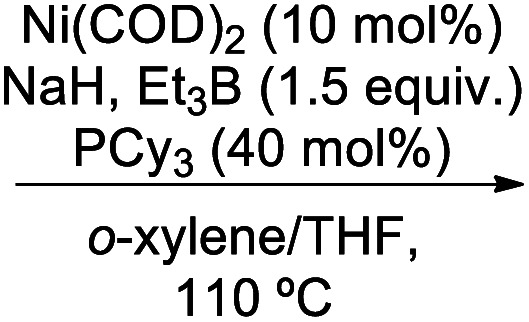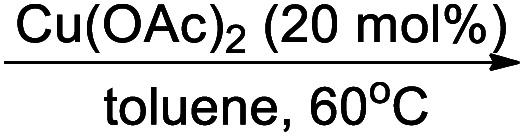Abstract
Bakuchiol is an emblematic meroterpene class of natural product extracted from Psoralea corylifolia. It has been reported to possess a broad range of biological and pharmacological properties and is considered as a leading biomolecule. It is highly desirable to devise an efficient approach to access bakuchiol and its chemical biology applications. In this review we provided structural features, isolation methods, various chemical routes and late-stage functionalization (LSF) approaches for bakuchiol and its derivatives. Moreover, this review encompasses the structure–activity relationships (SAR), value-added contributions and future perspectives of bakuchiol
The isolation methods, various chemical routes and late-stage functionalization approaches and structure–activity relationships of bakuchiol – a meroterpene class of natural product has been discussed in detail.
1. Introduction
Investigation of natural products plays a vital role in the field of drug research and development as a source of new effective life saving drugs.1 In general, the natural product research covers isolation, chemical synthesis and their biological properties. These studies are mutually beneficial and are often carried out independently.2 Bakuchiol is an meroterpenoid class of natural product (Fig. 1). Since its discovery and isolation in 1966,3 bakuchiol and its chemically modified analogues have been recognised to facilitate a multitude of biological properties like anticancer,4 antioxidant,5 estrogenic,6 antimicrobial,7 liver protective,8 human carboxylesterase 2 (hCE2) inhibitory,9 immune-suppressive activities10etc. Moreover, this scaffold is generating lot of interest in the skincare world because of its retinol like functionality.11 Bakuchiol is found to have no or little side effects when compared to retinoids, thus replacing the latter from the place of top anti-ageing molecule.12 This evidence suggests that bakuchiol has potential for further development, application and research.
Fig. 1. Structure of (+)-bakuchiol.
Like every natural products, the lack of suitable isolation and purification methods, low concentration in natural sources are increasing their demand and restricts the usage. Thus, organic chemists are now involved in the development of new synthetic routes for the production of bakuchiol and their analogues. The construction of its carbon tetra alkylated stereocenter is the key step in the total synthesis.13 This goal have been achieved through several strategies. This review gives an insight into the isolation, various synthetic routes and functionalization approaches in the production of bakuchiol and their analogues. Overall, this review throws light on the structure–activity relationship, and the important applications of bakuchiol to science and society foreseeing the future prospectives.
2. Occurrence and isolation
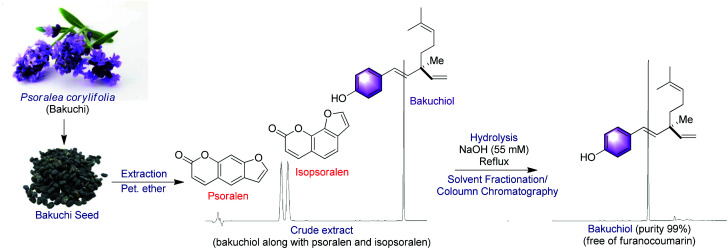
Bakuchiol (Syn. Chiba) was first extracted from the seeds of Psoralea corylifolia L. Medik (Bakuchi, now Cullen corylifolium L.) by a team of scientists at the Indian Institute of Chemical Technology (IICT). This plant belongs to Fabaceae family and it has long been a part of Indian and Chinese medicine.14 The other plant sources of bakuchiol includes Prosopis glandulosa,15aOtholobium pubescens,15bPimelea drupacea,15cUlmus davidiana,15dPiper longum,15eAerva sanguinolenta,15fFructus psoraleae,15gPsoralidium tenuiflorum,15hBridelia retusa,15iElaeagnus bockii,15jSpiraea formosana,15k and Nepeta angustifolia.15l Among these, P. corylifolia is currently the only natural source for obtaining bakuchiol on a larger scale.16 Bakuchiol is the most abundant compound in this species, which accounts for 6.24% weight of the dried seed.17 Other than bakuchiol, P. corylifolia serves as a source of several other meroterpenoids.18a–d
In general bakuchiol isolation was performed by cold or hot extraction with suitable solvents and purified through repeated column chromatography.19 There are number of factors which limits the use of bakuchiol due primarily to its low concentration in natural sources, as well as the co-existence of phototoxic agents such as psoralen, isopsoralen and other coumarin type compounds increasing the sensitivity of skin to ultra violet radiation and promotes skin cancer.20
However, Jia and Hong,21 developed improved method for the large scale isolation, purification and analysis of bakuchiol from P. corylifolia seeds. As illustrated by the following Scheme 1, the base hydrolysis opens up the lactone ring of the furanocoumarins (psoralen and isopsoralen), thereby converting them into the corresponding salts of carboxylic acids, which can then be easily separated from the remainder of the mixture by a water workup.22 The bakuchiol obtained in the organic layer appears as a dark blue spot under excitation at 254 nm and was obtained as a clear colourless oil. The extraction methods and techniques do play a major role in isolation of bakuchiol from P. corylifolia seeds. In solvent based extractions, bakuchiol percentage was found to be higher in non polar solvent like petroleum ether, suggesting that non polar solvents are best suitable one for the extraction of bakuchiol in large scale from the seeds of P. corylifolia.
Scheme 1. Method for isolation of natural bakuchiol.
The use of green solvents minimizes the risk during extraction and is safe to handle. Most organic solvents are flammable, volatile, and often toxic and are responsible for environmental pollution and the greenhouse effect. The green method of separation has gained remarkable interest among the scientific community in recent years. Extraction of bakuchiol with advanced methods was rarely explored. A study on ultrasonic assisted23 and supercritical CO2 extraction24 of bakuchiol reported highest yield when compared with other conventional methods. Further, many advanced extraction techniques (microwaves, ultrasonic, pressurized liquids, supercritical fluids, and electric fields etc.) showed many advantages,25 such as reduction in energy and solvent consumption, increased efficiency and selectivity, thereby meeting the increased market demands and eco-friendliness.
Therefore, more research efforts have to be taken for the development of effective green protocols to isolate bakuchiol from natural resources with high purity. The research areas such as (i) to improve and optimize existing processes (ii) to innovate in processes and procedures, and (iii) to find alternative solvents etc. has to be focused to develop an eco-friendly green and economically feasible protocol.
3. Biosynthesis
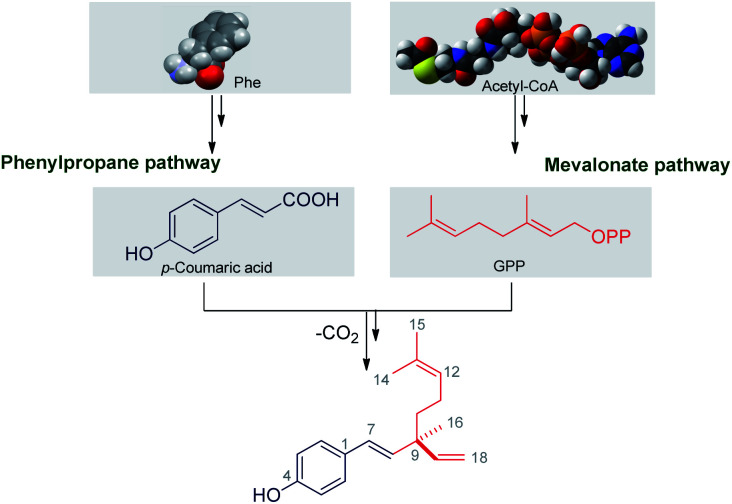
Bakuchiol has their own structural and chemical diversity, which is produced in nature from a mixed biosynthetic pathway involving combination of isoprene-based and amino acid based building blocks (Scheme 2). Looking to the structure, bakuchiol has aside chain atom, isoprenoid in nature and an aromatic ring, along with the two carbon side chains. Banerji and Chintalwar conducted experimental studies26a–d on two to three month-old mature plants of P. corylifolia using different labelled precursors. This systematic study showed that the aromatic ring of bakuchiol is derived from a phenylpropane pathway and monoterpene side chain is derived from mevalonate pathway (MVA). The distribution of labels in the isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) moieties are found to be equal. Leucine gets incorporated into bakuchiol with extensive randomisation.
Scheme 2. Biosynthesis of bakuchiol.
4. Structure–activity relationship (SAR)
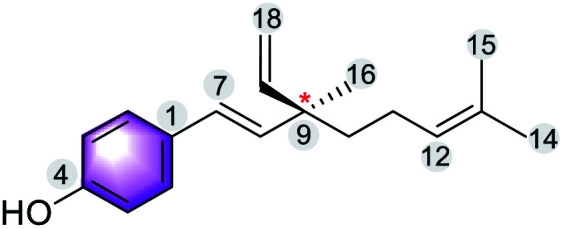
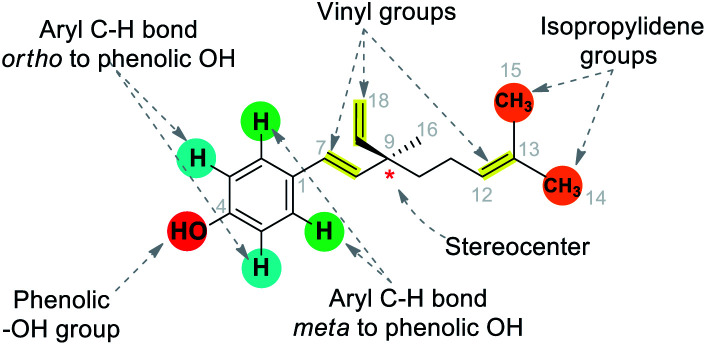
The multiple bioactivity of bakuchiol depends on its chemical entities. Structurally, bakuchiol (4-(3-ethenyl-3,7-dimethyl-1,6-octadienyl)-phenol) is partly phenolic and partly terpene (phenol unit in conjunction with terpene), hence it is a meroterpene combining those two structural elements in a single molecule. In other words, the structure consists of a single hydroxyl group on the aromatic ring and an unsaturated hydrocarbon chain (at para-position) with three olefinic linkages and one tetra-alkylated (all-carbon) quaternary stereocenter.3 One of the olefinic linkage (styryl) is conjugated with the aromatic ring and the absolute configuration (asymmetric stereocenter) is shown to possess (S)-chirality in its chemical structure.27 The long hydrophobic alkane chain of bakuchiol leads to its poor water solubility and druggability.28 Where as, the phenolic hydroxyl group of bakuchiol is able to combine with endogenous molecules (glucuronic acid and glycine) via covalent bonds, resulting in enhanced first-pass metabolism.29 The structural units and physicochemical properties (Lipinski's rule)30 of bakuchiol demonstrating its drug-likeness are illustrated in Table 1.
Chemical structure and physicochemical properties of (+)-(S)-bakuchiol.
| ||
|---|---|---|
| No. | Physicochemical properties | |
| 1 | 3D structure |
|
| 2 | Molecular mass | 256 Da |
| 3 | Molecular formula | C18H24O |
| 4 | Molecule class | Mono-terpenoid |
| 5 | H-bond acceptor | 1 |
| 6 | H-bond donor | 1 |
| 7 | Rotatable bond | 6 |
| 8 | Stereocenter | 1 |
| 9 | Molar refractivity | 84.10 |
| 10 | i log P | 3.54 |
| ||
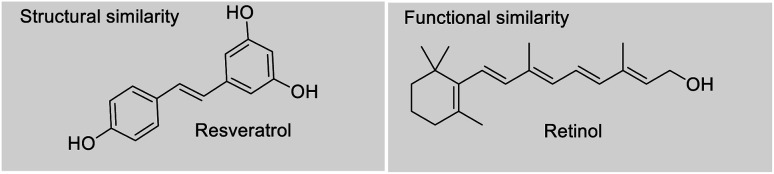
Depending upon the structural variation, more or less similar group of compounds shows divergent biological activities. There is a relationship between molecular structures and their biological activity, and this principle is referred to as structure activity relationship (SAR).31 The bioactivity of a bakuchiol molecule can often be predicted from its molecular entities (pharmacophores) using data about other similar molecules like resveratrol. Bakuchiol has structural similarities with resveratrol, both having a 4-hydroxystyryl moiety. Moreover, this styryl moiety linked pyrone, chromene and quinazoline derivatives reported potent biological properties.32 Therefore, the presence of similar pharmacophore in the bakuchiol moiety suggests that it may also show similar activities of resveratrol and related molecules.33
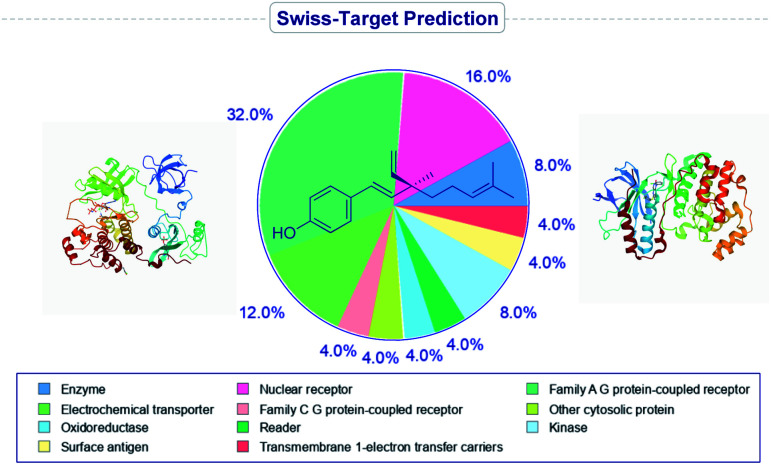
It has been found that the biological activity of bakuchiol depends significantly on the structural determinants such as (i) phenolic Csp2–OH group, (ii) vinyl groups (iii) stereoisomeric and (iv) terpenic chains. These structural features are essential in the interactions between substrate and its target protein/site. Swiss-Target Prediction34 provided a set of suggestions of potential molecular targets for bakuchiol (Fig. 2). There are limited reports on molecular docking of bakuchiol with different targets,35 and the details were described in Table 2. In addition, structural modification via late-stage functionalization (LSF) on Csp2–OH, phenolic C–H bonds, vinyl and isopropylidene groups of bakuchiol revealed their great potential and bioactivity (Table 3).41–46 We here described the structure–activity relationships of bakuchiol and its analogues.
Fig. 2. Predicted molecular targets of bakuchiol.
