Abstract
Background:
People with chronic low back pain display the altered movement pattern where the lumbar spine moves more readily into its available range of motion relative to other joints. A logical approach to treatment, therefore, would be to improve this pattern during functional activities.
Methods:
154 participants were randomized to receive 6 weeks of motor skill training or strength and flexibility exercise. Participants in the motor skill training group received person-specific training to modify their altered movement pattern during functional activities. Participants in the strength and flexibility group received exercises for trunk strength and trunk and lower-limb flexibility. At baseline, post-treatment and 6-months after treatment participants performed a test of picking up an object using their preferred pattern. Three-dimensional marker co-ordinate data were collected. A mixed-model repeated measures analysis of variance was used to examine the treatment group and time effects.
Findings:
Motor skill training:
Baseline early excursion values [mean (confidence interval)] were as follows: knee=11.1°(8.0,4.1), hip=21.2°(19.2,23.1), lumbar=11.3°(10.4,12.3). From baseline to post-treatment significant improvements in early excursion included: knee=+18.6°(15.4,21.8), hip=+10.8°(8.8,12.8), and lumbar =−2.0°(−0.1,−4.0). There were no significant changes from post-treatment to 6-month follow-up.
Strength and flexibility exercise:
Baseline early excursion values were as follows:knee=8.9°(5.8,11.9), hip=20.8°(18.9,22.8), and lumbar=11.2°(10.3,12.2) early excursion. There were no significant changes for knee, hip, and lumbar early excursion.
Interpretation:
Motor skill training was more effective than strength and flexibility exercise at changing and maintaining change to the altered movement pattern during a functional activity test of picking up an object.
Keywords: low back pain, chronic, functional activity, motor skill training, exercise, movement system
1. Introduction
At least 60-80% of adults experience low back pain (LBP) during their lifetime.5,7 Of those who have an initial episode, up to 75% report continued pain and limitation in function at 1 year.14,19 Thus, the majority of people who have an episode of LBP will transition to a fluctuating or persistent chronic course. In addition, the primary reason people with LBP seek medical care is a limitation in the performance of functional activities (e.g., reaching to the floor and picking up objects).4,27 Therefore, it is logical that treatment should be tailored towards improving the performance of functional activities limited due to LBP.
How people with LBP move during functional activities may contribute to why limitations in function are a primary problem. For example, people with chronic LBP often display an altered movement pattern in which the lumbar spine moves more readily into its available range of motion compared to other joints (e.g., knee and hip) that can contribute to the activity.17,18,25,33,38 The alteration is more prevalent in people with LBP compared to back healthy controls25,33,42 and, notably, is displayed across multiple functional activity tests.24 Importantly, the magnitude of the altered movement pattern is associated with a person’s LBP and LBP-related functional limitations.24,36 Thus, the altered movement pattern during functional activities is a relevant factor that may contribute to persistent functional limitation in people with LBP.
Exercise is a primary non-pharmacologic treatment for chronic LBP.23 Although exercise is often recommended,2,3,12,29 no specific exercise treatment is consistently more beneficial than another in the short-term and long-term.2,23,29 One potential reason for inconsistent effects is that the treatments tested do not directly address how people with LBP perform LBP-limited functional activities. Given the documented relevance of the altered movement pattern to limitations in functional activities24, a logical form of exercise-based treatment would target the altered pattern during performance of limited functional activities.35 Motor skill training (MST) is an exercise-based treatment that employs motor learning principles to promote the learning or relearning of motor skills.1,21,35 The overarching goal of MST applied to LBP-limited functional activities is to replace the person-specific, pain-provoking, altered movement pattern with an improved and symptom-free pattern.21,26,35 After a single session of MST, immediate improvement was reported in both the altered movement pattern and pain during the functional activity test of picking up an object (PUO).26 Further investigation is warranted to understand whether the altered movement pattern during the PUO activity can be improved over the short-term and long-term using MST.
