlated with IFN-β treatment and favorable outcomes in the disease flare state, we used an unbiased approach to detect differentially coexpressed genes in PD-1+CD8+ T-cell populations from these patients. We detected 170 DE genes between PD-1+ and PD-1−CD8+ T cells (Figure 3A, eTable 3, links.lww.com/NXI/A710). The upregulated DE genes included multiple coinhibitory molecule genes, such as CTLA4, TIGIT, and PDCD1. Of interest, CCR7 and SELL were both downregulated in PD-1+ cells, whereas differential markers PTPRC and CD27 were not found in the differential gene list, suggesting that differential genes coexpressed with PD-1 are distinct gene sets from differentiation subsets. We further assessed the specificity of these DE genes using data sets of PD-1+ and PD-1−CD8+ T cells from HSs18 and identified 14 genes, including PDCD1 (PD-1), as upregulated genes in PD-1+CD8+ T cells in HSs. Among the DE genes, 45 were specifically upregulated in PD-1+CD8+ T cells in patients with MS treated with IFN-β (eTable 4). Gene set enrichment analysis of these DE genes showed that chemokine receptors bind chemokines, costimulation by the CD28 family, and IL-10 signaling as the top-scored gene sets (Figure 3B). These results suggest that PD-1+CD8+ T cells are activated cells with the potential to migrate to inflammatory sites with a 2-facet role, facilitation and regulation, in inflammation.
Figure 3. PD-1+CD8+ T Cells From Patients Treated With IFN-β Harbor an Immune Regulatory Phenotype.
(A) Heatmap showing 170 DE genes between PD-1+ and PD-1– CD8+ T cells in the peripheral blood from patients with MS in remission treated with IFN-β. Upregulated DE genes in PD-1+CD8+ T cells include PDCD1, CTLA4, and TIGIT. Adjusted p < 0.05 with FDR correction. (B) Gene enrichment analysis of upregulated DE genes specific for patients with MS treated with IFN-β. Analysis was performed using the PANTHER overrepresentation test (Reactome version 65). The FDR-adjusted p values are plotted as bubbles such that the sizes indicate fold enrichment. DE = differentially expressed; FDR = false discovery rate; IFN = interferon; MS = multiple sclerosis; PD-1 = programmed cell death 1.
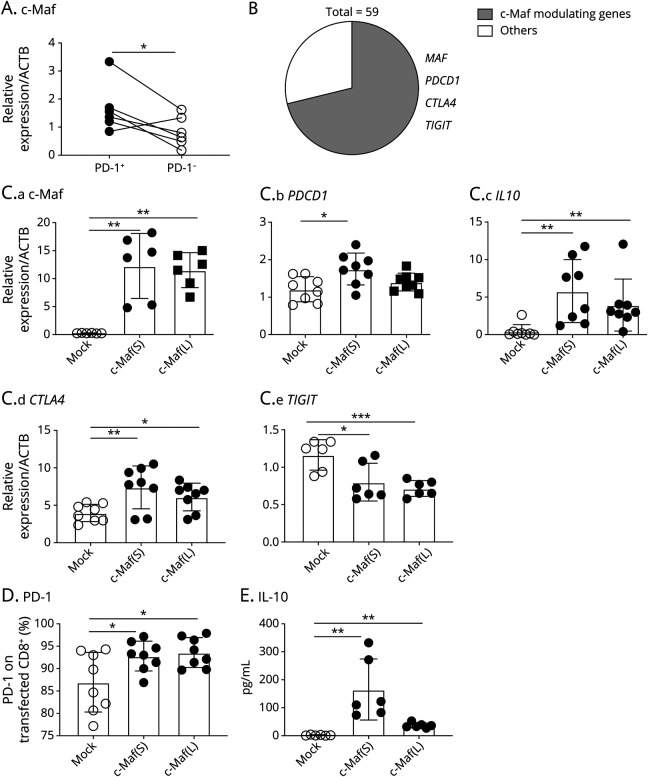
c-Maf Regulated a Majority of Regulatory Gene Modules in PD-1+CD8+ T Cells
To elucidate the deeper immune regulatory machinery within PD-1+CD8+ T cells, we analyzed the molecular mechanisms regulating the identified IFN-β–driven genes coexpressed with PD-1+ in CD8+ T cells in MS. To this end, we used a previously identified coinhibitory gene module found in multiple T-cell impairment states4 and identified several overlapping genes, including the transcription factor c-Maf (Figure 4A). We further used ChIP-seq data20 of human T cells immunoprecipitated by c-Maf and showed that approximately 70% (43 of 59) of upregulated DE gene loci showed potential genomic interaction with c-Maf, including c-Maf MAF (Figure 4B, eFigure 5, links.lww.com/NXI/A710). These data suggest that c-Maf in PD-1+CD8+ T cells could be a key regulator of immune regulation.