Molecular docking of bakuchiol with different targets.
| S. no. | Target | Structure | Hydrogen boning with residue | Hydrophobic bonding with residue | Reference |
|---|---|---|---|---|---|
| 1 | DNA polymerase (alpha) |
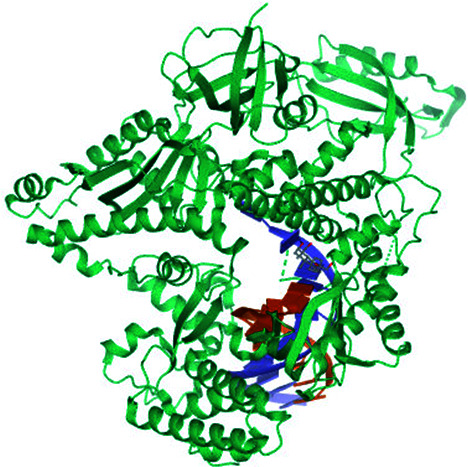
|
Asp1004 | DG111, DC11, Gly958, Tyr865 and Tyr957 | 35a |
| 2 | BthTX-II (2OQD) |
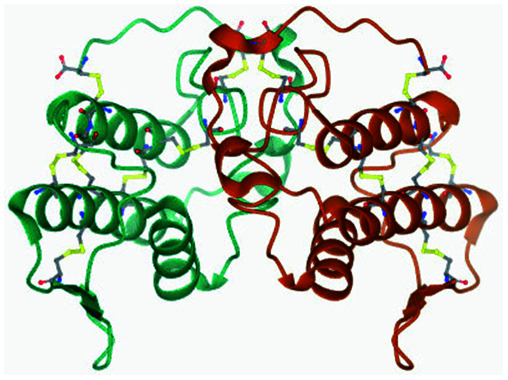
|
Asp48 and Cys44 | Gly6, Ala17, Ile18, Tyr21, Tyr27, Gly29, Trp30, and His47 | 35b |
| 3 | Transcriptional activator protein (LasR) |
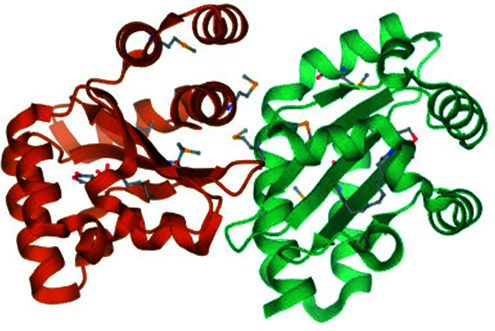
|
Asp73, Tyr56 and Tyr64 | Ala50, Ala 127, Ile52, Leu36, Leu125, Cys79, and Val76 | 30 |
| 4 | Estrogen receptor 1 alpha (Esr1) |
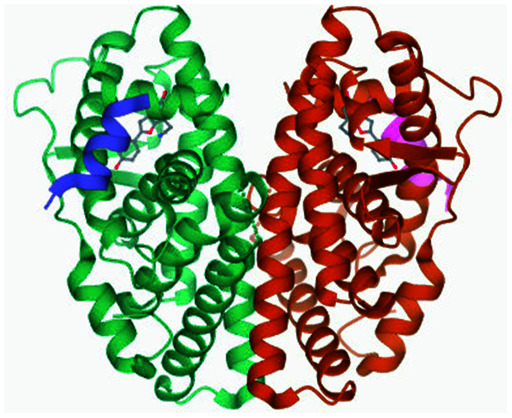
|
Glu353 and Arg394 | Leu391, Phe404, Leu387, Leu346, Met421, Ile424, Leu525 and Gly521 | 34 |
| 5 | Hematopoietic cell kinase (Hck) |
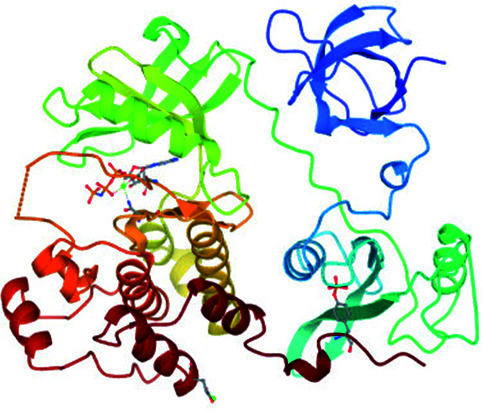
|
Met341 | Leu273, Phe278, Val281, Val323, Leu393 and Ala403 | 35c |
| 6 | Mitogen-activated protein kinasep (p38α) |
|
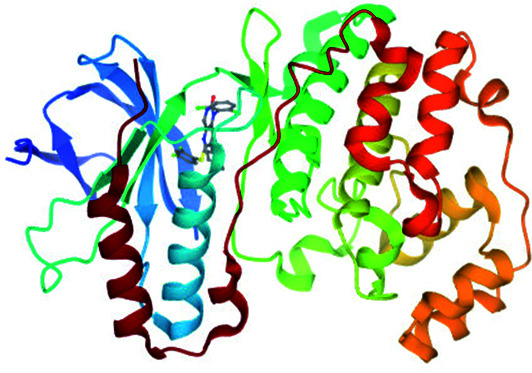
Met109 | Val30, Tyr35, Val38, Ala51, Leu75, Ile84, Leu167, and Leu171 | 35c |
| 7 | Tyrosinase inhibitors (2Y9X) |
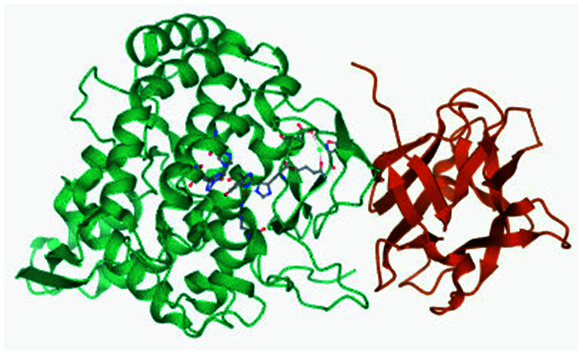
|
His60 | His84, His93, His243, His258, His294, His295, Phe291 and Phe263 | 35d |
Selected functionalized bakuchiol analogues and its functionalization sites and its up-regulated activity.
| S. no. | Selected modified analogues | Functionality and modified site | Up-regulated activity | Reference |
|---|---|---|---|---|
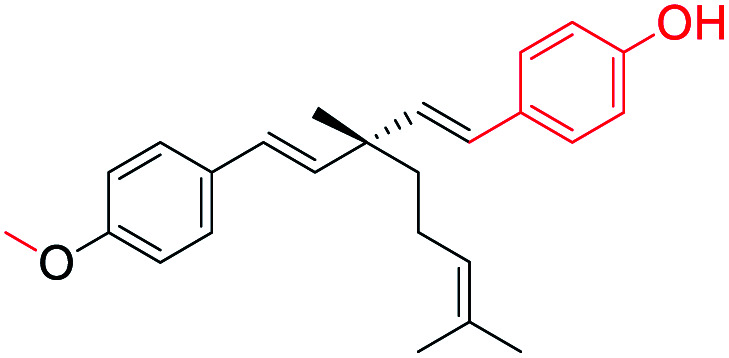
| 1 |
|
O-Methylation at phenolic –OH (site-I) and aryl group introduced at ethenyl group (site-III) | Anti-proliferative activity | 41 |
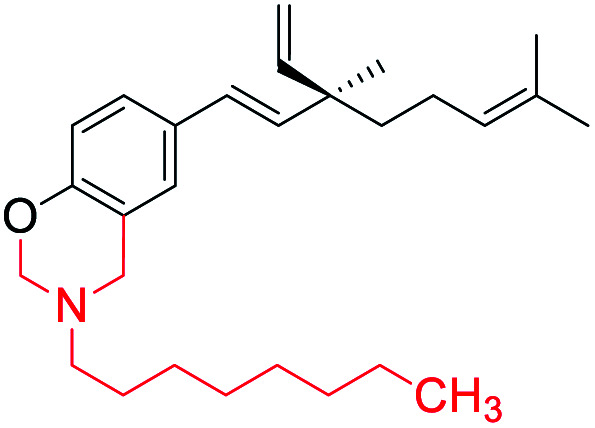
| 2 |
|
[1,3] oxazine ring introduced at phenolic –OH and phenolic o-CH (site-I) | Anti-proliferative activity | 44 |
| 3 |
|
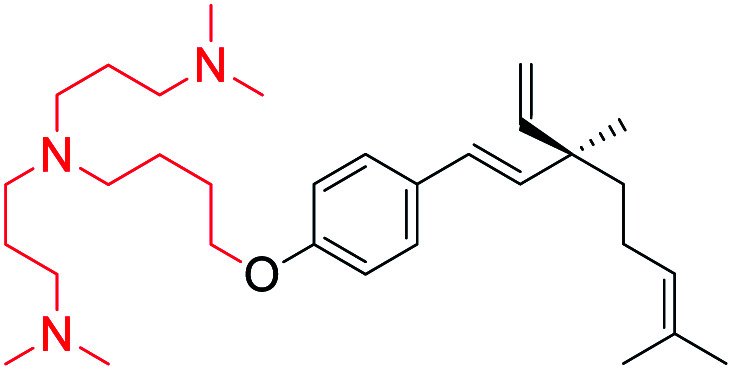
Cationic group introduced at phenolic –OH (site-I) | Anti-microbial activity | 46 |
| 4 |
|
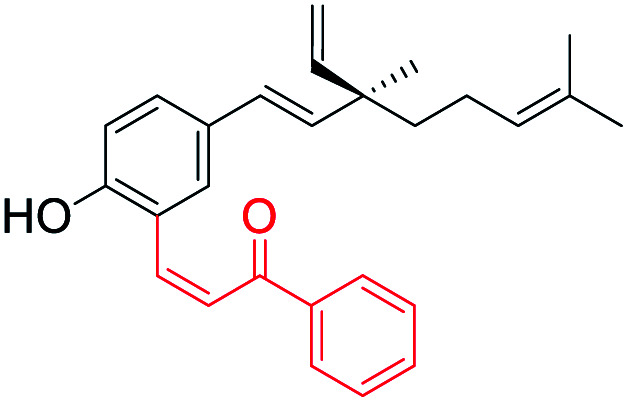
Electron withdrawing gp introduced at C(3) position (site-I) | Anti-proliferative activity | 42 |
| 5 |
|
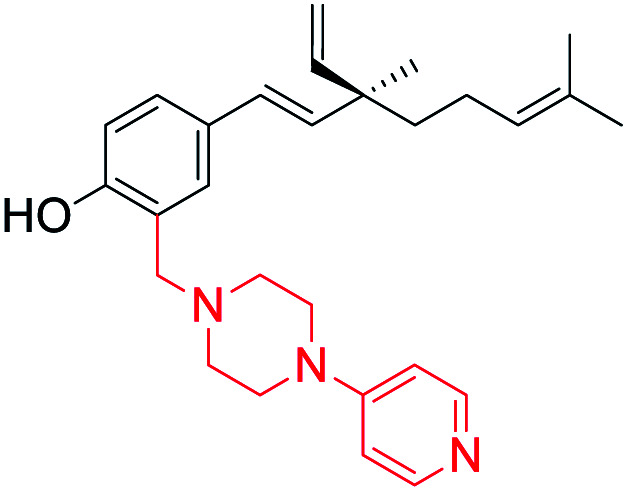
Pyridine substituted piperazine moiety introduced at C(3) position (site-I) | Anti-proliferative activity | 45 |
| 6 |
|
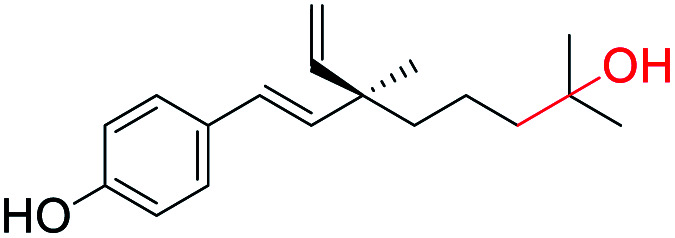
Hydroxylation at C(13) position (site-II) | Anti-proliferation activity | 43 |
| 7 |
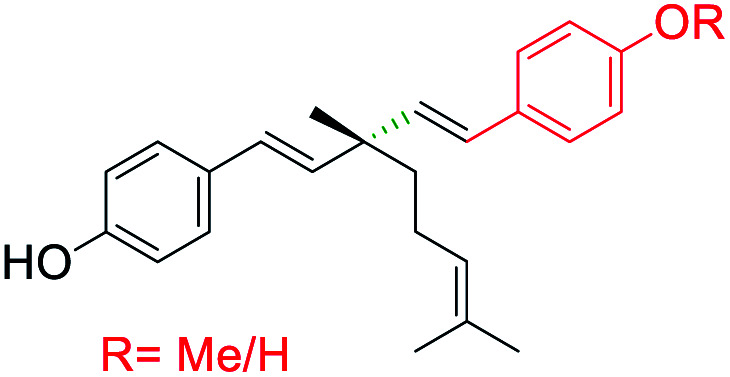
|
Aryl group introduced at ethenyl group (site-III) | Anti-microbial activity | 41 |
Bakuchiol is known to be very effective in the inhibition of oxidative stress. Many researchers reported the action of bakuchiol in preventing the mitochondrial lipid peroxidation and the protection of other enzymes from the oxidative stress.36a–c This action of bakuchiol in preventing the oxidative stress is highly co-related to its structure, as the phenoxy Csp2–OH group would contribute in trapping lipid peroxy radicals. The Csp2–OH group is conjugated to a double bond (like resveratrol) leading to a stronger stabilization of bakuchiol radical as compared to other phenolics. It has a terpenoid side-chain at the para-position of the phenoxy –OH group, providing an affinity for hydrophobic enviornments.36 From a proposed antioxidant mechanism of bakuchiol, it was found that the terpenoid chain with an easily abstractable hydrogen atom adjacent to its trisubstituted olefin linkage is giving it an enhanced antioxidant activity. The other two double bonds appear to be insignificant when comes to the antioxidant property. Bakuchiol can be considered to possess two antioxidant pharmacophores through the phenolic –OH group and the terpenoid functionality. The terpenoid substituent of bakuchiol, being an electron-donating group, is expected to reduce the bond dissociation enthalpy (BDE) of its phenolic moiety, which inturn enhances its antioxidant activity.
Thus, besides the phenolic group, the lipophilic alkenic side chain may also play a vital role in the antioxidant properties.37
Chen and co-workers (2008) found that C(12) and C(13) of bakuchiol was potential sites to be modified, that could improve the inhibitory activity and the introduction of conjugated groups to the C(3) position of the phenol led to higher immunosuppressive activity. The electron-withdrawing group –NO2 and electron-donating groups –CH2OH and –NH2 at C(3) position did not cause obvious change of the inhibitory effect but improved the cytotoxicity. The chiral quaternary center (C-9) also showed increased cytotoxicity.38
A study describing the structure–cytotoxic activity relationships clarified that an alkyl group was necessary for bakuchiol to be cytotoxically active against L929 cells. Moreover, this to study showed that double bond in the unsaturated hydrocarbon group exerted little influence on the cytotoxic activity.39 In another study, bakuchiol showed enantiomer-selective antiviral,40 and anticancer activities.41 They found that the S-isomer displayed far better activity than the R-isomer.