The purpose of this study was to compare the short-term and long-term effects of MST and strength and flexibility exercise (SFE) on movement of the knee, hip, and lumbar spine during the performance of the functional activity test of picking up an object. We chose the functional test of PUO since this is often reported as an activity that is difficult for people with LBP to perform. We hypothesized that MST would result in greater improvements in the altered movement pattern than SFE. Specifically, we hypothesized that after treatment the MST group would decrease early lumbar movement and increase early knee and hip movement, while the SFE group would not change the movement pattern across these regions. We also hypothesized that the improvements in the altered movement pattern in the MST group would be maintained in the 6 months after treatment.
2. Methods
2.1. Participants
This is a planned secondary analysis of kinematic data from 154 people with chronic7 non-specific28 LBP recruited as part of a single-blind, prospective, randomized controlled clinical trial.35 Recruitment was through word of mouth, flyers placed in the community, and ads and interviews through media and clinics in the region. Participants included were between 18-60 years of age, had chronic LBP for at least 1 year, experienced LBP but were not in an acute flare-up39, had a modified Oswestry Disability Questionnaire (MODQ) score of ≥ 20%, could stand and walk without assistance and could understand and sign a consent form. Participants were excluded if they had (1) a body mass index (BMI) >30, (2) any structural spinal deformity, (3) a spinal tumor or infection, (4) osteoporosis, (5) ankylosing spondylitis, (6) rheumatoid arthritis, (7) symptomatic disc herniation, (8) spondylolisthesis. Additional exclusion criteria can be found on Clinicaltrials.gov (NCT02027623).35 The study was approved by the Institutional Review Board and all participants provided written informed consent before enrolling in the study.
2.2. Data collection
Participants completed laboratory sessions at baseline, immediately following 6 weeks of treatment (post-treatment), and 6 months after treatment. For this planned secondary analysis we used a subset of self-report measures that were completed during the clinical trial to define clinical characteristics of our sample. These included a (1) demographic and LBP history questionnaire, (2) MODQ, (3) Numeric Rating Scale (NRS) for average (previous 7 days) and worst LBP symptoms (4) Fear-Avoidance Beliefs Questionnaire (FABQ)22,40 and (5) Short Form Health Survey (SF-36).20 Initially, a standardized examination was performed by a physical therapist to classify the person’s LBP.9,13,34,37 Classification was based on the person’s altered movements and alignments of the lumbar spine and LBP reports during clinical tests.11,38 Classification was used to aid the person-specific aspect of treatment within MST.
Reflective markers were placed on anatomical landmarks of the trunk, pelvis, and lower extremity, according to previously documented procedures.24,25 Anthropometric measurements were determined for each participant’s shank and trunk length. Shank length was measured as the vertical distance from the floor to fibular head. Trunk length was measured as the vertical distance between the marker superficial to the spinous process of the 7th cervical (C7) and the marker superficial to the spinous process of the 1st sacral (S1) vertebrae. Participants were instructed to perform three trials of a standardized functional activity test of picking up an object (PUO).24 Measures were obtained at baseline, immediately post-treatment and at 6-months post-treatment. For the PUO test, participants stood with their feet pelvis width apart and were told to pick up the container with both hands and return to the starting position. A 20 x 36 x 12 cm, lightweight container was placed at a height equal to the participant’s shank length, and a distance of 50% of trunk length. No instructions were given to the participant for how to pick up the object. Participants were given a maximum of 10 seconds to complete each movement trial. Marker co-ordinate data were collected for both a static standing trial and the PUO trials using a three-dimensional motion capture system (Vicon Motion Systems, LTD, Denver, CO) with a sampling rate of 120 Hz.