Figure 4. c-Maf Regulates IFN-β–Driven Immune Regulatory Phenotype in PD-1+CD8+ T Cells.
(A) Differences in c-Maf messenger RNA expression between PD1+CD8+ T cells and PD1−CD8+ T cells were analyzed by qRT-PCR. *p < 0.05, 2-sided paired t test. (B) Frequency of DE genes with potential c-Maf binding loci was assessed based on human c-Maf ChIP-seq data. (C) c-Maf was induced by lentiviral transfection in primary human CD8+ T cells from HSs that induced the expression of multiple coinhibitory molecules and IL-10 compared with the control vector (Mock) (n = 8 from 4 different individuals). Values are expressed as mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001, 2-sided t test or Mann-Whitney U test appropriately. Because c-Maf has 2 alternative transcriptional variants (short and long forms), we overexpressed either the short or long form of c-Maf in CD8+ T cells from HSs, c-Maf (S), or c-Maf (L), respectively. (D) PD-1 expression on c-Maf–expressing CD8+ T cells stimulated by anti-CD3 and anti-CD28 beads for 4 days. Summary plots are shown (n = 8 from 4 different individuals). Values are expressed as mean ± SD; *p < 0.05, 2-sided t-test. (E) After T-cell receptor stimulation, CD8+ T cells were further incubated with IL-2 for 3 days. Cultured supernatants were analyzed for the production of IL-10 (n = 6 from 3 different individuals). Values are expressed as mean ± SD; **p < 0.01, Mann-Whitney U test. DE = differentially expressed; HS = healthy subject; IFN = interferon; IL = interleukin; PD-1 = programmed cell death 1; qRT-PCR = quantitative real-time PCR.
Because c-Maf could be a potential key regulator of the regulatory function of PD-1+CD8+ T cells, we performed additional experiments to elucidate the functional role of c-Maf in CD8+ T cells. We performed lentiviral transfection, which allows primary human CD8+ T cells to express c-Maf, and found that c-Maf upregulated the expression of PDCD1 and CTLA4, but not that of TIGIT (Figure 4C). In line with this, c-Maf drove PD-1 expression on CD8+ T cells (Figure 4D). c-Maf induced immune regulatory cytokine IL-10 expression in CD8+ T cells, which was also compatible with the culture supernatants (Figure 4E).
c-Maf–Driven Humoral Factors of CD8+ T Cells Induced CD4+ T-Cell Death in Generalized Alloactivation of T Cells
Finally, we assessed the immune regulatory role of c-Maf in CD8+ T cells. To this end, we used an MLR and analyzed the impact of cultured supernatants from c-Maf–expressing CD8+ T cells on T-cell survival in an allogeneic MLR. Cultured supernatants from c-Maf–expressing CD8+ T cells showed significantly higher CD4+ T cell death than the control supernatant (Figure 5A). Furthermore, this difference in CD4+ T-cell death was not observed under IL-10R signal blockade (Figure 5B and C), suggesting that IL-10 from c-Maf–expressing CD8+ T cells facilitates activated CD4+ T-cell death.
Figure 5. IL-10 From c-Maf–Expressing CD8+ T Cells Induces T-Cell Death in Mixed Lymphocyte Reaction.
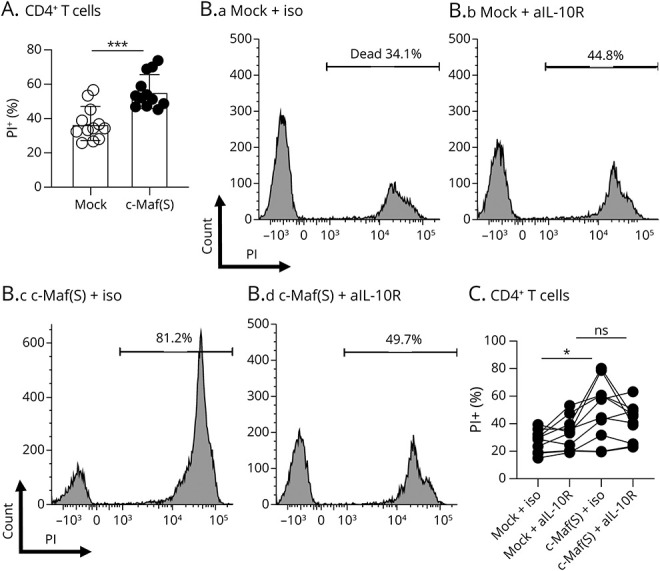
(A) PBMCs from 2 individuals were cocultured to induce MLR using cultured supernatants from CD8+ T cells with lentiviral c-Maf transfection or its control supernatants. The frequency of live PI– CD4+ T cells after culture was compared (N = 12 from 4 different individuals). Values are expressed as mean ± SD; ***p < 0.001, 2-sided t test. (B and C) MLR using the cultured supernatant of c-Maf or its control transfected CD8+ T cells treated with anti-IL-10R antibody (5 µg/mL) or an isotype. Representative FACS data (B) and summary plots (C) are shown. Values are connected for each individual (n = 10). *p < 0.05, repeated-measures ANOVA followed by the Tukey multiple comparison test. ANOVA = analysis of variance; FACS = fluorescence-activated cell sorting; IL = interleukin; PBMC = peripheral blood mononuclear cell; MLR = mixed lymphocyte reaction.