Reddy et al. evaluated the antibacterial activity of modified bakuchiol derivatives. From the SAR point of view, several inferences could be drawn such as (i) disturbing the phenolic character or the substitution pattern in the phenyl part of bakuchiol showed its high sensitivity towards modification with undesired results, (ii) modification at the isopropylidene group also showed discouraging results. These results points out the specificity of the structural modification and how it influences the different activities. However, modification at ethylene group at C(18) proved to be a good strategy to harvest very potent antibacterial molecules. This study also showed that reinforcement of an additional C(4) hydroxyphenyl or mono methoxyphenyl moiety (modified at C(18) position) plays an important role for the enhancement of activity. Disubstitution either in phenolic ring or in modified ring showed a decrease in the overall inhibitory activity against the tested pathogens. Later, they tested anticancer activity of bakuchiol analogs comprising of acyl, amino, halo, nitro, styryl and cyclized products. Results showed that addition of styryl moiety at vinyl group enhanced the anticancer activity.41
In another study on anti-proliferative activity against human liver cancer cell line (HepG2), the SAR indicate that (i) the substitution pattern in the phenolic part of bakuchiol showed sensitivity towards modification with promising results at C(3) position; (ii) modification at the isopropylidene group showed a moderate activity, (iii) the modification at terminal olefin resulted in greater potency when an electrophilic group was present. Furthermore, the study showed that addition of a hydroxyphenyl or mono methoxyphenyl moiety at C(4) plays an important role in the anti-proliferative activity.42 Similarly, Ryu and co-workers found that modification of double bond at isopropylidene group was a critical pharmacophore for the inhibitory activity on the proliferation of tumor cell lines such as A549, SK-OV-3 and SK-MEL-2.43 Sangwan et al. studied the structure–activity relationship between Mannich-type derivatives of bakuchiol (substitution at ortho position of phenol group) with four human cancer cell lines (MIA-Pa-Ca-2, HCT-116, MCF-7 and HL-60). It revealed that aliphatic primary amine analogs showed better anticancer effects than parent molecule as well as aromatic primary amine analogs. Among the aliphatic straight chain primary amine analogs, cytotoxic activity was found to be enhanced with increase in the number of carbon atoms along the chain length as better inhibitory effect was displayed.44 Later, they synthesized another set of ortho functionalized Mannich derivatives of bakuchiol. The compounds with free –OH and –COOH groups in aminoalkyl moiety of bakuchiol analogs enhanced the anticancer activity. Furthermore, N-substituted piperazine derivative with 4-pyridine substitution was found most potent and induces apoptosis in lung cancer cells (A549) via caspase activation and PARP-1 cleavage.45 Recently, Lin et al. (2021) designed and synthesized a series of new membrane-active O-alkylated bakuchiol derivatives by biomimicking the structure and function of cationic antibacterial peptides. They assumed that the incorporated basic cationic group could enhance the interaction between bakuchiol derivatives and negatively charged bacterial cell membrane via electrostatic interaction and then the isopentenyl lipid chain and the benzene ring can be inserted into the phospholipid bilayer of the bacterial cell membrane, which destroys the integrity of the bacterial cell membrane, leading to the leakage of bacterial intracellular contents and bacterial death.46 These findings indicate that bakuchiol represents an ideal bioactive scaffold for the synthesis of potent analogs and their SAR studies.
The late-stage functionalization (LSF) of bakuchiol is a powerful tool to speed up the synthesis of potent derivatives of bakuchiol. The recent developments in LSF on bakuchiol were described in Section 6. It has been found that the key functionalities such as phenolic –OH group, phenolic ortho-C–H bonds, vinyl and isopropylidene groups embodied in bakuchiol and it makes structural modification. The structurally modified analogues showed remarkably higher activity which depends upon the upcoming functional groups. During structural modification, there is a chance of elevation in hydrophilic character and the change of topology, which may effect its potential bioactivity profile.
5. Chemical synthesis
Since its discovery in 1966,3 several approaches towards the synthesis of bakuchiol have been developed. From the synthetic perspective, the stereochemistry is one of the most important feature and the construction of tetra-alkylated carbon stereocenter is the key step in the total synthesis and enantioselective construction of all-carbon quaternary stereocenters itself is considered a challenging task.48 Moreover, installation of alkenyl groups C(17–18) at sterically hindered positions49 also make additional level of difficulty in the total synthesis.
The stereoselective catalysis for the creation of all-carbon quaternary stereogenic centers in bakuchiol systems were developed in the last decade showing the contemporary interest of this molecule. Recently, enantioselective catalysis reflect the constant strive and creativity in the design and execution of carbon stereocenter. The Claisen rearrangement of geranyl enol ether, 1,4-addition of vinylcuprate species with α,β-unsaturated carbonyl compound, rearrangement of epoxy silyl ether, intramolecular diazosulfonate carbene insertion into a tertiary C–H bond, Cu-catalyzed SN2'-type allylic substitution, α-alkylation of α,β-unsaturated amide, allylmetalation, allylboration, hydroboration of allylic phosphonates with pinacolborane and diazo compounds with boronic acids are existing methods. Herein we sequentially discussed the various strategies and approaches.
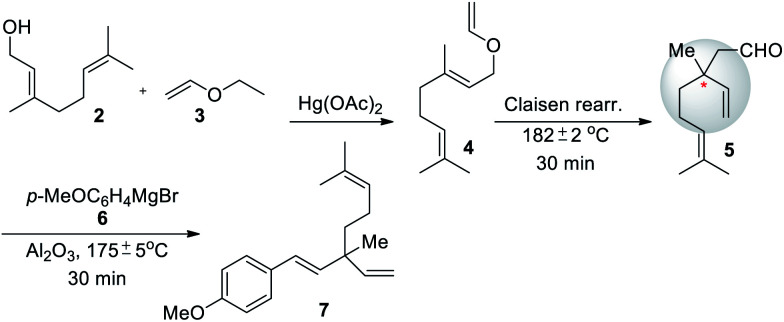
After the isolation and structural elucidation of bakuchiol from P. corylifolia, Damodaran and Dev (1967) reported a three-step synthesis of methyl ether derivative of bakuchiol starting from geraniol (Scheme 3).50 Initially, geraniol (2) is treated with ethyl vinyl ether 3 to affords corresponding geraniol enol ether 4 in 56% yield. Further, it undergo thermal reaction (Claisen rearrangement) to furnish aldehyde 5 along with quaternary carbon center, subsequently compound 5 undergoes reaction with p-methoxyphehyl magnesium bromide (6) to give alcohol and which on dehydration with alumina afford rec-methyl ether of bakuchiol (7).
Scheme 3. Damodaran and Dev's methods for rac-bakuchiol methyl ether synthesis.
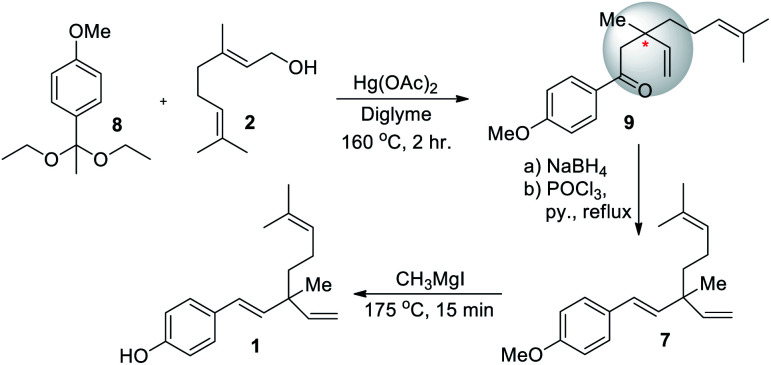
In the same year, Carnduff and Miller (1967)51 reported the first total synthesis of rac-bakuchiol. They also used geraniol (2) for the construction of terpene unit. In this rearrangement strategy, p-methoxyacetophenone diethyl acetal was heated (160 °C) with 2 and in the presence of mercuric acetate in diglyme to resulting methoxyacetophenone enol ether. Further, in situ Claisen rearrangement reaction of enol ether to form corresponding ketone with quaternary carbon center of bakuchiol (9). After reduction, elimination affords racemic bakuchiol methyl ether (7, 76%). Finally, removal of the methyl protecting group (demethylation) was achieved through 2 under reflux with Grignard reagent under solvent-free and high temperature conditions (Scheme 4).
Scheme 4. Carnduff and Miller's first total synthesis for rac-bakuchiol.
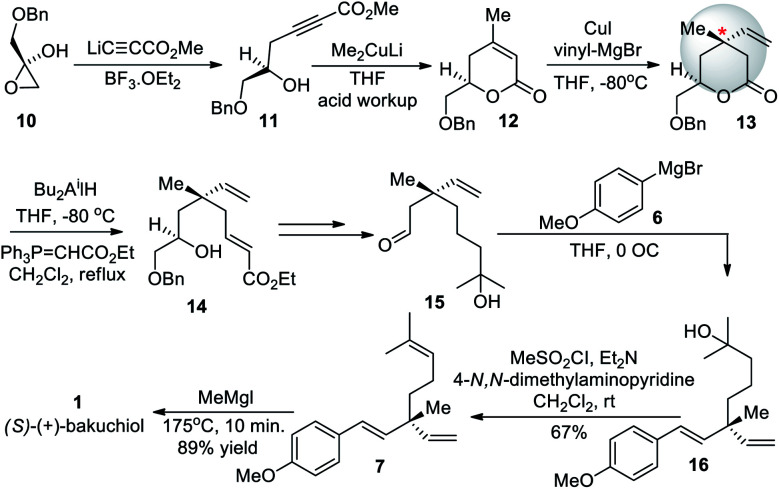
Later, Takano group (1990)52 demonstrated the first enantioselective synthesis of (+)-bakuchiol, starting from treatment of chiral (S)–O-benzylglycidol (10) with methyl lithiopropiolate in the presence of boron trifluoride etherate to form 11 which was then cyclized to form α-β-unsaturated-δ-lactone 12. The key step to form the chiral center of bakuchiol was the reaction of δ-lactone 12 with vinylmagnesium bromide in the presence of copper(i) iodide which proceeded in a stereoselective fashion from the stereoelectronically favorable face of the molecule to give the δ-lactone 13 with a quaternary center. This is the quaternary center that makes up the heart of the bakuchiol molecule. The total synthesis of enantio-enriched bakuchiol was completed by manipulating the molecule around the chiral center in a total of twelve steps with an overall yield of 16%. This method required the purchase of chiral starting material to facilitate the establishment of the chiral quaternary center of bakuchiol (Scheme 5).
Scheme 5. Takano and co-workers enantioselective synthesis.
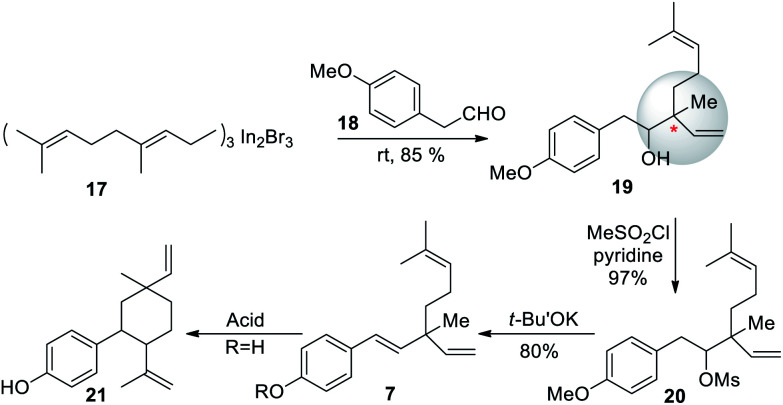
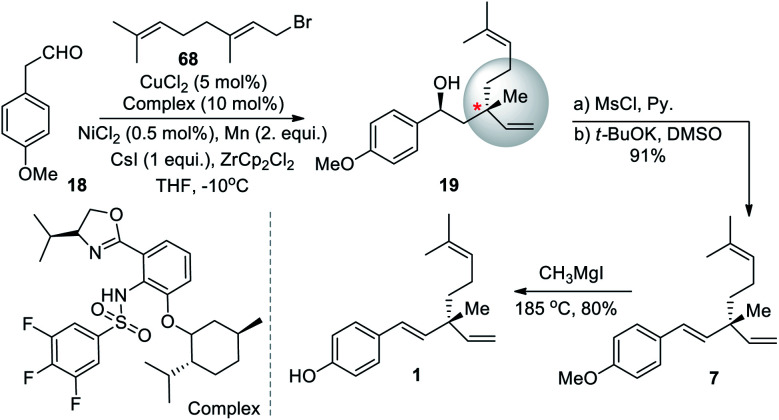
In 1991, a three-step strategy for rac-bakuchiol was developed by Araki and Bustugan,53 utilizing geranylindium reagent. Geranylindium sesquibromide 17 was reacted with 2-(4-methoxyphenyl)acetaldehyde (18) to form alcohol 19 in 85% yield (Scheme 6). The alcohol 19 was then reacted with mesyl chloride-pyridine to form compound 20. Potassium tert-butoxide was used to treat the crude mesylate (20) and gave the methyl ether 7. It was important to note from this synthesis that bakuchiol is an acid labile molecule. Upon treatment with acid, bakuchiol cyclizes to the p-methyl-8-ene (21, cyclobakuchiol) analogues.
Scheme 6. Araki and Bustugan synthesis via a geranylindium reagent.
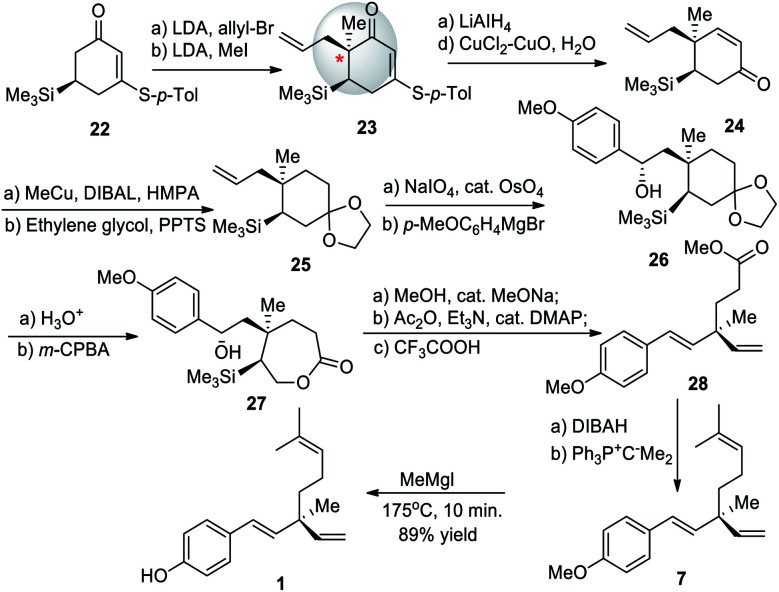
In 1999, a new synthesis of (+)-bakuchiol (1) was developed by the Asaoka group.54 This method used a silyl group to direct stereoselective alkylation to construct the chiral quaternary carbon center early in the synthetic pathway. Lithium diisopropylamide (LDA) followed by allyl bromide and LDA followed by methyl iodide were reacted with cyclohexenone 22 to yield cyclohexenone 23. Scheme 7 shows this chiral center being established in 28. After the chiral center was established, 28 was manipulated over a total of sixteen steps to yield 1 with an overall yield of 5%. This method required the purchase of a chiral starting material. The bulky silyl group acted as a chiral auxiliary to set the desired stereochemistry.
Scheme 7. Asaoka and co-workers synthesis via stereoselective alkylation using silyl group.
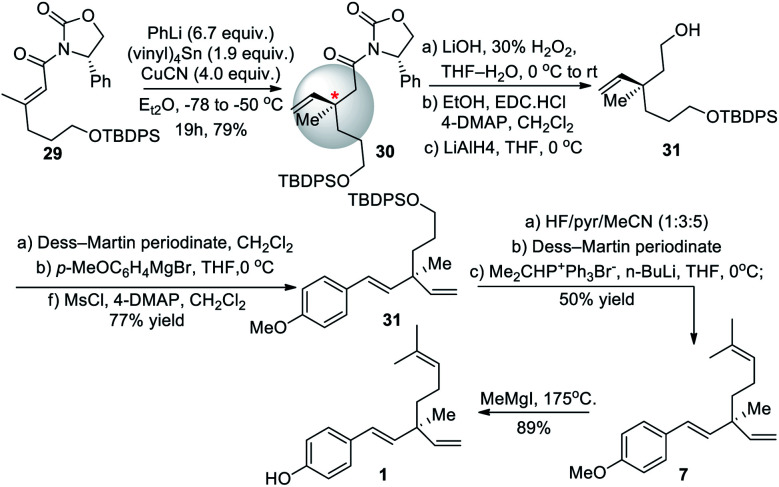
In 2008, Fukuyama and co-workers55 developed an efficient and facile methodology for constructing chiral all-carbon quaternary center via 1,4 addition using vinylcopper(i) reagents. They prepared chiral Michael acceptor 29 in 76% yield over three-steps (Scheme 8). Further, chiral Michael acceptor 29 was added to the copper lithium reagent made from vinyl tin in a diastereoselective manner to create the quaternary carbon center in 30. This method required the purchase of chiral phenyl oxazolidinone auxiliary to form 29 and stoichiometric amount (1.9 equiv.) of toxic (vinyl)4Sn to set the desired stereochemistry. The asymmetric addition yielded the desired R enantiomer of 30 in a ratio of 95 : 5 with the less favored S enantiomer of 30 giving an enantiomeric excess (ee) of 90%. This approach led to the total synthesis of desired product with an overall yield of 20% over ten steps.