Marker trajectory data were labeled using Nexus 2.7.1 (Vicon Motion Systems, LTD, Denver, CO). Next, a rigid body link segment model was created using custom programs written in Visual 3D 2020 (C-Motion Inc., Germantown, MD) and MATLAB 2017b software (MathWorks Inc., Natick, MA).24,45 Marker position data were filtered using a 4th order low-pass Butterworth filter with a cut-off frequency of 3 Hz. The cut-off frequency was based on residual analysis45 of similar movement tests.24,25 Vectors created between markers were used to describe joint angles (e.g., lumbar: T12, L3 and S1 markers) across movement time (Table 1).24,45 The lumbar spine angle was calculated as the displacement of the lumbar segment relative to the pelvis (i.e. CODA pelvis). The hip joint angle was defined as the displacement of the thigh segment relative to the pelvis. The knee joint angle was defined as the shank segment relative to the thigh segment. Early was defined as the 1st half of movement time for the descent phase of the PUO task.24–26 Initially a baseline joint angle was determined based on a 25 frame moving average for the knee, hip and lumbar spine. The start value was defined as the first joint angle that was 1 degree greater than baseline. The stop value was defined as the last joint angle value that was 98% of the absolute maximum. The 50% cutoff was determined based on total movement time from start to stop.
TABLE 1.
Marker set and segment definitions for movement testing.
| Markers | |||
|---|---|---|---|
| Segment | 1st | 2nd | 3rd |
| Lumbar Spine | S1 | T12 | L3 Lateral to L3 |
| Pelvis | Anterior superior iliac spines | Posterior superior iliac spines | -- |
| Thigh | Greater trochanter | Lateral & medial femoral condyle | Lateral Thigh Cluster |
| Shank | Lateral & medial femoral condyle | Lateral & medial malleolus | Lateral Shank Cluster |
S1 = spinous process of the 1st sacral vertebrae
T12 = spinous process of the 12th thoracic vertebrae
L3 = spinous process of the 3rd lumbar vertebrae
Lateral to L3 and thigh/shank clusters were used as additional tracking markers
All markers not on the spine were placed bilaterally
After the baseline data collection, participants were randomized to one of two treatment conditions, MST or SFE. Full details of the treatment conditions can be found on Clinicaltrials.gov (NCT02027623, doi:10.1001/jamaneurol.2020.4821).35 Briefly, MST involved practice to challenge participants to modify patient-specific, pain provoking, altered movement and alignment pattern during the performance of functional activities.35 The primary objectives of treatment were to train the participant to (1) reduce the amount of early lumbar spine movement related to the participant’s LBP classification (e.g., flexion), (2) increase the movement of other joints (e.g., knees and hips) and (3) avoid prolonged end range alignments of the lumbar spine in the specific direction related to the participant’s LBP classification.35 Physical therapists minimized extrinsic feedback during practice and training focused on problem solving by the participant to learn to perform the activities without increased LBP.35 MST was progressed within and between visits to match the participant’s motor capabilities.35 SFE focused on increasing the strength of all of the trunk muscles and improving trunk and lower limb flexibility in all planes following American College of Sports Medicine guidelines.6 SFE was progressed based on the participant’s ability to perform each exercise independently.10 All participants received 6, 1-hour treatment sessions, scheduled once/week for 6 weeks.35 We chose to use SFE because it is considered one of the primary non-pharmacologic and non-surgical treatments for chronic low back pain.23 Additional laboratory testing sessions were completed immediately post-treatment and 6 months after treatment. Both groups were told to adhere to their home program during both the active treatment and follow-up phase. Reproduction of symptoms during treatment was allowed as long as the person’s condition did not worsen. Additional treatment details can be found in the supplementary materials of the primary outcome manuscript (doi:10.1001/jamaneurol.2020.4821).35
2.3. Data analysis
Analyses were performed using R v3.5.3.31,43,44 The sample size of 154 participants was determined by a power analysis for detecting a minimal clinically important difference of 6 on the MODQ, which was the primary outcome measure for the clinical trial.35 Early excursion of the knee, hip, and lumbar spine was normally distributed based on the Shapiro-Wilk test (all p > 0.17). Descriptive statistics were calculated for participant demographics and self-report measures. A two-way, mixed effect analysis of variance (ANOVA) model was used to examine main effects of Treatment group (Tx), Time, and the Tx X Time interaction for early excursion of the knee, hip, and lumbar spine. When interactions were significant, a priori planned pairwise comparisons were examined using a Tukey’s HSD correction factor. Specifically, planned pairwise comparisons were examined for early movement for the knee, hip, and lumbar spine (1) between treatment groups at each time point and (2) within treatment groups from baseline to post-treatment and post-treatment to 6 month follow up.