Discussion
PD-1 has been suggested to not only be involved in persistent chronic infectious diseases or the cancer microenvironment but to also play an inhibitory role in long-term autoimmune disease pathology.3 In a previous study, the PD-1 expression in PBMCs from patients with MS was lower than that in PBMCs from healthy controls.24 In this study, we further extended the results by focusing on PD-1 expression on CD8+ T cells. We found that PD-1 expression was induced by IFN-β stimulation. During the acute phase of the disease, the proportion of PD-1+CD8+ T cells in the CSF was correlated with a good response to subsequent steroid therapy. These observations highlight the heterogeneous levels of inflammation, including the differences in intrinsic IFN-β–driving genes in each disease flare, which lead to different levels of PD-1 expression in CD8+ T cells.
We further annotated MAF as a key immune regulator gene coexpressed with PD-1. Furthermore, among the DE genes upregulated in PD-1+CD8+ T cells, c-Maf potentially interacted with known coinhibitory molecule genes, such as PDCD1, CTLA4, and TIGIT. c-Maf modulates the expression of not only PD-1 but also other coinhibitory molecules, such as Tim-3, and contributes to immune regulation in both cancer and autoimmunity.4,25 Of interest, we did not observe the coexpression of Tim-3 and PD-1 in this study that has been observed in brain lesion sites of MS.26 Because IFN-β suppresses the relapse of MS but does not prevent the progressive phase of the disease,27 another mutual assistance factor may be required to obtain sufficient immune regulation of CD8+ T cells.
Importantly, c-Maf induced the expression of PD-1 and the immune regulatory cytokine IL-10 in CD8+ T cells. Generally, CD8+ T cells play effector roles as killer T cells; however, c-Maf–expressing PD-1+CD8+ T cells may be commonly used to evaluate treatment responsiveness in stable disease states that harbor immune regulatory machinery. As a significant but not exclusive such mechanism, they induce cell death in activated pathogenic T cells via IL-10R signaling. Furthermore, because c-Maf has also recently been reported in human CD4+ T cells as a key regulator of not only immunoregulation but also tissue residency of the cells,20 further long-term and in-depth analysis could reveal the role of c-Maf in MS pathophysiology not only in the acute phase but also in the chronic progressive phase.
Our study has limitations related to the sample size and further prospective cohort studies with a larger sample size are needed. Another limitation was sample availability. Although we could obtain samples from untreated patients with MS at the early stage of the study, there have been no patients without treatment because we start disease-modifying therapy immediately after we diagnosed MS; thus, we performed in vitro experiments using samples from HSs. Compared with that, the coexpressed genes with PD-1 in CD8+ T cells that we found in patients with MS treated with IFN-β could reflect not only the therapeutic efficacy but also MS-specific phenotypes that require further investigations. Nevertheless, the statistically confirmed phenotypical and experimental results not only validated the role of PD-1 in CD8+ T cells in MS but also revealed a regulatory gene module within the PD-1+CD8+ T cells that was potentially useful for predicting treatment responsiveness and treatment targets.
It appears that overcoming the impairment of immune regulatory function may be a more appropriate strategy to improve the efficacy of individualized therapies for MS. Given the potential immunoregulators with a clinical aspect for predicting responsiveness to treatment, we found that PD-1+CD8+ T cells infiltrated into the CSF correlated with subsequent treatment response. Moreover, we found that type I IFN-driven genes were coexpressed in PD-1+CD8+ T cells, including multiple coinhibitory receptors, and IL-10 was predominantly regulated by the transcription factor c-Maf. These findings highlight the role of PD-1+CD8+ T cells and their c-Maf–driven gene sets in the treatment of MS, thus providing novel indicators for appropriate therapeutic decision making in MS cases.