Scheme 8. Fukuyama and co-workers synthesis via stereoselective alkylation using silyl group.
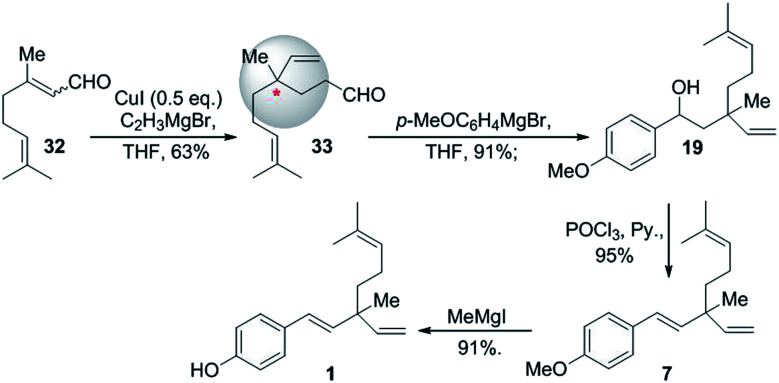
In the same year, Chen and Li (2008)56 demonstrated simple and convenient synthetic route for rac-bakuchiol. Here, they prepared key intermediate 33via 1,4 addition of citral (32) with vinylmagnesium bromide under Cu(i) catalyst. Further, attacked by Grignard reagent and other necessary rearrangements such as hydroxyl group was eliminated and methyl protecting group was removed to complete the synthesis of bakuchiol racemate in four steps with 50% of overall yield (Scheme 9).
Scheme 9. Chen and Li's synthesis via 1,4 addition on citral.
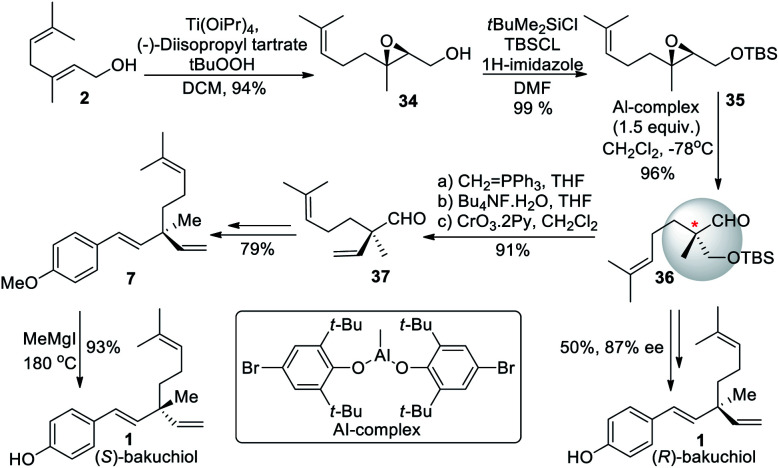
Li et al. (2008)57 synthesized (+)-bakuchiol (1) in ten steps, with an increased yield of 51%. The total synthesis started from geraniol (2), which undergo Sharpless epoxidation to form oxygen silyl ether compound 34. The –OH group of the compound was protected with a TBS group to form 35, followed by a rearrangement of the silyl ether to form a chiral quaternary carbon center 36 (Scheme 10). Further, aldehyde 37 undergoes Wittig reaction to remove TBS, oxidation, and Grignard reagent addition and elimination can get (S)-bakuchiol. They also synthesized its (R)-enantiomer in nine steps with an overall yield of 40%.
Scheme 10. Li and co-workers method of chiral center installation.
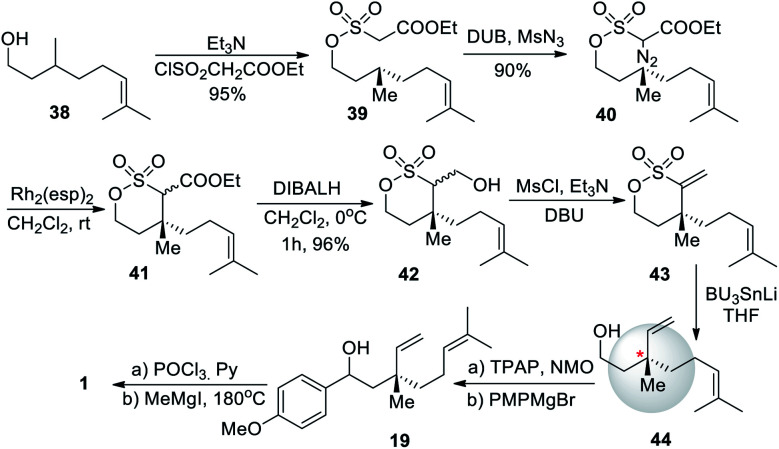
An intramolecular diazosulfonate C–H insertion to install the quaternary center was achieved by Novikov et al. (2009)58 This approach started with (−)-citronellol (38) as the starting material and was converted to citronellol based chiral δ-sulfone 43 in three-steps with high yields. It could be used in the total synthesis of bakuchiol with an yield of 45% (Scheme 11).
Scheme 11. Novikov and co-workers synthesis via intramolecular diazosulfonate C–H insertion.
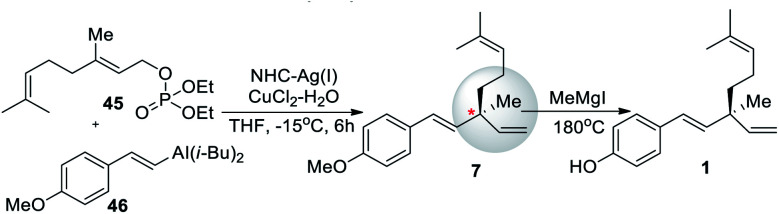
In 2010, Hoveyda et al.59 reported the synthesis of 1via Ni Catalyzed NCH–Cu enantioselective allylic substitution (EAS) reaction (Scheme 12). The synthesis was started with the phosphonation of geraniol (2) to yield 45, and β-selective Ni catalyst was attached to the terminal end of an alkyne followed by a hydroalumination to afford alkenyl aluminum reagent 46. The asymmetric substitution reaction of allyl phosphate 45, through the addition of hand azacarbene silver complex to control the stereo configuration of the chiral center of the product. This reaction was used for the synthesis of bakuchiol enantiomer (er 91 : 9). The synthetic route is very efficient (72% yield). However, the complex chiral silver azacarbene is difficult to prepare.
Scheme 12. Hoveyda co-workers synthesis via Ni catalyzed NCH–Cu EAS.
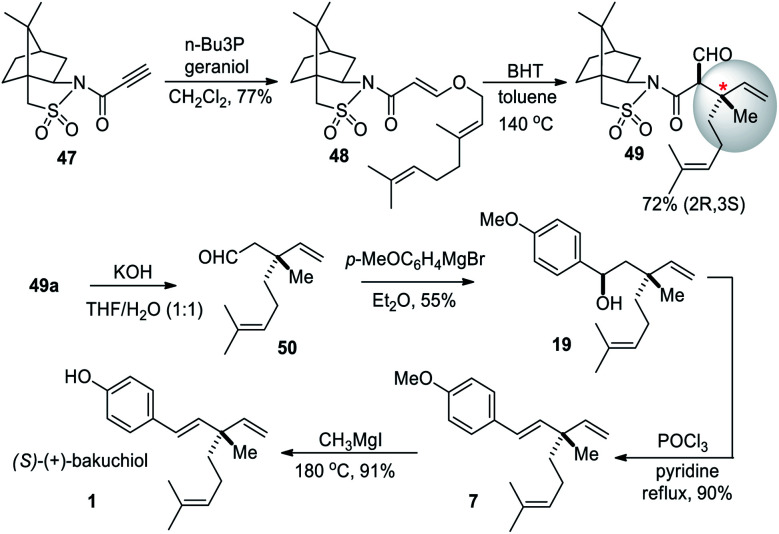
Tadano and co-workers (2012)60 utilized the Oppolzer's (sulfur-based) chiral auxiliaries for the asymmetric synthesis of (S)-bakuchiol. The chiral auxiliary-controlled asymmetric Claisen rearrangement was employed in this strategy (Scheme 13). Initially, the Michael addition reaction of N-propioloyl camphorsultam 47 with geraniol (2) afforded the adduct 48, which on heating with butylated hydroxytoluene (BHT) at 140 °C afforded the Claisen rearranged products 49a and 49b, respectively, as a mixture of diastereomers (dr 9 : 1) in good yield. The chiral auxiliary of the major isomer 49a was removed by hydrolysis under basic conditions with simultaneous decarboxylation which provided the desired enantiomerically pure aldehyde 50 in good yield. The aldehyde 50 was reacted with Grignard reagent, and the resultant alcohol 19 was dehydrated with POCl3 and pyridine. Finally, demethylation was achieved using MeMgI at high temperature to give the desired (+)-bakuchiol (1) in good yield.
Scheme 13. Tadano and co-workers synthesis via sulfur-based chiral auxiliaries-mediated Claisen rearrangement.
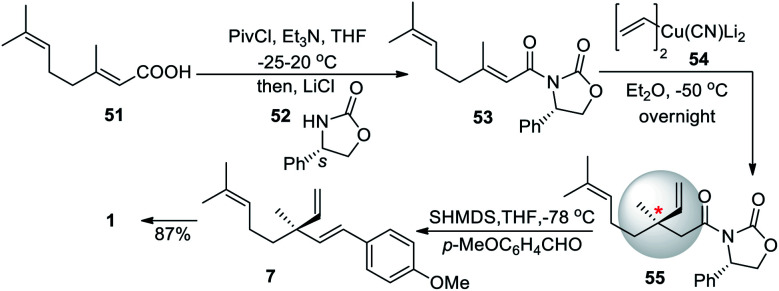
After the successful total synthesis in fourteen steps from but-3-yn-1-ol, Fukuyama and co-workers (2013)61 developed another asymmetric 1,4-addition reaction for chiral quaternary center, through introduction of chirality by the (2′S)-2′-phenyloxazolidinone auxiliary. A geranic acid (51) was treated with pivaloyl chloride and triethylamine, then (2′R)-2′-phenyloxazolidinone (52) was used as a chiral auxiliary to form compound 53. Further, 53 underwent an asymmetric 1,4 addition to form compound 55 which was reacted with sodium hexamethyl disilazane (SHMDS), followed by p-methoxy benzaldehyde addition to form methyl ether derivatives of bakuchiol (7). Which was further demethylated using the previously established method with an overall yield of 64% (Scheme 14).
Scheme 14. Esumi and co-workers synthesis via asymmetric 1,4-addition.
Lei et al. (2013),62 developed an asymmetric synthesis routes for (S)-bakuchiol through an intermediate 56 bearing a chiral all-carbon quaternary carbon center, which involves stereoselectively unconjugated alkylation of the α,β-unsaturated imide bearing Evans' auxiliary, Takai–Utimoto reaction, Negishi reaction, and Heck reaction as key steps (Scheme 15).
Scheme 15. Lei and co-workers approach using Evans' auxiliary.
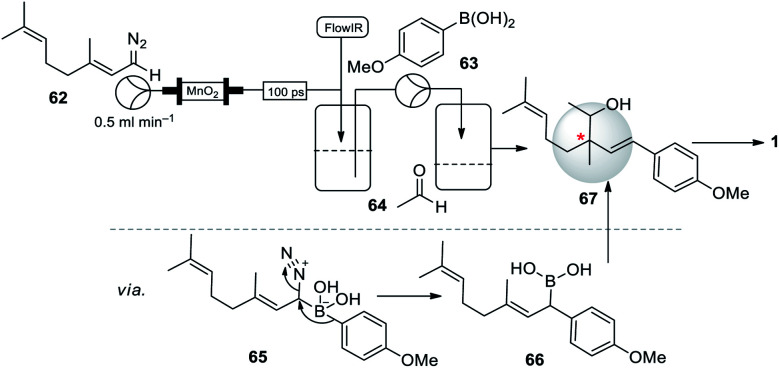
Interestingly, Battilocchio et al. (2016)63 disclosed the in situ preparation of reactive allylic and benzylic boronic acids, obtained by reacting flow-generated diazo compounds with boronic acids through C–C bond. They briefly demonstrated the utility of this method for the synthesis of bakuchiol precursor in one single operation. The geranial diazo 62 species directly added to the solution of 4-methoxy phenyl boronic acid (63) and aldehyde 64, the reaction gave a very selective addition to the boronic acid 63 to form a boronic acid intermediate 66, following a subsequent quench with aldehyde 64 to form desired precursor 67 (Scheme 16). Usually, the known route to this compound uses a multi-step sequence of reactions. The C–C bond formation in a controlled sequence and thus rapidly build molecular complexity in an iterative fashion is an important goal in modern chemical synthesis.
Scheme 16. Battilocchio and co-workers synthesis of bakuchiol precursor using an iterative coupling method.
Xiong and Zhang (2016)64 developed an asymmetric allylation of aldehyde with γ-disubstituted allylichalides to construct two contiguous stereogenic centers, including one quaternary center. A chromium salt and a chiral oxazoline sulfonamide ligand delivered the allylation products with good functional group tolerance and high diastereo and enantio selectivities. Under this mild reaction condition they used readily available allylation reagents. The synthetic potential of this method was demonstrated by transformation of an allylation product into the natural product (S)-bakuchiol. Under this catalytic system, commercially available 2-(4-methoxyphenyl)acetaldehyde (16) with geranyl bromide (68) proceeded smoothly to afford salicylic product 19 with 91% ee. Further, 19 was converted into dehydroxylated via a two-step sequence. The final demethylation of 7 furnished the desired product 1 (Scheme 17).
Scheme 17. Xiong and Zhang's method through chromium-catalyst.
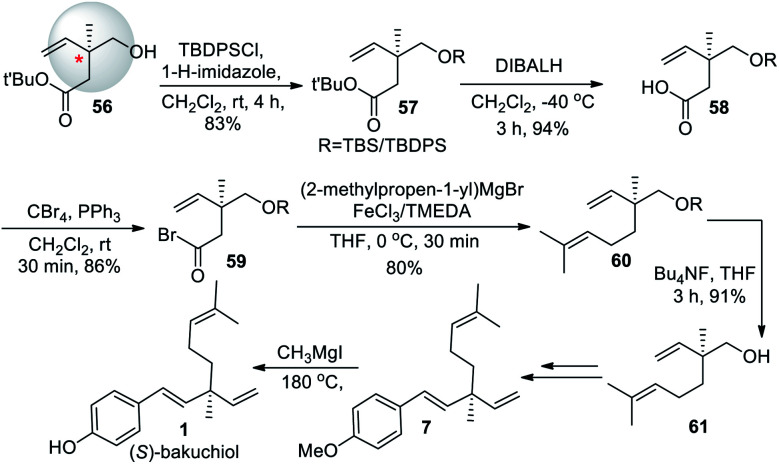
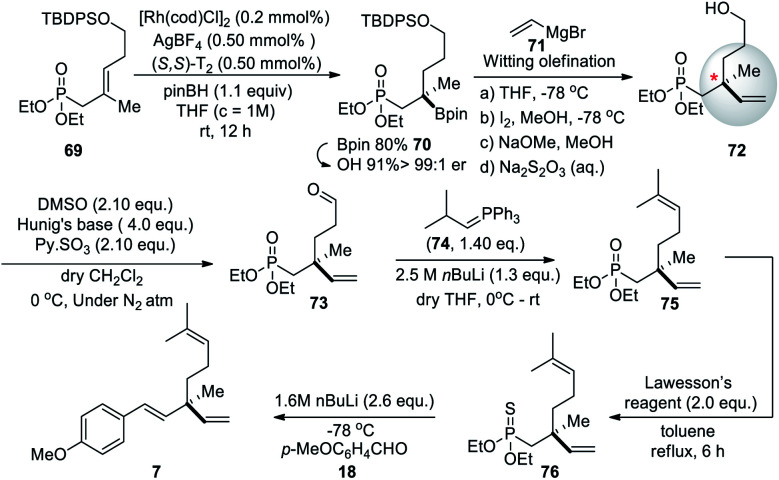
Highly enantioselective rhodium-catalyzed hydroboration of allylic phosphonates with pinacolborane affording chiral tertiary boronic esters strategy (Scheme 18) was developed by Chakrabarty and Takacs (2017).65 They are readily converted β-borylated phosphonates to chiral β- and γ-hydroxyphosphonates and aminophosphonates and to phosphonates bearing a quaternary carbon stereocenter. The utility of this strategy was illustrated by the synthesis of (S)-bakuchiol methyl ether (7). Here, catalytic asymmetric hydroboration of trisubstituted alkene 69 with (S,S)-T2 reaction affords chiral boronic ester 70 with enantioselectivity >99 : 1 er. Further it underwent oxidation to form chiral tertiary alcohol 72. The vinylation of chiral tertiary boronic ester 70 was achieved through cross-coupling with vinyl magnesium bromide (71). Later, they carried out the silyl ether deprotection of chiral vinyl posphonate followed by Parikh–Doering oxidation. The Witting olefination result chiral diene phosphoate formation. Further, transformation was carried out with some modifications of the original procedure described by E. J. Corey et al. because of the direct phosphonate olefination is limited in scope since β-hydroxy phosphonates lacking electron withdrawing substituents on the α-carbon are not prone to eliminate without activation. Treating phosphonate 75 with Lawesson's reagent affords thionophosphonate 76. Deprotonation by n-BuLi followed by the addition of 4-methoxy benzaldehyde (18) smoothly yields (S)-bakuchiol methyl ether 7 (Scheme 18).