3. Results
One hundred and fifty-four participants were enrolled in the study. Twenty-one participants dropped out over the study duration; Five were prior to treatment (MST = 3, SFE = 2). Participant characteristics for the sample are summarized in Table 2. At baseline, MST and SFE were similar in age, gender, body mass index, duration of LBP, medication use, MODQ scores, average and worst NRS scores, FABQ-work and physical scores, SF-36 physical and mental component scores.
TABLE 2.
Mean (95% confidence interval) for participant characteristics by treatment group
| Strength and Flexibility Exercise (n = 77) | Motor Skill Training (n = 77) | |
|---|---|---|
| Age (years) | 42.6 (40.0,45.3) | 42.5 (39.9,45.1) |
| Female, no. (%) | 52 (68) | 43 (56) |
| BMI (kg/m2) | 25.4 (24.7,26.1) | 26.1 (25.4,26.8) |
| Duration of LBP (years)e | 7.0 (11.0) | 7.0 (17.0) |
| LBP Medication Use (%) | 48 (62.3) | 45 (58.4) |
| Function (MODQ, 0-100%)a | 32.7 (30.4,35.0) | 32.5 (29.9,34.5) |
| Average Pain (NRS, 0-10)b | 4.7 (4.3,5.1) | 4.7 (4.3,5.0) |
| Worst Pain (NRS, 0-10)b | 6.3 (5.8,6.8) | 6.8 (6.4,7.2) |
| Fear-Work (FABQ-W, 0-42)c | 11.0 (9.2,12.9) | 11.7 (9.7,13.7) |
| Fear-Physical (FABQ-P, 0-24)c | 14.9 (13.6,16.3) | 14.1 (12.9,15.2) |
| SF-36 Physicald | 42.9 (41.4,44.4) | 40.7 (39.2,42.3) |
| SF-36 Mentalc | 48.8 (46.2,51.4) | 52.1 (50.0,54.1) |
modified Oswestry Disability Questionnaire scores range between 0% (no LBP-related functional limitation) and 100% (max limitation)
Patient report of average or worst pain in the prior 7 days on verbal numeric pain rating scale between 0 (no pain) and 10 (pain as bad as can be)
Fear-Avoidance Beliefs Questionnaire physical activity subscale score ranges from 0-24 and work subscale score ranges from 0-42 with higher scores indicating higher fear-avoidance
36-Item Short Form Health Survey (SF-36) Physical and Mental Component summary scores are scaled and normalized to have a mean of 50 and standard deviation of 10 in the normal 1998 US population
Data were not normally distributed, median (IQR) displayed
Prior to treatment, MST and SFE had similar knee [difference (CI) = 2.2° (−6.7, 2.5)], hip [difference (CI) = 0.4° (−2.9, 2.5)], and lumbar spine [difference (CI) = 0.1° (−1.4, 1.2)] early movement (Figure 1). After treatment MST increased early movement of the knee [Δ (CI) = +18.6° (14.6, 22.1)] and hip [Δ (CI) = +10.8° (8.5, 13.1)], and decreased early movement of the lumbar spine [Δ (CI) = −2.0° (−3.0, −1.0)]. SFE did not change early movement for the knee [Δ (CI) = −0.5° (−3.5, 2.5)], hip [Δ (CI) = +0.8° (−1.1, 2.7)], or lumbar spine [Δ (CI) = +0.2° (−0.6, 1.0)]. Six months after treatment MST maintained improvements of knee [Δ (CI) = 2.2° (−2.5, 6.7)], hip [Δ (CI) = 0.4° (−2.5, 2.9)], and lumbar spine [Δ (CI) = 0.1° (−1.2, 1.4)] early movement (Figure 1) obtained with treatment. In the SFE group, there was no significant change in early joint movements from post-treatment to the 6-month follow up (all p > 0.90).