Acknowledgment
The authors thank Prof. Kazuhiro Kobayashi for technical assistance in performing in the vitro experiments. They also thank their colleagues in the Division of Neurology at Kobe University Graduate School of Medicine for their help with specimen collection.
Glossary
- ANOVA
analysis of variance
- CIS
clinically isolated syndrome
- DE
differentially expressed
- EAE
experimental autoimmune encephalomyelitis
- EDSS
Expanded Disability Status Scale
- FACS
fluorescence-activated cell sorting
- HS
healthy subject
- IFN
interferon
- IL
interleukin
- IVMP
IV methylprednisolone
- MLR
mixed lymphocyte reaction
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cell
- PD-L1
PD-1 ligand 1
- qRT-PCR
quantitative real-time PCR
Appendix. Authors
Contributor Information
Shusuke Koto, Email: skotoh@med.kobe-u.ac.jp.
Ritsu Akatani, Email: rakatani@med.kobe-u.ac.jp.
Hiroko Nakano, Email: nakano@med.kobe-u.ac.jp.
Atsushi Hara, Email: ahara@med.kobe-u.ac.jp.
Kenji Sekiguchi, Email: sekiguch@med.kobe-u.ac.jp.
Riki Matsumoto, Email: matsumot@med.kobe-u.ac.jp.
Tatsushi Toda, Email: toda@m.u-tokyo.ac.jp.
Study Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (20H03562), Practical Research Project for Rare/Intractable Diseases by AMED (20ek0109436h0001, 21ek0109436h0002), and a Research Grant from the Uehara Memorial Foundation.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545-558. [DOI] [PubMed] [Google Scholar]
- 2.Saligrama N, Zhao F, Sikora MJ, et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 2019;572(7770):481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015;523(7562):612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chihara N, Madi A, Kondo T, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018;558(7710):454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192(7):1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroner A, Mehling M, Hemmer B, et al. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol 2005;58(1):50-57. [DOI] [PubMed] [Google Scholar]
- 7.Maurice C, Schneider R, Kiehl TR, et al. Subacute CNS demyelination after treatment with nivolumab for melanoma. Cancer Immunol Res 2015;3(12):1299-1302. [DOI] [PubMed] [Google Scholar]
- 8.Salama AD, Chitnis T, Imitola J, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med 2003;198(1):71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnus T, Schreiner B, Korn T, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci 2005;25(10):2537-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter LL, Leach MW, Azoitei ML, et al. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol 2007;182(1-2):124-134. [DOI] [PubMed] [Google Scholar]
- 11.Saresella M, Marventano I, Longhi R, et al. CD4+CD25+FoxP3+PD1- regulatory T cells in acute and stable relapsing-remitting multiple sclerosis and their modulation by therapy. FASEB J 2008;22(10):3500-3508. [DOI] [PubMed] [Google Scholar]
- 12.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996;8(5):765-772. [DOI] [PubMed] [Google Scholar]
- 13.Terawaki S, Chikuma S, Shibayama S, et al. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol 2011;186(5):2772-2779. [DOI] [PubMed] [Google Scholar]
- 14.Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 1993;43(4):662-667. [DOI] [PubMed] [Google Scholar]
- 15.Roy ER, Wang B, Wan YW, et al. Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J Clin Invest 2020;130(4):1912-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444-1452. [DOI] [PubMed] [Google Scholar]
- 18.Duraiswamy J, Ibegbu CC, Masopust D, et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol 2011;186(7):4200-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mi H, Muruganujan A, Huang X, et al. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat Protoc 2019;14(3):703-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aschenbrenner D, Foglierini M, Jarrossay D, et al. An immunoregulatory and tissue-residency program modulated by c-MAF in human TH17 cells. Nat Immunol 2018;19(10):1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao SS, Huntley MH, Durand NC, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014;159(7):1665-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford MP, Yan SX, Ortega SB, et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood 2004;103(11):4222-4231. [DOI] [PubMed] [Google Scholar]
- 23.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 1997;186(9):1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javan MR, Aslani S, Zamani MR, et al. Downregulation of immunosuppressive molecules, PD-1 and PD-L1 but not PD-L2, in the patients with multiple sclerosis. Iran J Allergy Asthma Immunol 2016;15(4):296-302. [PubMed] [Google Scholar]
- 25.Apetoh L, Quintana FJ, Pot C, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol 2010;11(9):854-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Nierop GP, van Luijn MM, Michels SS, et al. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol 2017;134(3):383-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Mantia L, Vacchi L, Di Pietrantonj C, et al. Interferon beta for secondary progressive multiple sclerosis. Cochrane Database Syst Rev 2012;1:CD005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.