Scheme 18. Chakrabarty and Takacs's strategies via rhodium-catalyzed hydroboration.
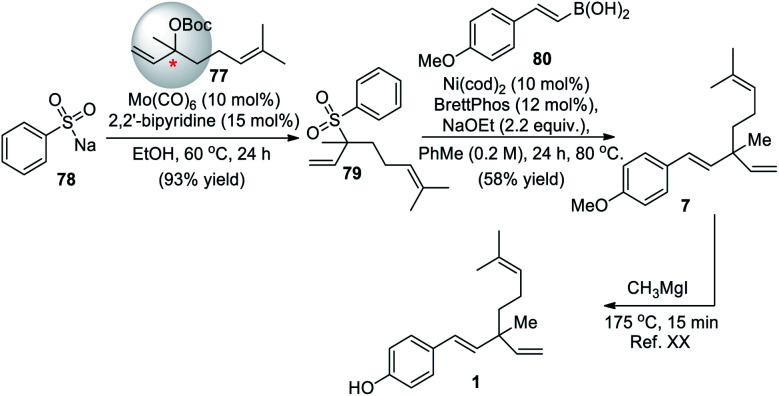
Recently, Khan and co-workers (2020)66 described regioselective molybdenum-catalyzed allylic substitution of tertiary allylic electrophiles. This was the first example of the use of sodium sulfonates as the hetero-nucleophile reagent with tertiaryallylic electrophiles to employ the group VI catalyst in allylic substitution of tertiary allylic electrophiles to form C–S bonds. Under the optimal condition tertiary allylic carbonate 77 were coupled with sulfinate salt 78, high yields of the branched allylic product 79 was obtained. This was the key intermediate for the formal synthesis of the rac-bakuchiol (Scheme 19). Further step involved a previously reported Suzuki–Miyaura cross-coupling reaction of tertiary allylic sulfone 79 with styryl boronic acid 80 to afford methyl ether derivative of rac-bakuchiol 7, and subsequent deprotection of phenol, thus completing the formal synthesis.
Scheme 19. Khan and co-worker's molybdenum-catalyzed allylic substitution.
Bakuchiol has a simple structure with a quaternary carbon chiral center. Being highly bioactive, it has attracted many organic chemists to investigate on its chemical synthesis, but most asymmetric synthetic routes involves chiral entities linked to carbon skeleton or it has been done racemically and there have been multiple methods or steps. The first chemical synthetic attempt was made by Dev group in 1967, the same year the first total synthesis was reported by Carnduff and Miller. From 1967 to 1990, no new synthetic routes reported on this molecule, due to the challenges behind the chemical synthesis. So far, the synthesis route of the Hoveyda group is the shortest, with only three steps, and the total yield is 72%, but the chiral silver azacarbene is not easy to prepare, which limits its application to a certain extent. This limitations hinders the development of bakuchiol and associated molecules in the meroterpenoid family.
A deep look into the advanced asymmetric routes is necessary for exploring the enantioselective synthesis of this molecule. The enantiomerically pure molecules can be obtained by chiral resolution, which involves enantiomerically pure substrate, reagent, solvent, or catalyst as starting material. We can choose suitable chiral auxiliaries for the enantioselective synthesis of bakuchiol. Chiral auxiliaries derived from naturally occurring compounds, such as amino acids, carbohydrates, and terpenes, are considered essential tools for the construction of highly complex molecules. Evans, Corey, Yamada, Enders, Oppolzer, and Kunz are the some of selected examples of auxiliaries, which are a powerful tool to obtain intermediates and final products of total synthesis. On bakuchiol, Oppolzer60 and Evans62 auxiliaries were successfully utilized for its enantioselective synthesis. The choice of the appropriate auxiliary for each reaction depends on some factors, and these must be taken into account in the development of new auxiliaries.
6. Late-stage functionalization (LSF)
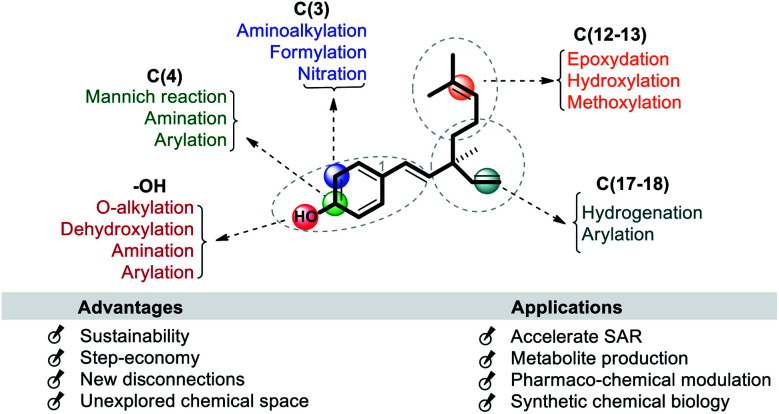
Bakuchiol is a small molecule having a multi-step chemical synthetic route as discussed in the above section. The production of its analogues is simple as the diversification or functionalization is started from its moiety through LSF. Which means, there is no need to introduce important functional groups (FG) in the very early steps of the synthesis. Several strategies and systems have been successfully developed for chemo/site-selective functionalization of bakuchiol via catalytic and non-catalytic reactions, C–H functionalizations, and functional group manipulations. It is envisioned that LSF will speed up the synthesis of its analogues and dramatically accelerate structural–activity relationships and improve the efficiency and stability. The chemo/site-selective functionalization is not easy and it have several challenges: (a) the nucleophilicity of the hydroxyl oxygen atom often results in O–H substitution in preference to C–H substitution, (b) the presence of multi-olefinic bonds often results in unfavorable reactions and (c) under suitable conditions bakuchiol may cyclize to the cyclobakuchiol derivatives. In this review we have described possible late-state modification sites on bakuchiol. They include (i) phenolic hydroxyl (Csp2–OH) group; (ii) phenolic C(4) carbon (iii) phenolic C(3)-H bond (iv) olefinic C(12–13) linkage at terpene chain and (v) alkenyl C(17–18) linkage at quaternary stereocenter (Scheme 20).
Scheme 20. Late-stage functionalization sites of bakuchiol.
The site-selective diversification of bakuchiol's phenolic group (sites i or ii) has been reported via either O–H substitution or C–O bond (ipso position) cleavage. In generally, phenols have a very reactive hydroxyl (Csp2–OH) group and a Csp2–O bond with a high bond dissociation energy (111 kcal mol−1) owing to p–π conjugation. Therefore, direct and selective replacement of C–O or O–H bond with new bonds represents an important and long-standing goal in LSF. These transformations have broad potential in synthesis because Csp2–O and O–H bonds are ubiquitous in natural products like bakuchiol.67 At the same time, achieving selectivity among different of these bonds remains a challenge.
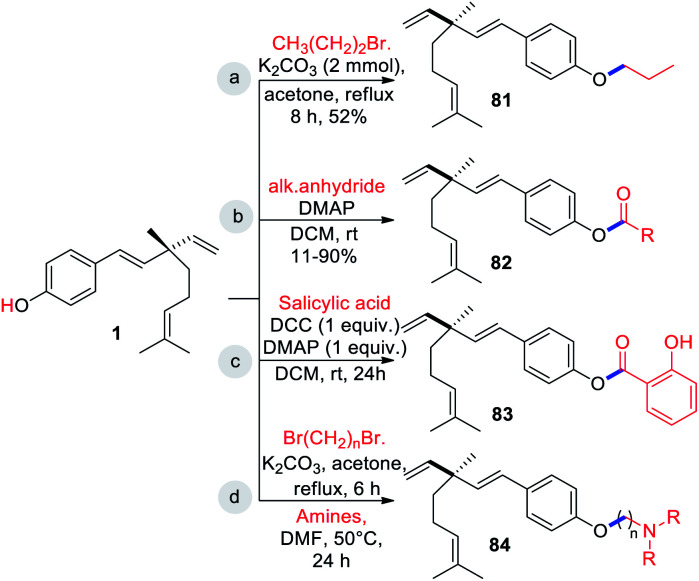
The presence of free Csp2–OH group (site-i) can be utilized for O-alkylations and esterifications with suitable coupling partners under catalytic or non-catalytic systems. In general such type of diversification was achieved through a classical method involving single or multi-step reactions. Some of the selected examples were outlined in Scheme 21. Notably, bromopropane, with anhydrous K2CO3 in acetone as the catalyst affords O-alkylated derivative 81 (Scheme 21a) and acyl derivatives 82 obtained by treating 1 with appropriate alkanoic anhydride in presence of catalytic amount of DMAP (Scheme 21b).47
Scheme 21. Synthetic routes for –OH functionalized bakuchiol derivatives.
Bakusylan (83) is an novel hybrid molecule prepared from salicylic acid with bakuchiol (1) via modified Steglich esterification method using DCC as condensing agent and DMAP as catalyst (Scheme 21c).68 Recently, Li and co-workers (2021) introduced a well-defined cationic amphiphilic groups under non-catalytic system.46 Initially they prepared key intermediate compounds by alkylation of compound 1 with the corresponding α,ω-dibromoalkanes. Further, intermediate or O-alkylated bakuchiols were treated with the corresponding amines to yield desired cationic amphiphilic derivatives 84 (Scheme 21d).
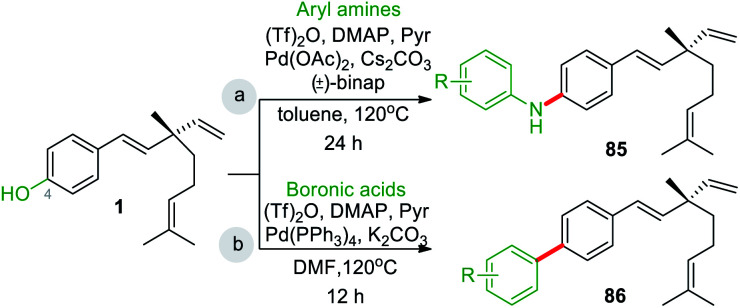
In last decades, great achievements have been made in developing cross-coupling reactions of arenols by catalytic Csp2–O activation and cleavage by first transforming them into more active species or weak C–O bonds such as aryl sulfonates,69 aryl esters or aryl carbamate derivatives,70 aryl alkyl ethers,71 and phenolic salts.72 In 2016, Gautam and co-workers42 conducted a protecting group manipulation on phenolic –OH group under (Tf)2O-DMAP catalytic system, which provides highly reactive triflated species. Further, Pd(OAc)2–Cs2CO3 system with aryl amines and Pd(PPh3)2–K2CO3 system with aryl boronic acids provided the desired aminated 85 and arylated 86 products respectively via ipso-C–O bond cleavage (Scheme 22).
Scheme 22. C(4) functionalization via ipso-C-O cleavage.
Functional group manipulation or pre-functionalization methods combined with separation and purification, which leads to additional steps, generates waste, and lowers the efficiency drastically, particularly for late-stage diversification. In this scenario, there is an demand in cross-coupling of free arenols (especially phenols) via C–O bond activation. Recently, several approaches have been explored on arenols for making various types of bonds such as C–C,73 C–N,74 C–S,75 C–B76 and C–P77 bonds (Table 4). Most reports directly use arenols as electrophiles, with some in situ formed intermediate. In addition, the basic systems remain speculative to some extent. All of these challenges leave many opportunities for the development of new C–O transformations, and a bright future for the direct use of arenols as modern electrophiles in cross-coupling reactions. These approaches and ideas may helpful diversification of phenolic Csp2–OH group of bakuchiol and its related compounds.
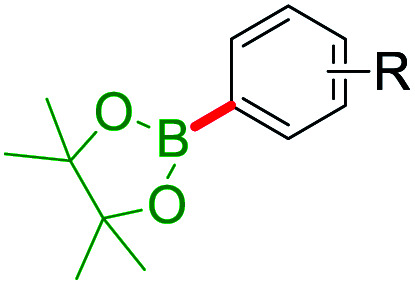
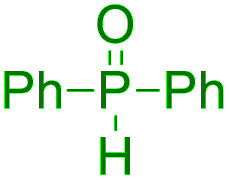
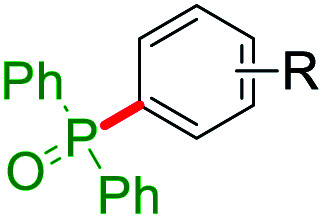
Selected ipso-C–O bond cleaving reaction on free arenols.
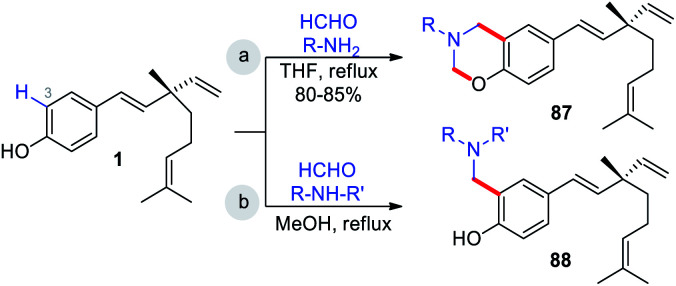
The Mannich reaction is an important, one-pot, multi-component, C–C bond forming reaction that is widely used in the syntheses of many biologically active and natural compounds.78 Gupta and co-workers44 reported that the Mannich type condensation-cyclization of bakuchiol with suitable primary amines and formaldehyde took place in the non-catalytic system and produced various dual functionalized 3,4-dihydro-2H-benzo[e][1,3]oxazinederivatives (Scheme 23a). Continuing their research into Mannich type reaction using bakuchiol, Gupta and groups,45 demonstrated the synthesis of ortho substituted aminoalkyl bakuchiol derivatives using secondary amines (Scheme 23b), which were obtained in good to moderate yield (80–85%).
Scheme 23. C(3)–H and phenolic –OH functionalization.