Figure 1.

Example of the altered pattern during the functional test of pick up an object.
4. Discussion
The purpose of this study was to compare the short- and long-term effects of MST and SFE on movement of the knee, hip and lumbar spine during the functional activity test of PUO in people with chronic LBP. As hypothesized, after 6 weeks of treatment we found MST resulted in a significant increase in early movement of the knee and hip and a decrease in early movement of the lumbar spine during the PUO test. Alternatively, SFE resulted in no change in early movement of the knee, hip and lumbar spine during the PUO test. We also hypothesized that the improvements in the altered movement pattern in the MST group would be maintained over the long-term. We found that the improved movement pattern obtained immediately post-MST was maintained 6 months after treatment. In addition, the movement pattern of the knee, hip and lumbar spine in the SFE group observed at baseline and post-SFE was similar 6 months after treatment. Therefore, MST directed at performance of a functional activity of PUO is superior to SFE in the ability to improve and maintain improvements in an altered movement pattern identified as important in people with chronic LBP.
Although MST targeting altered movement patterns during functional activities has not been widely studied in people with LBP, other exercise-based treatments have been examined. One treatment that has been extensively investigated in people with LBP is exercise to improve activation of the deep muscles to stabilize the spine (i.e., multifidus and transversus abdominis).8,15,16,32 In this intervention the motor skill targeted is isometric, continuous cocontraction of the deep muscles independent of superficial trunk muscle contraction.8,15,16,32 Typically, a final stage of the treatment is to progress to incorporating appropriate activation of these deep muscles during performance of light and heavy load functional activities. In contrast, rather than aiming to improve activation of specific deep muscles that stabilize the spine as the motor skill, the goal of MST in our clinical trial was to target the altered movement pattern shown to be relevant to the person’s LBP presentation during LBP-limited functional activities. Furthermore, we directly addressed functional activities from the beginning of training rather than starting with a more traditional exercise to improve activation of the deep muscles and then progressing to their use during functional activities. Given the observed short- and long-term improvements in the altered movement pattern during a functional activity test within MST and lack of change in the SFE group, these data further highlight the importance of using motor learning principles to directly address the person-specific altered movement pattern during functional activities.
Prior studies report a short-term change in movement-related variables (e.g., lumbar range of motion, lumbar movement velocity, etc.) in people with chronic LBP.41 However, studies that report durable change in movement (i.e., greater than 3 months) are scarce; short-term changes in movement are often not sustained.30,41 Results of the current trial suggest the improvements in the altered movement pattern within the MST group were maintained 6 months after treatment. A key component of MST during functional activities is the use of principles of motor learning to drive change. Participants problem-solved how to perform their LBP-limited activities without increased LBP while extrinsic feedback was minimized. Functional activity demands were repeatedly progressed to further facilitate learning across multiple activities. This aspect of MST, which is unique compared to previous trials examining change in movement, is likely to be contributing to the durable change in movement. Therefore, if a goal of treatment is to change a long-standing altered movement pattern associated with LBP, these data suggest a priority for treatment is to provide person-specific, challenging practice in a manner that promotes learning.
There were limitations to the study. First, the PUO test was standardized to the person. Specifically, participants were asked to stand with their feet pelvis width apart and the object was set at a standardized height and distance. We chose to standardize the test to minimize the potential effect of individual anthropometrics on the performance of the activity. However, the functional activity test of PUO may not be representative of how all people typically pick up objects during their day. Second, people with a BMI > 30 were excluded from this study. People were excluded based on this criterion to minimize skin artifact and thus ensure valid motion capture data. However, this exclusion reduces the ability to generalize findings to those with chronic LBP and a BMI > 30. Future work is warranted to understand these limitations and the generalizability of our findings.