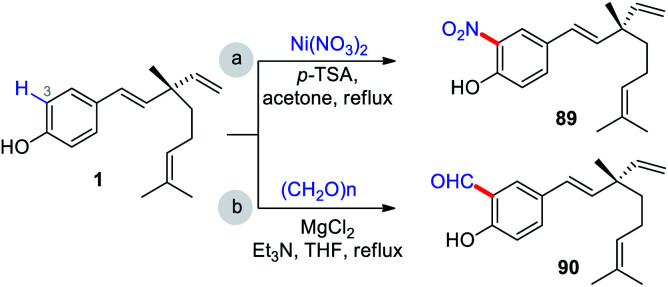
Highly regio-specific mono nitration of phenols and substituted phenols is accomplished employing a metal nitrate and a catalytic amount of p-toluenesulfonic acid (p-TSA) in acetone. An exclusive ortho-selectivity was observed with excellent yields.79 From this view point, Wu and co-workers adopted this p-TSA–Ni(NO3)2 catalytic system for nitration on bakuchiol (Scheme 24a) at C(3)-position. In another study, Chen and co-workers,47 used MgCl2–Et3N systems80 for formylation (Scheme 24b).
Scheme 24. C(3)–H nitration and formylation reaction.
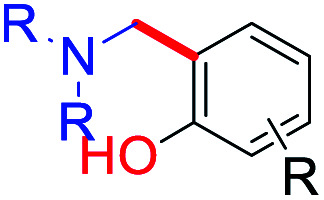
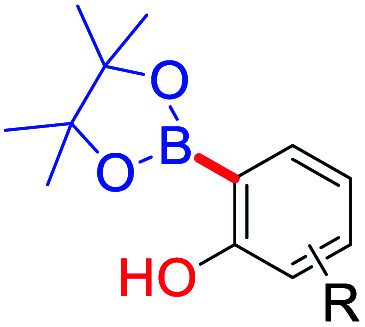
Likewise, several advanced site-selective ortho C–H functionalization of phenols have been explored extensively as an efficient, step- and/or atom-economic (Table 5).81 Therefore, we can adopt these existing methodologies (with or without modifications) on bakuchiol, thus may providing opportunities to explore its functionalities. Previously we developed several C–H functionalization methods on small molecules similarly.82 Notably, FeCl3–K2S2O8 system for para-site-selective oxidative quinone C–H arylation with phenols,82a AgNO3–TBHP system for chemo-selective quinone C–H alkylation with alkyl amides,82b and CrO3–H2O system for oxidative radical C–H/S–H cross-coupling of thiols with hydroquinones.82c
Selected coupling partners and catalytic systems for functionalization of phenolic ortho–CH bond.
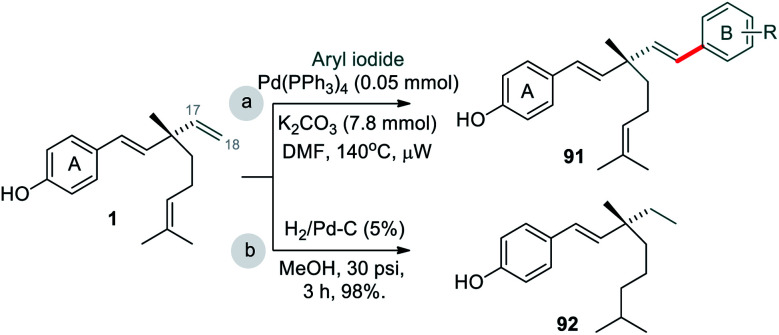
Reddy et al. (2010)41 developed an strategy for the functionalization of C(18) atom of the ethenyl group of bakuchiol (1) was modified (site-iii) by the introduction of substituted aryl groups through C–C bond formation via Heck and oxidative Heck coupling reactions (Scheme 25a). These coupling reactions resulted in the formation of optically pure (R)- and (S)-isomers and non chiral bisstyryl derivatives based on the nature of the substituents present at ring A and ring B of the two styryl moieties residing at C(9) stereogenic centre. Heck reaction afforded products exclusively with trans geometry at C(17)–C(18) double bond and this approach did not involve any change in the stereochemistry of parent molecule at C(9) chiral centre. The isolated yields of compounds were in the range of 48–97%. Moreover, they did hydrogenation at non-aryl part (Scheme 25b).42
Scheme 25. C(18)–H functionalization via Heck coupling and hydrogenation.
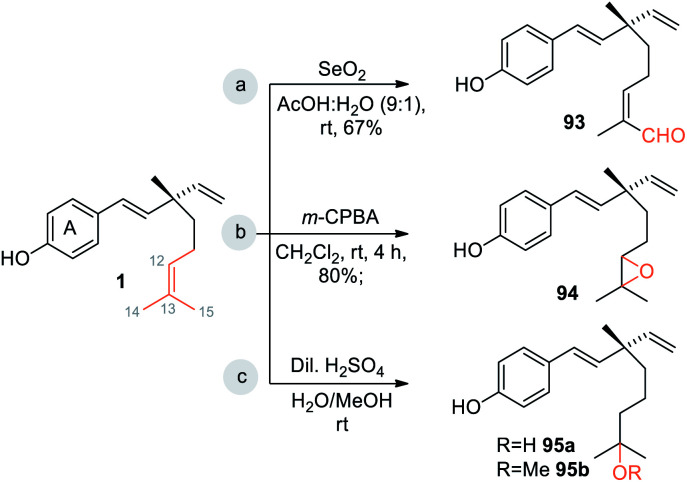
Bakuchiol (1) possesses a terpenic chain which is prone to further chemical modification. The conversion of one of the methyl group C(14) or C(15) into a formyl group and epoxidation (Scheme 26a/b)10 of the isopropylidene group was effected to afford compounds 93 and 94 respectively. Moreover, bakuchiol (1) affords C(13)-hydroxylated 95a or methoxylated 95b derivatives under the presence of dilute H2SO4 with water or methanol (Scheme 26c).47
Scheme 26. Functionalization at terpene chain.
7. Bakuchiol in cosmetology
Bakuchiol, the natural substitute for retinol, is a plant based natural compound playing a significant role in cosmetic and pharmaceutical industry without leaving any side effects.12 The properties such as anti-ageing, anti-pigmentation, anti-acne, anti-cancer, hepatoprotective, cardioprotective, hypoglycemic, hypolipemic, and antidepressant, antioxidant, anti-inflammatory and antimicrobial activities makes bakuchiol a superior component in both areas of cosmetology and pharmacology.11d,18b As mentioned earlier, bakuchiol is known to be functionally similar to retinol but do not have any structural similarities. Therefore, nowadays many cosmetic preparations are mainly using bakuchiol as an anti-ageing component, replacing retinol.11a
The human skin, the most visible indicator of age, is divided into three layers namely epidermis, dermis and hypodermis. The epidermis, the outer most skin layer, is a dynamic system whose metabolic activity is largely regulated by the integrity of the permeability barrier. Next to epidermis is the dermis layer composed mainly of tissues and blood vessels. Dermal connective tissue contains elastin and collagen; collagen fibers comprise the biggest volume of the skin and the bulk of its tensile strength, whereas elastin fibers contribute to elasticity and resilience. Epidermis is one of the major targets for the retinoic acids and its major activities are accomplished through the transcriptional regulation of specific genes. The Cellular Retinoic Acid Binding Protein (CRABP) transports the retinoic acids to the nucleus, thus mediating the genomic actions. Among the CRABP 1 and CRABP 2, the latter is highly expressed in the epidermis. There are six nuclear retinoic acid receptors namely RAR (α, β,γ) and RXR (α, β,γ), which aids in binding to response components in the DNA and thus carrying out transcription of specific genes. Chaudhuri et al. (2014)11a has demonstrated with the help of molecular techniques such as microarray, ELISA etc. that there is a close similarity on the gene expression profile of bakuchiol and retinol. Bakuchiol appears to target several cellular pathways similar to those targeted by retinoids, including the modulation of retinoic acid receptors genes and up regulation of collagen and extracellular matrix synthesis enzymes. At the same time, some genes up-regulated by retinol are not affected by bakuchiol, indicating a possible advantage of the latter in terms of side effects. Likely, retinol had no effects on CRBP II and CRBP IV, whereas bakuchiol showed significantly higher up-regulation. Furthermore, retinol causes down regulation of CRABP1 gene whereas bakuchiol causes up-regulation. Thus bakuchiol serves as a compound having similar but not identical gene expression pattern as compared with retinol, ideally, resulting in retinol-like beneficial effects, without having retinol-like detrimental side effects. Generally, all the topical retinoid treatment is associated with an antagonistic reaction commonly referred as “Retinoid Reaction”. Studies suggest that this reaction is likely the result of upregulation of inflammatory mediators in the skin.83
Cosmetic preparations with retinol applied directly to the skin results in burning, pruritus, redness, hives, dryness, increased keratinization of the epidermis, hypersensitivity to solar radiation, excessive sweating, hair loss, and dryness of the mucous membranes of the nose and eye.84 Apart from this, retinoids are known to increase the triglyceride and cholesterol in blood, muscle pain, bone decalcification and premature closure of articular cartilages.85 Thus, researchers gave bakuchiol a special attention, as it is completely safe compound having several significant advantages over retinol. Its outstanding photochemical and hydrolytic stability, high miscibility in various emollients and solubilizers makes it a highlighted compound in cosmetology. The high photostability allows its usage in day care products also.
8. Conclusion and future perspectives
Since the discovery of bakuchiol in 1966, it has been found to exhibit divergent biological properties and nowadays this molecule is a highlighted one in cosmetology due to its retinol like functionality. Considering its importance, researchers are in the quest of finding various natural sources for bakuchiol extraction and it revealed that P. corylifolia is currently the only natural source yielding this molecule on a larger scale. There are number of limitations associated with the isolation of this compound from P. corylifolia. Hence, ideas about the biosynthesis, structure and structure–activity relationship is necessary for the development of an effective and green protocol for isolation and synthesis.
The stereochemistry is one of the most important features in their chemical structure. Because, the use of racemic mixtures may contribute to the adverse effects of the molecule particularly they are associated with the inactive or active isomer. From the available literature, it revealed that the S-isomer displayed far better activity than the R-isomer. Therefore, its enantioselective synthesis is very important. The construction of tetra-alkylated carbon stereocenter bearing an alkenyl substituent is the key challenging step in the chemical synthesis. It has been done using various approaches, making use of chiral synthons and auxiliaries either commercial or developed in the laboratory. Most of the approaches yielded rec-bakuchiol. The first attempt at the synthesis of bakuchiol was done in 1967 by the Dev group and in the same year the first total synthesis was reported by Carnduff and Miller. From 1967 to 1990, there were no new approaches for bakuchiol, this was due to the challenges behind the chemical synthesis. Meantime the biological properties of this molecule were well established and it is considered as leading bioactive molecule. After that, scientists were put more efforts to design and synthesis of bakuchiol and its derivatives through LSF. We hope, the informations provided here could be helpful for researchers in understanding the trends and difficulties associated with this molecule.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support from Sreedhareeyam Farmherbs India Pvt. Ltd, Kerala India for carrying out this work.
References
- (a) Atanasov A. G. Zotchev S. B. Dirsch V. M. Supuran C. T. Nat. Rev. Drug Discovery. 2021;20(3):200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gaudencio S. P. Pereira F. Nat. Prod. Rep. 2015;32:779–810. doi: 10.1039/C4NP00134F. [DOI] [PubMed] [Google Scholar]; (c) Dossey A. T. Nat. Prod. Rep. 2010;27:1737–1757. doi: 10.1039/C005319H. [DOI] [PubMed] [Google Scholar]; (d) Blunt J. W. Copp B. R. Keyzers R. A. Munro M. H. G. Prinsep M. R. Nat. Prod. Rep. 2017;34:235–294. doi: 10.1039/C6NP00124F. [DOI] [PubMed] [Google Scholar]
- (a) Kuroda Y. Nicacio K. J. da Silva Jr I. A. Leger P. R. Chang S. Gubiani J. R. Deflon V. M. Nagashima N. Rode A. Blackford K. Ferreira A. G. Nat. Chem. 2018;9:938–945. doi: 10.1038/s41557-018-0084-x. [DOI] [PubMed] [Google Scholar]; (b) Hu P. Chi H. M. DeBacker K. C. Gong X. Keim J. H. Hsu I. T. Snyder S. A. Nature. 2019;569(7758):703–707. doi: 10.1038/s41586-019-1179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta G. Nayalca U. R. Dev S. Tetrahedron Lett. 1966:4561. doi: 10.1016/S0040-4039(00)70078-5. [DOI] [Google Scholar]
- (a) Majeed R. Reddy M. V. Chinthakindi P. K. Sangwan P. L. Hamid A. Chashoo G. Szxena A. K. Koul S. Eur. J. Med. Chem. 2012;49:55–67. doi: 10.1016/j.ejmech.2011.12.018. [DOI] [PubMed] [Google Scholar]; (b) Chen Z. Jin K. Gao L. Lou G. Jin Y. Yu Y. Lou Y. Eur. J. Pharmacol. 2010;643:170–179. doi: 10.1016/j.ejphar.2010.06.025. [DOI] [PubMed] [Google Scholar]; (c) Madrid A. Cardile V. González C. Montenegro I. Villena J. Caggia S. Graziano A. Russo A. Int. J. Mol. Sci. 2015;16:7944–7959. doi: 10.3390/ijms16047944. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jana F. Faini F. Lapier M. Pavani M. Kemmerling U. Morello A. Maya J. D. Jara J. Parra E. Ferreira J. Toxicol. Appl. Pharmacol. 2013;272:356–364. doi: 10.1016/j.taap.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Adhikari S. Joshi R. Patro B. S. Ghanty T. K. Chintalwar G. J. Sharma A. Chattopadhyay S. Mukheriee T. Chem. Res. Toxicol. 2003;16:1062–1069. doi: 10.1021/tx034082r. [DOI] [PubMed] [Google Scholar]
- (a) Mao H. Wang H. Ma S. Xu Y. Zhang H. Wang Y. Niu Z. Fan G. Zhu Y. Gao X. M. Toxicol. Appl. Pharmacol. 2014;274:180–189. doi: 10.1016/j.taap.2013.11.001. [DOI] [PubMed] [Google Scholar]; (b) Weng Z. B. Gao Q. Q. Wang F. Zhao G. H. Yin F. Z. Cai B. C. Chen Z. P. Li W. D. Mol. Cell. Endocrinol. 2015;417:103–113. doi: 10.1016/j.mce.2015.09.025. [DOI] [PubMed] [Google Scholar]
- (a) Madrid V. A. Díaz P. K. González T. C. Catalán M. K. Espinoza C. L. Nat. Prod. Res. 2015;29:586–588. doi: 10.1080/14786419.2014.955486. [DOI] [PubMed] [Google Scholar]; (b) Lau K. M. Wong J. H. Wu Y. O. Cheng L. Wong C. W. To M. H. Lau C. P. Yew D. T. Leung P. C. Fung K. P. Hui M. Nq T. B. Lau C. B. Phytomedicine. 2014;21:942–945. doi: 10.1016/j.phymed.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Choi J. M. Oh S. J. Lee J. Y. Jeon J. S. Ryu C. S. Kim Y. M. Lee K. Kim S. K. Chem. Res. Toxicol. 2015;28:872–885. doi: 10.1021/tx500504n. [DOI] [PubMed] [Google Scholar]
- Li Y. G. Hou J. Li S. Y. Lv X. Ning J. Wang P. Liu Z. M. Ren G. B. Ge J. Y. Yang L. Fitoterapia. 2015;101:99–106. doi: 10.1016/j.fitote.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Chen H. Du X. Tang W. Zhou Y. Zuo J. Feng H. Li Y. Bioorg. Med. Chem. 2008;16:2403–2411. doi: 10.1016/j.bmc.2007.11.054. [DOI] [PubMed] [Google Scholar]
- (a) Chaudhuri R. K. Bojanowski K. Int. J. Cosmet. Sci. 2014;36(3):221–230. doi: 10.1111/ics.12117. [DOI] [PubMed] [Google Scholar]; (b) Goldberg D. J. Robinson D. M. Granger C. J. Cosmet., Dermatol. Sci. Appl. 2019;18(3):806–814. doi: 10.1111/jocd.12896. [DOI] [PubMed] [Google Scholar]; (c) Bacqueville D. Maret A. Noizet M. Duprat L. Coutanceau C. Georgescu V. Touya S. B. Duplan H. Clin., Cosmet. Invest. Dermatol. 2020;13:359. doi: 10.2147/CCID.S235880. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d1) Jafernik K. Halina E. Ercisli S. Szopa A. Nat. Prod. Res. 2020;12:1–15. doi: 10.1080/14786419.2020.1837813. [DOI] [PubMed] [Google Scholar]; and cited therein.