5. Conclusions
Our findings suggest MST is more effective than SFE in improving and maintaining improvements of the altered movement pattern during functional activities commonly observed in people with chronic LBP. After 6 weeks of MST, people reduced early movement of the lumbar spine and increased early movement of the knee and hip joints with a functional activity test of PUO. These improvements were maintained 6 months after treatment. Alternately, the SFE group had no change in early movement of their knee, hip and lumbar spine across the study duration. Therefore, if a goal of treatment for people with chronic LBP is to improve and maintain the improvement of the altered movement pattern during performance of a functional activity, MST is superior to SFE.
Figure 2.
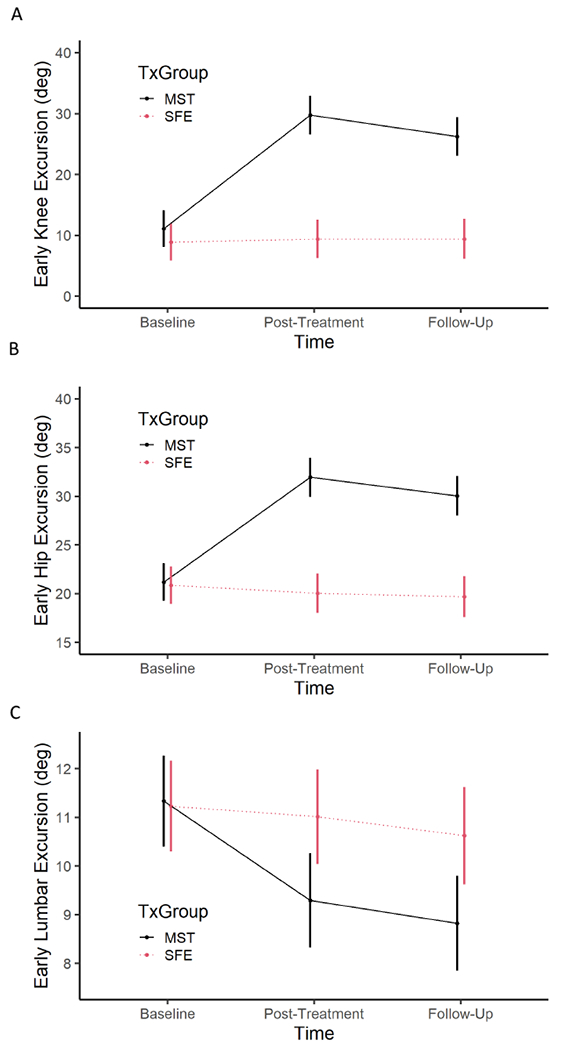
Early excursion (degrees) of the (A) knee, (B) hip, and (C) lumbar spine during a functional activity test of picking up an object. Outcomes and confidence intervals are displayed for motor skill training (MST) and strength and flexibility exercise (SFE) at baseline, post-treatment, and 6 month follow-up time points.
Highlights.
Motor skill training improved the altered movement pattern during a functional test.
Strength and flexibility exercise did not change the altered movement pattern.
Improvements in the pattern within motor skill training were maintained 6 months after treatment.
Acknowledgments:
The authors would like to thank Sara Putnam, Sara Francois and Jennifer Jarvis for their assistance with participant recruitment, data collection and data processing. The authors would also like to thank Kristen Ivy, Gabriel Dorn and Kara Ringkamp for their assistance with data processing.
Primary Funding Source:
This work was supported by the National Institutes of Health [R01HD047709, TL1TR002344].