- Spierings N. M. J. Cosmet., Dermatol. Sci. Appl. 2020;19(12):3208–3209. doi: 10.1111/jocd.13708. [DOI] [PubMed] [Google Scholar]
- Du X. L. Chen H. L. Feng H. J. Li Y. C. Helv. Chim. Acta. 2008;91(2):371–378. doi: 10.1002/hlca.200890041. [DOI] [Google Scholar]
- Zhang X. Zhao W. Wang Y. Lu J. Chen X. Am. J. Chin. Med. 2016;44:35–60. doi: 10.1142/S0192415X16500038. [DOI] [PubMed] [Google Scholar]
- (a) Labbé C. Faini F. Coll J. Connolly J. D. Phytochemistry. 1996;42(5):1299–1303. doi: 10.1016/0031-9422(96)00144-6. [DOI] [Google Scholar]; (b) Krenisky J. M. Luo J. Reed M. J. Carney J. R. Biol. Pharm. Bull. 1999;22(10):1137–1140. doi: 10.1248/bpb.22.1137. [DOI] [PubMed] [Google Scholar]; (c) Lystvan K. Belokurova V. Sheludko Y. Ingham J. L. Prykhodko V. Kishchenko O. Paton E. Kuchuk M. Plant Cell, Tissue Organ Cult. 2010;101(1):99–103. doi: 10.1007/s11240-009-9657-0. [DOI] [Google Scholar]; (d) Choi S. Y. Lee S. Choi W. H. Lee Y. Jo Y. O. Ha T. Y. J. Med. Food. 2010;13(4):1019–1023. doi: 10.1089/jmf.2009.1207. [DOI] [PubMed] [Google Scholar]; (e) Ohno O. Watabe T. Nakamura K. Kawagoshi M. Uotsu N. Chiba T. Yamada M. Yamaguchi K. Yamada K. Miyamoto K. Uemura D. Biosci., Biotechnol., Biochem. 2010;74(7):1504–1506. doi: 10.1271/bbb.100221. [DOI] [PubMed] [Google Scholar]; (f) Rao G. V. Kavitha K. Gopalakrishnan M. Mukhopadhyay T. J. Pharmacol. Res. 2012;5(1):174–176. [Google Scholar]; (g) Lau K. M. Fu L. H. Cheng L. Wong C. W. Wong Y. L. Lau C. P. Han S. Q. Chan P. K. Fung K. P. Lau C. B. Hui M. Am. J. Chin. Med. 2010;38(5):1005–1014. doi: 10.1142/S0192415X10008421. [DOI] [PubMed] [Google Scholar]; (h) Hsu P. J. Miller J. S. Berger J. M. Nat. Prod. Res. 2009;23(8):781–788. doi: 10.1080/14786410902840158. [DOI] [PubMed] [Google Scholar]; (i) Kai F. Pei H. Qiong G. Lin H. Xi-feng T. Ling-jie Z. Nat. Prod. Res. Dev. 2019;31(2):264. [Google Scholar]; (j) Lou F. M. Yang J. Bai Z. C. Wu B. F. China J. Chin. Mater. Med. 2006;31(12):988–989. [PubMed] [Google Scholar]; (k) Wu S. Hwang C. C. Kuo P. C. Damu A. G. Chou C. J. Chen C. F. Heterocycles. 2002;57(8):1495–1500. doi: 10.3987/COM-02-9509. [DOI] [Google Scholar]; (l) Meng N. Huang S. Dandan H. U. Yanli X. U. Wang Y. Wang J. Chin. Tradit. Pat. Med. 2017;39(5):976–980. [Google Scholar]
- Khushboo P. S. Jadhav V. M. Kadam V. J. Sathe N. S. Pharmacogn. Rev. 2010;4(7):69–76. doi: 10.4103/0973-7847.65331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Xiang Q. Chen Z. Anal. Methods. 2014;6:269–275. doi: 10.1039/C3AY41226A. [DOI] [Google Scholar]
- (a) Alam F. Khan G. N. Asad M. H. Phytother. Res. 2018;32(4):597–615. doi: 10.1002/ptr.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chopra B. Dhingra A. K. Dhar K. L. Fitoterapia. 2013;90:44–56. doi: 10.1016/j.fitote.2013.06.016. [DOI] [PubMed] [Google Scholar]; (c) Sangeetha S. Sarada D. V. J. Pharmacol. Res. 2012;5:1694–1695. [Google Scholar]; (d) Ruan B. Kong L. Y. Takaya Y. Niwa M. J. Asian Nat. Prod. Res. 2007;9(1):41–44. doi: 10.1080/10286020500289618. [DOI] [PubMed] [Google Scholar]
- (a) Katsura H. Tsukiyama R. I. Suzuki A. Kobayashi M. Antimicrob. Agents Chemother. 2001;45(11):3009–3013. doi: 10.1128/AAC.45.11.3009-3013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Haraguchi H. Inoue J. Tamura Y. Mizutani K. Planta Med. 2000;66(6):569–571. doi: 10.1055/s-2000-8605. [DOI] [PubMed] [Google Scholar]
- (a) Epstein J. H. Med. Surg. 1999;18(4):274–284. doi: 10.1016/s1085-5629(99)80026-1. [DOI] [PubMed] [Google Scholar]; (b) Jeong M. Hong T. Lee K. Hwangbo H. Kim M. Ma W. Zahn M. J. AOAC Int. 2015;98(4):902–906. doi: 10.5740/jaoacint.14-228. [DOI] [PubMed] [Google Scholar]
- Jia Q. and Hong M. F., US. Pat., 17/164,231, 2021
- Murray R. D. Jorge Z. D. Lawrie K. W. Tetrahedron. 1983;39(19):3159–3162. doi: 10.1016/S0040-4020(01)91559-5. [DOI] [Google Scholar]
- (a) Kaufmann B. Christen P. Phytochem. Anal. 2002;13(2):105–113. doi: 10.1002/pca.631. [DOI] [PubMed] [Google Scholar]; (b) Lang Q. Wai C. M. Talanta. 2001;53(4):771–782. doi: 10.1016/S0039-9140(00)00557-9. [DOI] [PubMed] [Google Scholar]; (c) Rezaeepour R. Heydari R. Ismaili A. Anal. Methods. 2015;7(7):3253–3259. doi: 10.1039/C5AY00150A. [DOI] [Google Scholar]; (d) Bucar F. Wube A. Schmid M. Nat. Prod. Rep. 2013;30(4):525–545. doi: 10.1039/C3NP20106F. [DOI] [PubMed] [Google Scholar]; (e) Xi J. Li Z. Fan Y. Crit. Rev. Food Sci. Nutr. 2021;61(10):1738–1750. doi: 10.1080/10408398.2020.1765308. [DOI] [PubMed] [Google Scholar]; (f) Dias A. L. de Aguiar A. C. Rostagno M. A. Ultrason. Sonochem. 2021;4:105584. doi: 10.1016/j.ultsonch.2021.105584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Khuranna D. Sharma S. Mir S. R. Aqil M. Ahmad A. Rehman M. U. Ahmad P. Alwahibi M. S. Elshikh M. S. Mujeeb M. Separations. 2020;7(3):48. doi: 10.3390/separations7030048. [DOI] [Google Scholar]; (b) Bhawna C. Dhingra A. K. Dhar K. L. Int. J. Curr. Pharmaceut. Clin. Res. 2013;5:13–16. [Google Scholar]; (c) Lewińska A. Domżał-Kędzia M. Maciejczyk E. Łukaszewicz M. Bazylińska U. Int. J. Mol. Sci. 2021;22:10091. doi: 10.3390/ijms221810091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar B. Sankar K. U. Biotechnol. Bioprocess Eng. 2009;14(1):112–117. doi: 10.1007/s12257-007-0210-x. [DOI] [Google Scholar]
- (a) Banerji A. Chintalwar G. J. Phytochemistry. 1983;22(9):1945–1957. doi: 10.1016/0031-9422(83)80019-3. [DOI] [Google Scholar]; (b) Banerji A. Chintalwar G. J. Phytochemistry. 1984;23:1605–1606. doi: 10.1016/S0031-9422(00)83449-4. [DOI] [Google Scholar]; (c) Banerji A. Chintalwar G. J. Indian J. Biochem. Biophys. 1989;26(6):394–396. [PubMed] [Google Scholar]; (d) Banerji A. Chintalwar G. J. Proc. Indiana Acad. Sci. 1884;93(7):1171–1178. doi: 10.1007/BF02863621. [DOI] [Google Scholar]
- (a) Sukh D. Prakasa A. S. A. Bhalla V. K. Nayak U. R. Tetrahedron. 1972;29:1127–1130. [Google Scholar]; (b) Prakasa Rao A. S. C. Bhalla V. K. Nayak U. R. Dev S. Tetrahedron. 1973;29:1127. doi: 10.1016/0040-4020(73)80072-9. [DOI] [Google Scholar]
- (a) Zhuang X. Zhong Y. Yuan M. Li H. J. Pharm. Biomed. Anal. 2013;75:18–24. doi: 10.1016/j.jpba.2012.11.001. [DOI] [PubMed] [Google Scholar]; (b) Zhang F. X. Qiu Z. C. Yao Z. H. Wong M. S. Yao X. S. Dai Y. Fitoterapia. 2016;109:31–38. doi: 10.1016/j.fitote.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Thomsen K. F. Bundgaard H. Int. J. Pharm. 1993;91:39–49. doi: 10.1016/0378-5173(93)90419-G. [DOI] [Google Scholar]
- Husain F. M. Ahmad I. Khan F. I. Al-Shabib N. A. H Baig M. Hussain A. Rehman M. T. Alajmi M. F. Lobb K. A. Front. Cell. Infect. Microbiol. 2018;8:351. doi: 10.3389/fcimb.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Jin K. Gao L. Lou G. Jin Y. Yu Y. Lou Y. Eur. J. Pharmacol. 2010;643(2–3):170–179. doi: 10.1016/j.ejphar.2010.06.025. [DOI] [PubMed] [Google Scholar]
- (a) Jaing J. B. Thesson D. P. Dusak B. A. Dexter D. L. Kang G. J. Hamel E. J. Med. Chem. 1990;33:1721–1728. doi: 10.1021/jm00168a029. [DOI] [PubMed] [Google Scholar]; (b) Zouhiri F. Danet M. Benard C. Normad-Boyle M. Mbemba G. d'Angelo J. Desmaele D. Tetrahedron Lett. 2005;6(13):2201–2205. doi: 10.1016/j.tetlet.2005.02.033. [DOI] [Google Scholar]; (c) Rocha-Periera J. Cunha R. Pinto D. C. G. Silva A. M. S. Nascimento M. S. J. Bioorg. Med. Chem. 2010;18(12):4195–4201. doi: 10.1016/j.bmc.2010.05.006. [DOI] [PubMed] [Google Scholar]; (d) Lee I. K. Han M. S. Lee M. S. Kim Y. S. Yun B. S. Bioorg. Med. Chem. Lett. 2010;20(18):5459–5461. doi: 10.1016/j.bmcl.2010.07.093. [DOI] [PubMed] [Google Scholar]
- (a) Sun N. J. Woo S. H. Cassady J. M. Snapka R. M. J. Nat. Prod. 1998;61:362–366. doi: 10.1021/np970488q. [DOI] [PubMed] [Google Scholar]; (b) Dong D. G. Zhang Y. Zhang S. L. Lv J. Guo E. M. ZFang Z. Cao Y. F. Int. J. Pharm. Sci. 2014;69:60–63. [PubMed] [Google Scholar]; (c) Choi S. Y. Lee S. Choi W. H. Lee Y. Jo Y. O. Ha T. Y. J. Med. Food. 2010;13(4):1019–1023. doi: 10.1089/jmf.2009.1207. [DOI] [PubMed] [Google Scholar]; (d) Feng J. Yang Y. Zhou Y. Wang B. Xiong H. Fan C. Jiang S. Liu J. Ma Z. Hu W. Li T. Apoptosis. 2016;21(5):532–545. doi: 10.1007/s10495-016-1225-6. [DOI] [PubMed] [Google Scholar]; (e) Hung L. M. Su M. J. Chen J. K. Free Radical Biol. Med. 2004;36(6):774–781. doi: 10.1016/j.freeradbiomed.2003.12.016. [DOI] [PubMed] [Google Scholar]; (f) Szekeres T. Fritzer-Szekeres M. Saiko P. Jäger W. Pharm. Res. 2010;27(6):1042–1048. doi: 10.1007/s11095-010-0090-1. [DOI] [PubMed] [Google Scholar]
- Xu K. Sha Y. Wang S. Chi Q. Liu Y. Wang C. Yang L. Cell Proliferation. 2019;52(5):12666. doi: 10.1111/cpr.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Zafar S. K. Iqbal S. Mumtaz M. Ali S. K. Ali S. T. J. Chem. Soc. Pak. 2018;40(6):1089–1092. [Google Scholar]; (b) Devi A. Namsa N. D. Doley R. Int. J. Biol. Macromol. 2020;165:1066–1078. doi: 10.1016/j.ijbiomac.2020.09.223. [DOI] [PubMed] [Google Scholar]; (c) Kim J. E. Kim J. H. Lee Y. Yang H. Heo Y. S. Bode A. M. Lee K. W. Dong Z. Oncotarget. 2016;7(12):14616. doi: 10.18632/oncotarget.7524. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Cheng M. Chen Z. Electrophoresis. 2017;38:486–493. doi: 10.1002/elps.201600367. [DOI] [PubMed] [Google Scholar]
- (a) Haraguchi H. Inoue J. Tamura Y. Mizutani K. Planta Med. 2000;66:569–571. doi: 10.1055/s-2000-8605. [DOI] [PubMed] [Google Scholar]; (b) Liu H. Guo W. Guo H. Zhao L. Yue L. Li X. Feng D. Luo J. Wu X. Cui W. Qu Y. Front. Pharmacol. 2020;11:712. doi: 10.3389/fphar.2020.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kim K. A. Shim S. H. Ahn H. R. Jung S. H. Toxicol. Appl. Pharmacol. 2013;269(2):109–120. doi: 10.1016/j.taap.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Adhikari S. Joshi R. Patro B. S. Ghanty T. K. Chintalwar G. J. Sharma A. Chattopadhyay S. Mukherjee T. Chem. Res. Toxicol. 2003;16(9):1062–1069. doi: 10.1021/tx034082r. [DOI] [PubMed] [Google Scholar]
- Chen H. Du X. Tang W. Zhou Y. Zuo J. Feng H. Li Y. Bioorg. Med. Chem. 2008;16(5):2403–2411. doi: 10.1016/j.bmc.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Iwamura J. Dohi T. Tanaka H. Odani T. Kubo M. J. Pharm. Soc. Jpn. 1989;109:962–965. doi: 10.1248/yakushi1947.109.12_962. [DOI] [PubMed] [Google Scholar]
- Shoji M. Arakaki Y. Esumi T. Kohnomi S. Yamamoto C. Suzuki Y. Takahashi E. Konishi S. Kido H. Kuzuhara T. J. Biol. Chem. 2015;46:28001–28017. doi: 10.1074/jbc.M115.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed R. Reddy M. V. Chinthakindi P. K. Sangwan P. L. Hamid A. Chashoo G. Saxena A. K. Koul S. Eur. J. Med. Chem. 2012;49:55–67. doi: 10.1016/j.ejmech.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Gautam L. N. Ling T. Lang W. Rivas F. Eur. J. Med. Chem. 2016;113:75–80. doi: 10.1016/j.ejmech.2016.02.034. [DOI] [PubMed] [Google Scholar]
- Cha M. R. Choi C. W. Lee J. Y. Kim Y. S. Yon G. H. Choi S. U. Ryu S. Y. Bull. Korean Chem. Soc. 2012;33:2378–2380. doi: 10.5012/bkcs.2012.33.7.2378. [DOI] [Google Scholar]
- Gupta N. Sharma S. Raina A. Dangroo N. A. Bhushan S. Sangwan P. L. RSC Adv. 2016;6:106150–106159. doi: 10.1039/C6RA23757F. [DOI] [Google Scholar]