Declarations of interest:
This project was funded by National Institutes of Health (R01HD047709. QLH was supported by a training grant from National Institutes of Health (TL1TR002344)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Boudreau SA, Farina D, Falla D. The role of motor learning and neuroplasticity in designing rehabilitation approaches for musculoskeletal pain disorders. Man Ther. 2010;15(5):410–414. [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Deyo R, Friedly J, et al. Noninvasive Treatments for Low Back Pain. 2016. AHRQ Publication No. 16-EHC004-EF. [PubMed] [Google Scholar]
- 3.Chou R, Deyo R, Friedly J, et al. Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166(7):493–505. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira ML, Machado G, Latimer J, Maher C, Ferreira PH, Smeets RJ. Factors defining care-seeking in low back pain -a meta-analysis of population based surveys. Eur J Pain. 2010;14(7):747 e741–747. [DOI] [PubMed] [Google Scholar]
- 5.Fourney DR, Andersson G, Arnold PM, et al. Chronic low back pain: a heterogeneous condition with challenges for an evidence-based approach. Spine. 2011;36:S1–S9. [DOI] [PubMed] [Google Scholar]
- 6.Franklin BA, Whaley MH, Howley ET, et al. Section III: Exercise Prescription. In: Johnson EP, Napora LS, eds. ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000:137–234. [Google Scholar]
- 7.Frymoyer JW. Back pain and sciatica. New Eng J Med. 1988;318(5):291–300. [DOI] [PubMed] [Google Scholar]
- 8.Gomes-Neto M, Lopes JM, Conceicao CS, et al. Stabilization exercise compared to general exercises or manual therapy for the management of low back pain: a systematic review and meta-analysis. Phys Ther Sport. 2017;23:136–142. [DOI] [PubMed] [Google Scholar]
- 9.Harris-Hayes M Reliability of examiners to classify LBP problems with the Movement System Impairment Classification System. American Association of Orthopedic and Manual Physical Therapy. 2007. [Google Scholar]
- 10.Harris-Hayes M, Holtzman GW, Early J, Van Dillen LR. Development and preliminary reliability testing of an assessment of patient independence in performing a treatment program: standardized scenarios. J Rehab Med. 2010;42(3):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris-Hayes M, Van Dillen LR. The inter-tester reliability of physical therapists classifying low back pain problems based on the movement system impairment classification system. PM&R. 2009;1(2):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden JA, van Tulder MW, Malmivaara AV, Koes BW. Meta-analysis: exercise therapy for nonspecific low back pain. Ann Intern Med. 2005;142(9):765–775. [DOI] [PubMed] [Google Scholar]
- 13.Henry SM, Van Dillen LR, Trombley AL, Dee JM, Bunn JY. Reliability of novice raters in using the movement system impairment approach to classify people with low back pain. Man Ther. 2013;18(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: what is the long-term course? A review of studies of general patient populations. Euro Spine J. 2003;12(2):149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electro and Kines. 2003;13(4):361–370. [DOI] [PubMed] [Google Scholar]
- 16.Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21(22):2640–2650. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman SL, Johnson MB, Zou D, Harris-Hayes M, Van Dillen LR. Effect of classification-specific treatment on lumbopelvic motion during hip rotation in people with low back pain. Man Ther. 2011;16(4):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman SL, Johnson MB, Zou D, Van Dillen LR. Differences in end-range lumbar flexion during slumped sitting and forward bending between low back pain subgroups and genders. Man Ther. 2012;17(2):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itz CJ, Geurts JW, van KM, Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Euro J Pain. 2013;17(1):5–15. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306(6890):1437–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanier VM, Lang CE, Van Dillen LR. Motor skill training in musculoskeletal pain: a case report in chronic low back pain. Disabil Rehabil. 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30(1):77–94. [DOI] [PubMed] [Google Scholar]