- Gupta N. Sharma S. Raina A. Bhushan S. Malik F. A. Sangwan P. L. ChemistrySelect. 2017;2(18):5196–5201. doi: 10.1002/slct.201700504. [DOI] [Google Scholar]
- Li H. Liu J. Liu C. F. Li H. Luo J. Fang S. Chen Y. Zhong R. Liu S. Lin S. J. Med. Chem. 2021;64:5603–5619. doi: 10.1021/acs.jmedchem.0c02059. [DOI] [PubMed] [Google Scholar]
- Wu C. Z. Liu D. C. Guo X. Dai Y. Ma T. Li H. M. Huo Q. Molecules. 2018;23(3):515. doi: 10.3390/molecules23030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Vetica F. de Figueiredo R. M. Orsini M. Tofani D. Gasperi T. Synthesis. 2015;47:2139–2184. doi: 10.1055/s-0034-1378742. [DOI] [Google Scholar]; (b) Quasdorf K. W. Overman L. E. Nature. 2014;516:181–191. doi: 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hawner C. Alexakis A. Chem. Commun. 2010;46:7295–7306. doi: 10.1039/C0CC02309D. [DOI] [PubMed] [Google Scholar]; (d) Trost B. M. Jiang C. Synthesis. 2006:369–396. doi: 10.1055/s-2006-926302. [DOI] [Google Scholar]; (e) Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis, ed. J. Christoffers and A. Baro, Wiley-VCH, Weinheim, 2005 [Google Scholar]
- Choudhury A. R. Manna M. S. Mukherjee S. Chem. Sci. 2017;8:6686–6690. doi: 10.1039/C7SC02232H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodaran N. P. Dev S. Tetrahedron Lett. 1967;30:2897. doi: 10.1016/S0040-4039(00)90883-9. [DOI] [Google Scholar]
- Carnduff J. Miller J. A. Chem. Commun. 1967;12:606–607. doi: 10.1039/C1967000606B. [DOI] [Google Scholar]
- Takano S. Shimazaki Y. Ogasawara K. Tetrahedron Lett. 1990;31:3325. doi: 10.1016/S0040-4039(00)89055-3. [DOI] [Google Scholar]
- Araki S. Bustugan Y. J. Chem. Soc., Perkin Trans. 1. 1991:2395–2397. doi: 10.1039/P19910002395. [DOI] [Google Scholar]
- Sakakiyama S. Yamamoto K. Asaoka M. Nat. Prod. Lett. 1999;14(1):1–4. doi: 10.1080/10575639908045426. [DOI] [Google Scholar]
- Esumi T. Shimizu H. Kashiyama A. Sasaki C. Toyota M. Fukuyama Y. Tetrahedron Lett. 2008;49(48):6846–6849. doi: 10.1016/j.tetlet.2008.09.106. [DOI] [Google Scholar]
- Chen H. Li Y. Lett. Org. Chem. 2008;5(6):467–469. doi: 10.2174/157017808785740499. [DOI] [Google Scholar]
- Du X. Chen H. Feng H. Li Y. Helv. Chim. Acta. 2008;91:371. doi: 10.1002/hlca.200890041. [DOI] [Google Scholar]
- Bequette J. P. Jungong C. S. Novikov A. V. Tetrahedron Lett. 2009;50:6963–6964. doi: 10.1016/j.tetlet.2009.09.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F. McGrath K. P. Lee Y. Hoveyda A. H. J. Am. Chem. Soc. 2010;132:14315–14320. doi: 10.1021/ja106829k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao K. I. Sakamoto S. Touati M. A. Kusakawa Y. Tadano K. I. Molecules. 2012;17(11):13330–13344. doi: 10.3390/molecules171113330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi T. Yamamoto C. Fukuyama Y. Synlett. 2013;14:1845–1847. doi: 10.1055/s-0033-1338968. [DOI] [Google Scholar]
- Xu Q. Q. Zhao Q. Shan G. S. Yang X. C. Shi Q. Y. Lei X. Tetrahedron. 2013;69(50):10739–10746. doi: 10.1016/j.tet.2013.10.064. [DOI] [Google Scholar]
- Battilocchio C. Feist F. Hafner A. Simon M. Tran D. N. Allwood D. M. Blakemore D. C. Ley S. V. Nat. Chem. 2016;4:360–367. doi: 10.1038/nchem.2439. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Zhang G. Org. Lett. 2016;18(19):5094–5097. doi: 10.1021/acs.orglett.6b02540. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S. Takacs J. M. J. Am. Chem. Soc. 2017;139(17):6066–6069. doi: 10.1021/jacs.7b02324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman M. Xu Y. Khan S. Zhang J. Khan A. Chem. Sci. 2020;21:5481–5486. doi: 10.1039/D0SC01763A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Qiu Z. Lv L. Li J. Li C. C. Li C. J. Chem. Sci. 2019;18:4775–4781. doi: 10.1039/C9SC00595A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dou Y. Liu J. Jiang J. Zhu Q. Chem.–Eur. J. 2019;28:6896–6901. doi: 10.1002/chem.201900530. [DOI] [PubMed] [Google Scholar]
- Ma S. Gobis K. Swindell W. R. Chaudhuri R. Bojanowski R. Bojanowski K. Clin. Exp. Dermatol. 2017;3:251–260. doi: 10.1111/ced.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Correa A. Martin R. J. Am. Chem. Soc. 2014;136:7253–7256. doi: 10.1021/ja5029793. [DOI] [PubMed] [Google Scholar]; (b) Alsabeh P. G. Stradiotto M. Angew. Chem., Int. Ed. 2013;52:7242–7246. doi: 10.1002/anie.201303305. [DOI] [PubMed] [Google Scholar]; (c) Alsabeh P. G. Stradiotto M. Angew. Chem., Int. Ed. 2013;125:7383–7387. doi: 10.1002/ange.201303305. [DOI] [PubMed] [Google Scholar]
- (a) Shimasaki T. Tobisu M. Chatani N. Angew. Chem., Int. Ed. 2010;49:2929–2932. doi: 10.1002/anie.200907287. [DOI] [PubMed] [Google Scholar]; (b) Shimasaki T. Tobisu M. Chatani N. Angew. Chem., Int. Ed. 2010;122:2991–2994. doi: 10.1002/ange.200907287. [DOI] [Google Scholar]; (c) Correa A. Leýn T. Martin R. J. Am. Chem. Soc. 2014;136:1062–1069. doi: 10.1021/ja410883p. [DOI] [PubMed] [Google Scholar]; (d) Takise R. Muto K. Yamaguchi J. Itami K. Angew. Chem., Int. Ed. 2014;53:6791–6794. doi: 10.1002/anie.201403823. [DOI] [PubMed] [Google Scholar]; (e) Takise R. Muto K. Yamaguchi J. Itami K. Angew. Chem., Int. Ed. 2014;126:6909–6912. doi: 10.1002/ange.201403823. [DOI] [PubMed] [Google Scholar]
- (a) Zarate C. Manzano R. Martin R. J. Am. Chem. Soc. 2015;137:6754–6757. doi: 10.1021/jacs.5b03955. [DOI] [PubMed] [Google Scholar]; (b) Leiendecker M. Hsiao C.-C. Guo L. Alandini N. Rueping M. Angew. Chem., Int. Ed. 2014;53:12912–12915. doi: 10.1002/anie.201402922. [DOI] [PubMed] [Google Scholar]; (c) Leiendecker M. Hsiao C.-C. Guo L. Alandini N. Rueping M. Angew. Chem., Int. Ed. 2014;126:13126–13129. doi: 10.1002/ange.201402922. [DOI] [PubMed] [Google Scholar]
- (a) Yu D.-G. Li B.-J. Zheng S.-F. Guan B.-T. Wang B.-Q. Shi Z.-J. Angew. Chem., Int. Ed. 2010;49:4566–4570. doi: 10.1002/anie.200907359. [DOI] [PubMed] [Google Scholar]; (b) Yu D.-G. Li B.-J. Zheng S.-F. Guan B.-T. Wang B.-Q. Shi Z.-J. Angew. Chem. 2010;122:4670–4674. doi: 10.1002/ange.200907359. [DOI] [Google Scholar]; (c) Yu D.-G. Shi Z.-J. Angew. Chem., Int. Ed. 2011;50:7097–7100. doi: 10.1002/anie.201101461. [DOI] [PubMed] [Google Scholar]; (d) Yu D.-G. Shi Z.-J. Angew. Chem., Int. Ed. 2011;123:7235–7238. doi: 10.1002/ange.201101461. [DOI] [Google Scholar]
- (a) Wenkert E. Michelotti E. L. Swingdell C. S. Tingoli M. J. J. Org. Chem. 1984;49:4894. doi: 10.1021/jo00199a030. [DOI] [Google Scholar]; (b) Chen G. J. Huang J. Gao L. X. Han F. S. Chem.–Eur. J. 2011;17:4038–4042. doi: 10.1002/chem.201003403. [DOI] [PubMed] [Google Scholar]; (c) . Yu D. G. Shi Z. J. Angew. Chem., Int. Ed. 2011;50:7097–7100. doi: 10.1002/anie.201101461. [DOI] [PubMed] [Google Scholar]
- (a) Yu D. G. Li B. J. Zheng S. F. Guan B. T. Wang B. Q. Shi Z. J. Angew. Chem., Int. Ed. 2010;49:4566. doi: 10.1002/anie.200907359. [DOI] [PubMed] [Google Scholar]; (b) Iranpoor N. Panahi F. Adv. Synth. Catal. 2014;356:3067–3073. doi: 10.1002/adsc.201400460. [DOI] [Google Scholar]; (c) Chen Z. Zeng H. Girard S. A. Wang F. Chen N. Li C. J. Angew. Chem., Int. Ed. 2015;54:14487–14491. doi: 10.1002/anie.201506751. [DOI] [PubMed] [Google Scholar]; (d) Chen Z. Zeng H. Gong H. Wang H. Li C. J. Chem. Sci. 2015;6:4174–4178. doi: 10.1039/C5SC00941C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Khakyzadeh V. Rostami A. Veisi H. Shaghasemi B. S. Reimhult E. Luque R. Xia Y. Darvishi S. Org. Biomol. Chem. 2019;17:4491–4497. doi: 10.1039/C9OB00313D. [DOI] [PubMed] [Google Scholar]; (b) Liu Y. L. Xu X. H. Qing F. L. Tetrahedron. 2018;74(40):5827–5832. doi: 10.1016/j.tet.2018.08.036. [DOI] [Google Scholar]
- Cao Z. C. Luo F. X. Shi W. J. Shi Z. J. Org. Chem. Front. 2015;2:1505–1510. doi: 10.1039/C5QO00243E. [DOI] [Google Scholar]
- Zhao Y.-L. Wu G.-J. Han F.-S. Chem. Commun. 2012;48:5868–5870. doi: 10.1039/C2CC31718D. [DOI] [PubMed] [Google Scholar]
- (a) Liras S. Davoren J. E. Bordner J. Org. Lett. 2001;3:703–706. doi: 10.1021/ol0070482. [DOI] [PubMed] [Google Scholar]; (b) Arend M. Westermann B. Risch N. Angew. Chem., Int. Ed. 1998;37:1044–1070. doi: 10.1002/(SICI)1521-3773(19980504)37:8<1044::AID-ANIE1044>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]; (c) Ito M. Clark C. W. Mortimore M. Goh J. B. Martin S. F. J. Am. Chem. Soc. 2001;123:8003–8010. doi: 10.1021/ja010935v. [DOI] [PubMed] [Google Scholar]
- Anuradha V. Srinivas P. V. Aparna P. Rao J. M. Tetrahedron Lett. 2006;47(28):4933–4935. doi: 10.1016/j.tetlet.2006.05.017. [DOI] [Google Scholar]
- Akselsen O. W. Skattebol L. Hansen T. V. Tetrahedron Lett. 2009;50:6339–6341. doi: 10.1016/j.tetlet.2009.08.101. [DOI] [Google Scholar]
- (a) Yu J. Li C. J. Zeng H. Angew. Chem., Int. Ed. 2021;60(8):4043–4048. doi: 10.1002/anie.202010845. [DOI] [PubMed] [Google Scholar]; (b) Ma B. Tang Z. Zhang J. Liu L. Chem. Commun. 2020;56(66):9485–9488. doi: 10.1039/D0CC04495D. [DOI] [PubMed] [Google Scholar]; (c) Dou Y. Liu J. Jiang J. Zhu Q. Chem.–Eur. J. 2019;25(28):6896–6901. doi: 10.1002/chem.201900530. [DOI] [PubMed] [Google Scholar]; (d) Battini N. Battula S. Ahmed Q. N. Eur. J. Org. Chem. 2016:658–662. doi: 10.1002/ejoc.201501423. [DOI] [Google Scholar]; (e) Yu C. Patureau F. W. Angew. Chem., Int. Ed. 2018;57:11807–11811. doi: 10.1002/anie.201804829. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chattopadhyay B. Dannatt J. E. Andujar-De Sanctis I. L. Gore K. A. Maleczka Jr R. E. Singleton D. A. Smith M. R. J. Am. Chem. Soc. 2017;139(23):7864–7871. doi: 10.1021/jacs.7b02232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Krishna T. P. A. Sakthivel P. Ilangovan A. Org. Chem. Front. 2019;6:3244–3251. doi: 10.1039/C9QO00623K. [DOI] [Google Scholar]; (b) Sakthivel P. Krishna T. P. A. Ilangovan A. Org. Biomol. Chem. 2020;18:3027–3031. doi: 10.1039/D0OB00323A. [DOI] [PubMed] [Google Scholar]; (c) Krishna T. P. A. Sakthivel P. Suresh C. Ilangovan A. RSC Adv. 2020;10:19454–19462. doi: 10.1039/D0RA01519A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Rajasekar S. Krishna T. P. A. Tharmalingam N. Ilangovan A. Mylonakis E. ChemistrySelect. 2019;4:2281–2287. doi: 10.1002/slct.201803816. [DOI] [Google Scholar]
- (a) Kim B. H. Lee Y. S. Kang K. S. Toxicol. Lett. 2003:65–73. doi: 10.1016/j.toxlet.2003.09.001. [DOI] [PubMed] [Google Scholar]; (b) Sorg O. Kuenzli S. Kaya G. Saurat J. H. J. Cosmet., Dermatol. Sci. Appl. 2005;4:237–244. doi: 10.1111/j.1473-2165.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- (a) Zasada M. Budzisz E. Adv. Dermatol. Allergol. 2019;36(4):392–397. doi: 10.5114/ada.2019.87443. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sah P. Agarwal D. Garg S. P. Indian J. Pharm. Sci. 2006;68(6):768–771. doi: 10.4103/0250-474X.31012. [DOI] [Google Scholar]; (c) Brecher A. R. Orlow S. J. J. Am. Acad. Dermatol. 2003;49(2):171–182. doi: 10.1067/S0190-9622(03)01564-0. [DOI] [PubMed] [Google Scholar]
- Cartmel B. Dziura J. Cullen M. R. Vegso S. Omenn G. S. Goodman G. E. Redlich C. A. Eur. J. Clin. Nutr. 2005;59:1173–1180. doi: 10.1038/sj.ejcn.1602229. [DOI] [PubMed] [Google Scholar]