- 23.Malfliet A, Ickmans K, Huysmans E, et al. Best Evidence Rehabilitation for Chronic Pain Part 3: Low Back Pain. J Clin Med. 2019;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marich AV, Hwang CT, Salsich GB, Lang CE, Van Dillen LR. Consistency of a lumbar movement pattern across functional activities in people with low back pain. Clin Biomech. 2017;44:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marich AV, Hwang CT, Sorensen CJ, van Dillen LR. Examination of the lumbar movement pattern during a clinical test and a functional activity test in people with and people without low back pain. Phys Med Rehabil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marich AV, Lanier VM, Salsich GB, Lang CE, Van Dillen LR. Immediate effects of a single session of motor skill training on the lumbar movement pattern during a functional activity in people with low back pain: a repeated-measures study. Phys Ther. 2018;98(7):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortimer M, Ahlberg G. To seek or not to seek? Care-seeking behaviour among people with low-back pain. Scand J Public Health. 2003;31(3):194–203. [DOI] [PubMed] [Google Scholar]
- 28.Nordin M, Weiser S, van Doorn JW, Hiebert R. Nonspecific low back pain. In: Rom WN, ed. Environmental and Occupational Medicine. 3rd ed. Philadelphia, PA: Lippincott-Raven Publishers; 1998:947–956. [Google Scholar]
- 29.Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–2803. [DOI] [PubMed] [Google Scholar]
- 30.Poitras S, Loisel P, Prince F, Lemaire J. Disability measurement in persons with back pain: a validity study of spinal range of motion and velocity. PM&R. 2000;83(10):1394–1400. [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. R: A language and environment for statistical computing. In: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 32.Richardson CA, Jull G, Hodges P, Hides J. Therapeutic Exercise for spinal segmental stabilization in low back pain: scientific base and clinical approach. 1999. [Google Scholar]
- 33.Scholtes SA, Gombatto SP, Van Dillen LR. Differences in lumbopelvic motion between people with and people without low back pain during two lower limb movement tests. Clin Biomech. 2009;24(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trudelle-Jackson E, Sarvaiya-Shah SA, Wang SS. Interrater reliability of a movement impairment-based classification system for lumbar spine syndromes in patients with chronic low back pain. JOSPT. 2008;38(6):371–376. [DOI] [PubMed] [Google Scholar]
- 35.Van Dillen LR, Lanier VM, Steger-May K, Wallendorf M, Norton BJ, Civello JM, Czuppon SL, Francois SJ, Roles K, Lang CE. Effect of motor skill training in functional activities vs strength and flexibility exercise on function in people with chronic low back pain – a randomized clinical trial. JAMA Neuro. 2021;78(4):385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Dillen LR, Sahrmann SA, Norton BJ, et al. Effect of active limb movements on symptoms in patients with low back pain. JOSPT. 2001;31(8):402–413. [DOI] [PubMed] [Google Scholar]
- 37.Van Dillen LR, Sahrmann SA, Norton BJ, et al. Reliability of physical examination items used for classification of patients with low back pain. Phys Ther. 1998;78(9):979–988. [DOI] [PubMed] [Google Scholar]
- 38.Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom NJ. Movement system impairment-based categories for low back pain: stage 1 validation. JOSPT. 2003;33(3): 126–142. [DOI] [PubMed] [Google Scholar]
- 39.Von Korff M Studying the natural history of back pain. Spine. 1994;19(18 Suppl):2041S–2046S. [DOI] [PubMed] [Google Scholar]
- 40.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. [DOI] [PubMed] [Google Scholar]
- 41.Wernli K, Tan J-S, O’Sullivan P, Smith A, Campbell A, Kent P. Does movement change when low back pain changes? A systematic review. JOSPT. 2020(0):1–48. [DOI] [PubMed] [Google Scholar]
- 42.Weyrauch SA, Bohall SC, Sorensen CJ, Van Dillen LR. Association between rotation-related impairments and activity type in people with and without low back pain. PM&R. 2015;96(8):1506–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickham H ggplot2: Elegant Graphics for Data Analysis. In: Springer-Verlag; 2016. [Google Scholar]
- 44.Wickham H, Francois R, Henry L, Muller K. dplyr: A Grammar of Data Manipulation. R package version 0.8.0.1. In:2019. [Google Scholar]
- 45.Winter DA. Biomechanics and Motor Control of Human Movement. 2 ed. New York: John Wiley & Sons; 1990. [Google Scholar]


